Abstract
Muscovy duck reovirus (MDRV) infection induces serious immunosuppression and intestinal injury in Muscovy ducklings with a high morbidity and mortality, and Astragalus polysaccharide (APS) pretreatment could efficiently protect ducklings from MDRV infection, although the underlying immunoregulatory mechanisms remain unclear. Thus, the objective of this study was to investigate effects of APS on the intestinal mucosal immunity in MDRV-infected Muscovy ducklings. A total of 190 1-day-old healthy Muscovy ducklings were randomly assigned to 3 groups (n = 50): normal control group, APS pretreatment for MDRV-infected group, and cohabitation infection group, then pretreated with 0.6 g/L APS or only drinking water followed by MDRV cohabitation infection with the remaining 40 artificially infected ducklings, respectively. At the 2, 3, 4, 6, 9 and 15 d after cohabitation infection, the intestinal samples were prepared to measure intestinal parameters including villus length, villus length/crypt depth (V/C) ratio, and wall thickness, together with counts of intraepithelial lymphocyte (IEL) and goblet cell (GC) by hematoxylin–eosin staining. Meanwhile, ileal secretory IgA (sIgA) and duodenal cytokine levels of IL-4, IL-6, IL-15, tumor necrosis factor-alpha, and interferon gamma were detected by the ELISA and radioimmunoassay, respectively. The results showed that APS significantly improved intestinal injuries of villi length, V/C ratio, and wall thickness of the small intestine infected with MDRV, effectively inhibited the reduction of IEL and GC caused by MDRV infection, subsequently increased sIgA and all the cytokine secretions at most time points, suggesting that APS pretreatment can effectively stimulate mucosal immune function by improving intestinal morphology and repair MDRV caused injures of small intestinal mucosal immune barrier in infected ducklings. Our findings lay the foundation for further application of APS in prevention and treatment of MDRV infection.
Key words: Muscovy duck reovirus, duckling, Astragalus polysaccharide
Introduction
Muscovy duck reovirus (MDRV), a member of the genus Orthoreovirus in the family Reoviridae, was isolated from sick ducks in France in 1972 (Gaudry et al., 1972; Malkinson et al., 1981). Muscovy duck reovirus infection has been reported in Muscovy ducklings in China since 1997, and the etiologic agent was isolated and first defined as MDRV by our group (Fujian Key Laboratory of Traditional Chinese Veterinary Medicine and Animal Health) in 2001 (Wu et al., 2001). Muscovy duck reovirus infection is characterized mainly by white necrotic foci in the liver and spleen of the sick ducklings, which caused severe damages to central immune organs, peripheral immune organs, and associated cellular and humoral immunoresponses, leading to immunosuppression (Marius-Jestin et al., 1988; Heffels-Redmann et al., 1992; Wu et al., 2001; Palya et al., 2003). As a kind of immunosuppressive disease with multimolecular structure in viral nucleic acid which is easier to genetic recombination, MDRV infection has led to severe economic loss in the Muscovy duck industry owing to the heavy morbidity and mortality (Liu et al., 2003; Yun et al., 2018).
Chinese herbal polysaccharide have been used as immunostimulants in veterinary and medical clinical fields owing to various biological activities such as immune modulatory and tumor inhibition, natural origin active, nonresidue, and low side (Jiang et al., 2010; Yu et al., 2018; Yin et al., 2019). Astragalus polysaccharide (APS) is the major active component of water-soluble heteropolysaccharide extracted from the stems or dried roots of traditional Chinese medicine Astragalus membranaceus, and the main components include heteropolysaccharide, dextran, neutral polysaccharide, and acidic polysaccharide (Zheng et al., 2020). It has been shown that there were 13 types of polysaccharides have β-D(1→6)-galactooligosaccharide branching β-D-(1→3)-galactose among the 14 types of polysaccharides isolated from Astragalus (Kiyohara et al., 2010). Astragalus polysaccharide is the most important natural active component in Astragalus membranaceus, and exerts multiple pharmacologic effects (Li et al., 2019). Recent studies have found that APS exerts multiple pharmacologic effects and strong extensive range of biological activities, including antioxidant, anti-inflammatory, antibacterial and antiviral, as well as profoundly affect the immune system (Wang et al., 2016; Meng et al., 2017). Previous study has shown that intestinal morphologic structure of poultry was severely damaged by MDRV infection, resulting in impaired intestinal mucosal barrier function of playing an important role in the immune system (Wu et al., 2018; Wu et al., 2019). As per new research, APS could enhance intestinal mucosal immune function (Shan et al., 2019) and enhance the immune response to several vaccines against viruses (Huang et al., 2008; Zhang et al., 2017). The preliminary work of our laboratory showed that APS pretreatment by drinking administration could efficiently protect Muscovy ducklings from MDRV infection, including alleviating clinical symptoms, reducing morbidity and mortality, and promoting recovery of sick Muscovy ducklings (Jiang et al., 2015). However, very few studies have evaluated the effects of APS on the intestinal mucosal immune of MDRV-infected ducks. Therefore, the present study aim was to investigate the effects of APS intake by drinking water on intestinal morphology, immunocyte number, secretory IgA (sIgA), and cytokine levels in intestinal tissues of MDRV-infected Muscovy ducklings.
Materials and methods
Astragalus Polysaccharide and Virus
Astragalus polysaccharide was purchased from Shengtai Company (Beijing, China), 70% content. Muscovy duck reovirus YB strains was isolated, identified, and preserved in our laboratory (median tissue culture infective dose [TCID50] = 10−7.56/0.1 mL, Reed and Muench method).
Ethics Statement
The animal protocols used in this work and all procedures of the experiment were performed in compliance with the laws and guidelines of the Fujian Agricultural and Forestry University Animal Care and Use Committee (Approval No: PZCASFAFU201604).
Animals and Experimental Design
A total of 190 1-day-old Muscovy ducklings with MDRV antigen–antibody–negative were selected and randomly allotted to 3 groups: normal control group (NCG), APS pretreatment for the MDRV-infected group (APG), and cohabitation infection group (CIG), with 50 ducklings in each group. The APG was pretreated with APS at dosage of 0.6 g/L in drinking water (Wu, 2010), whereas the other groups (NCG and CIG) were only supplemented with drinking water throughout the experimental trial. The remaining forty, 2-day-old healthy Muscovy ducklings were injected intramuscularly with 2,000 TCID50 of MDRV as an artificial challenge group, and 3 d later, half of them were selected to join fifty normal ducklings (CIG), with the other half joining fifty APS-pretreated ducklings (APG), at the ratio of 2:5 for cohabitation infection. Then, the following day was referred to as 1 d postinfection (dpi) (Wu et al., 2019). All the ducklings were provided with free food and water intake (ad libitum). Each group was raised in separate cages with the same conventional management condition.
Histology and Morphometric Analysis of the Small Intestine
At 2, 3, 4, 6, 9, and 15 dpi, 6 ducklings were randomly selected from each treatment group and euthanized by cervical dislocation. The mid-duodenum, jejunum, and ileum (approximately 1 cm) were taken immediately and rinsed, which were then fixed by immersion in 4% phosphate-buffered paraformaldehyde for 24 h. Then, the samples were washed with flowing water overnight. After washing, the samples were dehydration with a graded ethanol solution series and xylene and embedded in paraffin. A 5-μm section of each tissue was procured and stained with hematoxylin–eosin and then examined by light microscopy (TESA ETALON TCM100, Switzerland) under a magnification of 100×.
To measure morphometric parameters of intestinal structure, 5 sections with well oriented parts of each group were randomly selected for observation, and then, the villus length, crypt depth and intestinal wall thickness were measured and recorded from each section on randomly selected microscopic fields. Measurements were carried as follows: villus length was determined from the villous tip to the villous–crypt junction; crypt depth was measured from the opening to basing of crypt; wall thickness was measured from the outer of the intestine to the submucosa and muscular layer junction, involving the serosa layer and the muscular layer (Frankel et al., 1993).
Intraepithelial lymphocyte (IEL) and goblet cell (GC) in 5 randomly selected fields from each section were counted using a 400× magnification, and a total of 3 sections with well-oriented parts of each tissue sample were randomly counted. Measurements were carried as follows: the number of IEL per 100 columnar epithelial cells in the long intestinal villi of each section was counted and recorded. The number of GC per 100 columnar epithelial cells in 5 visual fields of each slice from 5 sections per ducking was counted and recorded. A total of 5 sections were recorded per duckling, and then took the average individually.
Determination of sIgA in Ileal Mucosa
The ileal mucosa (2.0 cm) was scraped and weighed, then was added to the prechilled PBS solution (pH 7.2) at a weight–volume ratio of 1:9 and mixed, followed by centrifuged at 10,000 rpm for 10 min at 4°C. Then, the supernatants were collected to detect the secretion of sIgA using specific ELISA kits (Jiancheng Bioengineering Institute, Nanjing, China) as per the manufacturer's instructions. Each sample was independently performed in triplicate.
Cytokines Levels in Duodenal Mucosa
The duodenal mucosa (5.0 cm) was scraped and weighed, then was add to the pre-chilled PBS solution (pH 7.2) with a weight–volume ratio of 1:2 and incubated at 4°C for 30 min. Subsequently, the samples were sonicated in an ultrasonic cleaner for 5 min followed by centrifuged at 8,000 rpm for 15 min at 4°C, and then, the supernatants were obtained. The concentrations of IL-4, IL-6, IL-15, tumor necrosis factor-alpha (TNF-α) and interferon gamma were measured using radioimmunoassay kits (Huaying Biotechnology Research Institute, Beijing, China). Each sample was independently performed in triplicate.
Statistical Analysis
All the statistical data were presented as the means ± SD, and statistical analysis was performed using a 1-way ANOVA with a Duncan's multiple-range model (software SPSS, version 17.0). The statistical significance was considered at P < 0.05.
Results
Changes in Intestinal Morphologic Structures of Ducklings
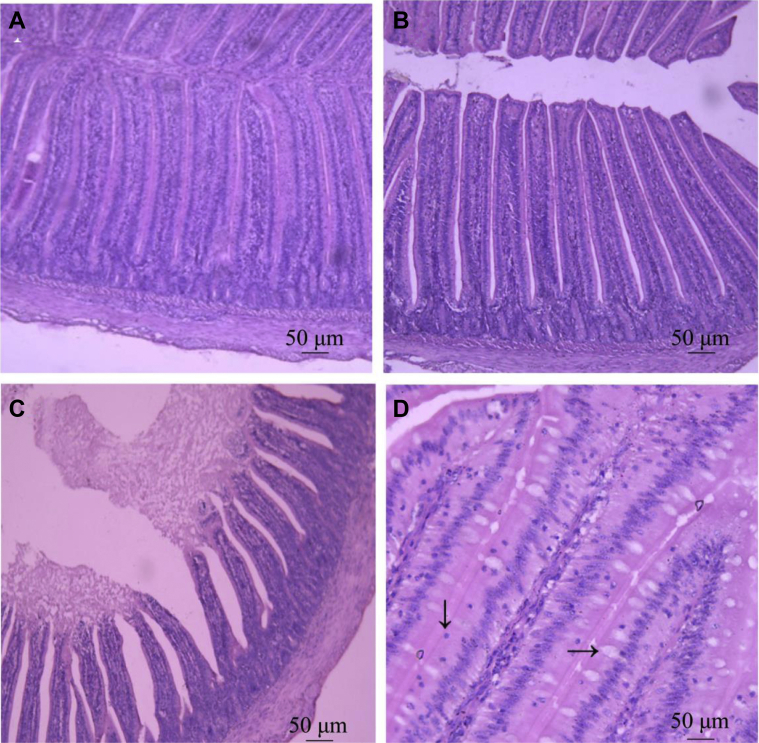
As shown in Figure 1, duodenal structure was normal, and the villi were intact and closely arranged in the NCG, but the villi exhibited incomplete structure, with loose and irregular arrangement, intestinal mucosa abscission, and villi atrophy in the CIG. The duodenal structure of APS-pretreated groups exhibited mild or no symptoms, remained normal with narrower villus space, longer and thicker villi in a closer and more orderly array than that in the CIG, signifying APS improve MDRV caused intestinal mucosal injuries.
Figure 1.
Effect of APS on duodenal morphologic structure in Muscovy ducklings. Duodenums from experimental groups at 15 dpi were sectioned and stained with HE, then examined by light microscopy (TESA ETALON TCM100, Switzerland) under a magnification of 100×. (A) Normal control group(NCG), 100×, (B) APS pretreatment for MDRV infected group (APG), (C) Cohabitation infection control group (CIG), (D) HE staining for observation of Intestinal intraepithelial lymphocyte (IEL, ↓) and goblet cells (GC, →), 400×. Abbreviations: APS, Astragalus polysaccharide; dpi, d postinfection; HE, hematoxylin–eosin stain.
Changes in Intestinal Morphologic Parameters of Ducklings
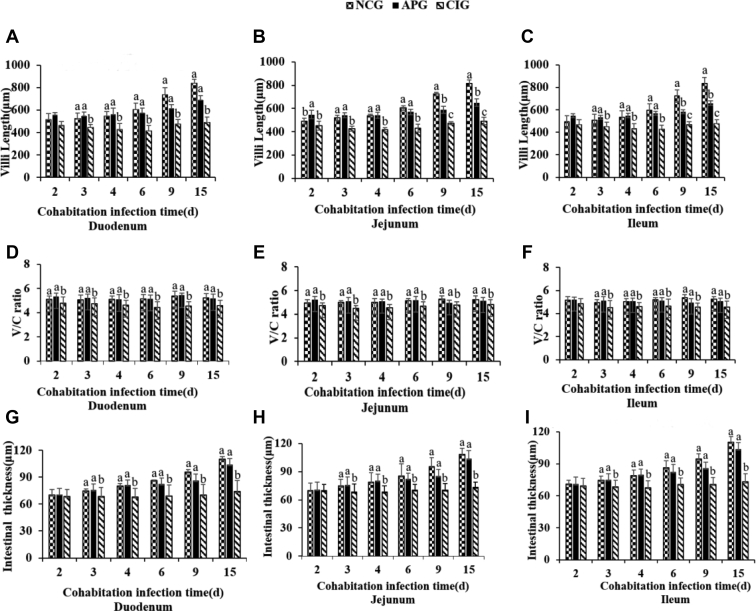
The morphologic parameters including villus length, V/C ratio, and the wall thickness of the small intestine are showed in Figure 2. Villus length of each small intestinal segment of ducklings in the APG and NCG gradually increased with age, whereas that in the CIG grew slowly or even atrophied. Villus length in the CIG decreased rapidly at 3 dpi or 4 dpi, and then stabilized at lower levels, which was significantly lower than those in the NCG and APG. In addition, compared with the NCG, ducklings of the CIG showed significant lower (P < 0.05) V/C ratio, especially at the intermediate stage of cohabitation infection. Importantly, no differences in villus length and V/C ratio of various intestine segments were observed between ducklings of the APG and NCG at most time points (P > 0.05). The wall thickness of 3 intestinal segments in the NCG and APG also increased with age, which were significantly higher than those in ducklings of the CIG after 3 dpi (P < 0.05). The results showed that APS may alleviate or antagonize the injuries of the small intestine in Muscovy ducklings infected with MDRV, by efficiently recovering villi growth, V/C ratio, and wall thickness.
Figure 2.
Effect of APS on intestinal villus length (A–C), V/C ratios (D–F), and wall thickness (G–I) in experimental Muscovy ducklings. Intestines from each experimental group (n = 6) were sectioned and stained with HE at 2, 3, 4, 6, 9, and 15 dpi, and then, 5 sections per group were selected for determination of villus length, V/C ratios, and wall thickness. Bars without the same superscripts (A–C) mean significant differences (P < 0.05). Abbreviations: APG, APS pretreatment for MDRV infected group pretreated with 0.6 g/L APS in drinking water; APS, Astragalus polysaccharide; CIG, cohabitation infection control group treated with drinking water and infected with 2,000 TCID50 of MDRV; dpi, d postinfection; HE, hematoxylin–eosin stain; NCG, normal control group supplemented with drinking water throughout the experimental trial; TCID50, median tissue culture infective dose.
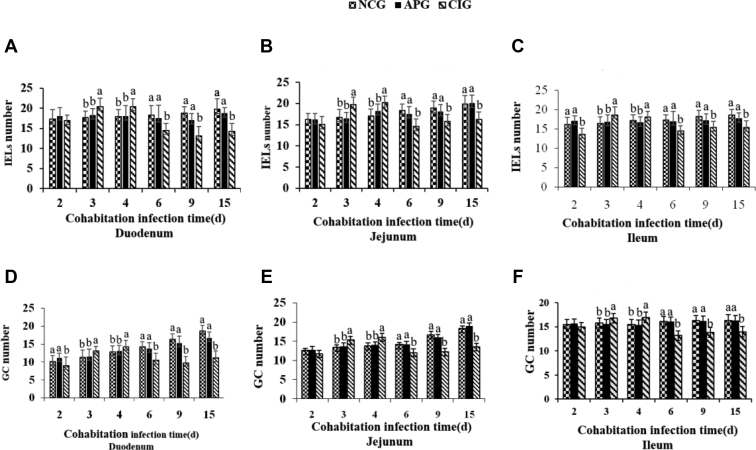
Data on immunocyte counts of ducklings, including IEL and GC, are shown in Figure 3. Compared with NCG ducklings, IEL and GC number in intestine segments of CIG ducklings peaked during 3 dpi to 4 dpi and decreased significantly afterward (P < 0.05). However, no differences in immunocyte number were observed between infected ducklings with APS treatment and the NCG ducklings during the trial (P > 0.05).
Figure 3.
Effect of APS on IEL number (A–C) and GC (D–F) number in experimental Muscovy ducklings. Sections stained with HE at 2, 3, 4, 6, 9 and 15 dpi were selected from each experimental group (n = 6) for intestinal immunocyte count, including IEL and GC. Bars without the same superscripts (a–c) mean significant differences (P < 0.05). Abbreviations: APG, APS pretreatment for the MDRV-infected group; APS, Astragalus polysaccharide; CIG, cohabitation infection group; GC, goblet cell; IEL, intraepithelial lymphocyte; NCG, normal control group.
Changes in Intestinal sIgA and Cytokine of Ducklings
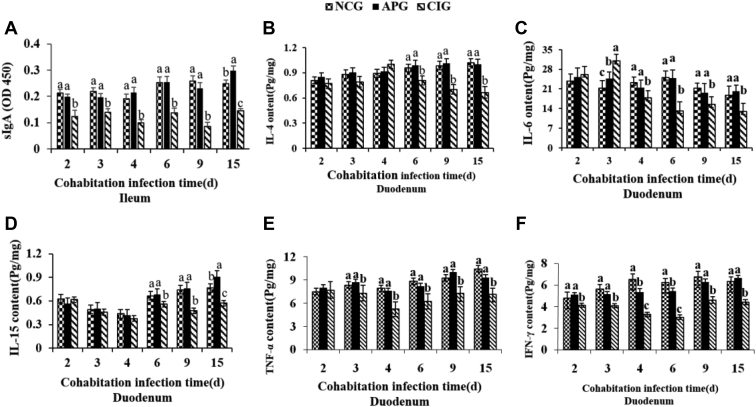
The dynamics of intestinal sIgA and cytokine concentrations in the ducklings of each group are showed in Figure 4. As shown in Figure 4A, ileal sIgA concentrations of the CIG reduced at all postinfection time points compared with control counterparts (P < 0.05), whereas sIgA productions in APG markedly enhanced at all the time points vs. the CIG. As for IL, IL-4 and IL-6 concentrations observably accumulated (P < 0.05) in the duodenal mucosa at 2 and 4 dpi, then significantly diminished (P < 0.05) from 6 dpi to 15dpi as compared with the control. In addition, MDRV infection significantly suppressed IL-15 level from 6 dpi to 15 dpi (P < 0.05) and APS notably increased IL-15 concentrations of infected ducklings at 15 dpi (P < 0.05). Moreover, MDRV infection also significantly suppressed (P < 0.05) the productions of TNF-α and interferon gamma from 3 dpi to 15 dpi compared with uninfected ones, whereas APS supplementation succeeded in normalizing the levels of TNF-α and interferon gamma to the control value, and no significant differences were found between the APG and NCG at most time points except the metaphase of experiment.
Figure 4.
Effect of APS on ileal sIgA and duodenal cytokines in experimental Muscovy ducklings from each experimental group (n = 6). Values are expressed as mean counts ±SD, different small letter superscripts mean significant difference (P < 0.05). Abbreviations: APG, APS pretreatment for the MDRV-infected group; APS, Astragalus polysaccharide; CIG, cohabitation infection group; NCG, normal control group; sIgA, secretory IgA.
Discussion
The intestinal mucosal barrier not only preserves the ability to digest and absorb nutrients but also acts as the first line of defense against most external pathogens (France and Turner, 2017). The mechanical barrier is an important part of intestinal mucosal barrier, which includes intestinal mucosal epithelial cells covered with mucus layer and the intercellular tight junctions, and protects against pathogen invasion as a first line of defense (Turner, 2009). In the present study, to evaluate the functions related to intestinal mucosal immune system of APS, we carried out a survey to study intestinal morphology including villus length, V/C ratio, thickness, and intestinal immunocyte count, together with sIgA production and cytokines secretion.
As villus length and crypt depth or V/C ratio are both important indicators of the maturity and functional capacity of intestine, long villi and high V/C ratios correspond to a relatively healthy intestinal system including high brush border enzyme activity (Hampson, 1986; Qin et al., 2019), and similarly, lower villus length relate to lower absorptive capability of small intestine (Turner, 2009). In the histologic survey, the villi height, V/C ratio, and thickness of MRDV-infected ducklings decreased significantly, while the aforementioned indexes of the APG efficiently recovered into approximate the control. The result was similar to the previous studies, which reported that APS could possess immunomodulating effect by increasing jejunal villus length and V/C ratio to maintain intestinal integrity of chickens (Wang et al., 2015; Shan et al., 2019). The result is in agreement with another report on weaned piglets whose jejunal villus length and V/C ratio was enhanced on feeding APS-supplemented diets (Yang et al., 2019). Similarly, piglets fed with APS had increased jejunal villus length (Wang et al., 2019). Furthermore, dietary APS has been found to decrease duodenum crypt depth of breeder cocks and jejunum crypt depth in broiler chickens, as well as increase the jejunum villus length and V/C ratios of breeder cocks together with the jejunum V/C radio of broiler chickens, suggesting that APS supplementation had transgenerational effects in promoting jejunal morphology of broilers (Li et al., 2018). All the results revealed that APS could stimulate villus length and V/C ratio, improve immune responses by recovering the aforementioned indexes that are closely related to innate immune barrier and the functions of digestion and absorption.
Valuation of intestinal immunocyte in the present study was also conducive. Intestinal IEL, a group of T lymphocytes locate at the basolateral side of the epithelial layer, interdigitated with the epithelial cells, are the front guardians in the immune system to encounter pathogens by releasing cytokines production immediately, such as IL-2, IL-7, IL-15, TNF-α, interferon gamma, interferon beta, and interferon alfa (Okazawa et al., 2004; Bhagat et al., 2008; Zhai et al., 2011). Goblet cell is a specialized secretory cell that not only secretes mucin, the major composition of the mucus layers, but also contains trefoil peptides, Fc-γ–binding protein, and other biologically active products, which are critical to maintain intestinal homeostasis and integrity of intestinal epithelium (Kim and Ho, 2010; Birchenough et al., 2015). Changes of quantity in the 2 immune-related cells are valuable for intestine mucosal immunity, as the IEL and GC are important parts to maintain epithelial integrity and defense against the invasion of pathogens or antigens (Kelly and Coutts, 2000; McCauley and Guasch, 2015). As proposed in the previous studies (Wu et al., 2018; 2019), MDRV infection caused remarkable decrease of IEL and GC number of the intestinal villi in the histologic trial. Such a depletion or significant reduction of the intestinal immunocyte number in infected ducklings can attribute to the apoptosis, necrosis, intestinal barrier injury, and impairment of the intestinal mucosal immune system (Curfs et al., 1997; Wu et al., 2019). Furthermore, in accordance with our previous study of HPS (Wu et al., 2018), APS pretreatment orally in the infected ducklings could maintain the IEL and GC number, ameliorate or alleviate intestinal mucosa injury in ducklings infected with MDRV, and guarantee mucosal integrity. Additional evidence exists in chickens in which the administration of APS elevated the number of jejunal IEL but had no difference in the number of GC (Wang et al., 2015). The present result is in agreement with another report on fish in which the microvilli length of intestine was light increased in the APS-treated vs. control groups (Zahran et al., 2014).
Secretory IgA, present in abundance in mucosal secretions, is a noninflammatory antibody mainly produced by goblet cells in the gastrointestinal tract. Secretory IgA promotes the excretion of antigens and pathogenic microorganisms from intestinal cavity though interdicting their access to epithelial receptors, wrapping them in mucus, and stimulating their removal via peristaltic and mucociliary activities (Mantis et al., 2011). As previously reported, intestinal sIgA production was significantly decreased in MDRV-infected ducklings than the control group, but the secretion was effectively augmented in the APG in comparison with the CIG (Wu et al., 2018). A recent study with chickens reported that sIgA antibody levels were significantly increased in the APS group compared with the other groups at most time points (Shan et al., 2019). These results have indicated that oral administration of APS could promote the intestinal mucosal immunization. The changes of sIgA secretion are potentially related to the destruction of GC in MDRV infection (Wu et al., 2018), and Mantis et al. (2011) have mentioned that the synthesis of sIgA also requires cytokines with immunoregulatory activity (Mantis et al., 2011).
Cytokines exert an important biological function in immunomodulatory function, antiviral response, and inflammatory reaction via being organized within a stratified regulatory network through complicated interactions, which may reflect anti-inflammation ability and antiviral response (Wang et al., 2018). Determination of dynamic cytokines secretions in the present study was also conducive. IL-6 mediating cellular and humoral immune responses plays a crucial role in determining the outcome of viral infection (Lan et al., 2015). Results showed that MDRV infection caused a rapid increase of the IL-4 and IL-6 concentrations in the early stage and then decreased, and the 2 concentrations in the CIG were lower than the NCG in the late stage, which may be in response to inflammation and infection (Wu et al., 2019). It seems that the invasion of the virus could evolve some ability against virus in the immune system by promoting IL-4 and IL-6. Present results showed that MDRV infection significantly suppressed the productions of IL-15, TNF-α and interferon gamma in the ducklings, whereas APS supplementation succeeded in normalizing various cytokines to the control value, suggesting that APS pretreatment could reverse the decrease of cytokines caused by MDRV infection. Astragalus polysaccharide could stimulate immune responses and improve the function of related cells to protect and repair themselves by promoting cytokines secretion during the process of MDRV inhibiting the proliferation and activity of intestinal immunocytes (Jiang et al., 2015). Likewise, a previous study with immunosuppressive mice reported that dietary supplementation of APS herbal formula significantly improved TNF, interferon gamma, IL-2, and IL-17A (Zhou et al., 2018). Previous reports also showed that oral supplements of APS numerically promoted expression of TNF, interferon gamma, IL-2, and IL-10 in broilers when compare with the corresponding vaccine group or blank control group (Yusuf et al., 2016). Astragalus polysaccharide significantly improved experimental 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats through downregulating TNF-α and IL-1β expressions (Yang et al., 2014). The polysaccharide could maintain cytokines levels in a dynamic balance by bidirectional regulation, thus boosting the immune system (Jiang et al., 2015). Multiple cytokines, which could help T cells and B cells to proliferate, are associated with cellular immune responses and humoral immunity, and they are equally important in intestinal stimulation of sIgA secretion to establish one of the many links between sIgA production, immunocyte immunity, and intestinal homeostasis (Loman et al., 1999; Newberry and Lorenz, 2005). In addition, the evidence exists in piglets in which feeding of APS significantly increased the intestinal barrier function by decreasing IL-1β, TNF-α levels and increasing toll-like receptor 4 and myeloid differentiation factor 88 and decreased NF-κB expression (Wang et al., 2019). In view of the intricacy of the interactions and dynamics of cytokines, it is very imperative to define such effects and mechanisms of APS on intestinal mucosa.
Conclusion
Supplementation of the APS studied herein resulted in improved intestinal mucosal morphology, related parameters and mucosal immunocyte number in infected ducklings. This was accompanied by a restoration at the least even improving intestinal sIgA, cytokine secretion, and immune-modulatory effects of the intestinal mucosa. Astragalus polysaccharide has the potential to be used in clinical prevention and control of MDRV infection or other animal diseases for the bidirectional immune-stimulatory effect. Moreover, further studies are needed to elucidate the specific mechanism of APS on intestinal mucosa.
Disclosures
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by grants from the Fujian Educational Bureau (Grant Number: JT180160), the Industry-University Cooperation Project in Fujian Province (Grant Number: 2019N5004), and Fujian Agriculture and Forestry University Science and Technology Innovation Foundation (NOS. CXZX2016013; CXZX2017054).
References
- Bhagat G., Naiyer A.J., Shah J.G., Harper J., Manavalan J.S. Small intestinal CD8+TCRγδ+NKG2A+ intraepithelial lymphocytes have attributes of regulatory cells in patients with celiac disease. J. Clin. Invest. 2008;118:281–293. doi: 10.1172/JCI30989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchenough G.M.H., Johansson M.E.V., Gustafsson J.K., Bergström J.H., Hansson G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015;8:712–719. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curfs J.H., Meis J.F., Hoogkampkorstanje J.A. A primer on cytokines: sources, receptors, effects, and inducers. Clin. Microbiol. Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France M.M., Turner J.R. The mucosal barrier at a glance. J. Cell Sci. 2017;130:307–314. doi: 10.1242/jcs.193482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel W.L., Zhang W., Afonso J., Klurfeld D.M., Don S.H., Laitin E., Deaton D., Furth E.E., Pietra G.G., Naji A., Rombeau J.L. Glutamine enhancement of structure and function in transplanted small intestine in the rat. JPEN J. Parenter. Enteral Nutr. 1993;17:47–55. doi: 10.1177/014860719301700147. [DOI] [PubMed] [Google Scholar]
- Gaudry D., Charles J.M., Tektoff J. A new disease expressing itself by a viral pericarditis in Barbary ducks. C.R. Acad. Hebd. Seances. Acad. Sci. D. 1972;274:2916–2919. [PubMed] [Google Scholar]
- Hampson D.J. Alteration in piglet small intestinal structure at weaning. Res. Vet. Sci. 1986;40:32–40. [PubMed] [Google Scholar]
- Heffels-Redmann U., Muller H., Kaleta E.F. Structural and biological characteristics of reoviruses isolated from Muscovy ducks (Cairina moschata) Avian Pathol. 1992;21:481–491. doi: 10.1080/03079459208418866. [DOI] [PubMed] [Google Scholar]
- Huang X., Wang D., Hu Y., Lu Y., Guo Z., Kong X., Sun J. Effect of sulfated astragalus polysaccharide on cellular infectivity of infectious bursal disease virus. Int. J. Biol. Macromol. 2008;42:166–171. doi: 10.1016/j.ijbiomac.2007.10.019. [DOI] [PubMed] [Google Scholar]
- Jiang J.X., Huang L.N., Jiang H.H., Fang J., Li D., Cheng X., Wu Y.J., Wu B.C. Effects of Astragalus polysaccharide on levels of cytokines in Muscovy ducks infected with Muscovy duck reovirus. Chin. Vet. Sci. 2015;45:873–880. [Google Scholar]
- Jiang M.H., Zhu L., Jiang J.G. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin. Ther. Targets. 2010;14:1367–1402. doi: 10.1517/14728222.2010.531010. [DOI] [PubMed] [Google Scholar]
- Kelly D., Coutts A.G.P. Early nutrition and the development of immune function in the neonate. Proc. Nutr. Soc. 2000;59:177–185. doi: 10.1017/s0029665100000197. [DOI] [PubMed] [Google Scholar]
- Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara H., Uchida T., Takakiwa M., Matsuzaki T., Hada N., Takeda T., Shibata T., Yamada H. Different contributions of side-chains in beta-D-(1-->3,6)-galactans on intestinal Peyer's patch-immunomodulation by polysaccharides from Astragalus mongholics bunge. Phytochemistry. 2010;71:280–293. doi: 10.1016/j.phytochem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Lan T., Chang L., Wu L., Yuan Y.F. IL-6 plays a crucial role in HBV infection. J. Clin. Transl. Hepatol. 2015;3:271–276. doi: 10.14218/JCTH.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.J., Lee L.H., Hsu H.W., Kuo L.C., Liao M.H. Molecular evolution of avian reovirus: evidence for genetic diversity and reassortment of the S-class genome segments and multiple cocirculating lineages. Virology. 2003;314:336–349. doi: 10.1016/s0042-6822(03)00415-x. [DOI] [PubMed] [Google Scholar]
- Li Y., Lei X., Guo W., Wu S., Duan Y., Yang X., Yang X. Transgenerational endotoxin tolerance-like effect caused by paternal dietary Astragalus polysaccharides in broilers' jejunum. Int. J. Biol. Macromol. 2018;111:769–779. doi: 10.1016/j.ijbiomac.2018.01.095. [DOI] [PubMed] [Google Scholar]
- Li S., Qi Y., Ren D., Qu D., Sun Y. The structure features and improving effects of polysaccharide from Astragalus membranaceus on antibiotic-associated diarrhea. Antibiotics (Basel) 2019;9:8. doi: 10.3390/antibiotics9010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman S., Jansen H.M., Out T.A., Lutter R. Interleukin-4 and interferon-gamma synergistically increase secretory component gene expression, but are additive in stimulating secretory immunoglobulin A release by Calu-3 airway epithelial cells. Immunology. 1999;96:537–543. doi: 10.1046/j.1365-2567.1999.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkinson M., Perk K., Weisman Y. Reovirus infection of young muscovy ducks (Cairina moschata) Avian Pathol. 1981;10:433–440. doi: 10.1080/03079458108418493. [DOI] [PubMed] [Google Scholar]
- Mantis N.J., Rol N., Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marius-Jestin V., Lagadic M., Menec Y.L., Bennejean G. Histological data associated with Muscovy duck reovirus infection. Vet. Rec. 1988;123:32–33. doi: 10.1136/vr.123.1.32. [DOI] [PubMed] [Google Scholar]
- McCauley H.A., Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015;21:492–503. doi: 10.1016/j.molmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Meng Q., Du X., Wang H., Gu H., Zhan J., Zhou Z. Astragalus polysaccharides inhibits cell growth and pro-inflammatory response in IL-1β-stimulated fibroblast-like synoviocytes by enhancement of autophagy via PI3K/AKT/mTOR inhibition. Apoptosis. 2017;22:1138–1146. doi: 10.1007/s10495-017-1387-x. [DOI] [PubMed] [Google Scholar]
- Newberry R.D., Lorenz R.G. Organizing a mucosal defense. Immunol. Rev. 2005;206:6–21. doi: 10.1111/j.0105-2896.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- Okazawa A., Kanai T., Nakamaru K., Sato T., Inoue N., Ogata H., Iwao Y., Ikeda M., Kawamura T., Makita S. Human intestinal epithelial cell-derived interleukin (IL)-18, along with IL-2, IL-7 and IL-15, is a potent synergistic factor for the proliferation of intraepithelial lymphocytes. Clin. Exp. Immunol. 2004;136:269–276. doi: 10.1111/j.1365-2249.2004.02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palya V., Glavits R., Dobos-Kovacs M., Ivanics E., Benko M. Reovirus identified as cause of disease in young geese. Secondary communication. Magy Allatorvosok. 2003;125:406–414. doi: 10.1080/030794502100007187. [DOI] [PubMed] [Google Scholar]
- Qin S.M., Zhang K.Y., Ding X.M., Bai S.P., Zeng Q.F. Effect of dietary graded resistant potato starch levels on growth performance, plasma cytokines concentration, and intestinal health in meat ducks. Poult. Sci. 2019;98:3523–3532. doi: 10.3382/ps/pez186. [DOI] [PubMed] [Google Scholar]
- Shan C., Sun B., Dalloul R.A., Zhai Z., Luan W. Effect of the oral administration of astragalus polysaccharides on jejunum mucosal immunity in chickens vaccinated against Newcastle disease. Microb. Pathog. 2019;135:103621. doi: 10.1016/j.micpath.2019.103621. [DOI] [PubMed] [Google Scholar]
- Turner J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- Wang Y., Chen Y., Du H., Yang J., Ming K., Song M., Liu J. Comparison of the anti-duck hepatitis A virus activities of phosphorylated and sulfated Astragalus polysaccharides. Exp. Biol. Med. 2016;242:344–353. doi: 10.1177/1535370216672750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Li Y., Shen J., Wang S., Yao J., Yang X. Effect of Astragalus polysaccharide and its sulfated derivative on growth performance and immune condition of lipopolysaccharide-treated broilers. Int. J. Biol. Macromol. 2015;76:188–194. doi: 10.1016/j.ijbiomac.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Wang X., Xue Y., Li Y., Liu F., Yan Y., Zhang H., Jin Q. Effects of Isatis root polysaccharide in mice infected with H3N2 swine influenza virus. Res. Vet. Sci. 2018;119:91–98. doi: 10.1016/j.rvsc.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Wang K., Zhang H., Han Q., Lan J., Chen G., Cao G., Yang C. Effects of astragalus and ginseng polysaccharides on growth performance, immune function and intestinal barrier in weaned piglets challenged with lipopolysaccharide. J. Anim. Physiol. Nutr. (Berl.) 2019;104:1096–1105. doi: 10.1111/jpn.13244. [DOI] [PubMed] [Google Scholar]
- Wu Y.J. Fujian Agriculture and Forestry University; Fuzhou: 2010. Immunoregulating Effects of Two Chinese Herbal Polysaccharides on Muscovy Duck Infecting of MDRV. [Google Scholar]
- Wu B., Chen J., Yao J., Chen Z., Chen W., Li G., Zeng X. Isolation and identification of Muscovy duck reovirus. J. Fujian Agric. Univ. 2001;30:227–230. [Google Scholar]
- Wu Y.J., Jiang H.H., Zhu E.P., Lia P., Wang Q.X., Zhou W.D., Qin T., Wu X.P., Wu B.C., Huang Y.F. Hericium erinaceus polysaccharide facilitates restoration of injured intestinal mucosal immunity in Muscovy duck reovirus-infected Muscovy ducklings. Int. J. Biol. Macromol. 2018;107:1151–1161. doi: 10.1016/j.ijbiomac.2017.09.092. [DOI] [PubMed] [Google Scholar]
- Wu Y.J., Liu Z.N., Zhu E.P., Li M.H., Jiang H.H., Luo Y., Wang Q.X., Wu X.P., Wu B.C., Huang Y.F. Changes in the small intestine mucosal immune barrier in Muscovy ducklings infected with Muscovy duck reovirus. Vet. Microbiol. 2019;233:85–92. doi: 10.1016/j.vetmic.2019.04.017. [DOI] [PubMed] [Google Scholar]
- Yang C.M., Han Q.J., Wang K.L., Xu Y.L., Lan J.H., Cao G.T. Astragalus and ginseng polysaccharides improve developmental, intestinal morphological, and immune functional characters of weaned piglets. Front. Physiol. 2019;10:418. doi: 10.3389/fphys.2019.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Lin H., Gong S., Chen P., Geng L., Zeng Y., Li D. Effect of Astragalus polysaccharides on expression of TNF-α, IL-1β and NFATc4 in a rat model of experimental colitis. Cytokine. 2014;70:81–86. doi: 10.1016/j.cyto.2014.07.250. [DOI] [PubMed] [Google Scholar]
- Yin M., Zhang Y., Li H. Advances in research on immunoregulation of macrophages by plant polysaccharides. Front. Immunol. 2019;10:145. doi: 10.3389/fimmu.2019.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Shen M., Song Q., Xie J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: a review. Carbohydr. Polym. 2018;183:91–101. doi: 10.1016/j.carbpol.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Yun T., Hua J., Ye W., Yu B., Chen L., Ni Z., Zhang C. Comparative proteomic analysis revealed complex responses to classical/novel duck reovirus infections in Cairna moschata. Sci. Rep. 2018;8:10079. doi: 10.1038/s41598-018-28499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf A.A., Sanpha K., Yu X., Zhang Y., Li G. Vaccination with Astragalus and ginseng polysaccharides improves immune response of chickens against H5N1 avian influenza virus. Biomed. Res. Int. 2016;2016:1–8. doi: 10.1155/2016/1510264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran E., Risha E., Abdelhamid F., Mahgoub H.A., Ibrahim T. Effects of dietary Astragalus polysaccharides (APS) on growth performance, immunological parameters, digestive enzymes, and intestinal morphology of Nile tilapia (Oreochromis niloticus) Fish Shellfish Immun. 2014;38:149–157. doi: 10.1016/j.fsi.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Zhai L., Li Y., Wang W., Wang Y., Hu S. Effect of oral administration of ginseng stem-and-leaf saponins (GSLS) on the immune responses to Newcastle disease vaccine in chickens. Vaccine. 2011;29:5007–5014. doi: 10.1016/j.vaccine.2011.04.097. [DOI] [PubMed] [Google Scholar]
- Zhang P., Wang J., Wang W., Liu X., Liu H., Li X., Wu X. Astragalus polysaccharides enhance the immune response to avian infectious bronchitis virus vaccination in chickens. Microb. Pathogenesis. 2017;111:81–85. doi: 10.1016/j.micpath.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Ren W., Zhang L., Zhang Y., Liu D., Liu Y. A review of the pharmacological action of Astragalus polysaccharide. Front. Pharmacol. 2020;11:349. doi: 10.3389/fphar.2020.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Liu Z., Long T., Zhou L., Bao Y. Immunomodulatory effects of herbal formula of astragalus polysaccharide (APS) and polysaccharopeptide (PSP) in mice with lung cancer. Int. J. Biol. Macromol. 2018;106:596–601. doi: 10.1016/j.ijbiomac.2017.08.054. [DOI] [PubMed] [Google Scholar]