Abstract
As the threat of arboviral diseases continues to escalate worldwide, the question of, “What types of human communities are at the greatest risk of infection?” persists as a key gap in the existing knowledge of arboviral diseases transmission dynamics. Here, we comprehensively review the existing literature on the socioeconomic drivers of the most common Aedes mosquito-borne diseases and Aedes mosquito presence/abundance. We reviewed a total of 182 studies on dengue viruses (DENV), chikungunya virus (CHIKV), yellow fever virus (YFVV), Zika virus (ZIKV), and presence of Aedes mosquito vectors. In general, associations between socioeconomic conditions and both Aedes-borne diseases and Aedes mosquitoes are highly variable and often location-specific. Although 50% to 60% of studies found greater presence or prevalence of disease or vectors in areas with lower socioeconomic status, approximately half of the remaining studies found either positive or null associations. We discuss the possible causes of this lack of conclusiveness as well as the implications it holds for future research and prevention efforts.
Keywords: Aedes-borne diseases, Zika virus, Dengue fever, Yellow fever, Chikungunya, Global health
1. Background
The global proliferation of Aedes aegypti (L.) and Aedes albopictus (Skuse) into novel regions represents a growing public health hazard due to their capacity of transmitting a variety of arboviral pathogens, including the emerging and re-emerging dengue viruses (DENV), chikungunya virus (CHIKV), yellow fever virus (YFVV), and Zika virus (ZIKV) [1]. These mosquitoes are abundant and particularly important in urban environments [2,3], where they use natural and artificial water-holding containers for rearing of larvae and feed nearly exclusively on humans [4]. The threat of vector-borne diseases has risen in recent decades due to the growth of cities, progression of climate change, and increase in globalization and international travel [5]. To date, few control techniques are sustainable, and recent global invasions of these vectors to new areas may further facilitate the proliferation of emerging viral pathogens [6,7], considerably increasing potential public health impacts. Although the biology, virology, and ecology of the vectors have been studied for decades, more recent research efforts have been made to examine arboviral diseases within the context of socioeconomic determinants of health.
Across both urban and rural environments, infrastructure quality disparities, discriminatory zoning practices, and differential exposure to social stressors place significant financial burdens on some neighborhoods over others [8,9]. Residents in these lower socioeconomic neighborhoods are more likely to be exposed to a wider variety of communicable diseases [[10], [11], [12], [13]], along with greater exposure to social costs, including poverty, institutional racism, crime, violence, isolation, and undesirable environmental conditions such as temperature extremes or weather events [14,15]. In contrast, there are also several examples of negative health outcomes associated with relatively high socioeconomic development. These so-called “Diseases of Affluence” include conditions such as diabetes mellitus, cardiovascular disease, and cancer, and these are largely non-communicable conditions associated with high-risk lifestyles and nutrition of highly developed regions [[16], [17], [18]]. Although numerous vector-borne diseases such as leishmaniasis, onchocerciasis, and Chagas disease have been labelled as “Diseases of Poverty” due to their strong association with socially vulnerable populations [19], to our knowledge the association between socioeconomic disparities and diseases caused by DENV, CHIKV, YFV, and ZIKV has yet to be examined in a systematic review process. A 2015 review on the relationship between DENV and poverty found only 12 studies on the subject, with inconclusive results regarding a directional effect [20]. Thus, our objective was to establish the first comprehensive review on the under-studied topic of the most common Aedes-borne diseases in the context of socioeconomic determinants of health. For each of the four diseases examined, in addition to Aedes occurrence, we aimed to describe the extent of negative, positive, or null relationships with socioeconomic factors. Overall, as a highly understudied subject with considerable public health consequence, we intend this review to serve as a catalyst for further investigations at both the local and global scale.
2. Methods
We systematically reviewed existing literatures using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21], starting with a literature search of the PubMed and Web of Science electronic databases on March 1, 2020. We used Boolean search strings for each of the five categories of this overall review analyses: DENV, CHIKV, YFV, ZIKV, and Aedes occurrence. For example, for dengue we used the search strings ‘Dengue’ AND ‘Poverty’ OR ‘Income’ OR ‘Socioeconomic’ OR ‘Socio-economic’ OR ‘Social class’ OR ‘Housing’ OR ‘Employment’ OR ‘Unemployment’ OR ‘Education’ OR ‘Community health services’. We repeated this for each disease. For the Aedes occurrence category, the first term of the search string was “‘aegypti’ OR ‘albopictus’” followed by the same socioeconomic search terms described above. No restrictions for the year of publication or geographic region were applied. If a single study was found for multiple searches because it covered multiple categories (i.e., examined both DENV and CHIKV), the study was included in both or more relevant categories, though only the conclusions specific to each category were analyzed (i.e., only the DENV results were analyzed in the DENV category, while the CHIKV results were analyzed in the CHIKV category).
After removing duplicates within each category using the semi-automatic count function in Mendeley [22], we screened the title and abstract of each study to assess basic subject matter relevance. Studies that met basic subject matter relevance of the review were then read in full and either included in the review or excluded based on meeting any one of six exclusion criteria [20]. First, included studies must not be burden of disease or economic studies. Second, they must not be review articles. Third, studies that did not involve actual disease or virus data were excluded. This includes risk modelling or assessment only studies. Eligible studies were required to use laboratory confirmed cases, clinically suspected cases, vector or host seropositivity, or antigen testing. Fourth, studies that did not involve actual socioeconomic data (e.g., housing quality, income, education) were excluded. Fifth, poor quality studies (small sample size, methodological concerns, or poor description of results) were excluded. Sixth, studies where the abstracts did not accurately reflect the conclusions in the full article were excluded. Excluded studies for the Aedes occurrence category were the same except that we excluded studies without actual entomological data (e.g., larval surveys, oviposition surveys) instead of excluding studies without actual disease or virus data. Although burden of disease studies and risk assessment studies are important in understanding the scope and scale of vector-borne disease, this review was intended to focus on empirical analysis of a current or past public health threat, rather than economic impact assessments or future disease risk estimations.
3. Results
The initial search yielded 1012 DENV studies (Fig. 1), 191 CHIKV studies (Fig. 2), 135 YFV studies (Fig. 3), 267 ZIKV studies (Fig. 4), and 581 Aedes occurrence studies (Fig. 5). After assessing each category's list of studies against the eligibility criteria, we included 99 DENV studies, 11 CHIKV studies, 0 YFV studies, 13 ZIKV studies, and 59 Aedes occurrence studies in the analysis (Appendices). Each study was reviewed for eligibility and analyzed independently by two reviewers. In addition to describing the country of study and socioeconomic metrics used, we determined the directional effect between socioeconomic factors and DENV/CHIKV/ZIKV or Aedes occurrence. There were negative (e.g., high disease rates or vector occurrence in low income areas), positive (e.g., high disease rates or vector occurrence in high income areas), or null (e.g. no association found between disease rates or vector occurrence) effects. Some studies had contrasting (i.e., more than one directional effect) or mediated conclusions (e.g., higher disease rates among children in areas of low income, yet higher disease rates among adults in areas of high income). In these circumstances, we further specified three additional directional effects: negative and positive, negative and null, or positive and null. For categories with no zeros values (i.e., having at least a study in every directional effect outcome), we ran a χ2 goodness-of-fit test to compare the distribution of effect directions.
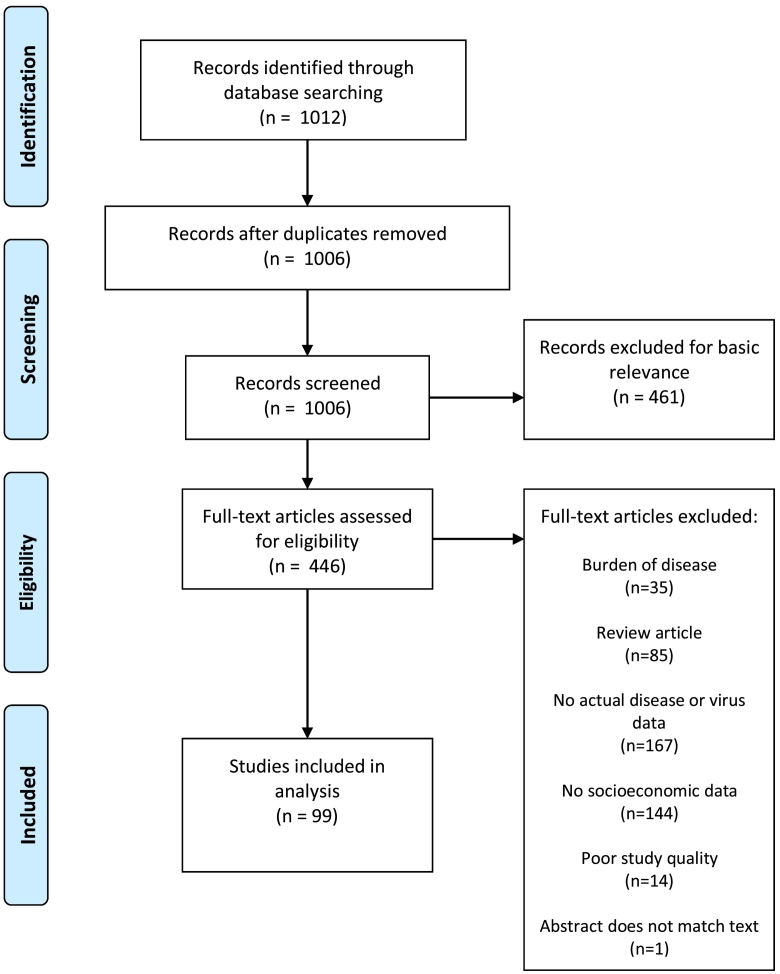
Fig. 1.
PRISMA flow diagram for DENV.
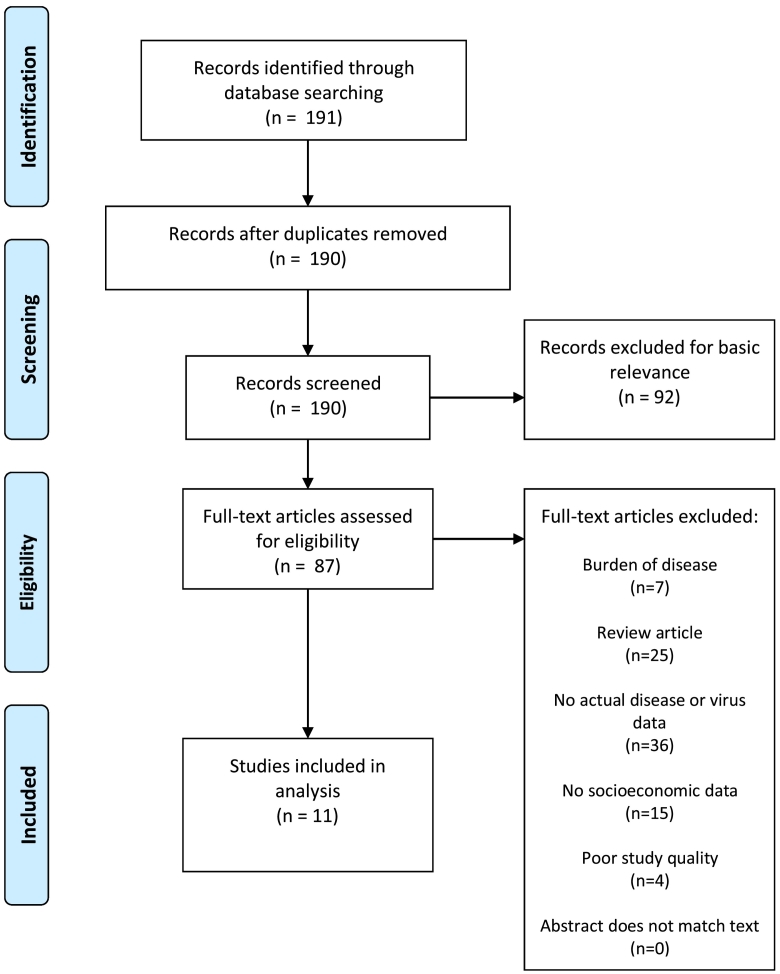
Fig. 2.
PRISMA flow diagram for CHIKV.
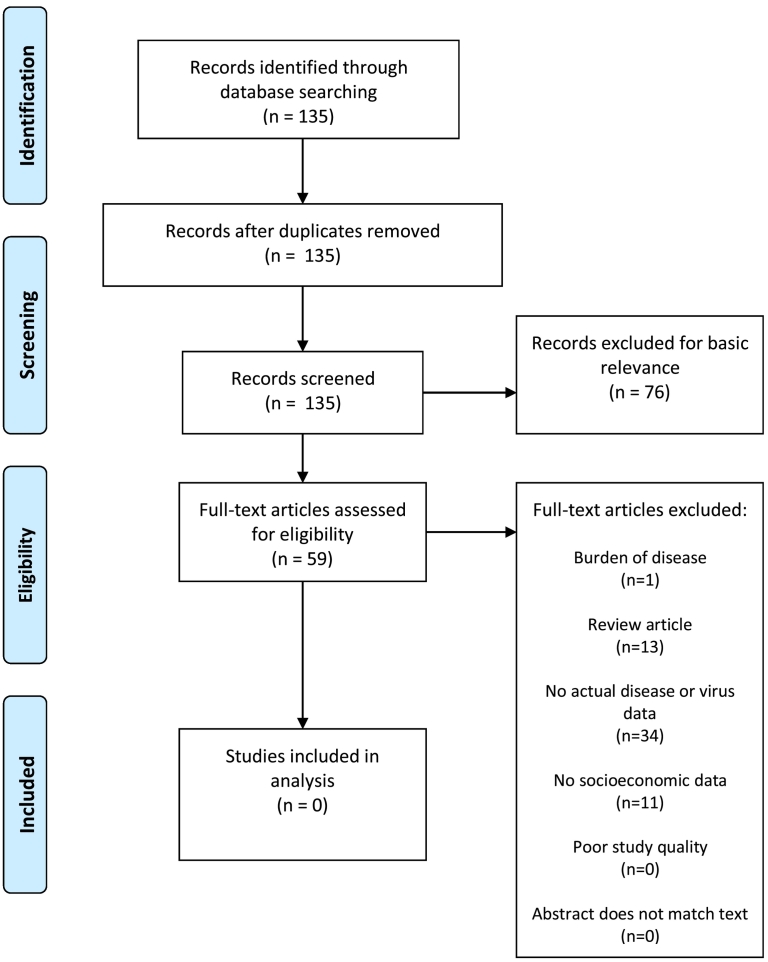
Fig. 3.
PRISMA flow diagram for YFV.
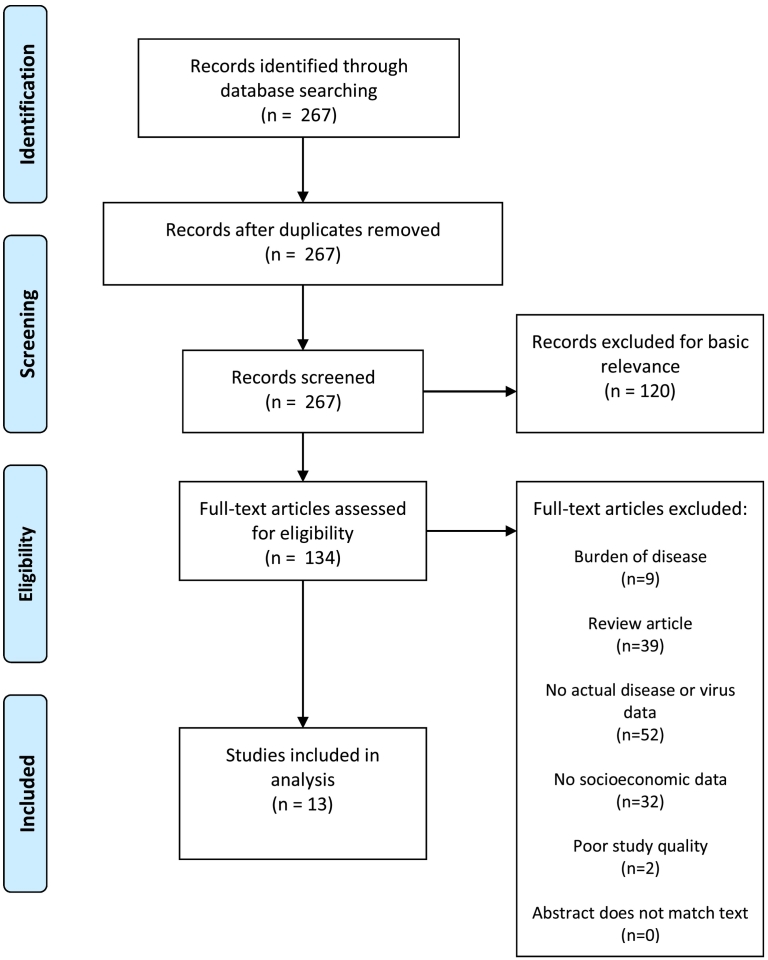
Fig. 4.
PRISMA flow diagram for ZIKV.
Fig. 5.
PRISMA flow diagram for Aedes presence or occurrence.
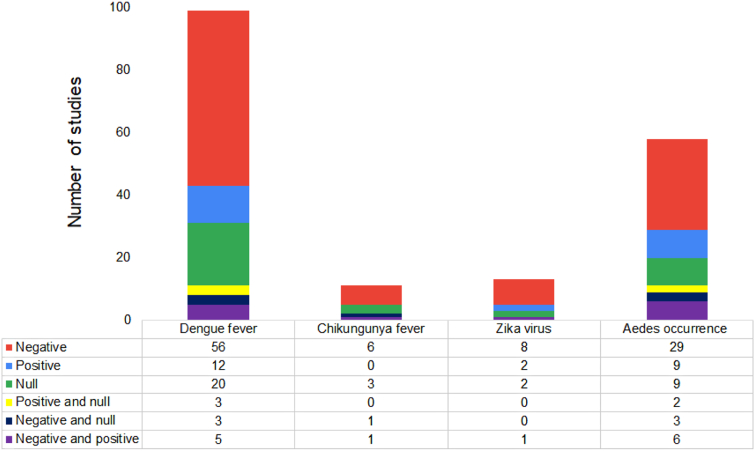
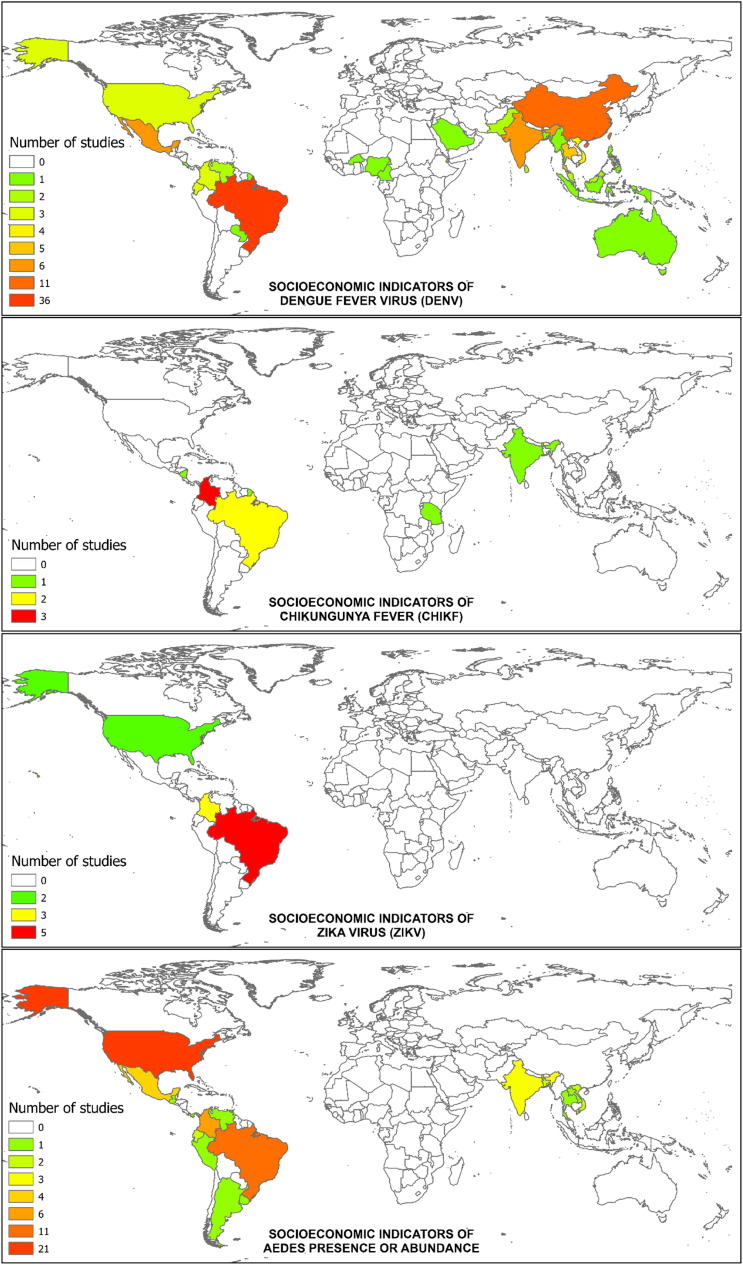
A total of 182 articles were included in the final analysis (Fig. 6). Of the 182 studies, the most (54.39%) were in the DENV category, followed by ZIKV and CHIKV. There were no articles that met the inclusion criteria in the YFV category. For the DENV, CHIKV, and ZIKV categories, Brazil represented the most common location of study, followed by Colombia. For Aedes, the United States was the most common setting (Fig. 7).
Fig. 6.
Number and directional effect of studies that empirically examine the relationship between socioeconomic metrics and either dengue fever, chikungunya fever, Zika virus, or Aedes occurrence. No studies on yellow fever met the eligibility criteria. A negative effect meant high disease rates or Aedes occurrence in low socioeconomic status areas, a positive effect meant high disease rates or Aedes occurrence in high socioeconomic status areas, and a null effect meant no association was found between socioeconomic factors and the disease or vector outcomes. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
Number of studies included for each country and category in the analysis.
DENV and Aedes occurrence categories had no zero values and the distributions of effect directions were statistically indistinguishable (P = 0.70).
3.1. Dengue
There were 99 (9.77% of the database search results) DENV studies that met the eligibility criteria. Of these, 36 (52.52%) were conducted in Brazil, followed by 11 (11.11%) in China. The most often used socioeconomic indicator was income, which appeared in 47 (47.47%) of the 99 studies. This was followed by education in 29 (29.29%) studies, and both garbage collection and gross domestic product (GDP) in 11 (11.11%) studies each.
Examples of greater presence or prevalence of DENV in lower socioeconomic areas alone were presented in 56 (56.56%) of the 99 papers, while positive association between DENV and socioeconomic status alone were presented in 12 (12.12%) studies. For papers with multiple contrasting results, five studies (5.05%) found both negative and positive associations between DENV and socioeconomic indicators, while both negative and null results, and both positive and null results were both found in 3 (3.03%) papers, respectively. The frequencies of observed effect directions were significantly different than the expected frequencies based on χ2 goodness-of-fit test (P < 0.01).
3.2. Chikungunya
There were 11 (6.11% of the database search results) CHIKV studies that met the eligibility criteria. Of the included 11 studies, three (25%) were conducted in Colombia, two in Brazil (18.18%), and each of the remaining nine in a separate country. Seven (63.63%) of these included income among their socioeconomic indicators, followed by water access and availability in four (36.36%) studies, housing type and education in three (25%) studies each. Six of the 11 (54.55%) found greater CHIKV prevalence or presence in lower socioeconomic areas, while none of these studies found a positive association. One (9.09%) study found both positive and negative associations, one (9.09%) found both positive and null associations, and three (25%) found no statistically significant association.
3.3. Zika
A total of 13 (4.87% of the database search results) studies met the eligibility criteria in the ZIKV category. Eight (72.72%) of the thirteen were conducted in Brazil, while a further three (25%) were conducted in Colombia, and the remaining two came (18.18%) from the United States. Income appeared as a socioeconomic indicator the most, at five (45.45%) studies. Out of the 13 studies, 8 (61.54%) found ZIKV prevalence or occurrence higher in lower socioeconomic areas, while the other two (15.38%) studies found higher ZIKV prevalence or occurrence in high socioeconomic areas. One (7.69%) study found both positive and negative associations and two (15.38%) found no statistically significant association.
3.4. Aedes occurrence
A total of 59 (10.15% of the database search results) studies met the eligibility criteria in the Aedes occurrence category. Of these, 22 (37.28%) were conducted in the United States, followed by 11 (18.64%) in Brazil, and five (8.47%) in Colombia. The most common socioeconomic indicator was income, which was used in 26 (44.06%) studies, followed by education in 18 (30.50%). Greater occurrence or abundance of Aedes mosquitoes in lower socioeconomic areas was demonstrated in 29 (50.00%) studies, while nine (15.52%) studies found a positive association, and nine (15.52%) found a null association. Both negative and positive results were illustrated in six (10.34) studies, whereas both negative and null results were found in three (5.17%) studies, and both positive and null results were found in two (3.45%). The frequencies of observed effect directions were significantly different than the expected frequencies (P < 0.01).
4. Discussion
Our results indicated a large variability regarding the relationship between socioeconomic factors and the most common Aedes-borne diseases and theoccurrence of their most common mosquito vectors. Although the largest share of studies in each category did indeed involve negative associations only, approximately half of the remaining studies included either positive or null associations. This lack of conclusiveness challenges the current practice of labeling DENV, CHIKV, or ZIKV as “Diseases of Poverty,” in a similar manner as malaria, tuberculosis, and HIV/AIDS [23]. Furthermore, approximately one third of the evaluated studies represented the only studies conducted in their respective countries, meaning that in many places the topic remains largely understudied.
There are several possible reasons for the lack of a strong consensus on the directional effect between socioeconomic indicators and either Aedes-borne disease or Aedes occurrence. The first is that rather than socioeconomic indicators consistently influencing Aedes-borne disease or Aedes occurrence in a linear relationship across all study sites, the expected effect direction may be highly situationally dependent. This highlights the importance of accounting for socioeconomic variation in any future study on these subjects, considering the bias that may be incurred when conducting research in a socioeconomically homogenous setting. In the absence of studies examining this topic in every threatened region, culture, and across all potential environmental conditions, extrapolations must be drawn from other cases. Based on the results of this review, effectively extrapolating results from other studies and justifying the devotion of limited public health resources may be difficult when the most likely effect globally only occurs approximately 50% of the time.
A second possible explanation for the lack of a singular effect is the simple ubiquity of the vectors and the difficulty in implementing comprehensive vector control. Combating the global spread of vector populations [24] has been difficult due to high resource costs for low budget institutions [[25], [26], [27]], varying effectiveness of the chemical agents [28,29], concerns of their environmental toxicity [30], and the burgeoning issue of insecticide resistance [31]. Even if vector control were to be heterogeneously distributed across socioeconomic gradients [32], the large-scale efficacy of control programs is often limited. Vector hotspots can be as small as 30 m across [33], meaning that even a single missed house in a neighborhood can result in persistent vector populations. In addition, production sources for adults can be extremely cryptic for container-breeding Aedes mosquitoes [34,35], making it nearly impossible to identify every possible habitat for larvicide treatment. Furthermore, source reduction of Aedes habitat typically requires the integration of the public's involvement [36]; therefore, the efficiency, extent, and variation of vector control educational campaigns may play a role in relationship outcome. To test these hypotheses, we recommend future studies focus on methods and outcomes of vector control between socioeconomically distinct areas. Despite the ubiquity of the vectors and viruses though, the geographic disparities we identified in the literature are striking. Southeast Asia, South Pacific Islands, and sub-Saharan Africa, in particular, represent key gaps in the literature and important areas for future empirical studies to take place.
We also hypothesize that the novelty of pathogens in many regions may limit consistent relationships to socioeconomic factors. With the arguable exception of DENV, which is currently endemic in 125 countries [37], both ZIKV and CHIKV have rapidly spread into new regions within the last decade at the continental scale [7,38,39]. The lack of viral immunity among human hosts would theoretically extend across a socioeconomic gradient, giving each individual human host similar susceptibility, though this does not account for other factors unrelated to the novelty of viruses that influence immunity [40]. Although a small number of the studies in our analysis involved antibody testing, larger scale serosurveillance efforts may help to identify the distribution of immunity across communities with socioeconomic disparities.
Another consequence of ZIKV and CHIKV recently emerging in novel regions is the possible lack of research on their epidemiology. This is evident when comparing the amount of eligible studies in our analysis for ZIKV and CHIKV compared to DENV, the latter of which is more abundant. We expect that in the future more research on socioeconomic determinants of these emerging diseases will be conducted, which can further validate conclusions of this review. The absence of any studies examining the association between socioeconomic indicators and YFV is perhaps not entirely surprising, given it is the only one of the four diseases with a widely available and highly effective vaccine. Subsequently, there have been numerous studies on socioeconomic disparities in YFV vaccine distribution [[41], [42], [43]] instead. However, with around 200,000 cases and 30,000 deaths a year attributed to YFV, 90% being in Africa [44], there is still a strong impetus to examine socioeconomic disparities in actual virus or disease burden. In addition, DENV, CHIKV, and ZIKV are mostly transmitted by urban-dwelling mosquitoes. The impact of socioeconomic variation on host vulnerability may be different when examining vectors and diseases associated more with rural landscapes.
The inability to derive a consensus between socioeconomic indicators and Aedes-borne disease or the prevalence of vector mosquitoes from this systematic review is concerning from a public health perspective. Had we established a strong and universal association between Aedes borne diseases or Aedes mosquitoes and socioeconomic indicators, public health resources could be more efficiently allocated towards the most needed communities. The inconclusiveness makes it difficult to determine the risk of Aedes-borne diseases across regions, making more people at risk, at least at the global scale. At the local scale, consistency may be easier to establish, which is why repeating the eligible studies in our analysis in different locations is important. As such, a helpful complement to our review may be accomplished by a future review on the types of studies that we specifically excluded, namely burden of disease studies or risk assessments. The burden of disease literature may serve to quantify the larger scale economic and health impacts of socioeconomic disparities in vector or virus occurrence, which may facilitate costs and benefit analyses of interventions. Likewise, a review on risk estimates may extend our conclusions into areas where the vectors and viruses are not present yet may be in the future.
Variation in environmental and cultural practices may have led to contrasting results between continents or regions, meaning that vulnerability in one location may not be comparable to vulnerability in others. While this may be a known component of Aedes-borne disease epidemiology, the widespread heterogeneity of the role of socioeconomics in vulnerability had not been previously described. Due to the myriad of variables that influence vector-borne disease risk in addition to socioeconomic conditions among host populations [[45], [46], [47]], a larger scale meta-analysis comparing the types of studies in this review across disparate regions can reveal key variable mediations. For instance, certain socioeconomic indicators may have a greater effect on infection rates only in regions of particular climatic conditions, cultural practices, or levels of public health oversight. This type of analysis will only be possible when the body of literature grows and becomes more evenly distributed across the regions of the world affected by Aedes-borne diseases.
5. Conclusion
In this review, we demonstrate that socioeconomic indicators of human host communities exhibit inconsistent associations with both Aedes-borne diseases and vector distribution. Thus, as further studies are published that explore the important confluence of social vulnerability and vector-borne disease, we emphasize the highly heterogeneous and complex nature of the coupled socio-epidemiological system of Aedes-borne diseases across the world. With risk increasing at a faster pace than prevention resources, identifying key socioeconomic indicators in each distinct jurisdiction should be the priority for both the academic and public health teams.
Declaration of Competing Interest
None.
All authors contributed equally to this study.
References
- 1.Gubler D.J. Vector Biology, Ecology and Control. 2010. The global threat of emergent/re-emergent vector-borne diseases; pp. 39–62. [DOI] [Google Scholar]
- 2.Li Y., Kamara F., Zhou G., Puthiyakunnon S., Li C., Liu Y. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tauil P.L. Urbanization and dengue ecology. Cad saude publica / Minist da Saude. Fund Oswaldo Cruz, Esc Nac Saude Publica. 2001;17(Suppl):99–102. (doi:S0102-311X2001000700018 [pii) [PubMed] [Google Scholar]
- 4.Ponlawat A., Harrington L.C., Chow E., Wirtz R.A., Scott T.W., Christophers S.R. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–849. doi: 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- 5.Gubler D.J. Dengue, urbanization and globalization: the unholy trinity of the 21 st century. Tropical Medicine Health. 2011 doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hennessey M., Fischer M., Staples J.E. Zika virus spreads to new areas - region of the Americas, may 2015-January 2016. Am. J. Transplant. 2016;16:1031–1034. doi: 10.1111/ajt.13743. [DOI] [PubMed] [Google Scholar]
- 7.Lancet The. Chikungunya--coming to America. Lancet. 2014;383:488. doi: 10.1016/S0140-6736(14)60167-7. [DOI] [PubMed] [Google Scholar]
- 8.Bullard R.D. Dismantling environmental racism in the USA. Local Environ. 1999;14:5–19. doi: 10.1080/13549839908725577. [DOI] [Google Scholar]
- 9.Müller N., Ignatieva M., Nilon C.H., Werner P., Zipperer W.C. 2013. Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities. Internet. [DOI] [Google Scholar]
- 10.Eisenhauer E. In poor health: supermarket redlining and urban nutrition. GeoJournal. 2001;53:125–133. doi: 10.1023/A:1015772503007. [DOI] [Google Scholar]
- 11.Galea S., Ahern J., Rudenstine S., Wallace Z., Vlahov D., Galea S. Urban built environment and depression: a multilevel analysis. J. Epidemiol. Community Health. 2005;59:822–827. doi: 10.1136/jech.2005.033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kjellstrom T., Friel S., Dixon J., Corvalan C., Rehfuess E., Campbell-Lendrum D. Urban environmental health hazards and health equity. J. Urban Health. 2007;84:i86–i97. doi: 10.1007/s11524-007-9171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray M., Edwards M.A., Abercrombie B., St Clair C.C. Poor health is associated with use of anthropogenic resources in an urban carnivore. Proc. Biol. Sci. 2015;282 doi: 10.1098/rspb.2015.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paradies Y., Ben J., Denson N., Elias A., Priest N., Pieterse A. Racism as a determinant of health: a systematic review and meta-analysis. PLoS One. 2015 doi: 10.1371/journal.pone.0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey Z.D., Krieger N., Agénor M., Graves J., Linos N., Bassett M.T. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017 doi: 10.1016/S0140-6736(17)30569-X. [DOI] [PubMed] [Google Scholar]
- 16.Van de Poel E., O’Donnell O., Van Doorslaer E. Urbanization and the spread of diseases of affluence in China. Econ. Hum. Biol. 2009 doi: 10.1016/j.ehb.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Al-Adawi S. Emergence of diseases of affluence in Oman: where do they feature in the health research agenda? Sultan Qaboos Univ. Med. J. 2006;6(2):3–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Ezzati M., Vander Hoorn S., Lawes C.M.M., Leach R., James W.P.T., Lopez A.D. Rethinking the “diseases of affluence” paradigm: global patterns of nutritional risks in relation to economic development. PLoS Med. 2005 doi: 10.1371/journal.pmed.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houweling T.A.J., Karim-Kos H.E., Kulik M.C., Stolk W.A., Haagsma J.A., Lenk E.J. Socioeconomic inequalities in neglected tropical diseases: a systematic review. PLoS Negl. Trop. Dis. 2016 doi: 10.1371/journal.pntd.0004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulligan K., Dixon J., Joanna Sinn C.-L., Elliott S.J. Is dengue a disease of poverty? A systematic review. Pathog Glob Health. 2015 doi: 10.1179/2047773214Y.0000000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendeley Ltd . Elsevier; 2019. Mendeley.https://www.mendeley.com/?interaction_required=true Available: [Google Scholar]
- 23.Singh A., Singh S. Diseases of poverty and lifestyle, well-being and human development. Mens Sana Monogr. 2008 doi: 10.4103/0973-1229.40567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraemer M.U.G., Sinka M.E., Duda K.A., Mylne A., Shearer F.M., Barker C.M. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife. 2015;4 doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Packierisamy P.R., Ng C.W., Dahlui M., Inbaraj J., Balan V.K., Halasa Y.A. Cost of dengue vector control activities in Malaysia. Am. J. Trop. Med. Hyg. 2015 doi: 10.4269/ajtmh.14-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luz P.M., Vanni T., Medlock J., Paltiel A.D., Galvani A.P. Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet. 2011 doi: 10.1016/S0140-6736(11)60246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yukich J.O., Lengeler C., Tediosi F., Brown N., Mulligan J.A., Chavasse D. Costs and consequences of large-scale vector control for malaria. Malar. J. 2008 doi: 10.1186/1475-2875-7-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harbison J.E., Runde A.B., Henry M., Hulsebosch B., Meresh A., Johnson H. An operational evaluation of 3 Methoprene Larvicide formulations for use against mosquitoes in catch basins. Environ Health Insights. 2018 doi: 10.1177/1178630218760539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcombe S., Chonephetsarath S., Thammavong P., Brey P.T. Alternative insecticides for larval control of the dengue vector Aedes aegypti in Lao PDR: insecticide resistance and semi-field trial study. Parasit. Vectors. 2018 doi: 10.1186/s13071-018-3187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brühl C.A., Després L., Frör O., Patil C.D., Poulin B., Tetreau G. Science of the Total Environment. 2020. Environmental and socioeconomic effects of mosquito control in Europe using the biocide bacillus thuringiensis subsp. israelensis (Bti) [DOI] [PubMed] [Google Scholar]
- 31.Moyes C.L., Vontas J., Martins A.J., Ng L.C., Koou S.Y., Dusfour I. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 2017 doi: 10.1371/journal.pntd.0005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mugisha A., McLeod A., Percy R., Kyewalabye E. Socio-economic factors influencing control of vector-borne diseases in the pastoralist system of south western Uganda. Trop. Anim. Health Prod. 2008 doi: 10.1007/s11250-007-9093-2. [DOI] [PubMed] [Google Scholar]
- 33.LaCon G., Morrison A.C., Astete H., Stoddard S.T., Paz-Soldan V.A., Elder J.P. Shifting patterns of Aedes aegypti fine scale spatial clustering in Iquitos, Peru. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandel K., Suman D.S., Wang Y., Unlu I., Williges E., Williams G.M. Targeting a hidden enemy: PyriproxYFVen autodissemination strategy for the control of the container mosquito Aedes albopictus in cryptic habitats. PLoS Negl. Trop. Dis. 2016 doi: 10.1371/journal.pntd.0005235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dieng H., Saifur R.G.M., Ahmad A.H., Che Salmah M.R., Aziz A.T., Satho T. Unusual developing sites of dengue vectors and potential epidemiological implications. Asian Pac. J. Trop. Biomed. 2012 doi: 10.1016/S2221-1691(12)60047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartumeus F., Costa G.B., Eritja R., Kelly A.H., Finda M., Lezaun J. Sustainable innovation in vector control requires strong partnerships with communities. PLoS Negl. Trop. Dis. 2019 doi: 10.1371/journal.pntd.0007204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray N.E.A., Quam M.B., Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clinical Epidemiology. 2013 doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezza G., Nicoletti L., Angelini R., Romi R., Finarelli A., Panning M. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 39.Fauci A.S., Morens D.M. Zika virus in the Americas - yet another arbovirus threat. N. Engl. J. Med. 2016;363:1–3. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 40.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 2010 doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aremu O. Socioeconomic differences in yellow fever vaccine uptake in Togo: a multilevel analysis. Int. J. Infect. Dis. 2018 doi: 10.1016/j.ijid.2018.04.4225. [DOI] [Google Scholar]
- 42.Branco F.L.C.C., Pereira T.M., Delfino B.M., Braña A.M., Oliart-Guzmán H., Mantovani S.A.S. Socioeconomic inequalities are still a barrier to full child vaccine coverage in the Brazilian Amazon: a cross-sectional study in Assis Brasil, acre, Brazil. Int. J. Equity Health. 2014 doi: 10.1186/s12939-014-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flamand C., Bailly S., Fritzell C., Pellerin S.F., Toure A., Chateau N. Vaccination coverage in the context of the emerging yellow fever threat in French Guiana. PLoS Negl. Trop. Dis. 2019 doi: 10.1371/journal.pntd.0007661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garske T., Van Kerkhove M.D., Yactayo S., Ronveaux O., Lewis R.F., Erin J. Yellow fever burden estimation: Summary Methods to estimate Yellow Fever Burden in Africa. World Heal Organ. 2012;11:1–2. http://www.who.int/csr/disease/yellowfev/YellowFeverBurdenEstimation_Summary2013.pDENV Available: [Google Scholar]
- 45.Dong Y., Manfredini F., Dimopoulos G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009 doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu-Helmersson J., Stenlund H., Wilder-Smith A., Rocklöv J. Vectorial capacity of Aedes aegypti: effects of temperature and implications for global dengue epidemic potential. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kramer L.D., Ciota A.T. Dissecting vectorial capacity for mosquito-borne viruses. Curr. Opin. Virol. 2015 doi: 10.1016/j.coviro.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]