Abstract
Hexavalent chromium (Cr(VI)) has carcinogenic, nephrotoxic, hepatotoxic, and neurotoxic effects. Exposure to Cr(VI) can also lead to hematological alterations and blood biochemical changes. The literature on Cr(VI) toxicity concerns mostly adult forms of vertebrates. In this study, an attempt was made to determine the effect on the developing chicken embryo of Cr(VI) in ovo administration. It was observed that chromium affected the hatchability of chicks in a dose-dependent manner. At a dose from 25 to 250 μg per egg, Cr(VI) resulted in a statistically significant reduction of hatchability. Chromium administrated at lower doses (1.56 and 2.5 μg per egg) caused a statistically insignificant increase of hatchability. However, chromium at a level of LD50 (15.6 μg per egg) or 1/10 LD50 (1.56 per egg) did not cause major changes in hematological parameters or plasma biochemical indices in newly hatched chicks. The same doses did not lead to any histopathological changes in the liver.
Key words: embryotoxicity, hematology, biochemical indices, histology, in ovo
Introduction
Chromium (Cr) is the 24th element in the periodic chart; it is situated between vanadium and manganese and has an average atomic weight of 52. It is the 21st most abundant element in the Earth's crust (Barnhart, 1997). Three thermodynamically stable forms of chromium, Cr(0), Cr(III), and Cr(VI), are used commercially and are present in the environment (Zhitkovich, 2011), especially hexavalent chromium, which is considered toxic, while trivalent chromium (Cr(III)) is an essential element. According to Pechova and Pavlata (2007), Cr(III) plays a role in insulin signaling and the metabolism of carbohydrate, lipid, and protein; it is also involved in stress regulation and in reproductive and immune functions. Chromium (VI) is a strong oxidizing agent, especially in acidic media; it is able to cross biological membranes and reacts with protein components and nucleic acids inside the cell while being reduced to Cr(III). According to Ray (2016), Cr(VI) in the blood is readily reduced to Cr(III), but excess Cr(VI) which is not reduced in plasma may enter erythrocytes and lymphocytes, inducing microcytic anemia in rodents. Toxic effects of Cr(VI) include mitochondrial injury and DNA damage of blood cells, thus leading to carcinogenicity. It is known that Cr(VI) is a human carcinogen that increases the risk of lung cancer (Costa and Klein, 2006; Urbano et al., 2008). According to Hamilton and Wetterhahn (1986), Cr(VI) caused DNA damage in the liver and blood cells of chick embryos. It was also demonstrated that excessive exposure to Cr(VI) can lead to pathophysiological and histopathological changes in the liver of chicks (Wang et al., 2017, 2020; Zhao et al., 2019). The median lethal dose (LD50) of hexavalent chromium was estimated as 50 to 150 mg/kg in mammals (Katz and Salem, 1993) and 164 mg per kg body weight in broilers (Zhao et al., 2019). However, doses of chromium which do not result in any measurable impact on adult animals may seriously affect embryos (Hui, 2002). Embryo-lethal effects of Cr(VI) have been observed in mammals (Trivedi et al., 1989) and birds (Kertesz and Fancsi, 2003). In very industrial regions, Cr content is up to 3.1 μg per g dry mass in the eggs of wild birds (Hui, 2002; Custer et al., 2007) and up to 4.5 μg per g dry mass in the eggs of poultry (Aendo et al., 2018). These values correspond to about 55 and 80 μg of Cr per hen egg, respectively. According to Venter et al. (2015), a dose of about 0.25 μg Cr per egg can be considered a “physiological dose”.
The avian embryo (in ovo model) is an approved biological model which can be used to reflect environmental pollution under laboratory conditions (Scanes and McNabb, 2003; Dżugan et al., 2011, 2012, 2018; Liu et al., 2015; Dżugan and Lis, 2016). The advantage of embryo-toxicological studies using an in ovo model is the lack of the mother's ontogenic biochemical influence on the embryo, as would be the case with in utero development in mammals (Scanes and McNabb, 2003). However, owing to the lack of a placenta in the avian egg, all substances are deposited until shell formation (Romanoff, 1960; Li-Chan and Kim, 2008) or, under laboratory conditions, after shell formation with the use of in ovo injection (Uni et al., 2005; Moran, 2007). It is worth mentioning that although avian embryos are relatively resistant to in ovo injection, their sensitivity to mechanical manipulation increases with the stage of development (Bruggeman et al., 2003).
The scientific data regarding the toxic effects of Cr(VI) on avian embryogenesis seem to be insufficient. Thus, the aim of this study was to determine whether Cr(VI) administrated in ovo affects the hatchability, blood parameters, and liver microstructure of newly hatched chicks.
Materials and methods
Experimental Design
According to Directive 2010/63/EU, the experimental and animal procedures used in this study did not need to be approved by the Local Animal Ethics Committee.
Determination of the Lethal Dose (LD50) of Chromium (VI)—Experiment 1
Hatching eggs (n = 360, weight (mean ± SD) 60.5 ± 5.42 g) of Ross 308 broiler chicken parental flock (Aviagen) were obtained from a commercial farm (Sławomir Domagała, Gołaczewy, Poland). The eggs were randomly divided into 6 groups (n = 30 eggs per group) and incubated in a Masalles 65 DIGIT incubator under standard conditions: from first to 18th day of incubation (E) at 37.8 ± 0.1°C, RH = 50 ± 2%; from E19 to E21 at 37.2 ± 0.1°C, RH = 60 ± 10%. On E5, the eggs were candled with an ovoscope to determine embryo development, and infertile and dead embryos eggs were rejected (n = 14). The remaining eggs with live embryos (n = 166, from 25 to 30 eggs per group) were used in further procedures (Table 1).
Table 1.
Chicken embryo mortality in various periods of development (E—day of incubation) and hatchability in the experiment carried out to determine the lethal dose (LD50) of hexavalent chromium (Cr(VI)) in chicken embryos.
| Dose of Cr(VI) [μg per egg] |
0.0 |
2.5 |
25.0 |
50.0 |
125.0 |
250.0 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group size | n | % | n | % | n | % | n | % | n | % | n | % |
| Injected eggs | 25 | 100.0 | 27 | 100.0 | 27 | 100.0 | 28 | 100.0 | 29 | 100.0 | 30 | 100.0 |
| Mortality between E5 – E7 | 4 | 16.0a | 2 | 7.4a | 15 | 55.6b | 25 | 89.3c | 29 | 100.0c | 30 | 100.0c |
| Mortality between E8 – E18 | 6 | 24.0b | 2 | 7.4a,b | 5 | 18.5a,b | 2 | 7.1a,b | 0 | 0.0a | 0 | 0.0a |
| Mortality between E19 – E21 | 2 | 8.0 | 4 | 14.8 | 2 | 7.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total mortality | 12 | 48.0a | 8 | 29.6a | 22 | 81.5b | 27 | 96.4b,c | 29 | 100.0b,c | 30 | 100.0b,c |
| Hatchability | 13 | 52.0c | 19 | 70.4c | 5 | 18.5b | 1 | 3.6a,b | 0 | 0.0a | 0 | 0.0a |
a-c—values in rows marked with different letters differ significantly (P < 0.05).
At E5, a hole (1.2 mm diameter) was aseptically drilled in the egg shell's air cell region using a G18 needle; 100 μL of physiological solution containing 0.0 (control), 2.5, 25.0, 50.0, 125.0, or 250.0 μg Cr(VI) per egg (as potassium dichromate K2Cr2O7, No. 483044, Sigma-Aldrich Ltd. Poznań, Poland) was injected into the albumen under the chorioallantoic membrane. The doses used in the experiment were based on the “physiological dose” (Venter et al., 2015) by multiplying it 10, 100, 200, 500, and 1,000 times. Next, the hole was sealed with hot wax and incubation was continued. The eggs were candled again on E7 and E18. All hatch debris and specimens removed during candling were analyzed embryopathologically to determine the development phase (Hamburger and Hamilton, 1951), malformations, and malpositions of dead embryos. The hatchability results allowed the lethal dose (LD50) to be determined using the Spearman-Kärber method (Hamilton et al., 1977).
Effects of Embryonic Exposure to Chromium (VI) on Blood Parameters and Liver Microstructure of Chicken—Experiment 2
Hatching eggs (n = 90, weight (mean ± SD) 61.2 ± 6.33 g) of the same stock as used in experiment 1 were randomly divided into 3 groups (n = 30 eggs per group). The incubation and injection procedures were performed in the same way as in experiment 1 with the proviso that doses were 0.0 (control), 1.56 (10% of the LD50 value established in the experiment 1; D1 group), and 15.6 μg of Cr(VI) (100% of the LD50; D2 group). Ten randomly selected chicks from each group were euthanized by decapitation, and samples of blood and liver were collected.
Tissue Sampling and Analysis
The blood was collected from the jugular vein into heparinized plastic tubes and subjected to hematological analyses or centrifuged using an MPW 250 centrifuge (MPW MED Instruments, Poland) (7,500 rpm for 5 min) to obtain plasma. The plasma was stored at −20°C until biochemical analysis.
Red blood cell (RBC) and white blood cell (WBC) counts were calculated using a Bürker hemocytometer in blood diluted 1:100 with Hayem solution. Hemoglobin (Hb) concentration was measured spectrophotometrically at 540 nm using the cyanmethemoglobin method. Hematocrit (Ht) value was determined after centrifugation of microhematocrit tubes at 12,000 rpm for 5 min. Mean erythrocyte volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration were calculated from Ht, Hb, and RBC using standard formulas.
The values of the following plasma parameters were evaluated by the colorimetric method with a Mindray BS-800M clinical chemistry analyzer: activities of alanine transaminase and aspartate transaminase (AST) and of alkaline phosphatase, and the concentrations of glucose, urea, total protein, and albumin.
Livers of the decapitated chickens were fixed in Bouin fixative for 2 d. The fixative was then rinsed from the tissues by immersing them in multiply changed 75% ethanol. The livers were dehydrated in a graded series of ethanol (70, 96, and 100%), cleared with toluene and embedded in Pathowax Plus tissue embedding medium (Mar-Four, Poland). Transverse sections of 7 μm were made on a Leica microtome and affixed to glass slides. Selected slides were treated with hematoxylin + eosin (H + E) for general cytology. Slides were analyzed using light microscopy.
Statistical Analysis
The hatchability results were analyzed by a z-test, whereas the results of biochemical and hematological investigations were subjected to a one-way ANOVA followed by Tukey's multiple range test. The relation between chromium dose and hatchability was determined by Spearman rank-order correlation. Statistical analyses were performed using Sigma-Stat 2.03 (SPSS Science Software Ltd., IL). The level of significance was set at α = 0.05.
Results
Hatchability
The hatchability of chicks gradually decreased from 52.0 and 70.4% in the group injected with 0 (control) and 2.5 μg Cr(VI) per egg, respectively, to 0% in the groups treated with 150 and 250 μg Cr(VI) per egg (Table 1). The observed relation between chromium dose and hatchability can be described by an inverse correlation (r = −0.928, P = 0.0167). The embryotoxic effect of a 25 μg Cr(VI) dose and higher appeared immediately after administration and resulted in mortality at the level of 55.6 to 100% of embryos in these groups. The lethal dose (LD50) was estimated as 15.6 μg Cr(VI) (Table 1). The results obtained in the second experiment were similar (Table 2).
Table 2.
Chicken embryo mortality at various stages of incubation (E—day of incubation) and hatchability in the experiment carried out to determine the effects on blood parameters and liver microstructure of embryonic exposure to hexavalent chromium (Cr(VI)) in one-day-old chicks.
| Dose of Cr(VI) [μg per egg] |
0.0 |
1.56 |
15.6 |
||||
|---|---|---|---|---|---|---|---|
| Group size | n | % | n | % | n | % | |
| Injected eggs | 20 | 100.0 | 20 | 100.0 | 30 | 100. | |
| Mortality | Mortality between E5 – E7 | 10 | 50.0b | 2 | 10.0a | 10 | 33.3a,b |
| MOrtality between E8 – E18 | 0 | 0.0 | 2 | 10.0 | 4 | 13.3 | |
| Mortality between E19 – E21 | 0 | 0.0 | 0 | 0.0 | 3 | 10.0 | |
| Total mortality | 10 | 50.0a,b | 4 | 20.0a | 17 | 56.7b | |
| Hatchability | 10 | 50.0a,b | 16 | 80.0b | 13 | 43.3a | |
a-c—values in rows marked with different letters differ significantly (P < 0.05).
Hematological and Blood Biochemical Parameters
Most hematological parameters did not significantly differ between the control and the Cr-exposed groups (Table 3). In the group treated with 15.6 μg (D2 group) only RBC was significantly lower (P = 0.039) compared with the control. Hematocrit showed a similar trend, whereas mean erythrocyte volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and WBC tended to increase in Cr-exposed individuals, but the differences were statistically insignificant. No significant differences were observed in the biochemical parameters, although activities of AST and alkaline phosphatase, glucose, and protein levels tended to decrease in Cr-exposed birds (Table 4).
Table 3.
Plasma biochemical parameters (mean ± SD) of one-day-old chicks (n = 10) in ovo injected with K2Cr2O7 solution.
| Cr(VI) [μg/egg] | AST [U/L] | ALT [U/L] | ALP [U/L] | Glucose [mg/dL] | Urea [mg/dL] | Total protein [g/L] | Albumin [g/L] |
|---|---|---|---|---|---|---|---|
| 0.0 | 189.6 ± 17.45 | 5.9 ± 0.99 | 2,982.9 ± 516.08 | 229.0 ± 11.98 | 27.4 ± 4.34 | 17.3 ± 2.49 | 8.0 ± 1.51 |
| 1.56 | 172.3 ± 22.01 | 5.6 ± 1.30 | 2,866.3 ± 722.89 | 223.1 ± 13.26 | 29.5 ± 2.14 | 17.4 ± 2.39 | 7.6 ± 1.77 |
| 15.6 | 175.5 ± 33.60 | 6.1 ± 1.89 | 2,725.0 ± 814.97 | 217.4 ± 31.56 | 27.1 ± 3.04 | 16.0 ± 3.07 | 7.6 ± 1.69 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; Cr, chromium.
Table 4.
Red blood cell parameters (mean ± SD) of one-day-old chicks (n = 8) injected in ovo with K2Cr2O7 solution.
| Dose of Cr(VI) [μg per egg] | RBC [106 μL] | Hb [g/L] | Ht [%] | MCV [fL] | MCH [pg] | MCHC [g/L] |
|---|---|---|---|---|---|---|
| 0.0 | 2.8 ± 0.25 | 166.7 ± 33.26 | 34.7 ± 5.81 | 124.9 ± 20.25 | 60.7 ± 15.10 | 497.2 ± 150.40 |
| 1.56 | 2.5 ± 0.48 | 156.5 ± 38.59 | 30.3 ± 5.19 | 121.4 ± 16.82 | 62.2 ± 10.18 | 517.3 ± 95.63 |
| 15.6 | 2.2 ± 0.401 | 167.7 ± 32.67 | 31.4 ± 3.85 | 145.0 ± 31.65 | 76.1 ± 12.37 | 541.7 ± 115.82 |
Abbreviations: Cr(VI), hexavalent chromium; Hb, hemoglobin; Ht, hematocrit; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean erythrocyte volume; RBC, red blood cell.
Value differs with the control (0.0 μg Cr(VI) per egg) (P < 0.05).
Liver Microstructure
The livers of 10 individuals from each of the 3 groups (the control and 2 experimental groups: D1 and D2) were dissected. Macroscopically, no visible differences were observed between organs from 2 experimental and the control groups.
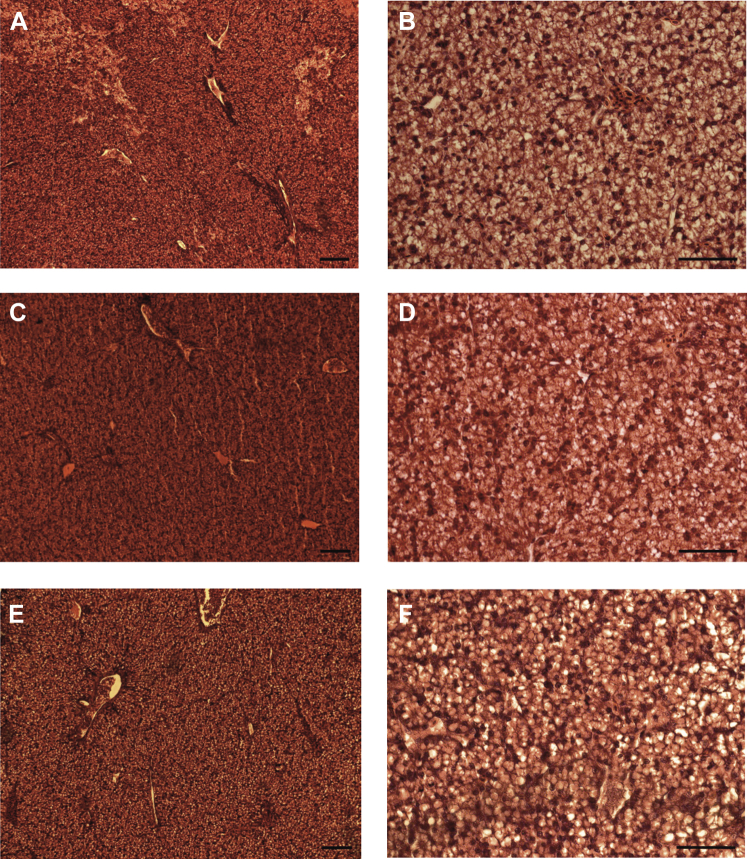
At the microscopic level, the livers (n = 5) were composed of capsule, hepatocytes, sinusoids, hepatic lobule, and portal triad. The hepatic portal vein, the proper hepatic artery, bile ductules, and lymphatic vessel were observed. No visible signs of negative processes such as inflammation, degeneration, necrosis, diapedesis, or central phlebectasia were noted in individuals treated with the lower dose of chromium (D1) or the higher dose (D2) in comparison with the control group (Figure 1). Thus, no differences were visible between experimental groups D1 and D2.
Figure 1.
Liver microstructure of one-day-old chicks injected in ovo with K2Cr2O7 solution. A—control group, magnitude 40×; B—control group, magnitude 100 x; C—group exposed to 1.56 μg/egg of Cr(VI), magnitude 40×; D—group exposed to 1.56 μg/egg of Cr(VI), magnitude 100×; E—group exposed to 15.6 μg/egg of Cr(VI), magnitude 40×; F—group exposed to 15.6 μg/egg of Cr(VI), magnitude 100×; scale bar = 100 μm.
Discussion
Our research has shown that Cr(VI) at higher doses (≥15.6 mg Cr per egg) led to a reduction in the hatchability of chicks. Similarly, Asmatullah and Shakoori (1998) observed dose-dependent mortality in chick embryos treated with Cr(VI). Reduced body size, microphthalmia, micromelia, everted viscera, abnormal and twisted neck, beak and spinal cord, isolated epicarditis, club foot, hemorrhage, and patchy feathers were observed in survivors. In the embryos treated with 25 μg/egg, the brain was not well developed. The authors noticed that the heart primordia protruded from neck region as a tubular structure. The somite development was abnormal. The neural tube was twisted and was not closed at the anterior region. In the eggs treated with higher doses of Cr(VI) (50 and 100 μg/egg), the observed pathological alterations were more severe (Asmatullah and Shakoori, 1998). Studies conducted by other authors have shown that Cr(VI) can also disrupt embryonic/fetal development in other vertebrates. Junaid et al. (1996) showed that Cr(VI) administration via drinking water during organogenesis in mice led to embryotoxic and fetotoxic effects. According to the authors, reduced fetal weight, retarded fetal development, and high incidences of dead fetuses and resorptions in treated mothers in the highest-dosed group (700 ppm of K2Cr2O7) were evident.
It should be noted that Cr(VI) administrated at low doses (1.56 and 2.5 mg Cr per egg) in our studies caused an increase in chick hatchability, which can be explained by the reduction of Cr(VI) to Cr(III) (Ray, 2016). This process does not appear to be efficient enough at higher doses of Cr(VI).
Our research showed that exposure to Cr(VI), despite having an effect on embryo mortality, did not lead to major changes in the hematological picture of newly hatched chicks, and did not significantly change the biochemical parameters of their plasma. Kumari et al. (2013) observed that broiler chicks fed with food containing 55.6 and 92.7 mg/kg of K2CrO4 for 15 to 30 d showed no changes in the values of hematological parameters; however, after 45 d of treatment, Hb, Ht, RBC, and WBC significantly decreased. Mohammed et al. (2014) reported that 0.5 mg/kg of dietary organic Cr(VI) significantly reduced glucose and cholesterol concentrations in broilers after 42 d of exposure, whereas alanine transaminases and AST activities, total protein, albumin, and globulin concentrations remained unaffected in groups treated with both inorganic and organic Cr(VI).
Conclusion
The analysis of our own results and literature data indicates that high levels of Cr(VI) may disturb embryonic development and result in reduced hatchability. However, no histopathological hepatic lesions or significant changes in hematological and blood biochemical parameters were observed in newly hatched chicks exposed to low doses of Cr(VI). Moreover, the lowest doses of Cr(VI) increased chick hatchability, probably as a result of endogenous reduction of Cr(VI) to Cr(III) and acted as micronutrient supplementation.
Acknowledgments
This research was supported by the Ministry of Science and Higher Education of the Republic of Poland (Subject No. 215-DZ06, University of Agriculture in Krakow). Mr. Michael Timberlake is acknowledged for professional language proofreading of the manuscript. Dr. Oksana Buchko participated in the experiments during a research internship within The Iwan Wyhowski Award founded by 21 Polish universities.
Disclosures
The authors declare no conflicts of interest.
References
- Aendo P., Netvichian R., Viriyarampa S., Songserm T., Tulayakul P. Comparison of zinc, lead, cadmium, cobalt, manganese, iron, chromium and copper in duck eggs from three duck farm systems in Central and Western, Thailand. Ecotoxicol. Environ. Saf. 2018;161:691–698. doi: 10.1016/j.ecoenv.2018.06.052. [DOI] [PubMed] [Google Scholar]
- Asmatullah S.N.Q., Shakoori A.R. Embryotoxic and teratogenic effects of hexavalent chromium in developing chicks of Gallus domesticus. Bull. Environ. Contam. Toxicol. 1998;61:281–288. doi: 10.1007/s001289900760. [DOI] [PubMed] [Google Scholar]
- Barnhart J. Occurrences, uses, and properties of chromium. Regulat. Toxicol. Pharmacol. 1997;26:3–7. doi: 10.1006/rtph.1997.1132. [DOI] [PubMed] [Google Scholar]
- Bruggeman V., Swennen Q., De Ketelaere B., Onagbesan O., Tona K., Decuypere E. Embryonic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in chickens: effects of dose and embryonic stage on hatchability and growth. Comp. Biochem. Physiol. C. 2003;136:17–28. doi: 10.1016/s1532-0456(03)00168-6. [DOI] [PubMed] [Google Scholar]
- Costa M., Klein C.B. Toxicity and carcinogenicity of chromium compounds in humans. Critic. Rev. Toxicol. 2006;36:155–163. doi: 10.1080/10408440500534032. [DOI] [PubMed] [Google Scholar]
- Custer T.W., Custer C.M., Eichhorst B.A., Warburton D. Selenium and metal concentrations in waterbird eggs and chicks at Agassiz National Wildlife Refuge, Minnesota. Arch. Environ. Contam. Toxicol. 2007;53:103–109. doi: 10.1007/s00244-006-0139-7. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union. L 276/33. [DOI] [PubMed] [Google Scholar]
- Dżugan M., Lis M.W. Cadmium-induced changes in hatchability and in the activity of aminotransaminases and selected lysosomal hydrolases in the blood plasma of Muscovy ducklings (Cairina moschata) Acta Vet. Hung. 2016;64:239–249. doi: 10.1556/004.2016.024. [DOI] [PubMed] [Google Scholar]
- Dżugan M., Lis M., Droba M., Niedziółka J.W. Effect of cadmium injected in ovo on hatching results and the activity of plasma hydrolytic enzymes in newly hatched chicks. Acta Vet. Hung. 2011;59:337–347. doi: 10.1556/AVet.2011.020. [DOI] [PubMed] [Google Scholar]
- Dżugan M., Lis M.W., Droba M., Niedziółka J.W. Protective effect of zinc on cadmium embryotoxicity and antioxidant status of blood plasma in newly hatched chicks. J. Environ. Sci. Health Part A. 2012;47:1288–1293. doi: 10.1080/10934529.2012.672133. [DOI] [PubMed] [Google Scholar]
- Dżugan M., Trybus W., Lis M.W., Wesołowska M., Trybus E., Kopacz-Bednarska A., Król T. Cadmium-induced ultrastructural changes in primary target organs of developing chicken embryos (Gallus domesticus) J. Trace Elem. Med. Biol. 2018;50:167–174. doi: 10.1016/j.jtemb.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in thedevelopment of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hamilton J.W., Wetterhahn K.E. Chromium (VI)-induced DNA damage in chick embryo liver and blood cells in vivo. Carcinogen. 1986;7:2085–2088. doi: 10.1093/carcin/7.12.2085. [DOI] [PubMed] [Google Scholar]
- Hamilton M.A., Russo R.C., Thurston R.V. Trimmed Spearman-Kärber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1977;11:714–719. [Google Scholar]
- Hui C.A. Concentrations of chromium, manganese, and lead in air and in avian eggs. Environ. Poll. 2002;120:201–206. doi: 10.1016/s0269-7491(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Junaid M., Murthy R.C., Saxena D.K. Embryoitoxicity of orally administered chromium in mice: exposure during the period of organogenesis. Toxicol. Lett. 1996;84:143–148. doi: 10.1016/0378-4274(95)03607-5. [DOI] [PubMed] [Google Scholar]
- Katz S.A., Salem H. The toxicity of chromium with respect to its chemical speciation: a review. J. Appl. Toxicol. 1993;13:217–224. doi: 10.1002/jat.2550130314. [DOI] [PubMed] [Google Scholar]
- Kertesz V., Fancsi T. Adverse effects of (surface water pollutants) Cd, Cr and Pb on the embryogenesis of mallard. Aquat. Toxicol. 2003;65:425–433. doi: 10.1016/s0166-445x(03)00155-3. [DOI] [PubMed] [Google Scholar]
- Kumari R.R., Kumar P., Mondal T.K. Effect of vitamin E and selenium on hematological parameters in sub-acute toxicity of hexavalent chromium in broiler chick. Nat. J. Physiol. Pharm. Pharmacol. 2013;3:157–161. [Google Scholar]
- Liu M., Liu Y., Cheng Z., Liu J., T, Chai T. Effects of chromic chloride on chick embryo fibroblast viability. Toxicol. Rep. 2015;2:555–562. doi: 10.1016/j.toxrep.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Chan E.C.Y., Kim H.O. Structure and chemical compositions of eggs. In: Mine Y., editor. Egg Bioscience and Biotechnology. John and Wiley Publishing; New York, NY: 2008. pp. 1–96. [Google Scholar]
- Mohammed H.H., El-Sayed B.M., Abd El-Razik W.M., Ali M.A., Abd El-Aziz R.M. The influence of chromium sources on growth performance, economic efficiency, some maintenance behaviour, blood metabolites and carcass traits in broiler chickens. Glob. Vet. 2014;12:599–605. [Google Scholar]
- Moran E.T., Jr. Nutrition of the developing embryo and hatchling. Poult. Sci. 2007;86:1043–1049. doi: 10.1093/ps/86.5.1043. [DOI] [PubMed] [Google Scholar]
- Pechova A., Pavlata L. Chromium as an essential nutrient: a review. Vet. Med. 2007;52:1–18. [Google Scholar]
- Ray R.R. Adverse hematological effects of hexavalent chromium: an overview. Interdisc. Toxicol. 2016;9:55–65. doi: 10.1515/intox-2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanoff A.L. MacMillan Co.; New York, NY: 1960. The Avian Embryo: Structural and Functional Development; p. 1305. [Google Scholar]
- Scanes C.G., McNabb A.F. Avian models for toxicological research. Avian Poult. Biol. Rev. 2003;14:21–52. [Google Scholar]
- Trivedi B., Saxena D.K., Murthy R.C., Chandra S.V. Embryotoxicity and fetotoxicity of orally administered hexavalent chromium in mice. Reprod. Toxicol. 1989;3:275–278. doi: 10.1016/0890-6238(89)90022-1. [DOI] [PubMed] [Google Scholar]
- Uni Z., Ferket P.R., Tako E., Kedar O. In ovo feeding improves energy status of late-term chicken embryo. Poult. Sci. 2005;84:764–770. doi: 10.1093/ps/84.5.764. [DOI] [PubMed] [Google Scholar]
- Urbano A.M., Rodrigues C.F.D., Alpoim M.C. Hexavalent chromium exposure, genomic instability and lung cancer. Gene Ther. Mol. Biol. 2008;12:219–238. [Google Scholar]
- Venter C., Oberholzer H.M., Taute H., Cummings F.R., Bester M.J. An in ovo investigation into the hepatotoxicity of cadmium and chromium evaluated with light- and transmission electron microscopy and electron energy-loss spectroscopy. J. Environ. Sci. Health Part A. 2015;47:1288–1293. doi: 10.1080/10934529.2015.1019804. [DOI] [PubMed] [Google Scholar]
- Wang Y., Hao J., Zhang S., Li L., Wang R., Zhu Y., Liu Y., Liu J. Inflammatory injury and mitophagy induced by Cr(VI) in chicken liver. Environ. Sci. Pollut. Res. 2020;27:22980–22988. doi: 10.1007/s11356-020-08544-3. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu Y., Wan H., Zhu Y., Chen P., Hao P., Cheng Z., Liu J. Moderate selenium dosing inhibited chromium (VI) toxicity in chick liver. J. Biochem. Mol. Toxicol. 2017;31:e21916. doi: 10.1002/jbt.21916. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhang H., Wu X., Zhang T., Shen K., Li L., Peng Y., Mehmood K., Zhou D. Metabonomic analysis of the hepatic injury suffer from hexavalent chromium poisoning in broilers. Environ. Sci. Poll. Res. 2019;26:18181–18190. doi: 10.1007/s11356-019-05075-4. [DOI] [PubMed] [Google Scholar]
- Zhitkovich A. Chromium in drinking water: sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011;24:1617–1629. doi: 10.1021/tx200251t. [DOI] [PMC free article] [PubMed] [Google Scholar]