Abstract
Coccidiosis, the parasitic disease caused by Eimeria spp., is controlled during broiler chicken production through the inclusion of in-feed anticoccidial medications. Live-coccidiosis vaccination has become an increasingly common alternative to these medications. Monitoring infections with Eimeria spp. in flocks can be accomplished through determining the concentration of oocysts excreted in the fecal material (i.e., oocysts per gram; OPG). The purpose of our study was to sample commercial Ontario broiler chicken flocks at various times of the year to determine weekly OPG counts for flocks that use either an in-feed anticoccidial medication or a live-coccidiosis vaccine. Weekly sampling of 95 flocks from placement to market permitted documentation of oocyst cycling patterns typical of conventional and antibiotic-free flocks, and variation of these patterns in summer and winter. Medicated flocks had higher and later peak oocyst shedding compared with vaccinated flocks. Flocks reared in the summer peaked in oocyst shedding earlier than flocks reared in the winter. Despite what appears to be poorer coccidiosis control in the medicated flocks, the performance data were similar for these flocks compared with vaccinated flocks. This is the first study describing typical patterns of parasite shedding in Ontarian commercial broiler chicken flocks; these data will provide a baseline of expected Eimeria spp. infections in Canadian broiler chicken flocks to ensure optimal coccidiosis prevention.
Key words: broiler chickens, coccidiosis monitoring, oocysts per gram, anticoccidial medications, coccidiosis vaccine
Introduction
Coccidiosis is an intestinal disease of production animals caused by parasites in the genus Eimeria. Historically, the disease has been problematic for the poultry industry, including broiler chicken production (Chapman et al., 2013). Both clinical and subclinical disease are responsible for decreasing profitability by increasing mortality and reducing feed efficiency, while incurring costs of disease prevention and intervention (Williams, 1999). The estimated global economic burden of the disease has been calculated to exceed USD $3 billion annually (Williams, 1999; Dalloul and Lillehoj, 2006).
The most popular coccidiosis prevention strategy for broiler chicken production over the last few decades has been the addition of anticoccidials in feed (Dalloul and Lillehoj, 2006; Chapman et al., 2013). Since the introduction of the first commercial anticoccidial medications (Grumbles et al., 1948; Chapman, 2009), the animal health industry has continued to respond to the threat posed by these parasites by developing products to prevent coccidiosis or reduce the severity of disease. Anticoccidials are classified into one of 2 categories, chemicals or ionophores, and are applied using a wide variety of combinations and timings (Peek and Landman, 2011; Chapman et al., 2013). The effectiveness of these anticoccidials led to their near ubiquitous use in the broiler chicken industry. Not surprisingly, such widespread and continuous use in the field generated varying levels of resistance against all commercially used products (Chapman, 1997; Martin et al., 1997; Stephan et al., 1997). As the industry shifts toward producing poultry without antibiotics, ionophores may no longer be acceptable for use because of their impacts on intestinal microbial communities (Dibner and Richards, 2005; Peek and Landman, 2011).
A number of production programs or marketing labels, such as raised without antibiotics (RWA in Canada), no antibiotics ever (NAE in USA), or antibiotic-free (ABF) have been introduced to address the concerns of scientific and medical communities regarding the overuse of antibiotics. An alternative coccidiosis prevention strategy used in such programs has been live-coccidiosis vaccines applied to chicks, which allows for the development of immunity to the Eimeria spp. present in the vaccine (Peek and Landman, 2011; Chapman et al., 2013; Price et al., 2015) after adequate cycling in the barn (Chapman et al., 2002; Velkers et al., 2012; Price et al., 2016).
Typically, diagnostic methods for Eimeria spp. are applied only when clinical coccidiosis is suspected (Johnson and Reid, 1970; Williams, 2005). In such cases, the intestinal tracts from a subsample of chickens in the flock are examined macroscopically for lesions typical of coccidiosis (Johnson and Reid, 1970). The severity of coccidiosis is related to the number of oocysts ingested because the parasites' life cycles are self-limiting (McDonald and Shirley, 2009; Price, 2012; Chapman et al., 2013). In nonclinical cases of coccidiosis, the severity of infection can be estimated by measuring the abundance of oocysts that are shed in the feces; this abundance is expressed as oocysts per gram (OPG) (Hodgson, 1970; Williams, 2001; Haug et al., 2008; Oden et al., 2012; Chapman et al., 2016; Parent et al., 2018).
By collecting samples systematically at predefined intervals, flock-specific shedding patterns have been sought after to provide detailed insights into the timing and abundance of Eimeria spp. cycling in each flock (Haug et al., 2008; Chapman et al., 2016; Parent et al., 2018).
The overall aim of this study was to document Eimeria spp. infection shedding patterns by chickens in commercial broiler production in Ontario, Canada. The specific objectives were to 1) establish benchmarks for, and compare OPG patterns in flocks reared under competing coccidiosis prevention strategies (medicated or vaccinated), 2) understand how external climate affects oocyst shedding patterns, and 3) determine if there are differences in performance between medicated and vaccinated flocks.
Materials and methods
Research Ethics
The University of Guelph Research Ethics Board approved the on-farm study (REB#16MR013). An institutional animal utilization protocol was not required because chickens were not handled or sampled. All handling of fecal and litter material was conducted in compliance with Biohazard Permit A-169-01-19-07 issued by the Biosafety Committee, University of Guelph.
Participant Enrollment and Study Duration for the Cohort (On-Farm) Study
Broiler producers were made aware of the study by word of mouth; representatives from feed and animal health companies and neighboring producers were the primary source of distribution. All commercial broiler production facilities in southern Ontario that were within 170 km of the University of Guelph, and were facilities registered with the Chicken Farmers of Ontario (provincial marketing board for chicken), were eligible to participate. Participating facilities, defined as those having one or more flocks sampled during the study period, were selected on the basis of their coccidiosis prevention program (medicated or vaccinated; defined below); facilities varied in their production inputs, such as size of operation, hatchery, feed mills and nutrition programs, and the size of bird grown. The amount of time and resources available for facility visits dictated the number of flocks included in the study; however, the sample collection period was extended to 2 yr to increase the number of flocks and to ensure that each season (summer and winter; defined below) was represented more than once. Participating facilities were required to be registered commercial production facilities (i.e., backyard flocks were ineligible). Participants were instructed to continue their normal protocols, and no change in coccidiosis prevention program or management was requested. A questionnaire was used to collect data on flock characteristics (breed, sex, number of birds placed) and performance parameters (mortality, days to market, feed consumption, live weight at processing, condemnations) for each flock from which a complete series of samples was collected; provision of these data was not a requirement for participation (i.e., the questionnaire was optional).
The first on-farm sampling was conducted in May 2016 and the last sampling was completed in April 2018. To reflect the seasonal differences in external environmental conditions, samples collected from flocks placed from May until early September were considered to be “summer” samples and those collected from flocks placed from December until early March were considered to be “winter” samples. Summer samples (May to October samplings) were obtained in 2016 and 2017, and winter samples (December to April samplings) were obtained in 2016–2017 and 2017–2018. Thus, there was a total of 4 sample collection periods during the study: summer 2016 [S16]; winter 2017 [W17]; summer 2017 [S17]; and winter 2018 [W18]. For each participating facility, only one flock per sample collection period was included, to a maximum of 4 flocks if the producer participated for the entire study period. Producers were free to join or leave the study at any time; however, during their enrollment, a full series of samples from each flock was obtained.
Sample Collection for the Cohort Study
Manual collection of 40 fecal droppings (pooled into a single sample) was performed using a standardized route for sampling within the barn. The collector walked the length of the barn 4 times, collecting 10 fresh droppings per pass (litter material and bedding was excluded), following paths that were approximately equally spaced across the barn. Both fecal and cecal droppings were obtained. Droppings were placed in either a ∼236 mL (8 oz.) wide-mouth plastic jar, or appropriately sized, robust, resealable bag (e.g., Ziploc freezer bag or similar). Samples were collected from each flock once per week (every seventh day) starting the seventh day after the day of chick placement until 5 wk of age, for a total of 5 pooled samples per flock. At each operation (i.e., farm), only one facility (i.e., house/barn) was sampled, and if it was a multistory facility, samples were collected from the ground floor (i.e., lower level or first floor).
The researcher showed each new participating producer how to perform sample collection during the first flock sampling (i.e., week 1) at that location. Thereafter, the researcher or participant collected the samples. If collected by the researcher, fresh samples were transported back to the University of Guelph in a cooler with an ice pack. Samples collected by the participants were stored on-farm until all required samples from the flock were collected, then shipped together by prepaid courier to the University of Guelph. If the facility had a refrigerator available, samples were stored at ∼4°C until shipping. If a refrigerator was not available, a premeasured volume of 1:50 diluted bleach preservative was added to the fecal material (approximately 1:1 v/v) and mixed to reduce bacterial growth (final concentration of sodium hypochlorite of ∼500 ppm).
Sample Processing
Samples were stored at the University of Guelph at 4°C for no more than 7 d after the day of collection before being processed. Fecal samples were processed to determine OPG counts through a modified McMaster counting chamber flotation using saturated salt solution, 100× magnification microscope, and a standard formula calculation (Long and Rowell, 1975) adjusted, when necessary, to account for bleach preservative. Some samples required additional dilutions when oocysts were too numerous to count; for such samples, up to three 10-fold dilutions using saturated salt were conducted so that 50 to 500 oocysts were observable within the counting grid. The calculated limit of detection using standard dilution and McMaster counting chambers was ∼17 OPG.
Medicated and Vaccinated Flocks
Medicated flocks were defined as those on a conventional feeding program that included the prophylactic use of antibiotics and anticoccidial medications in the feed. There was no standard program across these flocks with respect to the products used or the timing of administration. All flocks started on one anticoccidial product, then shuttled to a different product at 2 to 4 wk of age, depending on the program set by the feed mill.
Vaccinated flocks were defined as those from participating facilities that reared broilers under an RWA/NAE/ABF program that used neither in-feed antibiotics nor in-feed anticoccidials (i.e., none were on a bioshuttle or similar program that combined vaccination with anticoccidial medication). These flocks were vaccinated at the hatchery following standard industry protocols using one of the 2 commercial live-coccidiosis vaccines available in Canada: Coccivac B (Schering Plough, Kenilworth, NJ); or, Immucox III (Ceva Animal Health, Guelph, ON).
Data Analyses for the Cohort Study
The OPG and questionnaire data were manually entered into Microsoft Excel (Microsoft Office 2013, Microsoft Corporation, Redmond, WA), reviewed for accuracy, then uploaded to SAS 9.4 (SAS Institute Inc., Cary, NC) for analysis. Line graphs were used to illustrate OPG shedding patterns for individual flocks over the 5-week grow-out period; separate graphs were used for medicated and vaccinated flocks. In addition, using aggregated data, line graphs were used to illustrate OPG shedding patterns over the 5-week grow-out period, both overall (mean and median), and by season (summer and winter), for medicated and vaccinated flocks that were adjusted for facilities that had multiple flocks sampled.
The PROC MIXED program in SAS 9.4 was used to determine differences in OPG count data between prevention programs (medicated vs vaccinated) and age of sample collection (within and between prevention programs). The OPG data from the 5 weekly samplings (i.e., weeks 1–5) were classified as repeated measures in the analyses. The participating facility was classified as a random effect to account for repeated measures at the facility level (i.e., up to 4 flocks per facility). The predicted OPG count at the end of each week of age for the medicated and vaccinated flocks were estimated using the PROC MIXED program in SAS 9.4.
The PROC MIXED program in SAS 9.4 was used to determine differences in performance parameters (obtained from the questionnaire) between medicated and vaccinated flocks. The coccidiosis prevention program was the main effect, participating facility was a random effect, and flock characteristics and season were covariates. For all analyses, terms with a P-value ≤0.05 were considered to be significantly different. Post hoc power calculations were used to determine whether there was sufficient power to detect differences between coccidiosis prevention programs.
Adjusted feed conversion ratio (adj. FCR; Aviagen, 2018) was calculated using the flock-specific FCR and market weight, and the averaged market weight from all flocks using the following formula:
Results and discussion
In a 1970 study, Hodgson (1970) performed analysis on 100 individual droppings from broiler flocks to determine 95% confidence intervals for OPG counts and concluded that OPG counts based on 40 droppings would accurately represent the level of coccidial infection experienced by a flock at a given age. Hodgson also suggested that 50 droppings would provide a reliable estimate of flock OPG counts in a barn of 2,000 or more broilers (Hodgson, 1970). Based on our preliminary sampling methodology experiments (data not shown), and the experience of Parent et al. (2018), who pooled 20-25 droppings for each sampling of a flock, 50 droppings might be unnecessary to generate representative data in most field situations. The uniformity of broiler chicken growth and consistency in the barn environment in the 5 decades since Hodgson's (1970) study makes it possible that pooling a modest number of droppings may be appropriate for determining flock OPG counts.
The OPG counts in our study include oocysts of all Eimeria spp. shed by infected chickens without differentiating the individual species that might be present in each sample. There was no attempt to quantify individual Eimeria spp. For samples containing a single species, oocyst morphometrics can be used to tentatively identify the Eimeria sp. present (Tyzzer, 1929; Reid and Long, 1979; Mcdougald et al., 1997; Jenkins et al., 2017). However, in mixed-species infections typical in commercial flocks (Ogedengbe et al., 2011; Chapman et al., 2016), identification of most Eimeria spp. is unreliable due to the variability and overlap of oocyst morphometrics within and among Eimeria spp. (Joyner and Long, 1974; Long and Joyner, 1984; Ogedengbe et al., 2011). With typically 2 or more (and possibly up to 7) Eimeria spp. within a single field sample (Jenkins et al., 2017), the use of oocyst morphometrics for differentiating Eimeria spp. in the field samples from our study was considered unreliable.
Oocyst Shedding From Commercial Flocks
Characteristics of Participating Facilities and Flocks Sampled
A total of 45 facilities participated in this study. The coccidiosis prevention program of each participating facility did not change during the study period, with the exception of 2 facilities that switched from using coccidiosis vaccines to anticoccidial medications. From those 45 participating facilities, 95 broiler flocks (53 medicated; 42 vaccinated) were sampled over 4 sample collection periods (Table 1) to provide 461 pooled fecal samples that were processed to obtain OPG counts. Facilities received chicks from 4 different hatcheries, obtained feed from 6 different feed mills, and often used site-specific flock management, which could have included preferred barn environmental conditions, bedding material (i.e., pine-wood shavings or chopped straw), and feed and water additives. Flock characteristics (Table 2) and performance data were provided for 53 of 95 flocks. Most (>70%) of these were mixed-sex; the median flock size at placement was 22,554 birds, and breeds included Ross 708 and Cobb 500. All facilities followed Canadian broiler production regulations between flocks, which includes a complete clean out of all contaminated litter material after chickens are sent to market and provision of fresh bedding for the subsequent flock (Chicken Farmers of Canada, 2018). These regulated procedures result in nearly a complete removal of oocysts from the barn environment. By contrast, reusing litter for several consecutive flocks is the norm in American broiler production, and viable oocysts have been demonstrated in reused litter of typical American facilities before chick placement (Jenkins et al., 2019).
Table 1.
Season of collection and anticoccidial programs of Ontario flocks from which weekly fecal sample collections (n = 95) during the period of May 2016 to April 2018.
| Collection period1 | Medicated flocks | Vaccinated flocks | Total flocks |
|---|---|---|---|
| S16 (05/2016-10/2016) | 13 | 13 | 26 |
| W17 (12/2016-04/2017) | 20 | 14 | 34 |
| S17 (05/2017-10/2017) | 11 | 12 | 23 |
| W18 (12/2017-04/2018) | 9 | 3 | 12 |
| Totals | 53 | 42 | 95 |
Samples collected at 1 to 5 weeks of age consisted of a single pool of 40 manually collected fresh fecal droppings (n = 461).
S16 and S17: flocks placed from May until early September with sample collection from May to October. W17 and W18: flocks placed from December until early March with sample collection from December to April.
Table 2.
Characteristics of Ontario broiler flocks for which a performance questionnaire was completed by the participating producer (n = 53).
| Variable | Medicated flocks (n = 30) | Vaccinated flocks (n = 23) |
|---|---|---|
| Breed | ||
| Cobb 500 | 12 | 4 |
| Ross 708 | 18 | 19 |
| Sex | ||
| Cockerels | 6 | 1 |
| Mixed | 19 | 19 |
| Pullets | 5 | 3 |
| No. of birds placed | ||
| Mean | 21,785 | 26,281 |
| Median | 18,717 | 24,888 |
| Min | 7,750 | 7,956 |
| Max | 48,849 | 54,570 |
The characteristics of the pooled fecal samples collected at the end of the first, second, third, fourth, and fifth week of age are summarized in Table 3. The predicted OPG values obtained from the PROC MIXED analysis are outlined in Table 4. The values obtained from the model may not represent values expected from a production facility but permit the comparison between production strategies (medicated vs vaccinated flocks). Medicated flocks were predicted to have lower OPG counts than vaccinated flocks for weeks 1, 2, 3, and 4; medicated flocks were predicted to have higher OPG counts than vaccinated flocks for the fifth week of production.
Table 3.
Summary of the weight of fecal samples (n = 461) collected at the end of the first, second, third, fourth, and fifth week of age from Ontario broiler flocks.
| Broiler age (weeks) | Pooled Samples (n) |
Mean weight of 40 pooled droppings (g) | Median (g) | Min | Max | 95% CI |
|---|---|---|---|---|---|---|
| 1 | 89 | 43.1 | 35.5 | 7.9 | 152.0 | ±5.7 |
| 2 | 90 | 94.4 | 92.0 | 16.7 | 194.3 | ±6.9 |
| 3 | 95 | 136.3 | 127.7 | 52.0 | 289.5 | ±8.3 |
| 4 | 94 | 168.5 | 158.8 | 71.6 | 433.2 | ±11.3 |
| 5 | 93 | 167.2 | 163.8 | 74.4 | 399.1 | ±11.3 |
Each pooled sample consisted of a single pool of 40 manually collected fresh fecal droppings.
Table 4.
Oocyst per gram (OPG) predicted values obtained from the PROC MIXED analysis in SAS 9.4 for the 5 ages of medicated or vaccinated flocks including 95% confidence intervals and the P-value.
| Age (weeks) | Prevention program | Predicted OPG | Lower limit | Upper limit | P-value |
|---|---|---|---|---|---|
| 1 | Medicated | 10 | 5 | 23 | <0.001 |
| Vaccinated | 816 | 449 | 1,483 | ||
| 2 | Medicated | 166 | 91 | 302 | <0.001 |
| Vaccinated | 13,636 | 8,416 | 22,094 | ||
| 3 | Medicated | 1,305 | 685 | 2,490 | <0.001 |
| Vaccinated | 41,433 | 24,885 | 68,987 | ||
| 4 | Medicated | 5,110 | 28,12 | 9,287 | 0.002 |
| Vaccinated | 22,897 | 14,134 | 37,093 | ||
| 5 | Medicated | 9,962 | 4,513 | 21,989 | 0.0043 |
| Vaccinated | 2,301 | 1,268 | 4,176 |
Medicated Flocks—OPG Counts
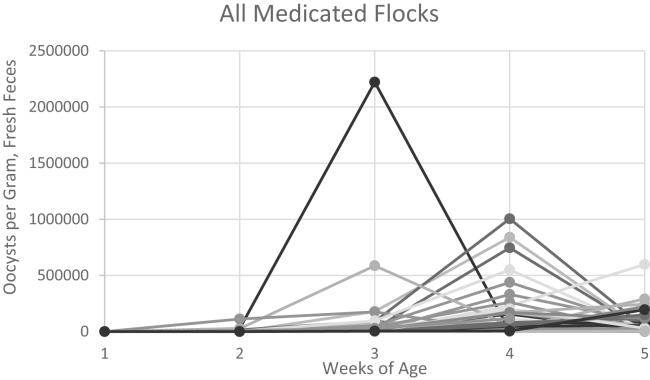
Oocyst shedding (weekly OPG counts) from all medicated flocks is illustrated in Figure 1. At week 1, most medicated flocks (43 of 47 sampled at week 1) did not have detectable oocysts. This finding can be explained by the removal of contaminated litter and addition of fresh bedding before chick placement. Most of the medicated flocks had maximal OPG counts at week 4 (45.3% of flocks) or week 5 (37.7%), whereas a few had maximal counts at week 3 (9.4%); the remaining 4 flocks (7.6%) had undetectable oocysts throughout the sample collection period (Figure 1). Such high oocyst shedding in medicated flocks shortly before marketing has been reported previously (Chapman et al., 2016). The maximal OPG count for each medicated flock (regardless of the week at which this maximal count was attained) ranged from undetectable to 2,220,591 (mean 222,371; median 116,853). In line with Parent et al. (2018), the range of OPG counts among the medicated flocks (Figure 1) was much wider than the range for the vaccinated flocks (Figure 2). Such variability can be explained by an individual flock's challenge (number of oocysts in the litter) and the susceptibility of the oocysts to the anticoccidial drugs administered to the flock (Bafundo et al., 2008). Depending on the history of drug use at a given facility, the degree of resistance of each Eimeria sp. present in the barn to the anticoccidial drugs in use could vary widely (McDonald and Shirley, 2009).
Figure 1.
Individual flock oocyst shedding patterns over the 5-week grow-out period for flocks on an anticoccidial medication program. Each line represents the 5 oocyst per gram (OPG) counts obtained from an individual flock (n = 53).
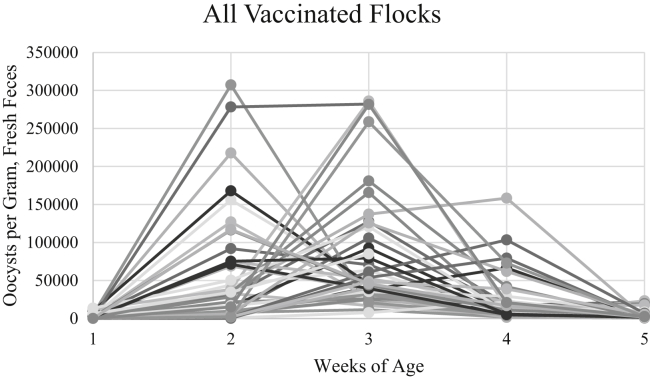
Figure 2.
Individual flock OPG shedding patterns over the 5-week grow-out period for flocks on a coccidiosis vaccination program. Each line represents the 5 oocyst per gram (OPG) counts obtained from an individual flock (n = 42).
Despite continuous in-feed anticoccidial medication, most medicated flocks (92.4%) showed increased oocyst shedding from week 3 onward (Figure 1); however, this varied among these flocks. Notably, the mean medicated flocks' OPG count at week 4 was higher than the mean vaccinated flocks' OPG counts at any week (Figure 3). Our finding of delayed, yet high, OPG counts in medicated flocks has been reported previously (Chapman et al., 2002; Williams, 2002; Jenkins et al., 2017; Parent et al., 2018), suggesting that resistance to anticoccidial medications is widespread and that at least some Eimeria spp. are totally refractory to control by some of the commonly used in-feed anticoccidials (Jenkins et al., 2019). Conversely, our observation that 29 of the 49 oocyst-positive medicated flocks had their maximal OPG count before week 5 suggests that the chickens in those flocks were developing immunity to the Eimeria spp. present while being provided anticoccidial medication (Chapman, 1999; Hu et al., 2000). Ionophorous anticoccidials have been documented to allow trickle infections to occur that support the development of immunity (Chapman, 1999; Hu et al., 2000), although protection takes longer to occur than in vaccinated flocks.
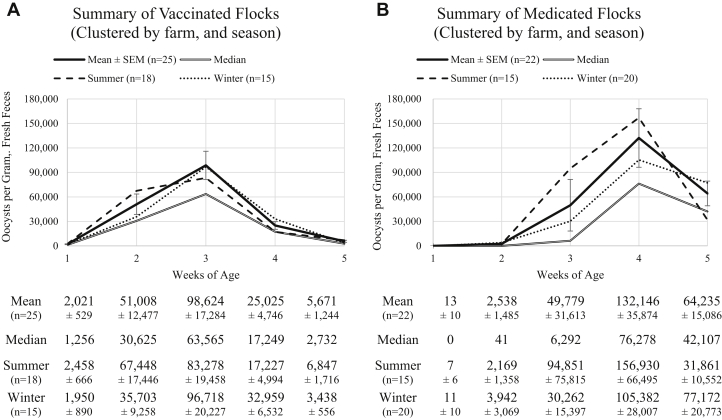
Figure 3.
(A) The aggregate data from the 42 flocks (clustered to 25 facilities) on a coccidiosis vaccination program. (B) The aggregate data from the 53 flocks (clustered to 22 facilities) that were on an anticoccidial medication program. Each graph includes the mean OPG ± SEM for each week of age, the median OPG count, the mean of the summer flocks, and the mean of the winter flocks. The table below the graph outlines the mean OPG counts ± SEM for the respective age and clustering. Abbreviation: OPG, oocyst per gram.
Vaccinated Flocks—OPG Counts
Oocyst shedding (weekly OPG counts) from all vaccinated flocks is illustrated in Figure 2. At week 1, most (38 of 42) vaccinated flocks had detectable oocysts, with OPG counts of up to 13,682. Oocyst shedding by vaccinated chicks at 5 to 8 d of age has been associated primarily with the ingestion of viable oocysts following live vaccine application at the hatchery (Long and Joyner, 1984; Price et al., 2016). Flocks shedding few or undetectable numbers of oocysts on day 7 might have been impacted by one of the following: 1) poor vaccine delivery (Jenkins et al., 2017); 2) poor vaccine ingestion by chicks (Price et al., 2015, 2016); 3) delayed access to feed after vaccination (Price et al., 2015); 4) administration of oocysts with greatly reduced or no viability (Cha et al., 2018); or 5) provision of starter ration containing anticoccidial medication, although such an event would be rare.
All vaccinated flocks showed an increase in OPG from week 1 to 2 (Figure 2) confirming that environmental cycling (Price et al., 2014) was successful; oocysts shed by vaccinated chicks had sporulated by the end of the first week and been ingested from the litter. Most vaccinated flocks had maximal OPG counts at week 2 (28.6% of flocks) or week 3 (59.5%), whereas a few had maximal counts at week 4 (11.9%). None of the vaccinated flocks had maximal counts at week 5, suggesting that all vaccinated flocks had developed at least partial protective immunity to the Eimeria spp. present by week 5 (Figure 2). The maximal OPG count for each vaccinated flock (regardless of the week at which this maximal count was attained) ranged from 11,608 to 307,359 (mean 110,133; median 88,406).
Seasonal Variation in OPG Counts
The mean and median OPG counts at the end of each week from medicated and vaccinated flocks (regardless of season) and mean counts from summer flocks (i.e., S16 and S17 combined) and winter flocks (i.e., W17 and W18 combined) are summarized in Figure 3; data are aggregated to the facility level. These aggregate data do not agree with the predicted values determined using the PROC MIXED model results summarized in Table 4; however, these aggregate data reflect more realistic OPG count shedding patterns expected to be observed by flocks on either prevention strategy than the model predictions.
Among vaccinated flocks, the mean OPG count was highest in week 3 for both summer and winter flocks; however, the mean count in week 2 was almost as high as week 3 for the summer flocks (Figure 3A). Before the onset of protective immunity, oocyst shedding is largely proportional to the number of infective, sporulated oocysts ingested by birds from the environment; this cycling (Price et al., 2014) is highly dependent on the environmental conditions in the barn. The external environment can certainly impact the environment inside the barn. Cold winter air holds less moisture than warm air, and barn heaters could create a dryer environment in the barn, especially during the first 2 wk after flock placement. Thereafter, chickens begin generating more moisture in the barn through respiration and defecation, allowing for more efficient Eimeria spp. cycling. Dry conditions impact oocyst sporulation success and shorten oocyst survival in the barn environment (Williams, 2005), which can thereby reduce the number of infective oocysts available for ingestion by chicks in their second week of life; this could explain the 1-week delay in cycling in the winter flocks.
Among medicated flocks, the mean OPG count was highest in week 4 for both summer and winter flocks, and the same general pattern was observed in both seasons, in that the count increased from week 2 to 4 then decreased from week 4 to 5, albeit the changes were much more dramatic in the summer flocks (Figure 3B). Notwithstanding, the mean count in week 4 was approximately one and a half times higher in summer flocks compared with winter flocks. The opposite was observed 1 wk later, in that the mean count in week 5 was approximately 2 times higher in winter flocks compared with summer flocks. These observations suggest that, similar to the vaccinated flocks, the drier environmental conditions in the winter delayed the cycling of parasites in the barn. However, owing to the typical seasonal rotation of anticoccidial medications in broiler production (Dalloul and Lillehoj, 2006), the medicated winter flocks would likely have been on a different program than the medicated summer flocks. Such a systematic change in drug program would have influenced the OPG counts due to the anticoccidial-specific sensitivity of the Eimeria spp. isolates (Chapman et al., 2016).
The range of temperatures in Ontario can range from −25°C (−13°F) in the winter to +35°C (95°F) in the summer (daily lows and daily highs in the respective season). This wide range of temperatures has certainly influenced building design for broiler barns in Canada and, consequently, the environmental conditions within. Therefore, these external factors must be taken into consideration when attempting to predict OPG cycling in broiler production in other climates.
Flock Performance
The results of the regression models (Table 5) showed that there was only a significant difference in mortality between medicated and vaccinated flocks after controlling for facility-level variation and flock and seasonal effects. Performance data were only available for approximately half (55.8%) of the 95 flocks; post hoc power calculations indicated that there was insufficient power (β < 0.80) to detect differences in performance between coccidiosis prevention programs (data not shown).
Table 5.
Flock performance data for medicated and vaccinated flocks for which data were available.
| Prevention strategy | Mortality (%) | Days to market | Mean weight at market (kg) | FCR1 | Adjusted FCR (target 2.42 kg) | Condemnations (%) |
|---|---|---|---|---|---|---|
| Medicated | 3.55 ± 0.57 (n = 20) | 40.1 ± 0.86 (n = 20) | 2.53 ± 0.05 (n = 20) | 1.681 ± 0.023 (n = 19) | 1.693 ± 0.021 (n = 19) | 1.17 ± 0.15 (n = 19) |
| Vaccinated | 5.47 ± 0.67 (n = 18) | 38.04 ± 0.46 (n = 18) | 2.40 ± 0.06 (n = 18) | 1.694 ± 0.030 (n = 13) | 1.721 ± 0.03 (n = 13) | 0.98 ± 0.05 (n = 17) |
| P value | 0.0189 | 0.085 | 0.077 | 0.886 | 0.433 | 0.3696 |
Mean values ± SEM for flocks clustered by facility.
FCR refers to the unadjusted calculated feed conversion ratio based on feed consumption and the total flock weight (no. of chickens × average weight) at market.
Medicated flocks had significantly lower mortality (3.55%) than vaccinated flocks (5.47%; P = 0.019). In addition to chemical or ionophore anticoccidials, in-feed antibiotics (e.g., bacitracin, virginiamycin, avilamycin) were used in all medicated flocks. The impact of the combination of in-feed antibiotics and ionophores on the enteric microbiota is likely responsible for the observed lower mortality in the medicated flocks (Dibner and Richards, 2005; Thabet et al., 2017; Kogut, 2019). Despite coccidiosis having a more severe impact on broiler metabolism in chickens nearing market age than younger chicks (Teeter et al., 2008; Price, 2012) and high OPG counts typical of medicated flocks, this impact was not reflected in increased mortalities.
Medicated flocks had a numerically lower adjusted FCR (1.693) than vaccinated flocks (1.721; P = 0.433). Part of this difference can be explained by the mean market weight of the medicated flocks (2.53 kg) that was higher than the target weight (2.40 kg) used for calculating the adjusted FCR. The higher mean market weight of the medicated flocks can be explained in part by production periods that averaged one and a half days longer (40 d) than vaccinated flocks (38 d) typically grown to smaller final body weights (∼2.2 kg). Despite the considerable Eimeria spp. challenge faced by most medicated flocks during the latter half of the grow-out period (Teeter et al., 2008; Price, 2012), growth-promoting in-feed antibiotics and ionophores supported strong growth performance over the production period.
Our study was designed to understand “where we are today” with oocyst cycling patterns in conventional (medicated) and RWA (vaccinated) broiler flocks reared in Canada; the observed cycling patterns provide a useful benchmark for recognizing desirable cycling patterns in future poultry production in which routine antimicrobial use has been largely eliminated. Our study has shown that RWA flocks had consistent and early oocyst output that resolved well before the fifth week of production in most cases.
As the market share of alternative production continues to grow, it will become prudent for the industry to learn best management practices from producers that have been successful at rearing RWA chickens, and from studies designed to investigate alternatives to antimicrobials in commercial flocks. As the Canadian broiler industry prepares for the removal of category III antibiotics for preventative use, both alternative and conventional flocks will want to continue to monitor Eimeria spp. infections to reduce the associated threat of necrotic enteritis.
Acknowledgments
The authors thank the producers who participated in the study, those who aided in the enrollment of producers, and those who provided technical assistance (J. Cobean, R. Imai, P. Kruth, A. Léveillé, and J. Whale of the University of Guelph), without whom this research would not have been successful. This research was funded through a grant from the National Science and Engineering Research Council (NSERC, Ottawa, Ontario, Canada, Grant ID: 400566) to J.R. Barta. Ryan P. Snyder was supported through the Ontario Veterinary College Graduate Student Stipend.
References
- Aviagen . 2018. Management Handbook. Accessed Nov. 2020. https://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross-BroilerHandbook2018-EN.pdf. [Google Scholar]
- Bafundo K.W., Cervantes H.M., Mathis G.F. Sensitivity of Eimeria field isolates in the United States: Responses of nicarbazin-containing anticoccidials. Poult. Sci. 2008;87:1760–1767. doi: 10.3382/ps.2008-00129. [DOI] [PubMed] [Google Scholar]
- Cha J.O., Zhao J., Yang M.S., Kim W.I., Cho H.S., Lim C.W., Kim B. Oocyst-shedding patterns of three Eimeria species in chickens and shedding pattern variation depending on the storage period of Eimeria tenella oocysts. J. Parasitol. 2018;104:18–22. doi: 10.1645/16-132. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. Anticoccidial drugs and their effects upon the development of immunity to Eimeria infections in poultry. Avian Pathol. 1999;28:521–535. doi: 10.1080/03079459994317. [DOI] [PubMed] [Google Scholar]
- Chapman H.D. A landmark contribution to poultry science — prophylactic control of coccidiosis in poultry. Poult. Sci. 2009;88:813–815. doi: 10.3382/ps.2008-00316. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Barta J.R., Blake D., Gruber A., Jenkins M., Smith N.C., Suo X., Tomley F.M. A selective review of advances in coccidiosis research. Adv. Parasitol. 2013;83:93–171. doi: 10.1016/B978-0-12-407705-8.00002-1. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Barta J.R., Hafeez M.A., Matsler P., Rathinam T., Raccoursier M. The epizootiology of Eimeria infections in commercial broiler chickens where anticoccidial drug programs were employed in six successive flocks to control coccidiosis. Poult. Sci. 2016;95:1774–1778. doi: 10.3382/ps/pew091. [DOI] [PubMed] [Google Scholar]
- Chapman H.D., Cherry T.E., Danforth H.D., Richards G., Shirley M.W., Williams R.B. Sustainable coccidiosis control in poultry production: the role of live vaccines. Int. J. Parasitol. 2002;32:617–629. doi: 10.1016/s0020-7519(01)00362-9. [DOI] [PubMed] [Google Scholar]
- Chicken Farmers of Canada . 2018. Chicken Farmers of Canada On-Farm Food Safety Assurance Program Manual. Accessed Nov. 2020. https://www.chickenfarmers.ca/wp-content/uploads/2014/07/OFFSAP-Manual-2014-with-2018-update.pdf. [Google Scholar]
- Dalloul R.A., Lillehoj H.S. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev. Vaccin. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth Promoters in Agriculture : history and Mode of Action. Poult. Sci. 2005;83:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Grumbles L.C., Delaplane J.P., Higgins T.C. Continuous feeding of low concentrations of sulfaquinoxaline for the control of coccidiosis in poultry. Poult. Sci. 1948;27:605–608. [Google Scholar]
- Haug A., Gjevre A.-G., Skjerve E., Kaldhusdal M. A survey of the economic impact of subclinical Eimeria infections in broiler chickens in Norway. Avian Pathol. 2008;37:333–341. doi: 10.1080/03079450802050705. [DOI] [PubMed] [Google Scholar]
- Hodgson J.N. Coccidiosis: oocyst counting technique for coccidiostat evaluation. Exp. Parasitol. 1970;28:99–102. doi: 10.1016/0014-4894(70)90073-1. [DOI] [PubMed] [Google Scholar]
- Hu J., Fuller L., McDougald L.R. Do anticoccidials interfere with development of protective immunity against coccidiosis in broilers? J. Appl. Poult. Res. 2000;9:352–358. [Google Scholar]
- Jenkins M.C., Parker C.C., O’Brien C.N., Ritter D. Viable Eimeria oocysts in poultry house litter at the time of chick placement. Poult. Sci. 2019;98:3176–3180. doi: 10.3382/ps/pez147. [DOI] [PubMed] [Google Scholar]
- Jenkins M.C., Parker C., Ritter D. Eimeria oocyst concentrations and species Composition in litter from commercial broiler farms during anticoccidial drug or live Eimeria oocyst vaccine control programs. Avian Dis. 2017;61:214–220. doi: 10.1637/11578-010317-Reg.1. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Joyner L.P., Long P.L. The specific characters of the Eimeria, with special reference to the coccidia of the fowl. Avian Pathol. 1974;3:145–157. doi: 10.1080/03079457409353827. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019;250:32–40. [Google Scholar]
- Long P.L., Joyner L.P. Problems in the identification of species of Eimeria. J. Protozool. 1984;31:535–541. doi: 10.1111/j.1550-7408.1984.tb05498.x. [DOI] [PubMed] [Google Scholar]
- Long P.L., Rowell J.G. Sampling broiler house litter for cooccidial oocysts. Br. Poult. Sci. 1975;16:583–592. doi: 10.1080/00071667508416233. [DOI] [PubMed] [Google Scholar]
- Martin A.G., Danforth H.D., Barta J.R., Fernando M.A. Analysis of Immunological and Cross-protection sensivities to anticoccidial drugs amoung five Geographical and Temporal strains of Eimeria maxima. Int. J. Parasitol. 1997;27:527–533. doi: 10.1016/s0020-7519(97)00027-1. [DOI] [PubMed] [Google Scholar]
- McDonald V., Shirley M.W. Past and future: vaccination against Eimeria. Parasitology. 2009;136:1477–1489. doi: 10.1017/S0031182009006349. [DOI] [PubMed] [Google Scholar]
- Mcdougald L.R., Fuller L., Mattiello R. A survey of coccidia on 43 poultry farms in Argentina. Avian Dis. 1997;41:923–929. [PubMed] [Google Scholar]
- Oden L.A., Lee J.T., Pohl S.K., Klein A.E., Anderson S.A., Dougherty S.D., Broussard C.T., Fitz-Coy S.H., Newman L.J., Caldwell D.J. Influence of diet on oocyst output and intestinal lesion development in replacement broiler breeders following live oocyst coccidiosis vaccination. J. Appl. Poult. Res. 2012;21:445–459. [Google Scholar]
- Ogedengbe J.D., Hunter D.B., Barta J.R. Molecular identification of Eimeria species infecting market-age meat chickens in commercial flocks in Ontario. Vet. Parasitol. 2011;178:350–354. doi: 10.1016/j.vetpar.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Parent E., Fernandez D., Boulianne M. The use of a live non-attenuated coccidiosis vaccine modifies Eimeria spp. excretion in commercial antibiotic-free broiler chicken flocks compared to conventional shuttle anticoccidial programs. Poult. Sci. 2018;97:2740–2744. doi: 10.3382/ps/pey140. [DOI] [PubMed] [Google Scholar]
- Peek H.W., Landman W.J.M. Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Vet. Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- Price K.R. Use of live vaccines for coccidiosis control in replacement layer pullets. J. Appl. Poult. Res. 2012;21:679–692. [Google Scholar]
- Price K.R., Freeman M., Van-Heerden K., Barta J.R. Shedding of live Eimeria vaccine progeny is delayed in chicks with delayed access to feed after vaccination. Vet. Parasitol. 2015;208:242–245. doi: 10.1016/j.vetpar.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Price K.R., Guerin M.T., Barta J.R. Success and failure: the role of relative humidity levels and environmental management in live Eimeria vaccination of cage-reared replacement layer pullets. J. Appl. Poult. Res. 2014;23:523–535. [Google Scholar]
- Price K.R., Hafeez M.A., Bulfon J., Barta J.R. Live Eimeria vaccination success in the face of artificial non-uniform vaccine administration in conventionally reared pullets. Avian Pathol. 2016;45:82–93. doi: 10.1080/03079457.2015.1125442. [DOI] [PubMed] [Google Scholar]
- Reid W.M., Long P.L. A diagnostic chart for nine species of fowl coccidia. Univ. Ga. Coll. Agric. Res. Rep. 1979;335:1–24. [Google Scholar]
- Stephan B., Rommel M., Daugschies A., Haberkorn A. Studies of resistance to anticoccidials in Eimeria field isolates and pure Eimeria strains. Vet. Parasitol. 1997;69:19–29. doi: 10.1016/s0304-4017(96)01096-5. [DOI] [PubMed] [Google Scholar]
- Teeter R.G., Beker A., Brown C., Broussard C., Fitz-Coy S., Radu J., Newman L.J. 2008. Transforming Coccidiosis-Mediated Lesion Scores into Production and Calorific Cost. Pages 18–21 in Proc 23rd World Poult. Congr., Bris. [Google Scholar]
- Thabet A., Zhang R., Alnassan A., Daugschies A., Bangoura B. Veterinary Parasitology Anticoccidial efficacy testing: in vitro Eimeria tenella assays as replacement for animal experiments. Vet. Parasitol. 2017;233:86–96. doi: 10.1016/j.vetpar.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Tyzzer E. Coccidiosis in gallinaceous birds. Am. J. Hyg. 1929;10:269–383. [Google Scholar]
- Velkers F.C., Bouma A., Stegeman J.A., De Jong M.C.M. Transmission of a live Eimeria acervulina vaccine strain and response to infection in vaccinated and contact-vaccinated broilers. Vaccine. 2012;30:322–328. doi: 10.1016/j.vaccine.2011.10.090. [DOI] [PubMed] [Google Scholar]
- Williams R.B. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int. J. Parasitol. 1999;29:1209–1229. doi: 10.1016/s0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Quantification of the crowding effect during infections with the seven Eimeria species of the domesticated fowl : its importance for experimental designs and the production of oocyst stocks. Int. J. Parasitol. 2001;31:1056–1069. doi: 10.1016/s0020-7519(01)00235-1. [DOI] [PubMed] [Google Scholar]
- Williams R.B. 2002. Anticoccidial Vaccines for Broiler Chickens: Pathways to Success. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Intercurrent coccidiosis and necrotic enteritis of chickens: Rational, integrated disease management by maintenance of gut integrity. Avian Pathol. 2005;34:159–180. doi: 10.1080/03079450500112195. [DOI] [PubMed] [Google Scholar]