Abstract
BACKGROUND
Many studies have investigated the progression of nonalcoholic fatty liver disease (NAFLD) and its predisposing risk factors, but the conclusions from these studies have been conflicting. More challenging is the fact that no effective treatment is currently available for NAFLD.
AIM
To determine the effects of proprotein convertase subtilisin/kexin type-9 (PCSK9) inhibitors on fatty infiltration of the liver.
METHODS
This retrospective, chart review-based study was conducted on patients, 18-year-old and above, who were currently on PCSK9 inhibitor drug therapy. Patients were excluded from the study according to missing pre- or post-treatment imaging or laboratory values, presence of cirrhosis or rhabdomyolysis, or development of acute liver injury during the PCSK9 inhibitor treatment period; the latter being due to false elevation of liver function markers, alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Radiographic improvement was assessed by a single radiologist, who read both the pre- and post-treatment images to minimize reading bias. Fatty infiltration of the liver was also assessed by changes in ALT and AST, with pre- and post-treatment levels compared by paired t-test (alpha criterion: 0.05).
RESULTS
Of the 29 patients included in the study, 8 were male (27.6%) and 21 were female (72.4%). Essential hypertension was present in 25 (86.2%) of the patients, diabetes mellitus in 18 (62.1%) and obesity in 15 (51.7%). In all, patients were on PCSK9 inhibitors for a mean duration of 23.69 ± 11.18 mo until the most recent ALT and AST measures were obtained. Of the 11 patients who received the radiologic diagnosis of hepatic steatosis, 8 (72.73%) achieved complete radiologic resolution upon use of PCSK9 inhibitors (mean duration of 17.6 mo). On average, the ALT level (IU/L) decreased from 21.83 ± 11.89 at pretreatment to 17.69 ± 8.00 at post-treatment (2-tailed P = 0.042) and AST level (IU/L) decreased from 22.48 ± 9.00 pretreatment to 20.59 ± 5.47 post-treatment (2-tailed P = 0.201).
CONCLUSION
PCSK9 inhibitors can slow down or even completely resolve NAFLD.
Keywords: Proprotein convertase subtilisin/kexin type-9 inhibitor, Fatty liver, Nonalcoholic fatty liver disease, Alanine aminotransferase, Aspartate aminotransferase, Imaging
Core Tip: This retrospective study evaluated the effects of proprotein convertase subtilisin/kexin type-9 (PCSK9) inhibitors on fatty infiltration of the liver. Among the 29 selected patients, 11 were found to have radiologic diagnosis of hepatic steatosis and 8 of those (72.73%) achieved complete radiologic resolution of the condition upon use of PCSK9 inhibitors for mean duration of 17.6 mo. Both alanine aminotransferase and aspartate aminotransferase levels showed a downward trend after PCSK9 inhibitors for mean duration of 23.69 ± 11.18 mo. These results highlight the potential benefit of PCSK9 inhibitors use for patients with nonalcoholic fatty liver disease.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a spectrum of fatty infiltration of the liver and is considered the most common chronic liver disease in the world[1]. Cases range from nonalcoholic fatty liver-a relatively benign condition-to nonalcoholic steatohepatitis (commonly known as NASH), which can eventually lead to the life-threatening condition of cirrhosis[1-3]. The continually increasing prevalence of NAFLD and numbers of cases progressing to cirrhosis, itself a major co-morbidity and emerging public health concern[4], have prompted many researchers to investigate the underlying mechanisms of NAFLD progression and the pre-disposing risk factors of such[3,5-7]. However, the conclusions from these studies have been conflicting. Even more challenging is the fact that there is currently no effective treatment available for NAFLD.
As some recent studies have implicated increased proprotein convertase subtilisin/kexin type-9 (PCSK9) synthesis and release in the pathogenic process of NAFLD[8,9], we hypothesized that PCSK9 inhibition could lead to improvement in fatty infiltration of the liver. Thus, the aim of this study was to assess improvement in fatty infiltration of the liver among patients on PCSK9 inhibitor drug therapy.
MATERIALS AND METHODS
Study design
This study was designed as a retrospective, chart-based review. After approval from the hospital’s Institutional Review Board, the database of the study site was searched for patients who were over 18 years in age and had received PCSK9 inhibitors anytime from January of 2015 until July of 2019.
Exclusion criteria
Patient records were excluded from the study according to: Missing pre- or post-treatment imaging; missing pre- or post-treatment laboratory values; diagnosis of cirrhosis, to avoid confounding by the inherently low alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels; signs of rhabdomyolysis that had developed any time during the PCSK9 inhibitor therapy, to avoid confounding by the inherently elevated ALT and AST; or presence of acute liver injury due to known cause, not thought to be related to PCSK9 inhibitor. For example, ‘Female B’ was started on PCSK9 inhibitors in February of 2018, and at that time showed ALT of 100 U/L and AST of 90 U/L. She experienced septic shock in May of 2018, due to pneumonia, and subsequent ischemic hepatitis (“shock liver”) with ALT of 3000 U/L and AST of 1850 U/L. Her case could not be regarded as “no improvement” or even of “worsening”, since a separate injury increased the levels of liver function enzymes.
Outcomes
Radiologic resolution of hepatic steatosis was the primary outcome, and improvement in liver biomarkers was the secondary outcome.
Radiologic resolution: Liver imaging scans [computed tomography (CT) scan, ultrasound (US)] that had been performed before the start of PCSK9 inhibitor therapy were compared with the most recent imaging scans, that had been performed at least 3 mo after the start of the PCSK9 inhibitor therapy. In order to minimize the confounding factors, a single radiologist worked independently to read the scans from before and after treatment. Where possible, attempts were made to compare CT scan with CT scan (or US to US scan), if both had been performed at the pre- and post-treatment times. Hepatic steatosis was defined by the following[10,11]: (1) ≤ 40 Hounsfield units (HU) on a non-contrast CT scan or during phase when the liver was not contrast-enhanced, such as a chest CT in the very early phase of contrast; (2) < 70 HU during portal venous phase image; and (3) Increased echogenicity on US of the liver parenchyma compared to the kidney, with loss of normal periportal echogenicity.
Liver biomarkers’ improvement: ALT and AST were used as the biomarkers to assess improvement of fatty infiltration of the liver, as ALT and AST can be elevated in fatty liver. Pretreatment levels of ALT and AST were compared with those measured post-treatment (at least 3 mo after the start of PCSK9 inhibitor therapy). The most recent ALT and AST measures obtained after the initiation of PCSK9 inhibitors were considered post-treatment values. For example, “Male A” had been started on PCSK9 inhibitors in January of 2015. Liver function tests were conducted during the treatment period (no interruptions in drug therapy), first in June of 2015 and then in January of 2016. For the study, the ALT and AST levels measured in January of 2016 were taken as the post-treatment levels since they were the most recent.
Data analysis
PCSK9 inhibitor use is currently low, due to its few indications as well as its cost. On top of that, selecting patients based on the strict selection criteria (in an attempt to minimize the influence of confounding factors) led to an anticipated small sample size. Radiologic improvement was reported in the form of descriptive data. Improvement in ALT and AST was assessed through paired t-test, with alpha criterion of 0.05.
RESULTS
Based on the strict selection criteria (in order to minimize confounders), 29 patients were included in this study.
Patient characteristics
The patient characteristics of the overall study population are summarized in Table 1. The majority of patients (n = 20; 68.96%) were started on PCSK9 inhibitors due to statin intolerance and need for lipid control, in particular lowering low-density lipoprotein (LDL) level. Six patients (20.68%) were able to tolerate their statin treatment but never achieved adequate control of their LDL levels, and therefore were started on PCSK9 inhibitors. No specific reason could be found for the initiation of PCSK9 inhibitors for the remaining 3 patients (10.36%).
Table 1.
Baseline characteristics of the study population
| Characteristic | Males | Females | Total |
| Sex | 8 | 21 | 29 |
| Race | |||
| Caucasian | 7 | 16 | 23 |
| African American | 1 | 4 | 5 |
| Other/unknown | 0 | 1 | 1 |
| Presence of | |||
| Diabetes | 3 | 15 | 18 |
| Hypertension | 8 | 17 | 25 |
| Obesity | 5 | 10 | 15 |
| Reason for PCSK-9 inhibitor initiation | |||
| Statin intolerance | 4 | 16 | 20 |
| Inadequate lipid control | 2 | 4 | 6 |
| Not known | 2 | 1 | 3 |
PCSK9: Proprotein convertase subtilisin/kexin type-9.
Table 2 provides a summary of the types of PCSK9 inhibitor used by the study population and the age at which the treatments were initiated.
Table 2.
Types of proprotein convertase subtilisin/kexin type-9 inhibitor used by the study population
| Males | Females | Total | |
| Type of PCSK9 inhibitor | |||
| Evolocumab | 5 | 17 | 22 |
| Alirocumab | 3 | 4 | 7 |
| Age in year at PCSK9 inhibitor initiation, mean ± SD | 67.7 ± 7.6 | 63.6 ± 9.6 | 64.8 ± 9.1 |
| Concomitant statin therapy during PCSK-9 therapy | 1 | 5 | 6 |
PCSK9: Proprotein convertase subtilisin/kexin type-9; SD: Standard deviation.
Primary endpoint
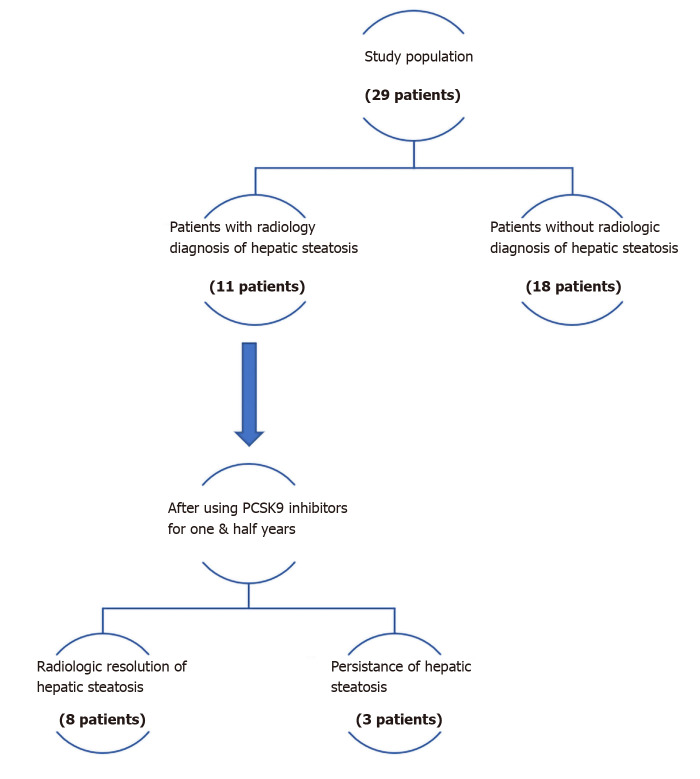
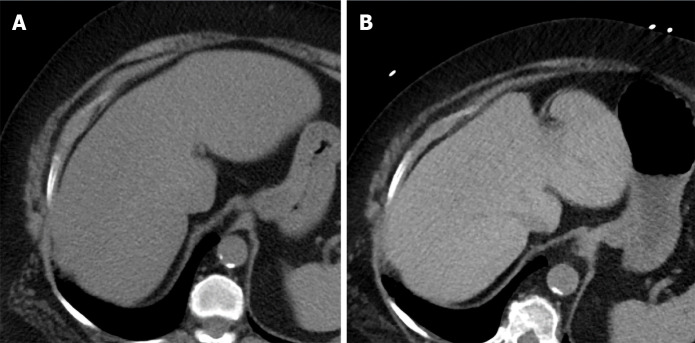
Pretreatment CT scan was compared with post-treatment CT scan for 26 (89.7%) of the patients. Pretreatment US was compared with post-treatment US for 2 patients, and only 1 patient required comparison of pretreatment CT scan with post-treatment US scan. As shown in Figure 1, 11 patients (37.9%) of the total study population had radiologic diagnosis of hepatic steatosis. Among these 11 patients, 8 (72.73%) achieved complete radiologic resolution of the hepatic steatosis after use of PCSK9 inhibitors for a mean duration of 17.6 mo. Figure 2A and B provide a comparison of CT images of liver, before and after treatment with PCSK9 inhibitor, from a patient who experienced complete resolution of hepatic steatosis.
Figure 1.
Flow diagram for primary outcome (resolution of hepatic steatosis). PCSK9: Proprotein convertase subtilisin/kexin type-9.
Figure 2.
Pre- vs post-treatment computed tomography scans of the liver. A: Liver computed tomography (CT) scan before treatment with proprotein convertase subtilisin/kexin type-9 (PCSK9) inhibitor, showing the liver to be homogeneously hypodense (< 40 HU) and the portal and hepatic veins to have unclear delineation from the surrounding parenchyma; B: Liver CT scan after treatment with PCSK9 inhibitor, showing the liver to have increased density and the portal and hepatic veins to be clear (i.e., linear hypodense structures, in contrast to the more hyperdense liver parenchyma).
Secondary endpoint
Only 2 patients (6.9%) of the total study population had abnormally elevated pretreatment AST levels. One patient had a pretreatment AST level of 41 IU/L, and the other patient had 44 IU/L; the upper limit of normal for AST at the study site is 40 IU/L. Post-treatment, AST level normalized for both patients (27 IU/L and 21 IU/L respectively). Although all other patients in the study had normal pretreatment ALT and AST levels, both markers showed a downward trend after PCSK9 use—showing a statistically significant reduction for ALT, as summarized in Table 3.
Table 3.
Effects of proprotein convertase subtilisin/kexin type-9 inhibitor on secondary outcome (alanine aminotransferase and aspartate aminotransferase) and lipid panel
| Parameter | Males | Females | Combined |
| Time elapsed in month from PCSK9 inhibitor initiation until most recent ALT and AST measurements | 18.4 ± 11.2 | 25.71 ± 10.74 | 23.69 ± 11.18 |
| Pre-treatment | Post-treatment | Sig (2-tailed)1 | |
| ALT in IU/L | 21.83 ± 11.89 | 17.69 ± 8.00 | 0.042 |
| AST in IU/L | 22.48 ± 9.00 | 20.59 ± 5.47 | 0.201 |
| LDL in mg/dL | 150.43 ± 44.69 | 90.89 ± 35.67 | 0.000 |
| Triglycerides in mg/dL | 220.07 ± 143.36 | 196.34 ± 140.73 | 0.447 |
| HDL in mg/dL | 48.59 ± 12.97 | 48.90 ± 16.27 | 0.884 |
Data are presented as mean ± standard deviation.
2-tailed P value for paired t-test. ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; PCSK9: Proprotein convertase subtilisin/kexin type-9.
DISCUSSION
Though the study population was small, our study showed not only a downward trend in the ALT and AST levels among patients who used PCSK9 inhibitors but also that 8 out of the 11 patients with hepatic steatosis achieved complete radiologic resolution. In general, it appeared that the patient needed to be on PCSK9 inhibitors for approximately 1.5 years to see reasonable radiologic improvement and for about 2 years to see a downward trend in ALT and AST levels.
PCSK9 is an enzyme synthesized mainly by the liver[12]. It binds to the LDL receptor (LDL-R) on the surface of hepatocytes, leading to the degradation of the LDL-R itself[13]. When LDL-R receptors are degraded, the result is an increase in plasma LDL-cholesterol levels. Evolocumab and alirocumab, both approved by the United States’ Federal Drug Administration (commonly referred to as FDA) in 2015, are fully humanized monoclonal antibodies that bind free plasma PCSK9, promoting degradation of this enzyme. Hence, the result is decreased degradation of LDL-R and increased up-take of the plasma LDL-cholesterol to liver; consequently, the levels of LDL in blood decrease. These PCSK9 inhibitors were initially approved by the FDA only for the treatment of familial hypercholesterolemia[14-16]. Subsequent approval provided for primary and secondary prevention of cardiovascular events in patients whose LDL-cholesterol was not at target level, despite being on optimal statin therapy, or who were intolerant to statins[17]. However, the long-term effects and mortality benefits are still unclear[17,18]. Some recent studies have shown that increased PCSK9 synthesis and release might be involved in NAFLD pathogenesis as well[8,9], which suggests that inhibition of PCSK9 may actually stop development or progression of NAFLD. Indeed, Theocharidou et al[9] demonstrated such, which prompted our interest in this research project. Despite the small sample size, our results, too, are promising.
Patients with NAFLD are four to five times more likely to develop cirrhosis and three to four times more likely to develop hepatocellular carcinoma, when compared to patients without NAFLD[19]. However, as of this writing, there is no effective treatment available for NAFLD. Weight loss is considered as the first step in the disease management[20]. While some pharmacologic therapy options are available, they have shown limited benefit. Vitamin E is recommended for NAFLD patients but only those without diabetes[21]. For NAFLD patients with type 2 diabetes, in particular, pioglitazone is recommended as an second line anti-diabetic medication, after metformin, due to the slight biochemical and histologic improvements seen with its use[22]. Since high-dose vitamin E is associated with an increase in all-cause mortality[23] and pioglitazone can cause both fluid retention and worsening of congestive heart failure[24], a benefit and risk assessment is necessary before initiation of either of these pharmacologic therapies. Atorvastatin and omega-3 fatty acids have also been studied among patients with NAFLD but remain of uncertain benefit to date.
Since 2010, several new therapies and clinical trials have come forward but their impact has been either limited by a weak degree of improvement or a less favorable side effect profile[25]. Most notable of all the new agents is obeticholic acid. In a multicenter, randomized, placebo-controlled phase-3 clinical trial of obeticholic acid which lasted from December 9, 2015 until October 26, 2018, the group treated with 25 mg obeticholic acid demonstrated improvement in the liver fibrosis endpoint but the NASH resolution endpoint was not met[26]. Moreover, more than half of the patients in the 25 mg treatment group experienced pruritis and 14% of patients experienced serious adverse events[26]. A recently published retrospective study by Zafar et al[27] showed increase in ALT and AST levels with the use of PCSK9 inhibitors, but of only 6.2 mg/dL and 5.8 mg/dL respectively. These are small increases and appear to be clinically insignificant. It is important to note as well that the follow-up time (since start of PCSK9 inhibitors) in that study was only 6 mo. A meta-analysis by Zhang et al[28] evaluated 25 randomized control trials, comprising a total sample of 12200 patients, and found that the PCSK9 inhibitors overall adverse effects profile was not significantly different than placebo. Rather, evolocumab was noted to reduce the rate of abnormal liver function, as was noted in our study. Hence, it can be concluded based on the available data that PCSK9 inhibitors are safe to use.
Important limitations of our study include the absence of a control population, a small sample size, the retrospective design, and single-center study design. Each of these limitations may impact the external validity. However, despite these limitations, the potential of PCSK9 inhibitors for NAFLD was supported by our findings of radiologic resolution of hepatic steatosis among 8 of 11 patients and of the downward trend of ALT and AST among the entire study population, despite having been within normal range pretreatment. This study, in conjunction with similar studies[9], can serve as the basis for prospective research to further delineate the potential of PCSK9 inhibitors for NAFLD and NASH.
CONCLUSION
PCSK9 inhibitors can slow down or even result in complete resolution of NAFLD. However, considering the limitations of this study, prospective studies are needed to validate these findings.
ARTICLE HIGHLIGHTS
Research background
Nonalcoholic fatty liver disease (NAFLD) is considered the most common chronic liver disease in the world and can be life-threatening, with some cases progressing to end-stage liver disease. Yet, there is no effective treatment available for it.
Research motivation
Proprotein convertase subtilisin/kexin type-9 (PCSK9) inhibitors have produced favorable effects on liver function in some studies. This prompted our interest in determining whether PCSK9 inhibitors can elicit a therapeutic effect on hepatic steatosis.
Research objectives
Radiologic resolution of hepatic steatosis was the primary outcome, and improvement in liver function biomarkers was the secondary outcome.
Research methods
This study was designed as a retrospective chart review and included the medical records of 29 adult patients (18 years and above in age) who had received PCSK9 inhibitors anytime from January 2015 to July 2019.
Research results
Among the total 29 patients, 11 were found to have radiologic diagnosis of hepatic steatosis. Eight of these eleven patients (72.73%) achieved complete radiologic resolution of hepatic steatosis after using PCSK9 inhibitors for a mean duration of 17.6 mo. Levels of both alanine aminotransferase and aspartate aminotransferase levels also showed a downward trend after use of PCSK9 inhibitors for about 2 years.
Research conclusions
PCSK9 inhibitors can slow down or even result in complete resolution of NAFLD.
Research perspectives
The findings from this study, in conjunction with those from similar studies, can serve as the basis for future prospective research to further explore the effects of PCSK9 inhibitors on hepatic steatosis. PCSK9 inhibitors may represent a significant breakthrough treatment for NAFLD, if prospective studies corroborate the current findings.
ACKNOWLEDGEMENTS
Data for this research project was obtained from Health System of the University of Kansas Medical Center, Kansas City, KS 66160, United States. The authors are grateful to the Department of Clinical Informatics at the University of Kansas Medical Center for their help in accessing the patient medical record database. Data extraction was conducted by the HERON automated data extraction tool.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Institutional Review Board of University of Kansas Medical Center (Kansas City, KS, United States).
Informed consent statement: In accordance with the retrospective design of the study, based upon chart reviews, no informed consent was required.
Conflict-of-interest statement: The authors declare having no financial relationships to disclose.
Manuscript source: Unsolicited manuscript
Peer-review started: June 9, 2020
First decision: September 18, 2020
Article in press: November 12, 2020
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ikura Y S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
Contributor Information
Muhammad Shafiq, General and Geriatric Medicine, University of Kansas Medical Center, Kansas City, KS 66160, United States. mshafiq@kumc.edu.
Timothy Walmann, Department of Diagnostic Radiology, University of Kansas Medical Center, Kansas City, KS 66160, United States.
Venkat Nutalapati, Department of Gastroenterology, University of Kansas Medical Center, Kansas City, KS 66160, United States.
Cheryl Gibson, General and Geriatric Medicine, University of Kansas Medical Center, Kansas City, KS 66160, United States.
Yousaf Zafar, Internal Medicine, NCH Health Care System, Naples, FL 34102, United States.
Data sharing statement
All relevant data has been provided in this article. No additional data is available.
References
- 1.Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: A prospective follow-up study with serial biopsies. Hepatol Commun. 2018;2:199–210. doi: 10.1002/hep4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Machado MV, Diehl AM. Pathogenesis of Nonalcoholic Steatohepatitis. Gastroenterology. 2016;150:1769–1777. doi: 10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: implications for prognosis and clinical management. J Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 5.Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol. 2009;51:371–379. doi: 10.1016/j.jhep.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, Chim AM, Yu J, Sung JJ, Chan HL. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Ruscica M, Ferri N, Macchi C, Meroni M, Lanti C, Ricci C, Maggioni M, Fracanzani AL, Badiali S, Fargion S, Magni P, Valenti L, Dongiovanni P. Liver fat accumulation is associated with circulating PCSK9. Ann Med. 2016;48:384–391. doi: 10.1080/07853890.2016.1188328. [DOI] [PubMed] [Google Scholar]
- 9.Theocharidou E, Papademetriou M, Reklou A, Sachinidis A, Boutari C, Giouleme O. The Role of PCSK9 in the Pathogenesis of Non-alcoholic Fatty Liver Disease and the Effect of PCSK9 Inhibitors. Curr Pharm Des. 2018;24:3654–3657. doi: 10.2174/1381612824666181010123127. [DOI] [PubMed] [Google Scholar]
- 10.Hamer OW, Aguirre DA, Casola G, Lavine JE, Woenckhaus M, Sirlin CB. Fatty liver: imaging patterns and pitfalls. Radiographics. 2006;26:1637–1653. doi: 10.1148/rg.266065004. [DOI] [PubMed] [Google Scholar]
- 11.Kodama Y, Ng CS, Wu TT, Ayers GD, Curley SA, Abdalla EK, Vauthey JN, Charnsangavej C. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 12.Ferri N, Tibolla G, Pirillo A, Cipollone F, Mezzetti A, Pacia S, Corsini A, Catapano AL. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis. 2012;220:381–386. doi: 10.1016/j.atherosclerosis.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Lo Surdo P, Bottomley MJ, Calzetta A, Settembre EC, Cirillo A, Pandit S, Ni YG, Hubbard B, Sitlani A, Carfí A. Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep. 2011;12:1300–1305. doi: 10.1038/embor.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 15.Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, Bolognese M, Wasserman SM. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380:1995–2006. doi: 10.1016/S0140-6736(12)61771-1. [DOI] [PubMed] [Google Scholar]
- 16.Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, Liu T, Mohanavelu S, Hoffman EB, McDonald ST, Abrahamsen TE, Wasserman SM, Scott R, Sabatine MS LAPLACE-TIMI 57 Investigators. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–2017. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt AF, Pearce LS, Wilkins JT, Overington JP, Hingorani AD, Casas JP. PCSK9 monoclonal antibodies for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2017;4:CD011748. doi: 10.1002/14651858.CD011748.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yadav K, Sharma M, Ferdinand KC. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors: Present perspectives and future horizons. Nutr Metab Cardiovasc Dis. 2016;26:853–862. doi: 10.1016/j.numecd.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Alexander M, Loomis AK, van der Lei J, Duarte-Salles T, Prieto-Alhambra D, Ansell D, Pasqua A, Lapi F, Rijnbeek P, Mosseveld M, Waterworth DM, Kendrick S, Sattar N, Alazawi W. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. doi: 10.1186/s12916-019-1321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A, Van Natta M, Clark J, Brunt EM, Kleiner DE, Hoofnagle JH, Robuck PR, NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35:66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 24.Goltsman I, Khoury EE, Winaver J, Abassi Z. Does Thiazolidinedione therapy exacerbate fluid retention in congestive heart failure? Pharmacol Ther. 2016;168:75–97. doi: 10.1016/j.pharmthera.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Pydyn N, Miękus K, Jura J, Kotlinowski J. New therapeutic strategies in nonalcoholic fatty liver disease: a focus on promising drugs for nonalcoholic steatohepatitis. Pharmacol Rep. 2020;72:1–12. doi: 10.1007/s43440-019-00020-1. [DOI] [PubMed] [Google Scholar]
- 26.Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, Newsome PN, Sheridan D, Sheikh MY, Trotter J, Knapple W, Lawitz E, Abdelmalek MF, Kowdley KV, Montano-Loza AJ, Boursier J, Mathurin P, Bugianesi E, Mazzella G, Olveira A, Cortez-Pinto H, Graupera I, Orr D, Gluud LL, Dufour JF, Shapiro D, Campagna J, Zaru L, MacConell L, Shringarpure R, Harrison S, Sanyal AJ REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. doi: 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 27.Zafar Y, Sattar Y, Ullah W, Roomi S, Rashid MU, Khan MS, Schmidt L. Proprotein convertase subtilisin/Kexin type-9 (PCSK-9) inhibitors induced liver injury - a retrospective analysis. J Community Hosp Intern Med Perspect. 2020;10:32–37. doi: 10.1080/20009666.2019.1710952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, Xie J, Kang LN, Xu B. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med. 2015;13:123. doi: 10.1186/s12916-015-0358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data has been provided in this article. No additional data is available.