Abstract
BACKGROUND
The implementation of nutritional strategies targeting several variables at once could benefit patients with cirrhosis. Non-alcoholic beer has different compounds that exert antioxidant, anti-inflammatory and nutritional properties.
AIM
To evaluate the effect of diet + exercise and non-alcoholic beer on nutritional status, endothelial function and quality of life in patients with cirrhosis.
METHODS
In this randomized open clinical trial, patients with cirrhosis were randomized into two groups: The intervention (non-alcoholic beer + diet + exercise) and control (water + diet + exercise) group. Treatment consisted of 330 mL non-alcoholic beer/day or the same amount of water, plus an individualized dietary plan and an exercise program with a pedometer-based bracelet to reach at least 5000 steps/d and > 2500 above the baseline during 8 wk. Endothelial function (flow-mediated dilation, plethysmography), biochemical and nutritional variables and quality of life (CLDQ) were evaluated.
RESULTS
Forty-three patients were included in the study, 21 in the control group and 22 in the intervention group. The mean age was 53.5 ± 7.8 years, 60% were women, the median MELD score was 8 (7-10) and most patients were Child-Pugh A (88%). Adherence to the interventions was > 90% in both groups, there were no adverse events and all biochemical parameters remained stable in both groups. Endothelial function improved in both groups. All measured nutritional parameters improved in the intervention group, compared to only 2 in the control group and quality of life improved in both groups; however, more domains improved in the intervention group.
CONCLUSION
The intervention consisting of non-alcoholic beer, diet and exercise seems to be safe and well tolerated in patients with cirrhosis, and shows improvement in nutritional status, endothelial function, and quality of life. These results need to be further confirmed.
Keywords: Hops, Portal hypertension, Sarcopenia, Diet, Cirrhosis, Antioxidants
Core Tip: Malnutrition is a frequent complication in patients with cirrhosis and it is associated with adverse outcomes. Diet and physical exercise are strategies that have shown a beneficial effect on nutritional status. On the other hand, non-alcoholic beer has several nutrients including vitamin B, minerals and flavonoids that make it an attractive "functional" supplement for patients with cirrhosis. The present study evaluated the effect of a multifactorial intervention that included diet, exercise and non-alcoholic beer, showing that it is safe and well tolerated. This intervention showed improvement in endothelial function, quality of life and nutritional status including muscle mass.
INTRODUCTION
Liver cirrhosis is a frequent disease, leading to poor quality of life and representing the 13th most common cause of death globally[1,2]. Most of the complications in cirrhosis arise from the development of portal hypertension, including ascites, variceal hemorrhage, hepatorenal syndrome and hepatic encephalopathy[2-4].
Malnutrition is a frequent complication in patients with cirrhosis, being present in up to 40%-90% of the population during the evolution of the disease[5]. Malnutrition involves different clinical manifestations, including sarcopenia and cachexia, having higher prevalence in the late stages of cirrhosis[5-8]. One of the most important facts regarding malnutrition in cirrhosis, is its association with mortality and the development of other complications, such as hepatic encephalopathy[9,10]. Therefore, any intervention aimed at improving the nutritional status could be helpful in the outcome in patients with cirrhosis and portal hypertension[11].
Nutritional therapy in patients with cirrhosis includes both the implementation of diets and physical exercise programs[12-16]. General recommendations of nutritional therapy are focused on providing a sufficient and non-restricted energy and protein supply, together with a high amount of fiber[17,18]. Other useful recommendations in this population include frequent meals and nocturnal supplementation with different nutrients, as well as the use of branched-chain amino acids (BCAAs)[19-22]. Nutritional supplementation with BCAAs has been extensively studied, and its benefits in patients with cirrhosis are widely recognized, specifically targeting protein metabolism[17,23-26]. However, implementation of this nutritional strategy is sometimes difficult due to various factors, including availability and tolerability, as well as high cost. The lack of other options in the different clinical settings, considering the factors mentioned above, renders mandatory searching for other options that could be helpful in the nutritional management of these patients.
On the other hand, non-alcoholic beer has several nutrients derived from its ingredients (yeast, xanthohumol and hops), including vitamin B, minerals and flavonoids[27], rendering it an attractive nutritional supplement in patients with cirrhosis. In addition to these effects, non-alcoholic beer has been shown to beneficially modify gut microbiota diversity[28], it also improves endothelial function and oxidative stress[29], and has been used in different clinical settings, including breastfeeding, and post-exercise rehydration among others[30,31]. Therefore, non-alcoholic beer can be regarded as a “functional” supplement. Physical exercise has proven to be a safe and effective intervention in cirrhosis, providing several benefits, for instance, improvement in nutritional status, quality of life and portal pressure[16,32,33].
Considering all the previous evidence, the use of a multifactorial intervention, including diet, exercise and a non-alcoholic beer as a supplement could be of benefit in patients with cirrhosis and portal hypertension, influencing the different components of malnutrition in this population. Therefore, the aim of this study was to evaluate the effect of a multifactorial intervention, with diet, exercise and non-alcoholic beer on nutritional parameters, endothelial function, quality of life and safety in patients with cirrhosis and portal hypertension.
MATERIALS AND METHODS
Patients and methods
This was a randomized open-clinical trial, performed at a third level center in Mexico City from March 2015 to September 2018 (Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán). The study protocol was approved by the local Research and Ethics Committee at the institution and registered at ClinicalTrials.gov. It was designed and conducted according to the principles of the Declaration of Helsinki and all patients signed the informed consent before study inclusion.
Inclusion criteria
The inclusion criteria were: (1) Patients with liver cirrhosis established by liver biopsy or the presence of markers of dysfunction in hepatocyte synthesis and portal hypertension (esophageal varices on endoscopy, ascites and other compatible features on ultrasonography); and (2) Patients aged 18-70 years.
Exclusion criteria
Patients with the following characteristics were excluded: (1) Genetic liver disease (hemochromatosis and Wilson´s disease); (2) Alcoholic liver disease (including current alcohol intake); (3) History of cancer (including hepatocellular carcinoma); (4) Systemic inflammatory response syndrome; or (5) Decompensation during the past 6 wk.
Intervention
Patients were randomized into 2 groups: (1) Control (diet + exercise + water); and (2) Intervention (diet + exercise + non-alcoholic beer). Randomization was carried out in blocks of 6 patients, using the web-based page randomization.com, and concealment was done with sequentially numbered, opaque, and sealed envelopes.
Randomization, enrollment, and assignment of patients to the groups were performed by different members of the study team.
Diet: Caloric intake was calculated using the Harris-Benedict equation to estimate the resting metabolic rate (RMR) plus 10% of the thermic effect of food and in addition, 20% of calories were added in order to prevent the catabolic effect of exercise. The proportion of macronutrients included was 60% of carbohydrates, 1.3-1.5g of protein/kg body weight/d, and the rest from lipids. Finally, sodium intake was restricted (60-90 mEq/d or 1.5-2 g/d of salt) in patients with ascites or edema.
In terms of distribution, 60% of the total caloric intake was obtained from carbohydrates of which 50% were complex carbohydrates, protein was calculated as 1.3-1.5g of protein/kg body weight/d, and the distribution of proteins was 50% vegetable protein and 50% animal protein, and the remaining calories were derived from fat, with less than 10% of saturated fat and 5%-15% of unsaturated fat.
The dietary plan was calculated according to the individual caloric requirement of each patient, with the same macronutrient distribution, as stated in the ESPEN guidelines. The plan was created and prescribed by a trained nutritionist specialized in the field of hepatology and liver transplantation.
The patients received a dietary plan based on exchangeable food sources, where they received the permitted portions of each food group according to the Mexican System of Equivalent Foods (SMAE), the food groups are vegetables, fruits, cereals (with and without fat), legumes, animal-origin foods (with different amounts of fat), milk, fats/oils (with and without protein) and sugars (with and without fat). The dietary plan included a list of all the permitted foods in each food group and the patients were only allowed to eat the food that was listed.
The foods that were excluded from the list were those normally excluded from the diet in patients with chronic liver disease; mainly, canned foods, cold meats such as sausages, ham, salami, etc., high fat meat, high fat dairy, and overly processed drinks.
Exercise: For the physical exercise program, each patient received a physical activity tracker, in the form of a bracelet-based accelerometer, able to measure the number of steps (i.e., functioning as a pedometer, PolarLoop, POLAR, Finland). During the 10-wk period of the study, the patients were allocated to a program that included the following: (1) Educational session: The first intervention that the patients received (both groups), was a 15-min presentation on general information related to cirrhosis and its complications, as well as the benefits, the indications and contraindications of physical exercise and non-alcoholic beer; (2) In the first 2 wk, the participants received a training program in order to learn how the device works and the appropriate use of the activity monitor tracker, as well as the correct record of the baseline physical activity in each patient, before starting the intervention; and (3) In the remaining 8 wk, the patient gradually increased their baseline physical activity, aiming to reach > 2500 steps/d above the average baseline level and a total number of steps of 5000/d. Intensity of exercise was set using the Borg Scale of perceived exertion (scale from 6 to 20), and each patient was trained to reach a target intensity of 10-12 (corresponding to light to moderate intensity and from 3 to 3-6 metabolic equivalents (METs))[34]. For training, we used a long hallway with a flat surface (Supplementary Figure 1) allowing the patient to use the monitor, learn how it works and how to reach the intensity of exercise through a chart depicting the required level of effort (Borg scale, Supplementary Figure 2). There should be no differences in exercise prescription between men and women according to the guidelines[34,35]. Nutritional requirements were adjusted according to sex.
Non-alcoholic beer nutritional supplementation: Each patient in the intervention group received 1 can (330 mL) of non-alcoholic beer daily (O´Doul´s®, St. Louis, MO, United States), which was indicated to be consumed together with food during lunch, for 8 wk. Caloric and nutritional intake derived from the non-alcoholic beer (0.4% alcohol, 90 kcal/350 mL, 0 g fat, 18 g carbohydrates/350 mL, 1.9 g protein/350 mL), was included in the daily caloric intake mentioned previously (detailed information regarding the components and ingredients of the non-alcoholic beer are shown in Supplementary Material Appendix 1). The control group received a 330 mL bottle of water, in order to control the possible adverse events resulting from the increased fluid intake.
Follow up during the study
Patients were followed for 10 wk (2 wk to determine the baseline physical activity and to adapt to the monitoring device, and 8 wk of intervention), they were evaluated onsite at weeks -2, 0, 4 and 8. In each visit, adverse events, vital signs and nutritional variables were evaluated, as well as adherence to the diet/exercise program and non-alcoholic beer/water consumption by counting the empty cans or water bottles that patients were asked to take back to the center.
At baseline and final evaluations, biochemical tests (liver function tests, serum electrolytes, creatinine, glucose, complete blood count and international normalized ratio (INR)) were performed in every patient, according to the standards of the central laboratory in our institution.
Outcome measurements
Nutritional status: At baseline evaluation, weight and height were measured and later weight at each subsequent visit.
Bioimpedance analysis (BIA) was performed using a monofrequency device at 50 kHz (RJL systems, Quantum IV), at baseline and final evaluations, after an overnight 8-h fasting period, with an empty bladder and removing any metal objects the patient may be carrying. After placing four electrodes, two in the right hand and two in the right foot, the measurement was performed to obtain resistance (R), reactance (Xc) and phase angle (PhA). Malnutrition was considered when PhA was below 4.9° as previously validated and standardized in patients with cirrhosis[36].
Anthropometry was performed at baseline and final evaluations, including arm circumference, mid-arm circumference (MAC), triceps skinfold thickness, thigh and calf circumferences, and handgrip strength.
Triceps skinfold thickness (TST) was measured on the non-dominant arm to the nearest mm using a Harpenden caliper and mid-arm circumference was measured on the non-dominant arm to the nearest 0.1 cm with a non-stretchable measuring tape. Mid-arm muscle circumference (MAMC), was calculated from the MAC and the TST using the formula MAMC (mm) = MAC (mm) - (3.14 TST in mm). Subjects were considered malnourished when TST and/or MAMC were below the 5th percentile.
Handgrip strength (HGS) was measured with the patient seated using a Jamar handgrip dynamometer (Patterson Medical, Warrenville, IL, United States). Measurements were made according to the manufacturer’s instructions and recorded to the nearest kg.
Quality of life: Quality of life was assessed with the chronic liver disease questionnaire (CLDQ), and the 36-item short form health survey (SF-36) in all the participants before and after completion of the study.
Neurocognitive function: At baseline and final evaluations, both the psychometric hepatic encephalopathy score (PHES) and critical flicker frequency (CFF, Hepatonorm Analyzer R&R Medi-Business Freiburg, Freiburg, Germany), were performed in all the participants.
Endothelial function: Endothelial function was measured using plethysmography. While the patient was sitting, the baseline plethysmography wave was recorded for 30 s, and thereafter ischemia was induced by insufflating an upper arm blood pressure cuff 30 mmHg above the systolic pressure for 5 min. Finally, a new wave was recorded for 120 s after deflating the arm cuff (post-ischemia wave). Every 30 s the plethysmography wave was recorded and contrasted with the baseline wave, and the maximal amplitude time (MAT) and the total time (TT) of the wave were calculated. Endothelial dysfunction was considered when the value of the MAT/TT ratio was < 30, as has been described previously[37]. The person who performed the study was unaware of the group status of the patient.
Statistical analysis
Sample size was estimated according to a previous study in patients with cirrhosis allocated to a physical exercise program, where nutritional status evaluated through BIA derived-phase angle, showed an improvement after exercise, from a baseline of 5.8º ± 0.89 to 6.0º ± 0.81 at the end of the study[33]; this difference was 0.2º ± 0.89 (3.4%), and for the present study a total change of 10% was expected (Δ = 0.58). Finally, with α y β error of 0.05 and 0.2, and a drop out of 10% the final number was 21 patients per group.
Normality of the data was evaluated with the Shapiro-Wilk test. Data are presented as mean ± SD, median (P25-P75) or frequencies. Results at baseline and final evaluations in each group (paired data) were analyzed with the Wilcoxon signed-rank test. For comparisons between groups, the Mann-Whitney U or Student´s t-test was used. Areas under the curve (AUC) were constructed with the repeated values (time 0’, 30’, 60’, 90’ and 120’), of the MAT/TT ratio obtained at baseline and final evaluations. In this analysis, only those patients receiving at least 1 d of intervention were included (modified intention to treat, mITT). Finally, to control for baseline differences, ANCOVA was performed. Statistical analysis was carried out with the package software SPSS version 20.0 (Armonk, NY, United States) and figures were created using GraphPad Prism 5.
RESULTS
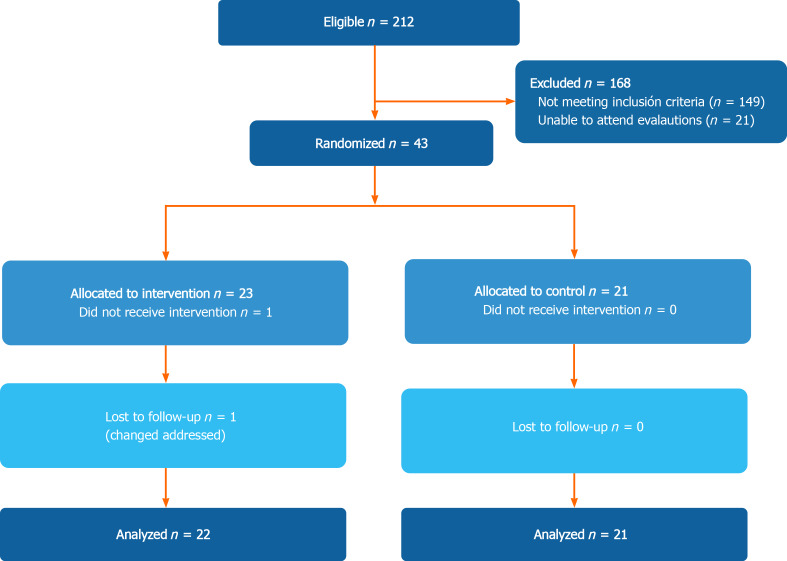
In total, 44 patients were included in the study (21 in the control group and 23 in the multifactorial intervention) (Figure 1). Only 1 patient in the intervention group was lost during follow-up. The final analysis included 21 patients in the control group, and 22 in the intervention group (mITT).
Figure 1.
CONSORT enrollment diagram.
In the total population, most of the patients were women (60.5%), mean age was 53.5 ± 7.8 years, and the main etiologies of cirrhosis were hepatitis C infection (32.6%), autoimmune hepatitis (20.9%) and non-alcoholic fatty liver disease (NAFLD) (18.6%). All patients were Child-Turcotte-Pugh (CTP) A (88.4%) and B (11.6%), with a median CTP score of 5 (5-6) and model for end-stage liver disease (MELD) score of 8 (7-10). The only baseline difference in biochemical parameters was a higher alkaline phosphatase level in the intervention group (P = 0.039) (Table 1). There were no differences between the groups regarding severity of disease, presence of complications or age.
Table 1.
Baseline characteristics of the study population
|
|
All (n = 43)
|
Control (n = 21)
|
Intervention (n = 22)
|
P
value
|
| Sex n (%) F/M | 26 (60.5)/17 (39.5) | 12 (46.2)/9 (52.9) | 14 (53.8)/8 (47.1) | 0.663 |
| Age (yr) | 53.5 ± 7.8 | 53.7 ± 8.2 | 53 ± 7.6 | 0.768 |
| BMI (kg/m2) | 29.5 ± 4.2 | 29.2 ± 3.7 | 29.8 ± 4.8 | 0.635 |
| Etiology of cirrhosis | ||||
| HCV n (%) | 14 (32.6) | 6 (28.5) | 8 (36.4) | 0.063 |
| AIH n (%) | 9 (20.9) | 3 (14.3) | 6 (27.3) | |
| NAFLD n (%) | 8 (18.6) | 5 (23.8) | 3 (13.6) | |
| Others1 n (%) | 12 (27.9) | 7 (33.4) | 5 (22.7) | |
| History of complications [n (%)] | ||||
| Variceal bleeding | 2 (4.7) | 2 (9.5) | 0 (0) | 0.233 |
| Encephalopathy | 1 (2.3) | 0 (0) | 1 (4.5) | 0.500 |
| Ascites | 5 (11.6) | 3 (14.3) | 2 (9.1) | 0.664 |
| Child-Pugh stage | ||||
| A | 38 (88.4) | 20 (95.2) | 18 (81.9) | 0.345 |
| B | 5 (11.6) | 1 (4.8) | 4 (18.1) | |
| Child-Pugh | 5 (5-6) | 5 (5-6) | 5 (5-6) | 0.617 |
| MELD score | 8 (7-10) | 8 (7.5-9.5) | 8.5 (7-10) | 0.524 |
| Biochemical parameters | ||||
| TB (mg/dL) | 1.19 (0.82-1.95) | 1.15 (0.75-1.44) | 1.28 (0.85-2.11) | 0.284 |
| ALT (U/L) | 43 (26-77) | 43 (30-86.5) | 43.1 (20.75-77) | 0.882 |
| AST (U/L) | 48 (37-93) | 47 (40-80) | 50.5 (32-98) | 0.881 |
| AP (mg/dL) | 142.2 ± 57.7 | 123.7 ± 44.1 | 159.8 ± 64.4 | 0.039 |
| Albumin (g/dL) | 4.0 (3.6-4.2) | 4.1 (3.8-4.3) | 3.9 (3.3-4.1) | 0.170 |
| Leukocytes (K/µL) | 4.2 (3.7-5.3) | 4.0 (3.7-5.15) | 4.75 (3.725-5.925) | 0.233 |
| Platelets (K/µL) | 80 (62-125) | 77 (61.5-125) | 93.5 (63.5-125) | 0.520 |
| Hemoglobin (g/dL) | 14.1 ± 1.94 | 14.2 ± 3.3 | 14.0 ± 2.4 | 0.755 |
| INR | 1.1 (1.1-1.2) | 1.1 (1.1-1.2) | 1.1 (1.1-1.2) | 0.870 |
| Glucose (mg/dL) | 91 (83-103) | 94 (83.5-109) | 91 (81-100.5) | 0.882 |
| Creatinine (mg/dL) | 0.72 (0.63-0.80) | 0.72 (0.60-0.81) | 0.71 (0.64-0.79) | 0.892 |
| Sodium (mmol/L) | 139 (137-141) | 140 (137.5-141) | 138 (137-140.2) | 0.290 |
| Potassium (mmol/L) | 4.08 ± 0.32 | 4.0 ± 0.37 | 4.1 ± 0.25 | 0.198 |
| CO2 (mmol/L) | 24 (21-26) | 23.0 (21.5-26) | 24.5 (21-27.25) | 0.518 |
Other etiologies included cryptogenic, primary biliary cholangitis and overlap syndrome (HAI/CBP). Data reported as mean ± standard deviation or median (p25-p75), according to the distribution of the data, or absolute frequency (percentage %). BMI: Body mass index; HCV: Hepatitis C virus; AIH: Autoimmune hepatitis; NAFLD: Non-alcoholic fatty liver disease; TB: Total bilirubin; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; AP: Alkaline phosphatase; INR: International normalized ratio.
The exercise program was successful in both groups, reaching an increase of almost 3000 steps/d after the intervention (P < 0.001 in both groups). In the biochemical parameters, only a mild improvement in transaminases and an increase in the number of platelets were observed in the control and intervention group, respectively. The remaining parameters such as the liver function tests, as well as creatinine, glucose, and serum electrolytes, showed no change compared to their baseline values (Table 2).
Table 2.
Changes in clinical and biochemical parameters
|
Control (n = 21)
|
|
Intervention (n = 22)
|
|
|||
|
|
Baseline
|
Final
|
P
value
|
Baseline
|
Final
|
P
value
|
| Number of daily steps | 8718 ± 2998 | 11391 ± 4298 | 0.000 | 8533 ± 4072 | 11142 ± 4055 | 0.000 |
| PHES score | -1 ± 2.2 | 0 ± 2.2 | 0.130 | -1 ± 2.1 | 0 ± 2.3 | 0.179 |
| CFF (Hz) | 43.9 ± 7.1 | 45.5 ± 5.8 | 0.238 | 44.5 ± 8.1 | 47.2 ± 6.4 | 0.174 |
| TB (mg/dL) | 1.15 (0.75-1.44) | 1.12 (0.85-1.28) | 0.985 | 1.28 (0.85-2.11) | 1.13 (0.8-1.865) | 0.068 |
| ALT (U/L) | 43 (30-86.5) | 41 (23.5-71) | 0.024 | 43.1 (20.75-77) | 36 (20.25-67.5) | 0.198 |
| AST (U/L) | 47 (40-80) | 45 (29.5-53.5) | 0.015 | 50.5 (32-98) | 44 (28-75.75) | 0.035 |
| Alkaline phosphatase (U/L) | 123.7 ± 44.1 | 105 (79.50-139) | 0.408 | 159.8 ± 64.4 | 159.9 ± 79.3 | 0.988 |
| Albumin (g/dL) | 4.1 (3.8-4.3) | 4 (3.75-4.2) | 0.668 | 3.9 (3.3-4.1) | 3.9 (3.475-4.3) | 0.501 |
| Leukocytes (K/µL) | 4.0 (3.7-5.15) | 3.9 (3-5) | 0.984 | 4.75 (3.725-5.925) | 4.9 (3.62-6.1) | 0.515 |
| Platelets (K/µL) | 77 (61.5-125) | 75 (59-106.5) | 0.278 | 93.5 (63.5-125) | 107 (78.5-150.25) | 0.046 |
| Hemoglobin (g/dL) | 14.2 ± 3.3 | 14.17 ± 1.52 | 0.968 | 14.0 ± 2.4 | 14.25 ± 2 | 0.834 |
| INR | 1.1 (1.1-1.2) | 1.1 (1.1-1.2) | 0.414 | 1.1 (1.1-1.2) | 1.1 (1.0-1.2) | 0.166 |
| Glucose (mg/dL) | 94 (83.5-109) | 88 (82-108) | 0.145 | 91 (81-100.5) | 87.5 (84.75-96.25) | 0.615 |
| Creatinine (mg/dL) | 0.72 (0.60-0.81) | 0.71 (0.6-0.84) | 0.403 | 0.71 (0.64-0.79) | 0.70 (0.62-0.79) | 0.723 |
| Na (mmol/L) | 140 (137.5-141) | 140 (139-141.5) | 0.118 | 138 (137-140.2) | 140 (138.5-141) | 0.061 |
| K (mmol/L) | 4.0 ± 0.37 | 4.16 ± 0.38 | 0.195 | 4.1 ± 0.25 | 4.2 ± 0.37 | 0.078 |
| CO2 (mmol/L) | 23.0 (21.5-26) | 24.0 (22-26) | 0.850 | 24.5 (21-27.25) | 24 (22.95-26.15) | 0.304 |
Data reported as mean ± standard deviation, or median (p25-p75), according to the distribution of the data. PHES: Psychometric hepatic encephalopathy score; CFF: Critical flicker frequency; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; INR: International normalized ratio.
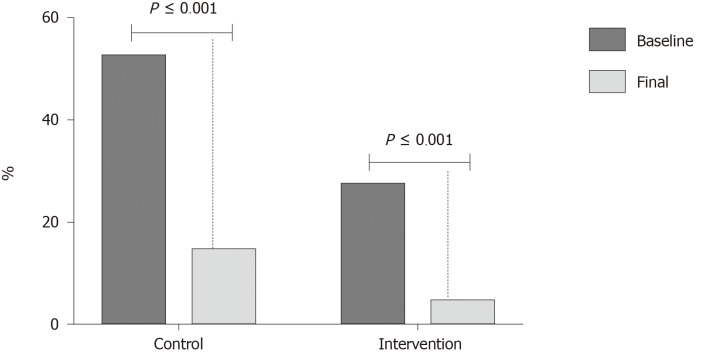
Of the subjects with endothelial dysfunction at the beginning of the study, in the control group 8 of 11 (72.7%) patients improved endothelial function at the end of the study, and 5 of 6 patients (83.3%) in the multi-intervention group. This change was significant in both groups (P < 0.001) (Figure 2).
Figure 2.
Changes in endothelial dysfunction at baseline and final evaluations. The figure shows the percentage of patients with endothelial dysfunction at baseline and final evaluation.
Endothelial function was further evaluated by the AUC of the MAT/TT ratio and an improvement was also seen in both groups; however, the behavior of the curves was different among groups (Table 3).
Table 3.
Endothelial function and hemodynamic variables
|
Control (n = 21)
|
P
value
|
Intervention (n = 22)
|
P
value
|
|||
|
Baseline
|
Final
|
|
Baseline
|
Final
|
|
|
| AUC | 103.13 (92.55-107.15) | 93.15 (86.53-99.61) | 0.021 | 98.04 (92.17-102.35) | 93.62 (85.06-99.60) | 0.046 |
| Systolic pressure (mmHg) | 120 (110-130) | 120 (120-129) | 0.418 | 120 (110-120) | 113 (110-120) | 0.513 |
| Diastolic pressure (mmHg) | 80 (60-80) | 80 (60-80) | 0.862 | 70 (67.5-80) | 70 (60-80) | 0.385 |
| Mean arterial pressure | 90 (76.693.3) | 93.3 (78.3-96) | 0.729 | 86.6 (82.5-93.3) | 83.3 (76.6-90.5) | 0.385 |
| Heart rate (bpm) | 62 (60-72.7) | 63 (60.25-80) | 0.079 | 69 (61.5-75.2) | 67 (61.5-71.25) | 0.235 |
Data reported as median (p25-p75). AUC: Area under the curve.
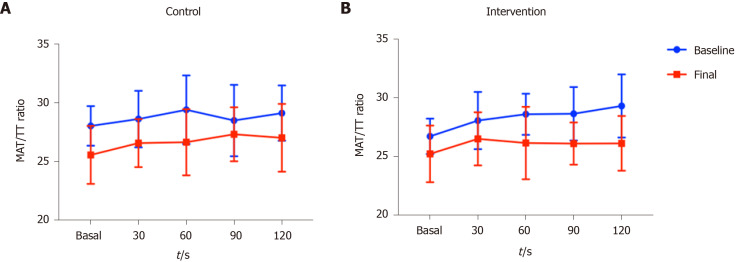
Figure 3 shows the changes in the MAT/TT ratio at baseline and final evaluations. Regarding the baseline evaluation, the behavior of the curve (repeated measurements over time) was similar in both groups, both showing higher MAT/TT ratio at time 120’ compared to time 0’, although a higher MAT/TT ratio was observed in the control group at time 0’. In terms of the final evaluation (end of interventions) a steadier curve was observed in the group receiving non-alcoholic beer, as well as a return to the time 0 values, showing better endothelial function, which was not observed in the control group.
Figure 3.
Area under the curve showing the behavior of endothelial function at baseline and final evaluations in the two groups. A: Control; B: Intervention. Data are presented as median (IQR). MAT: Maximal amplitude time; TT: Total time.
Hemodynamic variables including heart rate, diastolic, systolic, and mean arterial pressure remained stable throughout the study (Table 3).
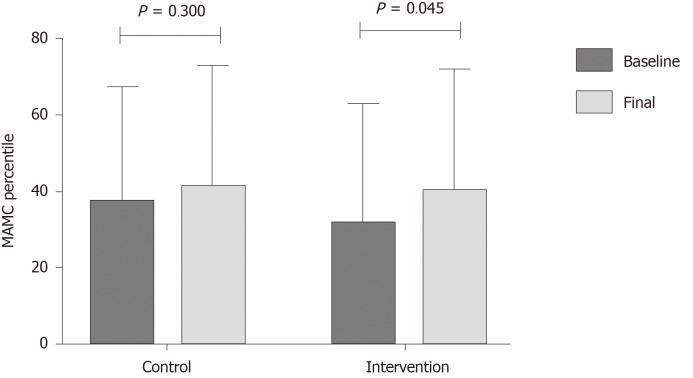
One of the most important results in this study was the improvement in nutritional status, where several nutritional markers (thigh circumference, HGS and sit-to-stand test), improved after the multifactorial intervention, compared to an increase only in the sit-to-stand test in the control group (Table 4). Adherence to the diet was evaluated at each visit, and it was found that in both groups adherence to the diet was > 90% in the first month, and > 95% in the following weeks. There was no statistical difference in the raw value of MAMC, but when this parameter was analyzed by the diagnosis of malnutrition with percentiles, a significant improvement was observed in the intervention group (P = 0.045) (Figure 4). There was a trend showing a more frequent improvement in PhA in the intervention group, where 63.6% of the patients receiving non-alcoholic beer had an improvement, compared to 47.6% of patients that did not receive non-alcoholic beer (P = 0.290, for the difference between the groups).
Table 4.
Changes in nutritional parameters at baseline and final evaluation
|
Control (n = 21)
|
P
value
|
Intervention (n = 22)
|
P
value
|
|||
|
Baseline
|
Final
|
|
Baseline
|
Final
|
|
|
| Phase angle | 6.0 ± 0.7 | 5.9 ±0.6 | 0.427 | 5.8 ± 0.7 | 5.9 ± 0.7 | 0.198 |
| MAMC (cm2) | 24.1 ± 3.1 | 23.9 ± 3.5 | 0.462 | 23.5 ± 3.4 | 23.6 ± 3.9 | 0.783 |
| TST (mm) | 25.5 ± 7.9 | 24.7 ± 4.5 | 0.397 | 26.6 ± 6.7 | 26.9 ± 6.3 | 0.782 |
| Thigh circumference (cm) | 53.1 ± 6.5 | 52.9 ± 7.0 | 0.758 | 51.7 ± 6.4 | 53.1 ± 4.6 | 0.033 |
| Handgrip strength (kg) | 19.9 ± 11.5 | 21 ± 11.2 | 0.121 | 18.1 ± 10.3 | 19.9 ± 10.5 | 0.048 |
| Sit-To-Stand test (s) | 24.2 ± 4.4 | 20.9 ± 6.9 | 0.001 | 23.3 ± 3.9 | 21.0 ± 6.2 | 0.045 |
Data reported as mean ± standard deviation. MAMC: Mid-arm muscle circumference; TST: Triceps skinfold thickness.
Figure 4.
Changes in mid-arm muscle circumference percentile throughout the study. Data are presented as mean percentile ± SD. MAMC: Mid-arm muscle circumference.
Almost all the individual components, as well as the overall score of the quality of life CLDQ score, improved after the intervention in the group that received non-alcoholic beer, compared to only minor changes in the control group (Table 5). Finally, in the SF-36 there was an improvement in the physical function role in both groups, in the general health role in the control group and in the vitality role in the multifactorial intervention group. Globally, the improvement in quality of life was higher in the group receiving non-alcoholic beer.
Table 5.
Changes in quality of life
|
Control (n = 21)
|
P
value
|
Intervention (n = 22)
|
P
value
|
|||
|
Baseline
|
Final
|
|
Baseline
|
Final
|
|
|
| CLDQ questionnaire | ||||||
| Abdominal symptoms | 5.4 ± 1.3 | 5.2 ± 0.8 | 0.592 | 5.0 ± 1.5 | 5.1 ± 1.2 | 0.982 |
| Fatigue | 4.7 ± 1.1 | 5.2 ± 0.8 | 0.063 | 4.2 ± 1.2 | 5.1 ± 1.2 | 0.000 |
| Systemic symptoms | 5.6 ± 1.0 | 5.6 ± 0.9 | 0.892 | 4.3 ± 1.2 | 5.0 ± 0.9 | 0.002 |
| Activity | 5.3 ± 1.2 | 5.3 ± 1.3 | 0.892 | 4.8 ± 1.2 | 5.5 ± 1.2 | 0.028 |
| Emotional function | 4.9 ± 0.9 | 5.3 ± 1.1 | 0.056 | 4.3 ± 1.3 | 5.3 ± 1.0 | 0.000 |
| Worry | 5.1 ± 1.1 | 5.3 ± 1.1 | 0.521 | 4.3 ± 1.8 | 5.2 ± 1.8 | 0.002 |
| CLDQ overall score | 5.2 ± 0.8 | 5.4 ± 0.9 | 0.204 | 4.6 ± 1.0 | 5.3 ± 0.8 | 0.001 |
| SF-36 questionnaire | ||||||
| Physical function | 80 (70-90) | 95 (80-95) | 0.005 | 72.5 (65-95) | 87.5 (70-95) | 0.007 |
| Physical role | 75 (25-100) | 100 (50-100) | 0.166 | 62.5 (25 -100) | 75 (25-100) | 0.468 |
| Body pain | 84 (66-90) | 84 (72-90) | 0.341 | 78 (61-84) | 78 (60-84) | 0.725 |
| General health | 40 (37-47) | 47 (35-52) | 0.044 | 40 (25-55) | 42 (32-52) | 0.375 |
| Vitality | 65 (50-70) | 70 (60-75) | 0.453 | 62.5 (50-75) | 70 (50-80) | 0.037 |
| Social function | 100 (75-100) | 88 (75-100) | 0.875 | 75 (63-100) | 88 (75-100) | 0.080 |
| Emotional role | 67 (33-83) | 67 (33-67) | 0.972 | 67 (33-72) | 67 (33-67) | 0.752 |
| Mental health | 72 (68-84) | 84 (64-88) | 0.868 | 76 (64-84) | 82 (68-92) | 0.073 |
Data reported as mean ± standard deviation, or median (p25-p75), according to the distribution of the data. CLDQ: Chronic liver disease questionnaire; SF-36: 36-item short form health survey.
In order to adjust for baseline differences among the groups, ANCOVA was also performed, controlling for baseline MAMC, number of steps, AUC for endothelial function, PhA, HGS, sit-to-stand test, and CLDQ (global score). The main results, including PhA, remained significant after adjustment for these baseline variables (Supplementary Material Appendix 2).
Adverse events
There were no reported adverse events or side effects derived from the intervention, this was evidenced by the fact that there were no changes in biochemical tests at the end of the intervention in either group, or worsening quality of life specifically of abdominal symptoms.
DISCUSSION
Nutritional status in cirrhosis has a central role in the prognosis of the disease, influencing both mortality (i.e., it has been associated with mortality) and the development of cirrhosis-related complications[38]. Therefore, any intervention able to positively modify malnutrition in patients with cirrhosis deserves special attention. In the present work, we studied the effect of a multi-level nutritional intervention, including diet, exercise, and non-alcoholic beer, in the nutritional status of patients with cirrhosis.
Even though there are studies addressing the effect of exercise and diet in cirrhosis[16,32], this is the first study showing the effect of adding a cheap, easy to access and consume and rational-based nutritional supplement (i.e., non-alcoholic beer). Although BCAA supplements play an important role in the nutritional management of patients with advanced cirrhosis[11], their use is limited in some clinical settings due to availability and cost, as well as poor palatability and gastrointestinal symptoms such as bloating, nausea and abdominal pain[39,40]. On the other hand, non-alcoholic beer has different compounds that can be useful in the nutritional management of patients with cirrhosis, and it is an attractive way of providing these compounds in those patients who usually have a limited variety of food. These were the main reasons for exploring the use of non-alcoholic beer in this population, together with a personalized diet and exercise, and involving a multidisciplinary group, as recommended in the guidelines[11].
First of all, the exercise program was created to be easy to follow, specifically by using pedometer-based bracelets to monitor exercise, and it was well understood and well implemented by the patients in both groups, allowing them to successfully reach the aimed number of steps per day (at least > 2500 per day above the baseline level), with an adherence > 90%. This is especially important because in this study, one of the main concerns was to provide a completely outpatient multifactorial approach, including an effective exercise program, in order to broaden the use of such an intervention in different clinical settings, and daily life, and therefore not limiting its use only into a very specialized research setting as has been shown in different studies[16,33].
Endothelial dysfunction has a central role in the pathophysiology of portal hypertension[41], thus interventions aimed to improve it have been the focus of treatment and research in cirrhosis. In the present study, an improvement in endothelial function was found in both groups, as a consequence of physical exercise. While these effects are expected after physical exercise, the complete picture of the benefits associated with the consumption of non-alcoholic beer, seems to bring additional benefits and enhance exercise performance, including nutritional status and body composition, as well as improvement in quality of life.
Several markers of nutritional status improved, which is the expected change for an effective program with exercise and diet, as has been previously demonstrated in several studies in cirrhotic populations[16,32]. However, the most prominent findings were observed in the group receiving non-alcoholic beer, where 3 important parameters of nutritional status (thigh circumference, HGS and sit to stand test) improved, compared to only the sit to stand test in the control group. In addition, more patients allocated to the group with non-alcoholic beer had an improvement in the phase angle, although no statistical difference was reached (P = 0.290). The overall results for nutritional status suggest a benefit for non-alcoholic beer, and, specifically addressing the results of the improvement in the non-alcoholic beer group, it is important to mention that being able to possibly modify the circumference of the thigh is of great relevance and taken together with the improvement of the two tests evaluating muscle function we can conclude that the intervention targeted the main issues of sarcopenia which is the amount of muscle and its function.
There are various reasons that can explain why non-alcoholic beer had an additive effect in these patients: Improvement in gut microbiota, and palatability of the meals (thus improving dietary intake); Better hydration and higher content of minerals, improving muscle function and therefore facilitating exercise performance; and The amount of micronutrients including vitamin B12, that is a known factor essential for muscle formation and can play a role as an antioxidant, improve exercise tolerance, and neuromuscular function. Given that patients in the control group received the same amount of water, the benefits mentioned above cannot be explained merely by hydration.
On the other hand, there was an improvement in quality of life assessed by the CLDQ and the SF-36 questionnaires, in both groups, but again the most prominent findings were observed in the group receiving non-alcoholic beer. It is noteworthy that all but one domain in the CLDQ improved in the non-alcoholic beer group, with no worsening of abdominal symptoms as could be expected for the intervention (bloating). There was improvement in the SF-36 only in 2 domains, including physical function, in both groups and in general health and vitality in the control and non-alcoholic beer group, respectively. A clear trend towards an improvement in social function and mental health was observed in the group receiving non-alcoholic beer. This point is extremely important, because usually patients with chronic diseases, including cirrhosis, have a lower quality of life that impedes them from social interaction with other people. Integration of these patients into their normal social environment, by allowing them to participate in activities such as physical exercise, as well as having a “normal” diet supplemented with non-alcoholic beer, can contribute to the benefits observed specifically in this group. Moreover, hops have been shown to improve symptoms of depression, anxiety and stress over a 4-week period partially explaining the results found in QoL[42].
Regarding the safety of the intervention, there were no reported side effects in any of the groups. In the biochemical tests, no changes in liver function tests, hemoglobin, leukocytes, creatinine or serum electrolytes were observed, and a trend towards a global improvement in those tests was noted after the study. In addition, there was no worsening of abdominal symptoms in the group receiving non-alcoholic beer, as might be expected as a consequence of the beverage.
The most important features in the present study are the design, controlling the amount of additional liquids in both groups, and proposing an easy to follow and reliable exercise protocol together with an affordable and simple nutritional supplement, involving a multidisciplinary group from different specialties (gastroenterology, hepatology, nutrition and cardiology).
There are some limitations in this study; first, the patients included in this study were compensated, therefore the findings are not applicable to patients with decompensated cirrhosis. Another potential limitation is the lack of computed tomography (CT) scan measurements; however, given the monthly evaluations performed in this study, CT scan measurements were not appropriate (due to radiation exposure), therefore it was decided to include other validated markers such as phase angle derived from BIA, that has been validated in cirrhosis against CT scanning with great sensitivity[43], and HGS, also validated in cirrhosis.
CONCLUSION
In conclusion, a multifactorial program including diet, monitored exercise and non-alcoholic beer is safe, well tolerated and results in improvements in endothelial function, nutritional status, and quality of life. The effects shown in this study should be confirmed with a longer duration of the intervention, and possibly with a larger amount of non-alcoholic beer.
ARTICLE HIGHLIGHTS
Research background
Non-alcoholic beer has been shown to positively modify gut microbiota diversity and improve endothelial function and oxidative stress, when used in different clinical settings, including breastfeeding and post-exercise rehydration. Thus, non-alcoholic beer can be regarded as a “functional” supplement. Additionally, physical exercise has proven to be a safe and effective intervention in cirrhosis, providing several benefits, for instance, improvement in nutritional status, quality of life and portal pressure.
Research motivation
Although nutritional therapy (diet + physical exercise) has beneficial effects in patients with cirrhosis, the availability and implementation of other nutritional strategies, such as supplements, is sometimes difficult due to different factors. Non-alcoholic beer has different compounds that exert antioxidant, anti-inflammatory and nutritional properties, and these properties are highly attractive in the treatment of patients with cirrhosis. Therefore, we hypothesize that it could be beneficial as a nutritional supplement in these patients.
Research objectives
The aim of the study was to evaluate the effect of diet + exercise and non-alcoholic beer on nutritional status, endothelial function, and quality of life in patients with cirrhosis.
Research methods
In this randomized open-clinical trial, eligible patients were randomized into two groups: (1) Control: Diet + physical exercise and (2) Intervention: Diet + physical exercise + non-alcoholic beer who were treated for 8 wk. The evaluated outcomes were nutritional status, endothelial function, and quality of life. For the analysis, only those patients receiving at least 1 d of intervention were included (modified intention to treat). Paired data were analyzed using the Wilcoxon signed-rank test. For comparisons between groups, Mann-Whitney U or Student´s t-test was used. Areas under the curve (AUC) were constructed for repeated measurements of endothelial function.
Research results
Forty-three patients were included in the study, 21 in the control group and 22 in the intervention group. The mean age was 53.5 ± 7.8 years, 60% were women, the median model for end-stage liver disease (MELD) score was 8 (7-10) and most patients were Child-Pugh A (88%). There were no adverse effects related to the consumption of non-alcoholic beer, or to the diet and exercise. Endothelial function improved in both groups. All the measured nutritional parameters improved in the intervention group, compared to only 2 in the control group and quality of life improved in both groups; however, more domains improved in the intervention group.
Research conclusions
A multifactorial program including the standard treatment diet and monitored exercise as well as a non-alcoholic beer is safe, well tolerated and results in improvements in endothelial function, nutritional status, and quality of life.
Research perspectives
Non-alcoholic beer represents a new nutritional strategy that has beneficial effects in patients with cirrhosis. However, the effects shown in this study should be confirmed with a longer duration of the intervention, and possibly with a larger amount of non-alcoholic beer. Our study did not include patients with decompensated cirrhosis, which limits the extrapolation of the results.
ACKNOWLEDGEMENTS
The authors would like to thank the interns involved for their technical support with data collection (Rodríguez JL, Moreno E, Méndez O and González S).
Footnotes
Institutional review board statement: The study protocol was approved by the local Research and Ethics Committee at the institution (No. Ref. 1605).
Clinical trial registration statement: This study is registered at https://clinicaltrials.gov. The registration identification number is NCT04041115.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: This study was supported by a research grant awarded in 2014 to Ricardo U. Macías-Rodríguez. The grant was derived from a call for scientific research from the Mexican Beer Council (MBC) and was evaluated by an independent scientific council (Research Committee on beer and health (RCBH)/ Consejo de Investigación sobre Salud y Cerveza A.C). Neither the MBC nor the members of the Committee (RCBH) had any influence in the design or development of the study, nor participated in the drafting of the manuscript, data analysis or data interpretation which was established a priori with a legally binding contract to ensure the independence of the research. The non-alcoholic beer used in this study does not belong to or is produced by the Mexican Beer Council (MBC). None of the authors have any actual or past relationship with the company producing the non-alcoholic beer.
CONSORT 2010 statement: The authors have read the CONSORT 2010 Statement, and the manuscript was prepared and revised according to the CONSORT 2010 Statement.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Association for the Study of Liver Diseases; European Association for the Study of the Liver; and European Society of Gastrointestinal Endoscopy.
Peer-review started: August 13, 2020
First decision: September 12, 2020
Article in press: October 29, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mandorfer M S-Editor: Gao CC L-Editor: Webster JR P-Editor: Li JH
Contributor Information
Ricardo U Macías-Rodríguez, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico; Liver Fibrosis and Nutrition Lab (LFN-Lab), MICTLÁN Network: Mechanisms of Liver Injury, Cell Death and Translational Nutrition in Liver Diseases-Research Network, Mexico City 14080, Mexico. ricardomacro@yahoo.com.mx.
Astrid Ruiz-Margáin, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Berenice M Román-Calleja, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
May E Espin-Nasser, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Nayelli C Flores-García, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Aldo Torre, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Gretel Galicia-Hernández, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Silvia L Rios-Torres, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Gabriela Fernández-del-Rivero, Department of Gastroenterology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Arturo Orea-Tejeda, Department of Cardiology, Instituto Nacional de Enfermedades Respiratorias, Mexico City 14080, Mexico.
Oscar A Lozano-Cruz, Department of Internal Medicine, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico.
Data sharing statement
Participant data that underlie the results reported in this article after deidentification (Text, tables, figures, appendices) and other documents (study protocol, statistical analysis plan and analytic code) are available on request from the corresponding author at ricardomacro@yahoo.com.mx.
References
- 1.Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650–2666. doi: 10.1016/j.cgh.2019.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 4.Poordad FF. Presentation and complications associated with cirrhosis of the liver. Curr Med Res Opin. 2015;31:925–937. doi: 10.1185/03007995.2015.1021905. [DOI] [PubMed] [Google Scholar]
- 5.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand AC. Nutrition and Muscle in Cirrhosis. J Clin Exp Hepatol. 2017;7:340–357. doi: 10.1016/j.jceh.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belarmino G, Torrinhas RS, Heymsfield SB, Waitzberg DL. Sarcopenia in liver cirrhosis: the role of computed tomography scan in the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol. 2015;27:1228. doi: 10.1097/MEG.0000000000000411. [DOI] [PubMed] [Google Scholar]
- 8.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061–8071. doi: 10.3748/wjg.v20.i25.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz-Margáin A, Macías-Rodríguez RU, Ampuero J, Cubero FJ, Chi-Cervera L, Ríos-Torres SL, Duarte-Rojo A, Espinosa-Cuevas Á, Romero-Gómez M, Torre A. Low phase angle is associated with the development of hepatic encephalopathy in patients with cirrhosis. World J Gastroenterol. 2016;22:10064–10070. doi: 10.3748/wjg.v22.i45.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21:113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Plauth M, Bernal W, Dasarathy S, Merli M, Plank LD, Schütz T, Bischoff SC. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trivedi HD, Tapper EB. Interventions to improve physical function and prevent adverse events in cirrhosis. Gastroenterol Rep (Oxf) 2018;6:13–20. doi: 10.1093/gastro/gox042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zenith L, Meena N, Ramadi A, Yavari M, Harvey A, Carbonneau M, Ma M, Abraldes JG, Paterson I, Haykowsky MJ, Tandon P. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014; 12: 1920-6. :e2. doi: 10.1016/j.cgh.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Hiraoka A, Michitaka K, Kiguchi D, Izumoto H, Ueki H, Kaneto M, Kitahata S, Aibiki T, Okudaira T, Tomida H, Miyamoto Y, Yamago H, Suga Y, Iwasaki R, Mori K, Miyata H, Tsubouchi E, Kishida M, Ninomiya T, Kohgami S, Hirooka M, Tokumoto Y, Abe M, Matsuura B, Hiasa Y. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2017;29:1416–1423. doi: 10.1097/MEG.0000000000000986. [DOI] [PubMed] [Google Scholar]
- 16.Macías-Rodríguez RU, Ruiz-Margáin A, Román-Calleja BM, Moreno-Tavarez E, Weber-Sangri L, González-Arellano MF, Fernández-Del-Rivero G, Ramírez-Soto K. Exercise prescription in patients with cirrhosis: Recommendations for clinical practice. Rev Gastroenterol Mex. 2019;84:326–343. doi: 10.1016/j.rgmx.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Ruiz-Margáin A, Macías-Rodríguez RU, Ríos-Torres SL, Román-Calleja BM, Méndez-Guerrero O, Rodríguez-Córdova P, Torre A. Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Rev Gastroenterol Mex. 2018;83:9–15. doi: 10.1016/j.rgmx.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Margáin A, Méndez-Guerrero O, Román-Calleja BM, González-Rodríguez S, Fernández-Del-Rivero G, Rodríguez-Córdova PA, Torre A, Macías-Rodríguez RU. Dietary management and supplementation with branched-chain amino acids in cirrhosis of the liver. Rev Gastroenterol Mex. 2018;83:424–433. doi: 10.1016/j.rgmx.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, Shiraishi K, Okuda H, Onji M, Kanazawa H, Tsubouchi H, Kato S, Kaito M, Watanabe A, Habu D, Ito S, Ishikawa T, Kawamura N, Arakawa Y Hepatic Nutritional Therapy (HNT) Study Group. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113–120. doi: 10.1016/j.nut.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Yamanaka-Okumura H, Nakamura T, Takeuchi H, Miyake H, Katayama T, Arai H, Taketani Y, Fujii M, Shimada M, Takeda E. Effect of late evening snack with rice ball on energy metabolism in liver cirrhosis. Eur J Clin Nutr. 2006;60:1067–1072. doi: 10.1038/sj.ejcn.1602420. [DOI] [PubMed] [Google Scholar]
- 21.Takeshita S, Ichikawa T, Nakao K, Miyaaki H, Shibata H, Matsuzaki T, Muraoka T, Honda T, Otani M, Akiyama M, Miuma S, Ozawa E, Fujimito M, Eguchi K. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr Res. 2009;29:89–93. doi: 10.1016/j.nutres.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Plank LD, Gane EJ, Peng S, Muthu C, Mathur S, Gillanders L, McIlroy K, Donaghy AJ, McCall JL. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48:557–566. doi: 10.1002/hep.22367. [DOI] [PubMed] [Google Scholar]
- 23.Nishida Y, Ide Y, Okada M, Otsuka T, Eguchi Y, Ozaki I, Tanaka K, Mizuta T. Effects of home-based exercise and branched-chain amino acid supplementation on aerobic capacity and glycemic control in patients with cirrhosis. Hepatol Res. 2017;47:E193–E200. doi: 10.1111/hepr.12748. [DOI] [PubMed] [Google Scholar]
- 24.Holeček M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition. 2017;41:80–85. doi: 10.1016/j.nut.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Park JG, Tak WY, Park SY, Kweon YO, Jang SY, Lee YR, Bae SH, Jang JY, Kim DY, Lee JS, Suk KT, Kim IH, Lee HJ, Chung WJ, Jang BK, Suh JI, Heo J, Lee WK. Effects of branched-chain amino acids (BCAAs) on the progression of advanced liver disease: A Korean nationwide, multicenter, retrospective, observational, cohort study. Medicine (Baltimore) 2017;96:e6580. doi: 10.1097/MD.0000000000006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi T, Taniguchi E, Sata M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr Clin Pract. 2013;28:580–588. doi: 10.1177/0884533613496432. [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Muniz FJ, Macho-González A, Garcimartín A, Santos-López JA, Benedí J, Bastida S, González-Muñoz MJ. The Nutritional Components of Beer and Its Relationship with Neurodegeneration and Alzheimer's Disease. Nutrients. 2019;11 doi: 10.3390/nu11071558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández-Quiroz F, Nirmalkar K, Villalobos-Flores LE, Murugesan S, Cruz-Narváez Y, Rico-Arzate E, Hoyo-Vadillo C, Chavez-Carbajal A, Pizano-Zárate ML, García-Mena J. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and β-cell function. Alcohol. 2020;85:77–94. doi: 10.1016/j.alcohol.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Vilahur G, Casani L, Mendieta G, Lamuela-Raventos RM, Estruch R, Badimon L. Beer elicits vasculoprotective effects through Akt/eNOS activation. Eur J Clin Invest. 2014;44:1177–1188. doi: 10.1111/eci.12352. [DOI] [PubMed] [Google Scholar]
- 30.Schneider C, Thierauf A, Kempf J, Auwärter V. Ethanol concentration in breastmilk after the consumption of non-alcoholic beer. Breastfeed Med. 2013;8:291–293. doi: 10.1089/bfm.2012.0156. [DOI] [PubMed] [Google Scholar]
- 31.Wijnen AH, Steennis J, Catoire M, Wardenaar FC, Mensink M. Post-Exercise Rehydration: Effect of Consumption of Beer with Varying Alcohol Content on Fluid Balance after Mild Dehydration. Front Nutr. 2016;3:45. doi: 10.3389/fnut.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, Macías-Rodríguez RU, Ferrando A, Kim WR. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl. 2018;24:122–139. doi: 10.1002/lt.24958. [DOI] [PubMed] [Google Scholar]
- 33.Macías-Rodríguez RU, Ilarraza-Lomelí H, Ruiz-Margáin A, Ponce-de-León-Rosales S, Vargas-Vorácková F, García-Flores O, Torre A, Duarte-Rojo A. Changes in Hepatic Venous Pressure Gradient Induced by Physical Exercise in Cirrhosis: Results of a Pilot Randomized Open Clinical Trial. Clin Transl Gastroenterol. 2016;7:e180. doi: 10.1038/ctg.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription 10th ed. Philadelphia, PA: Wolters Kluwer Health, 2018. [Google Scholar]
- 35.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription 9th ed. Philadelphia, PA: Thompson Wolters Kluwer/Lippincott Williams & Wilkins, 2014. [Google Scholar]
- 36.Ruiz-Margáin A, Macías-Rodríguez RU, Duarte-Rojo A, Ríos-Torres SL, Espinosa-Cuevas Á, Torre A. Malnutrition assessed through phase angle and its relation to prognosis in patients with compensated liver cirrhosis: a prospective cohort study. Dig Liver Dis. 2015;47:309–314. doi: 10.1016/j.dld.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 2010;4:351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishikawa H, Osaki Y. Liver Cirrhosis: Evaluation, Nutritional Status, and Prognosis. Mediators Inflamm. 2015;2015:872152. doi: 10.1155/2015/872152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marchesini G, Bianchi G, Merli M, Amodio P, Panella C, Loguercio C, Rossi Fanelli F, Abbiati R Italian BCAA Study Group. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 40.Charlton M. Branched-chain amino acid enriched supplements as therapy for liver disease. J Nutr. 2006;136:295S–298S. doi: 10.1093/jn/136.1.295S. [DOI] [PubMed] [Google Scholar]
- 41.Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926–931. doi: 10.1002/hep.510280405. [DOI] [PubMed] [Google Scholar]
- 42.Kyrou I, Christou A, Panagiotakos D, Stefanaki C, Skenderi K, Katsana K, Tsigos C. Effects of a hops (Humulus lupulus L.) dry extract supplement on self-reported depression, anxiety and stress levels in apparently healthy young adults: a randomized, placebo-controlled, double-blind, crossover pilot study. Hormones (Athens) 2017;16:171–180. doi: 10.14310/horm.2002.1738. [DOI] [PubMed] [Google Scholar]
- 43.Ruiz-Margáin A, Xie JJ, Román-Calleja BM, Pauly M, White MG, Chapa-Ibargüengoitia M, Campos-Murguía A, González-Regueiro JA, Macias-Rodríguez RU, Duarte-Rojo A. Phase angle from bioelectrical impedance for the assessment of sarcopenia in cirrhosis with or without ascites. Clin Gastroenterol Hepatol. 2020:Online ahead of print. doi: 10.1016/j.cgh.2020.08.066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Participant data that underlie the results reported in this article after deidentification (Text, tables, figures, appendices) and other documents (study protocol, statistical analysis plan and analytic code) are available on request from the corresponding author at ricardomacro@yahoo.com.mx.