Abstract
Glycerol monolaurate (GML), a member of medium-chain α-monoglycerides (MG), is proved to be beneficial for productive performance, feed efficiency, and health of broilers based on recent research. The present study aims to evaluate the effect of MG mixture rich in GML and glycerol monodecanoate on performance, intestinal development, serum parameters, carcass yield, and muscle composition in broilers. A total of 528 chicks were weighed and randomly assigned into 4 groups (22 chicks/replicate, 6 replicates/group) for a 56-d experiment. The control group received a basal diet containing 0 mg/kg MG (CON), and the treated groups fed basal diets containing 300 (MG300), 450 and 600 mg/kg MG. The results revealed that the BW (P < 0.05), ADG, and ADFI were notably increased in MG-containing groups during the finisher phase compared with the CON group. Remarkable intestinal improvements were observed in the duodenum and jejunum of MG-treated groups, but no statistical differences were obtained. Dietary MG significantly (P < 0.05) increased the serum high-density lipoprotein cholesterol, total protein, and superoxide dismutase content in broilers. Inclusion of 300 mg/kg MG in diet increased the eviscerated yield (P = 0.066), leg muscle (P < 0.01) and breast muscle yield (P = 0.083), and improved the fresh meat quality with reduced drip loss (P < 0.01) and pH decline (P < 0.01) compared with the CON group. Moreover, the saturated fatty acid (P = 0.073), flavor amino acid (P < 0.05), and total amino acid (P < 0.05) content was notably higher in the muscle of the MG300 group than that in the CON group. In summary, these findings revealed that mixed MG can be used as an effective and novel feed supplement to improve productive performance and quality of broilers.
Key words: feed supplement, performance, intestinal development, meat quality, muscle composition
Introduction
Medium-chain fatty acids (MCFA) are a group of aliphatic acids with 8 to 12 carbon atoms and naturally occur in edible fats such as coconut oil and milk fat, which have been considered as safe and green ingredients in the food industry (Dayrit, 2015). Recent studies reveal that MCFA and corresponding glycerides exert positive effect on productive performance, feed utilization, intestinal development, serum parameters, and meat and egg quality in the poultry industry (Wang et al., 2015; Zeitz et al., 2015; Khatibjoo et al., 2017; Zhao et al., 2019; Liu et al., 2020). Among them, medium-chain α-monoglycerides (MG) are regarded as promising feed supplements for broilers production. Many recently published literatures document that dietary glycerol monolaurate (GML) (C12:0) increases BW and feed efficiency, promotes health, and improves meat quality in broilers (Mustafa, 2018; Fortuoso et al., 2019; Valentini et al., 2020). However, little is known about the effect of dietary glycerol monodecanoate (GMD) (C10:0) and glycerol monocaprylin (C8:0) in the diet of broilers till now.
Poultry meat is widely consumed all over the world owing to low fat, low cholesterol, low calorie, low price, and high protein (Fan et al., 2018). However, there are increasingly growing complains from the consumers concerning the poor quality of meat produced in the modern commercial poultry industry. Poultry meat quality is determined by many aspects such as appearance, texture, water-holding capacity, pH, and freshness (Mir et al., 2017). Moreover, the meat composition including protein, fat, ash, moisture, fatty acids profile, amino acids profile, and flavor property is of great importance to meat quality (Mir et al., 2017). Meat quality is affected by a set of factors including breed, diet, nutrition, management, and processing. Except for genetic selection, dietary nutrition is one of the most critical strategies to improve meat quality, as the response of birds to their diets is closely related to the alterations in the growth of the skeleton, muscle, and fat deposition (Mir et al., 2017). It has been reported that feed supplements such as GML, (−)-hydroxycitric acid, and Lactobacillus johnsonii BS15 in the diet of broilers can alter the muscle protein, fat, fatty acids, and polyunsaturated fatty acids content in broilers (Lei et al., 2016; Peng et al., 2018; Valentini et al., 2020). Thus, nutritional intervention has been considered as an effective approach to improve meat quality in broilers (Nieto and Ros, 2012).
Glycerol monodecanoate is an MG of capric acid with melting point ranging from 48°C to 50°C, which presents white powder at 25°C; however, it melts with increasing temperature. As the temperature increases higher than 35°C, the solid fat content of GMD reduces rapidly and decreases to 0 at 50°C, which seriously hinders its application in the diet of farm animals. Compared with GMD, GML has a higher melting point (62°C–63°C) and stability and is easier to process in feed production. Moreover, it has been reported that synergistic antimicrobial effects are found when MG is used in combination in vitro (Batovska et al., 2009). Hence, a MG mixture rich in GML and GMD is selected in the present experiment by overall consideration. This study aims to 1) evaluate the effect of mixed MG on productive performance, intestinal development, and serum parameters in broilers and 2) explore the alteration of fresh meat quality and muscle composition by MG supplementation.
Material and methods
Experiment Design
A total of 528 1-day-old male yellow-feather broilers were weighed and randomly assigned into 4 groups (22 chicks/replicate, 6 replicates/group) for a 56-d experiment. The control group received a basal diet containing 0 mg/kg MG (CON), and the treated groups fed basal diets containing 300 (MG300), 450 (MG450), and 600 (MG600) mg/kg MG. α-Monoglyceride containing GML (CAS No. 142-18-7) and GMD (CAS No. 26402-22-2) with 95% purity was supplied by Hangzhou Kangyuan Food Technology Co., Ltd. (Hangzhou, Zhejiang, China) and was added to the diet by replacing the same energy amount of oil. The composition and nutrient level of the basal diet for the starter (1–28 d) and finisher (29–56 d) phases of broilers (Table 1) were formulated to meet nutrient requirements of the NRC (NRC, 1994). Each replicate was housed in an individual stainless-steel cage (200 cm long × 100 cm wide × 70 cm high) with mesh ground and equipped with nipple drinkers and feeders. All cages were randomly distributed in a farmhouse under constant lighting of 24 h. All birds were reared in an environmentally controlled net rearing system (31°C–36°C) during 1 to 14 d of age, where the temperature was gradually decreased to 26°C. Birds were fed ad libitum and given free access to water throughout the experiment. Birds were handled in accordance with the guidelines of the Animal Care and Use Committee of Zhejiang University (No. ZJU-BEFS-2016004), Hangzhou, China.
Table 1.
Composition and nutrient level of the basal diet for the starter (1–28 d) and finisher (29–56 d) phases of broilers.
| Items | Starter (day 1–28) | Finisher (day 29–56) |
|---|---|---|
| Ingredients, % | ||
| Corn | 56.90 | 59.66 |
| Soybean meal | 33.00 | 32.20 |
| Soybean oil | 3.00 | 3.90 |
| Fish meal | 3.00 | 0.00 |
| Limestone | 1.20 | 1.15 |
| Dicalcium phosphate | 1.45 | 1.65 |
| L-methionine | 0.15 | 0.14 |
| Sodium chloride | 0.30 | 0.30 |
| Vitamin–mineral premix1 | 1.00 | 1.00 |
| Calculated composition, % | ||
| ME (Mcal/kg) | 2.96 | 3.00 |
| Nonphytate phosphorus | 0.45 | 0.40 |
| Lysine | 1.17 | 1.00 |
| Methionine | 0.48 | 0.42 |
| Analyzed composition, % | ||
| CP | 21.50 | 19.00 |
| Calcium | 1.10 | 0.90 |
| Total phosphorus | 0.67 | 0.60 |
| Methionine + cystine | 0.80 | 0.65 |
Supplied per kilogram of diet: vitamin A, 12,500 IU; vitamin D3, 2,500 IU; vitamin E, 20.00 IU; vitamin K3, 5.00 mg; vitamin B1, 4.00 mg; vitamin B2, 6.00 mg; pantothenic acid, 15.30 mg; nicotinic acid, 30.00 mg; pyridoxine, 5.00 mg; biotin, 2.50 mg; folic acid, 1.50 mg; vitamin B12, 0.50 mg; ZnSO4·H2O, 212.52 mg; CuSO4·H2O, 33.18 mg; FeSO4·H2O, 247.75 mg; MnSO4 .H2O, 263.98 mg; Ca(IO3)2, 85.80 mg; Na2SeO3, 37.60 mg.
Growth Performance
Birds were weighed at 0, 28, 35, 42, 49, and 56 d of age by replicates. Feed consumption was recorded in the course of the whole experimental period for each replicate on a weekly basis, and the mortality were recorded to correct the feed conversion rate (FCR). The ADG and ADFI for each replicate were calculated subsequently.
Sample Collection and Preparation
Three randomly selected broilers from each replicate (3 broilers/replicate, 6 replicates/group) were weighed and slaughtered after fasting for 12 h at 56 d of age. Serum samples were collected from a wing vein in 5-mL vacuum tubes containing coagulant gel and were separated by centrifugation (2,000 × g for 15 min at 4°C) and stored at −80°C for further analysis. An approximately 4 cm length of the small intestine from each broiler was collected, duodenum (about 2 cm at the middle of the duodenal loop) and jejunum (about 2 cm at the midway between the point of entry of the bile duct and Meckel's diverticulum), and fixed in 10% buffered formalin for 24 h at 25°C and then embedded in paraffin. Breast muscle was isolated, rapidly frozen in liquid nitrogen, and transported to the laboratory in a dry-ice pack; then, part of them from each bird were freeze-dried (LGJ-10; Beijing Huaxing Technology Development Co., Ltd., Beijing, China) separately and ground to pass through a 40-mesh sieve and were stored at −20°C for the analysis of chemical composition and fatty acid and amino acid profile.
Intestinal Morphology
Tissues were sectioned at 5 μm thickness and stained with hematoxylin and eosin using standard protocols (Chwen et al., 2013). The villus length and crypt depth of stained tissue samples were analyzed by Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD).
Serum Parameters Determination
Total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol, glutamic oxalacetic transaminase, glutamic pyruvic transaminase, alkaline phosphatase, calcium, total protein, total antioxidant capacity, total superoxide dismutase (T-SOD), and malondialdehyde were determined using kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China) by following the manufacturer's instructions.
Carcass Characteristics and Fresh Meat Quality
Thirty-six randomly selected broilers from the CON and MG300 group (3 broilers/replicate, 6 replicates/group) were weighed (fasted live BW) and slaughtered after fasting for 12 h at 56 d of age. All broilers were electrically stunned for 11 s, then killed by exsanguination, and allowed to bleed for 90 s. Broilers were then scalded at 55°C for 90 s in a rotary scalder, picked for 30 s in a drum picker, and weighed (dressed weight). After removal of the crop, intestine, spleen, pancreas, bile, and reproductive organs, the rest of the body was weighed (half-eviscerated weight), and the eviscerated weight was then determined by removing the liver, heart, gizzard, abdominal fat, head, neck, lung, and stomach. Dressed percentage (100 × dressed weight/fasted live BW), half-eviscerated yield (100 × half-eviscerated weight/fasted live BW), and eviscerated yield (100 × eviscerated weight/fasted live BW) were calculated. Leg muscle (100 × leg breast weight/eviscerated weight) and breast muscle yield (100 × breast muscle weight/eviscerated weight) were calculated after removing bone, skin, and subcutaneous fat.
pH was measured using a digital pH meter (Testo 205, Germany) with a punch electrode and a temperature calibration device by inserting into the pectoralis major breast muscle after slaughtering in 45 min (pH1) and 24 h (pH24). Decline of pH = pH1 − pH24. Drip loss was determined as described by Zhou et al. (Zhou et al., 2019). Briefly, a slice of 2 × 2 × 6 cm muscle was cut from the right breast muscle perpendicular to the fiber direction and weighed, then hung in an airtight container that was stored at 4°C. Samples were taken away from the refrigerator and weighed again for the calculation of drip loss after 24-h storage.
Muscle Chemical Composition
Total protein (Association of Official Analytical Chemists [AOAC] 992.15), intermuscular fat (IMF) (AOAC 991.36), moisture (AOAC 985.14), and ash (AOAC 920.153) of breast muscle was measured in duplicate as per the AOAC (AOAC, 1990).
Muscle Fatty Acids Profile
Dry muscle (0.2 g) was weighed into ultrasound-cleaned 15-mL tubes in duplicate, then 3.2 mL formyl chlorid (acetyl chloride: anhydrous methanol, 1:10; v/v) and 3.2 mL n-hexane containing 1 mg/mL heptadecanoic acid (CAS: 506-12-7; Sigma, St. Louis, MO) were added before heating for 2 h at 75°C. After the tubes were cooled to 25°C, 4 mL of 7% potassium carbonate (v/v) was added before mixing and centrifuging at 3,000 × g for 5 min; then, the top layer was filtered through a 0.22-μm membrane filter before gas chromatography (Sukhija and Palmquist, 1989; Mrf et al., 2012). Analysis was conducted using GC-2014 (Shimadzu Corporation, Tokyo, Japan) with a column (DB-23, 60 m × 0.25 mm × 0.25 μm; Agilent, Santa Clara, CA). Nitrogen at a constant flow of 0.65 mL/min was the carrier gas. Flame ionization detector temperature and injector oven temperature was set at 250°C and 230°C, respectively. The temperature program of the oven was the same as that of our previous study (Liu et al., 2020). Certified reference standard (37 components mix, Stock No. Supelco 18919-1AMP; Sigma, St. Louis, MO) and heptadecanoic acid were used for the identification and quantification of fatty acids.
Muscle Amino Acids Profile
Dry muscle (20 mg) was dissolved in 1 mL 6 mol HCl in digestion tubes under pure nitrogen atmosphere, then were heated for 2 h at 150°C in a digestion furnace (SH60A; Jinan Hanon Instruments Co., Ltd., Jinan, China). Then, 10 μL digested samples or standard amino acids (Stock No. Supelco AA-S-18; Sigma, St. Louis, MO) was pipetted into 1.5-mL microcentrifuge tubes and dried under vacuum. Then, a total volume of 20 μL derivatization reagent (ethanol: water: triethylamine: phenyl-isothiocyanate, 7:1:1:1, v/v/v/v) was added and kept sealed for 30 min at 25°C under nitrogen atmosphere. The reagents were then removed under vacuum. The dried derivatized samples were dissolved in 50 μL mobile phase B (water: methanol, 1:4, v/v) and mixed thoroughly. Then, 450 μL mobile phase A (20 mmol/L sodium acetate containing 0.1% triethylamine, pH = 6.5) was added and mixed thoroughly. Then, the solution was filtered through a 0.22-μm membrane filter and 10 μL was injected to HPLC (Waters e2695; Waters Corporation, Milford, MA) equipped with a column (Ultimate AQ-C18, 4.6 mm × 250 mm × 5 μm; Welch Co., Shanghai, China). Identification and quantification of amino acids was aided with certified reference standard (Gheshlaghi et al., 2008; Liu et al., 2020).
Statistical Analysis
The data were collected and calculated individually for each group and checked for normality before statistical analysis. Statistical differences between more than 2 groups were evaluated using 1-way ANOVA by GraphPad Prism, version 8 (GraphPad Software, La Jolla, CA), and the differences among groups were examined by Tukey's test. Statistical differences between 2 groups were determined using unpaired 2-tailed t test (only for the data of carcass characteristics and fresh meat quality). P-value < 0.05 was considered significant, and 0.05 < P-value < 0.10 was discussed as tendencies.
Results
Growth Performance
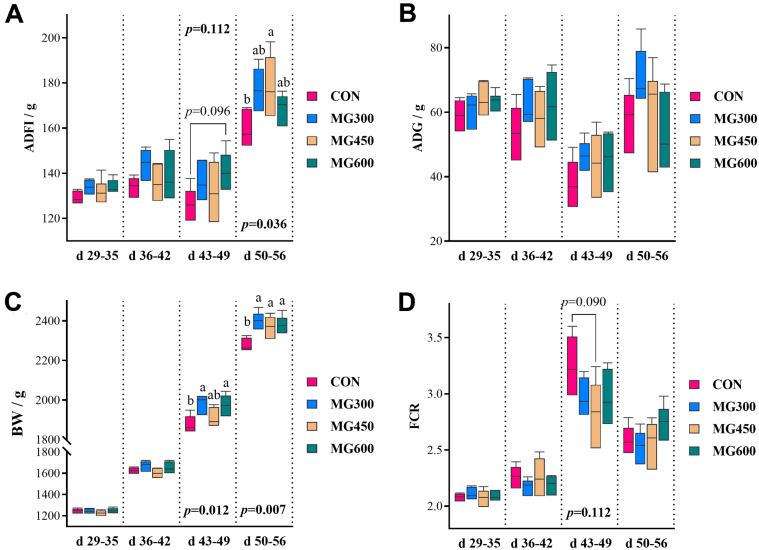
No significant differences were observed on growth performance during 1 to 28 d of age (Table 2). However, during 29 to 56 d of age, the ADFI was increased by 7.78% (P = 0.023), 5.10% (P = 0.160), and 6.08% (P = 0.074) in MG300, MG450, and MG600 groups, respectively, relative to that of the CON group, and the BW was 118.13, 87.2, and 95.26 g higher (P < 0.05) than that of the CON group, respectively. To figure out the effect of dietary MG on growth performance during the finisher phase (29–56 d of age), we analyzed and compared the data at the interval of a week (Figure 1). It was during 29 to 35 d of age that the ADFI and ADG were affected by MG supplementation, though the BW and FCR showed no changes. During 36 to 56 d of age, the ADFI, ADG, and BW were notably increased in MG-containing groups compared with the CON group, resulting in reduced FCR in treated groups. These results suggested that it was during 29 to 35 d of age that dietary MG exert beneficial effect on growth performance by stimulating the appetite of broilers.
Table 2.
Effect of dietary MG on growth performance in broilers.
| Items | CON | MG300 | MG450 | MG600 | SEM | P-value |
|---|---|---|---|---|---|---|
| 1–28 d of age | ||||||
| ADFI (g) | 56.233 | 56.912 | 55.825 | 57.944 | 0.314 | 0.076 |
| ADG (g) | 28.844 | 28.585 | 28.030 | 29.217 | 0.194 | 0.166 |
| BW (g) | 846.042 | 842.758 | 824.640 | 845.256 | 3.750 | 0.128 |
| FCR | 1.855 | 1.870 | 1.893 | 1.851 | 0.010 | 0.504 |
| 29–56 d of age | ||||||
| ADFI (g) | 136.866b | 147.515a | 143.846a,b | 145.187a,b | 1.325 | 0.027 |
| ADG (g) | 51.197b | 55.524a | 55.069a,b | 54.620a,b | 0.589 | 0.036 |
| BW (g) | 2,279.372b | 2,397.501a | 2,366.572a | 2,374.625a | 13.571 | 0.007 |
| FCR | 2.454 | 2.392 | 2.381 | 2.427 | 0.014 | 0.242 |
Means within a row lacking a common superscript differ (P < 0.05, n = 6).
Abbreviations: CON, basal diet; FCR, feed conversion rate; MG, α-monoglyceride; MG300, basal diet +300 mg/kg MG; MG450, basal diet +450 mg/kg MG; MG600, basal diet +600 mg/kg MG.
Figure 1.
Dynamic changes of growth performance in broilers during 29 to 56 d of age. (A) ADFI. (B) ADG. (C) BW. (D) FCR, feed conversion rate. Data were expressed as means ± SD (n = 6). Means at the same age with different letter differ significantly (P < 0.05). Abbreviations: CON, basal diet; MG, α-monoglyceride; MG300, basal diet +300 mg/kg MG; MG450, basal diet +450 mg/kg MG; MG600, basal diet +600 mg/kg MG.
Intestinal Morphology
Compared with the CON group, the villus length/crypt depth ratio (V/C ratio) of the duodenum was enhanced by 8.83, 26.42, and 18.16% in MG300, MG450, and MG600 groups, respectively. The villus length of the jejunum in MG300, MG450, and MG600 groups increased by 5.97, 5.75, and 7.12%, respectively, relative to that of the CON group. The V/C ratio of the jejunum in MG300 group increased by 15.18% compared with the CON group (Table 3), showing the highest values among groups. However, no significant differences were observed.
Table 3.
Effects of dietary MG on intestinal morphology in broilers.
| Items | CON | MG300 | MG450 | MG600 | SEM | P-value |
|---|---|---|---|---|---|---|
| Duodenum | ||||||
| Villus length (mm) | 1.749 | 1.691 | 1.722 | 1.965 | 0.039 | 0.051 |
| Crypt depth (mm) | 0.234 | 0.206 | 0.181 | 0.220 | 0.009 | 0.174 |
| V/C ratio | 7.836 | 8.557 | 9.906 | 9.259 | 0.384 | 0.272 |
| Jejunum | ||||||
| Villus length (mm) | 1.391 | 1.474 | 1.471 | 1.490 | 0.032 | 0.731 |
| Crypt depth (mm) | 0.202 | 0.198 | 0.208 | 0.225 | 0.007 | 0.631 |
| V/C ratio | 7.164 | 8.280 | 7.280 | 7.490 | 0.277 | 0.531 |
Abbreviations: CON, basal diet; MG, α-monoglyceride; MG300, basal diet +300 mg/kg MG; MG450, basal diet +450 mg/kg MG; MG600, basal diet +600 mg/kg MG; V/C, Villus length/Crypt depth ratio.
Serum Parameters
Compared with the CON group (Table 4), HDL-C, total protein, as well as the T-SOD in MG300 and MG450 groups were significantly increased (P < 0.05). No other significant changes were observed for the serum parameters measured in this experiment.
Table 4.
Effects of dietary MG on serum parameters in broilers.
| Items | CON | MG300 | MG450 | MG600 | SEM | P-value |
|---|---|---|---|---|---|---|
| TG (mmol/L) | 0.215a,b | 0.208a,b | 0.263a | 0.194b | 0.009 | 0.026 |
| TC (mmol/L) | 3.572 | 3.701 | 3.786 | 3.899 | 0.069 | 0.399 |
| HDL-C (mmol/L) | 4.321b | 4.988a | 4.948a | 5.379a | 0.009 | <0.001 |
| LDL-C (mmol/L) | 0.590 | 0.706 | 0.664 | 0.666 | 0.024 | 0.389 |
| GOT (U/L) | 54.085 | 58.791 | 53.681 | 57.790 | 1.311 | 0.412 |
| GPT (U/L) | 10.660 | 11.692 | 12.001 | 10.798 | 0.362 | 0.477 |
| AKP (U/L) | 657.616 | 768.514 | 662.836 | 767.175 | 58.744 | 0.846 |
| Ca (mmol/L) | 1.567 | 1.587 | 1.594 | 1.574 | 0.007 | 0.465 |
| Total protein (g/L) | 20.319b | 23.351a | 24.782a | 23.148a,b | 0.436 | <0.001 |
| T-AOC (U/mL) | 15.014a,b | 13.694b | 15.611a | 14.160a,b | 0.212 | 0.005 |
| T-SOD (U/mL) | 197.431c | 318.658a | 317.512a | 245.641b,c | 9.062 | <0.001 |
| MDA (nmol/L) | 2.168 | 2.515 | 2.312 | 2.263 | 0.056 | 0.116 |
Means within a row lacking a common superscript differ (P < 0.05, duodenum: n = 18).
Abbreviations: AKP, alkaline phosphatase; Ca, calcium; CON, basal diet; GOT, glutamic oxalacetic transaminase; GPT, glutamic-pyruvic transaminase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MDA, malondialdehyde; MG, α-monoglyceride; MG300, basal diet +300 mg/kg MG; MG450, basal diet +450 mg/kg MG; MG600, basal diet +600 mg/kg MG; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase; TC, total cholesterol; TG, triglycerides.
Carcass Characteristics and Fresh Meat Quality
Eviscerated yield, leg muscle, and breast muscle in MG300 group increased by 1.24% (P = 0.066), 6.84% (P = 0.004), and 5.53% (P = 0.083) compared with the CON group (Table 5). Drip loss and decline of pH in MG300 group decreased by 18.57% (P < 0.01) and 50.45% (P < 0.01), respectively, in relative to that of the CON group.
Table 5.
Carcass characteristics and fresh meat quality in the CON and MG300 groups.
| Item | CON | MG300 | SEM | P-value |
|---|---|---|---|---|
| Dressed percentage (%) | 91.087 | 91.254 | 0.128 | 0.538 |
| Half-eviscerated yield (%) | 86.828 | 87.163 | 0.155 | 0.301 |
| Eviscerated yield (%) | 79.132 | 80.106 | 0.269 | 0.066 |
| Leg muscle (%) | 16.820 | 17.966∗∗ | 0.225 | 0.004 |
| Breast muscle (%) | 14.115 | 14.891 | 0.225 | 0.083 |
| Drip loss (%) | 2.802 | 2.278∗ | 0.117 | 0.016 |
| pH1 | 5.700 | 5.670 | 0.023 | 0.538 |
| pH24 | 5.424 | 5.533∗∗ | 0.020 | 0.001 |
| Decline of pH | 0.275 | 0.136∗∗ | 0.028 | 0.005 |
Means within a row with asterisk indicates significant differences according to unpaired 2-tailed t test (∗P < 0.05, ∗∗P < 0.01).
Abbreviations: CON, basal diet; MG, α-monoglyceride; MG300, basal diet +300 mg/kg MG; pH1, muscle pH measured within 45 min after slaughter; pH24, muscle pH measured after storage at 4°C for 24 h.
Muscle Chemical Composition
No differences were observed in muscle chemical composition (Table 6), but notable higher IMF was observed in MG300 group than in the CON group.
Table 6.
Effect of dietary MG on muscle chemical composition in broilers.
| Items (%) | CON | MG300 | MG450 | MG600 | SEM | P-value |
|---|---|---|---|---|---|---|
| Total protein | 23.377 | 23.900 | 23.474 | 23.728 | 0.094 | 0.197 |
| Intermuscular fat | 0.572 | 0.672 | 0.576 | 0.589 | 0.022 | 0.331 |
| Moisture | 74.172 | 73.762 | 73.839 | 73.837 | 0.064 | 0.097 |
| Ash | 1.357 | 1.352 | 1.312 | 1.444 | 0.033 | 0.715 |
Abbreviations: CON, basal diet; MG, α-monoglyceride; MG300, basal diet +300 mg/kg MG; MG450, basal diet +450 mg/kg MG; MG600, basal diet +600 mg/kg MG.
Muscle Fatty Acids Profile
Muscle fatty acids profile (Table 7, CON group) was dominated by unsaturated fatty acids (UFA), which accounted for more than 56.68% in total fatty acids with C18:1n9c (24.92%), C18:2n6c (14.65%), C20:4n6 (5.38%), and C15:1n5 (5.12%) being the most representative. Palmitic acid (C16:0, 27.18%) and stearic acid (C18:0, 12.30%) were the most abundant saturated fatty acids (SFA). The concentration of C4:0 (P < 0.01), C12:0 (P < 0.01), C15:1n5 (P < 0.05), C17:1n7 (P = 0.055), C18:0 (P = 0.056), C18:3n3 (P < 0.01), C20:0 (P < 0.01), C20:2n6 (P < 0.05), and SFA (P = 0.073) in MG300 group was notably increased compared with the CON group. C4:0 (P < 0.05) and C12:0 (P < 0.073) content in MG450 group was higher than that of the CON group, and the C4:0 content in MG600 group (P < 0.01) was also increased.
Table 7.
Effect of dietary MG on muscle fatty acids profile in broilers.
| mg/g Dry material | CON | MG300 | MG450 | MG600 | SEM | P-value |
|---|---|---|---|---|---|---|
| C4:0 | 0.339b | 0.369a | 0.363a | 0.287c | 0.005 | <0.001 |
| C12:0 | 0.533b | 0.811a | 0.737a,b | 0.649a,b | 0.030 | 0.004 |
| C14:0 | 0.284 | 0.287 | 0.299 | 0.294 | 0.005 | 0.757 |
| C16:0 | 12.150 | 12.869 | 12.673 | 11.598 | 0.197 | 0.089 |
| C18:0 | 5.498a,b | 6.015a | 5.730a,b | 5.373b | 0.076 | 0.011 |
| C20:0 | 0.526b,c | 0.691a | 0.597a,b | 0.442c | 0.020 | <0.001 |
| SFA | 19.369a,b | 21.417a | 20.604a,b | 18.709b | 0.316 | 0.007 |
| C15:1n5 | 2.291b | 2.539a | 2.445a,b | 2.215b | 0.035 | 0.002 |
| C16:1n7 | 1.130 | 1.39 | 1.157 | 1.011 | 0.037 | 0.490 |
| C17:1n7 | 0.389a,b | 0.436a | 0.405a,b | 0.366b | 0.007 | 0.002 |
| C18:1n9c | 11.139 | 12.740 | 11.082 | 10.835 | 0.316 | 0.117 |
| C24:1n9 | 0.238a,b | 0.266a | 0.234a,b | 0.217b | 0.006 | 0.013 |
| MUFA | 15.187 | 17.195 | 15.618 | 14.645 | 0.371 | 0.076 |
| C18:2n6c | 6.555a,b | 7.163a | 6.531a,b | 6.108b | 0.143 | 0.062 |
| C18:3n3 | 0.283b,c | 0.369a | 0.318a,b | 0.226c | 0.010 | <0.001 |
| C20:2n6 | 0.213b,c | 0.265a | 0.249a,b | 0.185c | 0.008 | <0.001 |
| C20:3n6 | 0.462a,b | 0.553a | 0.498a,b | 0.397b | 0.016 | 0.004 |
| C20:4n6 | 2.398 | 2.273 | 2.387 | 2.351 | 0.063 | 0.899 |
| C22:6n3 | 0.244 | 0.247 | 0.246 | 0.238 | 0.005 | 0.904 |
| PUFA | 10.155a,b | 10.889a | 10.246a,b | 9.546b | 0.186 | 0.074 |
| UFA | 25.343a,b | 28.084a | 25.864a,b | 24.191b | 0.544 | 0.068 |
| EFA | 9.236 | 9.805 | 9.236 | 8.726 | 0.180 | 0.195 |
| SFA/UFA | 0.770 | 0.776 | 0.800 | 0.777 | 0.007 | 0.487 |
| TFA | 44.712a,b | 49.501a | 46.468a,b | 42.900b | 0.843 | 0.031 |
Means within a row lacking a common superscript differ (P < 0.05, n = 18).
Abbreviations: CON, basal diet; EFA, essential fatty acids (C18:2n6c + C18:3n3+C20:4n6); MG, α-monoglyceride; MG300, basal diet +300 mg/kg MG; MG450, basal diet +450 mg/kg MG; MG600, basal diet +600 mg/kg MG; MUFA, monounsaturated fatty acids (C15:1n5+C16:1n7+C17:1n7+C18:1n9c + C24:1n9); PUFA, polyunsaturated fatty acids (C18:2n6c + C18:3n3+C20:2n6+C20:3n6+C20:4n6+C22:6n3); SFA, saturated fatty acids (C4:0 + C12:0 + C14:0 + C16:0 + C18:0 + C20:0); UFA, unsaturated fatty acids (MUFA + PUFA); TFA, total fatty acids.
Muscle Amino Acids Profile
The aspartic acid (P < 0.01), glutamic acid (P < 0.01), serine (P < 0.05), proline (P < 0.01), and arginine (P < 0.05) content in MG300 group were significantly increased compared with the CON group, and the proline content (P < 0.01) in MG450 group was significantly increased. In summary, the total amino acids in MG300, MG450 and MG600 groups increased by 15.84% (P < 0.05), 9.18, and 1.21% (Table 8), respectively, and the flavor amino acid (FAA) content increased by 14.13% (P < 0.05), 6.84, and 0.60%, respectively, compared with the CON group. The essential amino acid content in MG300 and MG450 groups increased by 8.05 and 4.39%, respectively, compared with the CON group, but no significant differences were observed.
Table 8.
Effect of dietary MG on muscle amino acids profile in broilers.
| g/100 g dry material | CON | MG300 | MG450 | MG600 | SEM | P-value |
|---|---|---|---|---|---|---|
| Umami amino acids | ||||||
| Aspartic acid | 6.717b | 8.171a | 7.197a,b | 6.918b | 0.154 | 0.007 |
| Glutamic acid | 5.785b | 6.793a | 6.274a,b | 5.944b | 0.108 | 0.006 |
| Sweet amino acids | ||||||
| Serine | 1.723b | 2.019a | 1.937a,b | 1.822a,b | 0.040 | 0.045 |
| Glycine | 3.340 | 3.683 | 3.519 | 3.311 | 0.054 | 0.080 |
| Alanine | 4.983 | 5.140 | 5.127 | 4.661 | 0.086 | 0.189 |
| Σ FAA | 24.840b | 28.353a | 26.544a,b | 24.990a,b | 0.452 | 0.028 |
| EAA | ||||||
| Lysine | 5.460 | 6.153 | 5.405 | 6.108 | 0.163 | 0.245 |
| Methionine | 2.049 | 2.250 | 2.290 | 2.070 | 0.049 | 0.186 |
| Threonine | 2.288 | 2.546 | 2.491 | 2.334 | 0.052 | 0.266 |
| Leucine | 7.052 | 7.535 | 7.482 | 6.818 | 0.120 | 0.121 |
| Isoleucine | 4.403 | 4.800 | 4.597 | 4.290 | 0.084 | 0.192 |
| Arginine | 3.881b | 4.882a | 4.100a,b | 4.098a,b | 0.116 | 0.022 |
| Valine | 4.348 | 4.759 | 4.664 | 4.126 | 0.087 | 0.050 |
| Phenylalanine | 3.046 | 3.221 | 3.267 | 2.961 | 0.057 | 0.204 |
| Histidine | 2.510 | 2.937 | 2.706 | 2.530 | 0.066 | 0.114 |
| Σ EAA | 28.926 | 31.265 | 30.196 | 28.707 | 0.528 | 0.345 |
| Proline | 5.344c | 8.438a | 7.597a,b | 6.083b,c | 0.298 | <0.001 |
| Tyrosine | 2.144 | 2.147 | 2.305 | 2.070 | 0.070 | 0.704 |
| Cysteine | 1.093 | 1.229 | 1.346 | 0.881 | 0.075 | 0.162 |
| Σ AA | 66.216b | 76.705a | 72.304a,b | 67.024a,b | 1.335 | 0.022 |
Means within a row lacking a common superscript differ (P < 0.05, n = 18).
Abbreviations: Σ AA, sum of total amino acids; Σ EAA, sum of essential amino acids; Σ FAA, the sum of flavor amino acids (sweet and umami taste amino acids); CON, basal diet; MG, α-monoglyceride; MG300, basal diet +300 mg/kg MG; MG450, basal diet +450 mg/kg MG; MG600, basal diet +600 mg/kg MG.
Discussions
In the present study, we observed that inclusion of 300 to 600 mg/kg MG in diets led to a notable increase in feed intake and BW gain in broilers during the finisher phase. These results were consistent with those of previous studies in which broilers fed diets containing GML showed significant increased feed consumption and BW at the end of the experiment (Mustafa, 2018; Fortuoso et al., 2019). Feed intake is the primary factor driving the growth rate of broilers (Abdollahi et al., 2018), thus significantly increased ADFI induced by MG supplementation was considered to be the main reason for the improvement of growth performance in broilers in the present study. Moreover, the increased appetite of MG-supplemented groups during 29 to 35 d of age resulted in increased ADG and BW, and a reduction in FCR, subsequently, could also be regarded as a symbol of dietary MG started to affect the growth of broilers. Similar results were found in our previous study in which 400 and 800 mg/kg GML significantly increased the feed intake and BW in mice (Mo et al., 2019). Inconsistent with the present study, dietary MCFA were reported to reduce feed intake in broilers because of its organoleptic characteristics (intense goat-like odor of the nonesterified free fatty acids) that changed the taste of feed (Van der Aar et al., 2017). This discrepancy may derive from the composition of MCFA and its degree of esterification, diets, environment, and breeds. Mabayo et al. (Mabayo et al., 1992) demonstrated that MCFA could affect satiety and therefore feed intake in broilers by inducing the secretion of cholecystokinin and possibly other intestinal hormones, but effect of dietary MGs on feed intake warranted further study.
The small intestine, especially in the duodenum and jejunum, is the most important site for feed nutrient digestion and absorption in broilers. Better development of the small intestine results in better feed nutrient utilization of broilers, which has direct relationship with growth performance (Khosravinia, 2015). Villus length, crypt depth, and V/C ratio were the critical factors for assessing intestinal development, and longer villus, higher V/C ratio, and lower crypt depth led to higher mucosal surface area, contributing to increased digestive capacity in broilers (Zeitz et al., 2015; Wang et al., 2017). There were growing evidences that MCFA and corresponding glycerides could be used directly by the enterocytes for energy production in the small intestine and was helpful for supporting intestinal development and integrity of farm animals (Guillot et al., 1993; Van der Aar et al., 2017). Dietary GML increased the villus length in the jejunum and V/C ratio in the duodenum and ileum compared with those of the controls, resulted in higher apparent metabolic rate of CP, cuprum, and calcium, and reduced FCR in broilers in our unpublished work. Likewise, a previous study demonstrated that feeding medium-chain triglycerides to piglets before weaning improved growth performance with longer villus in the duodenum, jejunum, and ileum and a greater concentration of medium-chain triglycerides in blood plasma as energy source (Chwen et al., 2013). In the present study, there were remarkable intestinal improvement observed in the duodenum and jejunum of MG-treated groups, though no statistical differences were found. These findings indicated that the improved growth performance in the treated group may be partially explained by the better intestinal development by MG supplementation. Moreover, the significantly (P < 0.05) increased total protein level in the serum of the MG supplementation group suggested that the digestion of feed protein may be enhanced in broilers. This hypothesis was consistent with that of a previous study where dietary medium-chain triglycerides significantly increased the protein retention and the efficiency of protein utilization in broilers (Mabayo et al., 1993).
The alteration of serum lipid profile demonstrated that dietary MG could effectively improve lipid metabolism in broilers. It has been reported that MCFA and corresponding glycerides were related to a greater transport of excess cholesterol, resulting in lower serum total cholesterol levels (Zhang et al., 2016; Zhou et al., 2017). Application of MCFA in the diet of broilers decreased low-density lipoprotein cholesterol concentrations and increased HDL-C content compared with the control group (Shokrollahi et al., 2014). Recently, several studies documented that inclusion of MCFA reduced the serum total cholesterol, triglycerides, low-density lipoprotein cholesterol, and HDL-C in poultry, suggesting that dietary MCFA was better for blood lipid profile and health of birds (Wang et al., 2015; Saeidi et al., 2016). The present study obtained similar results by MG supplementation. The body usually keeps a balance between the production of free radicals and antioxidants; however, the balance is shifted toward free radicals and oxidative stress under various stressful conditions in the poultry production, restricting the growth performance of chickens (Wang et al., 2018). Serum T-SOD, total antioxidant capacity, and malondialdehyde content are key indicators to reflect the antioxidant status of broilers. The significantly increased T-SOD in treated groups indicated that MG supplementation enhanced the antioxidant capacity of broilers, which also contributed to the improvements of growth performance.
As a representative of MG-containing groups, the carcass characteristic of MG300 group was improved compared with the CON group, which indicated that dietary MG not only enhanced growth performance but also boosted lean meat production in broilers. The increase in eviscerated yield and leg muscle and breast muscle yield by MG supplementation may be associated with increased protein deposition in broilers, as the total protein in serum was notably increased. In addition, the fresh meat quality of raw meat was also enhanced by MG supplementation. The reduced drip loss demonstrated that the meat in MG300 group was of stronger water-binding potential and water-holding capacity, indicating that the tenderness, juiciness, firmness, and appearance of meat were enhanced (Mir et al., 2017). These findings were also supported by the decreased decline of pH in MG300 group after 24-h storage compared with the CON group because meat with high pH has a higher water-binding capacity than meat with lower pH. Moreover, the higher pH24 also indicated meat with longer shelf life, better tenderness, color, juiciness, and freshness.
There were increased IMF in the muscle of MG300 group than the CON group, associated with increased SFA and UFA; however, similar results were not observed in MG450 and MG600 groups. Higher IMF concentration in MG300 group enhanced the juiciness and tenderness of meat, proportionate to the fresh meat quality (drip loss and pH) measured in the present study. Intermuscular fat was also an important source of flavor precursor compounds, therefore the alterations may affect the overall flavor of broilers in MG300 group when cooking (Wen et al., 2018). Furthermore, muscle fatty acid profile in broilers reflects the nutritive values and healthcare actions (Jiang et al., 2016). In accordance with the World Health Organization, decrease of SFA content and increase of UFA content in a human diet were the effective nutrition and the prevention of chronic diseases (WHO, 2003). The present study found that 300 mg/kg MG increased both the content of UFA and SFA, but the SFA/UFA ratio was unchanged, suggesting that the nutritive values and healthcare efficacy of broilers muscle were influenced. It has been reported dietary GML at the level of 200 and 300 mg/kg in the diet of white-feather broilers reduced the IMF and SFA and increased the monounsaturated fatty acids and UFA/SFA ratio (Valentini et al., 2020). The discrepancy may due to the differences of MG, dosage, breeds, diets, and environment and needed further investigation.
Protein accounted for 18.4∼23.4% in raw poultry meat (Soriano-Santos, 2010), which played a major role in determining the quality of poultry meat. As protein is made up of amino acids, the composition and relative quantity of amino acids are of great importance to meat quality evaluation (Mir et al., 2017). Dietary MG at 300 and 450 mg/kg notably increased the total amino acids and essential amino acid content, implying that MG supplementation could promote the nutritive values of broilers. Moreover, the FAA in MG300 group was significantly enhanced relative to that of the CON group. Besides, the relative content of FAA including glutamic acid, aspartic acid, serine, glycine, and alanine accounted for about 34% in total amino acids of broilers. Furthermore, the FAA possess an umami (glutamic acid and aspartic acid) or sweet (serine, glycine, and alanine) taste, which had an important impact on the taste of meat (Bermúdez et al., 2014). Despite the fact that FAA contributing to basic taste (sweet and umami), they are also key flavor precursor substances in flavor formation of meat (Khan et al., 2015). Therefore, the increase of the FAA content was inevitable to influence the overall flavor of the meat.
In conclusion, mixed MG can be used as an effective and novel feed supplement to improve productive performance and meat quality of broilers in which 300 mg/kg MG tended to be more effective. In addition, it was worth noting that the alterations of growth performance, intestinal morphology, IMF, and fatty acid and amino acid profile in MG300 group differed a lot from those in MG450 and MG600 groups. This difference may derive from the dosage of MG used in the diets, and further experiment needed to be conducted to compare the effect of relative low supplementation levels (100–300 mg/kg) of MG on broilers.
Acknowledgments
This work was supported by the Technology and Achievement Transformation Project of Hangzhou, China (Grant NO. 20161631E01), Zhejiang University New Rural Development Research Institute Agricultural Technology Promotion Fund (Grant NO. 2017ZDNT006), Key Project of Natural Science Foundation of Zhejiang Province (Grant No. LD19C200001), Natural Science Foundation of China (31601561). We're especially grateful for Yanyun Ying, Tingting Bu, Jianli Wang, Hangzhou Guo and all people participating in the preparations and analysis of this study.
DISCLOSURES
The authors declare no conflict of interest.
References
- Abdollahi M.R., Zaefarian F., Ravindran V. Feed intake response of broilers: impact of feed processing. Anim. Feed Sci. Technol. 2018;237:154–165. [Google Scholar]
- AOAC . 15th ed. AOAC International Publisher; Washington DC: 1990. Official Methods of Analysis of the Association of the Official Analysis Chemists. [Google Scholar]
- Batovska D.I., Todorova I.T., Tsvetkova I.V., Najdenski H.M. Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: individual effects and synergistic relationships. Pol. J. Microbiol. 2009;58:43–47. [PubMed] [Google Scholar]
- Bermúdez R., Franco D., Carballo J., Sentandreu M.Á., Lorenzo J.M. Influence of muscle type on the evolution of free amino acids and sarcoplasmic and myofibrillar proteins through the manufacturing process of Celta dry-cured ham. Food Res. Int. 2014;56:226–235. [Google Scholar]
- Chwen L.T., Foo H.L., Thanh N.T., Choe D.W. Growth performance, plasma fatty acids, villous height and crypt depth of preweaning piglets fed with medium chain triacylglycerol.(Report) Asian-Australas J. Anim. Sci. 2013;26:700–704. doi: 10.5713/ajas.2012.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayrit F.M. The properties of lauric acid and their significance in coconut oil. J. Am. Oil Chem. Soc. 2015;92:1–15. [Google Scholar]
- Fan M.D., Xiao Q.F., Xie J.C., Cheng J., Sun B.G., Du W.B., Wang Y.X., Wang T.Z. Aroma compounds in chicken broths of Beijing Youji and commercial broilers. J. Agric. Food Chem. 2018;66:10242–10251. doi: 10.1021/acs.jafc.8b03297. [DOI] [PubMed] [Google Scholar]
- Fortuoso B.F., dos Reis J.H., Gebert R.R., Barreta M., Griss L.G., Casagrande R.A., de Cristo T.G., Santiani F., Campigotto G., Rampazzo L., Stefani L.M., Boiago M.M., Lopes L.Q., Santos R.C.V., Baldissera M.D., Zanette R.A., Tomasi T., Da Silva A.S. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: impact on health, performance and meat quality. Microb. Pathog. 2019;129:161–167. doi: 10.1016/j.micpath.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Gheshlaghi R., Scharer J.M., Moo-Young M., Douglas P.L. Application of statistical design for the optimization of amino acid separation by reverse-phase HPLC. Anal. Biochem. 2008;383:93–102. doi: 10.1016/j.ab.2008.07.032. [DOI] [PubMed] [Google Scholar]
- Guillot E., Vaugelade P., Lemarchal P., Re Rat A. Intestinal absorption and liver uptake of medium-chain fatty acids in non-anaesthetized pigs. Br. J. Nutr. 1993;69:431–442. doi: 10.1079/bjn19930045. [DOI] [PubMed] [Google Scholar]
- Jiang W.D., Wu P., Tang R.J., Liu Y., Kuang S.Y., Jiang J., Tang L., Tang W.N., Zhang Y.A., Zhou X.Q. Nutritive values, flavor amino acids, healthcare fatty acids and flesh quality improved by manganese referring to up-regulating the antioxidant capacity and signaling molecules TOR and Nrf2 in the muscle of fish. Food Res. Int. 2016;89:670–680. doi: 10.1016/j.foodres.2016.09.020. [DOI] [PubMed] [Google Scholar]
- Khan M.I., Jo C., Tariq M.R. Meat flavor precursors and factors influencing flavor precursors—a systematic review. Meat Sci. 2015;110:278–284. doi: 10.1016/j.meatsci.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Khatibjoo A., Mahmoodi M., Fattahnia F., Akbari-Gharaei M., Shokri A.N., Soltani S. Effects of dietary short- and medium-chain fatty acids on performance, carcass traits, jejunum morphology, and serum parameters of broiler chickens. J. Appl. Anim. Res. 2017;46:492–498. [Google Scholar]
- Khosravinia H. Effect of dietary supplementation of medium-chain fatty acids on growth performance and prevalence of carcass defects in broiler chickens raised in different stocking densities. J. Appl. Poult. Res. 2015;24:1–9. [Google Scholar]
- Lei L., Ni X., Dong Z., Wang H., Bo J., Yin Z., Pan K. Effect of a dietary probiotic, Lactobacillus johnsonii BS15, on growth performance, quality traits, antioxidant ability, and nutritional and flavour substances of chicken meat. Anim. Prod. Sci. 2016;57:920–926. [Google Scholar]
- Liu T., Li C., Li Y., Feng F. Glycerol monolaurate enhances reproductive performance, egg quality and albumen amino acids composition in aged hens with gut microbiota alternation. Agriculture. 2020;10:250. [Google Scholar]
- Mabayo R.T., Furuse M., Kita K., Okumura J. Improvement of dietary protein utilisation in chicks by medium chain triglyceride. Br. Poult. Sci. 1993;34:121–130. doi: 10.1080/00071669308417568. [DOI] [PubMed] [Google Scholar]
- Mabayo R.T., Furuse M., Yang S.-I., Okumura J.-I. Medium-chain triacylglycerols enhance release of cholecystokinin in chicks. J. Nutr. 1992;122:1702–1705. doi: 10.1093/jn/122.8.1702. [DOI] [PubMed] [Google Scholar]
- Mir N.A., Rafiq A., Kumar F., Singh V., Shukla V. Determinants of broiler chicken meat quality and factors affecting them: a review. Res. Rev. J. Food Sci. Technol. 2017;54:2997–3009. doi: 10.1007/s13197-017-2789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Q., Fu A., Deng L., Zhao M., Li Y., Zhang H., Feng F. High-dose glycerol monolaurate up-regulated beneficial indigenous microbiota without inducing metabolic dysfunction and systemic inflammation: new insights into its antimicrobial potential. Nutrients. 2019;11:1981. doi: 10.3390/nu11091981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrf L., Jks T., Kim E.J., Scollan N.D. Beef, chicken and lamb fatty acid analysis — a simplified direct bimethylation procedure using freeze-dried material. Meat Sci. 2012;92:863–866. doi: 10.1016/j.meatsci.2012.06.013. [DOI] [PubMed] [Google Scholar]
- Mustafa N.G. Biochemical trails associated with different doses of alpha-monolaurin in chicks. Adv. Anim. Vet. Sci. 2018;7:187–192. [Google Scholar]
- Nieto G., Ros G. Vol 12. IntechOpen; London, UK: 2012. Modification of fatty acid composition in meat through diet: effect on lipid peroxidation and relationship to nutritional quality - a review; pp. 239–258. (Lipid Peroxidation). [Google Scholar]
- NRC . 9th rev. ed. National Academies Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Peng M.L., Han J., Li L.L., Ma H.T. Metabolomics reveals the mechanism of (-)-hydroxycitric acid promotion of protein synthesis and inhibition of fatty acid synthesis in broiler chickens. Animal. 2018;12:774–783. doi: 10.1017/S175173111700221X. [DOI] [PubMed] [Google Scholar]
- Saeidi E., Shokrollahi B., Karimi K., Amiri-Andi M. Effects of medium-chain fatty acids on performance, carcass characteristics, blood biochemical parameters and immune response in Japanese quail. Br. Poult. Sci. 2016;57:358–363. doi: 10.1080/00071668.2016.1169508. [DOI] [PubMed] [Google Scholar]
- Shokrollahi B., Yavari Z., Kordestani A. Effects of dietary medium-chain fatty acids on performance, carcass characteristics, and some serum parameters of broiler chickens. Br. Poult. Sci. 2014;55:662–667. doi: 10.1080/00071668.2014.955836. [DOI] [PubMed] [Google Scholar]
- Soriano-Santos J. Wiley & Sons, Inc.; Hoboken, NJ: 2010. Handbook of Poultry Science and Technology: Chemical Composition and Nutritional Content of Raw Poultry Meat. pp. 467–489. [Google Scholar]
- Sukhija P.S., Palmquist D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1989;36:1202–1206. [Google Scholar]
- Valentini J., Da Silva A., Fortuoso B., Reis J., Gebert R., Griss L., Boiago M., Lopes L., Christ-Santos R., Wagner R. Chemical composition, lipid peroxidation, and fatty acid profile in meat of broilers fed with glycerol monolaurate additive. Food Chem. 2020;330:127187. doi: 10.1016/j.foodchem.2020.127187. [DOI] [PubMed] [Google Scholar]
- Van der Aar P.J., Molist F., van der Klis J.D. The central role of intestinal health on the effect of feed additives on feed intake in swine and poultry. Anim. Feed Sci. Technol. 2017;233:64–75. [Google Scholar]
- Wang H., Ni X., Qing X., Zeng D., Luo M., Liu L., Li G., Pan K., Jing B. Live probiotic lactobacillus johnsonii BS15 promotes growth performance and lowers fat deposition by improving lipid metabolism, intestinal development, and gut microflora in broilers. Front. Microbiol. 2017;8:1073. doi: 10.3389/fmicb.2017.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.H., Wang X.X., Li J.T., Chen Y.Q., Yang W.J., Zhang L.Y. Effects of dietary coconut oil as a medium-chain fatty acid source on performance, carcass composition and serum lpids in male broilers. Asian-Australas J. Anim. Sci. 2015;28:223–230. doi: 10.5713/ajas.14.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.Y., Wang Y.B., Xu H., Mei X.Q., Gong L., Wang B.K., Li W.F., Jiang S.Q. Direct-fed glucose oxidase and its combination with B. amyloliquefaciens SC06 on growth performance, meat quality, intestinal barrier, antioxidative status, and immunity of yellow-feathered broilers. Poult. Sci. 2018;97:3540–3549. doi: 10.3382/ps/pey216. [DOI] [PubMed] [Google Scholar]
- Wen C., Yan W., Zheng J., Ji C., Zhang D., Sun C., Yang N. Feed efficiency measures and their relationships with production and meat quality traits in slower growing broilers. Poult. Sci. 2018;97:2356–2364. doi: 10.3382/ps/pey062. [DOI] [PubMed] [Google Scholar]
- WHO Diet, nutrition and the prevention of chronic diseases. Eur. J. Clin. Nutr. 2003;45:619–627. [PubMed] [Google Scholar]
- Zeitz J.O., Fennhoff J., Kluge H., Stangl G.I., Eder K. Effects of dietary fats rich in lauric and myristic acid on performance, intestinal morphology, gut microbes, and meat quality in broilers. Poult. Sci. 2015;94:2404–2413. doi: 10.3382/ps/pev191. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang Y., Liu Y., Wang J., Xu Q., Yu X., Yang X., Liu Z., Xue C. Medium-chain triglycerides promote macrophage reverse cholesterol transport and improve atherosclerosis in ApoE-deficient mice fed a high-fat diet. Nutr. Res. 2016;36:964–973. doi: 10.1016/j.nutres.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Zhao M., Cai H., Liu M., Deng L., Li Y., Zhang H., Feng F. Dietary glycerol monolaurate supplementation for the modification of functional properties of egg white protein. J. Sci. Food Agric. 2019;99:3852–3859. doi: 10.1002/jsfa.9607. [DOI] [PubMed] [Google Scholar]
- Zhou S., Wang Y., Jacoby J.R.J., Jiang Y., Zhang Y., Yu L.L. Effects of medium-and long-chain triacylglycerols on lipid metabolism and gut microbiota composition in C57BL/6J mice. J. Agric. Food Chem. 2017;65:6599–6607. doi: 10.1021/acs.jafc.7b01803. [DOI] [PubMed] [Google Scholar]
- Zhou Y.M., Wen C., Qu H.M., Li J., Cheng Y.F., Chen Y.P., Zhao Y.R. Effects of dietary phytosterols on growth performance, antioxidant status, and meat quality in Partridge Shank chickens. Poult. Sci. 2019;98:3715–3721. doi: 10.3382/ps/pez059. [DOI] [PubMed] [Google Scholar]