Abstract
Carbapenem antibiotics were first introduced in the 1980s and have long been considered the most active agents for the treatment of multidrug-resistant gram-negative bacteria. Over the last decade, carbapenem-resistant Enterobacteriaceae (CRE) have emerged as organisms causing spontaneous bacterial peritonitis. Infections caused by CRE have shown a higher mortality rate than those caused by bacteria sensitive to carbapenem antibiotics. Current antibiotic guidelines for the treatment of spontaneous bacterial peritonitis are insufficient, and rapid de-escalation of empiric antibiotic treatment is not widely recognized. This review summarizes the molecular characteristics, epidemiology and possible treatment of spontaneous bacterial peritonitis caused by CRE.
Keywords: Spontaneous bacterial peritonitis, Carbapenem-resistant Enterobacteriaceae, Carbapenem-resistant Klebsiella pneumoniae, Cirrhosis
Core Tip: Carbapenem antibiotics were first introduced in the 1980s and have long been considered the most active agents for the treatment of multidrug-resistant gram-negative bacteria. Over the last decade carbapenem-resistant Enterobacteriaceae (CRE) have emerged as organisms causing spontaneous bacterial peritonitis (SBP). Infections caused by CRE have shown a higher mortality rate than those caused by bacteria sensitive to carbapenem antibiotics. Current antibiotic guidelines for the treatment of SBP are insufficient, and rapid de-escalation of empiric antibiotic treatment is not widely recognized. This review summarizes the molecular characteristics, epidemiology and possible treatment of SBP caused by CRE.
INTRODUCTION
Spontaneous bacterial peritonitis (SBP) is a common complication in patients with cirrhosis. It is defined as ascitic fluid infection in the absence of alternative surgically treatable sources of intra-abdominal infection[1]. SBP diagnosis relies on ascitic fluid polymorphonuclear cell count greater than or equal to 250 cells/mm3. Microbiological culture, either from ascitic fluid or the bloodstream, enables identification of the etiological pathogen[2,3]. Approximately 2.5% of all hospitalizations of patients with cirrhosis are for SBP, and the short-term mortality is about 25%[4]. In-hospital mortality remains a significant burden to the healthcare system, especially in patients with concurrent risk factors such as older age, female gender, hepatic encephalopathy, coagulopathy, variceal hemorrhage, sepsis, pneumonia and acute kidney injury[5].
Historically, the most frequent etiological agents remain gram-negative bacteria (GNB), especially Enterobacteriaceae spp. Although in recent times gram-positive bacteria (GPB) appear to be on the rise[6,7]. Today, SBP due to multidrug-resistant (MDR) bacteria represents a growing and complex healthcare problem. Infections caused by MDR-bacteria carry a high mortality rate in the cirrhotic patient[8]. This is likely due to difficulty in establishing an effective antibiotic regimen along with a depressed immune system[9].
SBP due to MDR bacteria proves to be a clinical challenge[10,11], and clinicians should consider reported resistance profiles for the decision-making process in deciding empiric antibiotic regimens[12]. Third generation cephalosporins that for decades have been used as the treatment of choice for community acquired-SBP should no longer be used as first-line therapy[13]. Carbapenem antibiotics, introduced in the 1980s, have long been considered the most active agents against MDR-GNB. Unfortunately, over the last decade carbapenem-resistant Enterobacteriaceae (CRE) have emerged as SBP causing bacteria[14] and have shown a higher mortality rate than infections caused by bacteria sensitive to carbapenem antibiotics[15]. Current antibiotic guidelines for the treatment of SBP are insufficient[9,16], and rapid de-escalation of empiric antibiotic treatment is not widely recognized[17]. This review summarizes the molecular characteristics, epidemiology, and possible treatment of SBP caused by CRE.
THE BURDEN OF CARBAPENEMASE-PRODUCING ENTEROBACTERIACE IN SPONTANEOUS BACTERIAL PERITONITIS
The health burden caused by cirrhosis corresponds to 14-26 new cases per 100000 individuals and results in 170000 deaths per year in Europe[18]. Cirrhotic patients have a higher susceptibility to infections caused by resistant bacteria (repeat hospitalizations and antibiotic exposure for long-term prophylaxis of SBP), and the management of these patients has become a major global health concern. In addition, antimicrobial resistance has emerged as a public health crisis. In the case of SBP, gram-positive cocci (methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci), extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae and CRE are emerging as the causative agents[19].
While resistant GPB can be common, the classes of resistant Enterobacteriaceae are much rarer and more devastating[20]. The spread of these pathogens is difficult to control because of a potential huge intestinal reservoir[21]. A recent single-center Italian study reported that the prevalence of extensively resistant (XDR) organisms increased from 16% between 2008-2009 to 36% between 2012-2013[22]. In patients with decompensated cirrhosis the major determinants of prognosis are bacterial infections, especially if caused by resistant pathogens. This can be shown to increase mortality rate four-fold[23]. The likely cause of resistant pathogens in cirrhotic patients is the inadequate long-term empirical prophylactic antibiotic treatment that they are prescribed. This results in antimicrobial resistance with life-threatening consequences. Between 11% and 45% of patients with SBP and spontaneous bacteremia are infected with organisms resistant to tigecycline, which is an antibiotic that seems to be effective in the majority of healthcare-associated and nosocomial infections[10]. The overall proportion of MDR bacteria in patients with nosocomial SBP was 22% to 73% of cases across multiple studies[24].
The high prevalence of MDR or XDR pathogens causing SBP are directly linked to high mortality rates. It is therefore not a surprise that we have been forced to incorporate empiric use of carbapenems. The rising global empiric administration of carbapenems has now created a selection pressure promoting the emergence of CRE[25]. It has furthermore been proven that the efficacy of empirical antibiotic therapy in nosocomial SBP is very low, ranging from 26% to 67.6%[26].
Piano et al[14] reported that even targeted therapy proved difficult for infection resolution. They described a case of SBP due to carbapenemase-producing Klebsiella pneumoniae (KPC) in a 57-year-old patient that was treated with meropenem for an extended period. The KPC found via nasal swab was susceptible to colistin and tigecycline but did not respond to treatment and ultimately led to death within 10 d. In 2015, Li et al[27] studied 31 patients affected by SBP both nosocomial and non-nosocomial acquired. Among these patients, four presented with KPC and two with Escherichia coli (E. coli) resistant to meropenem. While the E. coli cases were nosocomial-SBP, half of the KPC patients were found to be non-nosocomial, demonstrating spread of infection outside the nosocomial setting, which is where empiric treatment is more common.
Similar difficulties of treatment have been reported by Alexopoulou et al[28] in 2016. In this study the authors analyzed data from 130 patients affected by SBP. Meropenem showed a drug resistance rate of 30.7%. The 77% of pathogens resistant to meropenem were susceptible to colistin, while the 86% of GNB were susceptible to tigecycline. Only 54% of the pathogens resistant to meropenem were susceptible to tigecycline. All but one XDR bacteria were susceptible to a possible combination of colistin and tigecycline.
That same year, Lutz et al[29] described ninety-two SBP cases, three of which were Enterococcus faecium resistant to carbapenems. Tudorascu et al[30] found cases of carbapenem-resistant E. coli, KPC and carbapenem-resistant Enterobacter spp. In Italy, Salerno et al[31] reported one case of carbapenem-resistant E. coli and seven cases due to KPC. Béjar-Serrano et al[32] in 2019 reported a case of SBP caused by carbapenemase-producing Enterobacter cloacae (E. cloacae). Table 1 summarizes the findings of the studies mentioned above describing the total number of patients affected by SBP and the number of SBP caused by CRE. Furthermore, it describes the type of pathogen involved and if the SBP was nosocomial or non-nosocomial acquired.
Table 1.
Synthesis of a selection of the studies published on spontaneous bacterial peritonitis due to carbapenem-resistant Enterobacteriaceae producing pathogens
|
Ref.
|
Total number SBP/CRE SBP
|
CRE
|
CRE N-SBP/Total SBP
|
Not-N-SBP/Total SBP
|
| Piano et al[14], 2012 | 1/1 | K. pneumoniae | 1/1 | 0/1 |
| Li et al[27], 2015 | 31/6 | K. pneumoniae, E. coli | 2/4, 2/2 | 2/4, 0/2 |
| Alexopoulou et al[28], 2016 | 130/6 | K. pneumoniae, E. coli | 5/5, 1/1 | 0/5, 0/1 |
| Lutz et al[29], 2016 | 92/3 | E. faecium | 3/3 | 0/3 |
| Tudorascu et al[30], 2016 | 64/3 | K. pneumoniae, E. coli, Enterobacter | 1/1, 1/1, 1/1 | 0/1, 0/1, 0/1 |
| Salerno et al[31], 2016 | 56/8 | K. pneumoniae, E. coli | 5/7, 0/1 | 7/2, 1/1 |
| Béjar-Serrano et al[32], 2019 | 22/1 | E. cloacae | 1/1 | 0/1 |
CRE: Carbapenem-resistant Enterobacteriaceae; N: Nosocomial; SBP: Spontaneous bacterial peritonitis; CRE SBP: Spontaneous bacterial peritonitis due to carbapenem-resistant Enterobacteriaceae; CRE N-SBP: Nosocomial spontaneous bacterial peritonitis due to carbapenem-resistant Enterobacteriaceae; Not-N-SBP: Not nosocomial spontaneous bacterial peritonitis; K. pneumoniae: Klebsiella pneumoniae; E. coli: Escherichia coli; E. cloacae: Enterobacter cloacae; E. faecium: Enterococcus faecium.
MOLECULAR CHARACTERISTICS OF CARBAPENEMASE-PRODUCING ENTEROBACTERIACEAE CAUSING SPONTANEOUS BACTERIAL PERITONITIS
Enterobacteriaceae show two major types of antibiotic resistance. One mechanism involves the expression of ESBL, which render bacteria resistant to cephalosporins and monobactams. The other mechanism of resistance, which is even more troubling, is the expression of carbapenemases, which render bacteria resistant to almost all available β-lactams including the carbapenems[33]. These bacteria are called carbapenemase-producing CRE. Carbapenemases represent the most versatile family of β-lactamases, with a breadth of activity unrivaled by other β-lactam-hydrolyzing enzymes. Although known as “carbapenemases,” many of these enzymes recognize almost all hydrolyzable-lactams and are resilient against inhibition by all commercially viable β-lactamase inhibitors.
Carbapenemases are classified according to the degree of homology of the respective polypeptide chains. According to Ambler classification, four classes of enzymes are recognized. Classes A, C and D include the β-lactamases with serine at their active site, whereas molecular class B β-lactamases (MβLs) are all metalloenzymes with zinc at their active-site[34]. Currently, among the four classes of β-lactamases defined by the Ambler classification system, three have been identified to give resistance to carbapenems: (1) The class A of β-lactamases in which KPC is included; (2) The class B of metal-β-lactamases to which the imipenemase (IMP) and the Verona integron-encoded metal-β-lactamase [Verona imipenemase (VIM)] belong; and (3) The class D to which β-lactamases, such as oxicillinase oxacillin-hydrolyzing (OXA)-48, belong[35].
These enzymes are coded starting from specific genes that can be acquired in two ways: By transfer through plasmid or by clonal bacterial strain expansion[36]. Class A carbapenemases have a serine in the active state in position 70 and can hydrolyze carbapenemics, cephalosporins, penicillins and aztreonam while being inhibited by clavulanic acid and tazobactam. The enzymes KPC-1, KPC-2, KPC-3, Guiana-Extended-Spectrum (GES)-4, GES-5 and GES-6 have been found mainly in Klebsiella pneumoniae. Serratia marcescens (S. marcescens) enzyme (SME)-1, SME-2 and SME-3 have been found in S. marcescens; NMC-A and KPC-3 have been found in E. cloacae, and GES-5 has been found in E. coli[34]. These enzymes are summarized in Table 2.
Table 2.
Class A carbapenemase asset found in each pathogen
|
Pathogens
|
Class A carbapenemase
|
|||||||||
|
|
KPC-1
|
KPC-2
|
KPC-3
|
GES-4
|
GES-5
|
GES-6
|
SME-1
|
SME-2
|
SME-3
|
NMC-A
|
| K. pneumoniae | + | + | + | + | + | + | ||||
| S. marcescens | + | + | + | |||||||
| E. coli | + | |||||||||
| E. cloacae | + | + | ||||||||
KPC: Klebsiella pneumoniae; GES: Guiana-Extended-Spectrum; SME: Serratia marcescens enzyme; K. pneumoniae: Klebsiella pneumoniae; E. coli: Escherichia coli; E. cloacae: Enterobacter cloacae; S. marcescens: Serratia marcescens.
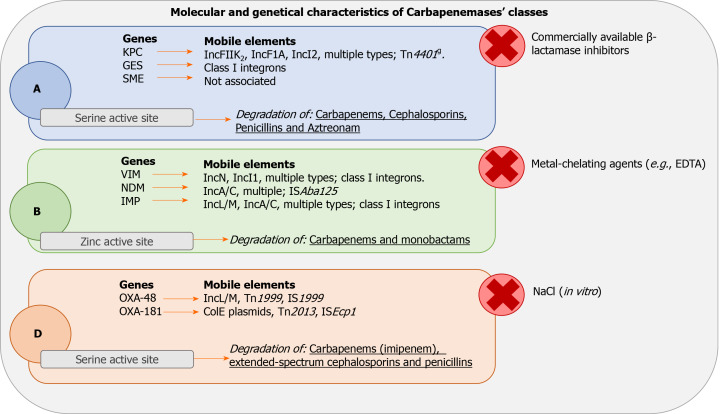
KPC and GES are associated with mobile elements. None have been reported yet for the SME genes[37,38]. Figure 1 illustrates the different kind of genes and mobile elements related to each class of carbapenemase with the site of action, the inhibitor substances and the antimicrobials hydrolyzed for each class of enzymes.
Figure 1.
Molecular characteristics, genetics and activity of carbapenems classes. A: Class A carbapenemases; B: Class B carbapenemases; D: Class D carbapenemases; GES: Guiana-Extended-Spectrum; SME: Serratia marcescens enzyme; KPC: Klebsiella pneumoniae carbapenemase; VIM: Verona imipenemase; NDM: New Delhi carbapenemase; IMP: Imipenemase; OXA: Oxacillinase.
Class B enzymes are characterized by resistance to beta-lactamase inhibitors. They share hydrolytic activity with Class A carbapenemases but are not effective against aztreonam. The hydrolysis mechanism depends on the activation of the active site by zinc ions. This feature makes them highly sensitive to inhibition by ethylene diamine tetraacetic acid, which is capable of chelating zinc and other cations. Although the amino acid homology of these proteases is poor (about 23%), all the class B carbapenemases show excellent zinc binding capacity and a well-preserved active site[39]. The B carbapenemases have been found, as described in Table 3, mainly in Klebsiella pneumoniae (IMP-1, IMP-1-like, IMP-4, VIM-1, VIM-2-like, VIM-4), E. coli (VIM-1, IMP-4, IMP-1-like), S. marcescens (IMP-1-like, VIM-2, VIM-2-like), E. cloacae (VIM-1, VIM-2, VIM-2-like, VIM-5, VIM-4, IMP-1-like, IMP-4, IMP-8) and Citrobacter freundii (IMP-1, IMP-1-like, VIM-2)[34]. Shown in Figure 1, these enzymes are associated with respective genes such as VIM, NMD and IMP. Furthermore, they are associated with several mobile elements (i.e. IncN, IncI1, multiple types; class I integrons, IncL/M, IncA/C)[37].
Table 3.
Class B carbapenemase asset found in each pathogen
|
Pathogens
|
Class B carbapenemase
|
|||||||||
|
|
IMP-1
|
IMP-1-like
|
IMP-4
|
IMP-8
|
VIM-1
|
VIM-1-like
|
VIM-2
|
VIM-2-like
|
VIM-4
|
VIM-5
|
| K. pneumoniae | + | + | + | + | + | + | + | |||
| S. marcescens | + | + | + | + | ||||||
| E. coli | + | + | + | |||||||
| E. cloacae | + | + | + | + | + | + | + | + | ||
| C. freundii | + | + | + | |||||||
IMP: Imipenemase; VIM: Verona imipenemase; K. pneumoniae: Klebsiella pneumoniae; E. coli: Escherichia coli; E. cloacae: Enterobacter cloacae; S. marcescens: Serratia marcescens; C. freundii: Citrobacter freundii.
Class D enzymes include oxacillin-hydrolyzing–β-lactamases identified mainly in Enterobacteriaceae and Pseudomonas aeruginosa[40]. Functionally they are penicillinases capable of hydrolyzing both oxacillin and cloxacillin. These enzymes are characterized by extreme variability in the amino acid sequence producing many enzyme variants that are only weakly inhibited by ethylene diamine tetraacetic acid and clavulanate[41]. The molecular structure was analyzed by detecting a homology with class A enzymes with serine in the active site in positions varying between 70 and 73 in the S-T-F-K tetrad[34]. The active site of the D carbapenemases is very efficient due to its small size and increased hydrophobicity due to the tyrosine and methionine residues present in position 112 and 223, respectively. The OXA carbapenemases have highly conserved structures in position 144-146 with sequence Y-G-N and in position 216-218 with sequence K-T-G. At present, 102 distinct OXA enzymes have been identified, of which at least 37 (9 broad spectrum enzymes) are to be considered carbapenemases. These 37 were then divided into 9 main subgroups based on an amino acid homology exceeding 92.5%[42]. Subgroups 1 and 2 share the substitution F with Y in the sequence Y-G-N that does not seem to improve the hydrolyzation of the imipenem compared to the other carbapenemases.
The mechanism of action is similar to other serine-carbapenemases but carbon dioxide seems to influence the kinetics of OXA-carbapenemases. In cases of high carbon dioxide concentrations, the carboxylation of lysine occurs in position 73 activating the serine at the catalytic site[43]. OXA carbapenemases act on penicillin, cephalosporin and imipenem with faster hydrolysis of imipenem than meropenem[44]. These enzymes, as described in Table 4, have been found mainly in Klebsiella pneumoniae (OXA-48, OXA-181, OXA-163), S. marcescens (OXA-48), E. coli (OXA-48, OXA-244, OXA-181) and E. cloacae (OXA-48). They are associated with OXA genes and several mobile elements (i.e. IncL/M, Tn1999, IS1999)[37], as reported extensively in Figure 1.
Table 4.
Class D carbapenemase asset found in each pathogen
|
Pathogens
|
Class D carbapenemase
|
|||
| OXA-48 | OXA-163 | OXA-181 | OXA-244 | |
| K. pneumoniae | + | + | + | |
| S. marcescens | + | |||
| E. coli | + | + | + | |
| E. cloacae | + | |||
OXA: Oxacillin-hydrolyzing; K. pneumoniae: Klebsiella pneumoniae; E. coli: Escherichia coli; E. cloacae: Enterobacter cloacae; S. marcescens: Serratia marcescens.
ANTIMICROBIAL MANAGEMENT OF SPONTANEOUS BACTERIAL PERITONITIS DUE TO CARBAPENEMASE-PRODUCING ENTEROBACTERIACEAE
Aminoglycosides, mainly amikacin and gentamicin, have been widely utilized in the era of limited treatment options for the management of CRE[45]. Overall, antimicrobial susceptibility for CRE varies[46]. These antibiotic agents require high dose daily administration with therapeutic drug monitoring to optimize their use[46-48]. Plazomicin is a newly marketed aminoglycoside. It is approved for the management of complicated urinary tract infections (cUTI) in patients with limited or no options for alternative treatment[49]. It has activity against GNB producing ESBL, KPC and AmpC[50,51]. Overall, it has poor activity against nonfermenting GNB[52,53].
Colistin is an old polymyxin widely utilized for the management of serious infections due to CRE[47,48,54,55]. Colistin resistance remains low among nonfermenting GNB but is increasing in Klebsiella spp. producing KPC enzymes[56]. Its role as monotherapy or within a combination regimen is still under discussion due to the absence of reliable data[48,57,58]. Fosfomycin is another old antibiotic utilized in the treatment of infections due to CRE in critically ill patients[59]. It has activity against GPB and GNB, including MDR strains such as CRE. However, during monotherapy rapid emergence of antibiotic resistance has been described[17,60,61]. High doses of tigecycline have been widely utilized as a last-resort option for the treatment of serious infections due to CRE. It is a glycylcycline with activity against a broad range of GPB and GNB including MDR strains but not Pseudomonas spp. or Proteus spp.[62].
Eravacycline is a synthetic fluorocycline antibiotic recently approved for the treatment of complicated intra-abdominal infections (cIAI). It has broad spectrum activity including MDR and XDR isolates with the exception of Pseudomonas spp. and Burkholderia spp. Overall, it has activity against GNB producing ESBL, KPC, AmpC, MβL and OXA enzymes[6,7,20,50]. Moreover, eravacycline is active against the most common tetracycline-resistance mechanisms such as efflux and ribosomal protection[63]. In IGNITE 1 and 4 clinical trials, it showed a high clinical and microbiological response with a favorable safety and tolerability profile in patients with cIAIs[64,65]. Eravacycline also has a high oral bioavailability that can facilitate a sequential antibiotic regimen (from intravenous to oral formulation) with patients being discharged home[66].
Among β-lactam antibiotics, ceftazidime/avibactam is a novel cephalosporin/β-lactamase inhibitor combination with activity against several GNB including strains producing ESBL, KPC, AmpC and some OXA enzymes (OXA-48)[50]. In phase 2 and 3 clinical trials, ceftazidime/avibactam demonstrated efficacy and safety in patients with cIAIs[67-69]. It was successfully used as salvage therapy in patients with severe infections due to CRE[70,71]. Of note, emergence of resistance during therapy has already been described[30]. The appropriate use of ceftazidime/avibactam in the management of CRE infections as monotherapy or part of combination regimen is still an open debate[72].
Meropenem/vaborbactam is a novel carbapenem/β-lactamase inhibitor combination with activity against GNB producing ESBL, KPC and AmpC but not MβL and OXA enzymes[50-52,73]. Meropenem/vaborbactam was approved for the treatment of bacteremic cUTI, cIAIs, hospital-acquired pneumonia including those associated to mechanical ventilators (hospital-acquired pneumonia and ventilator associated pneumonia) and for the treatment of all infections due to GNB where treatment options were limited. In TANGO 1 and 2 clinical trials, meropenem/vaborbactam was associated with high clinical and microbiological success[74,75]. In a sensitivity analysis of the TANGO 2 clinical trial among patients without prior antibiotic failure, meropenem/vaborbactam showed a significant higher clinical cure rate at the test-of-cure visit and a lower day-28 all-cause mortality than the best available therapy[75].
In a multicenter retrospective cohort study, meropenem/vaborbactam was found to have similar clinical success to ceftazidime/avibactam (69% vs 62%; P = 0.49)[76]. Although the propensity of meropenem/vaborbactam for development of resistance is lower than ceftazidime/avibactam, mechanisms of antibiotic resistance are described (porin mutations and increase in the blaKPC expression)[77,78]. Interestingly, an in vitro study showed a synergistic effect of meropenem/vaborbactam plus a ceftazidime/ avibactam combination against susceptible KPC strains but also against both meropenem/vaborbactam and ceftazidime/avibactam-resistant KPC isolates[79].
Imipenem/cilastatin/relebactam is another novel carbapenem/β-lactamase inhibitor combination with activity against GNB producing ESBL, KPC and AmpC enzymes[50-52]. Imipenem/cilastatin/relebactam was approved for the management of cUTIs and cIAIs in adult patients with limited or no available treatment options. In a phase 3 clinical trial (RESTORE-IMI 1), imipenem/cilastatin/relebactam was found as an effective and well-tolerated treatment agent for CRE infections[80].
Aztreonam/avibactam is a monobactam and β-lactamase inhibitor combination in the late form of development. It has activity against GNB producing ESBL, KPC, AmpC, MβL and some OXA enzymes (OXA-48)[50-52]. Cefiderocol is a siderophore cephalosporin recently approved for the treatment of cUTIs in adults. It has a broad spectrum of activity against GNB, including MDR Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter baumannii[50]. The approved drugs used to treat these CRE producing pathogens causing SBP are displayed with their advantages and disadvantages in Table 5.
Table 5.
Advantages and disadvantages of the antimicrobials used to treat spontaneous bacterial peritonitis due to gram-negative bacteria producing carbapenem-resistant Enterobacteriaceae
|
Antimicrobial agent
|
Advantages
|
Disadvantages
|
Ref.
|
| Aminoglycosides (i.e. Plazomicin) | Good activity against GNB producing ESβL, KPC, AmpC but not MβL enzymes | Heterogeneous susceptibility high dose (toxicity) | [49] |
| Polimixins (i.e. Colistin) | Low resistance emergence | Low efficacy for Klebsiella spp. producing KPC enzymes | [56] |
| Fosfomicyn | Moderate activity against MDR–CRE | Rapid emergence of antibiotic resistance | [59] |
| Glycylcycline (i.e. Tigecycline) | Good activity against MDR–CRE | High dose (toxicity) | [62] |
| Fluorocycline (i.e. Eravacycline) | Broad spectrum activity (even if MDR and XDR pathogens). Active against the most common tetracycline-resistance mechanisms. High oral bioavailability. Safety and tolerability | Not active on Pseudomonas spp. and Burkholderia spp. | [63- 65] |
| β-lactams/β-lactamase inhibitors (i.e. ceftazidime/avibactam) | Good activity against GNB producing ESβL, KPC, AmpC, OXA-48 and MβL. Safety and tolerability | Frequent emergence of antibiotic resistance | [67] |
| Carbapenem/β-lactamase inhibitors (i.e. meropenem/vaborbactam or Imipenem/cilastatin/relebactam) | Good activity against GNB producing ESβL, KPC and AmpC. Outcome improvement | Not active on GNB producing OXA-48 and MβL | [79] |
| Monobactam/β-lactamase inhibitor (i.e. aztreonam/avibactam) | Good activity against GNB producing ESβL, KPC, AmpC and OXA-48 | Recently approved | [50- 52] |
| Siderophore cephalosporin (i.e. Cefidecol) | Broad spectrum of activity against GNB, including MDR Enterobacteriaceae, Pseudomonas aeruginosa and A. baumannii | Recently approved | [50] |
GNB: Gram-negative bacteria; ESβL: Extended-spectrum β-lactamase; CRE: Carbapenem-resistant Enterobacteriaceae, KPC: Klebsiella pneumoniae; MβL: Molecular class B β-lactamases; MDR: Multidrug resistant; XDR: Extensively resistant.
Many more agents are in several phases of development: Cefepime/taniborbactam (phase 3), cefepime/enmetazobactam (phase 3), sulbactam/durlobactam (phase 3), sulopenem/etzadroxil/probenecid (phase 3), tebipenem pivoxil hydrobromide (phase 3), BOS-228 (phase 2), OP0595/RG6080 (phase 1), QPX-2015/QPX-7728 (phase 1), SPR206 (phase 1), SPR741 (phase 1), TP-6076 (phase 1) and WCK 5222 (phase 1).
CONCLUSION
SPB due to CRE is a major concern for hepatologists. Overall, CRE infections are associated with an increased risk of morbidity and mortality. Current antibiotic guidelines for the treatment of SBP caused by CRE are insufficient. This review summarizes the current molecular characteristics, epidemiology and possible treatment regimens for CRE causing SBP. Many new antibiotics are being introduced into clinical practice and others are still in the preclinical and clinical phases of development. Further research of these novel agents is required for appropriate use (microbiological activity and pharmacokinetic/pharmacodynamic parameters). A multidisciplinary approach (hepatologists, infectious diseases specialists, intensivists, microbiologists, pharmacists) is essential for the adequate placement of these newer anti-infective agents in therapy. In order to optimize antimicrobial treatments and preserve the antibiotic armamentarium, a careful knowledge of local microbiological epidemiology and antibiotic-resistant rates along with detailed antimicrobial stewardship programs must be applied.
Footnotes
Conflict-of-interest statement: All authors declare no conflicts-of-interest related to this article.
Manuscript source: Invited manuscript
Peer-review started: June 29, 2020
First decision: September 24, 2020
Article in press: October 23, 2020
Specialty type: Infectious diseases
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pop TL S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LL
Contributor Information
Marco Fiore, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, Naples 80138, Italy. marco.fiore@unicampania.it.
Sveva Di Franco, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Aniello Alfieri, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Maria Beatrice Passavanti, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Maria Caterina Pace, Department of Women, Child and General and Specialized Surgery, University of Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Stephen Petrou, Department of Emergency Medicine, Good Samaritan Hospital Medical Center, NY 11795, United States.
Francesca Martora, Department of Experimental Medicine, University of Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Sebastiano Leone, Division of Infectious Diseases, “San Giuseppe Moscati” Hospital, Avellino 83100, Italy.
References
- 1.Koulaouzidis A, Bhat S, Karagiannidis A, Tan WC, Linaker BD. Spontaneous bacterial peritonitis. Postgrad Med J. 2007;83:379–383. doi: 10.1136/pgmj.2006.056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karvellas CJ, Abraldes JG, Arabi YM, Kumar A Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Appropriate and timely antimicrobial therapy in cirrhotic patients with spontaneous bacterial peritonitis-associated septic shock: a retrospective cohort study. Aliment Pharmacol Ther. 2015;41:747–757. doi: 10.1111/apt.13135. [DOI] [PubMed] [Google Scholar]
- 3.Fiore M, Maraolo AE, Leone S, Gentile I, Cuomo A, Schiavone V, Bimonte S, Pace MC, Cascella M. Spontaneous peritonitis in critically ill cirrhotic patients: a diagnostic algorithm for clinicians and future perspectives. Ther Clin Risk Manag. 2017;13:1409–1414. doi: 10.2147/TCRM.S144262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iogna Prat L, Wilson P, Freeman SC, Sutton AJ, Cooper NJ, Roccarina D, Benmassaoud A, Plaz Torres MC, Hawkins N, Cowlin M, Milne EJ, Thorburn D, Pavlov CS, Davidson BR, Tsochatzis E, Gurusamy KS. Antibiotic treatment for spontaneous bacterial peritonitis in people with decompensated liver cirrhosis: a network meta-analysis. Cochrane Database Syst Rev. 2019;9:CD013120. doi: 10.1002/14651858.CD013120.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu B, Kim B, Limketkai BN, Sun J, Li Z, Woreta T, Chen PH. Mortality from Spontaneous Bacterial Peritonitis Among Hospitalized Patients in the USA. Dig Dis Sci. 2018;63:1327–1333. doi: 10.1007/s10620-018-4990-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiore M, Di Franco S, Alfieri A, Passavanti MB, Pace MC, Kelly ME, Damiani G, Leone S. Spontaneous bacterial peritonitis caused by Gram-negative bacteria: an update of epidemiology and antimicrobial treatments. Expert Rev Gastroenterol Hepatol. 2019;13:683–692. doi: 10.1080/17474124.2019.1621167. [DOI] [PubMed] [Google Scholar]
- 7.Fiore M, Maraolo AE, Gentile I, Borgia G, Leone S, Sansone P, Passavanti MB, Aurilio C, Pace MC. Current concepts and future strategies in the antimicrobial therapy of emerging Gram-positive spontaneous bacterial peritonitis. World J Hepatol. 2017;9:1166–1175. doi: 10.4254/wjh.v9.i30.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernández J, Prado V, Trebicka J, Amoros A, Gustot T, Wiest R, Deulofeu C, Garcia E, Acevedo J, Fuhrmann V, Durand F, Sánchez C, Papp M, Caraceni P, Vargas V, Bañares R, Piano S, Janicko M, Albillos A, Alessandria C, Soriano G, Welzel TM, Laleman W, Gerbes A, De Gottardi A, Merli M, Coenraad M, Saliba F, Pavesi M, Jalan R, Ginès P, Angeli P, Arroyo V European Foundation for the Study of Chronic Liver Failure (EF-Clif) Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol. 2019;70:398–411. doi: 10.1016/j.jhep.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Patel VC, Williams R. Antimicrobial resistance in chronic liver disease. Hepatol Int. 2020;14:24–34. doi: 10.1007/s12072-019-10004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández J, Bert F, Nicolas-Chanoine MH. The challenges of multi-drug-resistance in hepatology. J Hepatol. 2016;65:1043–1054. doi: 10.1016/j.jhep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Fiore M. Letter: the emergence of multi-drug resistant spontaneous bacterial peritonitis: a new challenge for the hepatologist? Aliment Pharmacol Ther. 2016;43:944–945. doi: 10.1111/apt.13539. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira JC, Carrera E, Petry RC, Deutschendorf C, Mantovani A, Barcelos STA, Cassales S, Schacher FC, Lopes AB, Alvares-da-Silva MR. High Prevalence of Multidrug Resistant Bacteria in Cirrhotic Patients with Spontaneous Bacterial Peritonitis: Is It Time to Change the Standard Antimicrobial Approach? Can J Gastroenterol Hepatol. 2019;2019:6963910. doi: 10.1155/2019/6963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiore M, Gentile I, Maraolo AE, Leone S, Simeon V, Chiodini P, Pace MC, Gustot T, Taccone FS. Are third-generation cephalosporins still the empirical antibiotic treatment of community-acquired spontaneous bacterial peritonitis? Eur J Gastroenterol Hepatol. 2018;30:329–336. doi: 10.1097/MEG.0000000000001057. [DOI] [PubMed] [Google Scholar]
- 14.Piano S, Romano A, Rosi S, Gatta A, Angeli P. Spontaneous bacterial peritonitis due to carbapenemase-producing Klebsiella pneumoniae: the last therapeutic challenge. Eur J Gastroenterol Hepatol. 2012;24:1234–1237. doi: 10.1097/MEG.0b013e328355d8a2. [DOI] [PubMed] [Google Scholar]
- 15.Martin A, Fahrbach K, Zhao Q, Lodise T. Association Between Carbapenem Resistance and Mortality Among Adult, Hospitalized Patients With Serious Infections Due to Enterobacteriaceae: Results of a Systematic Literature Review and Meta-analysis. Open Forum Infect Dis. 2018;5:ofy150. doi: 10.1093/ofid/ofy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiore M. Spontaneous bacterial peritonitis due to multidrug resistant bacteria: are the current guidelines outdated? Eur J Gastroenterol Hepatol. 2016;28:731. doi: 10.1097/MEG.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 17.Fiore M, Andreana L, Leone S. Treatment of spontaneous bacterial peritonitis: beyond the current international guidelines. Liver Int. 2016;36:918. doi: 10.1111/liv.13047. [DOI] [PubMed] [Google Scholar]
- 18.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulou A, Papadopoulos N, Eliopoulos DG, Alexaki A, Tsiriga A, Toutouza M, Pectasides D. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int. 2013;33:975–981. doi: 10.1111/liv.12152. [DOI] [PubMed] [Google Scholar]
- 20.Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkey PM. Multidrug-resistant Gram-negative bacteria: a product of globalization. J Hosp Infect. 2015;89:241–247. doi: 10.1016/j.jhin.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Merli M, Lucidi C, Di Gregorio V, Falcone M, Giannelli V, Lattanzi B, Giusto M, Ceccarelli G, Farcomeni A, Riggio O, Venditti M. The spread of multi drug resistant infections is leading to an increase in the empirical antibiotic treatment failure in cirrhosis: a prospective survey. PLoS One. 2015;10:e0127448. doi: 10.1371/journal.pone.0127448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arvaniti V, D'Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010; 139: 1246-1256, 1256.e1-1256. :e5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Fiore M, Maraolo AE, Gentile I, Borgia G, Leone S, Sansone P, Passavanti MB, Aurilio C, Pace MC. Nosocomial spontaneous bacterial peritonitis antibiotic treatment in the era of multi-drug resistance pathogens: A systematic review. World J Gastroenterol. 2017;23:4654–4660. doi: 10.3748/wjg.v23.i25.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA, Laxminarayan R. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14:742–750. doi: 10.1016/S1473-3099(14)70780-7. [DOI] [PubMed] [Google Scholar]
- 26.Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, Seva-Pereira T, Corradi F, Mensa J, Ginès P, Arroyo V. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 27.Li YT, Yu CB, Huang JR, Qin ZJ, Li LJ. Pathogen profile and drug resistance analysis of spontaneous peritonitis in cirrhotic patients. World J Gastroenterol. 2015;21:10409–10417. doi: 10.3748/wjg.v21.i36.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexopoulou A, Vasilieva L, Agiasotelli D, Siranidi K, Pouriki S, Tsiriga A, Toutouza M, Dourakis SP. Extensively drug-resistant bacteria are an independent predictive factor of mortality in 130 patients with spontaneous bacterial peritonitis or spontaneous bacteremia. World J Gastroenterol. 2016;22:4049–4056. doi: 10.3748/wjg.v22.i15.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutz P, Nischalke HD, Krämer B, Goeser F, Kaczmarek DJ, Schlabe S, Parcina M, Nattermann J, Hoerauf A, Strassburg CP, Spengler U. Antibiotic resistance in healthcare-related and nosocomial spontaneous bacterial peritonitis. Eur J Clin Invest. 2017;47:44–52. doi: 10.1111/eci.12701. [DOI] [PubMed] [Google Scholar]
- 30.Tudorașcu DR, Bărbulescu AL, Cârțână ET, Petrescu IO, Ciurea RN, Ciobanu D, Forțofoiu MC, Pădureanu V, Tica OS, Tudorache S, Petrescu F. Study of the Etiological Spectrum of Spontaneous Bacterial Peritonitis in a Group of Patients Suffering from Liver Cirrhosis. Curr Health Sci J. 2016;42:365–371. doi: 10.12865/CHSJ.42.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salerno F, Borzio M, Pedicino C, Simonetti R, Rossini A, Boccia S, Cacciola I, Burroughs AK, Manini MA, La Mura V, Angeli P, Bernardi M, Dalla Gasperina D, Dionigi E, Dibenedetto C, Arghittu M AISF Investigators. The impact of infection by multidrug-resistant agents in patients with cirrhosis. A multicenter prospective study. Liver Int. 2017;37:71–79. doi: 10.1111/liv.13195. [DOI] [PubMed] [Google Scholar]
- 32.Béjar-Serrano S, Del Pozo P, Fernández-de la Varga M, Benlloch S. Multidrug-resistant bacterial infections in patients with liver cirrhosis in a tertiary referral hospital. Gastroenterol Hepatol. 2019;42:228–238. doi: 10.1016/j.gastrohep.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016;80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20:440–458, table of contents. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeong SH, Kim HS, Kim JS, Shin DH, Kim HS, Park MJ, Shin S, Hong JS, Lee SS, Song W. Prevalence and Molecular Characteristics of Carbapenemase-Producing Enterobacteriaceae From Five Hospitals in Korea. Ann Lab Med. 2016;36:529–535. doi: 10.3343/alm.2016.36.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eda R, Nakamura M, Takayama Y, Maehana S, Nakano R, Yano H, Kitasato H. Trends and molecular characteristics of carbapenemase-producing Enterobacteriaceae in Japanese hospital from 2006 to 2015. J Infect Chemother. 2020;26:667–671. doi: 10.1016/j.jiac.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Logan LK, Weinstein RA. The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J Infect Dis. 2017;215:S28–S36. doi: 10.1093/infdis/jiw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Queenan AM, Shang W, Schreckenberger P, Lolans K, Bush K, Quinn J. SME-3, a novel member of the Serratia marcescens SME family of carbapenem-hydrolyzing beta-lactamases. Antimicrob Agents Chemother. 2006;50:3485–3487. doi: 10.1128/AAC.00363-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birgy A, Bidet P, Genel N, Doit C, Decré D, Arlet G, Bingen E. Phenotypic screening of carbapenemases and associated β-lactamases in carbapenem-resistant Enterobacteriaceae. J Clin Microbiol. 2012;50:1295–1302. doi: 10.1128/JCM.06131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans BA, Amyes SG. OXA β-lactamases. Clin Microbiol Rev. 2014;27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antunes NT, Fisher JF. Acquired Class D β-Lactamases. Antibiotics (Basel) 2014;3:398–434. doi: 10.3390/antibiotics3030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48:15–22. doi: 10.1128/AAC.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maveyraud L, Golemi-Kotra D, Ishiwata A, Meroueh O, Mobashery S, Samama JP. High-resolution X-ray structure of an acyl-enzyme species for the class D OXA-10 beta-lactamase. J Am Chem Soc. 2002;124:2461–2465. doi: 10.1021/ja016736t. [DOI] [PubMed] [Google Scholar]
- 44.Jeon JH, Lee JH, Lee JJ, Park KS, Karim AM, Lee CR, Jeong BC, Lee SH. Structural basis for carbapenem-hydrolyzing mechanisms of carbapenemases conferring antibiotic resistance. Int J Mol Sci. 2015;16:9654–9692. doi: 10.3390/ijms16059654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esposito S, Leone S, Carosi G. Analysis of current guidelines for intra-abdominal infections. J Chemother. 2009;21 Suppl 1:30–35. doi: 10.1179/joc.2009.21.Supplement-1.30. [DOI] [PubMed] [Google Scholar]
- 46.Zavascki AP, Klee BO, Bulitta JB. Aminoglycosides against carbapenem-resistant Enterobacteriaceae in the critically ill: the pitfalls of aminoglycoside susceptibility. Expert Rev Anti Infect Ther. 2017;15:519–526. doi: 10.1080/14787210.2017.1316193. [DOI] [PubMed] [Google Scholar]
- 47.Petrosillo N, Giannella M, Lewis R, Viale P. Treatment of carbapenem-resistant Klebsiella pneumoniae: the state of the art. Expert Rev Anti Infect Ther. 2013;11:159–177. doi: 10.1586/eri.12.162. [DOI] [PubMed] [Google Scholar]
- 48.Tumbarello M, Losito AR, Giamarellou H. Optimizing therapy in carbapenem-resistant Enterobacteriaceae infections. Curr Opin Infect Dis. 2018;31:566–577. doi: 10.1097/QCO.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 49.Eljaaly K, Alharbi A, Alshehri S, Ortwine JK, Pogue JM. Plazomicin: A Novel Aminoglycoside for the Treatment of Resistant Gram-Negative Bacterial Infections. Drugs. 2019;79:243–269. doi: 10.1007/s40265-019-1054-3. [DOI] [PubMed] [Google Scholar]
- 50.Leone S, Damiani G, Pezone I, Kelly ME, Cascella M, Alfieri A, Pace MC, Fiore M. New antimicrobial options for the management of complicated intra-abdominal infections. Eur J Clin Microbiol Infect Dis. 2019;38:819–827. doi: 10.1007/s10096-019-03533-y. [DOI] [PubMed] [Google Scholar]
- 51.Leone S, Cascella M, Pezone I, Fiore M. New antibiotics for the treatment of serious infections in intensive care unit patients. Curr Med Res Opin. 2019;35:1331–1334. doi: 10.1080/03007995.2019.1583025. [DOI] [PubMed] [Google Scholar]
- 52.Mo Y, Lorenzo M, Farghaly S, Kaur K, Housman ST. What's new in the treatment of multidrug-resistant gram-negative infections? Diagn Microbiol Infect Dis. 2019;93:171–181. doi: 10.1016/j.diagmicrobio.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Wright H, Bonomo RA, Paterson DL. New agents for the treatment of infections with Gram-negative bacteria: restoring the miracle or false dawn? Clin Microbiol Infect. 2017;23:704–712. doi: 10.1016/j.cmi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 54.Esposito S, Leone S, Noviello S. Management of severe bacterial infections. Expert Rev Anti Infect Ther. 2005;3:593–600. doi: 10.1586/14787210.3.4.593. [DOI] [PubMed] [Google Scholar]
- 55.Giacobbe DR, Saffioti C, Losito AR, Rinaldi M, Aurilio C, Bolla C, Boni S, Borgia G, Carannante N, Cassola G, Ceccarelli G, Corcione S, Dalla Gasperina D, De Rosa FG, Dentone C, Di Bella S, Di Lauria N, Feasi M, Fiore M, Fossati S, Franceschini E, Gori A, Granata G, Grignolo S, Grossi PA, Guadagnino G, Lagi F, Maraolo AE, Marinò V, Mazzitelli M, Mularoni A, Oliva A, Pace MC, Parisini A, Patti F, Petrosillo N, Pota V, Raffaelli F, Rossi M, Santoro A, Tascini C, Torti C, Trecarichi EM, Venditti M, Viale P, Signori A, Bassetti M, Del Bono V, Giannella M, Mikulska M, Tumbarello M, Viscoli C SITA GIOVANI (Young Investigators Group of the Società Italiana Terapia Antinfettiva) and the COLI-CROSS Study Group. Use of colistin in adult patients: A cross-sectional study. J Glob Antimicrob Resist. 2020;20:43–49. doi: 10.1016/j.jgar.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Durante-Mangoni E, Andini R, Zampino R. Management of carbapenem-resistant Enterobacteriaceae infections. Clin Microbiol Infect. 2019;25:943–950. doi: 10.1016/j.cmi.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 57.Zusman O, Altunin S, Koppel F, Dishon Benattar Y, Gedik H, Paul M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72:29–39. doi: 10.1093/jac/dkw377. [DOI] [PubMed] [Google Scholar]
- 58.Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L. Colistin alone vs colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18:391–400. doi: 10.1016/S1473-3099(18)30099-9. [DOI] [PubMed] [Google Scholar]
- 59.Esposito S, Leone S. Antimicrobial treatment for Intensive Care Unit (ICU) infections including the role of the infectious disease specialist. Int J Antimicrob Agents. 2007;29:494–500. doi: 10.1016/j.ijantimicag.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 60.Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev. 2016;29:321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dimopoulos G, Koulenti D, Parker SL, Roberts JA, Arvaniti K, Poulakou G. Intravenous fosfomycin for the treatment of multidrug-resistant pathogens: what is the evidence on dosing regimens? Expert Rev Anti Infect Ther. 2019;17:201–210. doi: 10.1080/14787210.2019.1573669. [DOI] [PubMed] [Google Scholar]
- 62.Noviello S, Ianniello F, Leone S, Fiore M, Esposito S. In vitro activity of tigecycline: MICs, MBCs, time-kill curves and post-antibiotic effect. J Chemother. 2008;20:577–580. doi: 10.1179/joc.2008.20.5.577. [DOI] [PubMed] [Google Scholar]
- 63.Scott LJ. Eravacycline: A Review in Complicated Intra-Abdominal Infections. Drugs. 2019;79:315–324. doi: 10.1007/s40265-019-01067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solomkin J, Evans D, Slepavicius A, Lee P, Marsh A, Tsai L, Sutcliffe JA, Horn P. Assessing the Efficacy and Safety of Eravacycline vs Ertapenem in Complicated Intra-abdominal Infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) Trial: A Randomized Clinical Trial. JAMA Surg. 2017;152:224–232. doi: 10.1001/jamasurg.2016.4237. [DOI] [PubMed] [Google Scholar]
- 65.Solomkin JS, Gardovskis J, Lawrence K, Montravers P, Sway A, Evans D, Tsai L. IGNITE4: Results of a Phase 3, Randomized, Multicenter, Prospective Trial of Eravacycline vs Meropenem in the Treatment of Complicated Intraabdominal Infections. Clin Infect Dis. 2019;69:921–929. doi: 10.1093/cid/ciy1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leone S, Stefani S, Venditti M, Grossi P, Colizza S, De Gasperi A, Scaglione F, Sganga G, Esposito S Italian Intra-abdominal Infections Working Group. Intra-abdominal infections: model of antibiotic stewardship in an era with limited antimicrobial options. Int J Antimicrob Agents. 2011;38:271–272. doi: 10.1016/j.ijantimicag.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Lucasti C, Popescu I, Ramesh MK, Lipka J, Sable C. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole vs meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, Phase II trial. J Antimicrob Chemother. 2013;68:1183–1192. doi: 10.1093/jac/dks523. [DOI] [PubMed] [Google Scholar]
- 68.Mazuski JE, Gasink LB, Armstrong J, Broadhurst H, Stone GG, Rank D, Llorens L, Newell P, Pachl J. Efficacy and Safety of Ceftazidime-Avibactam Plus Metronidazole Versus Meropenem in the Treatment of Complicated Intra-abdominal Infection: Results From a Randomized, Controlled, Double-Blind, Phase 3 Program. Clin Infect Dis. 2016;62:1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin X, Tran BG, Kim MJ, Wang L, Nguyen DA, Chen Q, Song J, Laud PJ, Stone GG, Chow JW. A randomised, double-blind, phase 3 study comparing the efficacy and safety of ceftazidime/avibactam plus metronidazole vs meropenem for complicated intra-abdominal infections in hospitalised adults in Asia. Int J Antimicrob Agents. 2017;49:579–588. doi: 10.1016/j.ijantimicag.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Tumbarello M, Trecarichi EM, Corona A, De Rosa FG, Bassetti M, Mussini C, Menichetti F, Viscoli C, Campoli C, Venditti M, De Gasperi A, Mularoni A, Tascini C, Parruti G, Pallotto C, Sica S, Concia E, Cultrera R, De Pascale G, Capone A, Antinori S, Corcione S, Righi E, Losito AR, Digaetano M, Amadori F, Giacobbe DR, Ceccarelli G, Mazza E, Raffaelli F, Spanu T, Cauda R, Viale P. Efficacy of Ceftazidime-Avibactam Salvage Therapy in Patients With Infections Caused by Klebsiella pneumoniae Carbapenemase-producing K. pneumoniae. Clin Infect Dis. 2019;68:355–364. doi: 10.1093/cid/ciy492. [DOI] [PubMed] [Google Scholar]
- 71.Temkin E, Torre-Cisneros J, Beovic B, Benito N, Giannella M, Gilarranz R, Jeremiah C, Loeches B, Machuca I, Jiménez-Martín MJ, Martínez JA, Mora-Rillo M, Navas E, Osthoff M, Pozo JC, Ramos Ramos JC, Rodriguez M, Sánchez-García M, Viale P, Wolff M, Carmeli Y. Ceftazidime-Avibactam as Salvage Therapy for Infections Caused by Carbapenem-Resistant Organisms. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01964-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onorato L, Di Caprio G, Signoriello S, Coppola N. Efficacy of ceftazidime/avibactam in monotherapy or combination therapy against carbapenem-resistant Gram-negative bacteria: A meta-analysis. Int J Antimicrob Agents. 2019;54:735–740. doi: 10.1016/j.ijantimicag.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 73.Petty LA, Henig O, Patel TS, Pogue JM, Kaye KS. Overview of meropenem-vaborbactam and newer antimicrobial agents for the treatment of carbapenem-resistant Enterobacteriaceae. Infect Drug Resist. 2018;11:1461–1472. doi: 10.2147/IDR.S150447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaye KS, Bhowmick T, Metallidis S, Bleasdale SC, Sagan OS, Stus V, Vazquez J, Zaitsev V, Bidair M, Chorvat E, Dragoescu PO, Fedosiuk E, Horcajada JP, Murta C, Sarychev Y, Stoev V, Morgan E, Fusaro K, Griffith D, Lomovskaya O, Alexander EL, Loutit J, Dudley MN, Giamarellos-Bourboulis EJ. Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection: The TANGO I Randomized Clinical Trial. JAMA. 2018;319:788–799. doi: 10.1001/jama.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, Mathers AJ, Bassetti M, Vazquez J, Cornely OA, Solomkin J, Bhowmick T, Bishara J, Daikos GL, Felton T, Furst MJL, Kwak EJ, Menichetti F, Oren I, Alexander EL, Griffith D, Lomovskaya O, Loutit J, Zhang S, Dudley MN, Kaye KS. Effect and Safety of Meropenem-Vaborbactam vs Best-Available Therapy in Patients with Carbapenem-Resistant Enterobacteriaceae Infections: The TANGO II Randomized Clinical Trial. Infect Dis Ther. 2018;7:439–455. doi: 10.1007/s40121-018-0214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ackley R, Roshdy D, Meredith J, Minor S, Anderson WE, Capraro GA, Polk C. Meropenem-Vaborbactam vs Ceftazidime-Avibactam for Treatment of Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.02313-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun D, Rubio-Aparicio D, Nelson K, Dudley MN, Lomovskaya O. Meropenem-Vaborbactam Resistance Selection, Resistance Prevention, and Molecular Mechanisms in Mutants of KPC-Producing Klebsiella pneumoniae. Antimicrob Agents Chemother. 2017;61:e01694–17. doi: 10.1128/AAC.01694-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/Avibactam, Meropenem/Vaborbactam, or Both? Clin Infect Dis. 2019;68:519–524. doi: 10.1093/cid/ciy576. [DOI] [PubMed] [Google Scholar]
- 79.Gaibani P, Ambretti S, Viale P, Re MC. In vitro synergistic activity of meropenem/vaborbactam in combination with ceftazidime/avibactam against KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2019;74:1457–1459. doi: 10.1093/jac/dky557. [DOI] [PubMed] [Google Scholar]
- 80.Motsch J, Murta de Oliveira C, Stus V, Köksal I, Lyulko O, Boucher HW, Kaye KS, File TM, Brown ML, Khan I, Du J, Joeng HK, Tipping RW, Aggrey A, Young K, Kartsonis NA, Butterton JR, Paschke A. RESTORE-IMI 1: A Multicenter, Randomized, Double-blind Trial Comparing Efficacy and Safety of Imipenem/Relebactam vs Colistin Plus Imipenem in Patients With Imipenem-nonsusceptible Bacterial Infections. Clin Infect Dis. 2020;70:1799–1808. doi: 10.1093/cid/ciz530. [DOI] [PMC free article] [PubMed] [Google Scholar]