Abstract
Both reticuloendotheliosis and Marek's disease are neoplastic diseases of chickens caused by reticuloendotheliosis virus (REV) and Marek's disease virus (MDV), respectively. The infection of REV or MDV may lead to clinical tumors and also result in immunosuppression and easily allow secondary infection by other pathogens. Here, we investigated a breeder flock of three-yellow chickens in southern China that had been vaccinated with CVI988/Rispens at hatching and had experienced depression, weakness, reduction in weight gain, and an increased death rate after 120 d of age. The morbidity and mortality were 20% and 10%, respectively, at 140 d of age when this infection was diagnosed. The necropsy of the birds revealed significant tumor-like lesions in the heart, liver, spleen, and ceca. Peripheral blood lymphocytes and tumor-like tissues were sampled for PCR detection and for histopathological observation, for virus isolation and the subsequent immunofluorescent assay on the cell cultures and for gene sequencing of the isolated viruses. A REV isolate GX18NNR1 and a MDV isolate GX18NNM5 were both recovered from the sampled bird. Further phylogenetic analysis based on the env gene of REV and the meq gene of MDV demonstrated that GX18NNR1 was closely related to the reference REV strain MD-2, which was isolated from a contaminated commercial turkey herpesvirus vaccine. In addition, the GX18NNM5 was found to belong to the Chinese very virulent MDV strains' cluster. The coinfection of REV and MDV may contribute to tumor outbreaks with high morbidity and mortality in three-yellow chicken flocks.
Key words: three-yellow chicken, reticuloendotheliosis virus, Marek's disease virus, coinfection, molecular analysis
Introduction
Along with Marek's disease virus (MDV) and avian leukosis virus (ALV), reticuloendotheliosis virus (REV) is one of the most common naturally occurring viruses that cause contagious, immunosuppressive, and neoplastic diseases in chickens (Nolan et al., 2016). Reticuloendotheliosis (RE) is a malignant disease of poultry most frequently affecting chickens, turkeys, and waterfowl (Bohls et al., 2006). Since the initial REV isolation where strain T was obtained in 1957 from a turkey with visceral lymphomas (Robinson and Twiehaus, 1974), some reports have described REV infections in different countries (Lopez-Osorio et al., 2017). In China, the virus was first isolated in 1986, and its infection has been reported in various places (Jiang et al., 2013; Bao et al., 2015). The REV genome contains 2 identical long terminal repeats and a complete set of genes including gag, pol, and env. The env gene is a major variation region which is related to the induction of neutralizing antibodies (Hu et al., 1981). Marek's disease (MD) is a lymphoproliferative disease of chickens caused by the highly infectious cell-associated alphaherpesvirus, MDV serotype 1 or simply MDV. Marek's disease virus can be clustered into different pathotypes, including mild (m), virulent (v), very virulent (vv), and very virulent plus (vv+) strains (Davison et al., 2009). Among several genes unique to MDV that have been identified and sequenced, the meq gene was reported to have the greatest possibility of being associated with viral oncogenicity and pathogenicity (Nolan et al., 2016).
Coinfection with REV and MDV has become an important epidemiologic situation in poultry production (Jiang et al., 2013; Li et al., 2019; Shi et al., 2019; Zhang et al., 2019). The immunosuppression caused by REV can decrease the antibody levels achieved by vaccinations and can then increase susceptibility to secondary infections (Sun et al., 2017). It is widely reported that the long terminal repeats of REV can integrate into the genome of MDV, which can promote the spread of MDV (Cui et al., 2010). In addition, REV can be transmitted by inoculation with the contaminated biologicals, such as the live commercial vaccines (Hertig et al., 1997; Li et al., 2013). Moreover, tumors with similar gross lesions caused by ALV have been the most prevalent clinical disease in China especially in the three-yellow chicken breeds in southern China (Wang et al., 2018; Li et al., 2019). These tumor diseases are hard to differentiate using only the gross lesions and need to be further diagnosed by laboratory techniques. Recently, cases of suspected tumor-like diseases have come to be more frequent in the yellow chicken and have caused serious losses. This is a serious problem for the yellow-chicken sector in China that produces annually 4.2 billion birds (Wei, 2019). In this study, we report an outbreak of severe tumors in a three-yellow chicken breeder flock caused by the coinfection of REV and vvMDV in Southern China.
Materials and methods
Case History
In 2018, 2,000 birds (16.7%, 2,000/12,000) in a three-yellow chicken breeder flock in Nanning, Guangxi Province, China, experienced depression, weakness, reduction in weight gain, and death beginning from the age of 120 d. The morbidity and mortality were 20% and 10%, respectively, at the age of 140 d when this disease was diagnosed. This flock had been vaccinated with the MD CVI988/Rispens vaccine at 1 d of age. The necropsy found significant tumor-like lesions in the heart, liver, spleen, and ceca.
Samples' Collection and Processing
An anticoagulation-treated (by adding sodium citrate) blood sample was collected from the bird of suspected disease and then the peripheral blood lymphocytes were separated for viral isolation according to the routine procedure (Buscaglia, 2013). Tissues of visceral organs with significant tumor-like lesions were sampled and divided into 2 parts: one part was processed for REV and/or ALV isolation (Bao et al., 2015; Wang et al., 2018). Briefly, the tissue samples were homogenized in the PBS (1:3 w/v) and freeze-thawed 3 times at −80 °C/room temperature. The supernatants were filtered through 0.22-μm filters and used for inoculation on the cell cultures. Another part was cut into a tissue cube 0.5 cm × 0.5 cm in size and fixed in 10% buffered formalin and then used for the histopathological observation according to routine procedures (Liu et al., 2019).
Cells and Chicken Embryos
A continuous chicken embryo fibroblast (CEF) DF-1 cell line, free of endogenous ALV, was used to isolate ALV following the procedures our group previously described (Wang et al., 2018; Li et al., 2019). Ten-day-old specific pathogen–free chicken embryos were used to prepare the CEF monolayer cell cultures and used for REV and MDV isolation (Cui et al., 2016).
Virus Isolation and Identification
For ALV and REV isolation, briefly, the supernatants of the tissue samples were inoculated into DF-1 and CEF monolayer cells for 2 h. Then, fresh Dulbecco's modified Eagle's medium with 1% fetal bovine serum was used for replacement. After 5–7 d of cell culture, the infected cells were frozen and thawed 3 times before the supernatants were inoculated onto the fresh DF-1 and CEF for 3 continuous passages, which were used to eliminate the possibility of detecting MDV. For MDV isolation, the harvested peripheral blood lymphocytes were inoculated onto the primary CEF grown in 6-well plates, and 2–3 continually blind passages were performed until the cytopathogenic effect was observed. The adherent DF-1 and CEF were examined by an immunofluorescent assay (IFA) using monoclonal antibody (mAb) (kindly provided by Dr. Zhizhong Cui, Shandong Agricultural University). In addition, mAb for REV (11B118), MDV serotype 1 (BA4), and ALV-p27 (IE5) were used at a 1:20 dilution as described previously (Cui et al., 1986; Lu and Lee, 1997).
Detecting, Cloning, and Sequencing of the Isolated Viruses
The total DNA was extracted from the cell cultures using a DNA extraction kit (TIANGEN, Beijing, China). To detect the oncogenic pathogens including MDV, REV, ALV, and CIAV, primers were used, which are listed in Table 1, as described previously (Yu et al., 2005; Cui et al., 2016). The specific PCR products were purified using a Universal DNA Purification Kit (TIANGEN, Beijing, China) according to the manufacturer's manual and then were ligated into pMD18-T vector (Takara, Dalian, China) according to the manufacturer's manual. Three clones of each sample were sent to accomplish the sequencing by Beijing Genomics Institute (BGI, Guangzhou, China). Sequence data have been submitted to the GenBank Nucleotide Sequence Database. GX18NNR1 and GX18NNM5 data were listed under Accession No. MN943308 and MN943299, respectively. The resulting nucleotide (nt) sequences were analyzed using the ClustalW (BioEdit version 7) software program with the DNASTAR software (DNAStar Inc., Madison, WI). Phylogenetic trees were constructed using the neighbor-joining method implemented in a MEGA program (version 6.0) with bootstrap values calculated from 1,000 replicates. The main features of the isolated REV and MDV strains are summarized in Tables 2 and 3, respectively.
Table 1.
Primers for PCR amplification of oncogenic pathogens.
| Pathogens | Types | Primer sequence 5’→3 | Annealing temperature | Length |
|---|---|---|---|---|
| CIAV-vp1 | Forward | GCATTC CGAGTGGTTACTATTCC | 55 °C | 842 bp |
| Reverse | CGTCTTGCCATCTTACAGTCTTAT | |||
| ALV-p27 | Forward | GGATGAGGTGACTAAGAAAG | 55 °C | 545 bp |
| Reverse | CGAACCAAAGGTAACACAC | |||
| MDV-meq | Forward | ATGTCTCAGGAGCCAGAGCC | 60 °C | 1,020 bp |
| Reverse | TCAGGGTCTCCCGTCACCTG | |||
| REV-env | Forward | CTCCTGACAACCAAGAAG | 53 °C | 1,825 bp |
| Reverse | AGCTCCCTCCCACATTC |
Table 2.
REV strains used in the construction of phylogenetic trees.
| Isolates | Origins | Years | Accession No. | Hosts |
|---|---|---|---|---|
| SNV | USA | 1,959 | DQ003591 | Chicken |
| ATCC-VR775 | USA | 1,972 | KF313137 | Duck |
| HA9901 | China | 1,999 | AY842951 | Chicken |
| APC-566 | USA | 2,005 | DQ387450 | Chicken |
| chicken/3337/05 | Taiwan, China | 2,005 | FJ439120 | Chicken |
| goose/3410/06 | Taiwan, China | 2,006 | FJ439119 | Goose |
| HLJ07I | China | 2,007 | GQ375848 | Chicken |
| ZD0708 | China | 2,007 | FJ496333 | Chicken |
| MD-2 | China | 2,008 | JX912710 | Vaccine |
| HLJR0901 | China | 2,009 | GQ415646 | Chicken |
| 1105 | China | 2,011 | JQ804915 | Duck |
| CY1111 | China | 2,011 | KJ909531 | Chicken |
| HA1101 | China | 2,011 | KF305089 | Chicken |
| SY1209 | China | 2,012 | KJ909530 | Chicken |
| 104865 | USA | 2,014 | KJ756349 | Turkey |
| GDBL1401 | China | 2,014 | KU204702 | Pigeon |
| IBD/C1605 | China | 2,016 | KX278301 | Vaccine |
| GX18NNR1 | China | 2,018 | MN943308 | Chicken |
Abbreviation: REV, reticuloendotheliosis virus.
Table 3.
Descriptions of MDV strains used in the study.
| Strains | Origins | Pathotypes | Years | Accession no. |
|---|---|---|---|---|
| CU-2 | USA | m1 | 1,968 | EU499381 |
| CVI988 | The Netherlands | att2 | 1,969 | DQ534538 |
| BC-1 | Australia | v4 | 1,970 | AY362707 |
| 814 | China | att | 1,980 | AF493551 |
| 595 | USA | vv+6 | 1,991 | AY362715 |
| MPF57 | Australia | v | 1,994 | EF523774 |
| 648A | USA | vv+ | 1,994 | AY362725 |
| G2 | China | vv5 | 1,995 | AF493556 |
| N | USA | vv+ | 1,997 | AF362718 |
| NEW | USA | vv+ | 1,999 | AF362719 |
| X | USA | vv+ | 1,999 | AY362723 |
| W | USA | vv+ | 1,999 | AY362723 |
| GX0101 | China | vv | 2,001 | JX844666 |
| L | USA | vv+ | 2,002 | AF362717 |
| 04CRE | Australia | v | 2,004 | EF523773 |
| 3004 | Russia | m | 2,007 | EU032468 |
| GX070092 | China | vv | 2,007 | HQ290416 |
| GXY2 | China | vv | 2,007 | HQ290372 |
| LCC | China | vv | 2,008 | KU744556 |
| MS01 | China | −3 | 2,009 | HQ638143 |
| DY01 | China | vv | 2,009 | HQ638141 |
| TQ20 | China | vv | 2,009 | HQ638151 |
| JM102 | USA | v | 2,010 | HM488348 |
| HNGS101 | China | vv | 2,011 | HF546084 |
| Anhui001 | China | vv | 2,013 | HG328238 |
| GX14PP03 | China | vv | 2,014 | KX506775 |
| QD2014 | China | vv | 2,014 | KP144354 |
| GX18NNM5 | China | − | 2,018 | MN943299 |
Abbreviation: MDV, Marek's disease virus.
Mild.
Attenuated.
Unknown.
Virulent.
Very virulent.
Very virulent plus.
Results
Histopathological Observations
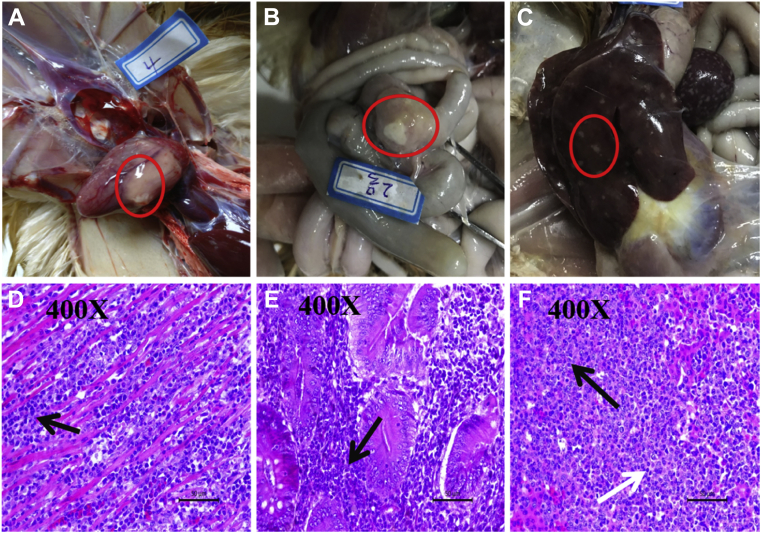
The histopathological observations of the tissue sections indicated that a large area of neoplastic lymphocyte cells was observed in the heart, ceca, and liver (Figures 1A–1C). Specifically, numerous lymphoid tumor nodules were composed of pleomorphic, neoplastic lymphoid cells, concentrated in the heart between the muscle fibers (Figure 1D). The infiltration of many diffuse lymphocytes was observed in the ceca (Figure 1E). The liver tissue had been infiltrated with both neoplastic lymphocytes and reticular cells (Figure 1F).
Figure 1.
Gross pathological changes and microscopic pathological changes (hematoxylin and eosin staining, 400×). White nodule in the heart (A); parenchymal nodular hyperplasia of the cecum (B); a lot of white diffused nodule in the swelled liver (C); the ceca and heart were infiltrated by a large number of pleomorphic, neoplastic lymphocytes (D and E). Two types of tumor cells exist in the same focus in a liver consisting of neoplastic lymphocytes and reticular cells (F).
Pathogen Detection and Identification
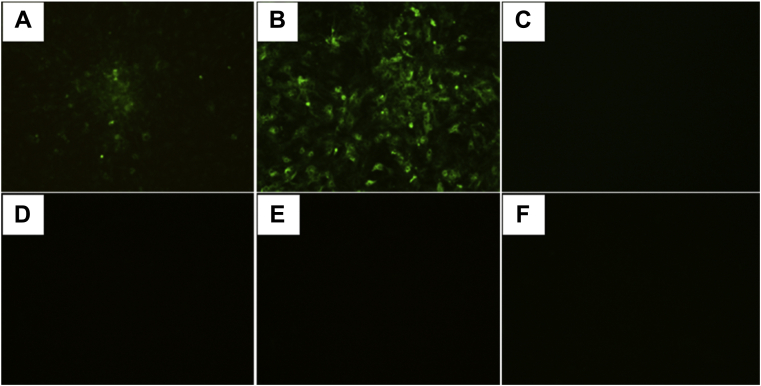
The PCR detection results using the cell cultures showed the samples were positive for MDV and REV but negative for ALV and CIAV, and the complete sequences of the MDV-meq and REV-env genes were successfully amplified. In addition, an IFA with specific mAb showed that an immunofluorescence signal was detected in MDV- and REV-infected cells, respectively (Figures 2A and 2B), whereas there was no fluorescence in the ALV-infected cells and the negative control (Figures 2C–2F). These data clearly showed that the sampled bird was coinfected with MDV and REV.
Figure 2.
IFA on CEF and DF-1 cell cultures infected with MDV, REV, and ALV. Plaques from the isolate GX18NNM5 were evident at 136 h (F2) after inoculation; specific staining of the viral plaques with MDV-1 gB-specific monoclonal antibody BA4 was observed (A, 100 ×), whereas no specific fluorescence was observed in the noninfected cells (D, 100 ×). Positive fluorescence was observed in the GX18NNR1-infected cells by the IFA with REV-specific monoclonal antibody 11B118 (B, 200 ×); no specific fluorescence was observed in the noninfected cells (E, 100 ×). No specific fluorescence was observed in the DF1 cells of both the inoculated cells (C, 200 ×) and the negative control (F, 100 ×) when the IFA was run by using the monoclonal antibody IE5 specific to ALV-p27. Abbreviations: ALV, avian leukosis virus; CEF, chicken embryo fibroblast; IFA, immunofluorescent assay; MDV, Marek's disease virus; REV, reticuloendotheliosis virus.
The GX18NNR1 Phylogenetically Resembled the Vaccine-Origin Strain MD-2
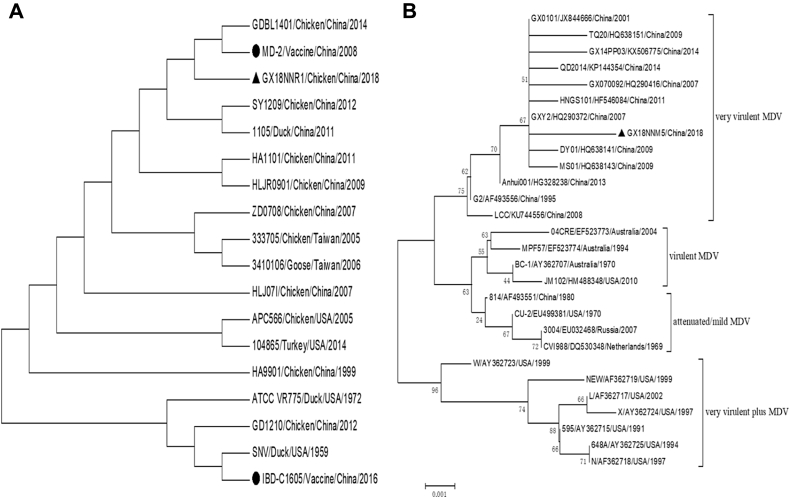
The phylogenetic relationships among the REV strains, which were isolated from chickens, geese, ducks, and wild birds in different countries and regions, were analyzed, and the nt similarity of GX18NNR1 compared with env gene of the reference strains was 95.8–99.9%. The phylogenetic tree based on the env gene sequences showed that most of the REV strains in China were in the same branch, and GX18NNR1 was most closely related to the vaccine-origin strain MD-2, which was isolated from a batch of commercial freeze-dried HVT vaccines in 2007, indicating that they may have the same origin (Figure 3A).
Figure 3.
Phylogenetic trees based on the env nucleotide sequences of REV (A) and meq nucleotide sequence of MDV (B). Numbers at nodes indicate bootstrap percentages obtained using 1,000 replicates. Abbreviations: MDV, Marek's disease virus; REV, reticuloendotheliosis virus.
The Isolate GX18NNM5 Belonged to vvMDV Strain
The comparisons of sequence similarities of nt and amino acid (AA) between GX18NNM5 and 27 other reference strains revealed that the GX18NNM5 has a higher similarity to vvMDV GX0101 and GXY2 strains in the meq gene (nt: 99.7%; AA: 99.1%). In addition, the AA mutations were found in the meq of GX18NNM5 at positions 71 (S→A), 77 (K→E), 80 (D→Y), 115 (V→A), 139 (T→A), 176 (P→R), and 217 (P→A), which were the same as the vvMDV field strains (Shamblin et al., 2004). As the numbers of 4-proline repeat (PPPP) in the proline-rich central region of the MEQ protein was found to have a significant negative association with the virulence of the MDV isolates (Renz et al., 2012), we found GX18NNM5 had the substitutions at both the positions 176 (P176R) and 217 (P217A), interrupting a stretch of the PPPP at position 2 (PPPP → PNPP) that may result in an increase in virulence (Shamblin et al., 2004). The phylogenetic tree showed that GX18NNM5 and the reference strains constitute 2 large lineages. One of the lineages contains 2 sublineages: the mild strains, vaccine strains, and the foreign v or vv+ strains' cluster. GX18NNM5 belonged to the Chinese vv strains' cluster in another lineage (Figure 3B).
Discussion
Reticuloendotheliosis virus not only spreads vertically but also spreads horizontally through feces and the contaminated feed and blood-sucking insects. In addition, the emergence of REV and the associated clinical problems are expanding and changing, which has brought new challenges for effective prevention and control of RE in the world poultry industry. In addition, there are many reports of chickens infected with REV at the time of vaccination or coinfection with other viruses (Fadly and Witter, 1997; Li et al., 2013; Zhang et al., 2019). Since the first description of MD in the 1907, it has spread rapidly all over the world but has been well-controlled by using the MD vaccine (Witter et al., 1979). However, recent studies showing the emergence of vv + MDV strains with further increases in the field virus strains suggested this was the main cause of breakthrough from the protection of vaccines (Bao et al., 2015; Sun et al., 2017). Reticuloendotheliosis virus–induced immunosuppression inhibited the host immune activity, thus reducing immune responses to stimulation with MD vaccines (Sun et al., 2017). Immunosuppression and pathogenicity induced by REV and the resultant coinfection can seriously impair the host immune resistance to MDV infection, inevitably resulting in more serious illness in the coinfected birds.
According to the epidemiological data obtained in our group from 2010 to 2017, the positive rate of coinfection of REV and MDV had remained at a low level (Li et al., 2019; Shi et al., 2019). However, since 2018, the number of coinfections in three-yellow chickens has increased dramatically (data not shown). In the case reported here, we confirmed that both GX18NNR1 and GX18NNM5 were found in the diseased birds via the virus isolation and identification including the IFA on the cell cultures and by isolates' gene sequencing. There were at least 3 possibilities for this case. First, it is due to REV contamination in poultry vaccines, such as the MD vaccine, which leads to decreased MDV vaccination efficacy, caused by coinfections of REV and MDV, which may also contribute to the tumor outbreak with high morbidity and mortality in the flock. Second, in the grandparent stocks of the broiler breeders that had been infected with REV, the virus might spread vertically, resulting in infection with REV and impairment of immune organs in younger chicks, leading to decreased immunity and immune failure of the MD vaccination (Witter et al., 1979; Sun et al., 2017). Third, the flock was infected with highly pathogenic MDV such as vvMDV or vv + MDV and broke through the protection of the vaccine, causing the chickens to be coinfected with other pathogens such as REV, as in this case.
In this study, an outbreak of severe tumors in a three-yellow chicken breeder flock caused by the coinfection of vvMDV and REV in southern China was diagnosed and analyzed. Because the coinfection occurs quite commonly in the field, it will be interesting to continue evaluating the role of REV in the pathogenesis of MD in vaccinated flocks. Finally, for the effective prevention and control of MD and RE, we should not only pay attention to the transmission and infection of MDV but also avoid the horizontal and vertical transmission of REV in the breeder flocks. Most importantly, REV contamination in poultry vaccines must be strictly eliminated.
Acknowledgments
This work was supported by the Guangxi Special Funding on Science and Technology Research [AA17204057], the Guangxi Program for Modern Agricultural Industry Technical System Construction-Chicken Industry [nycytxgxcxtd-19-03]. The manuscript was kindly reviewed by Dr. Richard Roberts, Aurora, CO 80014, USA.
Disclosures
The authors declare no conflicts of interest.
References
- Bao K.Y., Zhang Y.P., Zheng H.W., Lv H.C., Gao Y.L., Wang J.F., Gao H.L., Qi X.L., Cui H.Y., Wang Y.Q., Ren X.G., Wang X.M., Liu C.J. Isolation and full-genome sequence of two reticuloendotheliosis virus strains from mixed infections with Marek's disease virus in China. Virus Genes. 2015;50:418–424. doi: 10.1007/s11262-015-1191-z. [DOI] [PubMed] [Google Scholar]
- Bohls R.L., Linares J.A., Gross S.L., Ferro P.J., Silvy N.J., Collisson E.W. Phylogenetic analyses indicate little variation among reticuloendotheliosis viruses infecting avian species, including the endangered Attwater's prairie chicken. Virus Res. 2006;119:187–194. doi: 10.1016/j.virusres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Buscaglia C. Mixed infections of Marek's disease and reticuloendotheliosis viruses in layer flocks in Argentina. Avian Dis. 2013;57:569–571. doi: 10.1637/10398-100112-Case.1. [DOI] [PubMed] [Google Scholar]
- Cui Z., Lee L.F., Silva R.F., Witter R.L. Monoclonal antibodies against avian reticuloendotheliosis virus: identification of strain-specific and strain-common epitopess. J. Immunol. 1986;136:4237–4242. [PubMed] [Google Scholar]
- Cui N., Su S., Sun P., Zhang Y., Han N., Cui Z. Isolation and pathogenic analysis of virulent Marek's disease virus field strain in China. Poult. Sci. 2016;95:1521–1528. doi: 10.3382/ps/pew073. [DOI] [PubMed] [Google Scholar]
- Cui Z., Zhuang G., Xu X., Sun A., Su S. Molecular and biological characterization of a Marek's disease virus field strain with reticuloendotheliosis virus LTR insert. Virus Genes. 2010;40:236–243. doi: 10.1007/s11262-009-0437-z. [DOI] [PubMed] [Google Scholar]
- Davison A.J., Eberle R., Ehlers B., Hayward G.S., McGeoch D.J., Minson A.C., Pellett P.E., Roizman B., Studdert M.J., Thiry E. The order Herpesvirales. Arch. Virol. 2009;154:171–177. doi: 10.1007/s00705-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadly A.M., Witter R.L.J.A.D. Comparative evaluation of in vitro and in vivo assays for the detection of reticuloendotheliosis virus as a contaminant in a live virus vaccine of poultry. Avian Dis. 1997;41:695–701. [PubMed] [Google Scholar]
- Hertig C., Coupar B.E., Gould A.R., Boyle D.B. Field and vaccine strains of fowlpox virus carry integrated sequences from the avian retrovirus, reticuloendotheliosis virus. Virol. 1997;235:367–376. doi: 10.1006/viro.1997.8691. [DOI] [PubMed] [Google Scholar]
- Hu S.S., Lai M.M., Wong T.C., Cohen R.S., Sevoian M. Avian reticuloendotheliosis virus: characterization of genome structure by heteroduplex mapping. J. Virol. 1981;37:899–907. doi: 10.1128/jvi.37.3.899-907.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Qi X., Gao Y., Hua Y., Li K., Deng X., Wang Q., Zhang L., Chai H., Chen Y., Yin C., Gao H., Qin L., Wang Y., Qu Y., Chen Q., Fan Z., Wang X. Molecular characterization and phylogenetic analysis of the reticuloendotheliosis virus isolated from wild birds in Northeast China. Vet. Microbiol. 2013;166:68–75. doi: 10.1016/j.vetmic.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Li H., Wang P., Lin L., Shi M., Gu Z., Huang T., Mo M.L., Wei T., Zhang H., Wei P. The emergence of the infection of subgroup J avian leucosis virus escalated the tumor incidence in commercial Yellow chickens in Southern China in recent years. Transbound. Emerg. Dis. 2019;66:312–316. doi: 10.1111/tbed.13023. [DOI] [PubMed] [Google Scholar]
- Li J., Yang C., Li Q., Li H., Xia Y., Liu D., Yu K., Yang H. Complete genome sequence of reticuloendotheliosis virus strain MD-2, isolated from a contaminated Turkey herpesvirus vaccine. Genome Announc. 2013;1:713. doi: 10.1128/genomeA.00785-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Ma K., Liu M., Yang C., Huang X., Zhao Y., Qi K. Histologic findings and viral antigen distribution in natural coinfection of layer hens with subgroup J avian leukosis virus, Marek's disease virus, and reticuloendotheliosis virus. J. Vet. Diagn. Invest. 2019;31:761–765. doi: 10.1177/1040638719868274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Osorio S., Piedrahita D., Espinal-Restrepo M.A., Ramirez-Nieto G.C., Nair V., Williams S.M., Baigent S., Ventura-Polite C., Aranzazu-Taborda D.A., Chaparro-Gutierrez J.J. Molecular characterization of Marek's disease virus in a poultry layer farm from Colombia. Poult. Sci. 2017;96:1598–1608. doi: 10.3382/ps/pew464. [DOI] [PubMed] [Google Scholar]
- Lu C., Lee L.F. Monoclonal antibodies recognizing serotype 1 specific and group common epitopes on glucoprotein B molecule of Marek’s disease virus. Chin. J. Vet. Sci. 1997;4:5–8. [Google Scholar]
- Nolan L.K., Swayne D.E., Saif Y.M., Fadly A.M., Glisson J.R., Mcdougald L.R. Wiley; Hoboken, NJ: 2016. Diseases of Poultry; pp. 513–673. [Google Scholar]
- Renz K.G., Cooke J., Clarke N., Cheetham B.F., Hussain Z., Fakhrul Islam A.F., Tannock G.A., Walkden-Brown S.W. Pathotyping of Australian isolates of Marek's disease virus and association of pathogenicity with meq gene polymorphism. Avian Pathol. 2012;41:161–176. doi: 10.1080/03079457.2012.656077. [DOI] [PubMed] [Google Scholar]
- Robinson F.R., Twiehaus M.J. Isolation of the avian reticuloendothelial virus (strain T) Avian Dis. 1974;18:278–288. [PubMed] [Google Scholar]
- Shamblin C.E., Greene N., Arumugaswami V., Dienglewicz R.L., Parcells M.S.J.V.M. Comparative analysis of Marek's disease virus (MDV) glycoprotein-, lytic antigen pp38- and transformation antigen Meq-encoding genes: association of meq mutations with MDVs of high virulence. Vet. Microbiol. 2004;102:147–167. doi: 10.1016/j.vetmic.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Shi M., Li H., Wang W., Gu Z., Wang P., Lin L., Liang J., Wei P. Epidemiological Survey of three Kinds of avians tumor diseases of Local breed chickens in Guangxi and Surrounding areas in 2017. Chin Poult. 2019;41:73–76. [Google Scholar]
- Sun G.R., Zhang Y.P., Zhou L.Y., Lv H.C., Zhang F., Li K., Gao Y.L., Qi X.L., Cui H.Y., Wang Y.Q., Gao L., Pan Q., Wang X.M., Liu C.J. Co-infection with Marek's disease virus and reticuloendotheliosis virus increases illness Severity and Reduces Marek's disease vaccine efficacy. Viruses. 2017;9:158. doi: 10.3390/v9060158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Lin L., Li H., Yang Y., Huang T., Wei P. Diversity and evolution analysis of glycoprotein GP85 from avian leukosis virus subgroup J isolates from chickens of different genetic backgrounds during 1989-2016: Coexistence of five extremely different clusters. Arch. Virol. 2018;163:377–389. doi: 10.1007/s00705-017-3601-0. [DOI] [PubMed] [Google Scholar]
- Wei P. Challenge and Development Opportunity of Quality chicken Industry in China: a Review about 9 Issues on the Topic. Chin Poult. 2019;41:1–6. [Google Scholar]
- Witter R.L., Lee L.F., Bacon L.D., Smith E.J. Depression of vaccinal immunity to Marek's disease by infection with reticuloendotheliosis virus. Infect. Immun. 1979;26:90–98. doi: 10.1128/iai.26.1.90-98.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Cui Z., Shi J. Sequence analysis for the complete proviral genome of reticuloendotheliosis virus Chinese strain HA9901. Sci. China C Life Sci. 2006;42:340–348. doi: 10.1007/s11427-006-0149-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yu Z., Lan X., Zhang F., Wang Q., Li K., Pan Q., Gao Y., Qi X., Cui H.Y., Wang Y., Gao L., Wang X., Liu C. A high frequency of Gallid herpesvirus-2 co-infection with Reticuloendotheliosis virusis associated with high tumor rates in Chinese chicken farms. Vet. Microbiol. 2019;237:108418. doi: 10.1016/j.vetmic.2019.108418. [DOI] [PubMed] [Google Scholar]