Abstract
Background
Areal bone mineral density (BMD) of the lumbar spine by DXA is greater in Black compared to White adolescents. Bone strength is determined not only by BMD but also its microenvironment, and marrow adipose tissue (MAT) has been shown to be an important determinant of skeletal integrity, independent of BMD. Racial differences in volumetric BMD (vBMD) and MAT in adolescents and young adults with obesity are unknown.
Objective
To assess racial differences in lumbar vBMD and MAT in Black and White adolescents and young adults with obesity and to assess body composition determinants of bone parameters. We hypothesized that Blacks will have higher vBMD and lower MAT of the lumbar spine compared to Whites.
Methods
The study group comprised 77 adolescents/young adults, 25 Black and 52 White, (mean age 18.2 ± 2.5 years, range 13 to 24 years) with moderate to severe obesity (mean body mass index (BMI) 46.2 ± 7.3 kg/m2, range 35.5 to 69.7 kg/m2). Groups were similar in age, BMI, and sex distribution (p > 0.84). Subjects underwent QCT of the lumbar spine (L1−L2) for assessment of vBMD with the use of a calibration phantom and 1H-MRS/MRI for quantification of lumbar MAT content (L1-L2) and abdominal fat and thigh muscle mass. Groups were compared by Student's t-test or Wilcoxon test. Correlation analysis was performed to assess associations between bone parameters and body composition.
Results
Black adolescents/young adults with obesity had higher vBMD compared to Whites (p < 0.0001), while there was no significant difference in lumbar MAT (p = 0.64). There were also no significant differences in body composition measures between groups (p ≥ 0.28). An inverse association between MAT and vBMD was observed in Whites (r = −0.47, P = 0.001) but not in Blacks (p = 0.6). There were no significant associations between body composition measures and bone parameters (p > 0.1).
Conclusion
There are racial differences in lumbar vBMD in adolescents and young adults with moderate to severe obesity, with Blacks having higher vBMD than Whites, while there were no differences in MAT content. The known inverse association between BMD and MAT was only observed in Whites but not in Blacks, suggesting possible racial differences in stem cell differentiation into the bone and fat lineages.
Keywords: Race, Volumetric BMD, Quantitative computed tomography, Marrow adipose tissue, Proton MR spectroscopy, Lumbar spine, Adolescents, Obesity
Highlights
-
•
Volumetric BMD (vBMD) of the lumbar spine is higher in Black vs White adolescents/young adults with obesity.
-
•
There is no difference in lumbar marrow adipose tissue (MAT) between Black vs White adolescents/young adults with obesity.
-
•
The known inverse association between BMD and MAT is only observed in Whites, suggesting racial differences in stem cell differentiation.
1. Introduction
Racial differences in bone mineral density (BMD) and fracture risk have been described, with Black adults and adolescents having higher BMD and lower fracture risk compared to Whites (Baron et al., 1994; Liel et al., 1988; Wren et al., 2012). Bone strength is not only determined by BMD but also its microenvironment, and marrow adipose tissue (MAT) has been shown to be an important determinant of skeletal integrity, independent of BMD (Schellinger et al., 2001, Schellinger et al., 2004). Marrow adipocytes and osteoblasts originate from a common mesenchymal stem cell (Hu et al., 2018), and studies in adults across the weight spectrum have demonstrated an inverse association between MAT and BMD (Bredella et al., 2009, Bredella et al., 2011; Abdalrahaman et al., 2017; Singhal et al., 2015; Yu et al., 2017). MAT can be quantified non-invasively using proton MR spectroscopy (1H-MRS) (Bredella et al., 2009, Bredella et al., 2013), however, racial differences in MAT content in adolescents or adults with obesity have not been examined.
Childhood obesity is a serious public health threat and in addition to known cardiometabolic complications, children with obesity also have a higher incidence of fractures compared to children of normal weight (Valerio et al., 2012) despite having higher BMD (van Leeuwen et al., 2017). BMD is typically assessed using dual-energy x-ray-absorptiometry (DXA) which determines areal BMD and is susceptible to measurement errors due to increased soft tissues in obesity (Javed et al., 2009). Quantitative computed tomography (QCT) assesses volumetric bone mineral density (vBMD) and is less susceptible to measurement errors in obesity compared to DXA (Yu et al., 2012). Racial difference in vBMD and bone microarchitecture of the extremities have been described in adolescents and young adults of normal weight and with obesity (Campoverde Reyes et al., 2020; Misra et al., 2017; Popp et al., 2017; Putman et al., 2013). Those studies revealed higher vBMD and improved bone microarchitecture and strength estimates of the distal radius and tibia in Blacks compared to Whites (Campoverde Reyes et al., 2020; Misra et al., 2017; Popp et al., 2017; Putman et al., 2013), although these differences were less marked in moderate to severe obesity, suggesting that obesity may blunt the positive effect of race on bone endpoints (Campoverde Reyes et al., 2020). However, racial differences of vBMD of the lumbar spine, a critical site of skeletal integrity and future fracture risk, in adolescents and young adults with moderate to severe obesity is unknown.
Studies have suggested that body composition, the amount of lean versus fat mass, contributes to racial differences in BMD, with Blacks having higher lean mass compared to Whites (Rahman and Berenson, 2010; Travison et al., 2011). No studies have examined racial differences in adolescents and young adults with moderate to severe obesity and the contribution of fat and muscle mass to vBMD and MAT.
The purpose of this exploratory pilot study was to assess racial differences in lumbar vBMD and MAT in Black and White adolescents and young adults with obesity and to assess body composition determinants of bone parameters. We hypothesized that Blacks will have higher vBMD and lower MAT of the lumbar spine compared to Whites.
2. Methods
Our study was IRB-approved and complied with Health Insurance Portability and Accountability Act (HIPAA) guidelines. Written informed consent/assent was obtained after the nature of study procedures was fully explained.
2.1. Subjects
We evaluated baseline data from adolescents and young adults with moderate to severe obesity from an ongoing trial at the Massachusetts General Hospital. Inclusion criteria were ages 13 to 25 years and BMI ≥ 35 kg/m2. Exclusion criteria were pregnancy, weight > 250 kg (due to weight limitations of the MRI scanner) and contraindications to MRI, such as the presence of metallic implants or claustrophobia, history of medical disorders or medication known to affect bone metabolism, use of medication that cause weight gain if treated for less than six months or if the dose was not stable for at least two months prior to enrollment, untreated thyroid dysfunction or if the subject was on a stable dose of replacement levothyroxine for less than three months before study enrollment, history of smoking more than ten cigarettes per day or of substance abuse (per DSM-5).
Study participants were weighed in a hospital gown on a calibrated electronic scale. Height was measured in triplicate using a wall mounted stadiometer and BMI calculated (weight (kg)/height2 (m2)). Data on race were collected by self-report.
Clinical characteristics have been reported in a subset of study participants (Campoverde Reyes et al., 2020), however, no racial differences in vBMD of the lumbar spine or MAT have been reported.
2.2. Quantitative computed tomography (QCT)
Volumetric bone mineral density (vBMD) of the lumbar spine (L1-L2) was assessed by QCT using a 16-multidetector-row CT scanner (LightSpeed Pro, GE Healthcare, Waukesha, WI, USA). Subjects were placed supine in the CT scanner on a calibration phantom (Mindways Software, Inc., Austin, TX, USA), and helical scanning of the L1 and L2 vertebra was performed using the following parameters: 120 kV, 100 mA, slice thickness of 2.5 mm, FOV of 500 mm and table height of 144 mm.
Analysis of vBMD was performed using QCTPro software (Mindways Software, Inc., Austin, TX). An oval region of interest within trabecular bone of each vertebral body was placed manually, avoiding cortical bone and posterior veins. L1 and L2 results were averaged in each subject.
2.3. Proton MR spectroscopy
Subjects underwent proton MR spectroscopy (1H-MRS) of the 1st and 2nd lumbar vertebrae (L1-L2) on a 3.0 Tesla MR imaging scanner (Siemens Trio; Siemens Medical Systems, Erlangen, Germany). Single-voxel 1H-MRS data were acquired by using a point-resolved spatially localized spectroscopy pulse sequence without water suppression (TR/TE 3000/30, eight acquisitions, 1024 data). For each voxel placement, automated optimization of gradient shimming was performed.
Fitting of all 1H-MRS data was performed using LCModel (version 6.3-0K, Stephen Provencher, Oakville, Canada). Metabolite quantification was performed using eddy current correction and water scaling. A customized fitting algorithm for bone marrow analysis provided estimates for all lipid signals combined (0.9, 1.3, 1.6, 2.3, 5.2 and 5.3 ppm). Lipid resonances were scaled to unsuppressed water peak (4.7 ppm) and expressed in lipid-to-water ratios (LWR).
2.4. Magnetic resonance imaging
Single slice MRI (Siemens Trio; Siemens Medical Systems, Erlangen, Germany) was performed of the abdomen at the mid-portion of the 4th lumbar vertebra to determine abdominal adipose tissue and of the mid-thigh, equidistant to the femoral head and medial femoral condyle to determine muscle cross sectional areas (CSA) (axial T1-weighted fast spin-echo pulse sequence, TR 300 msec, TE 12 msec, echo train of 4, 10 mm slice thickness, 40 cm field of view, 512 × 512 matrix, 1 number of acquisitions). Abdominal adipose tissue CSA and mid-thigh muscle CSAs (cm2) were determined based on offline analysis of tracings obtained using commercial software (VITRAK, Merge/eFilm, Milwaukee, WI).
All QCT, 1H-MRS, and MRI acquisitions and analyses were performed blinded to the group assignment under the supervision of a musculoskeletal radiologist with 15 years of experience (M.A.B.).
2.5. Statistical analysis
Statistical analysis was performed using JMP software (version 11.0, SAS institute, Carey, NC). Variables were tested for normality of distribution using the Wilk–Shapiro test. Data are reported as means ± standard deviation when normally distributed and as median and interquartile range (IQR) for non-normally distributed data. Categorical variables are expressed using percentages and compared using the chi-squared test. Groups were compared using the Student's t-test for normally distributed data and the Wilcoxon test for non-normally distributed data. Correlation analysis between bone measures and body composition was performed and non-parametric Spearman rank correlation coefficients are reported. Multivariate standard least squares modeling was used to control for race and to assess whether there is an association between vBMD and race-adjusted MAT. Statistical significance was defined as a two-tailed p < 0.05.
3. Results
3.1. Subjects
Our study group included 77 adolescents/young adults (64 females, 13 males, mean age 18.2 ± 2.5 years, range 13 to 24 years) with moderate to severe obesity (mean BMI 46.2 ± 7.3 kg/m2, range 35.5 to 69.7 kg/m2). Twenty-five study subjects self-identified as Black and 52 as White. Groups were similar in age, sex distribution, weight, height, and BMI (Table 1).
Table 1.
Clinical characteristics of Black and White adolescents and young adults with moderate to severe obesity.
| Parameter | Blacks N = 25 | Whites N = 52 | p-value |
|---|---|---|---|
| Age (years) | 18.2 ± 2.6 | 18.1 ± 2.4 | 0.84 |
| Sex | 0.89 | ||
| Male | 4 (16%) | 9 (17.3%) | |
| Female | 21 (84%) | 43 (82.6%) | |
| Height (cm) | 165.2 ± 8.6 | 166.9 ± 7.7 | 0.42 |
| Weight (kg) | 126.8 (107.2–134.7)a | 123.4 (111.1–138.3)a | 1.00 |
| BMI (kg/m2) | 43.04 (40.4–50.4)a | 44.7 (40.5–49.2)a | 0.92 |
| QCT | |||
| Lumbar vBMD (mg/cm3)a | 223.5 ± 29.6 | 192.6 ± 30.2 | <0.0001 |
| Female vBMD (mg/cm3) | 226 ± 30 | 196 ± 29 | 0.0004 |
| Male vBMD (mg/cm3) | 211 ± 27 | 169 ± 31 | 0.047 |
| Lumbar vBMD Z-scorea | 1.3 ± 1.07 | 0.16 ± 1.2 | <0.0001 |
| Female vBMD Z-score | 1.60 ± 1.06 | 0.47 ± 1.13 | 0.0004 |
| Male vBMD Z-score | 0.90 ± 0.80 | −0.79 ± 1.22 | 0.04 |
| MRI/MRS | |||
| Lumbar MAT (LWR) | 0.34 (0.28–0.38)a | 0.36 (0.23–0.52)a | 0.64 |
| Female MAT (LWR) | 0.33 (0.28–0.37) | 0.36 (0.22–0.49) | 0.90 |
| Male MAT (LWR) | 0.35 (0.23–0.36) | 0.49 (0.38–0.63) | 0.12 |
| Abdominal adipose tissue (cm2) | 808.5 ± 191.5 | 852.7 ± 195.4 | 0.40 |
| Thigh muscle (cm2) | 178.5 ± 29.6 | 169.7 ± 28.2 | 0.28 |
Means ± SD; Median (interquartile range).
Student t-test was used to compare the two groups when normally distributed. Wilcoxon test was used when at least one of the groups was not normally distributed.
MAT: marrow adipose tissue; LWR: lipid to water ratio.
Significant after controlling for height.
3.2. Volumetric bone mineral density (vBMD)
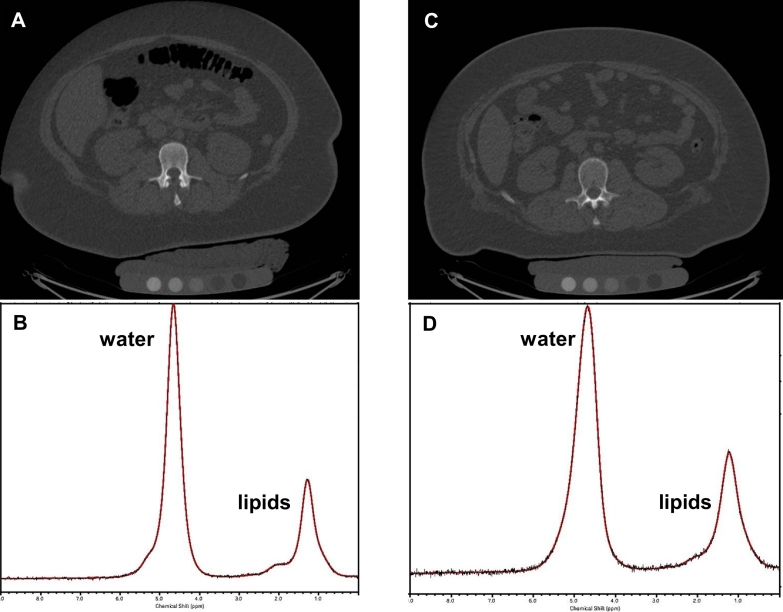
Lumbar spine vBMD was normal by BMD Z-score in Blacks and Whites. Blacks had higher vBMD and BMD Z-score of the lumbar spine compared to Whites (p < 0.0001) (Table 1 and Fig. 1).
Fig. 1.
A. QCT of L2 in a 15-year-old Black female with severe obesity (BMI 51 kg/m2) and volumetric BMD of 294 mg/cm3. B. Corresponding 1H MR spectrum of bone marrow at L2 shows 0.42 lipid to water ratio. C. QCT of L2 in a 15-year-old White female with severe obesity (BMI 53 kg/m2) and volumetric BMD of 133 mg/cm3. D. Corresponding 1H MR spectrum of bone marrow at L2 shows 0.43 lipid to water ratio.
CT images are presented using the same window and level. For purposes of visual comparison, the amplitude of unsuppressed water was scaled identically on the 1H MR spectra.
3.3. Marrow adipose tissue (MAT)
There was no significant difference in lumbar MAT between Blacks and Whites (p = 0.64) (Table 1 and Fig. 1).
3.4. Body composition
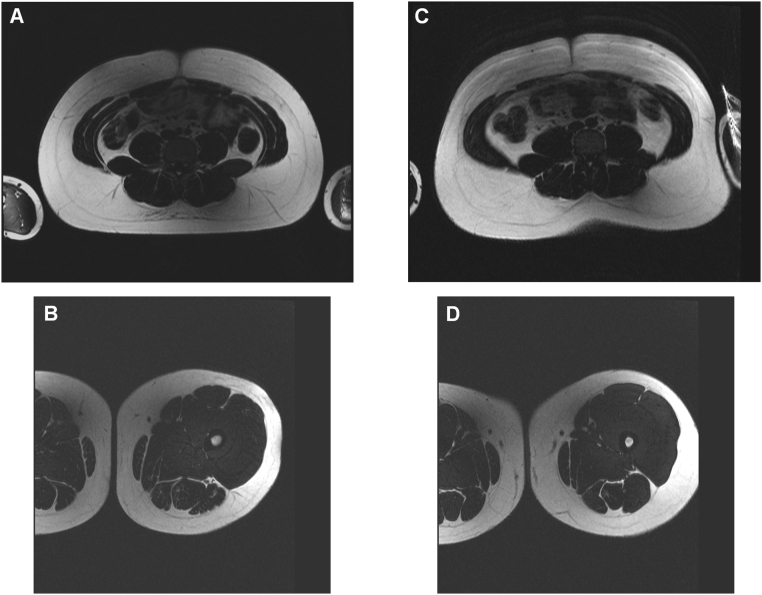
There were no significant differences in abdominal fat and thigh muscle CSAs between Blacks and Whites of similar BMI (p ≥ 0.28) (Table 1, Fig. 2).
Fig. 2.
A. MRI of the abdomen in a 16-year-old Black Male with severe obesity (BMI 40 kg/m2) showing abdominal fat cross sectional area of 570 cm2. B. Corresponding MRI of the mid-thigh showing muscle cross sectional area of 216 cm2. C. MRI of the abdomen in a 16-year-old White Male with severe obesity (BMI 40 kg/m2) showing abdominal fat cross sectional area of 572 cm2. B. Corresponding MRI of the mid-thigh showing muscle cross sectional area of 206 cm2.
3.5. Correlation analysis between bone parameters and body composition
There was an inverse association between vBMD and lumbar MAT in Whites (r = −0.47, P = 0.001) but not in Blacks (r = −0.12, p = 0.6). In the entire cohort, there was an inverse association between vBMD and MAT (r = −0.56, p < 0.0001), which remained significant after adjustment for race (p = 0.015).
There were no significant associations between body composition measures and bone parameters in either group (P > 0.1) (data not shown).
4. Discussion
Our exploratory pilot study shows that Black adolescents and young adults with moderate to severe obesity have higher vBMD by QCT compared to Whites of similar age, sex, BMI, and body composition. Contrary to our hypothesis, there was no significant difference in lumbar MAT content between the groups. Interestingly, the known inverse relationship between BMD and MAT was only observed in Whites but not in Blacks, suggesting possible racial differences in stem cell differentiation into the bone and fat lineages.
The amount of bone mass acquired during adolescence is a critical factor for determination of bone health and fracture risk later in life (Johnston Jr. and Slemenda, 1994). On average, more than 90% of bone mass is acquired by age 18 years and 99% by 25 years (Glastre et al., 1990; Teegarden et al., 1995). Bone accrual and skeletal integrity involve multiple factors such as sex, BMI, body composition, and nutritional status (Rahman and Berenson, 2010; Castro et al., 2005; Davies et al., 2005; Horlick et al., 2004). A positive association between BMD and weight has been consistently demonstrated (van Leeuwen et al., 2017), however, recent studies have revealed a higher incidence of fractures among adolescents with obesity despite higher BMD (Valerio et al., 2012), suggesting that excess fat accrual may be harmful for the skeleton and that parameters other than BMD contribute to fracture risk in these individuals.
Most studies have used DXA to assess areal BMD, which is less precise in obesity due to the excess soft tissue in the individual (Javed et al., 2009). QCT assesses vBMD and is more accurate in obesity than is DXA (Yu et al., 2012). Prior studies have shown higher vBMD in the peripheral skeleton (distal radius and tibia) in Blacks compared to Whites (Campoverde Reyes et al., 2020; Misra et al., 2017; Popp et al., 2017; Putman et al., 2013). Ours is the first study that has evaluated vBMD of the lumbar spine in adolescents and confirmed racial differences in lumbar vBMD with higher vBMD in Blacks compared to Whites of similar age, sex distribution, weight, height, BMI, abdominal fat and muscle mass.
Recent advances in the understanding of the bone-fat connection suggest MAT could function as an imaging biomarker of skeletal integrity (Devlin and Rosen, 2015; Gimble et al., 2006). Within bone marrow, osteoblasts and adipocytes arise from a common mesenchymal stem cell and many osteoporotic states, including old age, immobility and anorexia nervosa are associated with increased marrow adiposity (Schellinger et al., 2001, Schellinger et al., 2004; Bredella et al., 2009, Bredella et al., 2013; Trudel et al., 2012). Furthermore, higher MAT content has been demonstrated in adults with obesity compared to controls (Bredella et al., 2013), while we have shown lower MAT content of the lumbar spine in adolescents with obesity compared to normal weight controls (Singhal et al., 2019). However, no study has evaluated racial differences in MAT in adolescents with obesity. Advancements in MR imaging technology allow the non-invasive quantification of MAT content using 1H-MRS without ionizing radiation, which is particularly important when imaging adolescents and young adults. In our study, we did not find a significant difference in lumbar MAT content between Blacks and Whites, despite differences in vBMD. Several studies have shown an inverse association between MAT and BMD and this reciprocal relationship was observed in White adolescents/young adults in our study, while no such relationship was observed in Blacks, suggesting that obesity may disrupt the physiologic reduction of MAT in states of higher BMD. This is consistent with a prior study that showed that racial differences in BMD and bone microarchitecture as well as strength estimates were less pronounced in adolescents with moderate to severe obesity (Campoverde Reyes et al., 2020), suggesting that effects of obesity may blunt the effect of race on bone measures.
We did not observe racial differences in muscle mass and abdominal fat at similar BMI. Prior studies have shown higher lean mass in Blacks compared to Whites (Rahman and Berenson, 2010; Travison et al., 2011), suggesting that these changes might not be evident in moderate-severe obesity. There were also no associations between body composition parameters known to affect bone health and vBMD or MAT. One reason might be the limited range of fat and muscle depots in our study participants, as all had moderate to severe obesity. Another reason might be that in severe obesity, the effects of body composition measures on bone parameters are blunted.
Limitations of our study include its cross-sectional study design which does not allow assessment of causality. Another limitation is our sample size with fewer Blacks than Whites, which makes the study susceptible to type-2 error. Additionally, we did not investigate hormonal determinants involved in BMD and MAT regulation. Moreover, race was self-reported (Corbie-Smith et al., 2008). Strengths of our study include the assessment of vBMD of the lumbar spine and MAT content using advanced imaging methods.
In conclusion, our pilot study suggests that there are racial differences in lumbar vBMD in adolescents and young adults with moderate to severe obesity, with Blacks having higher vBMD than Whites, while there are no differences in MAT content. The known inverse relationship between BMD and MAT is only observed in Whites but not in Blacks, suggesting racial differences in stem cell differentiation into the bone and fat lineages. These findings are independent of body composition, which is known to influence bone health. Larger longitudinal studies are necessary to evaluate how racial differences in bone parameters contribute to fracture risk in adolescents and young adults with moderate-severe obesity.
Grant support
This work was supported by the following grants: National Institutes of Health (NIH) United States of America: R01DK103946-01A1 (MM, MAB), K23DK110419-01 (VS), P30DK040561 (VS, FCS), K24DK109940 (MAB), K24 HD071843 (MM), L30 DK118710 (FCS), P30DK057521 (VS).
CRediT authorship contribution statement
Miriam A. Bredella: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing - review & editing. Vibha Singhal: Investigation, Supervision, Writing - review & editing. Nazanin Hazhir Karzar: Investigation, Writing - review & editing. Abisayo Animashaun: Writing - review & editing. Amita Bose: Investigation, Writing - review & editing. Fatima Cody Stanford: Investigation, Supervision, Writing - review & editing. Brian Carmine: Investigation, Writing - review & editing. Madhusmita Misra: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing - original draft.
Declaration of competing interest
The authors do not have any conflicts of interests to disclose.
References
- Abdalrahaman N. The relationship between adiposity, bone density and microarchitecture is maintained in young women irrespective of diabetes status. Clin. Endocrinol. 2017;87(4):327–335. doi: 10.1111/cen.13410. [DOI] [PubMed] [Google Scholar]
- Baron J.A. Racial differences in fracture risk. Epidemiology. 1994;5(1):42–47. doi: 10.1097/00001648-199401000-00008. [DOI] [PubMed] [Google Scholar]
- Bredella M.A. Increased bone marrow fat in anorexia nervosa. J. Clin. Endocrinol. Metab. 2009;94(6):2129–2136. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella M.A. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19(1):49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredella M.A. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology. 2013;269(2):534–541. doi: 10.1148/radiol.13130375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoverde Reyes K.J. Bone density, microarchitecture and strength estimates in white versus African American youth with obesity. Bone. 2020;138:115514. doi: 10.1016/j.bone.2020.115514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J.P. Differential effect of obesity on bone mineral density in White, Hispanic and African American women: a cross sectional study. Nutrition & metabolism. 2005;2(1):9. doi: 10.1186/1743-7075-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbie-Smith G. Conceptualizing race in research. J. Natl. Med. Assoc. 2008;100(10):1235–1243. doi: 10.1016/s0027-9684(15)31470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J.H., Evans B.A., Gregory J.W. Bone mass acquisition in healthy children. Arch. Dis. Child. 2005;90(4):373–378. doi: 10.1136/adc.2004.053553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin M.J., Rosen C.J. The bone-fat interface: basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015;3(2):141–147. doi: 10.1016/S2213-8587(14)70007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J.M. Playing with bone and fat. J. Cell. Biochem. 2006;98(2):251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- Glastre C. Measurement of bone mineral content of the lumbar spine by dual energy x-ray absorptiometry in normal children: correlations with growth parameters. J. Clin. Endocrinol. Metab. 1990;70(5):1330–1333. doi: 10.1210/jcem-70-5-1330. [DOI] [PubMed] [Google Scholar]
- Horlick M. Prediction models for evaluation of total-body bone mass with dual-energy X-ray absorptiometry among children and adolescents. Pediatrics. 2004;114(3):e337–e345. doi: 10.1542/peds.2004-0301. [DOI] [PubMed] [Google Scholar]
- Hu L. Mesenchymal stem cells: cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 2018:19(2). doi: 10.3390/ijms19020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed F. Effect of fat on measurement of bone mineral density. Int J Body Compos Res. 2009;7(1):37–40. [PMC free article] [PubMed] [Google Scholar]
- Johnston C.C., Jr., Slemenda C.W. Peak bone mass, bone loss and risk of fracture. Osteoporos Int. 1994;4(Suppl. 1):43–45. doi: 10.1007/BF01623435. [DOI] [PubMed] [Google Scholar]
- Liel Y. The effects of race and body habitus on bone mineral density of the radius, hip, and spine in premenopausal women. J. Clin. Endocrinol. Metab. 1988;66(6):1247–1250. doi: 10.1210/jcem-66-6-1247. [DOI] [PubMed] [Google Scholar]
- Misra M. Racial differences in bone microarchitecture and estimated strength at the distal radius and distal tibia in older adolescent girls: a cross-sectional study. J. Racial Ethn. Health Disparities. 2017;4(4):587–598. doi: 10.1007/s40615-016-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp K.L. Bone mass, microarchitecture and strength are influenced by race/ethnicity in young adult men and women. Bone. 2017;103:200–208. doi: 10.1016/j.bone.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Putman M.S. Differences in skeletal microarchitecture and strength in African-American and white women. J. Bone Miner. Res. 2013;28(10):2177–2185. doi: 10.1002/jbmr.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M., Berenson A.B. Racial difference in lean mass distribution among reproductive-aged women. Ethn Dis. 2010;20(4):346–352. [PMC free article] [PubMed] [Google Scholar]
- Schellinger D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am. J. Neuroradiol. 2001;22(8):1620–1627. [PMC free article] [PubMed] [Google Scholar]
- Schellinger D. Bone marrow fat and bone mineral density on proton MR spectroscopy and dual-energy X-ray absorptiometry: their ratio as a new indicator of bone weakening. AJR Am. J. Roentgenol. 2004;183(6):1761–1765. doi: 10.2214/ajr.183.6.01831761. [DOI] [PubMed] [Google Scholar]
- Singhal V. Regional fat depots and their relationship to bone density and microarchitecture in young oligo-amenorrheic athletes. Bone. 2015;77:83–90. doi: 10.1016/j.bone.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal V. Marrow adipose tissue in adolescent girls with obesity. Bone. 2019;129:115103. doi: 10.1016/j.bone.2019.115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden D. Peak bone mass in young women. J. Bone Miner. Res. 1995;10(5):711–715. doi: 10.1002/jbmr.5650100507. [DOI] [PubMed] [Google Scholar]
- Travison T.G. Accounting for racial/ethnic variation in bone mineral content and density: the competing influences of socioeconomic factors, body composition, health and lifestyle, and circulating androgens and estrogens. Osteoporos. Int. 2011;22(10):2645–2654. doi: 10.1007/s00198-010-1520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel G. Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. J Appl Physiol (1985) 2012;112(11):1824–1831. doi: 10.1152/japplphysiol.00029.2012. [DOI] [PubMed] [Google Scholar]
- Valerio G. Prevalence of overweight in children with bone fractures: a case control study. BMC Pediatr. 2012;12:166. doi: 10.1186/1471-2431-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen J. Differences in bone mineral density between normal-weight children and children with overweight and obesity: a systematic review and meta-analysis. Obes. Rev. 2017;18(5):526–546. doi: 10.1111/obr.12515. [DOI] [PubMed] [Google Scholar]
- Wren T.A. Racial disparity in fracture risk between white and nonwhite children in the United States. J. Pediatr. 2012;161(6):1035–1040. doi: 10.1016/j.jpeds.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E.W. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J. Bone Miner. Res. 2012;27(1):119–124. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu E.W. Marrow adipose tissue composition in adults with morbid obesity. Bone. 2017;97:38–42. doi: 10.1016/j.bone.2016.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]