Abstract
Both American Trypanosomiasis (Chagas disease) and Human African Trypanosomiasis (HAT) are diseases caused by single-celled flagellate protozoan parasites. While cardiac complications such as conduction problems and heart failure are very common in Chagas disease there is little known about the long-term effects of Human African Trypanosomiasis (HAT) on cardiac sequelae in Sub-Saharan Africa, where heart failure has become an increasing problem and growing burden.
In the context of clinical trials conducted between 2004 and 2005 in the Democratic Republic of the Congo (DRC), the prevalence of HAT related signs and symptoms and an ECG were evaluated prior to the initiation of treatment.
The object of this follow-up study in 2017 was to assess the prevalence of cardiac sequelae in the same 51 first stage and 18 second stage HAT patients 12–13 years after their treatment by conducting a clinical examination and an ECG. A control group matched by age (± 5 years), sex and whenever possible form the same village was enrolled.
There were no significant differences in the prevalence of cardiac symptoms and in ECG findings between patients and their controls at the time of the follow-up evaluation. Repolarization changes disappeared or improved in 24.7% of HAT patients and were even less frequent than in the control group. Peripheral low voltage was the only parameter that increased over time in HAT patients and in three patients, new conduction problems in the ECG (ventricular bigeminy, RBBB, and bifascicular block) could be found, although none of these findings was clinically significant. However, the appearance of these conduction problems might represent an early indication of a HAT related cardiomyopathy or ongoing subclinical infection. This hypothesis would be supported by the findings of an older study in which antibodies (IFAT) against trypanosomiasis in 27% of Cameroonian patients with dilated cardiomyopathy compared to 2% in normal controls had been observed.
Keywords: Human African Trypanosomiasis, Sequelae, Myocarditis, Heart failure, Follow up, Sleeping sickness, Conduction problem
Highlights
-
•
In American Trypanosomiasis (Chagas)cardiac complications are common, but no significant cardiac sequelae was found in African Trypanosomiasis.
-
•
12 years after treatment none of the patients developed cardiac failure and there was no sudden death due to arrhythmia.
-
•
The appearance of cardiac conduction problems in the actual ECG could be an early indication of a HAT related cardiomyopathy.
-
•
Overall, as compared to Chagas disease, HAT seems to have minimal influence on the heart even over a long follow-up time.
1. Introduction
American Trypanosomiasis (Chagas' disease) and Human African Trypanosomiasis (HAT) are diseases caused by single-celled flagellate protozoan parasites. Arthropods serve as vectors for their transmission. Trypanosoma cruzi causes Chagas disease, Trypanosoma brucei gambiense (T. b. gambiense) the West African variant of HAT and Trypanosoma brucei rhodesiense the East African variant of HAT. Cardiac problems are the predominant symptoms in chronic Chagas disease and neuropsychiatric problems in African Trypanosomiasis [1].
The course of Chagas disease consists of three distinct phases. A first acute phase presents with fever, myalgia, headache and the Romaña sign (unilateral painless periorbital oedema at the site of parasite entry). It is followed by the second phase, which is asymptomatic and referred to as the indeterminate phase. The third phase occurs many years later with between 10 and 30% of patients developing chronic Chagas cardiopathy leading to congestive heart failure, conduction problems (mainly RBBB and RBBB+LAFB), apical aneurysms, mural thrombus formation and strokes [1].
The T. b. gambiense form of HAT is currently responsible for 96.7% of all cases of HAT. Its course consists of two distinct phases: an early or hemolymphatic stage followed by a late or meningoencephalitic stage. The disease has a chronic progressive course, lasting from months to years and leading to death if untreated [2]. Intermittent fever attacks, a persistent headache, pruritus, joint pain, and lymphadenopathy characterize the early stage. Sleeping disorders, motor weakness, a tremor, neuro-psychiatric abnormalities, a dyskinesia and epileptiform attacks are predominant symptoms of the late stage [[3], [4], [5]].
Due to the efforts of the endemically affected countries and of the international community, the incidence of HAT has decreased, but a diminishing of the efforts to eliminate the disease would increase the risk of a reemergence [6].
In the T. b. gambiense form of HAT cardiac involvement demonstrated by ECG alterations, was found in 35% - 71% of patients [[7], [8], [9]]. Histological post-mortem studies on hearts of HAT patients showed a clear infiltration of trypanosomes, inflammation, fibrosis and generalized lesions of the conducting system in 63–75% of those patients [10,11].
The most frequent ECG alterations were repolarization changes, peripheral low voltage and QTc prolongation [7]. In a prospective cohort study, symptoms of congestive heart failure such as exertional dyspnea (class II according to the New York Heart Association (NYHA) Classification), palpitations and cough were or tended to be more frequent in HAT patients than in the control group (19% vs 1.7%, p = 0.002; 18% vs.5%, p = 0.28 and 14% vs. 1.7%, p = 0.14, respectively). NT-proBNP values were significantly higher in patients than in controls, but still relatively low and it is questionable, if the shortness of breath was caused by heart failure. Clinical symptoms and signs as well as NT-proBNP improved after treatment but still remained higher than in the control group even after 3 months of HAT treatment [12]. Sudden death was observed anecdotally but the risk of fatal arrhythmias due to HAT has never been studied [9].
The objective of the current study is to assess the prevalence of cardiac sequelae in the T. b. gambiense form of HAT by performing a clinical examination and an ECG in patients 12–13 years after their treatment.
2. Materials and methods
The study design, selection of participants and the procedure to carry out the interview have been previously reported [13], only an abbreviated description is given here.
2.1. Study design and setting
The current study examines the prevalence of HAT related long-term cardiac sequelae and compares the cardiac signs and symptoms as well as ECG findings of HAT patients prior to initiation of HAT treatment to the possible occurrence and severity of the same signs, symptoms, and ECG findings in HAT patients 12 to 13 years after completion of HAT treatment. In addition, those patients' post treatment results were compared to a control group's results. The control group selected matched the HAT patient set by sex and age (±5 years) as previously described.
From 19 July to 14 September 2017, follow-up ECGs were performed at the same time as the clinical evaluation and interview in villages of the Vanga Health Zone (Kwilu Province) of the Democratic Republic of the Congo (DRC). The participating patients live in villages in remote rural areas accessible only by rough roads.
2.2. Participants
Second stage HAT patients received their treatment in the frame of a clinical trial conducted 2004 [12,14] and first stage HAT patients during clinical trials performed between 2003 and 2005 in Vanga [15,16]. At that time, clinical parameters and ECG results were documented and evaluated in 29 second stage and 96 first stage HAT patients.
A list of patients who participated in the above-mentioned clinical trials had been compiled and their places of residence were traced and recorded. The criteria for recruitment of study participants have been already described [13]. In short, recruitment was based on the number of eligible patients living per village and its accessibility (distance to Vanga hospital, road access and proximity to other participating villages). Potential candidates were only included in the study after having given their informed consent. Potential candidates falling within any one of the following exclusion criteria were not enrolled: history of severe chronic diseases such as tuberculosis, HIV, cancer, liver cirrhosis, or diabetes mellitus. If a candidate had died or could not be reached, a third-party history relayed by a family member or friend was recorded. Those candidates were not included in the analysis but discussed separately.
A control person matched by age (±5 years) and sex to each participating patient was also enrolled in the study. Family members were preferred as candidates for matched controls, however, in the case of no such candidates being available, alternative candidates such as hospital personnel or patients with minor surgical problems (i.e., herniotomy) were enrolled.
2.3. Sample size
Results from the cited previous studies were used for sample size calculations. If there were relevant differences in the prevalence rates of signs and symptoms between studies, the calculations were performed based on the results of both studies. The required sample sizes for an 80% power for ECG alterations (α = 0.05) varied from 30 patients (repolarisation changes) to 72 patients (QTc prolongation). It was planned to include 60 HAT patients in total, all accessible second stage HAT patients, and an identical number of controls matched for age (±5 years) and sex.
2.4. Study procedure
A short history and physical examination was performed on each HAT patient and each participant of the control group. Patients and control group members were asked the same questions concerning HAT symptoms in the same manner as in the former original studies and trials. The case report form used to compare signs and symptoms was substantially the same as that used in previous trials. The data collected during the follow up were compared to that before treatment initiation.
All ECG tracings were interpreted by two readers using standardized criteria as described below:
2.5. ECG interpretation
The PQ, QRS and QT intervals were measured manually by the principal investigator in three consecutive cycles. Mean values were calculated. Measures of the intervals were performed in lead II when feasible, with lead V2 or I as an alternative. QTc was calculated using the Bazett formula (QTc = QT/SQR (RR). Since a QTc longer than 500 ms is recognized as a predictor of torsades de pointes, QTc shorter than 440 ms for women and 460 ms for men were considered as normal in this analysis [17]. For the overall ECG interpretation, the following criteria were used: Right atrial hypertrophy (RAH): p > 2.5 mV; left atrial hypertrophy (LAH): p > 120 ms; right ventricular hypertrophy (RVH): Sokolow index right: R V1 and S V5 > 1.05 mV; left ventricular hypertrophy (LVH): Cornell voltage: R aVL and S V3 > 2.8 in men and > 2.0 in women; peripheral low voltage: RI and RII and RIII <1.6 mV; PR depression: > 0.8 mm; ST elevation: > 0.1 mV without a preceding notch, concave, arising from a deep S wave; ST depression: > 0,1 mV; repolarization changes: limb leads discordant in at least one lead, precordial leads negative in either V3, V4, V5 or V6; and early repolarization type: concave ST elevation, notch at the J point, positive T waves [7].
Changes in ECG alterations between the ECG before and 13 years after treatment initiation were analysed in every individual patient.
2.6. Statistical analysis
Patient's characteristics, symptoms and ECG parameters were quantified as counts and percentages, if they were of categorical nature and as means, standard deviation medians and interquartile ranges if continuous. Proportions were compared using the Fisher's exact test and the central values using the Mann-Whitney U test. The level of significance was set to alpha <0.05. SAS v9.4 (SAS Institute, Cary, NC, USA) was the software used for the statistical analysis.
2.7. Ethics statement
As stated before [13], the study protocol was approved by Ethikkommission Nordwest- und Zentralschweiz EKNZ (2017–00471; 12.4.2017) and Comité d'Éthique de l'Université Protestante au Congo (UPC) (CEUPC 0044; 13.6.2017). All participants provided their informed written consent prior to enrolment.
3. Results
96 first stage and 29 second stage HAT patients were recruited into several studies and trials between 2003 and 2005 [7,12,[14], [15], [16]]. The recruitment of participants for the present follow-up study took place during three visits to the villages of Kikongo Tango-Milundu, Nsalu and Mayoko-Nkai.
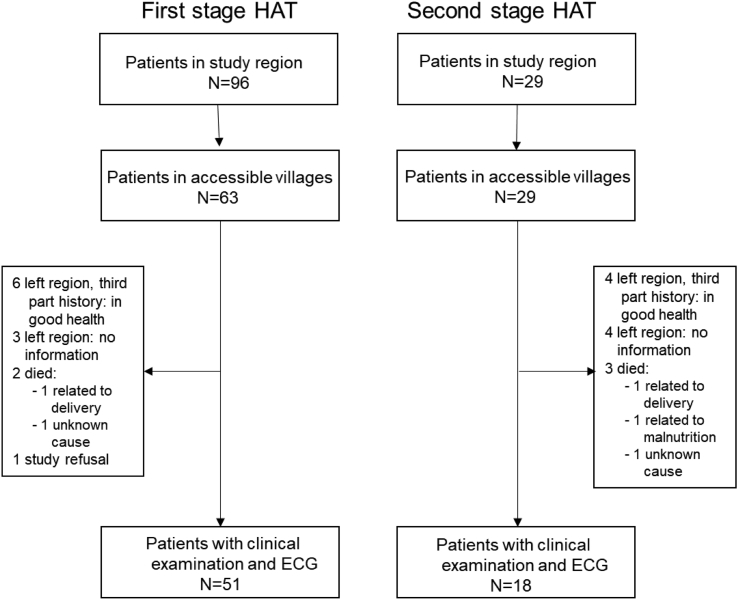
The flowchart of the study, including the number of participants, is shown in Fig. 1.
Fig. 1.
Study flow chart. Participants recruitment was previously described [13].
A clinical history and examination could be gathered and performed respectively from and on 75% (69 out of 92) of the eligible patients. 51 had previously been first stage and 18 second stage HAT patients. Ten out of 92 (11%) were not accessible but were in good health and did not have any obvious heart problem according to oral communication with relatives or friends. Five out of 92 (5%) had died: two of them in the peripartum period of causes unrelated to HAT; one due to malnutrition; one due to an unidentified disease with unspecified swelling and without fever; and the last due to unknown causes one month after HAT treatment. Although those deaths were probably not related to HAT or cardiac problems, such diagnoses cannot be ruled out with certainty. There was no history of a patient suffering from clinically diagnosed heart failure or irregular pulse and there was no history of a confirmed relapse in any of the patients followed up in this study. No information was available for eight out of 92 (9%) of the former patients.
Patients with second stage HAT were older (52.0 ± 15.8 years at the enrolment of the follow up study) than those with first stage (43.9 ± 12.5), p = 0.0700. The proportion of male participants was higher among second stage HAT patients (50.0% vs. 27.5%, p = 0.0810). None of the patients did take any medication at enrolment (especially no antihypertensive or cardiac treatment or other drugs influencing QTc). There were no comorbidities. Cardiac symptoms and signs such as dyspnea, chest pain or peripheral oedema had not been assessed in all previous trials, but only in the trial observing second stage HAT patients: Exertional dyspnea has been found in 5 out of 18 (28%) patients before treatment and in none 12–13 years later. Chest discomfort has been observed in 2 out of 18 (11%) before and in 1 out of 18 (5.5%) 12–13 years after treatment and peripheral oedema diminished from 3 out of 18 (17%) to none. There was no significant difference between the prevalence of cardiac symptoms between HAT stages, nor between patients and their controls at the time of the follow up (Table 1).
Table 1.
Cardiac symptoms and signs in HAT patients 13 years after treatment according to stage, compared to controls.
| Parameter/characteristic at 12–13 years follow-up | Stage I (n = 51) | Stage II (n = 18) | pa | Controls (n = 69) | pa, b |
|---|---|---|---|---|---|
| Demographic | |||||
| Gender: n (%) | |||||
| Female | 37 (72.5) | 9 (50.0) | 0.0917 | 46 (66.7) | 0.9999 |
| Male | 14 (27.5) | 9 (50.0) | 23 (33.3) | ||
| Age (years) (Mean ± SD) | 43.9 ± 12.5 | 52.0 ± 15.8 | 0.0744 | 48.9 ± 13.5 | 0.6685 |
| Heart rate and blood pressure | |||||
| Heart rate (beats/min) (Mean ± SD) | 79.9 ± 14.6 | 75.2 ± 18.4 | 0.2479 | 87.3 ± 88.4 | 0.5782 |
| Blood pressure, systolic [mmHg]: (Mean ± SD) | 112.2 ± 15.1 | 119.1 ± 21.5 | 0.2929 | 116.3 ± 21.2 | 0.6679 |
| Blood pressure, diastolic [mmHg]: (Mean ± SD) | 70.9 ± 10.2 | 70.8 ± 10.0 | 0.9471 | 71.1 ± 10.1 | 0.7853 |
| Symptoms and signs: n (%) | |||||
| Dyspnea | |||||
| Absent | 49 (96.1) | 18 (100.0) | 0.9999 | 67 (97.1) | 0.9999 |
| During physical activity | 2 (3.9) | 0 (0.0) | 2 (2.9) | ||
| Chest pain | |||||
| Absent | 50 (98.0) | 17 (94.4) | 0.4565 | 69 (100.0) | 0.4964 |
| During physical activity | 1 (2.0) | 1 (5.6) | 0 (0.0) | ||
| Abnormal cardiac rhythm | |||||
| Absent | 50 (98.0) | 17 (100.0) | 0.9999 | 68 (100.0) | 0.9999 |
| Irregular pulse rate | 1 (2.0) | 0 (0.0) | 0 (0.0) | ||
| Edema inferior limbs | |||||
| Absent | 51 (100.0) | 18 (100.0) | – | 68 (100.0) | – |
Mann-Whitney U test for continuous parameters and Fisher's exact test for categorical parameters.
For the comparison of 69 patients and 69 controls.
The mean QTc (Bazett corrected) was not prolonged in either of the HAT stages and was comparable to that of the control group. Values above the threshold of >440 msec for men and of >460 for women, associated with an elevated risk for arrhythmia, were observed in 9 out of 69 (13.0%) in HAT patients 12–13 years after treatment and in 13 out of 69 (18.8%) in the control group, p = 0.35. However, there was no QTc longer than 500 msec. Two patients developed bundle brunch blocks: one a right bundle brunch block (RBBB) and the other a bifascicular block (combination of RBBB and left anterior fascicular block = LAFB). In one patient, a ventricular bigeminy was observed. There were no significant differences in all ECG findings between HAT patients 12–13 years after treatment and their controls.
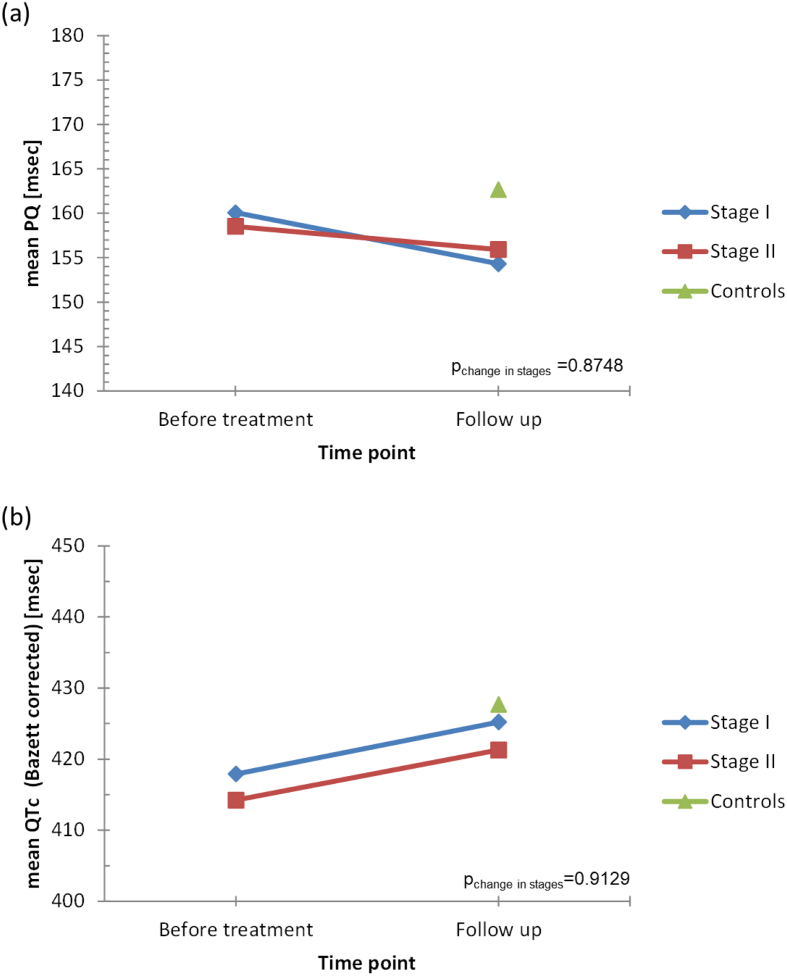
Changes in ECG parameters from baseline (i.e. before treatment) to the 12–13 year follow up are presented in Fig. 2 and in Table 3 for all patients and stratified by HAT stage. Repolarization changes disappeared or improved in 24.7% of HAT patients and were even lower or comparable to those in the control group. In only one patient was an appearance/deterioration of repolarization observed. 15.9% of HAT patients developed peripheral low voltage and the prevalence of low voltage was higher in the treated HAT group than in the control group (47–50% versus 28%, p = 0.0265) (Table 2).
Fig. 2.
PQ time (a) and corrected QTc time (b) in HAT patients before and 12–13 years after treatment and in a controls.
Table 3.
ECG changes in HAT patients between ECG before treatment to ECG 12–13 years after treatment by disease stage.
| ECG change: from before treatment to 10-13 yrs. follow-up | Category or level | All Patients (n = 69) |
Stage I (n = 51) |
(Stage II) n = 18) |
pa |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Repolarization Changes | Unchanged | 51 (73.9) | 36 (70.6) | 15 (83.3) | 0.095 |
| Aggravation | 1 (1.4) | 0 (0.0) | 1 (5.6) | ||
| No more | 14 (20.3) | 13 (25.5) | 1 (5.6) | ||
| Improvement | 3 (4.4) | 2 (3.9) | 1 (5.6) | ||
| Low Voltage | Unchanged | 57 (82.6) | 41 (80.4) | 16 (88.9) | 0.796 |
| New | 11 (15.9) | 9 (17.6) | 2 (11.1) | ||
| No more | 1 (1.5) | 1 (2.0) | 0 (0.0) | ||
| AV BLOCK I | Unchanged | 67 (97.1) | 50 (98.0) | 17 (94.4) | 0.461 |
| New | 1 (1.5) | 0 (0.0) | 1 (5.6) | ||
| No more | 1 (1.5) | 1 (2.0) | 0 (0.0) | ||
| AV BLOCK II | Unchanged | 69 (100.0) | 51 (100.0) | 18 (100.0) | – |
| Ventricular bigeminy | Unchanged | 68(98.6) | 51 (100.0) | 17 (94.4) | 0.261 |
| New | 1 (1.4) | 0 (0.0) | 1 (5.6) | ||
| RBBB | Unchanged | 68 (98.6) | 50 (98.0) | 18 (100.0) | 0.999 |
| New | 1 (1.5) | 1 (2.0) | 0 (0.0) | ||
| LBBB | Unchanged | 69 (100.0) | 51 (100.0) | 18 (100.0) | – |
| PR Depression | Unchanged | 67 (97.1) | 50 (98.0) | 17 (94.4) | 0.457 |
| No more | 2 (2.9) | 1 (2.0) | 1 (5.6) | ||
| Ventricular Hypertrophy | Unchanged | 66 (95.7) | 50 (98.0) | 16 (88.9) | 0.171 |
| New | 2 (2.9) | 1 (1.96) | 1 (5.6) | ||
| No more | 1 (1.5) | 0 (0.00) | 1 (5.6) | ||
| Bifascicular block (BBBB+LAHB) | Unchanged | 1 (50.0) | – | 1 (50.0) | |
| New | 1 (50.0) | – | 1 (50.0) | ||
| Left anterior fascicular(LAFB) | Unchanged | 2 (100.0) | – | 2 (100.0) |
Fisher's exact test.
Table 2.
ECG findings in HAT patients 12–13 years after treatment compared to a control group.
| Parameter/characteristic at 10–13 years follow-up | Stage I (n = 51) | Stage II (n = 18) | pa | Controls (n = 69) | pa, b |
|---|---|---|---|---|---|
| ECG intervals [msec] (Mean ± SD) | |||||
| PQ | 154.3 ± 22.1 | 155.9 ± 32.0 | 0.8153 | 162.6 ± 31.5 | 0.1331 |
| QRS | 82.7 ± 10.6 | 84.1 ± 15.0 | 0.9407 | 85.0 ± 13.3 | 0.3900 |
| Mean QTc (Bazett corrected) | 425.2 ± 29.1 | 421.2 ± 34.2 | 0.5753 | 427.6 ± 27.3 | 0.4930 |
| ECG findings: n (%) | |||||
| Rhythm | |||||
| Sinus | 51 (100.0) | 18 (100.0) | 0.9999 | 69 (100.0) | 0.999 |
| Atrial fibrillation | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Atrial flutter | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Premature atrial capture beats | |||||
| None | 48 (94.1) | 17 (94.4) | 0.9999 | 67 (97.1) | 0.6805 |
| Present | 3 (5.9) | 1 (5.6) | 2 (2.9) | ||
| Premature ventricular capture beats | |||||
| None | 47 (92.2) | 16 (88.9) | 0.3414 | 63 (91.3) | 0.9900 |
| Present | 4 (7.8) | 1 (5.6) | 6 (8.7) | ||
| Ventricular bigeminy | 0 (0.0) | 1 (5.6) | 0 (0.0) | ||
| Couplet/triplet | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Salves | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| AV block | |||||
| None | 51 (100.0) | 17 (94.4) | 0.2609 | 66 (95.7) | 0.6195 |
| AV block 1 | 0 (0.0) | 1 (5.6) | 3 (4.3) | ||
| AV block 2 | 0 (0.0) | (0.0) | 0 (0.0) | ||
| AV block 3 | 0 (0.0) | (0.0) | 0 (0.0) | ||
| Atrial hypertrophy | |||||
| None | 48 (94.1) | 17 (94.4) | 0.9999 | 68 (98.5) | 0.3658 |
| Right | 3 (5.9) | 1 (5.6) | 1 (1.5) | ||
| Left | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| RVH: Sokolow right | |||||
| Negative | 51 (100.0) | 16 (88.8) | 0.0652 | 66 (95.6) | 0.8332 |
| Positive | 0 (0.0) | 2 (11.1) | 3 (4.4) | ||
| LVH (Sokolow) | |||||
| Negative | 51 (100.00 | 18 (100.0) | 0.9999 | 67 (97.1) | 0.9999 |
| Positive | 0 (0.0) | 0 (0.0) | 2 (2.9) | ||
| Pathological q | |||||
| None | 51 (100.00) | 18 (100.0) | 68 (100.0) | – | |
| Ventricular block | |||||
| None | 49 (96.0) | 15 (83.4) | 0.1072 | 67 (97.0) | 0.4409 |
| Present: either RBBB or LBBB or LAFB or RBBB and LAFB | 2 (4.0) | 3 (16.6) | 2 (3.0) | ||
| RBBB | 1 (2.0) | 0 (0.0) | 1 (1.5) | ||
| LBBB | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| LAFB | 1 (2.0) | 2 (11.1) | 1 (1.5) | ||
| RBBB and LAFB | 0 (0.0) | 1 (5.6) | 0 (0.0) | ||
| Repolarization changes | |||||
| No | 44 (86.3) | 17 (94.4) | 0.6705 | 59 (85.5) | 0.8012 |
| Yes | 7 (13.7) | 1 (5.6) | 10 (14.5) | ||
| Peripheral low voltage | |||||
| Normal | 27 (52.9) | 9 (50.0) | 0.8487 | 50 (72.5) | 0.0265 |
| Low voltage | 24 (47.1) | 9 (50.0) | 19 (27.6) | ||
SD = standard deviation.
Mann-Whitney U test for continuous parameters and Fisher's exact test for categorical parameters.
For the comparison of 69 patients and 69 controls.
4. Discussion
Heart failure is an increasing problem and associated with a growing disease burden in Sub-Saharan Africa. Hypertensive cardiac disease, dilatative cardiomyopathy mostly of unknown etiology, peripartal cardiomyopathy, rheumatic and valvular heart disease are the main causes of the heart failure but in many patients the etiology remains unknown [18,19]. Parasitic infections including trypanosomiasis, leishmaniasis, schistosomiasis, toxocariasis, trichinellossis, cysticercosis and filariasis may cause cardiac pathology leading to heart failure [[20], [21], [22]].The presence of antibodies (IFAT) against trypanosomiasis in 27% of Cameroonian patients with dilated cardiomyopathy compared to 2% in normal controls may indicate that, untreated, subclinical infections of HAT may induce a chronic cardiomyopathy [23].
This is the first study, to our knowledge, to evaluate the long-term sequelae of HAT on the heart. In Gambiense Human African Trypanosomiasis, the cardiac effects include exertional dyspnea and occasionally conduction problems [7,8,12], but are usually mild and improve after treatment. The presented results show that heart failure was not observed in any patient12-13 years after treatment, but the cause of the death of two patients could not be determined by a third person history. On the contrary, dyspnea, chest pain and oedema disappeared in most patients and cardiac symptoms and signs were comparable in treated HAT patients and the control group.
Conduction problems are frequently found in Chagas patients. In HAT, histopathologic examinations showed infiltration of the conduction system [11,24]. Sudden death was observed [9]. Regarding the description of inflammation of the conduction system in histological examinations, the low number of described conduction problems is of interest: AV block I in 3.7–14%; AV block II in 1–2.5%; and AV block III in only one case report [8,9,25]. The QTc time is prolonged in HAT patients reaching a QTc prolongation with the potential risk for ventricular arrhythmia and sudden cardiac death (threshold of >440 msec for men and of >460 for women) in up to 12.5% [7,12,26]. An ECG analysis of 462 HAT patients showed that a QTc prolongation associated with an elevated risk for arrhythmia was observed in 11.1% of first stage and in 12.5% of second stage HAT patients. In one patient, the QTc time was longer than 500 msec [7]. In the present study, 12–13 years after treatment sudden death or irregular pulse rate (except in one patient) were not observed. The mean QTc time was normal and there were even fewer individuals with QTc prolongation in the patient group than in the control group. Only three HAT patients developed conduction problems in the ECG: one a RBBB; one a bifascicular block (RBBB and LAFB); and one a ventricular bigeminy. However, these blocks were not significantly more frequent than in the control group. Nevertheless, since the observation time was only 12–13 years and relevant conduction problems may occur in Chagas patients more than 30 years after the initial infection, the eventual development of conduction problems cannot be ruled out conclusively.
Repolarization changes disappeared or improved in 24.7% of HAT patients and were even less frequent than in the control group. Peripheral low voltage was the only parameter, which increased over time in HAT patients. In acute HAT myopericarditis, low voltage could be interpreted as pericardial involvement with pericardial effusion. However, 12–13 years after treatment and in the absence of additional signs of pericardial effusion low voltage has to be considered as an unspecific ECG alteration and should not be regarded as a relevant finding.
Most Chagas patients did not have an antiparasitic treatment during the acute phase. The persistence of trypanosomes is discussed as a possible cause of the development of Chagas cardiopathy. In contrast, all HAT patients had antiparasitic treatment, but the persistence of parasites in the skin [28], adipose tissue [29] or other body compartments [13] cannot be ruled out, even after initial treatment. Of note, a migrant leaving the endemic region 29 years ago developed a clinical HAT under immunosuppression [30].
There is limited knowledge on cardiac involvement in T. b. rhodesiense HAT patients. Peri-myocarditis may play an important factor in the clinical course and fatal outcomes [27,28]. Cardiac signs such as oedemas and hepatomegaly were often observed, but in the absence of a complete physical examination (including jugular veins), echocardiography or laboratory testing (i.e. brain natriuretic peptide) they could not be attributed to heart failure by certainty [29]. There are no studies on cardiopathy after successful treatment of T. b. rhodesiense HAT.
4.1. Limitations
The study was conducted only 12–13 years after initial treatment. Since in Chagas cardiopathy symptoms, signs and ECG alterations appear even decades after the initial infection, we cannot rule out that a cardiopathy could evolve in the following decades. The study was conducted in hut in villages without examination tables allowing a correct testing of the jugular veins or hepato-jugular reflux and no possibility to do laboratory examinations nor cardio sonography.
4.2. Conclusions
The present study shows that 12–13 years after treatment for HAT there are no clinical signs of heart failure, no significant arrhythmia nor QTc prolongation. However, even though unlikely, the appearance of conduction problems such as ventricular bigeminy, RBBB and RBBB+LAFB could be an indication of a first sign of a HAT related cardiopathy. The persistence of headache and neurologic symptoms many years after successful treatment and in untreated HAT patients [13,30], and the observation of reactivation of HAT (though without cardiac involvement) after immunosuppression in a patient even 3 decades after infection [31] may be an indication that the trypanosomes might cause not only in Chagas disease but also in HAT clinical signs and symptoms decades after the initial infection.
Overall, as compared to Chagas disease, HAT seems to have minimal influence on the heart even over a long follow-up time.
Funding
This work was supported by the “Stiftung für kardiovaskuläre Forschung Basel”, Spitalstrasse 2, 4056 Basel, Switzerland. This manuscript is original, has neither been published nor is it under consideration for publication elsewhere. We have no conflicts of interest to disclose.
Author’s statement
Sleeping hearts: 12 years after – A follow up study on cardiac findings due to sleeping sickness
We would like to submit the above-mentioned manuscript as an article in your journal.
Both American Trypanosomiasis (Chagas disease) and Human African Trypanosomiasis (HAT) are diseases caused by trypanosoma. While long-term cardiac complications such as conduction problems and heart failure are very common in Chagas disease there is little known about long-term cardiac sequelae of Human African Trypanosomiasis (HAT).
In the context of clinical trials conducted between 2004 and 2005 in the Democratic Republic of the Congo (DRC), the prevalence of HAT related signs and symptoms, and an ECG, were evaluated prior to the initiation of treatment. The object of this follow-up study in 2017 was to assess the prevalence of cardiac sequelae in the same 51 first stage and 18 second stage HAT patients 12–13 years after their treatment by conducting a clinical examination and an ECG. A control group was enrolled matched in age (by ±5 years), sex and whenever possible form the same village was enrolled.
In our findings at the time of the follow-up evaluation there were neither significant differences in the prevalence of cardiac symptoms nor in ECG findings between patients and their controls.
However there were some patients with a novel appearance of cardiac conduction problems such as ventricular bigeminy, RBBB and RBBB+LAFB in the actual ECG which could suggest an early indication of a HAT related cardiomyopathy or subclinical infection.
Overall, as compared to Chagas disease, HAT seems to have minimal influence on the heart even over a long follow-up period.
All the authors have read and agreed to the submitted version of the paper.
Declaration of Competing Interest
None.
References
- 1.Blum J.A., Zellweger M.J., Burri C., Hatz C. Cardiac involvement in African and American trypanosomiasis. Lancet Infect. Dis. 2008;8(10):631–641. doi: 10.1016/S1473-3099(08)70230-5. [DOI] [PubMed] [Google Scholar]
- 2.Blum J., Schmid C., Burri C. Clinical aspects of 2541 patients with second stage human African trypanosomiasis. Acta Trop. 2006;97(1):55–64. doi: 10.1016/j.actatropica.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Brun R., Blum J. Human African trypanosomiasis. Infect. Dis. Clin. N. Am. 2012;26(2):261–273. doi: 10.1016/j.idc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Buscher P., Cecchi G., Jamonneau V., Priotto G. Human African trypanosomiasis. Lancet. 2017;390(10110):2397–2409. doi: 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- 5.Kazumba L.M., Kaka J.T., Ngoyi D.M., Tshala-Katumbay D. Mortality trends and risk factors in advanced stage-2 human African Trypanosomiasis: a critical appraisal of 23 years of experience in the Democratic Republic of Congo. PLoS Negl. Trop. Dis. 2018;12(6) doi: 10.1371/journal.pntd.0006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mudji J., Benhamou J., Mwamba-Miaka E., Burri C., Blum J. The flipside of eradicating a disease; human African Trypanosomiasis in a woman in rural Democratic Republic of Congo: a case report. Trop. Med. Infect. Dis. 2019;4(4) doi: 10.3390/tropicalmed4040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blum J.A., Schmid C., Burri C., Hatz C., Olson C., Fungula B. Cardiac alterations in human African Trypanosomiasis (T.b. gambiense) with respect to the disease stage and Antiparasitic treatment. PLoS Negl. Trop. Dis. 2009;3(2):e383. doi: 10.1371/journal.pntd.0000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertrand E., Serie F., Rive J., Compaore P., Sentilhes L., Baudin L. Current aspects of the cardiac symptoms in African human trypanosomiasis due to Trypanosoma gambiense (apropos of 194 cases) Acta Cardiol. 1974;29(5):363–381. [PubMed] [Google Scholar]
- 9.Fouchet M., Gateff C. Development of cardiovascular involvement in African trypanosomiasis due to Trypanosoma gambiense. Med. Trop (Mars) 1968;28(5):583–590. [PubMed] [Google Scholar]
- 10.Poltera A.A., Owor R., Cox J.N. Pathological aspects of human African trypanosomiasis (HAT) in Uganda. A post-mortem survey of fourteen cases. Virchows Arch A Pathol. Anat. Histol. 1977;373(3):249–265. doi: 10.1007/BF00432240. [DOI] [PubMed] [Google Scholar]
- 11.Adams J.H., Haller L., Boa F.Y., Doua F., Dago A., Konian K. Human African trypanosomiasis (T.b. gambiense): a study of 16 fatal cases of sleeping sickness with some observations on acute reactive arsenical encephalopathy. Neuropathol. Appl. Neurobiol. 1986;12(1):81–94. doi: 10.1111/j.1365-2990.1986.tb00682.x. [DOI] [PubMed] [Google Scholar]
- 12.Blum J.A., Burri C., Hatz C., Kazumba L., Mangoni P., Zellweger M.J. Sleeping hearts: the role of the heart in sleeping sickness (human African trypanosomiasis) Tropical Med. Int. Health. 2007;12(12):1422–1432. doi: 10.1111/j.1365-3156.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- 13.Mudji J., Blum A., Grize L., Wampfler R., Ruf M.T., Cnops L. Gambiense human African Trypanosomiasis Sequelae after treatment: a follow-up study 12 years after treatment. Trop. Med. Infect. Dis. 2020;5(1) doi: 10.3390/tropicalmed5010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blum J.A., Schmid C., Hatz C., Kazumba L., Mangoni P., Rutishauser J. Sleeping glands? - the role of endocrine disorders in sleeping sickness (T.b. gambiense human African Trypanosomiasis) Acta Trop. 2007;104(1):16–24. doi: 10.1016/j.actatropica.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Burri C., Yeramian P.D., Allen J.L., Merolle A., Serge K.K., Mpanya A. Efficacy, safety, and dose of Pafuramidine, a new Oral drug for treatment of first stage sleeping sickness, in a phase 2a clinical study and phase 2b randomized clinical studies. PLoS Negl. Trop. Dis. 2016;10(2) doi: 10.1371/journal.pntd.0004362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pohlig G., Bernhard S.C., Blum J., Burri C., Mpanya A., Lubaki J.P. Efficacy and safety of Pafuramidine versus Pentamidine maleate for treatment of first stage sleeping sickness in a randomized, comparator-controlled, international phase 3 clinical trial. PLoS Negl. Trop. Dis. 2016;10(2) doi: 10.1371/journal.pntd.0004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moss A.J. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am. J. Cardiol. 1993;72(6):23B–25B. doi: 10.1016/0002-9149(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 18.Glezeva N., Gallagher J., Ledwidge M., O’Donoghue J., McDonald K., Chipolombwe J. Heart failure in sub-Saharan Africa: review of the aetiology of heart failure and the role of point-of-care biomarker diagnostics. Tropical Med. Int. Health. 2015;20(5):581–588. doi: 10.1111/tmi.12461. [DOI] [PubMed] [Google Scholar]
- 19.Agbor V.N., Essouma M., Ntusi N.A.B., Nyaga U.F., Bigna J.J., Noubiap J.J. Heart failure in sub-Saharan Africa: a contemporaneous systematic review and meta-analysis. Int. J. Cardiol. 2018;257:207–215. doi: 10.1016/j.ijcard.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 20.Nunes M.C., Guimaraes Junior M.H., Diamantino A.C., Gelape C.L., Ferrari T.C. Cardiac manifestations of parasitic diseases. Heart. 2017;103(9):651–658. doi: 10.1136/heartjnl-2016-309870. [DOI] [PubMed] [Google Scholar]
- 21.Kuenzli E., Neumayr A., Chaney M., Blum J. Toxocariasis-associated cardiac diseases--a systematic review of the literature. Acta Trop. 2016;154:107–120. doi: 10.1016/j.actatropica.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Hidron A., Vogenthaler N., Santos-Preciado J.I., Rodriguez-Morales A.J., Franco-Paredes C., Rassi A., Jr. Cardiac involvement with parasitic infections. Clin. Microbiol. Rev. 2010;23(2):324–349. doi: 10.1128/CMR.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsala M.P., Blackett K., Mbonifor C.L., Leke R., Etoundi J. Functional and immunologic involvement in human African trypanosomiasis caused by Trypanosoma gambiense. Bull. Soc. Pathol. Exot. Filiales. 1988;81(3 Pt 2):490–501. [PubMed] [Google Scholar]
- 24.Poltera A.A., Cox J.N., Owor R. Pancarditis affecting the conducting system and all valves in human African trypanosomiasis. Br. Heart J. 1976;38(8):827–837. doi: 10.1136/hrt.38.8.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damian M.S., Dorndorf W., Burkardt H., Singer I., Leinweber B., Schachenmayr W. Polyneuritis and myositis in Trypanosoma gambiense infection. Dtsch. Med. Wochenschr. 1994;119(49):1690–1693. doi: 10.1055/s-2008-1058888. [DOI] [PubMed] [Google Scholar]
- 26.Collomb H., Bartoli D. The heart in human African trypanosomiasis caused by Trypanosoma gambiense. Bull. Soc. Pathol. Exot. Filiales. 1967;60(2):142–156. [PubMed] [Google Scholar]
- 27.De Raadt P., Koten J.W. Myocarditis in Rhodesiense trypanosomiasis. East Afr. Med. J. 1968;45(3):128–132. [PubMed] [Google Scholar]
- 28.Koten J.W., De Raadt P. Myocarditis in Trypanosoma rhodesiense infections. Trans. R. Soc. Trop. Med. Hyg. 1969;63(4):485–489. doi: 10.1016/0035-9203(69)90036-4. [DOI] [PubMed] [Google Scholar]
- 29.Kuepfer I., Hhary E.P., Allan M., Edielu A., Burri C., Blum J.A. Clinical presentation of T.b. rhodesiense sleeping sickness in second stage patients from Tanzania and Uganda. PLoS Negl. Trop. Dis. 2011;5(3):e968. doi: 10.1371/journal.pntd.0000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamonneau V., Ravel S., Garcia A., Koffi M., Truc P., Laveissiere C. Characterization of Trypanosoma brucei s.l. infecting asymptomatic sleeping-sickness patients in cote d’Ivoire: a new genetic group? Ann. Trop. Med. Parasitol. 2004;98(4):329–337. doi: 10.1179/000349804225003406. [DOI] [PubMed] [Google Scholar]
- 31.Sudarshi D., Lawrence S., Pickrell W.O., Eligar V., Walters R., Quaderi S. Human African trypanosomiasis presenting at least 29 years after infection--what can this teach us about the pathogenesis and control of this neglected tropical disease? PLoS Negl. Trop. Dis. 2014;8(12):e3349. doi: 10.1371/journal.pntd.0003349. [DOI] [PMC free article] [PubMed] [Google Scholar]