Abstract
As the demand for alternatives to antibiotic growth promoters (AGP) increases in food animal production, phytobiotic compounds gain popularity because of their ability to mimic the desirable bioactive properties of AGP. Chestnut tannins (ChT) are one of many phytobiotic compounds used as feed additives, particularly in South America, for broilers because of its favorable antimicrobial and growth promotion capabilities. Although studies have observed the microbiological and immunologic effects of ChT, there is a lack of studies evaluating the metabolic function of ChT. Therefore, the objective of this study was to characterize the cecal metabolic changes induced by ChT inclusion and how they relate to growth promotion. A total of 200 day-of-hatch broiler chicks were separated into 2 feed treatment groups: control and 1% ChT. The ceca from all the chicks in the treatment groups were collected on day 2, 4, 6, 8, and 10 after hatch. The cytokine mRNA quantitative RT-PCR was determined using TaqMan gene expression assays for IL-1B, IL-6, IL-8, IL-10, and interferon gamma quantification. The cytokine expression showed highly significant increased expressions of IL-6 and IL-10 on day 2 and 6, whereas the other proinflammatory cytokines did not have significantly increased expression. The results from the kinome array demonstrated that the ceca from birds fed with 1% ChT had significant (P < 0.05) metabolic alterations based on the number of peptides when compared with the control group across all day tested. The increased expression of IL-6 appeared to be strongly indicative of altered metabolism, whereas the increased expression of IL-10 indicated the regulatory effect against other proinflammatory cytokines other than IL-6. The ChT initiate a metabolic mechanism during the first 10 d in the broiler. For the first time, we show that a phytobiotic product initially modulates metabolism while also potentially supporting growth and feed efficiency downstream. In conclusion, a metabolic phenotype alteration in the ceca of chickens fed ChT may indicate the importance of enhanced broiler gut health.
Key words: kinome array, feed additive, metabolism, phytobiotic
Introduction
By moving away from antibiotics in livestock production, there is a growing interest from the poultry industry to find plant-based alternatives to replace antibiotics in feed. There has been increased interest in using plant-based compounds or phytobiotics as antibiotic growth promoter (AGP) alternatives, including chestnut tannins (Castanea sativa) (Diaz Carrasco et al., 2018; Ren et al., 2019). Plant-based tannins can be categorized into 2 major groups: condensed tannins or hydrolyzable tannins (Huang et al., 2018). Tannins can be found in many plant species, mostly in the inedible portions of the plant such as the bark or wood (Brus et al., 2018; Molino et al., 2020). Owing to enhanced research, the previous knowledge that tannins possess antinutritional effects in livestock species is outdated and the benefits vary on poultry species and dosages in feed Schiavone et al., 2008). The bioactive compounds, polyphenols, found in tannins allow them to be effective immunomodulatory additives that promote human and animal health (Molino et al., 2020). While the mechanism of action is still not widely understood, the benefits of phytobiotics in livestock animals include antioxidation properties, stabilization of intestinal microbiome, and improvement of immune system through immunomodulatory effects (Lillehoj et al., 2018). Extensive studies performed on swine species exhibit strong evidence of phytobiotics as likely alternatives to antibiotics to improve growth performance and health (Michiels et al., 2010; Liu et al., 2013, 2014). Ruminant producers have increased interest in growth promotors because of the efficient utilization of energy during rumen fermentation (Nagaraja et al., 1997). The usage of tannins in the ruminant industry has been of major interest because of the tannins' ability to play a role in rumen protein utilization to improve feed efficiency (Min et al., 2003).
Previous studies evaluated the functionality of different tannin species against pathogenic infections across livestock species, showing antimicrobial activity in concentrations ranging between 0.5 and 1 kg/ton (Costabile et al., 2011; Liu et al., 2014; Lillehoj et al., 2018). Importantly, tannins in general have also been shown to improve feed efficiency, growth performance, and intestinal health, as seen when up to 0.2% chestnut tannins (ChT) are added into feed (Gai et al., 2010; Redondo et al., 2014). However, inclusions of 2% ChT in other avian studies did not improve growth performance and affected palatability. With all these studies conducted, the usage of tannins in production have shown potential to be efficient as growth promoter alternatives, given the quality and concentration provided (Redondo et al., 2014).
While the previously mentioned studies provide information about microbiological, immunologic, and performance data (Costabile et al., 2011; Redondo et al., 2015; Lillehoj et al., 2018), there is a lack of knowledge about the mechanism of the host metabolic interactions in the intestine, specifically the metabolic reaction to dietary ChT. Tannins exert proinflammatory immune responses but for a short-lived period and the anti-inflammatory responses follow soon after (Lopetuso et al., 2015). Tannins can also exert a synergistic effect with AGP alternatives to promote gut health (Redondo et al., 2014). Currently, there are proposed mechanisms for immunomodulatory activity based on different types of feed additives (Suresh et al., 2018; Ramah et al., 2020). While gene expression provides important information regarding pathogen interaction, there are disadvantages to genetic approaches including its inability to accurately predict the cellular phenotypes (Arsenault et al., 2017).
Studying phosphorylation events provides information on the mechanism of post-translational modification, which offers insight into cellular and tissue phenotypes (Arsenault et al., 2011; Manning et al., 2002). Peptide arrays for kinomic analysis have already been widely used across scientific disciplines (Diks et al., 2004; Parikh and Peppelenbosch, 2010; Arsenault et al., 2011). The kinome array provides functional phenotype data, indicating changes within tissue metabolism and immune response to an infection (Arsenault et al., 2013). By understanding the kinase activity, there are increased insights into identifying specific biomarkers to provide future therapeutic targets (Arsenault et al., 2011). Recent advancements have provided a species-specific peptide array for livestock species, including poultry (Arsenault et al., 2011; Arsenault et al., 2015). This integrated array demonstrates the importance of combined immunity and metabolic data on the animal's overall health and growth performance (Arsenault et al., 2015).
Therefore, the objective of this study was to determine the metabolic phenotype changes affected by ChT in the ceca. By using gene expression and kinome array, we were able to identify global metabolic phosphorylation-based events in the ceca of birds fed ChT at high doses compared with birds fed regular starter diets.
Materials and methods
Experimental Animals, Housing, and Treatments
All experiments conducted were in accordance with guidelines set by the USDA Animal Care and Use Committee (USDA ACUC #2019001), which meets all federal requirements as defined in the Animal Welfare Act, and the Human Care and Use of Laboratory Animals. A total of 200 male day-of-hatch broiler chicks were obtained from a local commercial hatchery and assigned to 2 treatment groups, totaling 50 chicks per pen: 1) control feed, normal starter feed (n = 50), and 2) 1% ChT inclusion feed (n = 50). The 1% ChT inclusion was determined to be most effective without compromising palatability for the birds. Chicks were randomly distributed into each group in pens with fresh pine shavings, water, and starter diet ad libitum. They were maintained in biosafety level units under 24 h of light from start to finish of the study. All treatments were fed a corn/soybean–based crumble diet, and they differ in AGP or tannins inclusion. The diets were formulated to meet or exceed broilers requirements (Table 1). The hydrolyzable ChT additive (Silvateam s.p.a., Buenos Aires, Argentina) contained 75% tannin content, supplemented with 94% DM, lignin, and sugars. The experimental process lasted 10 d per replicate experiment. This study was repeated 1 more time for a total of 2 replicate experiments.
Table 1.
Calculated composition of starter diets. The total basal diet contained 1,365 kcal/lb.
| Ingredients | % |
|---|---|
| Corn | 59.81 |
| SBM 48% | 33.84 |
| Monocalcium phosphate 21 | 1.56 |
| Soy oil | 2.09 |
| Choline chloride | 0.10 |
| Limestone | 1.56 |
| Salt | 0.33 |
| L-lysine HCL | 0.19 |
| DL-methionine | 0.28 |
| Vitamin premix | 0.13 |
| Mineral premix | 0.05 |
| L-Threonine | 0.05 |
| Calculated nutrients, % | 99.99 |
| Protein | 22.00 |
| Calcium | 0.90 |
| Available phosphorus | 0.45 |
| AMEn (Kcal/lb) | 1,365.00 |
| Digestible methionine | 0.59 |
| Digestible total sulfa amino acid | 0.88 |
| Digestible threonine | 0.77 |
| Digestible lysine | 1.18 |
| Choline | 1,256.87 |
| Sodium | 0.16 |
| Potassium | 0.84 |
| Chloride | 0.20 |
Sample Collection and Processing
On each necropsy day, 10 birds/group were euthanized via cervical dislocation and necropsied on day 2, 4, 6, 8, and 10 of each replicate experiment. Both the ceca were removed, flushed with PBS, and flash frozen in liquid nitrogen to preserve the kinase enzymatic activity. The frozen tissues were moved into a −80°C storage freezer until further processing.
Real-Time Quantitative RT-PCR
The immune portion was quantitated by gene expression studies, specifically through a TaqMan-based assay adapted from Eldaghayes et al. (2006). Total RNA was extracted using a Qiagen RNeasy Plus kit (Germantown, MD) and evaluated with a NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The ceca stored in RNALater were used for RNA isolation with the Qiagen RNeasy Plus Kit. The ceca were cut longitudinally to expose the lumen, and any remaining fecal matter was gently removed with forceps as to not disturb the mucosal layer. For each group, there were 10 ceca processed per replicate experiment for quantitative RT-PCR (qRT-PCR).
Cytokine mRNA expression levels were ascertained using RT-PCR with 28S as the reference gene. The RNA were stored at −80°C until plate setup. The cytokines IL-1B, IL-6, IL-8, IL-10, and interferon gamma were quantified using the method reported by Eldaghayes et al. (2006). Primer and probe sequences (Table 2) for amplification have been described previously by Kogut et al. (2003) and Kaiser et al. (2000). The plates were run in the Applied Biosystems ABI StepOne Plus PCR system (ThermoFisher Scientific, Waltham, MA) with the previously stated TaqMan Assay under the following conditions: 1 cycle of 48°C for 30 min, 95°C for 20 s, and 40 cycles of 95°C for 3 s and 60°C for 30 s. Results were calculated with the corrected 40- Ct method, as described in the study by Eldaghayes et al. (2006) and expressed in fold change values. Each sample was run in triplicate for technical replication.
Table 2.
Real-time quantitative RT-PCR primer and probe sequences.
| RNA target | Probe/prime sequence | Exon boundary | Accession no |
|---|---|---|---|
| 28S | |||
| Probe | 5′-(FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA)-3′ | X59733 | |
| F | 5′-GGCGAAGCCAGAGGAAACT-3′ | ||
| R | 5′-GACGACCGATTGCACGTC-3 | ||
| IL-1β | |||
| Probe | 5′-(FAM)-CCACACTGCAGCTGGAGGAAGCC-(TAMRA)-3′ | 5/6 | AJ245728 |
| F | 5′-GCTCTACATGTCGTGTGTGATGAG-3′ | ||
| R | 5′-TGTCGATGTCCCGCATGA-3′ | ||
| IL-6 | |||
| Probe | 5′-(FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-(TAMRA)-3′ | 3/4 | AJ250838 |
| F | 5′-GCTCGCCGGCTTCGA-3′ | ||
| R | 5′-GGTAGGTCTGAAAGGCGAACAG-3′ | ||
| IL-8 | |||
| Probe | 5′-(FAM)-CTTTACCAGCGCGTCCTACCTTGCGACA-(TAMRA)-3′ | 1/2 | AJ009800 |
| F | 5′-GCCCTCCTCCTGGTTTCAG-3′ | ||
| R | 5′-TGGCACCGCCAGCTCATT-3′ | ||
| IL-10 | |||
| Probe | 5′-(FAM)-CGACGATGCGGCGCTGTCA-(TAMRA)-3′ | 3/4 | AJ621614 |
| F | 5′-CATGCTGCTGGGCCTGAA-3′ | ||
| R | 5′-CGTCTCCTTGATCTGCTTGATG-3′ | ||
| Interferon gamma | |||
| Probe | 5′-(FAM)-TGGCCAAGCTCCCGATGAACGA-(TAMRA)-3′ | 3/4 | Y07922 |
| F | 5′-GTGAAGAAGGTGAAAGATATATCATGGA-3′ | ||
| R | 5′-GCTTTGCGCTGGATTCTCA-3′ |
Statistical Analysis for qRT-PCR
Cytokine mRNA expression for control and treated ceca from day 2, 4, 6, 8, and 10 were quantitated using a method described by Kaiser et al. (2000) and Moody et al. (2000). The 40-Ct values were calculated for each sample with averaged triplicates per sample. Statistical analysis was performed with SAS 9.4 (Cary, NC) based on the data collected from each trial for the qRT-PCR data. Each time frame had samples comparing 1% ChT–treated birds vs. control-fed birds. The Shapiro-Wilks test for normality was used to determine if the fold change within each group was parametric or nonparametric, with an alpha of 0.05. For all analyses, statistical significance was considered if P ≤ 0.05.
All data were found to be nonparametric and were summarized as median values. An ad hoc analysis using the Kruskal-Wallis test was conducted to determine where the statistical differences lie between the control and 1% ChT. The ceca samples for the IL-1B, IL-6, IL-8, IL-10, and interferon gamma were quantified using the 40-Ct method, as outlined by Eldaghayes et al. (2006). The results were reported in fold change values.
Chicken-Specific Kinome (Peptide) Array
For the phenotype readout, a peptide array was used to provide tissue immunometabolism information from the host. At 3 time points (day 4, 6, and 10), 3 whole ceca from 3 randomly selected birds—stored in −80°C—were defrosted for analysis (27 samples total for all 3 d). Each sample was weighed to obtain a consistent 40-mg sample for the array. The samples were homogenized with the Omni International Bead Ruptor Elite (Kennesaw, GA) in 100 μL of lysis buffer (20 mmol Tris-HCl pH 7.5, 150 mmol NaCl, 1 mmol EDTA, 1 mmol ethylene glycol tetraacetic acid, 1% Triton X-100, 2.5 mmol sodium pyrophosphate, 1 mmol Na3VO4, 1 mmol NaF, 1 μg/mL leupeptin, 1 g/mL aprotinin, and 1 mmol phenylmethylsulfonyl fluoride). All products were obtained from Sigma Aldrich (St. Louis, MO), unless indicated. After homogenization, the peptide array protocol was carried out according to Jalal et al. (2009) with alterations described in the study by Arsenault et al. (2017). The resulting tissue lysates were applied onto the PepStar peptide microarrays customized by JPT Peptide Technologies GmbH (Berlin, Germany).
Data Analysis: Kinome Array
Data normalization was performed for the kinome array, based on the study by Li et al. (2012) using the PIIKA2 online platform (http://saphire.usask.ca/saphire/piika/index.html), a tool designed for in silico analysis of phosphorylation sites (Trost et al., 2013). The array data were analyzed by conducting variance stabilization normalization and then performing t test, clustering and pathway analysis for statistical data. Gene ontology and Kyoto Encyclopedia of Genes and Genomes pathway analysis were performed by uploading the statistically significant peptide lists to the Search Tool for the Retrieval of Interacting Genes (STRING) (Szklarczyk et al., 2017).
Results
Real-Time Quantitative RT-PCR Results
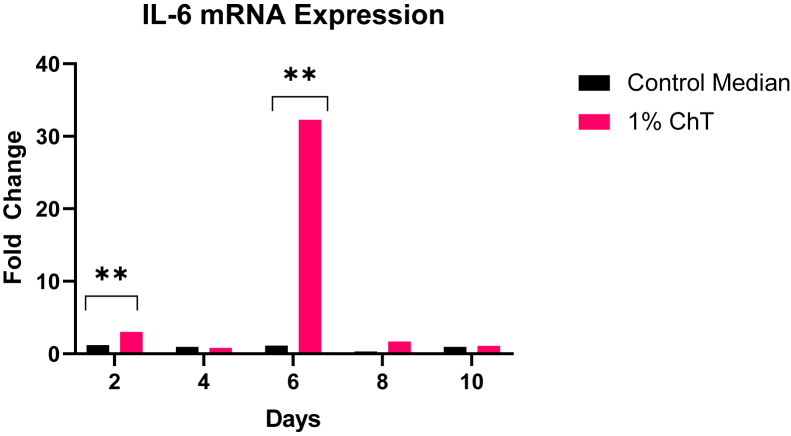
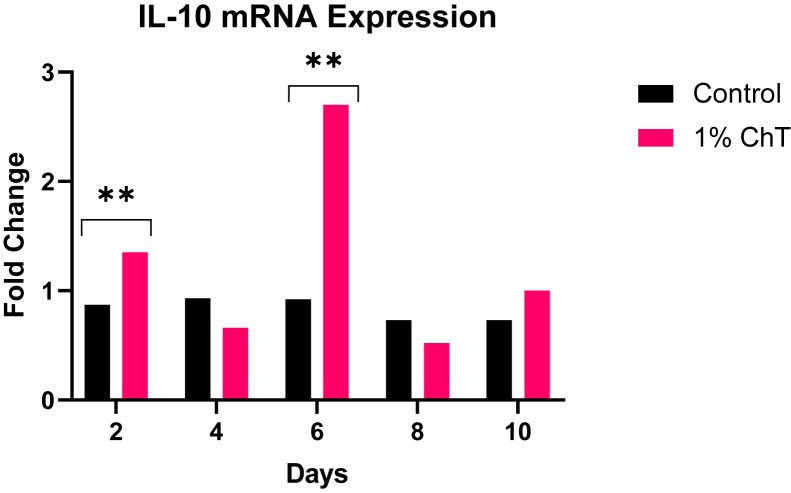
Across both replicate experiments, only IL-6 and IL-10 were found to have statistically significant increases in mRNA expression on day 2 and 6 across all day tested in the experiment. Figure 1 shows the mRNA expression levels of all tested day (day 2, 4, 6, 8, and 10) for IL-6. The results demonstrated that the expression levels of IL-6 were significantly increased after treatment with 1% ChT on day 2 and day 6 when compared with controls. There were no significant differences when compared with controls on day 4, 8, and 10. Figure 2 shows the mRNA expression levels of all tested day (day 2, 4, 6, 8, and 10) for IL-10. The results also demonstrated that the expression levels of IL-10 were significantly increased after treatment with 1% ChT on day 2 and day 6. However, there were no significant differences when compared with controls on day 4, 8, and 10.
Figure 1.
The fold change values based on day tested of the mRNA expression assay for IL-6. These data reflect the averaged replicate experiments (N = 200). The asterisks denote significant differences between the control and 1% ChT groups. Abbreviation: ChT, chestnut tannin.
Figure 2.
The fold change values based on day tested of the mRNA expression assay for IL-10. These data reflect the averaged replicate experiments (N = 200). The asterisks denote significant differences between the control and 1% ChT groups. Abbreviation: ChT, chestnut tannin.
Kinome Results
Using STRING-db (Szklarczyk et al., 2017), the resulting analysis of the kinome data showed distinct metabolic differences between the 1% ChT group and the control group. Each day is representative of 3 birds normalized and combined into representative data sets. The biological process terms generated from Gene Ontology for each data set include sets of molecular events with a defined beginning and end that pertain to the functioning of the integrated living units (Swaggerty et al., 2017). Based on the false discovery rate of the analysis, values P ≤ 0.01 were listed and considered statistically significant. The analysis of the kinome data showed distinct differences in the observed biological process between 1% CT–fed birds compared with the control birds. Table 3 summarizes the top 15 Gene ontology STRING-generated biological pathways of metabolic processes and the number of differentially phosphorylated peptides associated with them. Day 6 samples have the greatest number of primary biological processes related to metabolic pathways of the 3 d, indicated by the number of significant peptides. Day 4 had the most amount of fatty acid metabolic process present, but it decreased by day 6 and was not present by day 10.
Table 3.
The top 15 Gene ontology metabolic biological processes (BP) identified at day 4, 6, and 10 comparing 1% ChT–fed birds against control birds.
| Biological processes | Day 4 | Day 6 | Day 10 |
|---|---|---|---|
| Phosphate-containing compound metabolic process | 100 | 132 | 79 |
| Primary metabolic process | 145 | 187 | 122 |
| Protein metabolic process | 99 | 136 | 83 |
| Cellular metabolic process | 142 | 184 | 125 |
| Fatty acid metabolic process | 90 | 19 | - |
| Glucose metabolic process | 11 | 14 | 9 |
| Glycerolipid metabolic process | 18 | 28 | 16 |
| Cellular lipid metabolic process | 37 | 48 | 24 |
| Glycogen metabolic process | 8 | 9 | 6 |
| ATP metabolic process | 13 | 11 | 10 |
| Lipid metabolic process | 38 | 50 | 26 |
| Carbohydrate metabolic process | 21 | 25 | 18 |
| Hexose metabolic process | 13 | 15 | - |
| NAD metabolic process | 8 | 11 | 8 |
| Pyruvate metabolic process | 10 | 11 | 9 |
The hyphens indicate nonsignificant BP based on the FDR.
Abbreviation: ChT, chestnut tannin.
In addition, the top metabolic Kyoto Encyclopedia of Genes and Genomes pathways (Kanehisa et al., 2016) were obtained from the STRING-db, as summarized in Table 4, for the 1% ChT–fed group compared with the control group. The pathways of interest were those that showed significant changes at multiple time points. Day 6 samples have the greatest number of altered immune and metabolic pathways of the 3 d, indicated by the number of peptides and number of pathways shown in the tables. By day 10, the number of peptides is decreased, as well as the number of pathways.
Table 4.
Summarized table of Kyoto Encyclopedia of Genes and Genomes metabolic pathways at day 4, 6, and 10 comparing 1% ChT–fed birds and control birds.
| Metabolic pathways |
Day 4 |
Day 6 |
Day 10 |
|||
|---|---|---|---|---|---|---|
| Identified pathways | Number of peptides | P-value | Number of peptides | P-value | Number of peptides | P-value |
| PI3K-Akt signaling pathway | 34 | 1.15 × 10−22 | 44 | 4.28 × 10−29 | 24 | 1.11 × 10−13 |
| MAPK signaling pathway | 30 | 1.75 × 10−20 | 49 | 2.32 × 10−37 | 22 | 2.44 × 10−13 |
| Metabolic pathway | 28 | 1.39 × 10−05 | 32 | 4.67 × 10−05 | - | - |
| Insulin signaling pathway | 27 | 5.29 × 10−25 | 27 | 1.30 × 10−22 | 20 | 7.77 × 10−17 |
| AMPK signaling pathway | 21 | 1.19 × 10−18 | 20 | 7.95 × 10−16 | 16 | 3.16 × 10−13 |
| HIF-1 signaling pathway | 18 | 1.82 × 10−16 | 18 | 5.60 × 10−15 | 12 | 6.91 × 10−10 |
| mTOR signaling pathway | 18 | 6.72 × 10−14 | 18 | 2.23 × 10−12 | 18 | 5.53 × 10−14 |
| cAMP signaling pathway | 17 | 2.85 × 10−11 | 13 | 1.79 × 10−06 | - | - |
| Glucagon signaling pathway | 14 | 8.32 × 10−12 | 15 | 1.22 × 10−11 | 11 | 9.36 × 10−09 |
| VEGF signaling pathway | 12 | 7.40 × 10−12 | 17 | 7.06 × 10−17 | - | - |
| Calcium signaling pathway | 11 | 2.42 × 10−06 | 10 | 0.00012 | - | - |
| Glycolysis/Gluconeogenesis | 10 | 5.39 × 10−09 | 10 | 4.75 × 10−08 | - | - |
| GnRH signaling pathway | 10 | 4.43 × 10−08 | 14 | 3.03 × 10−11 | - | - |
| Adipocytokine signaling pathway | - | - | 18 | 3.60 × 10−17 | - | - |
The hyphens indicate less than 10 peptides and not significant for our analysis.
Abbreviation: ChT, chestnut tannin.
Discussion
The present study objective was to provide metabolic information on the effects of ChT when incorporated into the broiler feed. Tannins are already known to improve livestock health and growth performance, but the mechanism is still widely unknown (Lillehoj et al., 2018). To date, these results are the first of its kind to provide mechanistic information on how host intestine responds to tannins inclusion in the feed. With the qRT-PCR and kinome data, we were able to provide global post-translational perspectives on how intestine metabolic function is correlated in a tannins-fed diet.
Based on the qRT-PCR data, the cytokines of interest based on our results are the elevated expressions of IL-6 and IL-10 by day 6. As seen in Figure 1, the only notable fold changes (values higher than 2.0) are seen on day 2 and 6 with the 1% ChT group. This shows a significant upregulation of IL-6 and calls for speculation on its role when 1% ChT is included in the feed. A previous experiment by Ferro et al. (2005) concludes that IL-6 may need to work in tandem with other immunomodulatory cytokines (such as IL-1 family cytokines) to stimulate immune responses. In fact, McGeachy et al. (2007) discovered that IL-10 is upregulated by IL-6, revealing a dependent relationship between the 2 cytokines during an immune response. As shown in Figure 2, there was significant upregulation of IL-10 only on day 6 in the 1% ChT group, paralleling the IL-6 upregulation trend. This supports what was found in the previously mentioned studies, providing evidence of the IL-10 immunoregulatory properties. As the gene expression results show, the elevated IL-6 upregulation may be the peak time of modulation in the bird (day 6), with IL-10 regulating this proinflammatory response. What is important to note is the temporary peak of IL-6 expression: by day 10, the high level of expression is no longer present. This is beneficial for the birds because the ChT stimulate a response to prime the birds' immunity. Although IL-6 is known to have proinflammatory and anti-inflammatory properties based on the stimulus (Ferro et al., 2005), long-lasting IL-6 presence may contribute to chronic inflammation and tissue damage, which is undesirable for the birds and producers (Tanaka et al., 2014). There is also strong evidence of IL-6 involved in metabolic function, rather than just immune response. Flint et al. (2016) found that IL-6 induction stimulated a metabolic reprogramming which directly induced an immune suppression in mice. They predicted that this metabolic pathway of IL-6 helps prevent immunologic damage due to inflammation. Reviews by Pavlov and Tracey (2012) and Ghanemi and St Amand (2018) outline further proof of IL-6 and its direct linkage to metabolic pathways, especially affecting insulin pathways and fatty acid synthesis. These reviews discuss how IL-6 should be considered beyond its immunologic function owing to its effects on lipids, protein, and glucose metabolism.
The present study is the first one to demonstrate metabolic importance associated with ChT, as indicated by the number of post-translational modifications and significant P-values. Based on its known antimicrobial effects, ChT and other phytobiotics make ideal candidates for growth promotion and health improvements based its ability to retain bacterial diversity in the gut but reducing drug-resistant bacteria (Park et al., 2020). Chestnut tannins have already been known to affect Bifidobacterium in the ceca of mammals and chickens (Diaz Carrasco et al., 2018), which have been shown to alter carbohydrate metabolism and other metabolic processes downstream in the host by modifying enzymes and sugar transport pathways (Pokusaeva et al., 2011). Our research supports this through the direct alteration of the carbohydrate and primary metabolic pathways (Table 3). The main difference in bacterial taxa between control and tannins-fed birds was the increase in bacterial diversity over time within the tannins-fed group, especially with Lactobacillus and Enterococcus species (Diaz Carrasco et al., 2018). These bacterial species use sugar metabolism (such as hexose and glucose metabolism) and carbohydrate metabolism, which supports our findings in our tannins-fed group (Donohoe et al., 2011). Another study found older birds treated with tannins consistently had increased populations of order Clostridiales and family Ruminococcaceae, which have been of interest in the poultry industry as potential probiotic options (Diaz Carrasco et al., 2018). This is noteworthy evidence of how tannins-fed chickens could have increased members of the family Ruminococcaceae to alter the short chain fatty acids profile in the cecum toward butyrate production. The increased presence of these butyrate-producing communities would indicate the usage of carbohydrate metabolism, fatty acid metabolism, and glycerolipid metabolism downstream to ultimately benefit host physiology (Donohoe et al., 2011; Vital et al., 2014).
An interesting note by Diaz Carrasco et al. (2018) was the sensitivity effect that dietary tannins had on gram-positive bacteria, as also noted in rat studies with increased gram-negative bacteria in the gastrointestinal tract (Smith et al., 2004). Molino et al. (2018) have shown preliminary data on the importance of tannins in gut microbial fermentation and the nutritional importance in human application. This study provides additional new information on affected pathways in the intestine with the introduction of ChT in early broiler growth. Although the microbiota of young birds are more prone to fluctuating gut microbial communities, these results support the relevance of ChT in the feed to not only promote growth but to also provide more evidence as an alternative to antibiotics. Future studies in our group will look at growth promotion effects in depth by investigating the individual pathways that were altered with the introduction of ChT in the feed.
Therefore, with the current trend of removing antibiotics in feed, tannins are one of the promising feed additives owing to its ability to stimulate immunoregulatory effects and host modulation to prevent resistance, which is ideal as an alternative to antibiotics. This aims to reduce the immune response while redirecting energy toward growth (Arsenault et al., 2015). Ideal alternatives would promote health without risking loss of growth promotion or antibiotic resistance (Lillehoj et al., 2018). Chestnut tannins have already shown applicable results in reducing incidence of necrotic enteritis and Salmonella in livestock, although the effects on growth improvements varied based on dosages (Van Parys et al., 2010; Costabile et al., 2011; Diaz Carrasco et al., 2018). The results from this present study are providing further proof to what is currently in the literature of ChT as a potential alternative to antibiotics. The strong metabolic connection found in our study shows the promising nature of using ChTs as an antibiotic alternative because there would be promotion of growth on top of promotion of health. Our group has already conducted studies on the host–pathogen interactions of chickens using the kinome array (Swaggerty et al., 2004; Arsenault et al., 2014; Kogut et al., 2016), which shows the importance of viewing immunity and metabolism comprehensively instead of as individual units. With the increasing population, there will be a growing demand for poultry products especially cost-effective feed additives (Lillehoj et al., 2018). Therefore, identifying an alternative to AGP will be crucial in the future to keep up with increased demands for safe poultry products. Tannins might be a promising alternative to AGP because of the improvements in performance and microbiota, as seen in previous studies, and the current data support its metabolic modulation in the intestine.
In conclusion, it was observed that the influence of ChT in the diet alters gut immunometabolism of broilers. By focusing on the ChT-fed group compared with the control group, the results from these 2 replicate trials demonstrated the metabolic outcome when ChT are introduced into the diet. These are the first data in the literature demonstrating pathway data that support growth and health promotion with ChT. The objective of this study was to provide a global overview of ChT effect on the metabolism, providing mechanism information that is currently lacking in the literature. These data offer ChT not only as an immunoregulator but also a potential host-directed therapy option in disease studies. Phytobiotics can offer promising results because they can target metabolic and immunologic pathways of the host and may affect the pathogen. With the growing restriction of antibiotics in the feed, this study offers further evidence of ChT as an important alternative to antibiotics.
Acknowledgments
This research was funded by the United States Department of Agriculture, Agricultural Research Service Project number 3091-32000-034-00. The authors thank the animal care staff at the United States Department of Agriculture, Agriculture Research Service in College Station, TX.
References
- Arsenault R., Griebel P., Napper S. Peptide arrays for kinome analysis: new opportunities and remaining challenges. Proteomics. 2011;11:4595–4609. doi: 10.1002/pmic.201100296. [DOI] [PubMed] [Google Scholar]
- Arsenault R.J., Kogut M.H. Immunometabolism and the kinome peptide array: a new perspective and tool for the study of gut health. Front. Vet. Sci. 2015;2:44. doi: 10.3389/fvets.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault R.J., Lee J.T., Latham R., Carter B., Kogut M.H. Changes in immune and metabolic gut response in broilers fed β-mannanase in β-mannan-containing diets. Poult. Sci. 2017;96:4307–4316. doi: 10.3382/ps/pex246. [DOI] [PubMed] [Google Scholar]
- Arsenault R.J., Napper S., Kogut M.H. Salmonella enterica Typhimurium infection causes metabolic changes in chicken muscle involving AMPK, fatty acid and insulin/mTOR signaling. Vet. Res. 2013;44:35. doi: 10.1186/1297-9716-44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenault R.J., Trost B., Kogut M.H. A comparison of the chicken and Turkey proteomes and phosphoproteomes in the development of poultry-specific immuno-metabolism kinome peptide arrays. Front Vet. Sci. 2014;1 doi: 10.3389/fvets.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus M., Gradisnik L., Trapecar M., Skorjanc D., Frangez R. Beneficial effects of water-soluble chestnut (Castanea sativa Mill.) tannin extract on chicken small intestinal epithelial cell culture. Poult. Sci. 2018;97:1271–1282. doi: 10.3382/ps/pex424. [DOI] [PubMed] [Google Scholar]
- Costabile A., Sanghi S., Martin-Pelaez S., Mueller-Harvey I., Gibson G.R., Rastall R.A., Klinder A. Inhibition of Salmonella Typhimurium by tannins in vitro. J. Food Agric. Environ. 2011;9:119–124. [Google Scholar]
- Diaz Carrasco J.M., Redondo E.A., Pin Viso N.D., Redondo L.M., Farber M.D., Fernandez Miyakawa M.E. Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. Biomed. Res. Int. 2018 doi: 10.1155/2018/1879168. Article ID 1879168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diks S.H., Kok K., O’Toole T., Hommes D.W., van Dijken P., Joore J., Peppelenbosch M.P. Kinome profiling for studying lipopolysaccharide signal transduction in human peripheral blood mononuclear cells. J. Biol. Chem. 2004;279:49206–49213. doi: 10.1074/jbc.M405028200. [DOI] [PubMed] [Google Scholar]
- Donohoe D.R., Garge N., Zhang X. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13:517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldaghayes I., Rothwell L., Williams A., Withers D., Balu S., Davison F., Kaiser P. Infectious bursal disease virus: strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunol. 2006;19:83–91. doi: 10.1089/vim.2006.19.83. [DOI] [PubMed] [Google Scholar]
- Ferro P.J., Swaggerty C.L., He H., Rothwell L., Kaiser P., Kogut M.H. Recombinant chicken IL-6 does not activate heterophils isolated from day-old chickens in vitro. Dev. Comp. Immunol. 2005;29:375–383. doi: 10.1016/j.dci.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Flint T.R., Janowitz T., Connell C.M., Coll A.P., Jodrell D.I., Fearon D.T. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 2016;24:672–684. doi: 10.1016/j.cmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gai F., Gasco L., Schiavone A., Zoccarato I. Tannins: Types, Foods Containing, and Nutrition. Nova Science Publishers; Hauppauge, NY: 2010. Nutritional effects of chestnut tannins in poultry and rabbit; pp. 297–306. [Google Scholar]
- Ghanemi A., St Amand J. Interleukin-6 as a “metabolic hormone”. Cytokine. 2018;112:132–136. doi: 10.1016/j.cyto.2018.06.034. [DOI] [PubMed] [Google Scholar]
- Huang Q., Liu G., Hu T., Wang Y. Potential and challenges of tannins as an alternative to in-feedantibiotics for farm animal production. Anim. Nutr. 2018;4:137–150. doi: 10.1016/j.aninu.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal S., Arsenault R., Potter A.A., Babluk L.A., Gribel P.J., Napper S. Genome to kinome: species-specific peptide arrays for kinome analysis. Sci Signal. 2009;2:pl1. doi: 10.1126/scisignal.254pl1. [DOI] [PubMed] [Google Scholar]
- Kaiser P., Rothwell L., Galyov E.E., Barrow P.A., Burnside J., Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis, and Salmonella gallinarum. Microbiol. 2000;146:3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogut M.H., Rothwell L., Kaiser P. Differential regulation of cytokine gene expression by avian heterophils during receptor mediated phagocytosis of opsonized and non-opsonized Salmonella enteritidis. J. Interferon Cytokine Res. 2003;23:319–327. doi: 10.1089/107999003766628160. [DOI] [PubMed] [Google Scholar]
- Kogut M.H., Swaggerty C.L., Byrd J.A., Selvaraj R., Arsenault R.J. Chicken-specific kinome array reveals that Salmonella enterica serovar Enteritidis modulates host immune signaling pathways in the cecum to establish a persistence infection. Int. J. Mol. Sci. 2016;17:1207. doi: 10.3390/ijms17081207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Arsenault R.J., Trost B., Slind J., Griebel P.J., Napper S., Kusalik A. A systematic approach for analysis of peptide array kinome data. Sci. Signal. 2012;5:pl2. doi: 10.1126/scisignal.2002429. [DOI] [PubMed] [Google Scholar]
- Lillehoj H., Liu Y., Calsamiglia S., Fernandez-Miyakawa M.E., Gay C.G. Phytochemicals as antibiotic alternatives to promote growth and enhance host health. Vet. Res. 2018;49:76. doi: 10.1186/s13567-018-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Che T.M., Song M., Pettigrew J.E. Dietary plant extracts improve immune responses and growth efficiency of pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J. Anim. Sci. 2013;91:5668–5679. doi: 10.2527/jas.2013-6495. [DOI] [PubMed] [Google Scholar]
- Liu Y., Song M., Che T.M., Lee J.J., Bravo D., Maddox C.W., Pettigrew J.E. Dietary plant extracts modulate gene expression profiles in ileal mucosa of weaned pigs after an infection. J. Anim. Sci. 2014;92:2050–2062. doi: 10.2527/jas.2013-6422. [DOI] [PubMed] [Google Scholar]
- Lopetuso L.R., Scaldaferri F., Bruno G., Petito V., Franceschi F., Gasbarrini The therapeutic management of gut barrier leaking: the emerging role for mucosal barrier protectors. Eur. Rev. Med. Pharmacol. Sci. 2015;19:1068–1076. [PubMed] [Google Scholar]
- Manning G., Whyte D.B., Martinez R., Hunter T., Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- McGreachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., Cua D.J. TGF-b and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell–mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- Michiels J., Missotten J., Van Hoorick A., Dierick N. Effects of dose and formulation of carvacrol and thymol on bacteria and some functional traits of the gut in piglets after weaning. Arch. Anim. Nutr. 2010;64:136–154. doi: 10.1080/17450390903499915. [DOI] [PubMed] [Google Scholar]
- Min B.R., Barry T.N., Attwood G.T., McNabb W.C. The effect of condensed tannins on the nutritionand health of ruminants fed fresh temperate forages: a review. Anim. Feed Sci. Technol. 2003;106:3–19. [Google Scholar]
- Molino S., Fernandez-Miyakawa M., Giovando S., Rufian-Henares J.A. Study of antioxidant capacity and metabolization of quebracho and chestnut tannins through in vitro gastrointestinal digestion-fermentation. J. Func Foods. 2018;49:188–195. [Google Scholar]
- Molino S., Casanova N.A., Henares J.A.R., Fernandez Miyakawa M.E. Natural tannin wood extracts as a potential food ingredient in the food industry. J. Agric. Food Chem. 2020;68:2836–2848. doi: 10.1021/acs.jafc.9b00590. [DOI] [PubMed] [Google Scholar]
- Moody A., Sellers S., Bumstead N. Measuring infectious bursal disease virus RNA in blood by multiplex real-time quantitative RT–PCR. J. Virol. Methods. 2000;85:55–64. doi: 10.1016/s0166-0934(99)00156-1. [DOI] [PubMed] [Google Scholar]
- Nagaraja T.G., Newbold C.J., Van Nevel C.J., Demeyer D.I. Blackie Academic & Professional; London, UK: 1997. Manipulation of ruminal fermentation. Pages 523–632 in The Rumen Microbial Ecosystem. [Google Scholar]
- Parikh K., Peppelenbosch M.P. Kinome profiling of clinical cancer specimens. Infect Immun. 2010;75:5173–5182. doi: 10.1158/0008-5472.CAN-09-3989. [DOI] [PubMed] [Google Scholar]
- Park I., Oh S., Lillehoj E.P., Lillehoj H.S. Dietary supplementation with magnolia bark extract alters chicken intestinal metabolite levels. Front Vet. Sci. 2020;7:157. doi: 10.3389/fvets.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov V.A., Tracey K.J. The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat. Rev. Endocrinol. 2012;8:743–754. doi: 10.1038/nrendo.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokusaeva K., Fitzgerald G.F., van Sinderen D. Carbohydrate metabolism in Bifiobacteria. Genes Nutr. 2011;6:285–306. doi: 10.1007/s12263-010-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramah A., Yasuda M., Ohashi Y., Urakawa M., Abdelaleem N.M., El-Shewy E.A. Different doses of tannin reflect a double-edged impact on broiler chicken immunity. Vet. Immunol. Immunopathol. 2020;220:109991. doi: 10.1016/j.vetimm.2019.109991. [DOI] [PubMed] [Google Scholar]
- Redondo L.M., Chacana P.A., Dominguez J.E., Fernandez Miyakawa M.E. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014;5:118. doi: 10.3389/fmicb.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo L.M., Dominguez J.E., Rabinovitz B.C., Redondo E.A., Fernandez Miyakawa M.E. Hydrolyzable and condensed tannins resistance in Clostridium perfringens. Anaerobe. 2015;34:139–145. doi: 10.1016/j.anaerobe.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Ren H., Vahjen W., Dadi T., Saliu E.M., Boroojeni F.G., Zenrek J. Synergistic effects of probiotics and phytobiotics on the intestinal microbiota in young broiler chicken. Microorganisms. 2019;7:684. doi: 10.3390/microorganisms7120684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone A., Guo K., Tassone S., Gasco L., Hernandez E., Denti R., Zoccarato I. Effects of a natural extract of chestnut wood on digestibility, performance traits, and nitrogen balance of broiler chicks. Poult. Sci. 2008;87:521–527. doi: 10.3382/ps.2007-00113. [DOI] [PubMed] [Google Scholar]
- Smith A.H., Mackie R.I. Effect of condensed tannins on bacterial diversity and metabolic activity in the rat gastrointestinal tract. Appl. Environ. Microbiol. 2004;70:1104–1115. doi: 10.1128/AEM.70.2.1104-1115.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh G., Das R.K., Brar S.K., Rouissi T., Avalos Ramirez A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspective. Crit. Rev. Microbiol. 2018;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Swaggerty C.L., Kogut M.H., Ferro P.J., Rothwell L., Pevzner I.Y., Kaiser P. Differential cytokine mRNA expression in heterophils isolated from Salmonella-resistant and -susceptible chickens. Immunology. 2004;113:139–148. doi: 10.1111/j.1365-2567.2004.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaggerty C.L., Kogut M.H., He H., Genovese K.J., Johnson C., Arsenault R.J. Differential levels of cecal colonization by Salmonella Enteritidis in chickens triggers distinct immune kinome profiles. Front Vet. Sci. 2017;4:214. doi: 10.3389/fvets.2017.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szklarczyk D., Morris J.H., Cook H. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect. Biol. 2014;6:a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost B., Kindrachuk J., Määttänen P., Napper S., Kusalik A. Piika 2: an expanded, web-based platform for analysis of kinome microarray data. PLoS One. 2013;8:e80837. doi: 10.1371/journal.pone.0080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Parys A., Boyen F., Dewulf J., Haesebrouck F., Pasmans F. The use of tannins to control Salmonella Typhimurium infections in pigs. Zoonoses Public Health. 2010;57:423–428. doi: 10.1111/j.1863-2378.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- Vital M., Howe A.C., Tiedje J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio. 2014;5:e00889-14. doi: 10.1128/mBio.00889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]