Abstract
Oxidative stress could be prevented by antioxidant-loaded nanoparticles. The purpose of this study was to assess the effects of 10 (A10), 20 (A20), 30 (A30), 40 (A40), and 50 (A50) μM alpha-lipoic acid and alpha-lipoic acid nanostructured lipid carriers (ALN) at 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40), and 50 (ALN50) μM on post-thawed sperm quality, fertility, and apoptosis-related genes of rooster sperm. The extender supplemented with ALN30 led to higher total and progressive motility, straight-line velocity, and linearity in comparison to the control group. The ALN30 resulted in higher percentage of mitochondria activity and glutathione peroxidase level compared with control (P < 0.05). The extender supplemented with ALN30 led to lower percentage of apoptotic sperm, when compared with the control. CASPASE 3 expression in ALN30 was lower (P < 0.05) than the other groups. The results showed that BCL-2 mRNA expression of sperm was significantly (P < 0.05) higher in ALN30 compared with the other groups (P < 0.05). Higher percentages of fertility and hatchability rates were observed in ALN30 group. The results indicate that ALN30 could be regarded as a novel potential cryoprotectant for the cryopreservation of rooster semen. Therefore, nanostructured lipid carriers improve not only the active compound (such as alpha-lipoic acid) of biomedical applicability but also the potential for industrial application in sperm cryopreservation.
Key words: nanostructured lipid carriers, apoptosis, alpha-lipoic acid, sperm, gene
Introduction
Cryopreservation is extensively applied to the sperm cells of many animal species. Although cryopreservation technique proposes many benefits, such as reducing the optimum number of males in breeding programs, transportation of sperm, and supplying genetic resources, it causes damage to different sperm cell parts containing DNA, protein, and membrane lipids which may decrease sperm motility and eventually fertilization (Ogretmen and Inanan, 2014; Shahverdi et al., 2015). Sperm cryopreservation, including cooling and freezing, leads to ice formation and osmotic stress, therefore causes imbalances between antioxidant and pro-oxidant species, and it is proved that excessive reactive oxygen species (ROS) results in a lipoperoxidative damage (Luno et al., 2014; Daghigh Kia et al., 2016). An important factor for the decrease in sperm fertility following freezing is the intracellular generation of ROS through the univalent reduction of molecular oxygen which results in damages caused by oxidative stress (Johinke et al., 2014; Masoudi et al., 2019). There have been numerous research studies focusing on using antioxidants for improving the quality of post-thaw semen. Moreover, there is a lot of evidence that supplementing antioxidants to the freezing extenders can reduce the harmful effects of ROS (Santiani et al., 2014; Shojaeian et al., 2018).
Alpha-lipoic acid is a nonvitamin naturally existing in the mitochondria that plays a major role in antioxidant at cellular (Fayyaz et al., 2017). It enters the Kreb's cycle and develops the ATP production because of the effective nutrient utilization, being part of pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase enzyme systems as cofactor (Bilska and Wlodek, 2005; Fayyaz et al., 2017). As the alpha-lipoic acid is water soluble as well as fat soluble, it can act both extracellular and intracellular; therefore, it has suitable permeability across the cell membrane. It has been shown that alpha-lipoic acid can reduce the DNA damage of buck spermatozoa after freeze-thawing (Ibrahim et al., 2008), increase motility (Fayyaz et al., 2017), and decrease ROS production in buck spermatozoa (Ma et al., 2011). Therefore, using alpha-lipoic acid in freezing extenders can increase the sperm cryosurvival, function, and structure due to the enhanced energy production and decreased oxidative stress (Fayyaz et al., 2017). Nevertheless, a limitation of using alpha-lipoic acid, in terms of dietary, medical, and cosmetic applications, was observed because of its poor water solubility in aqueous solutions (Wang et al., 2014).
Encapsulation is defined as a useful technology in the nutritive, chemical, pharmaceutical, and cosmetic industries to ensure the progress in active ingredients of carrier systems (Ruktanonchai et al., 2009). Lipid nanoparticles such as nanostructured lipid carriers (NLC) are encouraging options in active substance delivery carriers. Consequently, NLC have been selected as delivery systems. The NLC are new active ingredients delivery systems which might be fabricated by combining any biocompatible substances (Li et al., 2010). In the present study, the manipulation of encapsulating it in NLC formulation was performed to avoid the problems of alpha-lipoic acid.
We hypothesized that enrichment of semen extender with alpha-lipoic acid loaded lipid nanoparticles before cryopreservation would improve the quality of rooster sperm, by reducing ROS through its antioxidant action. Therefore, the goal of the present study was to assess the effect of alpha-lipoic acid–loaded lipid nanoparticles on cryopreservation injuries of rooster sperm, evaluating post-thawing sperm quality.
Materials and methods
All chemical reagents were obtained from Sigma (St. Louis, MO), and Merck (Darmstadt, Germany). All protocols used in this study were approved by the Animal Welfare Committee of the Department of Animal Science, University of Tabriz.
Nanostructured Lipid Carriers Preparation
Hot homogenization procedure was used to make NLC. Alpha-lipoic acid was combined with liquid lipid (Miglyol), and the combination was included with melted solid lipid (Precirol). After that, the hot aqueous surfactant solution (Poloxamer 407) was included into the lipid phase under homogenization (Silent crusher M, Heidolph, Nuremberg, Germany) at 20,000 rpm for 15 min. The obtained nanoemulsion was cooled off to 24°C in lipid phase recrystallization, and the eventual formation of NLC was obtained. Afterward, the storage of alpha-lipoic acid–loaded nanostructured lipid carriers was performed at 4°C for further use (Najafi et al., 2019a).
Farm Management and Semen Collection
This study was performed using fifteen 28-wk-old Ross broiler breeder roosters which were kept in the research farm of Tabriz University, Iran. All the roosters were fed with the same diet that contained 12% crude protein, 2,750 kcal maintenance energy/kg, 0.7% calcium, and 0.35% available phosphorus. Semen samples were collected 2 times a week from roosters (5 replicates) based on the method of Burrows and Quinn (1937). Semen samples were delivered to laboratory at a temperature of 37°C within 15 min after collection for primary assessment. Ejaculates with the properties of volume of >0.2 mL, concentration of >3 × 109 sperm/mL, and motility of >80% were applied in this study. To eliminate individual differences and obtain sufficient sperm, samples with good quality were pooled and then divided into 11 equal aliquots.
Semen Processing and Cryopreservation
Beltsville extender was selected as the base medium (Table 1). Glycerol was added to this basic medium at 3.8% (v/v) and then combinations of Beltsville buffer and 10 (A10), 20 (A20), 30 (A30), 40 (A40) and 50 (A50) μM alpha-lipoic acid and alpha-lipoic acid–loaded nanostructured lipid carriers (ALN) at 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40), and 50 (ALN50) μM were included. The remaining aliquot was extended with nonsupplemented Beltsville, as control group. After dilution, semen samples were loaded into 0.25 mL French straws to obtain an approximate concentration of 100 × 106 sperm/straw. The straws were sealed with polyvinyl alcohol powder and subsequently equilibrated at 4°C for 3 h. After that, the straws were located 4 cm above the surface of the liquid nitrogen for 7 min in a cryobox then were placed in a tank of liquid nitrogen and stored (Najafi et al., 2019b). Each frozen straw was thawed at 37°C for 30 s in a water bath for immediate evaluation after thawing.
Table 1.
Composition of the Beltsville extender.
| Ingredient | Amount |
|---|---|
| Potassium citrate tribasic monohydrate (mM) | 2.08 |
| Sodium-L-glutamate (mM) | 51.28 |
| Magnesium chloride anhydrous (mM) | 0.35 |
| D-(−)-Fructose (mM) | 27.75 |
| Potassium phosphate dibasic trihydrate (mM) | 43.57 |
| Potassium phosphate monobasic (mM) | 5.14 |
| N-[Tris (hydroxymethyl) methyl]-2 (mM) | 13.95 |
| Sodium acetate trihydrate (mM) | 3.9 |
| Soybean lecithin (g/100 mL) | 1 |
| pH | 7.1 |
| Osmolality (mOsm/kg) | 310 |
Motility Analysis
Motility analysis was performed using a sperm analyzer (CASA) system IVOS 12 (Hamilton Thorne Inc., Beverly, MA). For this purpose, thawed sperm were diluted with PBS buffer, then 3 μL of diluted semen was placed onto a prewarmed chamber slide (37°C, Leja 4; 20 μm height; Leja Products, Luzernestraat B.V., Holland). Total of at least 200 cells were analyzed per sample. Total motility (%), progressive motility (%), straight-line velocity (μm/s), velocity average path (μm/s), curvilinear velocity (μm/s), and linearity (LIN, %) were evaluated (Mehdipour et al., 2016).
Plasma Membrane Functionality (Hypo-osmotic Swelling Test)
For evaluating membrane integrity of spermatozoa, hypo-osmotic swelling test was used as described by Mehdipour et al. (2017) with minor modifications. To perform the test, 10 μL of semen sample was mixed with 100 μL of a hypoosmotic solution (0.9 g fructose and 0.49 g sodium citrate in 100 μL distilled water; 100 mOsm/kg) at 37°C for 30 min. Then, 1 drop of the mixture was placed on a preheated microscope slide and mounted with a cover slip. Two hundred sperm cells were counted by a 400 magnification phase contrast microscope (20 microscopic fields were analyzed). The percentage of sperm with curl of the tail (hypo-osmotic swelling test) was recorded as intact plasma membrane feature.
Morphological Abnormalities Forms
Hancock's solution was used for assessing morphological features of sperm using phase-contrast microscopy ( × 1,000 magnification) (20 microscopic fields were analyzed) (as method described by Najafi et al. (2018a)). Then, 3 drops of semen were added to tubes containing 1 mL Hancock's solution. Next, 1 drop of the mixture was placed on a slide and was gently mounted with a coverslip. Two hundred sperm cells were evaluated to obtain the percent of abnormal sperm.
Lipid Peroxidation
Malondialdehyde (MDA) concentrations of semen samples were assessed as an index of lipid peroxidation by means of the thiobarbituric-acid reaction (Najafi et al., 2018b). To perform the assay, 1 mL of the diluted semen and 1 mL of cold 20% (w/v) trichloroacetic acid was mixed to precipitate the protein content. At the next step, it was pelleted by centrifuging (960 g for 15 min), and 1 mL of the supernatant was incubated with 1 mL of 0.67% (w/v) thiobarbituric acid in a boiling water bath at 100°C for 10 min (Fattah et al., 2017). Then, the samples were allowed to be cooled, and the absorbance was defined by a spectrophotometer (T80 UV/VIS PJ Instruments Ltd., UK) at 532 nm. The MDA levels were expressed as nmol/mL.
Total Antioxidant Capacity, Glutathione Peroxidase, and Superoxide Dismutase Determination
Glutathione peroxidase (GPx), superoxide dismutase (SOD), and total antioxidant capacity (TAC) were evaluated by commercial colorimetric assay kits (Randox Laboratories Ltd., London, U.K.). The procedures were performed by following the manufacturer's instructions.
Flow Cytometry Analysis
Flow cytometry examinations were performed using a BD FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA). An argon ion laser (488 nm) was used to excite fluorescent probes in the current study. Green fluresence from Annexin V/Fluorescein Isothiocyanate and Rhodamine 123 was identified by FL1 photodetector. Red fluresence from propidium iodide (PI) was recognized by FL3 photodetector. For evaluation of each sample, 10,000 events were acquired (Najafi et al., 2020). The data were analyzed by CellQuest 3.3 software (Becton Dickinson).
Apoptosis Status
Apoptosis status was identified by a Commercial Kit (IQP, Groningen, the Netherlands). The procedures were performed by following the manufacturers guide. First, sperm samples were washed in calcium buffer and re-adjusted to a concentration of 10 million sperm per milliliter. Subsequently, 10 microliters Annexin V-fluorescein isothiocyanate (0.01 mg/mL) was added to 100 μL of sperm suspension followed by incubation in dark for 20 min. Finally, 10 μL of PI (1 mg/mL) was added to sperm suspension and incubated for 10 min before assessment with flow cytometer (Ansari et al., 2017). The evaluated sperm were categorized into 4 groups: live cells (A−/PI−); live, early apoptotic (A+/PI−); and 2 categories of PI + dead cells, the late apoptotic or early necrotic cells (A+/P+); and late necrotic cells (A−/PI+). Late apoptotic and necrotic cells were distinctly classified together as dead cells (Mehdipour et al., 2018).
Mitochondrial Status
For evaluating the mitochondrial activity of sperm, Rhodamine 123 (R123; Invitrogen, Eugene, OR) and PI were used. At the first step, 5 μL of R123 (0.01 mg/mL) was added to the diluted semen samples (100 μL; 50 × 106 sperm/mL) and kept in the dark at room temperature. Then, 5 μL of PI solution (1 mg/mL) was added to the sample and evaluated by flow cytometry to define the total number of live sperm with active mitochondria (positive for R123 and negative for PI) (Nouri et al., 2018).
Artificial Insemination
Artificial insemination (AI) was implemented according to the technique of Najafi et al. (2019a) with a minor modification. For AI, 3 experimental groups (30 μM alpha-lipoic acid, 30 μM alpha-lipoic acid–loaded NLC and control group) were selected according to the results of in-vitro sperm parameters. Thirty breeder hens (Ross) were classified into 3 treatments (10 hens in each treatment), which were retained in different cages (70 × 70 × 85 cm). Glycerol removal was carried out by a discontinuous Accudenz column (equipped with a 12% (5 μL) layer and a 30% (0.5 μL) layer). After centrifugation of the samples (1,200 g; 20 min), the glycerol and extender maintained above the 12% layer, whereas the deposition of purified sperm was between the 2 layers of 12 and 30%. After Accudenz, up to 500 μL Beltsville extender was added to the semen layer. Finally, AI was carried out immediately after thawing twice a week (0.25 mL semen/hen; 100 × 106 sperm/per hen). Egg collection was done from the day after the final insemination to 5 d after the last AI. The collected eggs were incubated in the setter for 18 d and transferred to the hatcher for the last 3 d. Fertility rate was measured after 7 d of incubation by candling the eggs [(embryonated eggs/total eggs) × 100]. The hatchability percent of fertile eggs was assessed after 21 d [(hatched chick numbers/fertilized) × 100].
RNA Extraction and Real-Time Polymerase Chain Reaction
For the first step, primers were designed utilizing Primer3Plus online software on the basis of GenBank sequence of target genes and presented in Table 2. To assay the specificity of the primers, a BLAST analysis of the National Center for Biotechnology information's database was used. At the same time, GAPDH was selected as a useful endogenous control gene. Total RNA was extracted from semen samples using Trizol reagent (Invitrogen, Carlsbad, CA). For this purpose, the method stated by the manufacturer and quantified using ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) was used (Song et al., 2015). RNA was then transcribed into complementary DNA with the reverse transcription reagent kit (REVERTA-L RT reagents kit; code: K3-4-100-CE) by using thermal cycler. The process was done according to manufacturer's guidelines. The RT reaction was carried out in 20 μL of reaction combination at 37°C for 15 min and then stored at ≤ −20°С (Mahdieh et al., 2020).
Table 2.
Primer sequences used for quantitative real-time polymerase chain reaction.
| Gene | Primer sequence (5′–3′) | Product size (bp) | Accession no. |
|---|---|---|---|
| GAPDH | F: ATCACAGCCACACAGAAGACG | 120 | NM_204305.1 |
| R: GACTTTCCCCACAGCCTTAGC | |||
| CASPASE 3 | F: AACCAGCCTTTTCAGAGGTGAC | 119 | NM_204725.1 |
| R: CTGGTCCACTGTCTGCTTCAATA | |||
| BCL-2 | F: AACATTGCCACCTGGATGAC | 118 | NM_205339.2 |
| R: CGAACAAAGGCCTCATACTGT |
Quantification of all transcripts was performed using a ABI StepOnePlus Real-Time PCR Systems (Applied Biosystems, Foster City, CA) by the RealQ Plus 2x Master Mix Green Kit (Ampliqon, code: A325402). The reaction was implemented at 95°C for 15 min, followed by 40 cycles of denaturing, annealing, and elongating (95°C for 15 s, 61°C for 20 s, and 72°C for 30 s, respectively) (Mahdieh et al., 2020). The dissociation curves of PCR products were attained by a following cycle of 95°C for 15 s, 60°C for 1 min and 95°C for 15 s, and the specificity of reaction was defined when just one specific peak existed in the dissociation curve (Mahdieh et al., 2020). The efficiencies of the assays was ≥95% and standard curves R2 was 0.999. The quantitative PCR data were analyzed by the 2−ΔΔCt method.
Statistical Analysis
The data were definitely analyzed by SAS software (version 9.1). The Shapiro-Wilk test was selected for checking the normality of the data. At that point, the effects of the treatments were examined by linear mixed-effect models (PROC MIXED). Tukey's test was selected for comparing treatments while the models were significant. The fertility data were then analyzed by generalized linear models (PROC GENMOD, chi-square test). The significance level in this study was considered P < 0.05. The final results are presented as mean ± SEM.
Results
Several parameters such as motion and velocity parameters of rooster sperm supplemented with different levels of alpha-lipoic acid and ALN are shown in Table 3. The extender supplemented with ALN30 loaded NLC led to higher total and progressive motility, straight-line velocity, and LIN in comparison to the control group. Average path velocity and curvilinear velocity did not significantly change by different experimental treatments.
Table 3.
Effect of different levels of alpha-lipoic acid and ALN on motility parameters of rooster sperm assessed by CASA after freeze-thawing.
| Treatments | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Control | A10 | A20 | A30 | A40 | A50 | ALN10 | ALN20 | ALN30 | ALN40 | ALN 50 | SEM |
| TM (%) | 50.1b | 52.3c | 56.5b | 59.8b | 54.3b | 41.2c | 53.4b | 60.1b | 72.1a | 56.3b | 45.2b,c | 2.1 |
| PM (%) | 17.1c | 20.2b,c | 24.3b | 27.4a,b | 23.7b,c | 16.4c | 25.7a,b | 27.9a,b | 33.2a | 26.3a,b | 16.5c | 1.3 |
| VAP (μm/s) | 30.4 | 33.4 | 34.2 | 34.7 | 32.4 | 29.3 | 34.7 | 35.2 | 36.9 | 34.5 | 29.8 | 2.5 |
| VSL (μm/s) | 16.7b | 17.4b | 19.3a,b | 20.1a,b | 18.3a | 16.2b | 18.5a,b | 20.6a,b | 24.3a | 20.1a,b | 16.4b | 1.7 |
| VCL (μm/s) | 53.2 | 54.1 | 55.3 | 56.2 | 53.8 | 51.0 | 54.7 | 55.5 | 57.1 | 54.0 | 51.8 | 2.3 |
| LIN (%) | 31.4b | 32.5b | 35.0a,b | 35.9a,b | 33.9a,b | 31.3b | 33.9a,b | 36.2a | 42.5a | 36.1a,b | 31.4b | 2.1 |
Different superscripts within the same row indicate significant differences among groups (P < 0.05).
Extender supplemented with different levels of alpha-lipoic acid containing 10 (A10), 20 (A20), 30 (A30), 40 (A40), and 50 (A50) μM and alpha-lipoic acid loaded nanostructured lipid carriers (ALN) containing 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40), and 50 (ALN50) μM.
Abbreviations: LIN, linearity; PM, progressive motility; TM, total motility; VSL, straight-line velocity; VAP, average path velocity; VCL, curvilinear velocity.
Effect of different levels of alpha-lipoic acid and ALN on sperm abnormal morphology and membrane integrity are shown in Table 4. The results show that ALN30 provided better protective effect for membrane integrity. No significant difference was detected in abnormal morphology following the addition of alpha-lipoic acid, compared with the control group.
Table 4.
Effect of different levels of alpha-lipoic acid and ALN on plasma membrane functionality and abnormal forms of rooster thawed semen.
| Parameters | Treatments |
SEM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | A10 | A20 | A30 | A40 | A50 | ALN10 | ALN20 | ALN30 | ALN40 | ALN50 | ||
| Membrane integrity (%) | 45.2c,d | 47.2b,c | 51.2b,c | 58.3b | 50.2b,c | 38.7d | 48.1b,c | 57.3b | 69.3a | 52.1b,c | 40.1c,d | 1.9 |
| Abnormal forms (%) | 19.3 | 17.7 | 17.0 | 16.9 | 16.1 | 20.4 | 17.5 | 16.4 | 14.3 | 16.6 | 19.8 | 2.2 |
Different superscripts within the same row indicate significant differences among groups (P < 0.05).
Extender supplemented with different levels of alpha-lipoic acid containing 10 (A10), 20 (A20), 30 (A30), 40 (A40) and 50 (A50) μM and alpha-lipoic acid loaded nanostructured lipid carriers (ALN) containing 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40) and 50 (ALN50) μM.
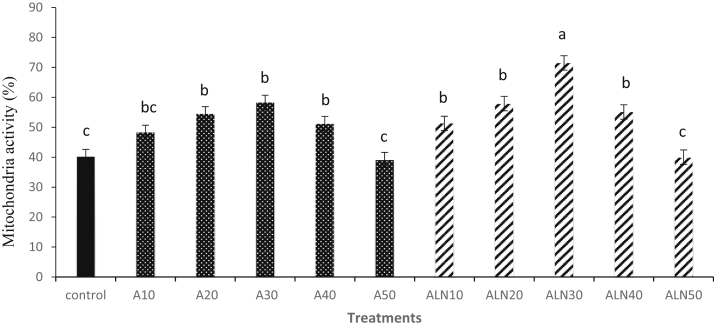
Percentages of mitochondria activity of frozen-thawed sperm using alpha-lipoic acid and ALN are shown in Figure 1. ALN30 resulted in higher percentage of mitochondria activity compared to the control (P < 0.05).
Figure 1.
Effect of different levels of alpha-lipoic acid and ALN on mitochondria activity of rooster thawed semen. Extender supplemented with different levels of alpha-lipoic acid containing 10 (A10), 20 (A20), 30 (A30), 40 (A40) and 50 (A50) μM and alpha-lipoic acid loaded nanostructured lipid carriers (ALN) containing 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40) and 50 (ALN50) μM. Columns with different superscript (a, b, and c) indicate differences (P < 0.05).
The highest percentage of live sperm and lowest dead sperm were detected in ALN30 group (Table 5). The extender supplemented with ALN led to lower percentage of apoptotic sperm, when compared with the control.
Table 5.
Effect of different levels of alpha-lipoic acid and ALN on live, apoptotic, and dead spermatozoa in rooster thawed semen, as assessed by flow cytometry.
| Parameters | Treatments |
SEM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | A10 | A20 | A30 | A40 | A50 | ALN10 | ALN20 | ALN30 | ALN40 | ALN50 | ||
| Live (%) | 45.3c | 52.2b,c | 55.3b,c | 60.1b | 52b | 40.2d | 53.6b,c | 59.1b | 70.2a | 55.2b,c | 41d | 2.6 |
| Early apoptosis (%) | 26a | 24.3a,b | 23.2a,b | 16.5b | 24.6a,b | 27.3a | 23.5ab | 19.5a,b | 14.2b | 22a,b | 27.1a | 1.9 |
| Dead (%) | 28.7a,b | 23.5b,c | 21.5c | 21.4c | 23.4b,c | 32.5a | 22.9c | 21.4c | 15.6d | 22.8c | 31.9a | 1.3 |
Viable (%, AnnexinV−/PI−)), apoptotic (%, AnnexinV+/PI−)) and dead (%, PI+) parameters were analyzed. Different superscripts within the same row indicate differences among groups (P < 0.05).
Extender supplemented with different levels of alpha-lipoic acid containing 10 (A10), 20 (A20), 30 (A30), 40 (A40) and 50 (A50) μM and alpha-lipoic acid loaded nanostructured lipid carriers (ALN) containing 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40) and 50 (ALN50) μM.
Abbreviation: PI, propidium iodide.
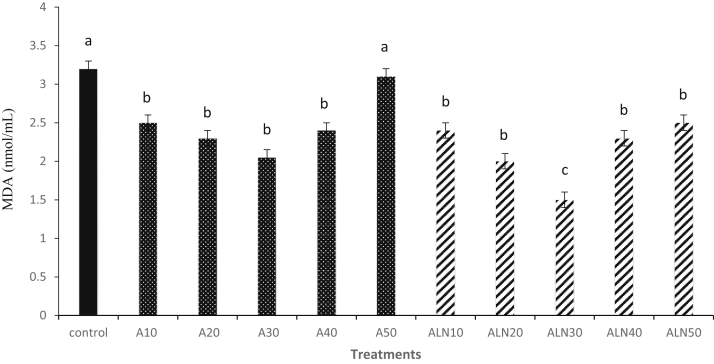
As presented in Figure 2, the MDA level was found to be lower (P < 0.05) in the ALN30 in comparison to the control group. The GPx activity significantly increased in the group with ALN30 when compared with the control group (Table 6). The SOD activity was significantly higher in the ALN30 (P < 0.05) compared with control (P < 0.05). The TAC was greater in the ALN30 compared with the control group (P < 0.05).
Figure 2.
Effect of different levels of alpha-lipoic acid and ALN on MDA level of rooster thawed semen. Extender supplemented with different levels of alpha-lipoic acid at 10 (A10), 20 (A20), 30 (A30), 40 (A40) and 50 (A50) μM and alpha-lipoic acid loaded nanostructured lipid carriers (ALN) at 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40) and 50 (ALN50) μM. Columns with different superscript (a, b, and c) indicate differences (P < 0.05). Abbreviation: MDA, malondialdehyde.
Table 6.
Effect of different levels of alpha-lipoic acid and ALN on malondialdehyde concentration (MDA), glutathione peroxidase (GPx), and superoxide dismutase (SOD) activities and total antioxidant capacity (TAC) of rooster thawed semen.
| Parameters | Treatments |
SEM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | A10 | A20 | A30 | A40 | A50 | ALN10 | ALN20 | ALN30 | ALN40 | ALN50 | ||
| GPx (U/mg protein) | 53.2c | 55.3b,c | 57.5b | 60.2a,b | 57.2a,b | 51.1c | 56.3b | 60.0a,b | 64.8a | 58.1a,b | 52.9c | 1.8 |
| SOD (U/mg) | 106.2 | 108.2 | 112.3 | 114.5 | 107.3 | 104.4 | 109.6 | 113.2 | 116.7 | 112.3 | 106.8 | 8.4 |
| TAC (mmol/l) | 1.2c,d | 1.3b,c,d | 1.4b,c | 1.7a,b | 1.4b,c | 1.0d | 1.3b,c,d | 1.6a,b | 1.8a | 1.5a,b,c | 1.3b,c,d | 0.1 |
Different superscripts within the same row indicate significant differences among groups (P < 0.05).
Extender supplemented with different levels of alpha-lipoic acid containing 10 (A10), 20 (A20), 30 (A30), 40 (A40) and 50 (A50) μM and alpha-lipoic acid loaded nanostructured lipid carriers (ALN) containing 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40) and 50 (ALN50) μM.
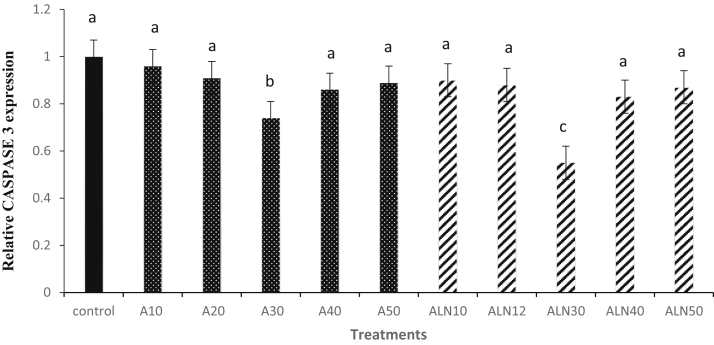
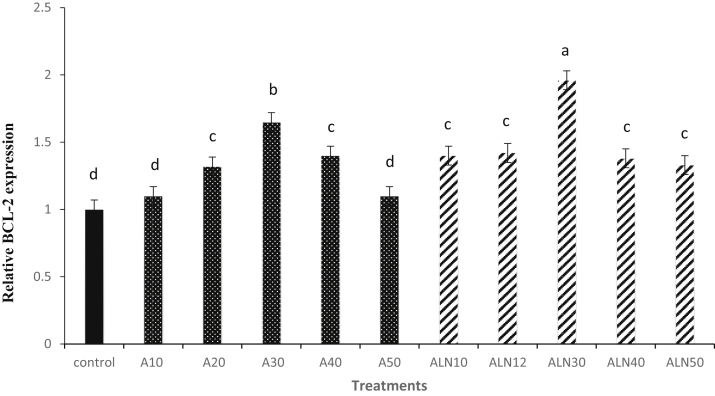
Results of CASPASE 3 mRNA expression have been shown in Figure 3. CASPASE 3 expression in ALN30 was lower (P < 0.05) than the other groups. The results in Figure 4 showed that BCL-2 mRNA expression of sperm was significantly (P < 0.05) higher in ALN30 compared with the other groups (P < 0.05).
Figure 3.
Effect of different levels of alpha-lipoic acid and ALN on relative CASPASE 3 expression of rooster thawed semen. Extender supplemented with different levels of alpha-lipoic acid at 10 (A10), 20 (A20), 30 (A30), 40 (A40) and 50 (A50) μM and alpha-lipoic acid loaded nanostructured lipid carriers (ALN) at 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40) and 50 (ALN50) μM. Columns with different superscript (a, b, and c) indicate differences (P < 0.05).
Figure 4.
Effect of different levels of alpha-lipoic acid and ALN on relative BCL-2 expression of rooster thawed semen. Extender supplemented with different levels of alpha-lipoic acid at 10 (A10), 20 (A20), 30 (A30), 40 (A40) and 50 (A50) μM and alpha-lipoic acid loaded nanostructured lipid carriers (ALN) at 10 (ALN10), 20 (ALN20), 30 (ALN30), 40 (ALN40) and 50 (ALN50) μM. Columns with different superscript (a, b, c, and d) indicate differences (P < 0.05).
Table 7 shows the fertility results of sperm in different levels of alpha-lipoic acid and ALN. The final percentage of fertility and hatchability rates were the highest (P < 0.05) in ALN30 semen group.
Table 7.
Effect of 30 μM alpha-lipoic acid, 30 μM alpha-lipoic acid-loaded NLC, and control group on fertility and hatchability rates of rooster semen after freeze-thawing.
| Parameters | Treatments |
||
|---|---|---|---|
| Control | ALN30 | A30 | |
| Fertilized eggs | 18 (36)b | 29 (58)a | 25 (50)a |
| Hatched eggs | 8 (16)c | 23 (46)a | 17 (34)b |
| Hatched eggs ratio (hatched/fertilized, %) | 44.4b | 79.31a | 68.0a |
Different superscripts letters within row are significantly different (P < 0.05).
Each experimental group contained 50 eggs initially. Extender supplemented with alpha-lipoic acid containing 30 (A30) and alpha-lipoic acid loaded nanostructured lipid carriers (ALN) containing 30 (ALN30) μM.
Discussion
In the current study, the effects of alpha-lipoic acid–loaded nanostructured lipid carriers on rooster sperm cryopreservation spermatozoa were evaluated. Several investigations revealed that lipid nanoparticles might be used for improving insolubility obstacles by delivering lipophilic substances (Fang et al., 2011). Alpha-lipoic acid is a compound which is poorly soluble in water and unstable, which make it an appealing candidate for development of a novel loaded nanostructured lipid carrier. Some properties of NLC formulation such as being biodegradable and biocompatible might possibly result in superiorities and simply eliminate or prevent the possible limitations.
It is proved that alpha-lipoic acid regulates metabolism and increases the availability of mitochondrial coenzymes, therefore improves the protection against free radicals which consequently reduces the prevalence of mitochondrial disorder, thus ensuring adequate ATP for sperm motility (Ma et al., 2011; Fayyaz et al., 2017; Ren et al., 2018). It was observed in the current study that alpha-lipoic acid improved sperm motility in ALN30. The observed improvement in sperm motility in the present research might be owing to energy modulation of spermatozoa related to alpha lipoic acid as it improves mitochondrial membrane potential of the sperm by functioning as an antioxidant. Furthermore, the addition of ALN30 to the freezing extender also ameliorated mitochondrial activity of rooster sperm, which is the same as the final results published by Ma et al. (2011). Even though alpha-lipoic acid has been confirmed to enhance sperm structural and morphological performance, but its higher concentration declined sperm quality. Totally, supplementing alpha-lipoic acid in semen extender improved the survival of sperm; therefore, it can be utilized in freezing extenders to maintain the sperm from cryopreservation damage (Fayyaz et al., 2017).
Alpha-lipoic acid known as a dithiol compound, reveals valuable effects on the cell which is under oxidative stress conditions because it shows strong synergistic effects with other antioxidants. It is demonstrated that alpha-lipoic acid reduces lipid peroxidation and protects the cells against unfavorable conditions such as radicals or toxic materials (Sandhya et al., 1995). Prahalathan et al. (2006) indicated that alpha-lipoic acid have effectively minimized the testicular toxicity caused by Adriamycin. Our findings are in agreement with Prahalathan et al. (2006) in ALN30 group on the parameters of the sperm motility and membrane integrity. In agreement to our findings, Ren et al. (2018) showed that supplementing alpha-lipoic acid in the extender led to beneficial effects on some goat sperm parameters such as motility, acrosome, and membrane integrity. However, Dominguez-Rebolledo et al. (2010) reported that alpha-lipoic acid shows that slight protective effect on the sperm of red deer.
In the percent study, ALN30 improved the sperm quality and TAC. However, Suleiman et al. (1996) showed that sperm MDA concentration increased when sperm motility was reduced. Bidmeshkipour et al. (2010) revealed that the TAC levels in the asthenospermic men seminal plasma were significantly lower than in healthy men. Moreover, they reported a positive correlation between TAC levels and sperm motility (Haghighian et al., 2015). In our study, ALN30 group significantly reduced in the lipid peroxidation and improved the antioxidant status. Ibrahim et al. (2008) reported an increase in goat sperm motility after adding alpha-lipoic acid into the semen. Alpha-lipoic acid is definitely a thiol carrying nucleophile, which generally reacts with endogenous electrophiles such as free radicals or chemically reactive metabolites. Alpha-lipoic acid is additionally demonstrated to generate the glutathione source through reduction of oxidized glutathione. Recent investigations have confirmed that alpha-lipoic acid might protect endogenous antioxidant enzymes by scavenging free radicals. For instance, alpha-lipoic acid could improve SOD, GSH, and CAT in rats (Abdou and Abdel-Daim, 2014). In the current study, we also observed that supplementing extenders with ALN30 enhanced GPx activity during the cryopreservation of sperm. There is a clear agreement with earlier studies which confirmed that alpha lipoic acid effectively protected sperm from the adverse effects of ROS (Ibrahim et al., 2008; Ma et al., 2011; Yeni et al., 2012). Though, the favorable antioxidant properties of alpha lipoic acid declined at high concentrations. As mentioned above, the addition of ALN30 enhanced GPx activity, which reduces ROS level of rooster sperm that attacks the sperm structure and mitochondria during cryopreservation. Therefore it is logical that in the current study, the mitochondria activity improved in the ALN30 group. In other reports, alpha-lipoic acid prohibited testicular damage caused by cadmium and cyclophosphamide by enhanced testicular antioxidants, improved enzymes such as superoxide dismutase, glutathione peroxidase, and glutathione reductase, and decreased MDA concentration in male rats (Selvakumar et al., 2005).
Another interesting result obtained in the present study was decreasing apoptosis along with the upregulation of bcl-2 by alpha-lipoic acid supplementation in the extender. In the present study, alpha-lipoic acid significantly reduced the percentage of apoptotic cells and the level of active caspase-3, which usually raises by the cryopreservation process. Consequently, it is predictable that alpha-lipoic acid can reduce apoptosis caused by oxidative stress following cryopreservation.
Our artificial insemination results showed that ALN30 supplemented to the extender was significantly better compared with the control group (P < 0.05). It might be because of the antioxidant capacity of alpha-lipoic acid which leads to a relative protection of sperm against oxidative damage caused during cryopreservation and artificial insemination. It was demonstrated in the present study that addition of ALN30 to the extender could enhance fertile and hatched eggs of frozen–thawed rooster sperm. Therefore, ALN30 could be actually a useful cryoprotectant in rooster sperm frozen extenders.
It was concluded from this study that higher motility, viability, mitochondria activity, membrane functionality, fertility, also lower lipid peroxidation and caspase-3 expression in rooster sperm was observed in ALN30 group after the freeze-thaw process. The results indicated that ALN30 might be considered as a novel potential cryoprotectant for the storage of rooster sperm in freezing media.
Acknowledgments
This research is supported by a research grant of the University of Tabriz (number 5783).
References
- Abdou R.H., Abdel-Daim M.M. Alpha-lipoic acid improves acute deltamethrin-induced toxicity in rats. Can. J. Physiol. Pharmacol. 2014;92:773–779. doi: 10.1139/cjpp-2014-0280. [DOI] [PubMed] [Google Scholar]
- Ansari M., Zhandi M., Kohram H., Zaghari M., Sadeghi M., Sharafi M. Improvement of post-thawed sperm quality and fertility of Arian rooster by oral administration of d-aspartic acid. Theriogenology. 2017;92:69–74. doi: 10.1016/j.theriogenology.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Bidmeshkipour A., Hosseinzadeh Colagar A., Gholinezhad Chari M., Biparva P. Seminal plasma total antioxidant capacity and vitamin-C levels in asthenozoospermia: a case-control study. Tehran Univ. Med. J. TUMS Publ. 2010;67:835–842. [Google Scholar]
- Bilska A., Wlodek L. Lipoic acid-the drug of the future. Pharmacol. Rep. 2005;57:570–577. [PubMed] [Google Scholar]
- Burrows W.H., Quinn J.P. The collection of spermatozoa from the domestic fowl and Turkey. Poult. Sci. 1937;16:19–24. [Google Scholar]
- Daghigh Kia H., Farhadi R., Ashrafi I., Mehdipour M. Anti-oxidative effects of ethanol extract of Origanum vulgare on kinetics, microscopic and oxidative parameters of cryopreserved Holstein bull spermatozoa. Iran. J. Appl. Anim. Sci. 2016;6:783–789. [Google Scholar]
- Dominguez-Rebolledo A.E., Fernandez-Santos M.R., Bisbal A., Ros-Santaella J.L., Ramon M., Carmona M., Martinez-Pastor F., Garde J.J. Improving the effect of incubation and oxidative stress on thawed spermatozoa from red deer by using different antioxidant treatments. Reprod. Fertil. Dev. 2010;22:856–870. doi: 10.1071/RD09197. [DOI] [PubMed] [Google Scholar]
- Fang Y.-P., Lin Y.-K., Su Y.-H., Fang J.-Y. Tryptanthrin-loaded nanoparticles for delivery into cultured human breast cancer cells, MCF7: the effects of solid lipid/liquid lipid ratios in the inner core. Chem. Pharm. Bull. 2011;59:266–271. doi: 10.1248/cpb.59.266. [DOI] [PubMed] [Google Scholar]
- Fattah A., Sharafi M., Masoudi R., Shahverdi A., Esmaeili V. L-carnitine is a survival factor for chilled storage of rooster semen for a long time. Cryobiology. 2017;74:13–18. doi: 10.1016/j.cryobiol.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Fayyaz M.H., Ahmad M., Ahmad N. Survival of buffalo bull spermatozoa: effect on structure and function due to alpha-lipoic acid and cholesterol-loaded cyclodextrin. Andrologia. 2017;49 doi: 10.1111/and.12652. [DOI] [PubMed] [Google Scholar]
- Haghighian H.K., Haidari F., Mohammadi-asl J., Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil. Steril. 2015;104:318–324. doi: 10.1016/j.fertnstert.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Ibrahim S.F., Osman K., Das S., Othman A.M., Majid N.A., Rahman M.P. A study of the antioxidant effect of alpha lipoic acids on sperm quality. Clin. (Sao Paulo) 2008;63:545–550. doi: 10.1590/S1807-59322008000400022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johinke D., de Graaf S.P., Bathgate R. Quercetin reduces the in vitro production of H2O2 during chilled storage of rabbit spermatozoa. Anim. Reprod. Sci. 2014;151:208–219. doi: 10.1016/j.anireprosci.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Li F., Wang Y., Liu Z., Lin X., He H., Tang X. Formulation and characterization of bufadienolides-loaded nanostructured lipid carriers. Drug Dev. Ind. Pharm. 2010;36:508–517. doi: 10.3109/03639040903264397. [DOI] [PubMed] [Google Scholar]
- Luno V., Gil L., Olaciregui M., Gonzalez N., Jerez R.A., de Blas I. Rosmarinic acid improves function and in vitro fertilising ability of boar sperm after cryopreservation. Cryobiology. 2014;69:157–162. doi: 10.1016/j.cryobiol.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Ma H., Quan F., Chen D., Zheng Y., Zhang B., Wang Y., Zhang Y. Protective function of alpha-lipoic acid on sperm motility and mitochondrial function during goat sperm-mediated gene transfer. Small Rumin. Res. 2011;99:191–198. [Google Scholar]
- Masoudi R., Sharafi M., Shahneh A.Z., Khodaei-Motlagh M. Effects of reduced glutathione on the quality of rooster sperm during cryopreservation. Theriogenology. 2019;128:149–155. doi: 10.1016/j.theriogenology.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Mehdipour M., Daghigh Kia H., Nazari M., Najafi A. Effect of lecithin nanoliposome or soybean lecithin supplemented by pomegranate extract on post-thaw flow cytometric, microscopic and oxidative parameters in ram semen. Cryobiology. 2017;78:34–40. doi: 10.1016/j.cryobiol.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Mehdipour M., Daghigh Kia H., Moghaddam G., Hamishehkar H. Effect of egg yolk plasma and soybean lecithin on rooster frozen-thawed sperm quality and fertility. Theriogenology. 2018;116:89–94. doi: 10.1016/j.theriogenology.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Mehdipour M., Kia H.D., Najafi A., V Dodaran H., Garcia-Alvarez O. Effect of green tea (Camellia sinensis) extract and pre-freezing equilibration time on the post-thawing quality of ram semen cryopreserved in a soybean lecithin-based extender. Cryobiology. 2016;73:297–303. doi: 10.1016/j.cryobiol.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Mahdieh M., Daghigh Kia H., Najafi A., Mohammadi H., Alvarez-Rodriguez M. Effect of crocin and naringenin supplementation in cryopreservation medium on post-thaw rooster sperm quality and expression of apoptosis associated genes. PLoS One. 2020;15:e0241105. doi: 10.1371/journal.pone.0241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi A., Kia H.D., Hamishehkar H., Moghaddam G., Alijani S. Effect of resveratrol-loaded nanostructured lipid carriers supplementation in cryopreservation medium on post-thawed sperm quality and fertility of roosters. Anim. Reprod. Sci. 2019;201:32–40. doi: 10.1016/j.anireprosci.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Najafi A., Kia H.D., Mehdipour M., Hamishehkar H., Alvarez-Rodriguez M. Effect of quercetin loaded liposomes or nanostructured lipid carrier (NLC) on post-thawed sperm quality and fertility of rooster sperm. Theriogenology. 2020;152:122–128. doi: 10.1016/j.theriogenology.2020.04.033. [DOI] [PubMed] [Google Scholar]
- Najafi A., Taheri R.A., Mehdipour M., Farnoosh G., Martinez-Pastor F. Lycopene-loaded nanoliposomes improve the performance of a modified Beltsville extender broiler breeder roosters. Anim. Reprod. Sci. 2018;195:168–175. doi: 10.1016/j.anireprosci.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Najafi A., Taheri R.A., Mehdipour M., Martínez-Pastor F., Rouhollahi A.A., Nourani M.R. Improvement of post-thawed sperm quality in broiler breeder roosters by ellagic acid-loaded liposomes. Poult. Sci. 2019;98:440–446. doi: 10.3382/ps/pey353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafi D., Taheri R.A., Najafi A., Rouhollahi A.A., Alvarez-Rodriguez M. Effect of Achillea millefolium-loaded nanophytosome in the post-thawing sperm quality and oxidative status of rooster semen. Cryobiology. 2018;82:37–42. doi: 10.1016/j.cryobiol.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Nouri H., Shojaeian K., Samadian F., Lee S., Kohram H., Lee J.I. Using resveratrol and epigallocatechin-3-gallate to improve cryopreservation of stallion spermatozoa with low quality. J. Equine Vet. Sci. 2018;70:18–25. [Google Scholar]
- Ogretmen F., Inanan B.E. Effect of butylated hydroxytoluene (BHT) on the cryopreservation of common carp (Cyprinus carpio) spermatozoa. Anim. Reprod. Sci. 2014;151:269–274. doi: 10.1016/j.anireprosci.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Prahalathan C., Selvakumar E., Varalakshmi P. Lipoic acid modulates adriamycin-induced testicular toxicity. Reprod Toxicol. 2006;21:54–59. doi: 10.1016/j.reprotox.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Ren F., Feng T., Dai G., Wang Y., Zhu H., Hu J. Lycopene and alpha-lipoic acid improve semen antioxidant enzymes activity and cashmere goat sperm function after cryopreservation. Cryobiology. 2018;84:27–32. doi: 10.1016/j.cryobiol.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Ruktanonchai U., Bejrapha P., Sakulkhu U., Opanasopit P., Bunyapraphatsara N., Junyaprasert V., Puttipipatkhachorn S. Physicochemical characteristics, cytotoxicity, and antioxidant activity of three lipid nanoparticulate formulations of alpha-lipoic acid. AAPS PharmSciTech. 2009;10:227. doi: 10.1208/s12249-009-9193-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhya P., Mohandass S., Varalakshmi P. Role of DL α-lipoic acid in gentamicin induced nephrotoxicity. Mol. Cell. Biochem. 1995;145:11–17. doi: 10.1007/BF00925707. [DOI] [PubMed] [Google Scholar]
- Santiani A., Evangelista S., Sepulveda N., Risopatron J., Villegas J., Sanchez R. Addition of superoxide dismutase mimics during cooling process prevents oxidative stress and improves semen quality parameters in frozen/thawed ram spermatozoa. Theriogenology. 2014;82:884–889. doi: 10.1016/j.theriogenology.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Selvakumar E., Prahalathan C., Mythili Y., Varalakshmi P. Beneficial effects of DL-α-lipoic acid on cyclophosphamide-induced oxidative stress in mitochondrial fractions of rat testis. Chem. Biol. Interact. 2005;152:59–66. doi: 10.1016/j.cbi.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Shahverdi A., Sharafi M., Gourabi H., Yekta A.A., Esmaeili V., Sharbatoghli M., Janzamin E., Hajnasrollahi M., Mostafayi F. Fertility and flow cytometric evaluations of frozen-thawed rooster semen in cryopreservation medium containing low-density lipoprotein. Theriogenology. 2015;83:78–85. doi: 10.1016/j.theriogenology.2014.07.044. [DOI] [PubMed] [Google Scholar]
- Shojaeian K., Nouri H., Kohram H. Does MnTBAP ameliorate DNA fragmentation and in vivo fertility of frozen-thawed Arabian stallion sperm? Theriogenology. 2018;108:16–21. doi: 10.1016/j.theriogenology.2017.11.019. [DOI] [PubMed] [Google Scholar]
- Song R., Yao X., Shi L., Ren Y., Zhao H. Effects of dietary selenium on apoptosis of germ cells in the testis during spermatogenesis in roosters. Theriogenology. 2015;84:583–588. doi: 10.1016/j.theriogenology.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Suleiman S.A., Ali M.E., Zaki Z.M.S., El-Malik E.M.A., Nasr M.A. Lipid peroxidation and human sperm motility: protective role of vitamin E. J. Androl. 1996;17:530–537. [PubMed] [Google Scholar]
- Wang J., Tang J., Zhou X., Xia Q. Physicochemical characterization, identification and improved photo-stability of alpha-lipoic acid-loaded nanostructured lipid carrier. Drug Dev. Ind. Pharm. 2014;40:201–210. doi: 10.3109/03639045.2012.753901. [DOI] [PubMed] [Google Scholar]
- Yeni D., Fidan A.F., Cigerci I.H., Konuk M., Avdatek F., Gundogan M. Effect of alpha-lipoic acid on sperm quality, reproductive tract measures in thinner exposed rats. Andrologia. 2012;44(Suppl 1):74–80. doi: 10.1111/j.1439-0272.2010.01140.x. [DOI] [PubMed] [Google Scholar]