Abstract
H9N2 avian influenza viruses (AIV) continue to circulate in vaccinated chicken flocks in China, which prompted us to investigate the differential immune protection factors induced by H9N2 AIV infection and immunization for analyzing the reason of protection deficiency of H9N2 AIV inactivated vaccine. In this study, we firstly explored virus-induced optimal immune responses in chicken after H9N2 AIV infection. And, we found that H9N2 hemagglutination inhibition (HI) antibody level, antiviral interferon-stimulated genes including 2′,5’-oligoadenylate synthetase-like and myxovirus resistance 1, CD8+ T cell response in peripheral blood lymphocytes (PBL) accompanied by the cytotoxicity-associated genes, including poly (ADP-ribose) polymerase and IFN-r play important roles in defending against H9N2 infection. Besides, we observed that vaccine immunization triggered the similar H9N2 HI antibody level as viral infection, the increase of CD4+ T cell percentage instead of CD8+ T cell percentage in PBL. Moreover, we further made a comparative analysis of immune-related gene expression profile in PBL and lung after H9N2 AIV infection and immunization, respectively. The results showed that vaccine immunization contributed to the up-regulation of Th2 cytokine. But the deficiency of cytotoxicity-associated genes induced by H9N2 AIV inactivated vaccine may be the potential key reason of protection deficiency. These findings provide evidence and direction for developing effective H9N2 AIV vaccines.
Key words: chicken, H9N2 AIV infection, vaccine immunization, T cell, immune-related gene
Introduction
H9N2 avian influenza viruses (AIV) are among the most commonly occurring in domestic poultry populations even under the long-term vaccination programs, resulting in great economic losses due to reduced egg production or high mortality associated with co-infection with other pathogens (Sun and Liu, 2015; Xu et al., 2018; Li et al., 2019). More seriously, H9N2 viruses could also serve as the gene donor for H7N9 and H10N8 viruses infecting humans (Sun and Liu, 2015; Wei et al., 2016). Hence, it warrants studies on the host immune key factors against H9N2 AIV infection for developing efficient vaccine.
In our previous study, we isolated and evaluated a selected candidate H9N2 AIV vaccine HN strain (A/Chicken/Hunan/HN/2015). And, we found that the inactivated oil-emulsion vaccine could not provide 100% protection even challenged with the same strain (Xu et al., 2018), which pushed us to investigate the differential immune protection factors induced by H9N2 AIV infection and immunization. Historically, researchers usually explored the expression of innate immune–related gene or inflammatory cytokine via extracting RNA of H9N2 infected chicken macrophage-like cell line (HD11), chicken embryo fibroblast cell line (DF1), or organ tissue (Nang et al., 2011; Liu et al., 2015; Qi et al., 2017). Few studies systematically identified the host immune response induced by AIV infection in important primary tissue cells such as chicken peripheral blood lymphocytes (PBL) and single cell suspensions of lung.
In this study, we firstly identified the host immune response in PBL and lung cell suspensions of H9N2 AIV infected or immunized SPF chickens, and then further compared their differential key immune protection factors for analyzing the reason of protection deficiency of H9N2 AIV inactivated vaccine.
Materials and methods
Ethics Statement
All animal research projects were sanctioned by the South China Agriculture University Institutional Animal Care and Use Committee (Identification code: 2019180, 27 November 2019). All animal procedures were performed according to the regulations and guidelines established by this committee and international standards for animal welfare.
Virus and Experimental Animal Infection
Low pathogenic avian influenza H9N2 subtype HN strain (A/Chicken/Hunan/HN/2015) is isolated and identified in our lab, which is the current epidemic virus belonging to h9.4.2.5 lineage (Xu et al., 2018). For the animal infection experiment, a total of twenty-eight 2-wk-old, specific pathogen–free (SPF) White Leghorn chickens (Guangdong Da Hua Nong Animal Health Products Co., Ltd., Guangdong, China) were randomly assigned to 2 groups, namely, a H9N2-infected group and control group, each with 14 chickens per group which were reared separately in negative-pressure isolators. After feeding for 2 wk, 4-wk-old SPF chickens were inoculated intranasally at a dose of 0.2 mL (107 EID50/0.2 mL) of strain HN. The control group was inoculated with 0.2 mL PBS alone. At 3, 5, and 7 d postinfection (DPI), 3 chickens in each group were humanely sacrificed. And the liver, kidney, spleen, duodenum, jejunum, ileum, and lung were aseptically collected at each time point, respectively. Oropharyngeal and cloacal swabs from 4 chickens in each group were collected from 1 DPI to 12 DPI. Sera from 4 chickens in each group were collected from 3 DPI to 35 DPI. Collected organs and swabs were titered by EID50 assay (Reed and Muench, 1938).
Vaccine Preparation and Animal Immunization
The inactivated oil-emulsion vaccine was prepared using H9N2 subtype HN strain (A/Chicken/Hunan/HN/2015) according to the previously described (Stone et al., 1978). The titer of the seed virus was 108.3 EID50/0.1 mL. And the seed virus was inactivated with 0.2% volume ratio of formaldehyde at 37°C for 48 h and then injected into 10 SPF eggs. The embryo allantoic fluid was collected to check residual infectious virus after 2 generations of blind transmission. And then, the determined inactivated vaccine was fully emulsified with white oil and twain 80 (inactivated vaccine: white oil: twain 80 = 1:2:0.035). 12 mL H9N2 AIV inactivated vaccine was prepared. And the antigen content of vaccine preparation was about 2 × 108.3 EID50 per 600 μL. For the animal immunization experiment, a total of twenty 4-wk-old, SPF White Leghorn chickens (Guangdong Da Hua Nong Animal Health Products Co., Ltd., Guangdong, China) were randomly assigned to 2 groups, namely, a H9N2-immunization group and control group each with 10 chickens per group which were reared separately in negative-pressure isolators. After feeding for 1 wk, 5-wk-old SPF chickens were injected subcutaneously at the backside of the neck of 600 μL prepared vaccine. The control group was inoculated with 600 μL PBS at the same way. At 13 and 28 d postimmunization (DPIm), 2 chickens in each group were humanely sacrificed. And, the lung was aseptically collected at each time point, respectively. Sera from 5 chickens in each group were collected from 3 DPIm to 35 DPIm.
Hemagglutination-Inhibition Assay
The hemagglutination inhibition (HI) antibody titers of the sera were determined using 1% chicken red blood cells as previously described (Edwards, 2006).
Lymphocyte Isolation
Five days before infection, each time point after infection, 5 d before immunization, and each time point after immunization, heparinized blood samples from individual chickens were collected to isolate peripheral blood lymphocytes (PBL) as previously described (Dai et al., 2020). Single cell suspensions of lung were obtained in RPMI 1640 medium (Gibco, Carlsbad, CA) according to the manufacturer's instructions of tissue mononuclear cell kit (Haoyang, Tianjin, China). Briefly, Dice lung lobes into slurry in petri dish using curved scissors while immersed in digestion media containing 0.002% DNase I (Sigma-Aldrich, St. Louis, MO) and 0.1% Collagenase Type 4 (Sigma-Aldrich) for 20 min at 37°C. Filter digested tissue through 190 μM metal mesh and then isolated lymphocytes with the tissue separation medium in the kit, as described before (Dai et al., 2020). Cell viability and counting was performed using Trypan Blue and a Neubauer hemocytometer (Sigma-Aldrich). And, the chicken PBL and tissue single cell suspensions were frozen in liquid nitrogen for later study.
Flow Cytometry
The 3 × 105 cells of PBL were simultaneously incubated with APC-conjugated mouse anti-chicken CD3+, FITC-conjugated mouse anti-chicken CD4+, and PE-conjugated mouse anti-chicken CD8α+ monoclonal antibodies (SouthernBiotech, Birmingham, AL) in the dark at 4°C for 30 min. After 3 washes with PBS, the labeled cells were analyzed by flow cytometer (CytoFLEX; Beckman Coulter, Brea, CA) within 12 h. The data were analyzed by the software of FlowJo, V10 (TreestarInc, Ashland, OR).
Analysis Expression of Immune Related Gene by Quantitative Real-Time Polymerase Chain Reaction
Total RNA was extracted from 4 × 106 cells of PBL or lung cell suspensions for analysis the expression of immune-related gene by quantitative real-time polymerase chain reaction. The primers and method have been described in our previously published paper (Dai et al., 2020). Data analyses were performed using the 2−△△C t method (Livak and Schmittgen, 2001).
Statistical Analyses
Statistical comparisons were made by GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA). The results were presented as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ns indicates not significant.
Results
Monitor of H9N2 Virus Shedding, Replication, HI Antibody Level, and T Lymphocyte Percentage After Infection
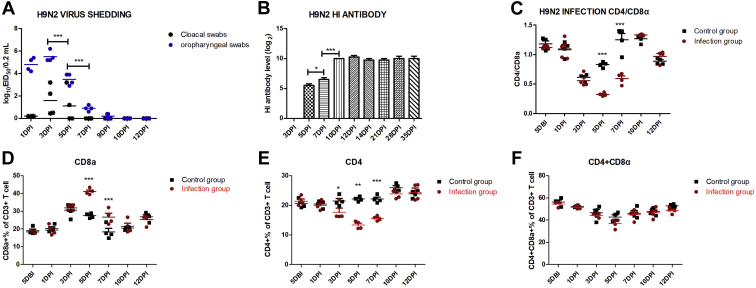
To investigate the infection of HN strain in chickens, HI antibody-negative SPF chickens were inoculated intranasally with 107EID50 of virus. In the control group, H9N2 virus shedding, H9N2 HI antibody, and virus titer in different organs were all negative at all time points (data not shown). In the H9N2 infected group, the virus shedding lasted for about 9 d. Specifically, the viral load of oropharyngeal swabs peaked at 3 DPI, had fallen off since 5 DPI, and disappeared at 10 DPI. The cloacal swabs were detected virus positive from 1 DPI to 5 DPI and negative since 7 DPI (Figure 1A). The viral load of oropharyngeal swabs was much higher than that of cloacal swabs from 1 DPI to 5 DPI (P < 0.001). Besides, the virus titer could be detected in various organs, was highest in lung and peaked at 3DPI (Table 1). These results indicated that HN strain reached a replication peak in chicken at 3 DPI, significantly decreased at 5 DPI and 7 DPI, and was eliminated at 10 DPI.
Figure 1.
Monitor of H9N2 virus shedding, HI antibody level, and T lymphocyte percentage after infection. Four chickens of infected and control groups were randomly selected for sampling to detect the viral load of cloacal swabs and oropharyngeal swabs (A), H9N2 HI antibody level (B), the percentage of CD3+CD8+ T cell (D), the percentage of CD3+CD4+T cell (E), the percentage of CD3+CD4+CD8+ T cell (F), and the ratio of CD3+CD4+ / CD3+CD8+ (C). Each sample collected 1 × 105 cells for flow cytometric analysis. H9N2 virus shedding and H9N2 HI antibody in the control group were all negative at all time points (data not shown). The one-way or two-way ANOVA was used for statistical comparison. ns P > 0.05, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations: DPI, days postinfection; HI, hemagglutination inhibition.
Table 1.
Virus titer in different organs.
| DPI | Liver | Kidney | Spleen | Duodenum | Jejunum | Ileum | Lung |
|---|---|---|---|---|---|---|---|
| 3DPI | 0.4,0.9 (2/3) | 2.2,0.4 (2/3) | 0.2,0.2 (2/3) | 2.4,0.2 (2/3) | 2.2,1.2 (2/3) | 0.9,0.9 (2/3) | 3.5 ± 0.29 (3/3) |
| 5DPI | 0/3 | 1.1,0.9 (2/3) | 0/3 | 0.2,0.2(2/3) | 0.2,0.2 (2/3) | 0.2,0.2 (2/3) | 1.15 ± 0.25 (3/3) |
| 7DPI | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0/3 | 0.4,0.4 (2/3) |
Abbreviation: DPI, days post infection.
No. of chickens positive for virus/total no. of chickens tested. The virus titers were presented in log10EID50/0.1 mL or Expressed as mean titers [log10EID50/0.1 mL] ± SEs.
Additionally, we found that there was an obviously negative correlation between virus replication timeline and host immune response timeline. For instance, at 3 DPI, the peak of virus proliferation, we have not detected antivirus adaptive immune response, including HI antibody level and the percentage increase of CD8+T cell and CD4+T cell in the infection group. Conversely, at 5 DPI and 7 DPI when the virus shedding and replication dramatically decreased, we detected significantly increasing H9N2 HI antibody level and CD8+T cell percentage. But, the ratios of CD4+/CD8+ were obviously downregulated in this stage (Figures 1B–1D and Supplementary Figure 1). And, the percentage of CD4+T cell was distinctly reduced from 3 DPI to 7 DPI (Figure 1E and Supplementary Figure 1). These results implies that the antibody-namely, humoral immunity and the CD8+T cell response were the potential key factor to defend against H9N2 AIV infection.
Monitor of T Lymphocyte Percentage and HI Antibody After H9N2 AIV Inactivated Vaccine Immunization
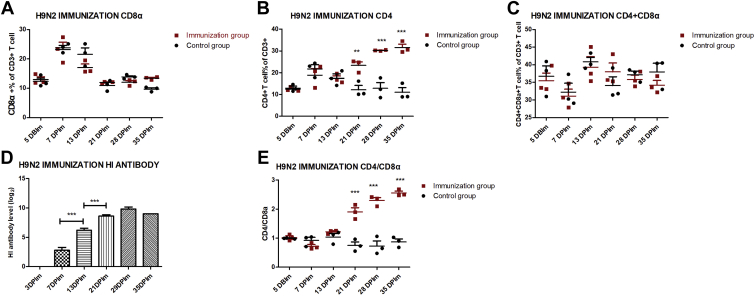
To explore the adaptive immune response induced by H9N2 AIV-inactivated vaccine immunization, HI antibody negative SPF chickens were injected subcutaneously about 2 × 108.3 EID50 vaccine. In the control group, H9N2 HI antibody was negative from 3DPIm to 35DPIm (data not shown). In the H9N2-immunization group, H9N2 HI antibody level was detected starting from 7DPIm, then gradually significant increase and peaking at 29 DPIm (Figure 2D). Compared with the control group, the percentage of CD4+T cell and the ratios of CD4+/CD8+ in the H9N2-immunization group were remarkably up-regulated from 21 DPIm to 35 DPIm (Figures 2B and 2E, and Supplementary Figure 1). On the contrary, there was no obvious difference about the percentage of CD8+T cell and CD4+ CD8+ double-positive T cells (Figures 2A and 2C, and Supplementary Figure 1). These results indicated that inactivated vaccine may trigger humoral immunity and the CD4+T cell response but not the CD8+T cell response.
Figure 2.
Monitor of T lymphocyte percentage and HI antibody after H9N2 AIV inactivated vaccine immunization. Five chickens of immunized and control groups were randomly selected for sampling to detect the HI antibody level (D). In the control group, H9N2 HI antibody was negative from 3 d post immunization (DPIm) to 35 DPIm (data not shown). From 5 d before immunization (DBIm) to 35 DPIm, peripheral blood lymphocytes (PBL) derived from 3 chickens of immunized and control groups were isolated to detect the percentage of CD3+CD8+ T cell (A), the percentage of CD3+CD4+T cell (B), the percentage of CD3+CD4+CD8+ T cell (C), and the ratio of CD3+CD4+/CD3+CD8+ (E). The one-way or two-way ANOVA was used for statistical comparison. ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations: AIV, avian influenza viruses; HI, hemagglutination inhibition.
Monitor of Immune-Related Genes Expression in PBL After H9N2 AIV Infection and Immunization, Respectively
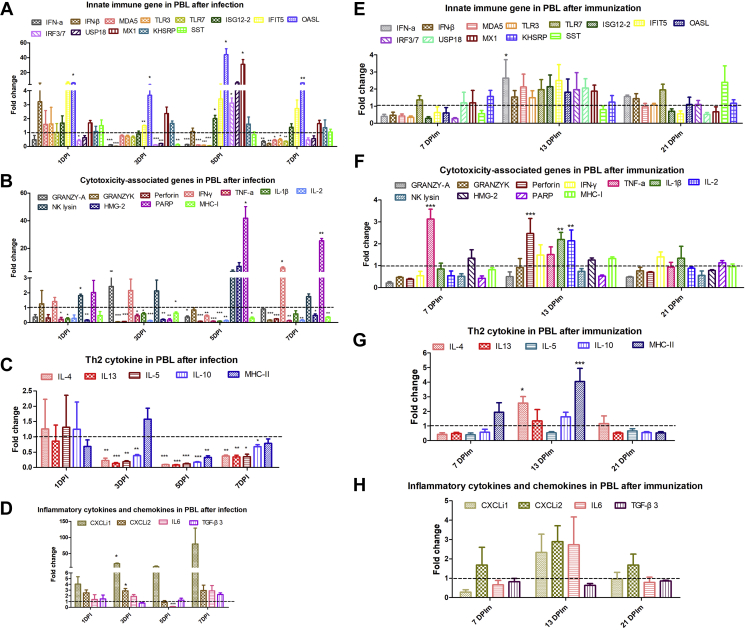
We further monitored immune-related genes expression in PBL at various time points for analyzing the effective immune protection factors after H9N2 AIV infection and immunization, respectively. And, we observed that H9N2 AIV infection resulted in a more than 20-fold upregulation of poly (ADP-ribose) polymerase (PARP) at 5 DPI (P < 0.05) and 7 DPI (P < 0.01) and greater than 4-fold upregulation of interferon-γ (IFN-γ) at 7 DPI (P < 0.05) (Figure 3B). Besides, 4 kinds of interferon-stimulated genes (ISG) including IFN-stimulated gene 12 (ISG12) (P < 0.05), 2′,5’-oligoadenylate synthetase-like (OASL) (P < 0.05), interferon response factor 7 (P < 0.05), and myxovirus resistance 1 (MX1) (P < 0.05) were obviously increased after H9N2 AIV infection at 5 DPI (Figure 3A). Especially, the upregulated OASL and MX1 were both more than 20-fold. Moreover, H9N2 AIV infection triggered a more than 10-fold upregulation of CXCLi1 (P < 0.05) and greater than 2-fold upincrease of CXCLi2 (P < 0.05) at 3 DPI (Figure 3D). However, the detected T helper (Th) 2 cytokine including interleukin-13 (IL-13), IL-4, IL-5, and IL-10 showed no increased expression (Figure 3C).
Figure 3.
Monitor of immune-related genes expression in PBL after H9N2 AIV infection and immunization, respectively. Expressions of immune-related gene in PBL were detected by quantitative real-time polymerase chain reaction (qRT-PCR). In the H9N2 AIV infected and immunization experiments, the total RNA of PBL was extracted derived from 3 chickens of each group. And, the data were collected from 3 biological samples in each group, each sample performed in triplicate. The results were presented as means ± SEM, and the paired t test was used for statistical comparison. ns P > 0.05, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations: AIV, avian influenza viruses; IFN-γ, interferon-γ; IFIT5, interferon-induced proteins with tetratricopeptide repeats 5; IRF7, interferon response factor 7; IL, interleukin; KHSRP, K-homology splicing regulatory protein; MX1, myxovirus resistance 1; OASL, 2′,5’-oligoadenylate synthetase-like; PARP, poly (ADP-ribose) polymerase; PBL, peripheral blood lymphocyte; SST, somatostatin; TLR, Toll-like receptor.
Conversely, H9N2-inactivated vaccine induced the different expression profile of immune-related genes. Specifically, the transcriptional expression level of cytotoxicity genes, including tumor necrosis factor-α (TNF-α) (P < 0.001) at 7 DPIm, Perforin (P < 0.001), IL-1β (P < 0.01), and IL-2 (P < 0.01) at 13 DPIm, and Th2 cytokines, IL-4 (P < 0.05) and MHC-II (P < 0.001), and IFN-α (P < 0.05) at 13 DPIm were significantly upregulated after vaccine immunization (Figures 3F and 3G). But no ISG genes were induced by immunization and all the fold change values of immune genes were below 6, which indicated that the changes of immune-related genes induced by immunization were not as violent as viral infection.
Monitor of Immune-Related Genes Expression in Lung After H9N2 AIV Infection and Immunization, Respectively
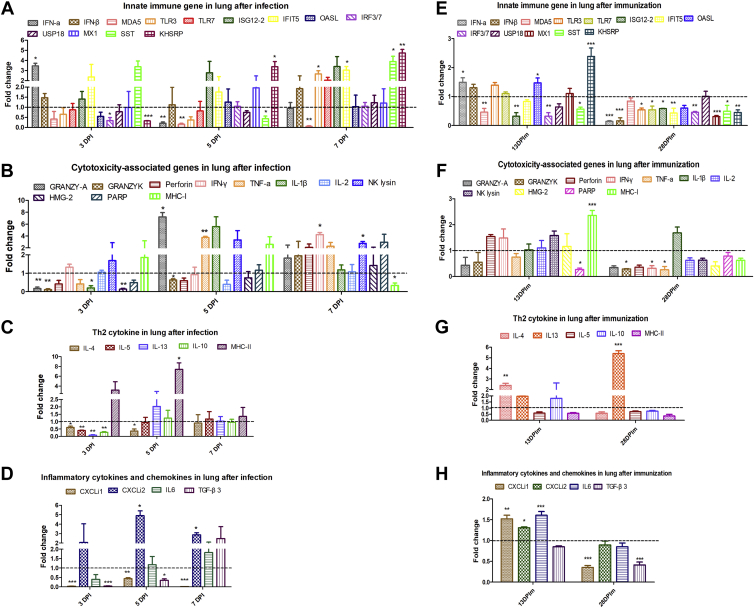
Given that lung was an important target organ for AIV infection, we also monitored immune-related genes expression in lung at various time points after H9N2 AIV infection and immunization, respectively. The transcriptional expression level of cytotoxicity genes, including granzyme A (P < 0.05) and TNF-α (P < 0.01) at 5 DPI, IFN-γ (P < 0.05) and NK lysin (P < 0.05) at 7 DPI (Figure 4B), and innate immune genes, including IFN-α (P < 0.05) at 3 DPI, K-homology splicing regulatory protein at 5 DPI (P < 0.05) and 7 DPI (P < 0.01), and Toll-like receptor 3, interferon-induced proteins with tetratricopeptide repeats 5, and somatostatin at 7 DPI (P < 0.05) (Figure 4A), and chemokines CXCLi2 (P < 0.05) at 5 DPI and 7 DPI (Figure 4D), and MHC-II (P < 0.05) at 5DPI (Figure 4C) were significantly upregulated after H9N2 AIV infection.
Figure 4.
Monitor of immune-related genes expression in lung after H9N2 AIV infection and immunization, respectively. Expressions of immune-related gene in lung were detected by qRT-PCR. In the H9N2 AIV-infected experiment, the total RNA of lung cell suspensions was extracted derived from 3 chickens of infected and control groups, respectively. In the H9N2 AIV immunization experiment, the total RNA of lung cell suspensions was extracted derived from 2 chickens of immunized and control groups, respectively. The data were collected from 3 biological samples in each group, each sample performed in triplicate. The results were presented as means ± SEM, and the 2-way ANOVA was used for statistical comparison. ns P > 0.05, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations: AIV, avian influenza viruses; IFN-γ, interferon-γ; IFIT5, interferon-induced proteins with tetratricopeptide repeats 5; IRF7, interferon response factor 7; IL, interleukin; KHSRP, K-homology splicing regulatory protein; MX1, myxovirus resistance 1; OASL, 2′,5’-oligoadenylate synthetase-like; PARP, poly (ADP-ribose) polymerase; PBL, peripheral blood lymphocyte; SST, somatostatin; TLR, Toll-like receptor.
In addition, the mRNA expression level of IFN-α (P < 0.05), OASL (P < 0.05), K-homology splicing regulatory protein (P < 0.001) (Figure 4E), MHC-I (P < 0.001) (Figure 4F), IL-4 (P < 0.01) (Figure 4G), CXCLi1 (P < 0.01), CXCLi2 (P < 0.05), IL-6 (P < 0.001) (Figure 4H) at 13 DPIm, and IL-13 (P < 0.001) at 28 DPIm (Figure 4G)were significantly increased after vaccine immunization.
Discussion
Usually, vaccination as a strategy is used to reduce the economic impact of AIV in poultry industry. Up to now, most commercial AIV vaccines are whole virus–inactivated vaccine. Previously, we verified that the vaccine of H9N2 AIV epidemic HN strain provided better protection from the current prevailing H9N2 viruses infection than the classical vaccine strain SS (A/chicken/Guangdong/SS/94). However, HN strain vaccine still could not totally prevent the replication of H9N2 virus at 5 DPI even challenged with the same strain (Xu et al., 2018). This phenomenon remaindered us standard vaccines inducing neutralizing antibodies may be not sufficiently valid to eliminate H9N2 virus. It is imperative to identify the differential immune protection factors induced by H9N2 AIV infection and immunization.
In our previous study, we established an infectious model to study virus-induced optimal immune responses in chicken (Dai et al., 2020). In this study, we took use of the same infection model to investigate the dynamic change of H9N2 AIV replication and induced immune response. And, we found that at 5 DPI and 7 DPI when the virus shedding and replication in lung dramatically decreased, H9N2 HI antibody level and upregulated CD8+T cell percentage in PBL were starting to be detected. Meanwhile, we observed that apoptosis protein PARP (Pinkoski and Green, 2003) and IFN-r were significantly increased at this period, which may be associated with the stage of activation of cytotoxic T lymphocytes in H9N2 AIV-infected PBL (Dai et al., 2019). Besides, H9N2 AIV infection triggered more than 20-fold upregulated expression of 2 chicken antiviral ISG, OASL (Tag-El-Din-Hassan et al., 2012), and MX1 (Sasaki et al., 2013) at 5 DPI. And, obvious upregulation of chemokine CXCLi1 and CXCLi2 at 3 DPI after infection may be related to the circulation or migration of immune cells to sites of infection (Dai et al., 2020). Accordingly, H9N2 HI antibody level, antiviral ISG including OASL and MX1, CD8+ T cell response in PBL accompanied by the cytotoxicity-associated genes, including PARP and IFN-r, may have been the key factor to defend against H9N2 infection.
Similarly, the HI antibody level, T cell percentage, and immune-related gene expression were detected in PBL after H9N2 AIV-inactivated vaccine immunization. And, the similar HI antibody level was detected. Vaccine immunization promoted the increase of CD4+ T cell percentage instead of CD8+ T cell percentage in PBL. Also the ratios of CD4+/CD8+ were remarkably upregulated after immunization and obviously downregulated after infection, respectively, which suggested that vaccine immunization caused immune enhancement but viral infection induced immune suppression (Xue et al., 2013; Yang et al., 2018; Fu et al., 2019; Dai et al., 2020). Compared with viral infection, vaccine immunization triggered different immune gene expression profile in PBL. For instance, cytotoxicity genes including TNF-α, perforin, IL-1β, and IL-2 were increased, but the fold change values were far lower than that of the viral infection-induced upregulation of PARP and IFN-r. Besides, upregulated Th2 cytokines, IL-4, and MHC-II in PBL contributing to the humoral immune response were induced by vaccine immunization instead of viral infection. Conversely, when more than 20-fold upregulated expressions of 2 ISG (MX1 and OASL) were induced by viral infection, no increased ISG genes were detected by immunization. Taken together, vaccine immunization may be inclined to induce the humoral immune response and CD4+ T cell response producing Th2 cytokines.
Given that lung was an important target organ for AIV infection, we also compared and analyzed the immune-related genes expression profile in lung after H9N2 AIV infection and immunization, respectively. And, we found that both H9N2 AIV infection and immunization could induce the expressions of innate immune related gene and chemokine in lung. However, upregulated cytotoxicity-associated genes including granzyme A, TNF-α, IFN-γ, and NK Lysin were induced by viral infection instead of vaccine immunization in lung. Conversely, upregulated Th2 cytokines including IL-4 and IL-13 were induced by vaccine immunization instead of viral infection in lung. These results further confirmed that viral infection mainly boosted obvious CD8+ T cell response, but vaccine immunization was most apt to promote CD4+ T cell response. Therefore, we infer that the protection deficiency of H9N2 AIV inactivated vaccine may be ascribed to induce deficiency of CD8+ T cell response, which needs improvement in future vaccine development.
In summary, this study investigated host immune key factors against viral infection in H9N2 AIV-infected SPF chickens. We found that H9N2 HI antibody level, antiviral ISG, and CD8+ T cell response played important roles in defending against H9N2 infection. Besides, we further made a comparative analysis of key immune protection factors in H9N2 AIV-infected and immunized SPF chicken and deduced that deficiency of CD8+ T cell response induced by H9N2 AIV-inactivated vaccine was the potential key reason of protection deficiency. These findings provide evidence and direction for developing effective H9N2 AIV vaccines.
Acknowledgments
This work was supported by the National Natural Science Foundation Grants (31830097 and 31802174), National Key Research and Development Plan of China (2017YFD500701-4).
DISCLOSURES
The authors declare that they have no conflicts of financial interest.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.09.080.
Supplementary data
Supplementary Figure 1.
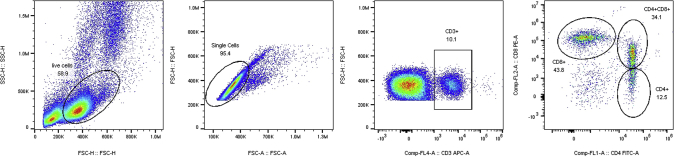
Gating strategy for analysis of T lymphocyte percentage in PBL with the CD3+ (APC), CD4+ (FITC) and CD8α+ (PE) antibodies.
References
- Dai M., Li S., Shi K., Liao J., Sun H., Liao M. Systematic identification of host immune key factors influencing viral infection in PBL of ALV-J infected SPF chicken. Viruses. 2020;12:114. doi: 10.3390/v12010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M., Xu C., Chen W., Liao M. Progress on chicken T cell immunity to viruses. Cell. Mol. Life Sci. 2019;76:2779–2788. doi: 10.1007/s00018-019-03117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. OIE laboratory standards for avian influenza. Dev. Biol. (Basel) 2006;124:159–162. [PubMed] [Google Scholar]
- Fu L., Wang X., Zhai J., Qi W., Jing L., Ge Y., Gao X., Liu C., Lv X., Zheng S. Changes in apoptosis, proliferation and T lymphocyte subtype on thymic cells of SPF chickens infected with reticuloendotheliosis virus. Mol. Immunol. 2019;111:87–94. doi: 10.1016/j.molimm.2019.04.003. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu M., Sun Q., Zhang H., Zhang H., Jiang S., Liu S., Huang Y. Genotypic evolution and epidemiological characteristics of H9N2 influenza virus in Shandong Province, China. Poult. Sci. 2019;98:3488–3495. doi: 10.3382/ps/pez151. [DOI] [PubMed] [Google Scholar]
- Liu A.L., Li Y.F., Qi W., Ma X.L., Yu K.X., Huang B., Liao M., Li F., Pan J., Song M.X. Comparative analysis of selected innate immune-related genes following infection of immortal DF-1 cells with highly pathogenic (H5N1) and low pathogenic (H9N2) avian influenza viruses. Virus Genes. 2015;50:189–199. doi: 10.1007/s11262-014-1151-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nang N.T., Lee J.S., Song B.M., Kang Y.M., Kim H.S., Seo S.H. Induction of inflammatory cytokines and Toll-like receptors in chickens infected with avian H9N2 influenza virus. Vet. Res. 2011;42:64. doi: 10.1186/1297-9716-42-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkoski M.J., Green D.R. Granzyme A: the road less traveled. Nat. Immunol. 2003;4:106–108. doi: 10.1038/ni0203-106. [DOI] [PubMed] [Google Scholar]
- Qi X., Liu C., Li R., Zhang H., Xu X., Wang J. Modulation of the innate immune-related genes expression in H9N2 avian influenza virus-infected chicken macrophage-like cells (HD11) in response to Escherichia coli LPS stimulation. Res. Vet. Sci. 2017;111:36–42. doi: 10.1016/j.rvsc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- Sasaki K., Yoneda A., Ninomiya A., Kawahara M., Watanabe T. Both antiviral activity and intracellular localization of chicken Mx protein depend on a polymorphism at amino acid position 631. Biochem. Biophys. Res. Commun. 2013;430:161–166. doi: 10.1016/j.bbrc.2012.11.053. [DOI] [PubMed] [Google Scholar]
- Stone H.D., Brugh M., Hopkins S.R., Yoder H.W., Beard C.W. Preparation of inactivated oil-emulsion vaccines with avian viral or Mycoplasma antigens. Avian Dis. 1978;22:666–674. [PubMed] [Google Scholar]
- Sun Y., Liu J. H9N2 influenza virus in China: a cause of concern. Protein Cell. 2015;6:18–25. doi: 10.1007/s13238-014-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tag-El-Din-Hassan H.T., Sasaki N., Moritoh K., Torigoe D., Maeda A., Agui T. The chicken 2'-5' oligoadenylate synthetase A inhibits the replication of West Nile virus. Jpn. J. Vet. Res. 2012;60:95–103. [PubMed] [Google Scholar]
- Wei Y., Qi L., Gao H., Sun H., Pu J., Sun Y., Liu J. Generation and protective efficacy of a cold-adapted attenuated avian H9N2 influenza vaccine. Sci. Rep. 2016;6:30382. doi: 10.1038/srep30382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Ye H., Qiu W., Lin H., Chen Y., Zhang H., Liao M. Phylogenetic classification of hemagglutinin gene of H9N2 avian influenza viruses isolated in China during 2012-2016 and evaluation of selected candidate vaccine strains. Poult. Sci. 2018;97:3023–3030. doi: 10.3382/ps/pey154. [DOI] [PubMed] [Google Scholar]
- Xue M., Shi X., Zhao Y., Cui H., Hu S., Cui X., Wang Y. Effects of reticuloendotheliosis virus infection on cytokine production in SPF chickens. PLoS One. 2013;8:e83918. doi: 10.1371/journal.pone.0083918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Li G., Zhao Z., Huang Z., Fu J., Song M., Lin S., Zhu R. Taishan Pinus massoniana Pollen Polysaccharides enhance immune responses in chickens infected by avian Leukosis virus Subgroup B. Immunol. Invest. 2018;47:443–456. doi: 10.1080/08820139.2018.1435689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.