Abstract
This study was designed to evaluate the effects of γ-irradiated Astragalus polysaccharides (IAPS) on growth performance, cecal microbiota populations, and concentrations of cecal short-chain fatty acids of immunosuppressed broilers. A total of 144 one-day-old broiler chicks were randomly assigned into 3 groups: nontreated group (control), cyclophosphamide (CPM)-treated groups fed either a basal diet or the diets containing 900 mg/kg IAPS, respectively. On day 16, 18, and 20, broilers in the control group were intramuscularly injected with 0.5 mL sterilized saline (0.75%, wt/vol), and those in the CPM and IAPS groups were intramuscularly injected with 0.5 mL CPM (40 mg/kg of BW). The trial lasted 21 d. Compared with the control group, CPM treatment decreased the broiler average daily gain (ADG) and feed intake (P < 0.05) but did not affect the overall microbial diversity and compositions, as well as the concentrations of cecal acetate, propionate, and butyrate in cecum of broilers (P > 0.05). Dietary IAPS supplementation increased broiler ADG, Shannon index, and decreased Simpson index (P < 0.05). Specifically, broilers fed diets containing IAPS showed lower abundances of Faecalibacterium, Bacteroides, and Butyricicoccus and higher proportions of Ruminococcaceae UCG-014, Negativibacillus, Shuttleworthia, Sellimonas, and Mollicutes RF39_norank, respectively (P < 0.05). The IAPS treatment also increased butyrate concentration (P < 0.05) and tended to elevate acetate concentration (P = 0.052) in cecal digesta. The results indicated that IAPS are effective in increasing the cecal beneficial bacteria and short-chain fatty acids production, contributing to improvement in the growth performance of immunosuppressive broilers. These findings may expand our knowledge about the function of modified Astragalus polysaccharides in broiler chickens.
Key words: broiler, γ-irradiated Astragalus polysaccharides, cecum, microbiota, short-chain fatty acids
Introduction
The gastrointestinal tracts (GIT) of broilers house a complex and dynamic microbial community that generally comprised of 15 phyla and 288 genera bacteria, making contribution to the nutrient absorption, short-chain fatty acids (SCFA) synthesis, inhibition of intestinal pathogens, and the development of host immune system (Sekirov et al., 2010; Xiao et al., 2017). Meanwhile, the composition of the intestinal microbiota is dependent on the diet variation, pathological environment, and antibiotic therapy (Cisek and Binek, 2014). Broilers suffer from overcrowding, environmental ammonia, physiological stress, and typically virus infection are potentially under the condition of immunosuppression, which shift the microbial structure and ultimately disrupt the intestinal function (Hoerr, 2010). Some plant polysaccharides that reach the distal gastrointestinal tract can be fermented by gut microbiota and further regulate the intestinal microenvironment (Zhang et al., 2018a).
Astragalus polysaccharides (APS) is one of the main active components of the traditional Chinese medicinal herb Astragalus membranaceus (Fisch.) Bunge (family Fabaceae) originated in Northern China (Li et al., 2019a). The major monosaccharides existed in Astragalus are mannose, D-glucose, D-galactose, xylose, and L-arabinose (Kallon et al., 2013). The biological activities of polysaccharides are closely related to their molecular weight, specific spatial structures, and the degree of polymerization (Choi and Kim, 2013). Among these, molecular weight is one of the most critical factors mediating the bioactivities of polysaccharides (Choi and Kim, 2013). The γ-irradiation is an ionic, nonthermal method of preservation which is applied in modifications of macromolecular polysaccharides through crosslinking, grafting, and degradation (Choi and Kim, 2013; Ren et al., 2018). Our previous studies have indicated that γ-irradiation decreased the molecular weight and the viscosity of natural APS and increased its water solubility (Ren et al., 2018; Li et al., 2019a). Besides, γ-irradiated Astragalus polysaccharides (IAPS) increased growth performance, promoted intestinal development, and maintained a better immune function of broiler chickens under the immunosuppression status (Li et al., 2019a, 2019b). These findings indicated that IAPS possessed stronger immunomodulatory effect than natural APS. However, whether the protective effects of IAPS for maintaining better growth performance and immune function of immunosuppressive broilers is also closely related to its moderating effect to gut microbiota populations is still unknown. Therefore, the purpose of this study was to evaluate the effects of dietary supplementation with IAPS on cecal microbial composition and diversity and concentrations of SCFA in cecum of immunosuppressed broilers.
Materials and methods
Preparation of IAPS
Natural APS with a determined purity of 87.64% was purchased from Tianjin Sainuo Pharmaceutical Co., Ltd. (Tianjin, China). The sample modification was carried out using a BFT-IV cobalt-60 source irradiator (BINE High-Tech Co., Ltd., Beijing, China) at an ambient temperature of 25 ± 0.5°C in XiYue Irradiation Technology Co., Ltd. (Nanjing, China). The Co-60 gamma source strength was 2 × 106 Ci, and the irradiation dose was 25 kGy, with a rate of 4 Gy/s. Then, IAPS samples were stored at −20°C for further use.
Birds and Experimental Design
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. A total of 144 one-day-old healthy Arbor Acres broiler chicks (Shengnong Co., Ltd., Jiangsu, China) with a similar initial weight (44.81 ± 0.44 g) were randomly allocated into 3 dietary treatments devoid of antibiotics: 1) control group, 2) cyclophosphamide (CPM) group, and 3) IAPS group. Each treatment consists of 6 replicates (1 replicate per cage; cage size, 100 cm × 60 cm × 40 cm) with 8 broilers per replicate. Broilers in the control and CPM groups were fed with a basal diet. Birds in IAPS group were fed with the same basal diets containing 900 mg/kg IAPS. The whole experiment lasted 21 d. On day 16, 18, and 20, all birds in the control group were injected with 0.5 mL sterile saline (0.75%, wt/vol) at pectoralis major muscle, and the birds in CPM and IAPS treatment groups were intramuscularly injected with 0.5 mL CPM solution (40 mg/kg of BW) in the same manner (Li et al., 2019a). All broilers were housed in 3-tier wired cages for the 21-day feeding trial and had free access to feed and water during the entire rearing period. The ingredient composition and nutrient levels of the basal diet are presented in Table 1. The basal diet was formulated to meet or exceed the nutrient requirements of broiler chickens recommended by the National Research Council (NRC, 1994). All diets were fed in mash form. The temperature of the experimental room was maintained at 33°C for the first week and then decreased by 3°C each week until it reached 25°C. On 21 d, body weight and feed consumption for each replicate were recorded to calculate the average daily gain (ADG), average daily feed intake (ADFI), and feed to gain ratio (F/G, g: g).
Table 1.
Ingredient composition and calculated nutrient levels of the basal diets, on an as-fed basis.
| Ingredient (%) | 1–21 d |
|---|---|
| Corn | 57.61 |
| Soybean meal (44% crude protein) | 31.00 |
| Corn gluten meal (60% crude protein) | 3.29 |
| Soybean oil | 3.11 |
| Dicalcium phosphate | 2.00 |
| Limestone | 1.20 |
| Salt | 0.30 |
| L-Lysine HCl | 0.34 |
| DL-Methionine | 0.15 |
| Premix1 | 1.00 |
| Calculated Nutrient levels (%) | |
| Metabolizable energy (MJ/kg) | 12.56 |
| Crude protein | 21.00 |
| Calcium | 1.00 |
| Total phosphorus | 0.70 |
| Available phosphorus | 0.46 |
| Lysine | 1.20 |
| Methionine | 0.50 |
| Methionine + Cysteine | 0.85 |
Premix provided per kilogram of diet: vitamin A, 12,000 IU; vitamin D3, 2,500 IU; vitamin E, 20 IU; menadione sodium bisulfate, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; calcium pantothenate, 10 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12, 0.013 mg; choline chloride, 400 mg; Fe, 80 mg; Cu, 8 mg; Mn, 110 mg; Zn, 60 mg; I, 1.1 mg; Se, 0.3 mg.
Sample Collection
At 21 d, one broiler with similar weight close to the average weight of their replicate was selected, electrically stunned (50 V, alternating current, 400 Hz for 5 s each bird), and then slaughtered via exsanguination of the left carotid artery. Thereafter, carcasses were dissected, and the digesta of 2 ceca were ligated with cotton thread, removed, and collected in the sterile freezing tubes. All samples were stored at −80°C for the further analysis.
Genomic DNA Extraction, PCR Amplification, and Illumina MiSeq Sequencing
Total bacterial genomic DNA of the cecal digesta (0.30 g) was extracted according to a bead-beating method (Satokari et al., 2001). The DNA concentration was measured using a NanoDrop spectrophotometer (ND-1000UV-Vis; ThermoFisher Scientific, Wilmington, DE) and diluted to 1 ng/μL with sterile water. The V4-V5 hypervariable regions of the bacteria 16S rRNA gene were amplified by PCR using primer pair 341 F/806R (341F: CCTAYGGGRBGCASCAG, 806R: GGACTACNNGGGTATCTAAT). All PCR components contained 15 μL of Phusion High-Fidelity PCR Master Mix (New England Biolabs, Beverly, MA), 0.2 μmol of forward and reverse primers, 10 ng of template DNA, and double-distilled water filled to 30 μL. The thermal cycling condition was as follows: 95°C for 2 min; 25 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, followed by 72°C for 5 min. The PCR products were purified with the GeneJET Gel Extraction Kit (Thermo Fisher Scientific, Waltham, MA). Then, the DNA library was constructed using purified amplicons and sequenced on an Illumina MiSeq platform and 250 bp/300 bp paired-end reads were generated.
Sequencing Data Processing
The paired-end sequence reads were merged using FLASH (Version 1.2.7, http://ccb.jhu.edu/software/FLASH/) (Magoč and Salzberg, 2011). Sequence analyses were performed using UPARSE software (Version 7.0.1001, http://drive5.com/uparse/) with the UPARSE-OTU and UPARSE-OTUref algorithms (Edgar, 2013). The unique sequence set was classified into operational taxonomic units (OTU) when sequences were at 97% similarity. Taxonomic classification (form phylum to genus level) of these OTU sequences was performed with the Ribosomal Database Project classifier (version 2.2 http://sourceforge.net/projects/rdpclassifier/) with the default 80% confidence threshold (Wang et al., 2007). To determine the community diversity of cecal microbiota, alpha diversity analysis was made using the OTU table. Diversity indices (Shannon, Simpson) and richness estimators (Chao1) of each sample was calculated by MOTHUR (version 1.31.2) (Schloss et al., 2009). The principal coordinate analysis was applied to estimate β-diversity based on the Weighted UniFrac distance metric.
SCFA Analysis
The concentrations of acetate, propionate, and butyrate in cecal digesta was measured using gas chromatography after metaphosphoric acid derivation as previously described with minor modifications (Zhang et al., 2018b). Briefly, 0.20 g of thawed sample was diluted with 2 mL double-distilled water in a sterile screw-capped tube, then homogenized, and centrifuged at 5,000 × g for 10 min at 4°C. A volume of 1 mL of supernatant was then transferred to another Eppendorf tube and mixed with 0.2 mL, 25% (wt/vol) ice-cold metaphosphoric acid solution. Subsequently, this solution was kept at −20°C overnight and centrifuged at 12,000 × g for 10 min before analysis. The supernatant was then filtered with a 0.22 μm membrane, and an injection volume of 0.4 μL of sample solution was analyzed on a capillary column gas chromatograph (GC-14B; Shimadzu, Kyoto, Japan) equipped with a capillary column (Agilent HP-5MS; 30 m × 0.32 mm × 0.25 μm, Agilent Technologies Inc., Santa Clara, CA) and a flame ionization detector. The carrier gas was nitrogen at a flow rate of 2.0 mL/min. The column temperature program: held at 90°C for 2 min and then heated at 25°C/min to 250°C (held for 5 min). The concentrations of acetate, propionate, and butyrate were calculated and expressed as μmol per g of wet cecal digesta.
Statistical Analysis
The data of growth performance, microbial diversity, the relative abundances of bacteria, and the SCFA concentrations in cecal digesta were analyzed by one-way analysis of variance (ANOVA) using SPSS 20.0 statistical software (version 20.0, SPSS Inc., Chicago, IL). The growth performance data were analyzed with the replicate (each replicate in one cage) as the experimental unit, and other data were analyzed with the individual sampled bird from each replicate as the experimental unit (n = 6). The differences among treatments were examined using Duncan's multiple range tests. All results were presented by mean values and the standard error of the mean (SEM). Differences were considered significant as P < 0.05, unless otherwise stated.
Results
Growth Performance
As shown in Table 2, CPM stimulation decreased the ADG and ADFI of 21-day broilers compared with the control group, whereas dietary supplementation with 900 mg/kg IAPS improved ADG of CPM-treated broilers (P < 0.001). No significant difference on F/G ratio was observed among control, CPM, and IAPS groups (P > 0.05).
Table 2.
Effect of 60Co γ-ray irradiated Astragalus polysaccharides (IAPS) on the growth performance of cyclophosphamide (CPM) immunosuppressed broilers from 1 to 21 d.
| Items2 | Treatments1 |
SEM | P-value | ||
|---|---|---|---|---|---|
| Control | CPM | IAPS | |||
| ADG (g/day·bird) | 34.26a | 31.92b | 33.55a | 0.29 | <0.001 |
| ADFI (g/day·bird) | 49.27a | 46.82b | 47.79a,b | 0.40 | 0.024 |
| F/G (g:g) | 1.44 | 1.47 | 1.43 | 0.01 | 0.245 |
a,bDifferent letters in the mean value of the same row indicate a significant difference (P < 0.05). Data are represented as means with pooled SEM (n = 6).
Control, broilers were fed a basal diet and injected with saline; CPM, CPM-injected broilers were fed a basal diet; IAPS, CPM-injected broilers were fed a basal diet containing 900 mg/kg IAPS.
ADG, average daily gain; ADFI, average daily feed intake; F/G, feed to gain ratio.
The Quality Analysis of Sequencing Data
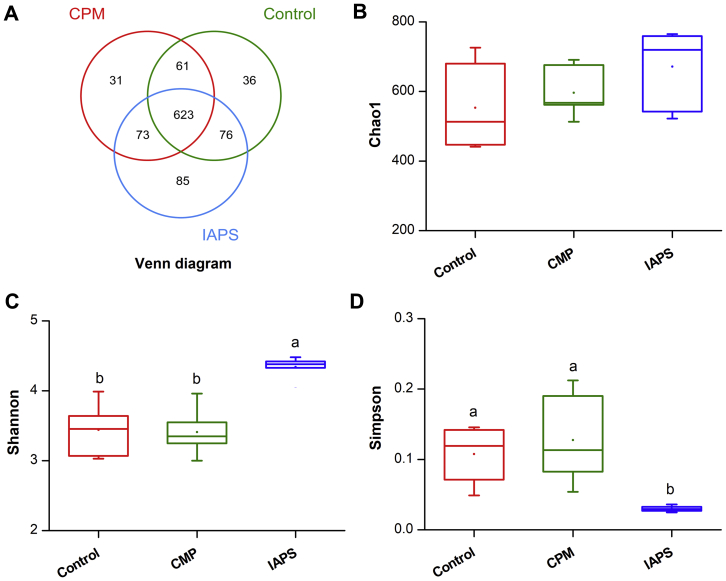
Following the removal of low-quality or chimeric sequences, the average number of reads generated from the cecal digesta sample per bird was 42,914 (28,898-55,187). The estimate of Good's coverage reached greater than 99.6% for all cecal samples. A Venn diagram (Figure 1A) was constructed to illustrate the shared and unique OTU distribution among all groups. The Venn diagram (Figure 1A) showed that 623 OTU were shared by all 3 groups, and the unique OTU numbers corresponding to control, CPM, and IAPS were 36, 31, and 85, respectively.
Figure 1.
OTU analysis and alpha diversity index analysis among groups. (A) Venn diagram based on OTU and Box plot of (B) Chao1 index, (C) Shannon index, and (D) Simpson index intergroup differences among groups. Means without a common letter significantly differ (P < 0.05). Control, broilers were fed a basal diet and injected with saline; CPM, CPM-injected broilers were fed a basal diet; IAPS, CPM-injected broilers were fed a basal diet containing 900 mg/kg IAPS. Abbreviations: CPM, cyclophosphamide; IAPS, 60Co γ-ray irradiated Astragalus polysaccharides.
Microbial Diversity
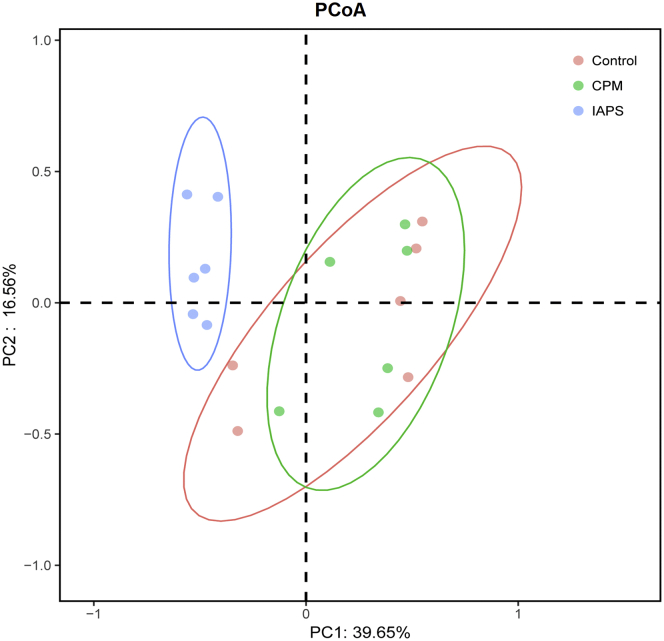
The bacterial α diversity indices among the groups are presented in Figure 1. The Chao1, Shannon, and Simpson indices were not affected by the CPM challenge compared with the control group (P > 0.05; Figures 1B–1D). By contrast, IAPS treatment increased Shannon index and decreased Simpson index in comparison with the control and CPM groups (P < 0.01; Figures 1C and 1D). The principal coordinate analysis reflected the β diversity by revealing overall microbial profiles of all the groups. As shown in Figure 2, we noted that bacterial communities in cecal samples collected form the CPM and control groups clustered more closely, whereas the sample form birds fed IAPS diet clustered separately from the bacteria of the control and CPM groups.
Figure 2.
Principal coordinate analysis (PCoA) plot of cecal microbiota composition based on unweighted UniFrac in broilers. Control, broilers were fed a basal diet and injected with saline; CPM, CPM-injected broilers were fed a basal diet; IAPS, CPM-injected broilers were fed a basal diet containing 900 mg/kg IAPS. Abbreviations: CPM, cyclophosphamide; IAPS, 60Co γ-ray irradiated Astragalus polysaccharides.
Microbial Community Compositions
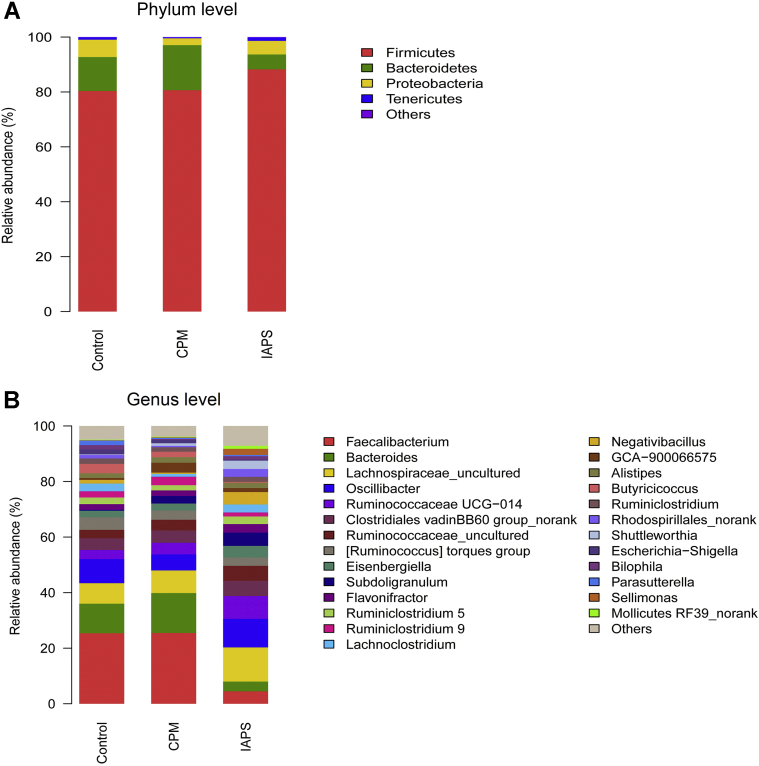
To assess the differences induced by CPM and IAPS in the bacterial community members of the cecal microbiota, taxonomic compositions were analyzed at the phylum and genus levels. Phylum level microbiota analysis showed that Firmicutes, Bacteroidetes, Proteobacteria, and Tenericutes were the most 4 dominants, together accounting for over 99.80% of the total sequences of broiler cecal microbiota in all groups (Figure 3A and Table 3). No significant difference was found in the relative abundance of all bacterial community at phylum level among 3 treatment groups (P > 0.05). Nevertheless, IAPS treatment had a tendency for decreasing the relative abundance of Bacteroidetes in comparison with other groups (P = 0.097).
Figure 3.
Composition of cecal microbiota of 21-day broilers at (A) phylum and (B) genus levels. Control, broilers were fed a basal diet and injected with saline; CPM, CPM-injected broilers were fed a basal diet; IAPS, CPM-injected broilers were fed a basal diet containing 900 mg/kg IAPS. Abbreviations: CPM, cyclophosphamide; IAPS, 60Co γ-ray irradiated Astragalus polysaccharides.
Table 3.
Effect of 60Co γ-ray irradiated Astragalus polysaccharides (IAPS) on the cecal microbiota composition and abundances of cyclophosphamide (CPM) immunosuppressed broilers on the phylum level.
| Items | Treatments1 |
SEM | P-value | ||
|---|---|---|---|---|---|
| Control | CPM | IAPS | |||
| Firmicutes (%) | 80.36 | 80.65 | 88.22 | 2.82 | 0.462 |
| Bacteroidetes (%) | 12.41 | 16.43 | 5.48 | 2.03 | 0.097 |
| Proteobacteria (%) | 6.29 | 2.49 | 4.93 | 0.94 | 0.262 |
| Tenericutes (%) | 0.87 | 0.33 | 1.23 | 0.17 | 0.166 |
Data are represented as the means with pooled SEM (n = 6).
Control, broilers were fed a basal diet and injected with saline; CPM, CPM-injected broilers were fed a basal diet; IAPS, CPM-injected broilers were fed a basal diet containing 900 mg/kg IAPS.
At the genus level, CPM treatment had no significant influence on overall bacterial compositions compared with the control group except for decreasing the relative abundance of Negativibacillus (P < 0.001; Figure 3B and Table 4). Compared with the control and CPM groups, IAPS group was characterized by lower relative abundances of Faecalibacterium, Bacteroides, and Butyricicoccus, higher proportions of Ruminococcaceae UCG-014, Negativibacillus, Shuttleworthia, Sellimonas, and Mollicutes RF39_norank, respectively (P < 0.05).
Table 4.
Effect of 60Co γ-ray irradiated Astragalus polysaccharides (IAPS) on the cecal microbiota composition and abundances of cyclophosphamide (CPM) immunosuppressed broilers on the genus level.
| Items | Treatments1 |
SEM | P-value | ||
|---|---|---|---|---|---|
| Control | CPM | IAPS | |||
| Faecalibacterium (%) | 25.37a | 25.48a | 4.46b | 3.01 | <0.001 |
| Bacteroides (%) | 10.68a,b | 14.41a | 3.56b | 1.89 | 0.049 |
| Lachnospiraceae_uncultured (%) | 7.35 | 8.10 | 12.26 | 1.21 | 0.208 |
| Oscillibacter (%) | 8.66 | 5.77 | 10.31 | 1.06 | 0.215 |
| Ruminococcaceae UCG-014 (%) | 3.26b | 4.19b | 8.17a | 0.74 | 0.006 |
| Clostridiales vadinBB60 group_norank (%) | 4.24 | 4.49 | 5.50 | 0.82 | 0.818 |
| Ruminococcaceae_uncultured (%) | 3.03 | 3.85 | 5.40 | 0.53 | 0.187 |
| [Ruminococcus] torques group (%) | 4.53 | 3.30 | 3.01 | 0.46 | 0.376 |
| Eisenbergiella (%) | 2.38 | 2.53 | 4.19 | 0.59 | 0.406 |
| Subdoligranulum (%) | 0.32 | 2.72 | 4.75 | 0.81 | 0.071 |
| Flavonifractor (%) | 2.03 | 2.01 | 3.06 | 0.40 | 0.504 |
| Ruminiclostridium 5 (%) | 2.42 | 1.89 | 2.77 | 0.37 | 0.642 |
| Ruminiclostridium 9 (%) | 2.21 | 3.02 | 1.43 | 0.36 | 0.203 |
| Lachnoclostridium (%) | 2.78 | 1.00 | 2.87 | 0.41 | 0.097 |
| Negativibacillus (%) | 1.35b | 0.49c | 4.46a | 0.37 | <0.001 |
| GCA-900066575 (%) | 0.76 | 3.55 | 1.45 | 0.52 | 0.053 |
| Alistipes (%) | 1.73 | 2.02 | 1.91 | 0.38 | 0.957 |
| Butyricicoccus (%) | 3.34a | 1.95a,b | 0.31b | 0.45 | 0.011 |
| Ruminiclostridium (%) | 1.98 | 1.62 | 1.82 | 0.34 | 0.924 |
| Rhodospirillales_norank (%) | 1.24 | 0.59 | 2.83 | 0.62 | 0.302 |
| Shuttleworthia (%) | 0.22b | 0.96b | 2.99a | 0.38 | 0.002 |
| Escherichia-Shigella (%) | 1.71 | 1.16 | 0.41 | 0.34 | 0.304 |
| Bilophila (%) | 1.60 | 0.37 | 1.18 | 0.31 | 0.270 |
| Parasutterella (%) | 1.55 | 0.43 | 0.47 | 0.26 | 0.087 |
| Sellimonas (%) | 0.14b | 0.18b | 2.20a | 0.33 | 0.006 |
| Mollicutes RF39_norank (%) | 0.18b | 0.20b | 1.07a | 0.13 | <0.001 |
a-cDifferent letters in the mean value of the same row indicate a significant difference (P < 0.05). Data are represented as the means with pooled SEM (n = 6).
Control, broilers were fed a basal diet and injected with saline; CPM, CPM-injected broilers were fed a basal diet; IAPS, CPM-injected broilers were fed a basal diet containing 900 mg/kg IAPS.
The Concentrations of SCFA in Cecal Digesta
The concentrations of cecal SCFA are presented in Table 5. No significant statistic differences on the concentrations of cecal acetate, propionate, and butyrate were observed between the CPM and control groups (P > 0.05). Feeding diets containing IAPS significantly increased butyrate concentration (P < 0.05) and tended to elevate acetate concentration (P = 0.052) in cecal digesta of CPM-injected broilers in comparison with those in the control and CPM treatments.
Table 5.
Effect of 60Co γ-ray irradiated Astragalus polysaccharides (IAPS) on the concentrations of cecal short-chain fatty acids of cyclophosphamide (CPM) immunosuppressed broilers.
| Items | Treatments1 |
SEM | P-value | ||
|---|---|---|---|---|---|
| Control | CPM | IAPS | |||
| Acetate (μmol/g) | 34.59 | 31.78 | 50.64 | 3.52 | 0.052 |
| Propionate (μmol/g) | 2.08 | 2.69 | 4.14 | 0.65 | 0.128 |
| Butyrate (μmol/g) | 2.88a,b | 2.45b | 4.36a | 0.31 | 0.019 |
a,bDifferent letters in the mean value of the same row indicate a significant difference (P < 0.05). Data are represented as the means with pooled SEM (n = 6).
Control, broilers were fed a basal diet and injected with saline; CPM, CPM-injected broilers were fed a basal diet; IAPS, CPM-injected broilers were fed a basal diet containing 900 mg/kg IAPS.
Discussion
Previous evidences indicated CPM-induced immunosuppression resulted in damaged immune system, altered gut microbiota, and even reduced growth performance of broilers (Yang et al., 2011; Li et al., 2019a). Herein, we found a decrease of growth performance, as characterized by the decreased ADG and ADFI in birds of CPM group compared with the control group, whereas dietary supplementation with 900 mg/kg IAPS improved ADG of CPM-treated broilers, indicating that IAPS had growth-promoting effect in broilers under immunosuppressive status.
The gastrointestinal microbiota exists in a symbiotic relationship with its host through the regulation of digestion, intestine development, nutrients absorption, as well as the innate and adaptive immune system (Mahmood and Guo, 2020). In reverse, the composition of microbiota is chiefly affected by health status of host and indigestible feed ingredients. Previously, immunosuppressive models were respectively established on chickens and mice via CPM injection, both of which exhibited poorer richness of intestinal bacteria (Yang et al., 2011; Huo et al., 2020). Whereas, no significant differences in richness and abundance between control and CPM groups were observed in the current study. We thus speculated CPM injection–induced microbes perturbation is very limited, resulting in a recover to the initial state instantly. By feeding diets supplemented with IAPS, the numbers of OTUs were amplified compared with the control and CPM group, suggesting that the bacterial communities were enriched and tend to be diversification by degrading IAPS. It was reported that pectic heteropolysaccharides of Camellia sinensis L. increased the indexes of Chao and Shannon of fecal microbiota in CPM treated mice, indicating that prebiotics could improve the richness and evenness of microorganisms under immunocompromised condition (Chen et al., 2019). Similarly, it was noted that broilers in the IAPS group had higher diversity of microbial communities than those both in the control and CPM groups. This is generally thought to be desirable for ecological stability. Species-rich communities contain high functional response diversity, allowing functionally similar species to fill the niche when compromised by an environmental disturbance (Lozupone et al., 2012). In this regard, functional redundancy in the diverse microbial communities can confer resilience through a wide repertoire of responses to chaotical fluctuation or biodiversity loss because of blooms of subpopulations, consequently avoiding pathogen infection and transmission (Bäckhed et al., 2005; Keesing et al., 2010). Besides, the abundant species are less susceptible to overpopulation and invasion caused by eutrophication because there are limited dietary nutrients for different species specialized to each potential resources that would be used in a more efficient way (Lozupone et al., 2012).
Previously, 915 species-equivalent OTU0.03 were detected in the chicken sequence collections, where Firmicutes, Bacteroidetes, and Proteobacteria represented 713, 172, and 157 OTU0.03, respectively (Wei et al., 2013). In general terms, the ratio of Firmicutes to Bacteroidetes is of significant relevance in the gut microbiota composition as well as the capacity for energy harvest by host (Kasai et al., 2015). Study on broiler chickens drew the results that Firmicutes and Bacteroidetes accounted for 71.36% and 23.40% in fat chickens and 53.44% and 41.09% in lean chickens, respectively (Hou et al., 2016). In this experiment, IAPS tended to decrease the relative abundance of cecal Bacteroidetes of broilers, which was in line with their increased body weight gain compared with other groups. It is documented that Bacteroidetes are involved in many important metabolic activities, including fermentation of carbohydrates, induction of critical glycolytic enzymes in the enterocytes, utilization of nitrogenous substances, biotransformation of bile acids, and prevention of pathogen colonization (Rubio et al., 2015). However, some members of this phylum have strong pathogenic behaviors through producing polymer-degrading enzymes that recorded as virulence factors targeting host cellular components, contributing to the destroyed extracellular matrix of animals (Thomas et al., 2011). It was reported that birds fed with Achyranthes bidentata polysaccharides had a reduced level of cecal Bacteroidetes and an enhanced immune systematic activity (Liu et al., 2018). This revealed the prerequisite process for these undigested macropolymers exerting immunomodulatory functions are participating in microbial fermentation and improving the structure of intestinal microflora.
The pattern of a strengthened biodiverse microecosystem influencing host well-being is depended on the functional roles played by individual species and those who are amplified or lost from intestinal communities (Keesing et al., 2010). The phylum Firmicutes, genus Faecalibacterium is generally considered as one of the most important bacteria that had great benefits for host by producing formate, D-lactate, and butyrate through glucose fermentation, therefore providing energy for colonic epithelium cells, inhibiting inflammation, and modulating oxidative stress (Zhou et al., 2018). It is noteworthy that Faecalibacterium showed limited ability to utilize polysaccharides including arabinogalactan, xylan, and soluble starch and was adapted to be cross-fed by other members of the intestinal microflora (Ferreira-Halder et al., 2017). Consequently, the number of Faecalibacterium is susceptible to the modulated microflora that inhibits its growth by producing adverse substances when exposed to polysaccharides (Thompson-Chagoyán et al., 2007). This partially elucidated the decreased relative abundance of Faecalibacterium in broilers fed IAPS found in this study. Furthermore, Faecalibacterium showed less response to reduced dietary carbohydrate and was in weak relationship with fecal butyrate concentration, illustrating the contribution of this bacteria genus to fecal butyrate formation was small (Duncan et al., 2007). Bacteroides, belonging to phylum Bacteroidetes, is a Gram-negative, nonspore forming, obligate anaerobic bacteria which represents one of the predominant anaerobic genera in the chicken cecum (Houston et al., 2010). It is known for its fermentative end products in form of different types of SCFA and glycans that aid the mutualism by utilizing complex polysaccharides (Comstock, 2009). While some species of Bacteroides genus harbor particular pathogenic potential in facilitating epithelial penetration of intestinal bacteria and inducing diarrhea through producing enterotoxin to the lateral surface of enterocyte (Wells et al., 1996). Guo et al. (2004) observed that feeding diets supplemented with different polysaccharide extracts reduced the number of harmful Bacteroides spp. in Apramycin-treated broiler chickens. Likewise, we found that birds consuming IAPS had a lower abundance of Bacteroides compared with the CPM group. It can be inferred the effects of prebiotics on particular beneficial regulations are made at the expense of some intestinal bacteria, for example, Bacteroides (Macfarlane et al., 2006).
Genus Ruminococcaceae UCG-014 from phylum Firmicutes is recognized as a SCFA producer that autochthonously inhabits in the cecum and colon (Donaldson et al., 2016; Tian et al., 2019). Generally, members of Ruminococcaceae are equipped with endo-1,4-beta-xylanase and cellulase genes that are responsible for the degradation of diverse cellulose and hemicellulose components of plant material (Biddle et al., 2013). It has been reported that the growth of Ruminococcaceae is amplified by degrading fucoidan from Laminaria japonica, which subsequently modify the microbial structure of mice (Shang et al., 2016). Genus Negativibacillus in phylum Firmicutes is a kind of bacillus with a Gram-negative cell wall structure (Ricaboni et al., 2016). Zhao et al. (2019) figured out that Negativibacillus performed in line with Ruminococcaceae UCG-014, both of which were enriched in a growing abundance microecological environment. Similar results were obtained in the present study after feeding broilers with IAPS, where there were higher diversity of microbial communities accompanied with increased relative abundance of Ruminococcaceae and Negativibacillus. Some previous studies found that the abundance of Ruminococcaceae and Negativibacillus had positive correlation with body weight gain of male rats and obese mice, respectively (Yan et al., 2017; Wang et al., 2019). Similarly, birds from IAPS group showed a higher ADG in this study. This might ascribe to the butyrate secreting property of Ruminococcaceae UCG-014 which contributed to provide energy for enterocytes and improve gut health by influencing intestinal barrier function and immune system (Fukui, 2017; Louis and Flint, 2017).
Phylum Firmicutes, genus Shuttleworthia, is recognized as saccharolytic bacteria that produce acetate, butyrate, and lactate as end-products of glucose fermentation (Downes et al., 2002). Our current results demonstrated that feeding IAPS increased the body weight gain of birds and the relative abundance of Shuttleworthia in the cecum. In line with this, González-Recio et al. (2018) pointed out that Shuttleworthia had a significant Spearman correlation with feed efficiency value. It is speculated that Shuttleworthia could participate in carbohydrate and lipid metabolism and regulate endocrine system via secreting SCFA, which potentially contributes to the weight gain of the host (Lee et al., 2017). Genus Sellimonas, belonging to phylum Firmicutes, is a gram positive and obligately anaerobic bacterial genus that involved in host nutrient metabolism. In this experiment, the relative abundance of Sellimonas was elevated in IAPS treatment. This was similar to the result found by Zhang et al. (2020) who exhibited that diets containing 8% resistant starch increased the relative abundance of Sellimonas. It is presumably the highly frequent genes directed by Sellimonas that coded metabolisms including amino acid and carbohydrate transport as well as energy production and conversion, conducing to better growth performance of broilers (Muñoz et al., 2020).
The SCFA are predominantly the by-product of the intestinal microbial fermentation of nondigestible carbohydrates, basically composed of acetate, propionate, and butyrate, which differ in their targeting tissue distribution and microbial origin that selectively affected by dietary composition and gut physiology (Louis and Flint, 2017). Acetogens, like enteric bacteria, are obligately anaerobic bacteria that use reductive acetyl-CoA and via Wood-Ljungdahl pathway as the main mechanism for energy conservation and acetate synthesis (Ragsdale and Pierce, 2008). Metagenome prediction analysis of the bioreactor suggests microorganisms in the Ruminococcaceae family were mainly involved in acetate production and consumption and were showed being positively associated with plasma acetate levels and subcutaneous adipose tissue in obese subjects (Esquivel-Elizondo et al., 2017; Moreno-Navarrete et al., 2018). Acetate could drain into liver through portal vein where it is oxidized in the tricarboxylic acid cycle or used as a substrate for synthesis of cholesterol, ketone bodies, and long-chain fatty acids (den Besten et al., 2013). Whereas butyrate is the preferred energy source for colonocytes and is locally consumed (Bedford and Gong, 2018). The biological functions of butyrate contain activating intestinal gluconeogenesis, inhibiting inflammation and carcinogenesis, reinforcing various components of the colonic defence barrier and decreasing oxidative stress (Hamer et al., 2008; De Vadder et al., 2014). Ruminococcaceae UCG-014 and Shuttleworthia that express butyryl-CoA:acetate CoA-transferase and butyrate kinase contribute to butyrate formation (Louis and Flint, 2017; O'Hara et al., 2018). Moreover, the relative abundance of Ruminococcaceae in CPM-treated mice could be enhanced by administration of polysaccharides from purple sweet potato (Tang et al., 2017). In this trial, broilers injected with CPM presented higher concentrations of acetate and butyrate after feeding IAPS. Similar results exhibited that the concentrations of SCFA in immunosuppressive mice were inversely regulated by dietary polysaccharides extracted from C. sinensis L. and fruits of Lycium barbarum (Chen et al., 2019; Ding et al., 2019). This indicated that SCFA could be promoted by specific beneficial bacteria via fermenting polysaccharides and further improve the host immunity and metabolism.
Conclusion
In summary, intramuscularly injection with CPM resulted in poorer growth performance of broilers but did not affect the overall microbial richness and diversity, as well as the SCFA concentrations in cecal digesta. In contrast, dietary supplementation with 900 mg/kg IAPS increased the microbial diversity, enriched the proportion of beneficial bacteria in the host, and elevated SCFA production in cecum of broilers, contributing to the subsequent improvement in growth performance. These findings may expand our knowledge about the function of modified Astragalus polysaccharides in broiler chickens.
Acknowledgments
This work was financed by the National Key Research and Development Program of China (2017YFD0500505), the National Natural Science Foundation of China (31601957), the Earmarked Fund for Jiangsu Agricultural Industry Technology System (JATS[2020]407), and the Jiangsu Overseas Visiting Scholar Program for University Prominent Young & Middle-aged Teachers and Presidents (2018).
Disclosures
The authors declare that there are no conflicts of interest.
References
- Bäckhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Bedford A., Gong J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018;4:151–159. doi: 10.1016/j.aninu.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddle A., Stewart L., Blanchard J., Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. [Google Scholar]
- Chen D., Chen G., Ding Y., Wan P., Peng Y., Chen C., Ye H., Zeng X., Ran L. Polysaccharides from the flowers of tea (Camellia sinensis L.) modulate gut health and ameliorate cyclophosphamide-induced immunosuppression. J. Funct. Foods. 2019;61:103470. [Google Scholar]
- Choi J.I., Kim H.J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohydr. Polym. 2013;97:358–362. doi: 10.1016/j.carbpol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Cisek A.A., Binek M. Chicken intestinal microbiota function with a special emphasis on the role of probiotic bacteria. Pol. J. Vet. Sci. 2014;17:385–394. doi: 10.2478/pjvs-2014-0057. [DOI] [PubMed] [Google Scholar]
- Comstock L.E. Importance of glycans to the host-bacteroides mutualism in the mammalian intestine. Cell Host Microbe. 2009;5:522–526. doi: 10.1016/j.chom.2009.05.010. [DOI] [PubMed] [Google Scholar]
- De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell. 2014;156:84–96. doi: 10.1016/j.cell.2013.12.016. [DOI] [PubMed] [Google Scholar]
- den Besten G., Lange K., Havinga R., van Dijk T.H., Gerding A., van Eunen K., Müller M., Groen A.K., Hooiveld G.J., Bakker B.M., Reijngoud D.J. Gut-derived short-chain fatty acids are vividly assimilated into host carbohydrates and lipids. Am. J. Physiol. Gastrointest. Liver Physiol. 2013;305:G900–G910. doi: 10.1152/ajpgi.00265.2013. [DOI] [PubMed] [Google Scholar]
- Ding Y., Yan Y., Chen D., Ran L., Mi J., Lu L., Jing B., Li X., Zeng X., Cao Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019;10:3671–3683. doi: 10.1039/c9fo00638a. [DOI] [PubMed] [Google Scholar]
- Donaldson G.P., Lee S.M., Mazmanian S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016;14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes J., Munson M.A., Radford D.R., Spratt D.A., Wade W.G. Shuttleworthia satelles gen. nov., sp. nov., isolated from the human oral cavity. Int. J. Syst. Evol. Microbiol. 2002;52:1469–1475. doi: 10.1099/00207713-52-5-1469. [DOI] [PubMed] [Google Scholar]
- Duncan S.H., Belenguer A., Holtrop G., Johnstone A.M., Flint H.J., Lobley G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007;73:1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- Esquivel-Elizondo S., Ilhan Z.E., Garcia-Peña E.I., Krajmalnik-Brown R. Insights into butyrate production in a controlled fermentation system via gene predictions. mSystems. 2017;2 doi: 10.1128/mSystems.00051-17. e00051-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Halder C.V., Faria A.V.S., Andrade S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best. Pract. Res. Clin. Gastroenterol. 2017;31:643–648. doi: 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Fukui H. Gut microbiome-based therapeutics in liver cirrhosis: basic consideration for the next step. J. Clin. Transl. Hepatol. 2017;5:249–260. doi: 10.14218/JCTH.2017.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Recio O., Guasch I., Bach A. Challenges and limitations for improving feed efficiency from metagenomics data. Proc. World Congr. Genet. Appl. Livest. Prod. 2018;1:93. [Google Scholar]
- Guo F.C., Williams B.A., Kwakkel R.P., Li H.S., Li X.P., Luo J.Y., Li W.K., Verstegen M.W. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on the cecal microbial ecosystem in broiler chickens. Poult. Sci. 2004;83:175–182. doi: 10.1093/ps/83.2.175. [DOI] [PubMed] [Google Scholar]
- Hamer H.M., Jonkers D., Venema K., Vanhoutvin S., Troost F.J., Brummer R.J. Review article: the role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Hoerr F.J. Clinical aspects of immunosuppression in poultry. Avian Dis. 2010;54:2–15. doi: 10.1637/8909-043009-Review.1. [DOI] [PubMed] [Google Scholar]
- Hou Q., Kwok L.Y., Zheng Y., Wang L., Guo Z., Zhang J., Huang W., Wang Y., Leng L., Li H., Zhang H. Differential fecal microbiota are retained in broiler chicken lines divergently selected for fatness traits. Sci. Rep. 2016;6:37376. doi: 10.1038/srep37376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston S., Blakely G.W., McDowell A., Martin L., Patrick S. Binding and degradation of fibrinogen by Bacteroides fragilis and characterization of a 54 kDa fibrinogen-binding protein. Microbiology. 2010;156:2516–2526. doi: 10.1099/mic.0.038588-0. [DOI] [PubMed] [Google Scholar]
- Huo W.Y., Feng Z.P., Hu S.Y., Cui L.J., Qiao T., Dai L., Qi P., Zhang L.G., Liu Y., Li J.Z. Effects of polysaccharides from wild morels on immune response and gut microbiota composition in non-treated and cyclophosphamide-treated mice. Food Funct. 2020;11:4291–4303. doi: 10.1039/d0fo00597e. [DOI] [PubMed] [Google Scholar]
- Kallon S., Li X., Ji J., Chen C., Xi Q., Chang S., Xue C., Ma J., Xie Q., Zhang Y. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. J. Anim. Sci. Biotechnol. 2013;4:22. doi: 10.1186/2049-1891-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai C., Sugimoto K., Moritani I., Tanaka J., Oya Y., Inoue H., Tameda M., Shiraki K., Ito M., Takei Y., Takase K. Comparison of the gut microbiota composition between obese and non-obese individuals in a Japanese population, as analyzed by terminal restriction fragment length polymorphism and next-generation sequencing. BMC Gastroenterol. 2015;15:100. doi: 10.1186/s12876-015-0330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesing F., Belden L.K., Daszak P., Dobson A., Harvell C.D., Holt R.D., Hudson P., Jolles A., Jones K.E., Mitchell C.E., Myers S.S., Bogich T., Ostfeld R.S. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–652. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.C., Kil D.Y., Sul W.J. Cecal microbiome divergence of broiler chickens by sex and body weight. J. Microbiol. 2017;55:939–945. doi: 10.1007/s12275-017-7202-0. [DOI] [PubMed] [Google Scholar]
- Li S., Ren L., Zhu X., Li J., Zhang L., Wang X., Gao F., Zhou G. Immunomodulatory effect of γ-irradiated Astragalus polysaccharides on immunosuppressed broilers. Anim. Sci. J. 2019;90:117–127. doi: 10.1111/asj.13133. [DOI] [PubMed] [Google Scholar]
- Li S., Wang X.F., Ren L.N., Li J.L., Zhu X.D., Xing T., Zhang L., Gao F., Zhou G.H. Protective effects of γ-irradiated Astragalus polysaccharides on intestinal development and mucosal immune function of immunosuppressed broilers. Poult. Sci. 2019;98:6400–6410. doi: 10.3382/ps/pez478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Wang X., Ou S., Arowolo M.A., Hou D.X., He J. Effects of Achyranthes bidentata polysaccharides on intestinal morphology, immune response, and gut microbiome in yellow broiler chickens challenged with Escherichia coli K88. Polymers (Basel) 2018;10:E1233. doi: 10.3390/polym10111233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis P., Flint H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017;19:29–41. doi: 10.1111/1462-2920.13589. [DOI] [PubMed] [Google Scholar]
- Lozupone C.A., Stombaugh J.I., Gordon J.I., Jansson J.K., Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane S., Macfarlane G.T., Cummings J.H. Review article: prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2006;24:701–714. doi: 10.1111/j.1365-2036.2006.03042.x. [DOI] [PubMed] [Google Scholar]
- Magoč T., Salzberg S.L. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinform. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood T., Guo Y. Dietary fiber and chicken microbiome interaction: where will it lead to? Anim. Nutr. 2020;6:1–8. doi: 10.1016/j.aninu.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Navarrete J.M., Serino M., Blasco-Baque V., Azalbert V., Barton R.H., Cardellini M., Latorre J., Ortega F., Sabater-Masdeu M., Burcelin R., Dumas M.E., Ricart W., Federici M., Fernández-Real J.M. Gut microbiota interacts with markers of adipose tissue browning, insulin action and plasma acetate in morbid obesity. Mol. Nutr. Food Res. 2018;62:1700721. doi: 10.1002/mnfr.201700721. [DOI] [PubMed] [Google Scholar]
- Muñoz M., Guerrero-Araya E., Cortés-Tapia C., Plaza-Garrido Á., Lawley T.D., Paredes-Sabja D. bioRxiv; 2020. Comprehensive Genome Analyses of Sellimonas intestinalis, a Potential Biomarker of Homeostasis Gut Recovery. Accessed Apr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. 9th rev. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- O'Hara E., Kelly A., McCabe M.S., Kenny D.A., Guan L.L., Waters S.M. Effect of a butyrate-fortified milk replacer on gastrointestinal microbiota and products of fermentation in artificially reared dairy calves at weaning. Sci. Rep. 2018;8:14901. doi: 10.1038/s41598-018-33122-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale S.W., Pierce E. Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation. Biochim. Biophys. Acta. 2008;1784:1873–1898. doi: 10.1016/j.bbapap.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Wang X., Li S., Li J., Zhu X., Zhang L., Gao F., Zhou G. Effect of gamma irradiation on structure, physicochemical and immunomodulatory properties of Astragalus polysaccharides. Int. J. Biol. Macromol. 2018;120:641–649. doi: 10.1016/j.ijbiomac.2018.08.138. [DOI] [PubMed] [Google Scholar]
- Ricaboni D., Mailhe M., Vitton V., Andrieu C., Fournier P.E., Raoult D. “Negativibacillus massiliensis” gen. nov., sp. nov., isolated from human left colon. New Microbes New Infect. 2016;17:36–38. doi: 10.1016/j.nmni.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio L.A., Peinado M.J., Ruiz R., Suárez-Pereira E., Ortiz Mellet C., García Fernández J.M. Correlations between changes in intestinal microbiota composition and performance parameters in broiler chickens. J. Anim. Physiol. Anim. Nutr. (Berl) 2015;99:418–423. doi: 10.1111/jpn.12256. [DOI] [PubMed] [Google Scholar]
- Satokari R.M., Vaughan E.E., Akkermans A.D., Saarela M., de Vos W.M. Bifidobacterial diversity inhumanfeces detected by genus-specific PCR and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2001;67:504–513. doi: 10.1128/AEM.67.2.504-513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: opensource, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I., Russell S.L., Antunes L.C., Finlay B.B. Gut microbiota in health and disease. Physiol. Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Shang Q., Shan X., Cai C., Hao J., Li G., Yu G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016;7:3224–3232. doi: 10.1039/c6fo00309e. [DOI] [PubMed] [Google Scholar]
- Tang C., Sun J., Zhou B., Jin C., Liu J., Kan J., Qian C., Zhang N. Effects of polysaccharides from purple sweet potato on immune response and gut microbiota composition in normal and cyclophosphamide treated mice. Food Funct. 2017;9:937–950. doi: 10.1039/c7fo01302g. [DOI] [PubMed] [Google Scholar]
- Thomas F., Hehemann J.H., Rebuffet E., Czjzek M., Michel G. Environmental and gut bacteroidetes: the food connection. Front. Microbiol. 2011;2:93. doi: 10.3389/fmicb.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Chagoyán O.C., Maldonado J., Gil A. Colonization and impact of disease and other factors on intestinal microbiota. Dig. Dis. Sci. 2007;52:2069–2077. doi: 10.1007/s10620-006-9285-z. [DOI] [PubMed] [Google Scholar]
- Tian B.M., Zhao J.H., An W., Zhang J.W., Cao X., Mi J.L., Zhao J.S., Zhang Y.X., Li J.X. Lycium ruthenicum diet alters the gut microbiota and partially enhances gut barrier function in male C57BL/6 mice. J. Funct. Foods. 2019;52:516–528. [Google Scholar]
- Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Pan Y., Wang L., Zhou H., Song G., Wang Y., Liu J., Li A. Optimal dietary ferulic acid for suppressing the obesity-related disorders in leptin-deficient obese C57BL/6J-ob/ob mice. J. Agric. Food Chem. 2019;67:4250–4258. doi: 10.1021/acs.jafc.8b06760. [DOI] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Wells C.L., van de Westerlo E.M., Jechorek R.P., Feltis B.A., Wilkins T.D., Erlandsen S.L. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterol. 1996;110:1429–1437. doi: 10.1053/gast.1996.v110.pm8613048. [DOI] [PubMed] [Google Scholar]
- Xiao Y., Xiang Y., Zhou W., Chen J., Li K., Yang H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017;96:1387–1393. doi: 10.3382/ps/pew372. [DOI] [PubMed] [Google Scholar]
- Yan H., Lu J., Wang Y., Gu W., Yang X., Yu J. Intake of total saponins and polysaccharides from Polygonatum kingianum affects the gut microbiota in diabetic rats. Phytomedicine. 2017;26:45–54. doi: 10.1016/j.phymed.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Yang X.J., Li W.L., Feng Y., Yao J.H. Effects of immune stress on growth performance, immunity, and cecal microflora in chickens. Poult. Sci. 2011;90:2740–2746. doi: 10.3382/ps.2011-01591. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu Y., Li J., Xing T., Jiang Y., Zhang L., Gao F. Dietary resistant starch modifies the composition and function of caecal microbiota of broilers. J. Sci. Food Agric. 2020;100:1274–1284. doi: 10.1002/jsfa.10139. [DOI] [PubMed] [Google Scholar]
- Zhang Y.J., Liu Q., Zhang W.M., Zhang Z.J., Wang W.L., Zhuang S. Gastrointestinal microbial diversity and short-chain fatty acid production in pigs fed different fibrous diets with or without cell wall-degrading enzyme supplementation. Livest. Sci. 2018;207:105–116. [Google Scholar]
- Zhang T., Yang Y., Liang Y., Jiao X., Zhao C. Beneficial effect of intestinal fermentation of natural polysaccharides. Nutrients. 2018;10:1055. doi: 10.3390/nu10081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li K., Luo H., Duan L., Wei C., Wang M., Jin J., Liu S., Mehmood K., Shahzad M. Comparison of the intestinal microbial community in ducks reared differently through high-throughput sequencing. Biomed. Res. Int. 2019;2019:9015054. doi: 10.1155/2019/9015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Yan Y., Mi J., Zhang H., Lu L., Luo Q., Li X., Zeng X., Cao Y. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of Chinese wolfberry. J. Agric. Food Chem. 2018;66:898–907. doi: 10.1021/acs.jafc.7b05546. [DOI] [PubMed] [Google Scholar]