Abstract
Purpose:
To retrospectively review the ability of direct bilirubin serum level to predict mortality and complications in patients undergoing transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC) and compare it to the predictive value of the currently utilized total bilirubin serum level.
Materials and methods:
A total of 219 patients who underwent TACE for 353 hepatocelluar carcinomas (HCC) at a single institution were included. There were 165 men and 54 women, with a mean age of 61.4 ± 7.6 (SD) [range: 27–86 years]. The patients’ electronic medical records were evaluated and they were divided into cohorts based on total bilirubin (< 2, 2–3, and > 3 mg/dL) as well as direct bilirubin (< 1 and 1–2 mg/dL).
Results:
Direct bilirubin serum level was significantly greater in the cohort of patients who did not survive as compared to those who survived 6 months ([0.58 ± 0.46 (SD) mg/dL; range: < 0.1–1.8 mg/dL] vs. [0.40 ± 0.31 (SD) mg/dL; range: < 0.1–1.6 mg/dL], respectively) (P = 0.04) and 12 months ([0.49 ± 0.38 (SD) mg/dL; range: < 0.1–1.8 mg/dL] vs. [0.38 ± 0.32 (SD) mg/dL; range: < 0.1–1.6 mg/dL], respectively) (P = 0.03). While total bilirubin serum level was not significantly different in those who did not and did survive 6 months ([1.54 ± 0.99 (SD) mg/dL; range: 0.3–3.9 mg/dL] vs. [1.27 ± 0.70 (SD) mg/dL; range: 0.3–3.75 mg/dL], respectively) (P = 0.16), it was significantly different when evaluating 12 months survival ([1.46 ± 0.87 (SD) mg/dL; range: 0.3–3.9 mg/dL] vs. [1.22 ± 0.65 (SD) mg/dL; range: 0.3–3.9 mg/dL]) (P = 0.03). Akaike information criterion (AIC) analysis revealed that direct bilirubin level more accurately predicted overall survival (AIC = 941.19 vs. 1000.51) and complications (AIC = 352.22 vs. 357.42) than total bilirubin serum levels.
Conclusion:
Direct bilirubin serum level appears to outperform total bilirubin concentration for predicting complications and overall survival in patients undergoing TACE. Patients with relatively maintained direct bilirubin levels should be considered for TACE, particularly in the setting of bridging to transplant.
Keywords: Chemoembolization, Hepatocellular carcinoma, Bilirubin, Cohort studies, Ethiodized oil
Transarterial chemoembolization (TACE) is a mainstay of loco-regional therapy in patients with hepatocellular carcinoma (HCC) who cannot undergo thermal ablation or surgical resection and has been shown to provide a survival benefit in large randomized controlled trials [1,2]. One factor to consider when evaluating patients for TACE is baseline liver function, most commonly reflected by total bilirubin serum level. Prior studies have found that an elevated total bilirubin serum level is predictive of both complications and poorer survival in patients undergoing TACE for HCC [3–10]. This has led the national comprehensive cancer network (NCCN) to recommend not performing TACE in patients with a total bilirubin serum level > 3 mg/dL [11].
Patients who are not considered candidates for TACE have limited treatment options. They may receive systemic therapy, however even if deemed a candidate for such treatments, these agents have shown modest survival benefits in advanced HCC [12,13]. Many of these patients remain listed for liver transplantation, and as such would be candidates for curative therapy, if they could be downsized or maintained within Milan criteria through bridging with TACE [14–17].
Given the limited treatment options for patients in this demographic, several authors have treated high-risk patients (i.e., those with a total bilirubin serum level > 3 mg/dL) with TACE, and discovered that in some instances this can be done safely [18–21]. One possible explanation for the discrepancy in results when treating patients with HCC and elevated total bilirubin serum levels with TACE may relate to technique, with superselective therapies providing improved outcomes [22]. However, improving the ability to determine patients’ hepatic reserve is also of importance.
Total bilirubin consists of two different subgroups (i.e., direct and indirect bilirubin). Direct, or conjugated bilirubin, represents the bilirubin that has undergone conjugation with glucuronic acid by the enzyme glucuronyltransferase within the liver. As such, the direct bilirubin has already been metabolized by the liver and made water soluble while unconjugated or indirect bilirubin has not. Unconjugated bilirubin may reflect Gilbert syndrome or hemolysis and have little to do with hepatic reserve. At times in cirrhosis, the unconjugated or indirect bilirubin is initially elevated and as the disease progresses direct or conjugated bilirubin elevates [23]. Thus, it is reasonable to assume that those patients with an elevated total bilirubin concentration but a relatively normal direct bilirubin maintain greater hepatic reserve than those with an elevated direct bilirubin concentration. Therefore, those with relatively normal direct bilirubin but an elevated total bilirubin concentration may have sufficient reserve to safely tolerate TACE. This would have the potential to provide significant improvement in patient selection for TACE, particularly in the setting of bridging to transplant.
The goal of this retrospective review was to evaluate the ability of direct bilirubin serum level to predict mortality and complications in patients who undergo TACE for HCC, and compare it to the predictive value of the currently utilized total bilirubin serum level.
Materials and methods
Patients
After Institutional Review Board approval, all patients who underwent TACE for HCC between 1/1/2011 and 12/31/2016 at a single institution were reviewed. A total of 255 consecutive patients received TACE for the treatment of 404 HCCs. However, 36 patients who underwent treatment of 51 lesions were excluded because they were lost to follow up (32 patients), had insufficient available data (3 patients), or were under the age of 18 (1 patient) (Fig. 1).
Figure 1.
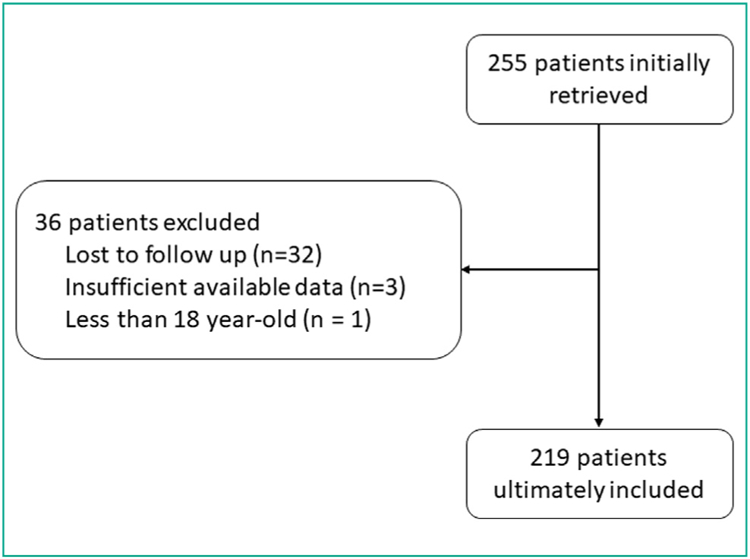
Flow diagram of included patients.
The final study population included 219 patients who underwent TACE for the treatment of 353 HCCs. There were 165 (165/219; 74.9%) men and 54 (54/219; 25.1%) women with a mean age of 61.4 7.6 (SD) years [range: 27–86 years]. A total of 71 TACEs (71/353; 20.1%) were performed with drug eluting beads (DEB-TACE) and 282 (282/353; 79.9%) were performed with classic TACE (cTACE). One and six patients respectively did not have total or direct bilirubin serum level value available for review respectively and were therefore excluded from those portions of the analysis. Demographic data for the patients are Table 1.
Table 1.
Demographic data of 219 patients who underwent transarterial chemoembolization for the treatment of hepatocellular carcinoma.
| Variable | |
|---|---|
| Age (years) | 61.4 ± 7.6 [27–86] |
| Sex | |
| Male | 165 (165/219; 75.3%) |
| Female | 54 (54/219; 24.7%) |
| HCC size (cm) | 3.8 ± 3.6 [1–33] |
| AST (U/L) | 102 ± 60 [26–198] |
| ALT (U/L | 92 ± 64 [25–201] |
| CR (mg/dL) | 1.0 ± 0.9 [0.4–10.7] |
| INR | 1.2 ± 0.2 [0.9–1.8] |
| Albumin (g/dL) | 3.2 ± 0.6 [1.1–5.2] |
| Total bilirubin (mg/dL) | 1.3 ± 0.7 [0.2–4.2] |
| Direct bilirubin (mg/dL) | 0.4 ± 0.3 [< 0.1–2] |
| MELD score | 10.3 ± 3.1 [1–22] |
| Number of TACE treatments | 1.4 ± 0.7 [1–6] |
| Selectivity of treatment | |
| Hemiliver | 29 (29/353; 8.1%) |
| > Sectional but < Hemiliver | 50 (50/353; 14.2%) |
| Sectional | 140 (140/353; 39.7%) |
| Segmental | 134 (134/353; 38%) |
HCC: hepatocellular carcinoma; TACE: Transarterial chemoembolization; Na: Sodium; INR: International normalization ratio; MELD: Model for end-stage liver disease. Quantitative variables are expressed as mean ± standard deviations, followed by ranges in brackets. Qualitative variables are expressed as raw numbers. Numbers in parentheses are proportions followed by percentages
TACE procedure
The TACE procedure has been previously described [24,25], however, in brief percutaneous arterial access was gained through the common femoral artery under ultrasound guidance. The celiac artery was then catheterized utilizing a 5-French (Fr) base catheter, most commonly a Cobra 2 (Terumo), and a microcatheter, most commonly a 2.8-Fr Pregreat® (Terumo), was utilized to gain more peripheral access into the liver. The preforming physician attempted to treat the lesion in as selective a manor as possible and cone beam CT was utilized at the operators’ discretion. Utilization of cTACE or DEB-TACE was at the operators’ preference. In the setting of cTACE 50 mg of doxorubicin and 10 mg of mitomycin C was mixed with ethiodized oil (Lipiodol®, Guerbet) at a 1:2 ratio. The chemotherapeutic agent was followed by particle embolization, most commonly 100–300 µm Embosphere® (Merit Medical), with a target embolization endpoint of complete or near stasis. In the setting of DEB-TACE, two vials of drug eluting beads (LC beads, Boston Scientific) were loaded with 50 mg of doxorubicin each. Each vial of beads was diluted in 10 cc of normal saline and 10 cc of contrast. For DEB-TACE the embolization endpoint was near stasis.
Data collection
The electronic medical records were reviewed for demographic data, including gender, age, and cause of cirrhosis. Preprocedural laboratory values such as creatinine serum level (CR), international normalized ratio (INR), total bilirubin serum level, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and albumin were recorded, as was the model for endstage liver disease (MELD) score. Tumoral factors were also evaluated including size of lesion and number of TACE treatments required. The selectivity of TACE performed was also evaluated and divided into 4 categories, segmental (delivery to less than a Couinaud section), sectional (delivery to 1 entire section), greater than sectional but less than hemiliver (delivery to > 1 section but less than a hemiliver), and hemiliver. Finally, the type of TACE (DEB-TACE or cTACE) was recorded.
Radiologic response was evaluated utilizing the European Association for the Study of the Liver (EASL) criteria [26]. Overall survival (OS) was calculated and considered to be the survival from the date of TACE until death and was censored for transplantation. Time to progression (TTP) was evaluated and considered to be time from TACE to progression as defined by EASL. Overall radiologic response (ORR) was considered to be positive if patients had a partial or complete response by EASL criteria. Radiologic response was evaluated after initial TACE as well as after maximal response following multiple TACE treatments when applicable. TACE was performed per the on demand model. Complications were reviewed and recorded. To further determine complication profiles, ratio of total bilirubin, AST, and ALT were calculated at 1 day and 1 month by dividing the values at these post-procedural time points by the pretreatment values. Change in total bilirubin, AST, and ALT were also evaluated at one month by subtracting the pretreatment values from the one-month post-treatment values. Complication grades for non-laboratory events were defined per the Society of Interventional Radiology reporting standards [27], biological value escalations at 1 day and 1 month were graded based on the National Cancer Institute (NCI) Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0 [28].
Statistical analysis
For analysis patients were divided into total bilirubin serum levels chosen a priori: < 2, 2–3, and > 3 mg/dL as well as direct bilirubin < 1 and 1–2 mg/dL. Two-sample t-tests, one-way ANOVAs, and Fisher exact tests were utilized as appropriate. The continuous total and direct bilirubin values were used for the prediction portion of the analysis. A multinomial model utilizing Akaike Information Criterion (AIC) was utilized to determine whether direct or total bilirubin better predicted whether or not a subject had any complications at 1 month. To determine which measure was a better predictor for time to progression and overall survival, AIC was calculated from the Cox regression models. Linear regression models using root mean squared error (RMSE) were utilized to determine whether direct or total bilirubin more accurately predicted changes in liver enzymes. Smaller AIC and RSME numbers indicate superior prediction. R Version 3.4.1 was used for the analysis and a P-value < 0.05 were considered to indicate significant differences.
Results
The selectivity of TACE was hemiliver in 29 (29/353, 8.1%), less than hemiliver but greater than sectional in 50 (50/353, 14.2%), sectional in 140 (140/353, 39.7%) and segmental in 134 (134/353, 38%) patients. Radiologic response following TACE is reported in Table 2; pretreatment total bilirubin or direct bilirubin, did not vary significantly by radiographic response. The comparison of the total and direct bilirubin serum level for those who lived and did not live 6 and 12 months are reported in Table 3.
Table 2.
Radiologic response as measured by the European Association of the Study of the Liver (EASL) criteria of 353 hepatocellular carcinomas treated with transarterial chemoembolization.
| Variable | Progressive disease | Stable disease | Partial response | Complete response |
|---|---|---|---|---|
| Initial response | 8 (8/353; 2.2%) | 64 (64/353; 18.1%) | 85 (85/353; 24.1%) | 196 (196/353; 55.5%) |
| Max response | 8 (8/353; 2.2%) | 38 (38/353; 10.8%) | 77 (77/353; 21.8%) | 230 (230/353; 65.2%) |
| ORR by total bilirubin | ||||
| Total bilirubin < 2 | Total bilirubin 2–3 | Total bilirubin > 3 | P-value | |
| Initial response | 0.58 | |||
| Yes | 236 (236/293; 80.5%) | 34 (34/45; 75.6%) | 9 (9/12; 75%) | |
| No | 57 (57/293; 19.5%) | 11 (11/45; 24.4%) | 3 (3/12; 25%) | |
| Max response | ||||
| Yes | 259 (259/293; 88.4%) | 36 (36/45; 80%) | 10 (10/12; 83.3%) | |
| No | 34 (34/293; 11.6%) | 9 (9/45; 20%) | 2 (2/12; 16.7%) | 0.22 |
| ORR by direct bilirubin | ||||
| Direct bilirubin < 1 | Direct bilirubin 1–2 | P-value | ||
| Initial response | 0.78 | |||
| Yes | 255 (255/318; 80.2%) | 17 (17/20; 85%) | ||
| No | 63 (63/318; 19.8%) | 3 (3/20; 15%) | ||
| Max response | >0.99 | |||
| Yes | 278 (278/318; 87.4%) | 18 (18/20; 90%) | ||
| No | 40 (40/318; 12.6%) | 2 (2/20; 10%) | ||
ORR: Overall radiologic response. Variables are expressed as raw numbers. Numbers in parentheses are proportions followed by percentages.
Table 3.
Univariate analysis of total and direct bilirubin serum level for survival at 6 and 12 months.
| Six month survival | |||
|---|---|---|---|
| Variable | Did not survive (n = 29) | Survived (n = 191) | P-value |
| Mean total bilirubin serum level (mg/dL) | 1.54 ± 0.99 [0.3–3.75] | 1.27 ± 0.70 [0.3–3.9] | 0.16 |
| Mean direct bilirubin (mg/dL) | 0.58 ± 0.46 [< 0.1–1.8] | 0.40 ± 0.31 [< 0.1–1.6] | 0.04 |
| 12 month survival | |||
| Variable | Did not survive (n = 78) | Survived (n = 142) | P-value |
| Mean total bilirubin (mg/dL) | 1.46 ± 0.87 [0.3–3.9] | 1.22 ± 0.65 [0.3–3.9] | 0.03 |
| Mean direct bilirubin (mg/dL) | 0.49 ± 0.38 [< 0.1–1.8] | 0.38 ± 0.32 [< 0.1–1.6] | 0.03 |
Variables are expressed as means ± standard deviations, followed by ranges in brackets.
Direct bilirubin serum level was significantly greater in the cohort of patients who did not survive than in those who survived at 6 months ([0.58 ± 0.46 (SD) mg/dL; range: < 0.1–1.8 mg/dL] vs. [0.40 ± 0.31 (SD) mg/dL; range: < 0.1–1.6 mg/dL], respectively) (P = 0.04) and at 12 months ([0.49 ± 0.38 (SD) mg/dL; range: < 0.1–1.8 mg/dL] vs. [0.38 ± 0.32 (SD) mg/dL; range: < 0.1–1.6 mg/dL], respectively) (P = 0.03).
Total bilirubin serum level was not available for one patient, who was excluded from this portion of the analysis. Mean total bilirubin serum level was not significantly different between patients who did not survive (1.54 ± 0.99 [SD] mg/dL; range: 0.3–3.9 mg/dL) and those who did survive (1.27 ± 0.70 [SD] mg/dL; range: 0.3–3.75 mg/dL) at 6 months (P = 0.16). By contrast, mean total bilirubin serum level was significantly greater in patients who did not survive at 12 months (1.46 ± 0.87 [SD] mg/dL; range: 0.3–3.9 mg/dL) than in those who did not survive at 12 months (1.22 ± 0.65 [SD] mg/dL; range: 0.3–3.9 mg/dL) (P = 0.03).
Next patients were divided according to total bilirubin serum level at the time of TACE, (total bilirubin < 2 mg/dL (179/219, 81.7%), 2–3 mg/dL (30/219, 13.7%), and > 3 mg/dL (10/219, 4.5%). A logistic regression analysis revealed that the 2–3 mg/dL total bilirubin group did not differ significantly in 6 months survival compare to < 2 mg/dL group (OR 0.86; P = 0.80). However, patients with total bilirubin ≥ 3 mg/dL were less likely to survive 6 months as compared to those with total bilirubin < 2 mg/dL (OR 0.20; P = 0.02). When using the same model for direct bilirubin after dividing into two cohorts (direct bilirubin < 1 (193/220, 87.7%) or 1–2 (17/220, 12.3%) mg/dL), the 1–2 mg/dL cohort were less likely to survive 6 months (OR 0.32; P = 0.04). Patients with total bilirubin between 2–3 mg/dL did not significantly vary in 12 month survival as compared to those with a total bilirubin < 2 mg/dL (OR 0.63; P = 0.25) However, patients with a total bilirubin ≥3 mg/dL did have significantly poorer 12 month survival as compared to patients with a total bilirubin < 2 mg/dL (OR 0.21; P = 0.03). Patients with a direct bilirubin < 1 mg/dL 12 month survival did not significantly vary when compared to those with a direct bilirubin of 1–2 mg/dL (OR 0.45; P = 0.11).
Finally, the ability to predict overall survival following TACE of direct and total bilirubin concentration was compared utilizing an AIC model. This analysis revealed that direct bilirubin level (AIC = 941.19) more accurately predicted overall survival than total bilirubin (AIC = 1000.51). If the type of TACE utilized was considered than direct bilirubin (AIC = 579.8) continued to outperform total bilirubin (AIC = 627.2) in the cTACE only cohort and also those who underwent DEB-TACE (AIC = 96.7 for direct vs. AIC = 103.5 for total bilirubin).
Complications are reported in Table 4. The most common complication was fatigue occurring in 37 (37/219,16.9%) patients. When evaluating whether direct or total bilirubin was most predictive of complications the AIC model was again utilized. This analysis again revealed that direct bilirubin (AIC = 352.22) outperformed total bilirubin (AIC = 357.42). Direct bilirubin continued to outperform total bilirubin when only cTACE (direct bilirubin AIC = 518.4 vs. total bilirubin AIC = 526.5) or DEB-TACE (direct bilirubin AIC = 112.7 vs. total bilirubin AIC = 114.6) were considered. Complications were also evaluated through a change in liver enzymes. The change in liver enzymes at 1 day and 1 month as well as the ratio of enzymes at 1 month are reported in Table 5. The RMSE analysis to identify whether total or direct bilirubin serum level more accurately predicted changes in enzymes is reported in Table 6. Total bilirubin serum level outperformed direct bilirubin serum level in the ability to predict enzymatic change at 1 day and 1 month. Grade of complications are described in Table 7. There were no significant differences in grade of complications, either enzyme based or non-laboratory based, by either total or direct bilirubin.
Table 4.
Complications experienced at 1 month in 219 patients who underwent transarterial chemoembolization of 353 hepatocellular carcinomas.
| Complication | |
|---|---|
| Fatigue | 37 (37/219; 16.9%) |
| Pain requiring analgesics | 15 (15/219; 6.8%) |
| Ascites development | 15 (15/219; 6.8%) |
| Alopecia | 5 (5/219; 2.3%) |
| Hepatic encephalopathy | 5 (5/219; 2.3%) |
| Other | 13 (13/219; 5.9%) |
Variables are expressed as raw numbers. Numbers in parentheses are proportions followed by percentages.
Table 5.
Change in liver enzymes at 1 day and 1 month in 219 patients undergoing transarterial chemoembolization for hepatocellular carcinoma.
| Total bilirubin serum level | ||||
|---|---|---|---|---|
| Variable | TB < 2 (n = 179) |
TB 2–3 (n = 30) |
TB > 3 (n = 10) |
P-value |
| Change AST, 1 day (U/L) | 4.4 ± 8 [0.1–65.9] | 1.8 ± 1.7 [0.2–10.1] | 2.8 ± 3.1 [0.8–11.8] | 0.5 |
| Change ALT, 1 day (U/L) | 2.9 ± 5.2 [0.1–60.7] | 1.6 ± 1.7 [0.2–11.8] | 2 ± 1.9 [0.8–7.4] | 0.1 |
| Change AST, 1 month (U/L) | 1.2 ± 1.2 [0–13] | 0.9 ± 0.3 [0–1.3] | 1.1 ± 0.3 [0.8–1.6] | 0.32 |
| Change ALT, 1 month (U/L) | 1.1 ± 1.6 [0.1–18.3] | 0.8 ± 0.3 [0–1.7] | 0.8 ± 0.3 [0.13–1.2] | 0.47 |
| Direct bilirubin serum level | ||||
| Variable | DB < 1 (n = 197) |
DB 1–2 (n = 17) |
P-value | |
| Change AST, 1 day (U/L) | 3.7 ± 6.1 [0.1–65.9] | 2.3 ± 2.8 | 0.36 [0.9–11.8] | |
| Change ALT, 1 day (U/L) | 2.6 ± 5.1 [0.1–60.7] | 2.3 ± 3.0 | 0.85 [0.7–11.8] | |
| Change AST, 1 month (U/L) | 1.1 ± 1.0 [0–13] | 1.0 ± 0.4 | 0.79 [0–1.7] | |
| Change ALT, 1 month (U/L) | 1.0 ± 1.3 [0.1–18.3] | 0.8 ± 0.4 | 0.59 [0–1.6] | |
AST: aspartate aminotransferase; ALT: alanine transaminase; TB: total bilirubin; DB: direct bilirubin. Quantitative variables are expressed as mean ± standard deviations, followed by ranges in brackets.
Table 6.
Root men square error (RMSE) evaluation of total versus birect bilirubin serum levels ability to predict enzymatic change.
| Response variable | RMSE (total bilirubin) | RMSE (direct bilirubin) |
|---|---|---|
| Change in AST 1 day | 7.1598 | 7.3576 |
| Change in AST 1 month | 1.1117 | 1.1245 |
| Change in ALT 1 day | 4.7640 | 4.8887 |
| Change in ALT 1 month | 1.4433 | 1.4711 |
AST: aspartate aminotransferase; ALT: alanine transaminase. RMSE modeling to compare the ability of pre-transarterial chemoembolization total or direct bilirubin ability to predict post treatment change in AST and ALT at 1 day and 1 month time points.
Table 7.
Complication by grade in 219 patients treated with transarterial chemoembolization for hepatocellular carcinoma.
| Total bilirubin | ||||
|---|---|---|---|---|
| Variable | TB < 2 (n = 179) |
TB 2–3 (n = 30) |
TB > 3 (n = 10) |
P-value |
| Non-laboratory based complications | 0.66 | |||
| None | 125 (125/179; 69.8%) | 18 (18/30; 60%) | 8 (8/10; 80%) | |
| Grade 1 | 43 (43/179; 24%) | 8 (8/30; 26.7%) | 2 (2/10; 20%) | |
| Grade 2 | 11 (11/179; 6.1%) | 4 (4/30; 13.3%) | 0 (0/10; 0%) | |
| Grade 3 | 2 (2/179; 1.1%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 4 | 0 (0/179; 0%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 5 | 0 (0/179; 0%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Total bilirubin change at 1 day | 0.71 | |||
| None | 174 (174/179; 97.1%) | 29 (29/30; 96.7%) | 10 (10/10; 100%) | |
| Grade 1 | 3 (3/179; 1.7%) | 1 (1/30; 3.3%) | 0 (0/10; 0%) | |
| Grade 2 | 1 (1/179; 0.6%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 3 | 1 (1/179; 0.6%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 4 | 0 (0/179; 0%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Total bilirubin change at 1 montha | 0.12 | |||
| None | 172 (172/179; 96%) | 28 (28/30; 93.4%) | 9 (9/10; 90%) | |
| Grade 1 | 5 (5/179; 2.8%) | 1 (1/30; 3.3%) | 1 (1/10; 10%) | |
| Grade 2 | 0 (0/179; 0%) | 1 (1/30; 3.3%) | 1 (1/10; 10%) | |
| Grade 3 | 1 (1/179; 0.6%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 4 | 1 (1/179; 0.6%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| AST change at 1 daya | 0.63 | |||
| None | 99 (99/179; 55.3%) | 21 (21/30; 70%) | 5 (5/10; 50%) | |
| Grade 1 | 28 (28/179; 15.7%) | 4 (4/30; 13.3%) | 1 (1/10; 10%) | |
| Grade 2 | 21 (21/179; 11.7%) | 3 (3/30; 10%) | 2 (2/10; 20%) | |
| Grade 3 | 35 (35/179; 19.6%) | 2 (2/30; 6.7%) | 3 (3/10; 30%) | |
| Grade 4 | 8 (8/179; 4.5%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| AST change 1 month | 0.66 | |||
| None | 163 (163/179; 91.1%) | 30 (30/30; 100%) | 9 (9/10; 90%) | |
| Grade 1 | 9 (9/179; 5%) | 0 (0/30; 0%) | 1 (1/10; 10%) | |
| Grade 2 | 5 (5/179; 2.8%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 3 | 1 (1/179; 1.1%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 4 | 0 (0/179; 0%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| ALT change 1 daya | 0.48 | |||
| None | 140 (140/179; 78.2%) | 28 (28/30; 93.4%) | 7 (7/10; 70%) | |
| Grade 1 | 15 (15/179; 8.4%) | 0 (0/30; 0%) | 2 (2/10; 20%) | |
| Grade 2 | 18 (18/179; 10%) | 1 (1/30; 3.3%) | 1 (1/10; 10%) | |
| Grade 3 | 9 (9/179; 5.1%) | 1 (1/30; 3.3%) | 0 (0/10; 0%) | |
| Grade 4 | 0 (0/179; 0%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| ALT change 1 month | 0.99 | |||
| None | 174 (174/179; 97.2%) | 30 (30/30; 100%) | 10 (10/10; 100%) | |
| Grade 1 | 4 (4/179; 2.2%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 2 | 0 (0/179; 0%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 3 | 1 (1/179; 0.6%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Grade 4 | 0 (0/179; 0%) | 0 (0/30; 0%) | 0 (0/10; 0%) | |
| Direct Bilirubin | ||||
| Variable | DB < 1 (n = 197) |
DB 1–2 (n = 17) |
P-value | |
| Non-laboratory based complicationsa | 0.58 | |||
| None | 135 (135/197; 68.5%) | 11 (11/17; 64.7%) | ||
| Grade 1 | 47 (47/197; 23.9%) | 6 (6/17; 35.3%) | ||
| Grade 2 | 15 (15/197; 7.6%) | 0 (0/17; 0%) | ||
| Grade 3 | 2 (2/197; 1%) | 0 (0/17; 0%) | ||
| Grade 4 | 0 (0/197; 0%) | 0 (0/17; 0%) | ||
| Grade 5 | 0 (0/197; 0%) | 0 (0/17; 0%) | ||
| Total bilirubin change at 1 day | 0.99 | |||
| None | 191 (191/197; 97%) | 17 (17/17; 100%) | ||
| Grade 1 | 4 (4/197; 2%) | 0 (0/17; 0%) | ||
| Grade 2 | 1 (1/197; 0.5%) | 0 (0/17; 0%) | ||
| Grade 3 | 1 (1/197; 0.5%) | 0 (0/17; 0%) | ||
| Grade 4 | 0 (0/197; 0%) | 0 (0/17; 0%) | ||
| Total bilirubin change at 1 montha | 0.69 | |||
| None | 189 (189/197; 96%) | 15 (15/17; 88.2%) | ||
| Grade 1 | 5 (5/197; 2.5%) | 2 (2/17; 11.8%) | ||
| Grade 2 | 1 (1/197; 0.5%) | 1 (1/17; 5.9%) | ||
| Grade 3 | 1 (1/197; 0.5%) | 0 (0/17; 0%) | ||
| Grade 4 | 1 (1/197; 0.5%) | 0 (0/17; 0%) | ||
| AST change at 1 daya | 0.69 | |||
| None | 111 (111/197; 56.3%) | 11 (11/17; 64.7%) | ||
| Grade 1 | 30 (30/197; 15.2%) | 3 (3/17; 17.6%3) | ||
| Grade 2 | 23 (23/197; 11.7%) | 2 (2/17; 11.8%) | ||
| Grade 3 | 35 (35/197; 17.8%) | 2 (2/17; 11.8%) | ||
| Grade 4 | 5 (5/197; 2.5%) | 0 (0/17; 0%) | ||
| AST change 1 month | 0.32 | |||
| None | 183 (183/197; 92.9%) | 15 (15/17; 88.2%) | ||
| Grade 1 | 9 (9/197; 4.6%) | 1 (1/17; 5.9%) | ||
| Grade 2 | 3 (3/197; 1.5%3) | 1 (1/17; 5.9%) | ||
| Grade 3 | 2 (2/197; 1%) | 0 (0/17; 0%) | ||
| Grade 4 | 0 (0/197; 0%) | 0 (0/17; 0%) | ||
| ALT change 1 daya | 0.95 | |||
| None | 157 (157/197; 79.7%) | 14 (14/17; 82.4%) | ||
| Grade 1 | 15 (15/197; 7.6%) | 1 (1/17; 5.9%) | ||
| Grade 2 | 19 (19/197; 9.6%) | 1 (1/17; 5.9%) | ||
| Grade 3 | 9 (9/197; 4.6%) | 1 (1/17; 5.9%) | ||
| Grade 4 | 0 (0/197; 0%) | 0 (0/17; 0%) | ||
| ALT change 1 month | 0.99 | |||
| None | 192 (192/197; 97.5%) | 17 (17/17; 100%) | ||
| Grade 1 | 4 (4/197; 2%) | 0 (0/17; 0%) | ||
| Grade 2 | 0 (0/197; 0%) | 0 (0/17; 0%) | ||
| Grade 3 | 1 (1/197; 0.5%) | 0 (0/17; 0%) | ||
| Grade 4 | 0 (0/197; 0%) | 0 (0/17; 0%) | ||
AST: aspartate aminotransferase; ALT: aminotransferase; TB: total bilirubin; DB: direct bilirubin. Variables are expressed as raw numbers. Numbers in parentheses are proportions followed by percentages.
Indicates some patients had more than one complication during multiple treatments of their HCC.
Discussion
Our study found that direct bilirubin serum level was greater in patients not surviving 6 months, than those who did, on both univariate and logistic regression analysis. Furthermore, direct bilirubin outperformed total bilirubin in the AIC model suggesting it is a better predictor of hepatic reserve and post-TACE survival. Direct bilirubin seems to particularly excel at differentiating patients who will survive only short periods of times (more or less than 6 months), a meaningful ability when trying to bridge patients to transplant. This may suggest that using only total bilirubin would exclude some patients from undergoing TACE who could benefit from this treatment, particularly if only needing to be bridged to transplant for a relatively short period of time. Maintaining patients within Milan criteria is particularly important in regions of the world where transplant wait list times are lengthy. In this setting, disease often presents or progresses during this wait time and the disease must be controlled in order for the patient to receive orthotopic liver transplantation. If total bilirubin alone is utilized, the findings of this paper would suggest that those patients with lower direct bilirubin levels are suffering from under treatment.
The ability of a patient to gain benefit from TACE for HCC treatment is in part based on hepatic reserve. This has traditionally been judged in large part by total serum bilirubin concentration, with several studies demonstrating that a level > 3 mg/dL is associated with greater morbidity and mortality [3–10,29,30]. However, the limited treatment options in this patient population has led several authors to attempt TACE in this patient cohort, with promising results [18–21]. This would suggest more sophisticated patient selection tools are needed. Direct bilirubin, which many regard as a more reliable liver function test may provide this tool [23].
Another important consideration in high-risk patients is complications. Here again, direct bilirubin concentration outperformed total bilirubin in the AIC modeling. While total bilirubin did outperform direct bilirubin in terms of predicting changes in AST and ALT on RMSE modeling at 1 day and 1 month, these findings are of limited benefit by themselves. The value of AST and ALT in this setting is their correlation with complications. This would suggest that again direct bilirubin is more accurate in predicting post TACE outcomes. Neither total bilirubin nor direct bilirubin was found to predict complication grade. This may be secondary to the limited sample size, or a result of the focus on superselective therapy when performing TACE.
Other authors have evaluated methods other than total bilirubin, which may help to determine hepatic reserve as well. Indocyanine green has been evaluated for its ability to predict outcomes following TACE [31]. However, while minimally invasive, this does require extra testing, a disadvantage as compared to the use of direct bilirubin, which simply requires further analysis of standard blood work. Others have found that young patients with small lesions and relatively low body mass indexes tend to be more tolerant of treatment than older patients with more extensive disease and elevated body mass indexes [32]. However, while these factors are important, they fail to address the primary concern of hepatic reserve.
This study has a number of limitations including its retrospective design. There were a limited number of patients with elevated bilirubin treated with TACE and this limitation is compounded by the fact that not all variables were available for all patients. Furthermore, the study was performed at a quaternary referral center, which may not reflect all practices. Finally, the quarternary referral pattern means that some complications may have been missed as the patients may have presented to outside hospitals.
In conclusion, direct bilirubin concentration appears to be more accurate at predicting patients’ ability to tolerate TACE than total bilirubin concentration. Patients with hyperbilirubinemia but relatively maintained direct bilirubin levels should be considered for TACE, especially in the setting of bridging to transplant. However, prospective studies are required to confirm this and to shed further light on cut off points for direct bilirubin.
Acknowledgements
Research reported in this publication was supported by NIH grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of interest
Shamar Young is consultant for BTG/Boston Scientific Group. The other authors declare that they have no competing interest.
References
- [1].Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 2002;359:1734–9. [DOI] [PubMed] [Google Scholar]
- [2].Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164–71. [DOI] [PubMed] [Google Scholar]
- [3].Bouchard-Fortier A, Lapointe R, Perreault P, Bouchard L, Pomier-Layrargues G. Transcatheter arterial chemoembolization of hepatocellular carcinoma as a bridge to liver transplantation: a retrospective study. Int J Hepatol 2011;2011:974514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chan AO, Yuen MF, Hui CK, Tso WK, Lai CL. A prospective study regarding the complications of transcatheter intraarterial lipiodol chemoembolization in patients with hepatocellular carcinoma. Cancer 2002;94:1747–52. [DOI] [PubMed] [Google Scholar]
- [5].Ray JRCE, Brown AC, Green TJ, Winston H, Curran C, Kreidler SM, et al. Survival outcomes in patients with advanced hepatocellular carcinoma treated with drug-eluting bead chemoembolization. AJR Am J Roentgenol 2015;204:440–7. [DOI] [PubMed] [Google Scholar]
- [6].Garwood ER, Fidelman N, Hoch SE, Kerlan RK Jr, Yao FY. Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and synthetic hepatic dysfunction. Liver Transpl 2013;19:164–73. [DOI] [PubMed] [Google Scholar]
- [7].Huang YS, Chiang JH, Wu JC, Chang FY, Lee SD. Risk of hepatic failure after transcatheter arterial chemoemblization for hepatocellular carcinoma: predictive value o the monoethylglycinexylidide test. Am J Gastroenterol 2002;97:1223–7. [DOI] [PubMed] [Google Scholar]
- [8].Jeon SH, Park KS, Kim YH, Shin YS, Kang MK, Jang BK, et al. Incidence and risk factors of acute hepatic failure after transcatheter arterial chemoembolization for hepatocellular carcinoma. Korean J Gastroenterol 2007;50:176–82. [PubMed] [Google Scholar]
- [9].Nouso K, Ito Y, Kuwaki K, Kobayashi Y, Nakamura S, Ohashi Y, et al. Prognostic factors and treatment effects for hepatocellular carcinoma in child C cirrhosis. Br J Cancer 2008;98:1161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Olivo M, Valeenza F, Buccellato A, Scala L, Virdone R, Sciarrino E, et al. Transcatheter arterial chemoemblisation for hepatocellular carcinoma in cirrhosis: survivial rate and prognostic factors. Dig Liver Dis 2010;42:515–9. [DOI] [PubMed] [Google Scholar]
- [11].National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: hepatobiliary cancers version 2; 2012. [Accessed 2/29/2019] http://www.nccn.org/professionals/physiciangls/pdf/hepatobiliary.pdf. [DOI] [PubMed]
- [12].Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [13].Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of Sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [14].Balogh J, Victor D 3rd, Aham EH, Burroughs SG, Boktour M, Saharia A, et al. Hepatocellular carcinoma: a review. J Hepatocell Carcinoma 2016;3:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beal EW, Dittmar KM, Hanje AJ, Michaels AJ, Conteh L, Davidson G, et al. Pretransplant locoregional therapy for hepatocellular carcinoma: evaluation of explant pathology and overall survival. Front Oncol 2016;6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Frenette CT, Osorio RC, Stark J, Fok B, Boktour MR, et al. Conventional TACE and drug-eluting bead TACE as locoregional therapy before orthotopic liver transplantation: comparison of explant pathologic response. Transplantation 2014;98:781–7. [DOI] [PubMed] [Google Scholar]
- [17].Vogl TJ, Naquib NN, Nour-Eldin NE, Rao P, Emami AH, Zangos S, et al. Review on transarterial chemoembolization in hepatocellular carcinoma: palliative, combined, neoadjuvant, bridging, and symptomatic indications. Eur J Radiol 2009;72:505–16. [DOI] [PubMed] [Google Scholar]
- [18].Thorat A, Lee CF, Wu TH, Chan KM, Chou HS, Lee WC. Safety of transarterial chemoemblization as bridging therapy in HCC patients with hyperbilirubinemia on the waiting list for liver transplantation: a center experience. Hepatogastroenterology 2013;60:2076–9. [PubMed] [Google Scholar]
- [19].Kiely JM, Rilling WS, Touzios JG, Hieb RA, Franco J, Saeian K, et al. Chemoembolization in patients at high risk: results and complication. J Vasc Interv Radiol 2006;17:47–53. [DOI] [PubMed] [Google Scholar]
- [20].Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Ishikawa T, et al. Is there a survival benefit in interventional radiology for hepatocellular carcinoma in patients with Child-Pugh C liver cirrhosis? A multicenter study. Hepatol Res 2016;46:521–8. [DOI] [PubMed] [Google Scholar]
- [21].Massani M, Stecca T, Ruffolo C, Bassi N. Should we routinely use DEBTACE for unresectable HCC? cTACE versus DEBTACE: a single-center survival analysis. Updates Surg 2017;69:67–73. [DOI] [PubMed] [Google Scholar]
- [22].Bouvier A, Ozenne V, Aube C, Boursier J, Vullierme MP, Thouveny F, et al. Transarterial chemoembolization: effect of selectivity on tolerance, tumour response and survival. Eur Radiol 2011;21:1719–26. [DOI] [PubMed] [Google Scholar]
- [23].Ohkubo A Bilirubin metabolism in liver cirrhosis. Nihon Risho 1994;52:138–44. [PubMed] [Google Scholar]
- [24].Young S, Sanghvi T, Rubin N, Hall D, Roller L, Charaf Y, et al. Transarterial chemoembolization of hepatocellular carcinoma: propensity score matching study comparing survival and complications in patient with nonalcoholic steatohepatitis versus other causes cirrhosis. Cardiovasc Intervent Radiol 2019, http://dx.doi.org/10.1007/s00270-019-023630x. [DOI] [PubMed]
- [25].Young S, Craig P, Golzarian J. Current trends in the treatment of hepatocellular carcinoma with transarterial embolization: a cross-sectional survey of techniques. Eur Radiol 2019;29:3287–95. [DOI] [PubMed] [Google Scholar]
- [26].Llovet JM, Ducreux M, Lencioni R, Di Bisceglie AM, Galle PR, Dufour JF, et al. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- [27].Khalilzadeh O, Baerlocher MO, Shyn PB, Connolly BL, Devane AM, Morris CS, et al. Proposal of a new adverse event classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol 2017;28: 1432–7. [DOI] [PubMed] [Google Scholar]
- [28].Grigorian A, O’Brien CB. Hepatotoxicity secondary to chemotherapy. J Clin Transl Hepatol 2014;2:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dhamija E, Paul SB, Gamanagatti SR, Acharya SK. Biliary complications of arterial chemoembolization of hepatocellular carcinoma. Diagn Interv Imaging 2015;96:1169–75. [DOI] [PubMed] [Google Scholar]
- [30].Boulin M, Delhom E, Pierredon-Foulongne MA, Cercueil JP, Guiu B. Transarterial chemoembolization for hepatocellular carcinoma: an old method, now flavor of the day. Diagn Interv Imaging 2015;96:607–15. [DOI] [PubMed] [Google Scholar]
- [31].Shalimar, Jain S, Gamanagatti SR, Kedia S, Thakur B, Nayak B, et al. Role of indocyanine green in predicting post-transarterial chemoembolization liver failure in hepatocellular carcinoma. J Clin Exp Hepatol 2018;8:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Eltawil KM, Berry R, Abdolell M, Molinari M. Analysis of survival preditors in a prospective cohort of patients undergoing transarterial chemoembolization for hepatocellular carcinoma in a single Candian centre. HPB (oxford) 2012;14:162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]


