CLINICAL SIGNIFICANCES OF SLEEP APNEA AND AF
Atrial fibrillation (AF) is the most common arrhythmia clinically and is associated with significant morbidity and mortality. Emerging clinical evidence suggests that sleep disordered breathing (SDB) is an independent risk factor for AF.1 SDB is characterized by repetitive episodes of shallow breathing or apnea during sleep, which may cause an intermittent hypoxemia. Patients with SDB are linked to increased comorbidities of aging, heart failure (HF), hypertension, and coronary artery disease, which are also known as the AF risk factors. Clinical data further suggest that patients with SDB suffer from an increased AF recurrence rate following radiofrequency catheter ablation as well as a reduced efficacy of pharmacological antiarrhythmic therapies. A continuous positive airway pressure therapy has been shown to reduce the AF recurrent rate after ablation procedures and prevent the aggravation of AF.1 Unfortunately, eligibility for continuous positive airway pressure intervention in patients with SDB is rather selective, which significantly limits the success rate of AF intervention in patients with SDB. To date, an understanding of the underlying molecular and electrophysiological mechanisms of SDB-associated AF remains scarce.
NOVEL FINDINGS OF CAMKII-DEPENDENT LATE SODIUM CURRENT IN SDB-ASSOCIATED AF
Multiple pathological alterations concurrent with SDB have been reported, including increases in atrial wall stress due to altered intrathoracic pressure during apnea, sympathetic activation, and reactive oxygen species (ROS).2 Notably, these changes are known to contribute to enhanced activation of CaMKII (Ca2+/calmodulin-kinase type II), which is a well-known proarrhythmic molecule.3-6
In this issue of Circulation Research, Lebek et al1 reported an increased ROS production along with enhanced CaMKII activity in atrial myocytes from patients with SDB. In their well-conducted studies, the authors demonstrated a dominant effect of CaMKII-dependent late sodium current (INa,L) on enhanced AF risk in patients with SDB. Moreover, this INa,L enhancement is concordantly linked to the severity of SDB as indicated by an increased apnea-hypopnea index. This INa,L enhancement was also found in persistent AF reported by this same research group.7 Recently, a report using a mouse model suggested that CaMKII phosphorylates NaV1.5 channels (at the site of serine-571) to increase pathogenic INa,L.8 In the current article,1 the authors conducted a unique bed-to-bench translational study in humans. They performed a series of molecular biology and electrophysiology studies using isolated atrial myocytes and multicellular atrial trabeculae preparations from 113 coronary artery bypass graft patients (50% w SDB). Their results suggest that levels of NaV1.5 expression may be less important, but CaMKII phosphorylation at the serine-571 site is critical to enhanced INa,L in SDB atrial myocytes. Of note is the authors’ demonstration for the first time that NaV1.5 phosphorylation of the serine-571 site was increased in patients with AF with SDB, independent from predisposed AF. Utilization of this specific phospho-biomarker in future studies could, therefore, serve to further unlock this CaMKII-dependent pathway in SDB-associated AF genesis.
In their studies, the authors also showed increased arrhythmogenic activities in SDB atrial trabeculae preparations as indicated by premature atrial contractions and post-pause contraction (reflecting an increased sarcoplasmic reticulum [SR] Ca2+ leak). Increased frequency of pacing-induced early afterdepolarizations and delayed afterdepolarizations and spontaneous afterdepolarization events in SDB atrial myocytes further support the enhanced arrhythmogenic activities. Strikingly, such enhanced arrhythmic activities in both atrial myocytes and atrial trabeculae preparations were markedly reduced by either INa,L inhibition (tetrodotoxin), or CaMKII inhibition (AIP [autocamtide-2-related inhibitory peptide]) or SERCA (sarco/endoplasmic reticulum Ca2+-ATPase) inhibition (thapsigargin), suggesting that the enhanced INa,L, diastolic SR Ca2+ leak, and SR Ca2+ load could be interrelated or act independently. These could either be explained by well-established CaMKII-dependent phosphorylation of RyR2 (ryanodine receptor type 2) channels3,5 or could be attributed to INa,L-promoted SR Ca2+ mishandling, with the possible involvement of NCX (Na+/Ca2+ exchanger). Although this study did not reveal the intricate underlying mechanisms, potential molecular targets, such as NCX, likely play vital roles according to previous studies and are of great future interest. Additionally, potential roles of CaMKII-dependent INa,L in the different arrhythmic susceptibility between atria and ventricles in SDB, as the authors noticed,1 could be a possible point of interest to explore further.
This current study is important for its clinically well-characterized coronary artery bypass graft patients. As described by the authors, the selected SDB groups concurrently possessed a clinical status including AF and HF in association with significantly enhanced CaMKII activity. However, a directed post hoc analysis after exclusion of HF and AF patients showed that INa,L enhancement and arrhythmogenic activities are significantly associated with SDB but independent of the covariance of HF and AF. Intriguingly, further extraction of other covariates including age, sex, body mass index, existing AF, existing HF, diabetes mellitus, and creatinine levels did not lessen the described relationship between SDB and CaMKII-dependent proarrhythmic atrial activities. Moreover, the link between SDB and proarrhythmic alterations could be disrupted by acute inhibition of either CaMKII or INa,L. It is clear that this report lays an important foundation for further mechanistic and clinical investigations to validate the specific contribution of CaMKII-dependent atrial arrhythmogenic remodeling independent from other clinical comorbidities in patients with SDB.
CLINICAL SIGNIFICANCE, NOVELTY, LIMITATIONS, AND FUTURE DIRECTIONS
The high prevalence of SDB and SDB-associated high AF risk clearly highlight the clinical significance of the current report by Lebek et al.1 Their findings are important because they are the first to demonstrate that CaMKII-dependent enhancement of INa,L drives arrhythmic activities in patients with SDB. However, the specific influence of converging mechanisms between SDB and AF still require further validation from a larger patient population. As preemptively stated by the authors, this study was not designed to isolate the pathophysiological factor(s) leading to an activation of CaMKII in patients with SDB. Therefore, future efforts devoted toward these ends hold significant promise to advance our understanding of the multifactorial progression towards SDB-associated AF.
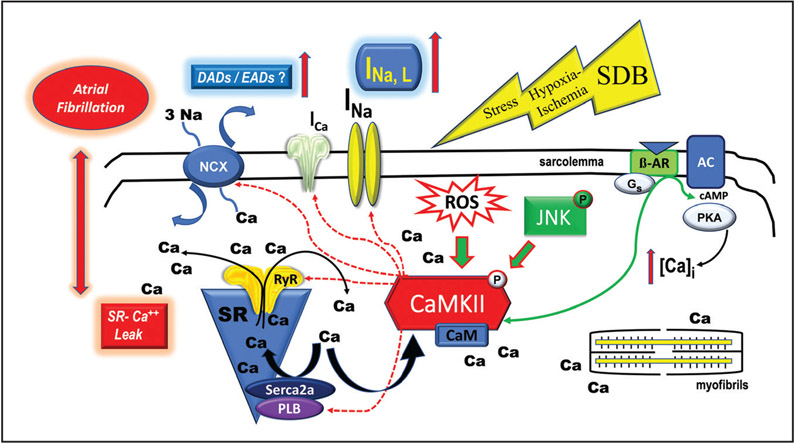
Lebek et al1 reported that increased ROS production in atrial myocytes from patients with SDB are concordant with increased CaMKII activation. Although CaMKII-dependent Nav1.5 phosphorylation in patients with SDB is a plausible mechanism for AF genesis, detailed underlying molecular mechanisms of CaMKII activation in SDB remain elusive. While oxidation of CaMKII was found to promote CaMKII activation and lead to Ca2+ mishandling and AF,6, 9 knowing the status of oxidized CaMKII and whether oxidized CaMKII is the cause of CaMKII activation in SDB would further strengthen their conclusion. To highlight the rapidly expanding topic (Figure), our lab recently reported that the activation of stress-response JNK (c-Jun N-terminal kinase) can directly activate CaMKII independent of ROS.3,4 These findings demonstrated a novel form of kinase-to-kinase crosstalk between JNK and CaMKII that causes arrhythmias by driving abnormal diastolic SR Ca2+ activities. And this kinase crosstalk could explain the link between arrhythmias and cellular stresses from aging, alcohol abuse, obesity, HF, and beyond. In addition, JNK also promotes the formation of a reentrant substrate by downregulating gap junction connexin43, thus causing slow conduction and facilitating triggered activities for AF development.10 In the context of ischemia, as occurs in patients with SDB, gap junction remodeling could be an important reentrant substrate of AF. Thus, it would be interesting to know the intricate crosstalk between ROS, JNK, CaMKII along with their subsequent contribution to the generation of triggered activities and reentrant substrate in SDB. Additionally, AF is linked to elevated inflammation. Interestingly, patients with SDB showed increased circulating C-reactive protein, a biomarker for inflammation, due to an increased TNF-α (tumor necrosis factor α/NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells) signaling pathway related to intermittent hypoxia. And this pathway can be suppressed by the continuous positive airway pressure intervention suggesting the possible involvement of inflammation.11 Another related point of interest is that hypersensitized RyR2 channels in SDB could be regulated by other mechanisms. For example, direct oxidation of RyR was suggested to enhance SR Ca2+ leak. With an increased ROS production in SDB, oxidation of RyR2 could, at least in part, contribute to the SR Ca2+ mishandling. Also, patients with SDB exhibit increased sympathetic responses, which could activate the PKA (protein kinase A) pathway.12 The contributions relative to the PKA-dependent RyR2 and INa,L in SDB-associated AF require elaboration in future studies.
Figure. Schematic outlines triggered arrhythmic activities through the relevant mechanisms of coupled kinases and ion channels.
Physiological and pathological regulation of ca2+ dynamics occur in myocytes through a number of kinases including the CaMKII (Ca2+/calmodulin-kinase type-II), PKA (protein kinase A), JNK (c-Jun N-terminal kinase) signaling pathways. Sleep disordered breathing (SDB) facilitates proarrhythmic activities and atrial fibrillation through a CaMKII-dependent dysregulation of INa,L and sarcoplasmic reticulum (SR) Ca2+ mishandling. SDB may act as a cellular stressor manifesting its affects via hypoxia-ischemic input which could involve the PKA and JNK pathways or other yet to be identified pathways. β-AR indicates β-adrenergic receptor; AC, adenylyl cyclase; c-AMP, cyclic adenine monophosphate; CaM, calmodulin; DAD, delayed afterdepolarization; EAD, early afterdepolarization; INa,L, late Na channel; ICa, Ca2+ current; NCX, Na+/Ca2+ exchanger; PLB, phospholipase B; and ROS, reactive oxygen species.
Although the treatment strategies for AF have been improved with the technological advancements and the refinement of ablation procedures, advancing our understanding of the underlying mechanisms of AF genesis is clearly needed in order to exploit new and effective therapeutic targets. Despite the fact that results from animal models have shown an antiarrhythmic effect of INa,L inhibition by ranolazine (a INa,L inhibitor),7,8 the current study demonstrates a potential means by which novel therapeutic targets and strategies could be exploited to modulate CaMKII activity or INa,L to treat AF for patients with SDB.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health (HL113640, AA024769, and HL146744 to X. Ai).
Footnotes
Disclosures
None.
REFERENCES
- 1.Lebek S, Pichler K, Reuthner K, Trum M, Tafelmeier M, Mustroph J, Camboni D, Rupprecht L, Schmid C, Maier L, et al. Enhanced CaMKII-dependent late Ina induces atrial proarrhythmic activity in patients with sleep-disordered breathing. Circ Res. 2020;126:603–615. doi: 10.1161/CIRCRESAHA.119.315755 [DOI] [PubMed] [Google Scholar]
- 2.May AM, Van Wagoner DR, Mehra R. OSA and cardiac arrhythmogenesis: mechanistic insights. Chest. 2017;151:225–241. doi: 10.1016/j.chest.2016.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan J, Zhao W, Thomson JK, Gao X, DeMarco DM, Carrillo E, Chen B, Wu X, Ginsburg KS, Bakhos M, et al. Stress signaling JNK2 crosstalk with CaMKII underlies enhanced atrial arrhythmogenesis. Circ Res. 2018;122:821–835. doi: 10.1161/CIRCRESAHA.117.312536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan J, Thomson JK, Zhao W, Gao X, Huang F, Chen B, Liang Q, Song LS, Fill M, Ai X. Role of stress kinase JNK in binge alcohol-evoked atrial arrhythmia. J Am Coll Cardiol. 2018;71:1459–1470. doi: 10.1016/j.jacc.2018.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89 [DOI] [PubMed] [Google Scholar]
- 6.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, et al. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sossalla S, Kallmeyer B, Wagner S, Mazur M, Maurer U, Toischer K, Schmitto JD, Seipelt R, Schöndube FA, Hasenfuss G, et al. Altered Na(+) currents in atrial fibrillation effects of ranolazine on arrhythmias and contractility in human atrial myocardium. J Am Coll Cardiol. 2010;55:2330–2342. doi: 10.1016/j.jacc.2009.12.055 [DOI] [PubMed] [Google Scholar]
- 8.Glynn P, Musa H, Wu X, Unudurthi SD, Little S, Qian L, Wright PJ, Radwanski PB, Gyorke S, Mohler PJ, et al. Voltage-gated sodium channel phosphorylation at Ser571 regulates late current, arrhythmia, and cardiac function in vivo. Circulation. 2015;132:567–577 doi: 10.1161/CIRCULATIONAHA.114.015218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Finet JE, Wolfram JA, Anderson ME, Ai X, Donahue JK. Calcium/calmodulin-dependent protein kinase II causes atrial structural remodeling associated with atrial fibrillation and heart failure. Heart Rhythm. 2019;16:1080–1088. doi: 10.1016/j.hrthm.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan J, Thomson JK, Zhao W, Wu X, Gao X, DeMarco D, Kong W, Tong M, Sun J, Bakhos M, et al. The stress kinase JNK regulates gap junction Cx43 gene expression and promotes atrial fibrillation in the aged heart. J Mol Cell Cardiol. 2018;114:105–115. doi: 10.1016/j.yjmcc.2017.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryan S, Taylor CT, McNicholas WT. Systemic inflammation: a key factor in the pathogenesis of cardiovascular complications in obstructive sleep apnoea syndrome? Thorax. 2009;64:631–636. doi: 10.1136/thx.2008.105577 [DOI] [PubMed] [Google Scholar]
- 12.Hegyi B, Bányász T, Izu LT, Belardinelli L, Bers DM, Chen-Izu Y. β-adrenergic regulation of late Na+ current during cardiac action potential is mediated by both PKA and CaMKII. J Mol Cell Cardiol. 2018;123:168–179. doi: 10.1016/j.yjmcc.2018.09.006Author Queries [DOI] [PMC free article] [PubMed] [Google Scholar]