Abstract
An experiment was conducted to estimate the nutritional requirements of calcium (Ca) and available phosphorus (aP) for Japanese quails (Coturnix coturnix japonica) in the egg-laying stage (64–168 D). The experiment was an entirely randomized design, in a factorial scheme (Ca = 1.70, 2.40, 3.10, and 3.80% and aP = 0.15, 0.30, 0.45, and 0.60%), with 3 replicates and 10 quails per experimental unit. No interactions were found for quail body weight and daily feed intake (DFI) (P > 0.05). However, body weight decreased linearly (P < 0.05) as the levels of Ca increased, whereas DFI exhibited a quadratic effect (P < 0.05) for both Ca and aP. The lowest values of DFI were estimated in 2.79 and 0.36% for Ca and aP, respectively. Egg production, egg mass, and feed conversion ratio per egg dozen presented significant interaction in which all of them had a quadratic effect (P < 0.05), with estimations for maximum yield in feed containing 2.74, 2.71, 2.75, and 2.74% Ca and 0.40, 0.39, 0.39, and 0.40% aP. The concentration of Ca in the eggshell increased linearly as per the levels of Ca studied, having a quadratic effect for aP levels, with a maximum point of 0.44%. In relation to the bone parameters, there was a linear interaction for Ca and aP in bone density and bone resistance (BR) of the femur, with a quadratic effect in BD estimating 2.84 and 0.50% for Ca and aP, respectively. In BR, the estimation was 3.27% Ca, with linear increase for aP. The BR of the tibiotarso increased linearly as per the Ca levels, obtaining the same Ca concentration response in this bone. As conclusion, when considering the estimations obtained through overlapped contour plots, the best responses to the effects of Ca and aP on productive characteristics were estimated at 2.68% Ca and 0.38% aP to produce feed for egg-laying Japanese quails.
Key words: bone resistance, egg shell quality, nutrition
Introduction
There is a concern with nutrition of the Japanese bird because little is known about this farming activity under Brazilian conditions. Because the Japanese quail has been successfully introduced in many countries under a range of climatic conditions (Jeke et al., 2018), new information is required, especially regarding genetic material and rearing systems. The Brazilian Tables of Poultry and Swine (Rostagno et al., 2017) recommends on average 3.16% calcium and 0.33% available phosphorus (aP); however, other authors recommend 2.5 to 2.5% calcium and 0.15 to 0.35% aP (Costa et al., 2007, Costa et al., 2010, Silva, 2009).
Limited information on nutritional requirements can compromise food costs, in addition to underestimating or overestimating their needs, causing losses to the sector (Souza et al., 2017). From the nutritional point of view, minerals are among the most important ingredients composing 5% of animal body. Among all minerals, calcium and phosphorus stand out as they contribute to making up a large portion of animal skeleton (80–85%) and also participate in the formation of egg shells and muscle structure, thus being essential for the proper functioning of animal body (McDowell, 1992).
Calcium derived from bone is needed during the final stages of shell formation (Bain et al., 2016), so bone structure is of paramount importance.
The quality of the egg is also related to the nutrition of the birds, in which calcium and phosphorus have great impact. In the shell formation, the mineral phase corresponds to 95% and the organic phase to 3.5% (Gautron et al., 2001). The chemical composition (by weight) of by-product eggshell has been reported as follows: calcium carbonate (94%), magnesium carbonate (1%), calcium phosphate (1%), and organic matter (4%) (Stadelman, 2000), making calcium an essential mineral for the quality of egg shells. Sohail and Roland (2002) have stated that phosphorus is also a required mineral for metabolism and in eggshell formation. Furthermore, excess calcium influences the availability of other minerals, and excess phosphorus negatively affects eggshell quality (Silversides et al., 2006). This occurs because phosphorus is responsible for blood acidosis during the formation of the egg (Bertechini, 2014). In addition to being considered the third most expensive ingredient in the monogastric diet (Pinheiro et al., 2015), the excess of phosphorus in animal feed can cause serious problems to the environment because of excessive elimination in the feces (Delezie et al., 2015).
This binomial is directly associated to the quality of the eggs because low-quality eggs, caused by poor eggshell formation, soft shells, and cracked shells can cause great economic loss (Kira et al., 1996). Consequently, the adequate levels of minerals, in association with correct management, tend to decrease loss and increase production.
New research should be conducted to verify the requirements of commercial egg-laying Japanese quail lineages. Thus, the present study aimed to estimate the optimum levels of calcium (Ca) and aP in animal feed to obtain maximum yield from egg-laying quails, from the beginning of production (64 D) up to 168 D of age. Furthermore, during this period, the impact of these minerals on the quality of the eggs and the bones of the quails was assessed.
Material and methods
The experiment was conducted in the Coturniculture sector of the Experimental Farm of Iguatemi, belonging to the State University of Maringá. All the procedures used were in accordance with the norms of the Ethics Committee on Animal Experimentation of the State University of Maringá (Protocol No. 5250070515/2015).
Animals, Experimental Design, and Diets
The birds were acquired from a commercial breeder at 1 D of age (commercial lineage – Vicami) and raised from the first to the 42nd day with conventional corn and soybean meal feed, in accordance with the nutritional recommendations of Rostagno et al. (2011). Subsequently, 480 female laying quails, 43-day-old, were selected, transferred to cages, and evaluated from 64 D of age when they reached maximun laying rate.
The experiment was conducted in a completely randomized design and 4 × 4 factorial scheme (Ca = 1.70, 2.40, 3.10, 3.80% × aP = 0.15, 0.30, 0.45, 0.60%), totaling 16 treatments with 3 replicates of 10 quails each. The initial average weight of the quails was 120.57 g, and the birds were kept in a conventional building, containing galvanized-wire cages, with nipple drinkers and trough type feeders. During the entire experiment (from day 43–day 168), feed and water were supplied at will (ad libitum). The temperature and air humidity levels were collected at 8 am, which registered minimum and maximum temperatures and air humidity levels with averages of 24°C to 16°C and 87 to 57%, respectively.
The lighting program began at 43 D of age, supplying 14 h of light. Every week, 30 min of light was added until it reached 17 total hour of natural + artificial light. An automatic timer controlled the lighting program with a light intensity of 21 lux.
Experimental diets were formulated in a corn and soybean meal base, considering the recommendations and chemical composition values of feed proposed by Rostagno et al. (2011), except for Ca and aP (Table 1).
Table 1.
Percentage and nutritional calculate composition of the basal diet for the Japanese quail in the laying phase with levels of calcium and available phosphorus.
| Diet | Quantity (g/kg) |
|---|---|
| Corn | 545.03 |
| Soybean meal 45% | 301.24 |
| Soybean oil | 27.79 |
| Dicalcium phosphate x limestone x inert blend1 | 110.83 |
| Vitamin/mineral suplement2 | 4.00 |
| Salt | 3.25 |
| DL-Methionine | 4.03 |
| L-Lysine HCl | 2.67 |
| L-Threonine | 0.68 |
| L-Tryptophan | 0.38 |
| BHT | 0.10 |
| Calculated values | |
| Metabolizable energy (kcal/kg) | 2.900 |
| Crude protein (%) | 18.800 |
| Lysine digestible (%) | 1.120 |
| Met.+cyst. digestible (%) | 0.900 |
| Threonine digestible (%) | 0.658 |
| Tryptophan digestible (%) | 0.230 |
| Chlorine (%) | 0.286 |
| Sodium (%) | 0.146 |
| Potassium (%) | 0.710 |
| Electrol. balance mEq/kg | 164 |
Abbreviation: BHT, butyl hydroxy toluene.
Dicalcium phosphate and limestone–diluted soft sand at the levels of 1.70, 2.40, 3.10, 3.80% calcium and 0.15, 0.30, 0.45, 0.60% available phosphorus.
Vitamin/Mineral supplementation (garante levels per kg of product); Vitamin A, 3,125 IU/g; Vitamin D3, 625,000IU/g; Vitamin K3, 975 mg; Vitamin B1, 1,225 mg; Vitamin B2, 2,200 mg; Vitamin B6, 2,062.500 mg; Vitamin B12, 6,250 mg; Vitamin E, 15,645IU; Calcium pantothenate, 14,843.750 mg; Niacin, 15,312.500 mg; Folic acid, 416.667 mg; Biotin, 62.500 mg; Choline, 81,375.00 mg; Antioxidant, 1,250 mg.
Performance
Starting on day 64, during 5 productive cycles (21 D), the quails were evaluated, and the eggs were collected daily (8 am) with the intent of calculating the egg production (%) and egg mass (EM) (g eggs/bird/day). The quails and the feed were weighed at the end of each cycle to determine their respective body weight (BW), daily feed intake (DFI) (g), and feed conversion ratio per egg and per dozen egg. The dead birds were counted daily to correct the feed consumption and to determine the viability of each experimental unit.
Internal and External Quality of the Eggs
The internal and external quality of the eggs was conducted in the last 3 D of each cycle, along with average weight measurements of all viable eggs (soft, broken, and cracked eggs were excluded). Specific gravity was performed on all eggs as per the methodology described by Hamilton (1982). For this, the eggs were immersed in saline solution (NaCl) with densities 1.050; 1.055; 1,060; 1.065; 1.70; 1.075; 1.080; 1.085; and 1.090 g/cm³, placed in growing order, from the first and so on until the eggs floated in the solution.
For all other quality assessments, 3 eggs within the average weight were chosen and evaluated. They were cut, and their internal content was placed on a plate of dark glass to measure the height (mm) and diameter (mm) of the egg yolk and albumen, which was conducted using a digital caliper (accurate to 0.02 mm; digimess). The height of the yolk was determined at its highest point and the height of the albumen at the region closest to the yolk. Furthermore, the diameter was obtained by calculating the averages of 2 traversing measurements of the yolk. Using these data, the internal quality of the eggs was assessed, determining the yolk index and the (Haugh, 1937).
Afterward, the yolk and albumen were separated to conduct weight assessments using a precision scale (model BL3200H; Shimadzu; accurate to 0.001 g). In addition, the shells were washed (shell membranes have not been removed) and, after they dried, they too were weighed. These data enabled us to quantify the percentages of yolk, albumen, and shell in relation to the weight of the egg.
The shells were also used for thickness measurements (mm), which was determined at 4 different points in the equatorial region using a thickness gauge (model 700-118 “Quick Mini;” Mitutoyo). Furthermore, the shell weight per unit of surface area (SWUSA) was determined by using the formula adapted from Rodrigues et al. (1996), were SWUSA = (shell weight (g)/3.9782 × egg weight (g)) × 100. Finally, were used to determine dry matter and concentration of mineral and calcium matter, in accordance with the methodologies described by AOAC (2005).
Serum Biochemical Profile
To quantify the serum levels of calcium, phosphorus, albumin, total protein, and alkaline phosphatase, blood was collected from 2 birds at the end of the experiment (week 24). The quails selected were within the average replicate weight (±5%). Before collection, they were submitted to a 6-h fasting solid.
Blood was collected through the ulnar vein and centrifuged (3,000 rpm for 15 min). The serum obtained was stored at −20°C until spectrophotometer analysis (Bioplus 2000 model) using commercial kits (Gold Analisa Diagnóstica Ltda).
Bone Variables
The same birds used for blood sampling were sacrificed and had their left tibiotarsus and femurs collected and frozen (−20°C) until analyses. To determine the Seedor index (Seedor et al., 1996; where Seedor index = bone weight (mg)/bone length (mm)), the adjacent tissues were removed and the bones were weighed on a precision scale and their length was measured using a digital caliper. Then, the bones were immersed in petroleum ether for 24 h for degreasing and later dried in a forced ventilation oven at 55°C for 72 h for future assessments.
Radiographic optical density measurements were with the bones placed under periapical film containing an aluminum scale and were then radiographed (dental x-rays, model Spectro 70X electronic; Dabi Atlante). The processing of radiographic films was conducted using an automatic processor, with a work period of about 150 s, operating with Kodak RP X-Omat solutions. Then, they were scanned and saved as a progressive JPEG file. The readings of the x-rays for determining the density of the bones were performed using the “Adobe Photoshop CS6” software and its “Histogram” tool, which is based on a gray scale. The optical radiographic density was obtained by comparing the area of 3 central bone points (10 pixels × 10 pixels) with one point of the third step of the aluminum scale.
The resistance analyses were conducted in the Mechanic Laboratory of Soils of the Civil Engineering using a press for assays of simple compressive strength. The bones were positioned leaning on the epiphysis region. Force was applied in the central region using a probe with the speed of 5 mm/s and a load of 500 N in which the applied force was measured at the time before bone rupture.
After determining bone resistance, dry matter and mineral, calcium, and phosphorus matter concentration were measured, following the methodologies described by AOAC (2005).
Statistical Analysis
The statistical analysis of data was conducted in R studio (R Core Team, Version 3.5.3, 2019) statistics program, and the package used was Agricolae. The model described in the following was used to test the effects:
where Yijk = variable measured in experimental unit k, fed with a diet containing level i of calcium and level j of aP; β0 = general constant; βi = calcium effect; βj = effect of aP; βiβj = effect of the interaction between calcium and aP; Ɛijk = random error associated with each observation.
The effects were tested, and then, normality of residuals was checked. When factor effect was significant (P < 0.05), the polynomial regression analyses were conducted with the intent of estimating the best data adjustment model in which the nutritional requirement was determined in accordance with to the quadratic model, as proposed by Sakomura and Rostagno (2016).
An overlapping contour graph was drawn using yield variables with a quadratic effect by R software, as proposed by Oliveira-Bruxel (2016). The region where the responses of the equations are within their limits is shown, and the graph contour lines show the intersection to estimate the corresponding values on the axes that represent Ca and aP.
Results
Performance
In accordance with the levels of the studied minerals (Table 2), no significant interaction nor effects were observed for viability and age at the first egg. Body weight of the quails and DFI had no significant interaction. However, BW did have decreasing linear effect as per the levels of Ca. Daily feed intake exhibited a quadratic effect for Ca and for aP in which the lowest estimated levels were 2.79 and 0.37%, respectively.
Table 2.
Performance of the Japanese quail in the laying phase (64–168 D of age) with levels of calcium and available phosphorus.
| Ca (%) |
1.70 |
2.40 |
3.10 |
3.80 |
SE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aP (%) | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | |
| IBW (g) | 160.07 | 163.02 | 162.08 | 162.80 | 157.63 | 161.87 | 162.93 | 160.70 | 158.26 | 159.33 | 162.40 | 160.78 | 157.40 | 157.87 | 157.40 | 160.60 | 0.571 |
| FBW (g) | 174.09 | 177.86 | 177.84 | 181.33 | 174.19 | 181.05 | 172.16 | 170.73 | 172.54 | 168.26 | 173.76 | 173.56 | 171.20 | 171.98 | 171.36 | 164.88 | 0.787 |
| DFI (g/bird/day) | 32.50 | 29.55 | 30.11 | 31.61 | 29.37 | 25.56 | 25.30 | 29.97 | 28.70 | 24.54 | 24.99 | 29.10 | 30.63 | 30.37 | 29.46 | 31.52 | 0.385 |
| EP (%) | 82.80 | 86.79 | 86.62 | 88.38 | 84.17 | 96.55 | 96.82 | 93.95 | 88.37 | 96.96 | 96.81 | 87.34 | 85.00 | 88.49 | 89.33 | 83.69 | 0.770 |
| EM (g eggs/bird/day) | 7.79 | 8.86 | 9.05 | 8.75 | 8.85 | 10.87 | 10.92 | 9.97 | 9.49 | 10.53 | 10.76 | 8.99 | 8.06 | 8.93 | 8.93 | 8.04 | 0.153 |
| FCEM | 4.19 | 3.33 | 3.33 | 3.61 | 3.34 | 2.35 | 2.32 | 3.01 | 3.02 | 2.33 | 2.32 | 3.24 | 3.81 | 3.40 | 3.30 | 3.92 | 0.087 |
| FCDZ | 0.63 | 0.49 | 0.52 | 0.54 | 0.63 | 0.39 | 0.40 | 0.44 | 0.49 | 0.38 | 0.38 | 0.53 | 0.57 | 0.51 | 0.49 | 0.62 | 0.012 |
| Viability (%) | 96.67 | 90.00 | 100.00 | 96.67 | 80.00 | 96.67 | 100.00 | 83.33 | 90.00 | 93.33 | 96.67 | 90.00 | 96.67 | 100.00 | 96.67 | 90.00 | 1.412 |
| Age at first egg (days) | 48.00 | 49.67 | 48.00 | 50.00 | 50.00 | 50.00 | 46.67 | 47.67 | 50.33 | 49.00 | 51.33 | 48.67 | 53.33 | 49.00 | 50.33 | 51.67 | 0.580 |
| Regression equation | R2 |
P value |
Estimate |
|||
|---|---|---|---|---|---|---|
| Ca | aP | Ca x aP | Ca | aP | ||
| FBW = 183.8740 − 3.7544Ca | 0.30 | <0.0001 (L) | 0.1047 (NS) | 0.0745 | - | - |
| DFI = 61.9427 −20.0890Ca + 3.5990Ca2 −48.4681 aP + 65.2500aP2 | 0.76 | <0.0001 (Q) | <0.0001 (Q) | 0.7139 | 2.79 | 0.37 |
| EP = 19.4562 + 38.4014Ca-6.3618Ca2+123.74541 aP-124.0833aP2-28.7406Ca∗aP | 0.72 | <0.0001 (Q) | <0.0001 (Q) | 0.0107 | 2.74 | 0.40 |
| EM = −5.5681 + 8.81452Ca −1.5259Ca2 + 23.08904 aP −24.7130P2 -1.3432Ca∗aP | 0.85 | <0.0001 (Q) | <0.0001 (Q) | 0.0081 | 2.71 | 0.39 |
| FCEM = 11.9447 −5.1687Ca +0.8890Ca2 −13.6504Pd +15.1204P2 +0.7235Ca∗aP | 0.90 | <0.0001 (Q) | <0.0001 (Q) | 0.0024 | 2.75 | 0.39 |
| FCDZ = 1.6380 −0.5819Ca + 0.0948Ca2 −2.3777 aP+ 2.4537aP2 + 0.1597Ca∗aP | 0.82 | <0.0001 (Q) | <0.0001 (Q) | 0.0004 | 2.74 | 0.40 |
Abbreviations: aP, available phosphorus; DFI, daily feed intake; EM, egg mass; EP, egg production; FBW, final body weight; FCDZ, feed conversion ratio per dozen egg; FCEM, feed conversion ratio per egg mass IBW, initial body weight; L, linear effect; NS, nonsignicant; Q, quadratic effect.
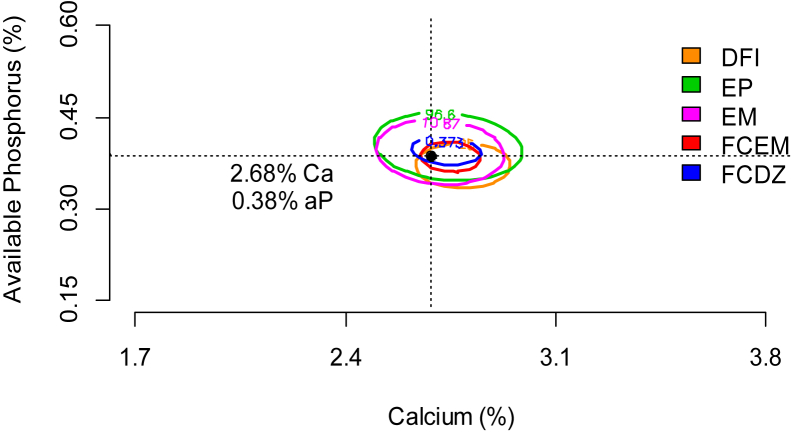
Egg production, EM, feed conversion ratio per EM, and feed conversion ratio per egg dozen exhibited significant interaction in which all of them had a quadratic effect concerning the levels of Ca and aP. The estimations for maximum egg production and EM were obtained when using feed containing 2.74 Ca and 0.40% aP and 2.71% Ca and 0.39% aP, respectively. The results for feed conversion ratio per EM and feed conversion ratio per egg dozen were obtained by using 2.75 and 2.74% Ca and 0.39 and 0.40% aP, respectively. The performance variables showed similar responses when Ca and aP levels were raised, reaching values very close to the maximum yield of quails in the egg-laying stage. However, using the overlapped contour plot, the viable estimations were 2.68% Ca and 0.38% aP (Figure 1). This point on the graph indicates these independent variables showing the best results.
Figure 1.
Overlapping contour graph of daily feed intake (DFI), egg production (EP), egg mass (EM), feed conversion ratio per egg mass (FCEM), and feed conversion ratio per dozen egg (FCDZ) indicating the estimated levels of calcium and available phosphorus (aP) of Japanese laying quails.
Internal and External Quality of the Eggs
Egg weight (EW) had no significant interaction with none of the studied minerals, however, which exhibited a quadratic effect for both minerals. The optimal point was estimated at 2.69% Ca and 0.38% aP (Table 3).
Table 3.
Egg quality of the Japanese quail in the laying phase (64–168 D of age) with levels of calcium and available phosphorus.
| Ca (%) |
1.70 |
2.40 |
3.10 |
3.80 |
SE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aP (%) | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | |
| SG (g/mL) | 1.072 | 1.066 | 1.077 | 1.070 | 1.071 | 1.072 | 1.074 | 1.071 | 1.073 | 1.070 | 1.064 | 1.072 | 1.069 | 1.074 | 1.077 | 1.077 | 0.001 |
| Haugh units | 91.39 | 93.82 | 94.33 | 90.75 | 93.94 | 95.72 | 95.44 | 94.06 | 93.45 | 95.94 | 96.47 | 93.77 | 91.48 | 93.60 | 93.89 | 92.02 | 0.275 |
| Yolk Index | 0.46 | 0.44 | 0.46 | 0.46 | 0.47 | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 | 0.47 | 0.46 | 0.45 | 0.46 | 0.46 | 0.47 | 0.002 |
| EW (g) | 9.40 | 10.21 | 10.45 | 9.90 | 10.50 | 11.26 | 11.28 | 10.61 | 10.74 | 10.86 | 11.12 | 10.30 | 9.48 | 10.10 | 9.99 | 9.62 | 0.093 |
| % Yolk | 30.70 | 31.36 | 31.25 | 30.82 | 30.49 | 31.31 | 30.69 | 30.61 | 30.26 | 31.24 | 30.85 | 30.70 | 30.43 | 30.40 | 30.63 | 30.53 | 0.080 |
| % Albumen | 62.13 | 62.13 | 61.72 | 61.97 | 62.19 | 61.22 | 61.90 | 61.92 | 62.28 | 61.54 | 61.60 | 61.71 | 62.15 | 61.90 | 61.58 | 61.83 | 0.103 |
| % Shell | 6.68 | 7.05 | 6.96 | 6.71 | 7.33 | 7.92 | 7.88 | 7.46 | 7.47 | 7.88 | 7.72 | 7.55 | 7.13 | 7.70 | 7.68 | 7.30 | 0.060 |
| SWUSA | 3.15 | 3.63 | 3.62 | 3.21 | 3.73 | 3.96 | 3.90 | 3.81 | 3.83 | 3.97 | 3.90 | 3.81 | 3.18 | 3.94 | 3.91 | 3.52 | 0.041 |
| ST (mm) | 0.187 | 0.209 | 0.207 | 0.187 | 0.220 | 0.224 | 0.220 | 0.220 | 0.219 | 0.232 | 0.206 | 0.218 | 0.201 | 0.206 | 0.224 | 0.211 | 0.002 |
| MM (%DM) | 67.63 | 77.55 | 83.46 | 69.87 | 83.03 | 87.22 | 86.73 | 76.24 | 78.66 | 83.37 | 82.40 | 85.00 | 69.77 | 79.74 | 76.51 | 71.35 | 1.024 |
| Ca shell (%DM) | 31.56 | 36.40 | 37.85 | 31.50 | 34.32 | 38.14 | 37.07 | 38.11 | 35.52 | 37.40 | 39.53 | 37.03 | 36.61 | 37.54 | 38.15 | 41.34 | 0.451 |
| Regression equation | R2 |
P value |
Estimate |
|||
|---|---|---|---|---|---|---|
| Ca | aP | Ca x aP | Ca | aP | ||
| EW = 2.4236 + 5.1720Ca −0.9596Ca2 + 10.0686 aP −13.1204aP2 | 0.78 | <0.0001 (Q) | <0.0001 (Q) | 0.4445 | 2.69 | 0.38 |
| % Yolk = 30.3117−0.2317Ca + 6.9039 aP −8.8704aP2 | 0.25 | 0.0150 (L) | 0.0078 (Q) | 0.7416 | - | 0.38 |
| % Shell = 2.0943 + 3.0669Ca −0.5106Ca2 + 6.7558 aP −8.8056aP2 | 0.85 | <0.0001 (Q) | <0.0001 (Q) | 0.7684 | 3.00 | 0.38 |
| SWUSA = 0.0758 + 2.0316Ca −0.3503Ca2 +5.6383 aP −7.2407aP2 | 0.80 | <0.0001 (Q) | <0.0001 (Q) | 0.5057 | 2.90 | 0.39 |
| ST = 0.0342 + 0.1128Ca −0.0196Ca2 + 0.1861 aP −0.2407aP2 | 0.71 | <0.0001 (Q) | <0.0001 (Q) | 0.5618 | 2.88 | 0.39 |
| Ca shell = 24.6708 + 1.8158Ca + 39.0158 aP −44.6944aP2 | 0.41 | 0.0003 (L) | 0.0076 (Q) | 0.2352 | - | 0.44 |
Abbreviations: aP, available phosphorus; EW, egg weight; L, linear effect; MM, mineral matter; Q, quadratic effect; SG, specific gravity; ST, shell thickness; SWUSA, shell weight per unit of surface area.
Moreover, none of the evaluated parameters of internal and external qualities of the eggs showed significant interaction between the levels of Ca and aP. However, the percentage of shell, shell weight per unit of surface area (SWUSA), and shell thickness (ST) exhibited independent quadratic responses for each mineral. The percentage of the shell enabled to estimate the levels of Ca and aP is 3.0% and 0.38%, respectively. The SWUSA had its maximum point with 2.90% Ca and 0.39% aP, whereas in ST, it was with 2.88% Ca and also 0.39% aP.
The concentration of calcium in the eggshell had a quadratic effect in accordance with the aP levels, its maximum point being 0.44% aP and increasing linearly as per the levels of Ca studied.
The percentage of the yolk reduced linearly in accordance with the levels of Ca, wheras aP had a quadratic effect and its optimum levels were found to be 0.38%.
The Haugh unit, yolk index, and percentage of albumen are variables related to the internal quality of eggs and exhibited no interactions nor significant differences for the levels of Ca and aP used in the present study. The same was found for mineral matter results.
Serum Biochemical Profile
The levels of total calcium exhibited a linear increase in parallel to the levels of Ca used in the diet (Table 4). The levels of phosphorus, albumin, total protein, and alkaline phosphatase did not present a significant difference.
Table 4.
Serum biochemical profile of the Japanese quail in the laying phase (64–168 D of age) with levels of calcium and available phosphorus.
| Ca (%) |
1.70 |
2.40 |
3.10 |
3.80 |
SEA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aP (%) | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | |
| TP (g/dL) | 4.15 | 3.98 | 4.28 | 4.43 | 4.17 | 4.57 | 4.30 | 4.12 | 4.62 | 4.25 | 3.79 | 4.36 | 4.48 | 4.48 | 4.22 | 4.38 | 0.049 |
| Albumin (g/dL) | 1.35 | 1.50 | 1.28 | 1.32 | 1.18 | 1.33 | 1.27 | 1.15 | 1.27 | 1.15 | 1.08 | 1.25 | 1.30 | 1.56 | 1.32 | 1.30 | 0.019 |
| AP (U/L) | 607.5 | 783.3 | 576.7 | 365.7 | 475.2 | 705.3 | 625.5 | 533.3 | 374.8 | 690.5 | 613.4 | 579.7 | 494.2 | 897.8 | 548.5 | 273.5 | 23.896 |
| Ionic Ca (mg/dL) | 7.45 | 9.46 | 9.34 | 8.86 | 8.73 | 8.72 | 8.41 | 8.17 | 9.68 | 9.00 | 7.75 | 8.68 | 7.86 | 11.00 | 9.05 | 8.56 | 0.141 |
| Total Ca (mg/dL) | 14.63 | 16.78 | 17.63 | 17.76 | 16.90 | 15.98 | 16.62 | 15.80 | 18.57 | 18.33 | 16.33 | 16.38 | 15.78 | 20.61 | 20.96 | 17.05 | 0.289 |
| Phosphorus (mg/dL) | 7.72 | 7.93 | 9.98 | 9.42 | 10.12 | 11.98 | 8.03 | 6.83 | 12.02 | 11.12 | 11.80 | 10.62 | 10.39 | 8.59 | 8.07 | 9.43 | 0.257 |
| Regression equation | R2 |
P value |
Estimate |
|||
|---|---|---|---|---|---|---|
| Ca | aP | Ca x aP | Ca | aP | ||
| Total Ca = 8.5293 + 2.4489Ca | 0.17 | 0.0077 (L) | 0.1344 | 0.1851 | - | - |
Abbreviations: AP, alkaline phosphatase; L, linear effect; TP, total protein.
Bone Variables
The data obtained regarding the bone variables in the egg-laying stage show that the levels of Ca and aP had no significant effect on the Seedor index, mineral matter, and the concentration of phosphorus in either of the bones analyzed. They had no effect on the concentration of calcium in the femur and on the bone density (BD) of the tibiotarsus (Table 5).
Table 5.
Bone variables of the Japanese quail in the laying phase (64–168 D of age) with levels of calcium and available phosphorus.
| Ca (%) |
1.70 |
2.40 |
3.10 |
3.80 |
SEA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aP (%) | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | 0.15 | 0.30 | 0.45 | 0.60 | |
| Femur | |||||||||||||||||
| SI (mg/mm) | 12.50 | 13.13 | 14.40 | 12.41 | 13.67 | 14.74 | 14.71 | 12.89 | 14.85 | 15.67 | 15.45 | 14.41 | 12.76 | 13.25 | 13.1 | 13.11 | 0.170 |
| BD (mm Eq/Al) | 1.85 | 1.82 | 1.95 | 1.82 | 1.96 | 2.04 | 2.04 | 1.95 | 1.98 | 2.01 | 2.02 | 2.09 | 1.73 | 1.81 | 1.92 | 1.93 | 0.020 |
| BR (kgf) | 26.47 | 38.03 | 35.67 | 36.00 | 37.7 | 32.48 | 42.97 | 39.44 | 38.75 | 45.79 | 43.11 | 43.73 | 41.37 | 35.36 | 34.85 | 35.27 | 0.790 |
| MM (%DM) | 49.76 | 56.73 | 58.33 | 52.48 | 60.67 | 63.56 | 60.75 | 57.55 | 59.83 | 60.02 | 61.11 | 58.85 | 54.71 | 55.22 | 55.99 | 52.64 | 0.590 |
| CCa (%DM) | 19.32 | 22.43 | 19.64 | 23.32 | 23.92 | 22.8 | 21.84 | 23.75 | 22.19 | 22.86 | 23.76 | 24.60 | 21.00 | 21.11 | 22.91 | 23.3 | 0.260 |
| CP (%DM) | 10.55 | 10.64 | 10.23 | 10.32 | 10.36 | 10.47 | 10.52 | 10.64 | 10.2 | 10.72 | 11.17 | 10.30 | 9.55 | 9.57 | 9.91 | 9.71 | 0.070 |
| Tibiotarsus | |||||||||||||||||
| SI (mg/mm) | 10.87 | 11.32 | 13.16 | 11.92 | 11.43 | 13.36 | 12.61 | 12.87 | 12.18 | 12.93 | 13.49 | 12.3 | 11.37 | 11.52 | 11.69 | 10.77 | 0.14 |
| BD (mm Eq/Al) | 1.87 | 1.83 | 2.05 | 1.82 | 1.88 | 1.93 | 1.92 | 1.98 | 1.94 | 2.02 | 1.94 | 2.10 | 1.92 | 1.84 | 2.03 | 1.98 | 0.01 |
| BR (kgf) | 31.32 | 34.18 | 33.01 | 32.62 | 46.38 | 36.91 | 41.87 | 35.68 | 42.99 | 44.17 | 41.18 | 43.72 | 36.24 | 44.84 | 46.31 | 34.91 | 0.80 |
| MM (%DM) | 55.30 | 60.33 | 59.97 | 58.07 | 60.36 | 56.95 | 60.7 | 56.71 | 54.42 | 55.09 | 56.8 | 57.64 | 54.93 | 55.69 | 55.01 | 58.5 | 0.40 |
| CCa (%DM) | 22.79 | 16.91 | 12.85 | 21.61 | 26.85 | 22.72 | 19.6 | 26.11 | 25.19 | 21.97 | 23.88 | 22.16 | 22.88 | 24.82 | 23.72 | 23.9 | 0.49 |
| CP (%DM) | 10.07 | 10.40 | 10.28 | 10.20 | 10.47 | 10.77 | 10.66 | 10.67 | 10.44 | 10.45 | 11.10 | 10.53 | 9.30 | 9.40 | 10.13 | 9.74 | 0.08 |
| Regression equation | R2 |
P value |
Estimate |
|||
|---|---|---|---|---|---|---|
| Ca | aP | Ca x aP | Ca | aP | ||
| BD femur = 0,8709 + 0,8257Ca −0,1659Ca2 +0,1004 aP −0,7455aP2+ 0,2290Ca∗aP | 0.78 | <0.0001 (Q) | 0.0507 (Q) | 0.0005 | 2.84 | 0.50 |
| BR femur = −24,2559 + 38,4325Ca −5,6735Ca2 + 44,9438 aP −13,7442Ca∗aP | 0.46 | 0.0002 (Q) | 0.0029 (L) | 0.0075 | 3.27 | |
| BR tibiotarsus = 28,2055 + 3,6009Ca | 0.14 | 0.0148 (L) | 0.4292 (NS) | 0.6088 | - | - |
| CCa tibiotarsus = 12.71709 + 2.80180451Ca | 0.13 | 0.0183 (L) | 0.4354 (NS) | 0.6888 | - | - |
Abbreviations: BD, bone density; BR, bone resistance; CCA, concentration of calcium; CP, concentration of phosphorus; L, linear effect; MM, mineral matter; NS, nonsignificant; Q, quadratic effect; SI, Seedor index.
Nonetheless, they did exhibit linear interaction for Ca and aP in BD and bone resistance (BR) of the femur. Bone density of the femur was also influenced, with a quadratic effect, by the levels of Ca and aP, whereas BR exhibited a quadratic effect for Ca and a linear increase for aP. The optimum levels for BD were estimated with the levels at 2.84% and 0.50% for Ca and aP, respectively.
Discussion
The consumption of Ca is related to the ingestion of feed because birds generally have the capacity of regulating the consumption of Ca to meet their requirements (Classen and Scott 1982). Therefore, high levels of Ca tend to reduce the consumption of feed. Similarly, birds can also regulate consumption as per the levels of energy and protein in the diet (Costa et al., 2010). Excess of Ca in feed has a neutralizing effect on the intestine, causing an increase in pH (Aguda et al., 2015), also impairing the use of P, which occurs because of formation of insoluble complexes with the Ca, in the digestive tract (Handi et al., 2015).
In the present experiment, DFI was minimized to 2.79% for Ca and 0.37% for aP. After these levels, we observed an imbalance in consumption, which occurred without impaired performance because the other variables were not impaired.
Amoah et al. (2012), studying the levels of Ca and aP, also reported lower feed consumption when Ca was increased and no response with rises in aP. Garcia et al. (2000), studying laying quails, found similar results; these authors observed lower feed consumption for the higher levels of Ca and a quadratic effect for aP in which the highest consumption occurred at 0.36%, different from the present study. Other authors found no significant differences in feed intake in regard to the levels of Ca and aP studied (Costa et al., 2007; Ribeiro et al., 2016), which proves the need for further studies to clarify the true effect of these mineral on DFI.
Calcium influences the absorption of phosphate; therefore, it is necessary to establish an adequate proportion so that optimal use of these minerals occurs. In the present study, the behavior of DFI was influenced in a similar way as per the Ca and aP levels, that is consumption was reduced, which was influenced by the capacity to regulate consumption until reaching the optimal point. After, the high levels of Ca and aP promoted an increase in consumption, associated to the formation of insoluble salts in which the excess of Ca produces imbalance with other minerals, especially P, increasing its requirement (Schoulten et al., 2002) and justifying this consequential increase.
Egg production considers the production of eggs and the number of quails producing and is extremely important to evaluate the productivity index. It was observed that this variable was not negatively influenced even as DFI was reduced, which supports the hypothesis that birds regulate their consumption to meet their needs and maintain this level of consumption, so egg-laying yield is not impaired (Scott et al., 1982). This behavior is extended to EM as well.
Although the NRC (1994) reference was elaborated a while ago, it recommends a nutritional requirement of 2.5% Ca and 0.35% aP. In the Brazilian Tables of Poultry and Swine (Rostagno et al., 2017), on the other hand, the avarage recommended requirement is 3.16% Ca and 0.33% aP, which in comparison to this study, are higher for Ca and lower for aP. Even when considering the lighter birds (190 g), the recommended requirements by the authors are 2.990% Ca and 0.309% aP, which are also different from the present study.
The genetic material used can explain this difference. In this experiment, the BW and EM are inferior to those used in the Brazilian Tables, which considers these parameters to determine the needs of the birds. It is important to emphasize that although this study has lower levels of these variables, this caused no harm to egg yield, which reached optimum results. This evidence supports that the differences of needs among studies must be associated to the genetic material in use because the birds were in a state of comfort during the experiment, even though they were exposed to ambient temperature.
The Silva (2009) study recommends dividing the egg-laying stage into 2 phases. The first phase would reach the yield peak, using 2.95% Ca and 0.35% aP, which also estimates superior Ca levels but similar levels of aP.
The use efficiency of these minerals depends on quantity and the interaction that occurs between the 2. The NRC (1994) establishes 7:1 Ca: aP ratio, while Rostagno et al. (2017) recommend 9.6:1 and (Silva, 2009) suggest 8:1. In this study, we found an optimum ratio of 7.05:1, which supports the aforementioned explanation regarding the BW of the birds.
We believe that the result obtained for EW may be associated with the egg-laying rate, which presented the same positive response to the increase, up to a certain limit, of mineral levels. Thus, an excess of calcium only causes a positive impact on the perfomance of the birds in some situations (Masukawa et al., 2001).
Increase in shell percentage was associated to increase of EW. This result is controversial in the literature that discusses commercial egg-laying hens. Rutz et al. (2007) reported that from the beginning to the end of the productive cycle, EW increases as laying hens grow older; however, no proportional increase was observed in egg shells. This difference in the result can be associated with the fact that shows quails exhibit different behavior in the production of eggs and better shell resistance.
Among the variables regarding the external quality of eggs, specifically the quality of the egg shells, only specific gravity was not affected by different levels of minerals. However, although it is commonly used, specific gravity considered an indirect method, and ST, the percentage of shell in relation to the weight of the egg, and SWUSA are all considered direct methods to evaluate the quality of the shell (Baião and Cançado, 1997).
In poultry farming, the direct economic loss is associated with shell quality and cracking rates of eggs. Consequently, quality of the shell is the main concern of egg-laying industries because of the economic loss associated by the incidence of poor quality.
This study shows the influence Ca and aP have on the improvement of shell quality of eggs produced by quails in the interval from 64 to 168 D of age. Souza et al. (2016), who worked with quails at the end of production, found the same result. These authors stated that a Ca level of 3.85% increased ST, which was higher than what we observed in the present study. This difference is probably because the foregoing authors studied quails at the end of their production cycles. Still, they highlight the importance of these minerals during the entire productive stage.
To avoid loss of shell quality, farmers must avoid high levels of inorganic phosphorus because the excess prevents the release of Ca from the bone and proper shell mineralization (Bertechini, 2014). According to Mello (2015), approximately 30% of the calcium present in the egg shells comes from the bones, and this is owing to the fact that in the uterus, where the deposition of calcium carbonate in the egg occurs, there is no storage of calcium. Souza et al. (2015), while using 5 levels of Ca (2.95, 3.25, 3.55, 3.85, and 4.15%) and a 14-day storage period, obtained better internal quality indexes as the levels of Ca increased.
Total calcium is a good indicator of ionized calcium because 50 to 60% of Ca present in the blood is linked to plasmatic proteins or to citrate and phosphate; however, the remaining ionized calcium is responsible for controlling physiological actions (Becker, 2008).
Although of low relevance (R2 = 0.17), this linear increase in total calcium may be associated with the physiological conditions the birds were kept because they were submitted to a 6-h dietary fast. During fasting (am), the quails had eggs in their uterus; therefore, they needed higher quantities of available Ca, maintaining constant the total calcium level in circulation. Consequently, the complex form of Ca works as a supply in serum, highly available for ionization (Pizzolante, 2000) and then used in shell formation and for bone absorption, which is also a mineral source for the egg shell formation. Therefore, birds receiving diets with higher levels of calcium mobilized larger amounts of calcium. These results are in agreement with those of the study by Pelicia et al. (2009), who also reported that the increase of the Ca level in feed promoted an increase in the level of blood Ca.
This increase in calcium found in serum can also be associated to the need for a higher deposition of the mineral in the egg shell, as observed in this experiment, where the higher the levels of Ca the higher the deposition of this mineral in the shell.
Nonsignificance for ionic calcium and variables used for measurement (total protein and albumin), as well as phosphorus concentration, can also be associated to the efficient homeostasis of these minerals in which parathyroid hormone and vitamin D3 regulate the absorption of calcium in case of deficiency, and calcitonin does the opposite, acting when there is an increase in Ca concentration. This emphasizes that the metabolism of P is associated with the metabolism of Ca.
Bone densitometry can precisely detect the gain and loss variations of bone mass and can indicate bone mineralization (Barreiro et al., 2009). Almeida Paz (2006) monitored the bone development of heavy matrices by bone densitometry and observed that BD values of the tibiotarsus oscillates less than the femur, indicating that the former absorbs more minerals, as well as provides them more easily when needed. The results of the foregoing authors are in agreement with the result of the present study in which only the femur was affected by the different levels of minerals studied.
Mineral BD can be measured using techniques, such as the mineral composition of the bone, bone resistance, and Seedor index (Almeida Paz and Bruno, 2006). For BR of the femur, the best result was found when the level of Ca was at 3.27%. Nonetheless, BR of the tibiotarsus increased linearly as the levels of Ca increased, obtaining the same responses for calcium concentration in the bone. These responses may be associated with the level of total Ca in the blood, which in addition to increasing the concentration of this mineral in the egg shell, and promoted an increase in bone absorption.
The bone is a complex tissue composed of organic and inorganic matrices, which offers mechanical support and resistance, therefore, bone resistance is conditioned by levels of minerals, but also by the organic structure of the bone (Rath et al., 2000). Bone flexibility is represented by the area and measures bone deformity as a function of the force applied to it (Gopinger et al., 2017).
The results indicate that adequate levels of Ca in feed support bone quality, demonstrating better mineralization. According to Whitehead and Fleming (2000), when there is no bone structure loss, these bones, especially the tibiotarsus, become less fragile and less susceptible to fracture.
Conclusion
It was verified that Ca and aP requirements for obtaining better yield in egg-laying Japanese quails were 2.68% Ca and 0.38% aP, a ratio of 7:1 between those minerals for the early stage of laying (64–168 D of age).
The estimates were obtained through the use of overlapping contour graphs, where the point selected in the graph indicated the best levels when combining the multiple responses of the performance variables, allowing considering all variables with equal importance.
Therefore, this work will help to establish support for Japanese quail nutrition in laying, demonstrating its benefits and advantages, as well as presenting the interrelationship of Ca and aP metabolism in quails. There was an improvement in the quality of the shell, suggesting that with the storage time, there will be an improvement in the quality of the egg; for this, new experiments should be performed.
Acknowledgments
The authors acknowledge the National Council for Scientific and Technological Development (CNPq) and the Araucária Foundation (FA) for granting financial resources and the National Council for the Improvement of Higher Education (CAPES) for the financial support during this study.
Disclosures
The authors declare no conflicts of interest or other relationships with other people or organizations that could inappropriately influence or be perceived to influence this study.
References
- Aguda A.Y., Sekoni A.A., Omage J.J. Requirement of calcium and available phosphorus for laying Japanese quail birds (Coturnix coturnix japonica) in Nigeria. J. Anim. Poult. Sci. 2015;4:31–38. [Google Scholar]
- Almeida Paz I.C.L., Bruno L.G.D. Bone mineral density: review. Braz. J. Poult. Sci. 2006;8:69–73. [Google Scholar]
- Almeida Paz I.C.L. Univ. Estadual Paulista; Botucatu: 2006. Avaliação da densidade mineral óssea em matrizes pesadas por meio da técnica de densitometria óptica em imagens radiográficas. PhD Thesis. [Google Scholar]
- Amoah J.K., Martin E.A., Barroga A.J., Garillo E.P., Domingo I. Calcium and phosphorus requirements of Japanese quail layers. J. Appl. Biosci. 2012;54:3892–3900. [Google Scholar]
- Baião N.C., Cançado S.U. Fatores que afetam a qualidade da casca do ovo. Caderno Técnico de Veterinária. 1997;21:43–59. [Google Scholar]
- Bain M.M., Nys Y., Dunn I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles.What are the challenges? Br. Poult. Sci. 2016;57:330–338. doi: 10.1080/00071668.2016.1161727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro F.R., Sagula A.L., Junqueira O.M., Pereira G.T., Baraldi-Artoni S.M. Densitometric and biochemical values of broiler tibias at different ages. Poult. Sci. 2009;88:2644–2648. doi: 10.3382/ps.2008-00079. [DOI] [PubMed] [Google Scholar]
- Becker C. Diseases of calcium metabolism and metabolic bone disease. Am. Coll. Phys. Med. 2008;1:1–18. [Google Scholar]
- Bertechini A.G. Exigências de minerais para aves. In: Sakomura N.K., Silva J.H.V., Costa F.G.P., Fernandes J.B.K., Hauschild L., editors. Nutrição de Não Ruminantes. FUNEP; Jaboticabal, Brazil: 2014. pp. 375–388. [Google Scholar]
- Classen H.L., Scott T.A. Self selection of calcium during the rearing and early laying periods of White Leghorn pullets. Poult. Sci. 1982;61:2065–2074. doi: 10.3382/ps.0612065. [DOI] [PubMed] [Google Scholar]
- Costa C.H.R., Barreto S.L.T., Moura W.C.O., Reis R.S., Leite C.D.S., Maia G.V.C. Níveis de fósforo e cálcio em dietas para codornas japonesas em postura. R. Bras. Zootec. 2007;36:2037–2046. [Google Scholar]
- Costa C.H.R., Barreto S.L.T., Umigi R.T., Lima H.J.D., Araujo M.S., Medina P. Balanço de cálcio e fósforo e estudo dos níveis desses minerais em dietas para codornas japonesas (45 a 57 semanas de idade) R. Bras. Zootec. 2010;39:1748–1755. [Google Scholar]
- Delezie E., Bierman K., Nollet L., Maertens L. Impacts of calcium and phosphorus concentration, their ratio, and phytase supplementation level on growth performance, foot pad lesions, and hock burn of broiler chickens. J. Appl. Poult. Res. 2015;24:115–126. [Google Scholar]
- Gautron J., Hincke M.T., Mann K., Panhéleux M., Bain M., McKee M.D., Solomon S.E., Nys Y. Ovotransferrin is a matrix protein of the hen eggshell membranes and basal calcified layer. Connect Tissue Res. 2001;42:255–267. doi: 10.3109/03008200109016840. [DOI] [PubMed] [Google Scholar]
- Garcia J., Murakami A.E., Martins E.M., Furlan A.C. Exigências nutricionais de cálcio e fósforo para codornas japonesas (Coturnix coturnix japonica) em postura. Acta Sci. Anim. Sci. 2000;22:733–739. [Google Scholar]
- Gopinger E., Krabbe E.L., Surek D., Lopes L.S., Avila V.S. Live Performance, carcass, and bone quality responses of grower and finisher broilers to dietary metabolizable energy levels. Rev. Bras. Ciênc. Avíc. 2017;19:559–566. [Google Scholar]
- Hamilton R.M.G. Methods and factors that affect the measurement of egg shell quality. Poult. Sci. 1982;61:2022–2039. [Google Scholar]
- Hamdi M., López-Vergé S., Manzanilla E.G., Barroeta A.C., Pérez J.F. Effect of different levels of calcium and phosphorus and their interaction on the performance of young broilers. Poult. Sci. 2015;94:2144–2151. doi: 10.3382/ps/pev177. [DOI] [PubMed] [Google Scholar]
- Haugh R.R. The Haugh unit for measuring egg quality. U. S. Egg Poult. Mag. 1937;43:552–555. [Google Scholar]
- Horwitz W., Latimer G.W. 18th ed. AOAC International; Gaithersburg, MD: 2005. Official Methods of Analysis. [Google Scholar]
- Jeke A., Phiri C., Chitiindingu K., Taru P. Nutritional compositions of Japanese quail (Coturnix coturnix japonica) breed lines raised on a basal poultry ration under farm conditions in Ruwa, Zimbabwe. Cogent Food Agric. 2018;4:473009. [Google Scholar]
- Kira K.C., Murakami A.E., Furlan A.C. Conferência Apinco de Ciência e Tecnologia Avícolas. FACTA. Curitiba; PR, Brazil: 1996. Utilização de diferentes fontes de cálcio para poedeiras comerciais; p. 26. [Google Scholar]
- Masukawa Y., Moraes V.M.B., Ariki J., Bruno L.D.G. Níveis de cálcio da dieta sobre o desempenho e a qualidade da casca de ovos de codornas japonesas. Ars Vet. 2001;17:144–148. [Google Scholar]
- McDowell L.R. 1st ed. Acad Press; San Diego, CA: 1992. Calcium and Phosphorus – Minerals in Animal and Human Nutrition. 524. [Google Scholar]
- Mello J.F. Univ. Estadual de Maringá; Maringá: 2015. Influência dos níveis de cálcio e fósforo na dieta de matrizes de codornas japonesas, no desempenho produtivo e no desenvolvimento ósseo embrionário da progênie. PhD Diss. [Google Scholar]
- National Research Council . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Oliveira- Bruxel T.M. Univ. Estadual de Maringá; Maringá: 2016. Exigência de energia metabolizável e lisina digestível para codornas japonês.as (Coturnix coturnix japonica) PhD Thesis. [Google Scholar]
- Pelicia K., Garcia E.A., Faitarone A.B.G., Silva A.P., Berto D.A., Molino A.B., Vercese F. Calcium and available phosphorus levels for laying hens in second production cycle. Braz. J. Poult. Sci. 2009;11:39–49. [Google Scholar]
- Pinheiro S.R.F., Oliveira R.G., Goulart K.B., Pires A.V., Gonçalves F.M., Drumond E.S.C., Costa L.S., Carvalho D.C.O. Fósforo disponível na ração de codornas de corte em fase de crescimento. Rev. Bras. Saúde Prod. Anim. 2015;16:818–826. [Google Scholar]
- Pizzolante C.C. Univ. Federal de Lavras; Lavras: 2000. Estabilidade da fitase e sua utilização na alimentação de frangos de corte. PhD Thesis. [Google Scholar]
- Rath N.C. Factors regulating bone maturity and strength in poultry. Poult. Sci. 2000;79:1024–1032. doi: 10.1093/ps/79.7.1024. [DOI] [PubMed] [Google Scholar]
- R Core Team – Version 3.5.3 . R Foundation for Statistical Computing; Vienna, Austria: 2019. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Ribeiro C.L.N., Barreto S.L.T., Reis R.S., Muniz J.C.L., Viana G.S., Ribeiro Junior V., Mendonça M.O., Ferreira R.C., Degroot A.A. The effect of calcium and available phosphorus levels on performance, egg quality and bone characteristics of Japanese quails at end of the egg-production phase. Rev. Bras. Ciênc. Avíc. 2016;18:33–40. [Google Scholar]
- Rodrigues P.B., Bertechini A.G., Oliveira B.C., Teixeira A.S., Oliveira A.I.G. Fatores nutricionais que influenciam a qualidade do ovo no segundo ciclo de produção. Níveis de aminoácidos sulfurosos totais. R. Bras. Zootecnia. 1996;25:248–260. [Google Scholar]
- Rostagno H.S., Albino L.F.T., Donzele J.L., Gomes P.C., Oliveira R.F., Lopes D.C., Ferreira A.S., Barreto S.L.T., Euclides R.F. 3rd ed. Dep. de Zootec.; UFV, Viçosa, MG, Brazil: 2011. Tabelas Brasileiras para Aves e Suínos- Composição de Alimentos e Exigências Nutricionais. 252. [Google Scholar]
- Rostagno H.S., Albino L.F.T., Hannas M.I., Donzele J.L., Sakomura N.K., Perazzo F.G., Saraiva A., Teixeira M.L., Rodrigues P.B., Oliveira R.F., Barreto S.L.T., Brito C.O. 4th ed. Dep. de Zootec.; UFV, Viçosa, MG, Brazil: 2017. Tabelas Brasileiras para Aves e Suínos - Composição de Alimentos e Exigências Nutricionais. 488. [Google Scholar]
- Rutz F., Anciuti M.A., Xavier E.G., Roll V.F.B., Rossi P. Avanços na fisiologia e desempenho reprodutivo de aves domésticas. Rev. Bras. Reprod. Anim. 2007;31:307–317. [Google Scholar]
- Sakomura N.K., Rostagno H.S. 2nd ed. FUNEP; Jaboticabal, SP, Brazil: 2016. Métodos de Pesquisa em Nutrição de Monogástricos. 262. [Google Scholar]
- Scott M.L., Nesheim M.C., Young R.J. 3rd ed. ML Soctt and Associates; Ithaca, NY: 1982. Nutrition of the Chickens. 562. [Google Scholar]
- Schoulten N.A., Teixeira A.S., Bertechini A.G., Freitas R.T.F., Conte A.J., Silva U.H. Efeito dos níveis de cálcio sobre a absorção de minerais em dietas iniciais para frangos de corte suplementadas com fitase. Ciênc. Agrotec. 2002;26:1313–1321. [Google Scholar]
- Seedor T., Watanabe E., Kadowaki W. Effect of dietary and arginine levels on bone development in broiler chicks. Anim. Sci. Tech. 1996;67:7–13. [Google Scholar]
- Silva J.H.V. 2nd ed. 2009. Tabelas para Codornas Japonesas e Europeias. 107 FUNEP, Jaboticabal, SP, Brazil. [Google Scholar]
- Silversides F.G., Scott T.A., Korver D.R., Afsharmanesh M., Hruby M. A study on the interaction of xylanase and phytase enzymes in wheat-based diets fed to commercial white and brown egg laying hens. Poult. Sci. 2006;85:297–305. doi: 10.1093/ps/85.2.297. [DOI] [PubMed] [Google Scholar]
- Sohail S.S., Roland D.A. Influence of dietary phosphorus on performance of HyLine W36 hens. Poult. Sci. 2002;81:75–83. doi: 10.1093/ps/81.1.75. [DOI] [PubMed] [Google Scholar]
- Souza D.S., Calixto L.F.L., Lemos M.J., Filho C.A.S., Pinho T.P., Machado C.A., Melo I.A., Togashi C.K. Quail performance and egg quality at the end of production fed with varying levels of calcium. Semina: Ciênc. Agrár. 2016;37:2395–2406. [Google Scholar]
- Souza C.S., Barreto S.L.T., Vieites F.M., Calderano A.A., Moraes G.H.K., Oliveira M.G.A. Cálcio e fósforo na nutrição de codornas japonesas em postura. Sci. Anim. Health. 2017;3:260–281. [Google Scholar]
- Souza D.S., Filho C.A.S., Pinho T.P., Azevedo V.M., Oliveira S.M., Calixto L.F.L. Níveis de cálcio na manutenção da qualidade interna de ovos de codornas japonesas após armazenamento. Rev. Bras. Saúde Prod. Anim. 2015;16:139–148. [Google Scholar]
- Stadelman W.J. Encyclopedia of Food Science and Technology. 2nd ed. FJ Francis; New York, NY: 2000. Eggs and egg products; pp. 593–599. [Google Scholar]
- Whitehead C.C., Fleming R.H. Osteoporosis in cage layers. Poult. Sci. 2000;7:1033–1041. doi: 10.1093/ps/79.7.1033. [DOI] [PubMed] [Google Scholar]