Abstract
The aim of this study was to investigate the effects of osteocalcin (OCN) on fatty liver hemorrhagic syndrome (FLHS) in aged laying hens. Thirty 68-week-old White Plymouth laying hens were randomly assigned into conventional single-bird cages, and the cages were randomly allocated into one of 3 treatments (n = 10): normal diet (ND + vehicle, ND + V), high-fat diet (HFD + vehicle, HFD + V), and HFD + OCN (3 μg/bird, 1 time/2 d, i.m.) for 40 d. At day 30, oral glucose tolerance tests (OGTT) and insulin tolerance tests (ITT) were performed. At the end of experiment, the hens were euthanized followed by blood collection. The plasma aspartate transaminase (AST), alkaline phosphatase (ALP), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured using an automatic biochemistry analyzer. Pathological changes in the liver were examined under both light and transmission electron microscopes. The plasma inflammatory factors including interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α) were analyzed by ELISA, and the gene expressions of these inflammatory factors in the liver were analyzed by real-time PCR. The level of oxidative stress was evaluated using malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) assay kits, respectively. The results showed that HFD + V hens had more severe liver hemorrhage and fibrosis than ND + V hens (P < 0.05). The ultramicrostructural examination showed that hepatocytes of HFD + V hens exhibited necrotic pyknosis showing great intracellular electron, mitochondrial swelling, shrunk nucleus, and absence of autolysosomes. Osteocalcin mitigated HFD + V–induced pathological changes in aged laying hens. High-fat diet + OCN hens had higher insulin sensitivity; lower liver concentrations of MDA (P = 0.12) but higher GSH-Px (P < 0.05); and lower blood TNF-α concentrations (P < 0.05) and mRNA expressions (P < 0.05) than HFD + V hens. These results suggest OCN functions in preventing the FLHS process in old laying hens through inhibiting excessive energy diet-induced metabolic disorder, oxidative stress, and related pathological damage.
Key words: high-fat diet, fatty liver hemorrhagic syndrome, osteocalcin, metabolic disorder, hen
Introduction
Fatty liver hemorrhagic syndrome (FLHS) is a critical noninfectious metabolic disease, causing sudden death of birds resulted from liver rupture and hemorrhage (Trott et al., 2014; Rozenboim et al., 2016). Fatty liver hemorrhagic syndrome is one of the main causes of death in laying hens (Shini et al., 2019), associated with approximately 40% of the necropsied hens, and can be up to 74% in the caged laying hens (Trott et al., 2014). Several factors have been related to FLSH, such as nutritional, metabolic, hormonal, environmental, and genetic factors (Trott et al., 2014; Rozenboim et al., 2016), but excess energy intake is the fundamental player as that FLHS can be induced by high-fat diets (HFD) (Zhang et al., 2008; 2018; Choi et al., 2012; Jiang et al., 2014; Sanchez-Polo et al., 2015) or high-fat and low-protein (HFLP) diets (Jiang et al., 2013a,b; Rozenboim et al., 2016; Gao et al., 2019; Peng et al., 2019; Robinson and Kiarie, 2019).
The hen liver plays a major role in the synthesis and metabolism of fat. The synthesis of fat in birds is much greater in the hepatic tissue than in the adipose tissue (Zaefarian et al., 2019). In addition, commercial hens have been selected for high production and feed efficiency, by which hen liver is more susceptible to metabolic disorder–induced injury. Hen FLHS has been used as a model of mammal nonalcoholic fatty liver disease (NAFLD) (Ayala et al., 2009; Sanchez-Polo et al., 2015; Tsai et al., 2017; Wu et al., 2019; Zhuang et al., 2019). Although the pathophysiological mechanism of hen FLSH remains unclear, it could be similar to the ones identified in NAFLD, such as insulin resistance, hepatic oxidation stress, inflammatory reaction, and autophagy (Ayala et al., 2009; Cai et al., 2011; Wu et al., 2019; Zhuang et al., 2019).
Osteocalcin (OCN), a major noncollagenous protein in bone matrix, is mainly synthesized by osteoblasts (Gundberg et al., 1984; Jiang et al., 2013a). A fraction of OCN is released into blood as uncarboxylated OCN (ucOCN) and carboxylated OCN (cOCN) (Li et al., 2016; Mizokami et al., 2017). For example, the total circulating OCN concentration is approximately 300 ng/mL in adult wild-type mice, whereas the ucOCN is only 5∼10 ng/mL (Ferron et al., 2008; Ferron et al., 2010a). However, ucOCN has been known as the “active form” with an energy-regulating function in rodent (Lee et al., 2007; Ferron et al., 2008; 2010b) and clinical research (Kanazawa et al., 2009; Strapazzon et al., 2011; Lacombe et al., 2020). In humans, ucOCN has been used for preventing various metabolic diseases including type 2 diabetes (Ferron et al., 2008; 2012) and NAFLD (Lee et al., 2007; Gupte et al., 2014; Du et al., 2016). In rodents, ucOCN (3 or 30 ng/g/d) significantly increases glucose tolerance and insulin sensitivity in mice fed a regular die. In addition, administration of 30 ng/g/d recombinant OCN can partially restore insulin sensitivity and glucose tolerance in HFD fed mice and prevent the development of type 2 diabetes (Ferron et al., 2012). Uncarboxylated OCN also prevents NAFLD in mice fed a western-style high-fat high-cholesterol diet, resulting from developing insulin resistance, reducing hepatic damage including steatosis, ballooning degeneration, and fibrosis, and inhibiting expressions of proinflammatory and profibrotic genes through the regulation of the nuclear factor-like 2 and or c-Jun N-terminal kinase pathways (Gupte et al., 2014; Du et al., 2016).
In hens, the OCN synthesis and release are affected by the sexual maturation process and egg production. It is 200∼300 ng/mL at 6 wk old, decreases to about 150 ng/mL at 16 wk, and then sharply declines to below 50 ng/mL after hens starting to lay eggs, even cannot be detected in some laying hens (Fleming et al., 2003). The average of total blood OCN concentration is only 10∼30 ng/mL in 70 wk or older laying hens (Fleming et al., 2003; Jiang et al., 2013a). Compared with it in mice (about 300 ng/mL), the circulating total OCN concentration is far lower in adult laying hens (Ferron et al., 2010a). Therefore, it is possible that OCN in hens has some specific functions which have not releaved in mice. Previously, we have shown that the alteration of serum OCN concentration is correlated with the fatty liver disorder in HFLP diet fed hens (Jiang et al., 2013b). In the present study, the mechanism of OCN in promoting liver health of high-fat diet fed laying hens was further investigated.
Materials and methods
All procedures in this experiment were approved by the Animal Ethics Committee of the Southwest University, Chongqing, China (the permission number: IACUC-20190725023).
Experimental Design
Thirty healthy 68-week-old White Plymouth Rock laying hens were randomly assigned into conventional single-bird cages (40 cm × 35 cm × 35 cm each). After 2 wk for hens to adapt their rearing environment, the cages were randomly assigned into one of 3 treatments (n = 10 per treatment): normal diet + vehicle (ND + V, Saline, 1 time/every other day, i.m.), HFD + vehicle (HFD + V) (Table 1), and HFD + ucOCN treatment (3 μg/bird, 1 time/every other day, i.m.) (HFD + OCN) for 40 d (75.7 wk of age). The selected dose was based on the studies conducted in mice (Ferron et al., 2008; Gupte et al., 2014; Du et al., 2016). The recombinant chicken ucOCN solution (MyBioSource, San Diego, CA) was freshly diluted in saline solution at a concentration of 6 μg/mL. During the experimental period, hens received light for 16 h/d. Feed and water were provided ad libitum.
Table 1.
Laying hen feeding recipes.
| Ingredient | Normal diet | High-fat diet |
|---|---|---|
| Corn (%) | 62.6 | 49 |
| Soybean (%) | 25.7 | 28.5 |
| Shell powder (%) | 7.4 | 7.4 |
| Soybean oil (%) | 1.3 | 9.8 |
| Zeolite powder | 0 | 2.3 |
| 3% premix1 (%) | 3 | 3 |
| Nutrition composition | ||
| Energy (MJ/kg) | 11.2 | 13.0 |
| Crude protein (%) | 16.5 | 16.5 |
| Calcium (%) | 3.5 | 3.5 |
Premix supplied the following per kilogram of feed: vitamin A: 220,000 to 330,000 IU, vitamin D3: 55,000 to 85,000 IU, vitamin E: ≥320 mg, vitamin K3: 40 to 140 mg, vitamin B1: ≥75 mg, vitamin B2: ≥155 mg, vitamin B6: ≥75 mg, thiamine nitrate: ≥ 80 mg, calcium pantothenate: ≥ 155 mg, nicotinamide: ≥ 850 mg, iodine: 5 to 15, iron 2,000 to 6,000 mg, zinc: 2,400 to 4,830 mg, manganese: 2,930 to 4,820 mg, copper: 267 to 667 mg, selenium: 5 to 15 mg, calcium: ≥ 8%, total phosphorus: ≥3.3%, sodium chloride: 7 to 14%, methionine: ≥2.3%.
Sample Collection
Body weight (BW) was measured individually at day 0, 20, and 40 during the study. Feed intake was recorded and calculated on days 19 to 20 and 39 to 40, respectively (Jiang et al., 2013b). At the end of the experiment, each hen was anesthetized with pentobarbital sodium (30 mg/mL) within 2 min after removed from its cage. A 10 mL of blood sample from each hen (n = 10) was collected via cardiac puncture into a plasma separator tube with EDTA and then centrifuged at 3,000 × g for 15 min at 4°C for collecting plasma. After blood collection, the chickens were euthanized immediately.
After gross liver pathological examination (please see the following), the whole abdominal fat pad, liver, and pancreas weight of each hen were collected, and relative weight of the abdominal fat pad, liver, and pancreas mass was calculated using the following formula: the relative weight = the tissue (organ) weight (g)/the body weight (kg). A piece of the left lob liver (1 mm × 1 mm × 1 mm) sample at the same location was collected from each hen and fixed with 2.5% glutaraldehyde for transmission electron microscope analysis. The rest of left lob liver was fixed in 10% formalin until analysis. And, a piece of right lob liver sample was stored at −80°C for real-time PCR and western-blotting analyses. The rest of right lob live was stored at −20°C for liver fat content analysis by the Soxhlet method (Jiang et al., 2013b).
Liver Hemorrhage Score
The liver hemorrhage was scored from 0 to 5: 0, indicating normal liver; 1, less than 10 subcapsular petechial or ecchymotic hemorrhages; 2, more than 10 subcapsular petechial or ecchymotic hemorrhages; 3, more than 1/3 but less than 1/2 of liver has subcapsular ecchymosis or ecchymosis;4, more than 1/2 liver but less than 3/4 of liver has subcapsular ecchymosis or ecchymosis; 5, more than 3/4 of liver has subcapsular ecchymosis or ecchymosis (Diaz et al., 1999; Rozenboim et al., 2016).
Metabolic Analysis
At day 30, oral glucose tolerance tests (OGTT) were performed following 16 h overnight fasting. Five birds per group were orally given glucose at 2 g/kg BW, and then the comb's blood glucose level was measured at 0, 15, 30, 60, 120, and 240 min using a blood glucose meter (Sinocare Inc., Changsha, China). Insulin tolerance tests (ITT) were performed on the rest 5 birds per group after 16 h of fasting. Bovine insulin (100 μg/kg BW) (Coolaber, Beijing, China) was administered by abdominal subcutaneous injection (s.c.), and then the comb's blood glucose level was measured at 0, 15, 30, 60, 120, and 240 min. The concentration of plasma insulin was measured on day 40 in 75.7-week-old laying hens by ELISA kit (Xiamen Huijia Biotechnology Co., Ltd., Fujian, China).
Blood Parameters Analysis
The concentrations of plasma aspartate transaminase (AST), alkaline phosphatase (ALP), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured using the relative commercial kits via an automatic biochemistry analyzer (Olympus AU400, Japan). And the concentrations of interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α) were analyzed using the relative ELISA kits (Xiamen Huijia Biotechnology Co., Ltd., Fujian, China).
Liver Microstructural and Ultramicrostructure Analyses
The Light Microscopic Analysis. The liver samples were processed by followed a routine tissue preparation procedure used in our laboratory. Briefly, the samples were dehydrated with serially diluted ethanol, transparentized with benzene, embedded in paraffin, and then sectioned at 3 μm using a sliding microtome (Leica RM2235; Leica microsystems, Wetzlar, Germany). The sections were stained with hematoxylin and eosin or Masson's trichromatic staining for light microscopic examinations (Leica DM500; Leica microsystems, Wetzlar, Germany). The Masson's trichromatic stained fibrosis areas were analyzed by Image J software (National Institutes of Health).
The Electronic Microscopic Analysis. The ultrathin sections of liver samples were prepared and analyzed at the Wuhan Servicebio technology Co., Ltd. (Wuhan, China). Briefly, liver samples were fixed with 2.5% glutaraldehyde and rinsed by 0.1 mol phosphoric acid buffer (pH7.4) for 15 min, and repeated 3 times, then fixed again with 1% osmic acid·0.1 mol phosphoric acid buffer. The fixed sections were dehydrated with serially diluted ethanol, permeated with acetone and SPI-pon812 embedding agent (1:1; SPI Supplies, West Chester, PA), and embedded with SPI-Pon812 embedding agent, then sectioned at 60 to 80 nm using ultra microtome (Leica UC7; Leica microsystems, Wetzlar, Germany). The slices were stained using double staining method: 2% saturated alcohol solution of uranium acetate and lead citrate, 15 min for each. The specimens were analyzed under the transmission electron microscope (HT7700; Hitachi, Japan).
Oxidative Damage Factors Concentration of Liver
The concentrations of malondialdehyde (MDA) and glutathione peroxidase (GSH-Px) in the liver were measured by using the MDA and GSH-Px assay kit, respectively (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer's instructions.
Real-Time PCR
Total RNA of each liver samples was extracted using Trizol reagent (Invitrogen); and its quality was examined using NanoPhotometer (P330, Implen, Munich, Germany). The cDNA of IL-1, IL-6, and TNF-α and GAPDH (Hu et al., 2008) was synthesized from 1 μg of total RNA using NovoScript Plus All-in-one 1st Strand cDNA Synthesis SuperMix (gDNA Purge) (Novoprotein Scientific Inc., Jiangsu, China). The GAPDH was used as a housekeeping gene. The quantities of mRNA expression of IL-1, IL-6, and TNF-α relative to GAPDH mRNA expression were determined using the 2−ΔCt method by fluorescent quantitative real-time PCR, where ΔCt = Cttarget gene − Cthousekeeping gene. The sequences of primers were designed using the Primer 5.0, synthesized by the Invitrogen Biotechnology (Shanghai, China), and presented in Table 2.
Table 2.
Real-time PCR primers and amplified PCR product size.
| Gene | GenBank ID | PCR primers sequence (5′-3′) | PCR products (bp) |
|---|---|---|---|
| IL-1 | NM_204524.1 | F: GGTCAACATCGCCACCTACA R: CATACGAGATGGAAACCAGCAA |
86 |
| IL-6 | NM_204628.1 | F: AAATCCCTCCTCGCCAATCT R: CCCTCACGCTCTTCTCCATAAA |
106 |
| TNF-α | NM_204267.1 | F: GGACAGCCTATGCCAACAAG R: ACACGACAGCCAAGTCAACG |
168 |
| GAPDH | NM_204305.1 | F: TTGACGTGCAGCAGGAACAC R: ATGGCCACCACTTGGACTTT |
124 |
Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL-1, interleukin-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha.
Statistical Analyses
The data were analyzed by using SPSS 20.0 (IBM Corp.). A one-way ANOVA was used to analyze the differences between treatments. The experiment unit was the cage (n = 10). Post hoc multiple comparisons were performed using LSD's test. Data normality was checked. Values were expressed as mean ± SEM, and P-value < 0.05 was considered statistically significant.
Results
Osteocalcin Effect on HFD-Induced Gross Organ Changes and Liver Damage
Compared with the controls, both HFD + V and HFD + OCN birds had higher absolute and relative abdominal fat pad weights (P < 0.05; Table 3), whereas HFD + V birds but not HFD + OCN birds also had a lower relative liver weight (P < 0.05). There were no treatment effects on BW, feed intake, pancreas weight, and liver fat content (P > 0.05; Table 3). However, compared with controls, OCN administration alleviated HFD-induced hen liver hemorrhage damage (P = 0.05).
Table 3.
The effects of osteocalcin on body weight, feed intake, abdominal fat pad, pancreas, and liver weights in high-fat diet fed aged laying hens.
| Item | ND + V | HFD + V | HFD + OCN | SEM | P |
|---|---|---|---|---|---|
| 0 d BW (g) | 1,569.8 | 1,602.9 | 1,607.7 | 0.06 | 0.81 |
| 20 d BW (g) | 1,603.0 | 1,665.1 | 1,673.6 | 0.07 | 0.59 |
| 40 d BW (g) | 1,557.8 | 1,687.7 | 1,655.9 | 0.08 | 0.23 |
| 20 d feed intake (g) | 95.66 | 87.50 | 88.18 | 8.11 | 0.56 |
| 40 d feed intake (g) | 97.51 | 89.43 | 91.05 | 8.26 | 0.91 |
| Fat pad weight (g) | 37.37b | 67.32a | 62.97a | 8.48 | 0.003 |
| Relative fat pad mass (g/kg) | 23.61b | 39.19a | 37.66a | 4.11 | 0.001 |
| Liver weight (g) | 40.90 | 37.85 | 40.72 | 3.03 | 0.54 |
| Relative liver mass (g/kg) | 26.19a | 22.31b | 24.71a,b | 1.44 | 0.04 |
| Pancreas weight (g) | 3.46 | 3.28 | 3.46 | 0.27 | 0.76 |
| Relative pancreas mass (g/kg) | 2.22 | 1.94 | 2.09 | 0.14 | 0.17 |
| Liver hemorrhage score | 0.8a | 1.67b | 1.0a,b | 0.92 | 0.05 |
| Fat content of liver (%) | 21.11 | 17.06 | 18.20 | 0.8 | 0.15 |
a, bMean ± SEM with different small letter in the same row differ significantly (n = 10, P < 0.05).
Abbreviations: BW, body weight; HFD + OCN, high-fat diet + osteocalcin; HFD + V, high-fat diet + vehicle; ND + V, normal diet + vehicle.
Osteocalcin Effect on Biochemical Parameters of Liver Function and Blood Lipids
Both HFD + V and HFD + OCN hens had higher plasma ALP activity than the control hens (P < 0.05; Table 4). However, there were no treatment effects on plasma AST activity and TC, TG, LDL-C, and HDL-C concentrations (P > 0.05).
Table 4.
The effects of osteocalcin on liver function and lipid biochemical indexes in high-fat diet fed aged laying hens.
| Parameters | ND + V | HFD + V | HFD + OCN | SEM | P |
|---|---|---|---|---|---|
| AST (U/L) | 157.40 | 147.06 | 142.41 | 9.82 | 0.32 |
| ALP (U/L) | 285.1b | 1,139.7a | 978.3a | 42.79 | 0.008 |
| TC (mmol/L) | 2.75 | 2.07 | 2.97 | 0.64 | 0.35 |
| TG (mmol/L) | 12.06 | 11.98 | 12.59 | 2.28 | 0.96 |
| LDL-C (μmol/L) | 312.08 | 325.45 | 321.27 | 9.77 | 0.40 |
| HDL-C (μmol/L) | 198.15 | 202.86 | 200.73 | 5.80 | 0.73 |
a, b Mean ± SEM with different capital letter in the same row differ significantly (n = 10, P < 0.05).
Abbreviations: ALP, alkaline phosphatase; AST, aspartate transaminase; HDL-C, high density lipoprotein cholesterol; HFD + OCN, high-fat diet + osteocalcin; HFD + V, high-fat diet + vehicle; ND + V, normal diet + vehicle; LDL-C, low density lipoprotein cholesterol; TC, total cholesterol; TG, triglyceride.
Osteocalcin Effect on Hepatic Fibrosis, Pyknosis, and Autophagy
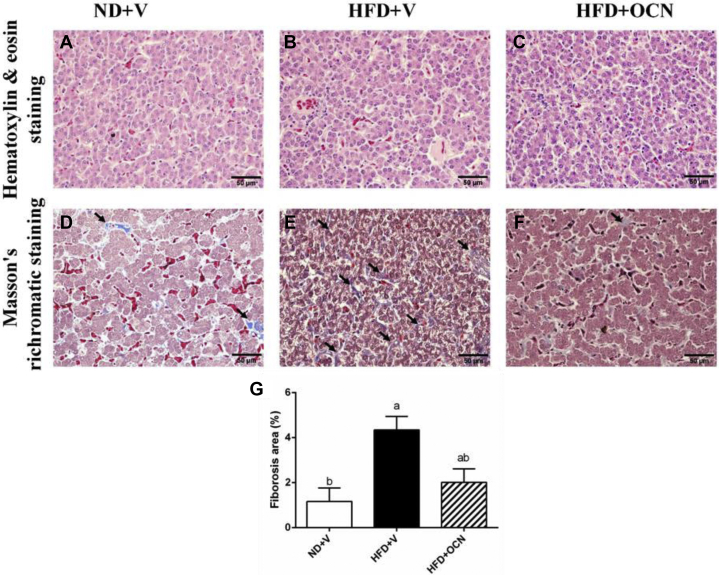
There was no treatment effect on the liver microstructure observed with hematoxylin and eosin staining (Figures 1A–C) except that the fibrosis areas; excessive amount of scar tissue was significantly increased (P < 0.05) in the HFD + V group compared with the controls (Figures 1D–G), which the HFD negative effect was reduced by OCN administration although without significance (P = 0.14).
Figure 1.
The effects of a high-fat diet and osteocalcin on the pathological changes of the liver in aged hens. (A–C) Examples of liver light microscopical structures were examined with HE staining. (D–F) The hepatica fibrosis change was observed by Masson's trichromatic staining. (G) Percentage of the fibrosis areas. Bar = 50 μm. →: fibrosis. a, bMean ± SEM with different small letter differ significantly (P < 0.05, n = 10). Abbreviations: HE, hematoxylin and eosin; HFD + OCN, high-fat diet + osteocalcin; HFD + V, high-fat diet + vehicle; ND + V, normal diet + vehicle.
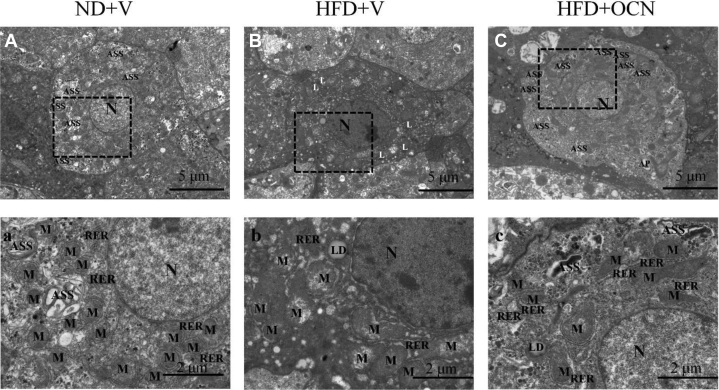
The transmission electron microscopic image observation indicated compared with the controls (Figures 2A, 2a), the HFD + V (Figures 2B, 2b) hens' hepatocytes had more cellular damage with hepatocyte apoptosis evidenced as nuclear pyknosis, higher intracellular electron, and great mitochondrial swelling with decreased or disappeared of crista and increased dilated rough endoplasmic reticulum; and autolysosomes were replaced by lysosomes. In HFP + OCN hens, OCN administration prevented the damage effects of HFD on hepatocytes; and autolysosomes exhibited similarly to those found in the controls (Figures 2C, 2c).
Figure 2.
The effects of a high-fat diet and osteocalcin on the hepatocytic ultrastructures in aged hens. (A, B, C) Examples of electron micrographs showing hepatocytic ultrastructural features. (a, b, c) High power micrographs of the relative areas outlined by the squares. Bar = 5 or 2 μm, respectively. Abbreviations: AP, autophagosome; ASS, autolysosome; HFD + OCN, high-fat diet + osteocalcin; HFD + V, high-fat diet + vehicle; L, lysosome; LD, lipid droplet; M, mitochondria; N, nucleus; ND + V, normal diet + vehicle; RER, endoplasmic reticulum.
Osteocalcin Alleviates HFD-Induced Insulin Resistance
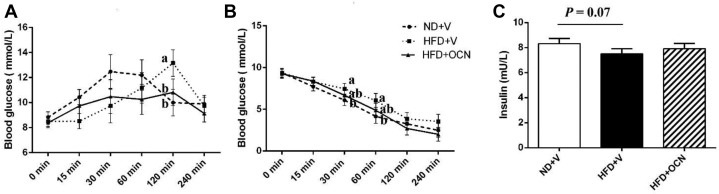
The treatment significantly affected the OGTT testing outcomes with a unique time-dependent changed pattern of blood glucose concentrations. In the controls, the blood glucose concentration was sharply increased at 15 min after oral glucose challenge, reached a maximum (12.48 mmol/L) at 30 min, maintained at the similar level until 60 min, then sharply reduced at 120 min and maintained at the level up to 240 min (Figure 3A). In the HFD + V group, the blood glucose concentration reached its peak (12.48 mmol/L vs. 13.16 mmol/L; control vs. HFD + V) was delated until 120 min (30 min vs. 120 min; control vs. HFP + V hens), and then sharply diminished to the control level at 240 min. The effects of HFD on blood glucose concentration were decreased by administration of OCN. In HFD + OCN group, the concentration of blood glucose was relative stable, and the peak level was 10.82 mmol/L only at 120 min, which was similar to the level of ND + V group at the same point tine (P > 0.05) but significantly lower than HFD + V group (P < 0.05).
Figure 3.
The effects of a high-fat diet and osteocalcin on glucose tolerance and insulin sensitivity outcomes in aged hens. (A) The outcomes of glucose tolerance test. (B) The outcomes of the insulin tolerance test. (C) Plasma insulin concentrations. a, b Mean ± SEM with different small letter differ significantly (P < 0.05, n = 10). Abbreviations: HFD + OCN, high-fat diet + osteocalcin; HFD + V, high-fat diet + vehicle; ND + V, normal diet + vehicle.
There were treatment effects on ITT outcomes. High-fat diet + V hens had the highest blood glucose level from 15 min to 240 min after administrated insulin among treatments (Figure 3B). Compared with the controls, the blood glucose levels were significantly increased between 30 and 60 min in HFD + V hens (P < 0.05), whereas HFD + OCN group was intermediate (P > 0.05). In addition, the plasma insulin concentration in HFD + V hens but not in HFD + OCN hens had a downward trend compared with the control hens (P = 0.07; Figure 3C).
Osteocalcin Effects on Liver Oxidative Stress and Inflammatory Reaction
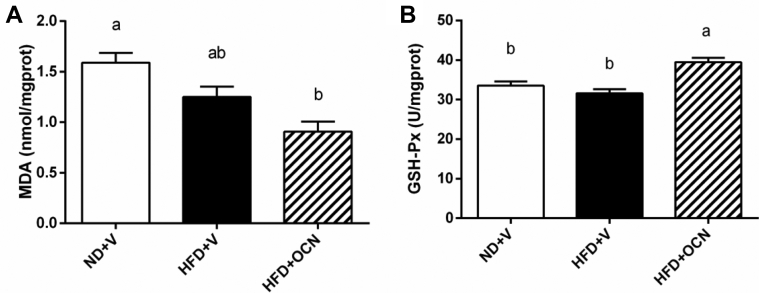
Compared with the control group, liver MDA concentration in HFD + OCN group was lower (P < 0.05; Figure 4A) but GSH-Px concentration was higher (P < 0.05; Figure 4B). And, compared with the HFD + V group, the liver GSH-Px was increased (P < 0.05) in the HFD + OCN group.
Figure 4.
The effects of a high-fat diet and osteocalcin on hepatic oxidative damage in aged hens. (A) The changes of malondialdehyde concentrations. (B) The changes of glutathione peroxidase concentrations. a, b Mean ± SEM with different small letter differ significantly (P < 0.05, n = 10). Abbreviations: GSH-Px, glutathione peroxidase; HFD + OCN, high-fat diet + osteocalcin; HFD + V, high-fat diet + vehicle; MDA, malondialdehyde; ND + V, normal diet + vehicle.
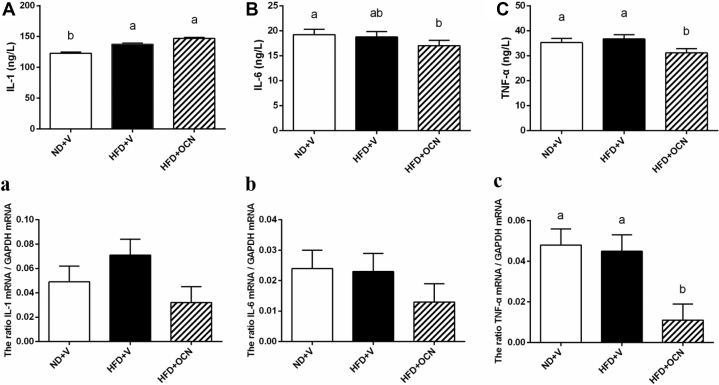
Compared with the control group, the plasma IL-1 (Figure 5A) concentration was increased in both the HFD + V (P < 0.05) and HFD + OCN (P < 0.05) groups, but IL-6 (P < 0.05; Figure 5B) and TNF-α (P < 0.05; Figure 5C) concentrations were reduced in the HFD + OCN group but not in the HFD + V group. Moreover, compared with the control group, the gene expression of TNF-α in the liver was significantly decreased by OCN administration compared with the ND + V (P < 0.05; Figure 5c) and HFD + V (P < 0.05) groups. There was no treatment effect on the liver IL-1 and IL-6 mRNA expressions (P > 0.05; Figures 5a, 5b).
Figure 5.
The effects of a high-fat diet and osteocalcin on plasma concentrations and liver mRNA expressions of inflammatory factors in aged hens. (A) The change of plasma IL-1. (B) The change of plasma IL-6. (C) The change of plasma TNF-α. (a) The change of liver IL-1 mRNA expression. (b) The change of liver IL-6 mRNA expression. (c) The change of liver TNF-α mRNA expression. a, b Mean ± SEM with different small letter differ significantly (P < 0.05, n = 10). Abbreviations: HFD + OCN, high-fat diet + osteocalcin; HFD + V, high-fat diet + vehicle; ND + V, normal diet + vehicle; IL-1, interleukin-1; IL-6, interleukin-6; TNF-α, tumor necrosis factor-alpha.
Discussion
Hens fed a HF diet (Zhang et al., 2008; 2018; Choi et al., 2012; Jiang et al., 2014) or HFLP diet (Jiang et al., 2013b; Rozenboim et al., 2016; Gao et al., 2019; Peng et al., 2019; Robinson and Kiarie, 2019) have been created for studying the pathogenesis of FLHS in laying hens or as an animal model for human NAFLD investigation. The pathological characters of the FLHS include hepatic fat accumulation with liver hemorrhage or rupture. In the current experiment, however, the liver weight, especially relative liver weight, was reduced without change of fat content in hens fed HFD. These results may indicate that the HFD did not induce hepatic lipidosis in aged hens. However, HFD fed hens had severe liver hemorrhages and significant heavier abdominal fat pad. Chicken stores energy as neutral fats mainly in the adipocytes of the abdominal fat pad (Alvarenga et al., 2011). Although it was not observed hepatic lipidosis in HFD-fed hens, the results from a previous study have shown that the abdominal fat weight is positively correlated with the liver fat percentage in broilers (Liang et al., 2015). The different findings could be related to multiple factors such as the genetic background of chickens and their age. Similar to the current findings, Trott et al. (2014) reported that 48% of 76 FLHS backyard chickens hardly had hepatic fat accumulation. Rozenboim et al. (2016) reported that both HFD and HFLP diets reduced liver fat content without effect on liver mass in 100-week-old hens, whereas the diets significantly increased liver fat content in 42-week-old hens. Therefore, the hepatic lipidosis may not be generalizable in FLHS chickens. In addition, the reduced liver mass in HFD + V hens may be because the hepatic apoptosis and nuclear pyknosis evidenced by the ultramicrostructural analyses, indicating severer liver damage. Ultramicrostructural analysis also revealed that HFD inhibits hepatic autophagy but OCN reversed the effect. Autophagy, a self-degradative process, is a critical biological function for the degradation of damaged intracellular components by lysosomes (Amir and Czaja, 2011; Ni et al., 2012; Kwanten et al., 2016; Mao et al., 2016). Hepatocytes' autophagic function is affected by hepatocytic lipid accumulation by which it reduces the infusion efficiency between autophagosomes and lysosomes, leading to suppressed autophagy (Singh et al., 2009). The hepatocytes' autophagy is also inhibited by insulin resistance-associated hepatocyte oxidative stress, inflammatory reaction, and apoptosis (Amir and Czaja, 2011; Kwanten et al., 2016; Wu et al., 2018). Autophagy has been potentially linked to fibrogenesis (Amir and Czaja, 2011). The hypothesis is further supported by the pathological changes with fibrosis in the livers of HFD-induced FLHS hens. In humans, similar to the pathological findings in HFD-induced FLHS of laying hens, NAFLD is an accumulating liver damage which includes fatty (buildup of fat), steatohepatitis, fibrosis, and cirrhosis of the liver (Sharma and Arora, 2020). Therefore, we consider that OCN prevents or reduces the HFD promoting the fat metabolic disorder–associated steatohepatitis and/or fibrosis of FLHS seen in aged hens.
Plasma ALP and AST activities have been used as indicators of liver damage; and plasma TC, TG, LDL-C, and HDL-C are the biomarkers of blood lipid metabolism. There biological factors have been used for diagnosis of FLHS in chickens (Yousefi et al., 2005; Zhang et al., 2008) and NAFLD in humans (Shi et al., 2013; Zhuang et al., 2015). In the current experiment, ALP but not AST activity was increased in HFD + V hens compared with the controls. Similarly, Rozenboim et al. (2016) analyzed the changes of ALP and AST in both 26-week-old and 84-week-old laying hens fed HFD, low-protein diet, or HFLP at 5, 10, and 15 wk. The plasma ALP value was significantly higher in young hens at week 5 whereas the value of AST became higher at week 15 post-fed HFLP diets than in the controls; while there were not diet effects on both ALP and AST concentrations in 84-week-old hens at any tested time points. Choi et al. (2012) and Robinson and Kiarie (2019) also reported that the blood concentration of AST and ALP were not difference between controls and FLSH hens. In the present study, there were also no treatment effects on the concentrations of TC, TG, LDL-C, and HDL-C, which may reveal that HFD causes liver damage without hepatic lipidosis. These biochemistry indicators of liver function and blood lipid metabolism have been considered as limited diagnostic tools for FLHS (Rozenboim et al., 2016).
Insulin is the only hyperglycemic hormone released by the pancreatic β cells in humans and various animals including chickens. Insulin resistance plays a key role in both human NAFLD and hen FLHS (Gariani et al., 2013; Zhuang et al., 2019). In the present study, after fed 40 d of HFD, blood glucose tolerance, insulin sensitivity, and insulin concentration were decreased in hens, but these changes were alleviated by OCN administration. Similar to our results, Zhuang et al. (2019) reported that FLHS hens have higher blood glucose than the control hens detected by both OGTT and ITT. In addition, HFD-induced NAFLD-like damage in mice exhibits the positive results during both OGTT and ITT analyses, and these changes can be revised by oral administration or intramuscular injection of OCN (Gupte et al., 2014; Du et al., 2016; Yasutake et al., 2016). The changes of insulin concentrations identified in the present study may indicate that OCN administration may prevent or reduce HFD-caused pancreatic β cell damage (Ferron et al., 2008, 2012).
Insulin resistance will lead to further hepatic damage by triggering oxidative stress and inflammatory reaction (Gariani et al., 2013; Zhuang et al., 2019). Oxidative stress is caused by reactive oxygen compounds such as MDA which is a toxic molecule and has been used as an index of lipid peroxidation in humans and animals (Czauderna et al., 2011; Konieczka et al., 2014; Du et al., 2016). GSH-Px is an important peroxidase, functionally as an index of antiperoxidation ability (Yuan et al., 2012). In the present study, the steeply decreased MDA and increased GSH-Px in HFD + OCN hens indicate that OCN inhibits hepatic oxidative stress in HFD-induced FLHS hens. Interestingly, there is a similar metabolic milieu between FLHS in laying hens and NAFLD in mice and humans; and oxidative stress in NAFLD can be alleviated by OCN administration (Du et al., 2016).
As proinflammatory factors, IL-1, IL-6, and TNF-α have been used for evaluating inflammatory and infectious reaction and related immunity in humans and various animals including chickens (Yang et al., 2014; Koronowicz et al., 2016; Huang et al., 2017). Tumor necrosis factor-alpha plays an important role in the pathogenesis of mammals' NAFLD (Wigg et al., 2001) and has a close relationship with the liver damage including steatosis, fibrosis, and apoptosis (Tomita et al., 2006; Zhang et al., 2010). In the present study, the massively reduced TNF-α concentrations in plasma and mRNA expression in the liver, along with the similar trend changes of IL-6, suggest that OCN functions in preventing hepatic inflammatory reaction. However, OCN elevated the blood IL-1 concentration but potentially reduced liver IL-1 mRNA expression. The reason of the different effects of OCN on blood IL-6 and IL-1 concentration did not examine in the present study, but it could be related the ones proposed in humans. In the patients with systemic sclerosis, for example, the expressions of inflammatory factors including IL-1 and IL-6 are based on the extension of the lesions and the disease activity periods (Alecu et al., 1998). Bartekova et al. (2018) reported that the different in the expressions of IL-1 and IL-6 as well as other cytokines in heart diseases may be due to differences in the nature, duration, and degree of cardiac dysfunction. Similarly, Sevimli et al. (2013) reported that vitamin A–treated chickens (5-week-old layers) with Freund's adjuvant–induced amyloid arthropathy had increased concentrations of TNF-α and IL-6 but decreased IL-1 compared with the controls.
Conclusion
The results of this study suggest that HFD promotes FLHS development in aged hens, whereas OCN alleviates HFD-induced liver damage, reducing hepatocytic hemorrhage and fibrosis, inhibits metabolic disorders including insulin resistance, oxidation stress, and activates hepatocyte autophagy.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (No.31702307) and Fundamental Research Funds for the Central Universities (XDJK2019B013).
Disclosures
The authors declare no conflicts of interest.
References
- Alecu M., Geleriu L., Coman G., Galajescu L. The interleukin-1, interleukin-2, interleukin-6 and tumour necrosis factor alpha serological levels in localised and systemic sclerosis. Rom. J. Intern. Med. 1998;36:251–259. [PubMed] [Google Scholar]
- Alvarenga R.R., Zangeronimo M.G., Pereira L.J., Rodrigues P.B., Gomide E.M. Lipoprotein metabolism in poultry. Worlds Poult. Sci. J. 2011;67:431–440. [Google Scholar]
- Amir M., Czaja M.J. Autophagy in nonalcoholic steatohepatitis. Expert Rev. Gastroenterol. Hepatol. 2011;5:159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala I., Castillo A.M., Adánez G., Fernández-Rufete A., Pérez B.G., Castells M.T. Hyperlipidemic chicken as a model of non-alcoholic steatohepatitis. Exp. Biol. Med. 2009;234:10–16. doi: 10.3181/0807-RM-219. [DOI] [PubMed] [Google Scholar]
- Bartekova M., Radosinska J., Jelemensky M., Dhalla N.S. Role of cytokines and inflammation in heart function during health and disease. Heart Fail. Rev. 2018;23:733–758. doi: 10.1007/s10741-018-9716-x. [DOI] [PubMed] [Google Scholar]
- Cai Y., Song Z., Wang X., Jiao H., Lin H. Dexamethasone-induced hepatic lipogenesis is insulin dependent in chickens (Gallus gallus domesticus) Stress. 2011;14:273–281. doi: 10.3109/10253890.2010.543444. [DOI] [PubMed] [Google Scholar]
- Choi Y.I., Ahn H.J., Lee B.K., Oh S.T., An B.K., Kang C.W. Nutritional and hormonal induction of fatty liver syndrome and effects of dietary lipotropic factors in egg-type male chicks. Asian-Australas. J. Anim. Sci. 2012;25:1145–1152. doi: 10.5713/ajas.2011.11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czauderna M., Kowalczyk J., Marounek M. The simple and sensitive measurement of malondialdehyde in selected specimens of biological origin and some feed by reversed phase high performance liquid chromatography. J. Chromatogr. B. 2011;879:2251–2258. doi: 10.1016/j.jchromb.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Diaz G.J., Squires E.J., Julian R.J. The use of selected plasma enzyme activities for the diagnosis of fatty liver-hemorrhagic syndrome in laying hens. Avian Dis. 1999;43:768–773. [PubMed] [Google Scholar]
- Du J., Zhang M., Lu J., Zhang X., Xiong Q., Xu Y., Bao Y., Jia W. Osteocalcin improves nonalcoholic fatty liver disease in mice through activation of Nrf2 and inhibition of JNK. Endocrine. 2016;53:701–709. doi: 10.1007/s12020-016-0926-5. [DOI] [PubMed] [Google Scholar]
- Ferron M., Hinoi E., Karsenty G., Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M., McKee M.D., Levine R.L., Ducy P., Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–575. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M., Wei J., Yoshizawa T., Del Fattore A., DePinho R.A., Teti A., Ducy P., Karsenty G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron M., Wei J.W., Yoshizawa T., Ducy P., Karsenty G. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem. Biophys. Res. Commun. 2010;397:691–696. doi: 10.1016/j.bbrc.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming R.H., McCormack H.A., McTeir L., Whitehead C.C. Effects of dietary particulate limestone, vitamin K3 and fluoride and photostimulation on skeletal morphology and osteoporosis in laying hens. Br. Poult. Sci. 2003;44:683–689. doi: 10.1080/00071660310001643688. [DOI] [PubMed] [Google Scholar]
- Gao X., Liu P., Wu C., Wang T., Liu G., Cao H., Zhang C., Hu G., Guo X. Effects of fatty liver hemorrhagic syndrome on the AMP-activated protein kinase signaling pathway in laying hens. Poult. Sci. 2019;98:2201–2210. doi: 10.3382/ps/pey586. [DOI] [PubMed] [Google Scholar]
- Gariani K., Philippe J., Jornayvaz F.R. Non-alcoholic fatty liver disease and insulin resistance: from bench to bedside. Diabetes Metab. 2013;39:16–26. doi: 10.1016/j.diabet.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Gundberg C.M., Hauschka P.V., Lian J.B., Gallop P.M. Osteocalcin: isolation, characterization, and detection. Methods Enzymol. 1984;107:516–544. doi: 10.1016/0076-6879(84)07036-1. [DOI] [PubMed] [Google Scholar]
- Gupte A.A., Sabek O.M., Fraga D., Minze L.J., Nishimoto S.K., Liu J.Z., Afshar S., Gaber L., Lyon C.J., Gaber A.O., Hsueh W.A. Osteocalcin protects against nonalcoholic steatohepatitis in a mouse model of metabolic syndrome. Endocrinology. 2014;155:4697–4705. doi: 10.1210/en.2014-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Ni Y., Ren L., Dai J., Zhao R. Leptin is involved in the effects of cysteamine on egg laying of hens, characteristics of eggs, and posthatch growth of broiler offspring. Poult. Sci. 2008;87:1810–1817. doi: 10.3382/ps.2008-00040. [DOI] [PubMed] [Google Scholar]
- Huang X.Y., Ansari A.R., Huang H.B., Zhao X., Li N.Y., Sun Z.J., Peng K.M., Zhong J., Liu H.Z. Lipopolysaccharide mediates immunopathological alterations in young chicken liver through TLR4 signaling. BMC. Immunol. 2017;18:12. doi: 10.1186/s12865-017-0199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Cheng H.W., Hester P.Y., Hou J.F. Development of an enzyme-linked immunosorbent assay for detection of chicken osteocalcin and its use in evaluation of perch effects on bone remodeling in caged White Leghorns. Poult. Sci. 2013;92:1951–1961. doi: 10.3382/ps.2012-02909. [DOI] [PubMed] [Google Scholar]
- Jiang S., Cheng H.W., Cui L.Y., Zhou Z.L., Hou J.F. Changes of blood parameters associated with bone remodeling following experimentally induced fatty liver disorder in laying hens. Poult. Sci. 2013;92:1443–1453. doi: 10.3382/ps.2012-02800. [DOI] [PubMed] [Google Scholar]
- Jiang S., Cui L.Y., Hou F.J., Shi C., Ke X., Yang L.C., Ma X.P. Effects of age and dietary soybean oil level on eggshell quality, bone strength and blood biochemistry in laying hens. Br. Poult. Sci. 2014;55:653–661. doi: 10.1080/00071668.2014.949624. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Yamaguchi T., Yamamoto M., Yamauchi M., Kurioka S., Yano S., Sugimoto T. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2009;94:45–49. doi: 10.1210/jc.2008-1455. [DOI] [PubMed] [Google Scholar]
- Konieczka P., Rozbicka-Wieczorek A.J., Więsyk E., Smulikowska S., Czauderna M. Improved derivatization of malondialdehyde with 2-thiobarbituric acid for evaluation of oxidative stress in selected tissues of chickens. J. Anim. Feed. Sci. 2014;23:190–197. [Google Scholar]
- Koronowicz A.A., Banks P., Szymczyk B., Leszczyńska T., Master A., Piasna E., Szczepański W., Domagała D., Kopeć A., Piątkowska E., Laidler P. Dietary conjugated linoleic acid affects blood parameters, liver morphology and expression of selected hepatic genes in laying hens. Br. Poult. Sci. 2016;57:663–673. doi: 10.1080/00071668.2016.1192280. [DOI] [PubMed] [Google Scholar]
- Kwanten W.J., Martinet W., Francque S.M. Autophagy in Current Trends in Cellular Physiology and Pathology. InTech; London, UK: 2016. Autophagy in non-alcoholic fatty liver disease (NAFLD) pp. 455–483. [Google Scholar]
- Lacombe J., Al Rifai O., Loter L., Moran T., Turcotte A.F., Grenier-Larouche T., Tchernof A., Biertho L., Carpentier A.C., Prud'homme D., Rabasa-Lhoret R., Karsenty G., Gagnon C., Jiang W., Ferron M. Measurement of bioactive osteocalcin in humans using a novel immunoassay reveals association with glucose metabolism and beta-cell function. Am. J. Physiol. Endocrinol. Metab. 2020;318:E381–E391. doi: 10.1152/ajpendo.00321.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N.K., Sowa H., Hinoi E., Ferron M., Ahn J.D., Confavreux C., Dacquin R., Mee P.J., McKee M.D., Jung D.Y., Zhang Z., Kim J.K., Mauvais-Jarvis F., Ducy P., Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhang H., Yang C., Li Y., Dai Z. An overview of osteocalcin progress. J. Bone Miner. Metab. 2016;34:367–379. doi: 10.1007/s00774-015-0734-7. [DOI] [PubMed] [Google Scholar]
- Liang M.J., Wang Z.P., Xu L., Leng L., Wang S.Z., Luan P., Cao Z.P., Li Y.M., Li H. Estimating the genetic parameters for liver fat traits in broiler lines divergently selected for abdominal fat. Genet. Mol. Res. 2015;14:9646–9654. doi: 10.4238/2015.August.14.27. [DOI] [PubMed] [Google Scholar]
- Mao Y., Yu F., Wang J., Guo C., Fan X. Autophagy: a new target for nonalcoholic fatty liver disease therapy. Hepat. Med. 2016;8:27–37. doi: 10.2147/HMER.S98120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizokami A., Kawakubo-Yasukochi T., Hirata M. Osteocalcin and its endocrine functions. Biochem. Pharmacol. 2017;132:1–8. doi: 10.1016/j.bcp.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Ni H.M., Williams J.A., Yang H., Shi Y.H., Fan J., Ding W.X. Targeting autophagy for the treatment of liver diseases. Pharmacol. Res. 2012;66:463–474. doi: 10.1016/j.phrs.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng G., Huang E., Ruan J., Huang L., Liang H., Wei Q., Xie X., Zeng Q., Huang J. Effects of a high energy and low protein diet on hepatic and plasma characteristics and Cidea and Cidec mRNA expression in liver and adipose tissue of laying hens with fatty liver hemorrhagic syndrome. Anim. Sci. J. 2019;90:247–254. doi: 10.1111/asj.13140. [DOI] [PubMed] [Google Scholar]
- Robinson S., Kiarie E.G. Production and metabolic consequences of high-energy and low-crude-protein diet fed to 49-wk-old Shaver white leghorn without or with top-dressed organic selenium. Can. J. Anim. Sci. 2019;99:848–857. [Google Scholar]
- Rozenboim I., Mahato J., Cohen N.A., Tirosh O. Low protein and high-energy diet: a possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016;95:612–621. doi: 10.3382/ps/pev367. [DOI] [PubMed] [Google Scholar]
- Sanchez-Polo M.T., Castells M.T., García-Pérez B., Martín A., Adánez G., Ayala I. Effect of diet/atorvastatin on atherosclerotic lesions associated to nonalcoholic fatty liver disease in chickens. Histol. Histopathol. 2015;30:1439–1446. doi: 10.14670/HH-11-639. [DOI] [PubMed] [Google Scholar]
- Sevimli A., Bülbül T., Bülbül A., Yagci A. Chicken amyloid arthropathy: serum amyloid A, interleukin-1beta, interleukin-6, tumour necrosis factor-alpha and nitric oxide profile in acute phase (12th hour) Pol. J. Vet. Sci. 2013;16:241–247. doi: 10.2478/pjvs-2013-0034. [DOI] [PubMed] [Google Scholar]
- Sharma P., Arora A. Clinical presentation of alcoholic liver disease and non-alcoholic fatty liver disease: spectrum and diagnosis. Transl. Gastroenterol. Hepatol. 2020;5:19. doi: 10.21037/tgh.2019.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F.Y., Leng J., Cao W.J., Tan Z.J., Meng W.J., Wang S.Z., Xu Y.Y. Fatty liver disease index: a simple screening tool to facilitate diagnosis of nonalcoholic fatty liver disease in the Chinese population. Dig. Dis. Sci. 2013;58:3326–3334. doi: 10.1007/s10620-013-2774-y. [DOI] [PubMed] [Google Scholar]
- Shini A., Shini S., Bryden W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: impact of production system. Avian Pathol. 2019;48:25–34. doi: 10.1080/03079457.2018.1538550. [DOI] [PubMed] [Google Scholar]
- Singh R., Kaushik S., Wang Y., Xiang Y., Novak I., Komatsu M., Tanaka K., Cuervo A.M., Czaja M.J. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strapazzon G., De Toni L., Foresta C. Serum undercarboxylated osteocalcin was inversely associated with plasma glucose level and fat mass in type 2 diabetes mellitus. Osteoporos. Int. 2011;22:1643–1644. doi: 10.1007/s00198-010-1322-2. [DOI] [PubMed] [Google Scholar]
- Tomita K., Tamiya G., Ando S., Ohsumi K., Chiyo T., Mizutani A., Kitamura N., Toda K., Kaneko T., Horie Y., Han J.Y., Kato S., Shimoda M., Oike Y., Tomizawa M., Makino S., Ohkura T., Saito H., Kumagai N., Nagata H., Ishii H., Hibi T. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott K.A., Giannitti F., Rimoldi G., Hill A., Woods L., Barr B., Anderson M., Mete A. Fatty liver hemorrhagic syndrome in the backyard chicken: a retrospective histopathologic case series. Vet. Pathol. 2014;51:787–795. doi: 10.1177/0300985813503569. [DOI] [PubMed] [Google Scholar]
- Tsai M.T., Chen Y.J., Chen C.Y., Tsai M.H., Han C.L., Chen Y.J., Mersmann H.J., Ding S.T. Identification of potential plasma biomarkers for nonalcoholic fatty liver disease by integrating transcriptomics and proteomics in laying hens. J. Nutr. 2017;147:293–303. doi: 10.3945/jn.116.240358. [DOI] [PubMed] [Google Scholar]
- Wigg A.J., Roberts-Thomson I.C., Dymock R.B., McCarthy P.J., Grose R.H., Cummins A.G. The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 2001;48:206–211. doi: 10.1136/gut.48.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Tang H., Wang H. The anti-oxidation and mechanism of essential oil of paederia scandens in the NAFLD model of chicken. Animals. 2019;9:E850. doi: 10.3390/ani9100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W.K.K., Zhang L., Chan M.T.V. Autophagy, NAFLD and NAFLD-related HCC. Adv. Exp. Med. Biol. 2018;1061:127–138. doi: 10.1007/978-981-10-8684-7_10. [DOI] [PubMed] [Google Scholar]
- Yang K.T., Lin C., Liu C.W., Chen Y.C. Effects of chicken-liver hydrolysates on lipid metabolism in a high-fat diet. Food Chem. 2014;160:148–156. doi: 10.1016/j.foodchem.2014.03.052. [DOI] [PubMed] [Google Scholar]
- Yasutake Y., Mizokami A., Kawakubo-Yasukochi T., Chishaki S., Takahashi I., Takeuchi H., Hirata M. Long-term oral administration of osteocalcin induces insulin resistance in male mice fed a high-fat, high-sucrose diet. Am. J. Physiol. Endocrinol. Metab. 2016;310:E662–E675. doi: 10.1152/ajpendo.00334.2015. [DOI] [PubMed] [Google Scholar]
- Yousefi M., Shivazad M., Sohrabi-Haghdoost I. Effect of dietary factors on induction of fatty liver-hemorrhagic syndrome and its diagnosis methods with use of serum and liver parameters in laying hens. Int. J. Poult. Sci. 2005;4:568–572. [Google Scholar]
- Yuan D., Zhan X.A., Wang Y.X. Effect of selenium sources on the expression of cellular glutathione peroxidase and cytoplasmic thioredoxin reductase in the liver and kidney of broiler breeders and their offspring. Poult. Sci. 2012;91:936–942. doi: 10.3382/ps.2011-01921. [DOI] [PubMed] [Google Scholar]
- Zaefarian F., Abdollahi M.R., Cowieson A., Ravindran V. Avian liver: the forgotten organ. Animals. 2019;9:E63. doi: 10.3390/ani9020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Chen D., Yu B. Effect of different dietary energy sources on induction of fatty liver-hemorrhagic syndrome in laying hens. Int. J. Poult. Sci. 2008;7:1232–1236. [Google Scholar]
- Zhang W., Kudo H., Kawai K., Fujisaka S., Usui I., Sugiyama T., Tsukada K., Chen N., Takahara T. Tumor necrosis factor-alpha accelerates apoptosis of steatotic hepatocytes from a murine model of non-alcoholic fatty liver disease. Biochem. Biophys. Res. Commun. 2010;391:1731–1736. doi: 10.1016/j.bbrc.2009.12.144. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu Z., Liu R., Wang J., Zheng M., Li Q., Cui H., Zhao G., Wen J. Alteration of hepatic gene expression along with the inherited phenotype of acquired fatty liver in chicken. Genes. 2018;9:199. doi: 10.3390/genes9040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X., Sun F., Li L., Jiang D., Li X., Sun A., Pan Z., Lou N., Zhang L., Lou F. Therapeutic effect of metformin on chemerin in non-obese patients with non-alcoholic fatty liver disease (NAFLD) Clin. Lab. 2015;61:1409–1414. doi: 10.7754/clin.lab.2015.150211. [DOI] [PubMed] [Google Scholar]
- Zhuang Y., Xing C., Cao H., Zhang C., Luo J., Guo X., Hu G. Insulin resistance and metabonomics analysis of fatty liver haemorrhagic syndrome in laying hens induced by a high-energy low-protein diet. Sci. Rep. 2019;9:10141. doi: 10.1038/s41598-019-46183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]