Abstract
Aims
Left atrial (LA) remodelling is a common feature of many cardiovascular pathologies and is a sensitive marker of adverse cardiovascular outcomes. The aim of this study was to establish normal ranges for LA parameters derived from coronary computed tomographic angiography (CCTA) imaging using a standardised image processing pipeline to establish normal ranges in a previously described cohort.
Methods
CCTA imaging from 193 subjects recruited to the Budapest GLOBAL twin study was analysed. Indexed LA cavity volume (LACVi), LA surface area (LASAi), wall thickness and LA tissue volume (LATVi) were calculated. Wall thickness maps were combined into an atlas. Indexed LA parameters were compared with clinical variables to identify early markers of pathological remodelling.
Results
LACVi is similar between sexes (31 ml/m2 v 30 ml/m2) and increased in hypertension (33 ml/m2 v 29 ml/m2, p = 0.009). LASAi is greater in females than males (47.8 ml/m2 v 45.8 ml/m2 male, p = 0.031). Median LAWT was 1.45 mm. LAWT was lowest at the inferior portion of the posterior LA wall (1.14 mm) and greatest in the septum (median = 2.0 mm) (p < 0.001). Conditions known to predispose to the development of AF were not associated with differences in tissue thickness.
Conclusions
The reported LACVi, LASAi, LATVi and tissue thickness derived from CCTA may serve as reference values for this age group and clinical characteristics for future studies. Increased LASAi in females in the absence of differences in LACVi or LATVi may indicate differential LA shape changes between the sexes. AF predisposing conditions, other than sex, were not associated with detectable changes in LAWT.
Clinical trial registration:http://www.ClinicalTrials.gov/NCT01738828.
Abbreviations: AF, atrial fibrillation; BSA, body surface area; CCTA, cardiac computed tomography; DZ, dizygotic; LA, left atrium; LAA, left atrial appendage; LACV, left atrial cavity volume; LASA, left atrial surface area; LATV, left atrial tissue volume; LAWT, left atrial wall thickness; MZ, monozygotic; PV, pulmonary vein
Keywords: Computed tomography (CT), Left atrium, Tissue thickness
1. Introduction
The left atrium (LA) is a thin walled structure with an operating pressure of 4 – 12 mmHg [1], in health. A common feature of many cardiovascular pathologies is an increase in left atrial pressure. The increased LA load can lead to remodelling and is a sensitive marker of adverse cardiovascular events [2,3]. Remodelling in the LA is associated with atrial fibrillation (AF), the most common sustained adult arrhythmia, which confers a large burden of mortality and morbidity and imposes a huge healthcare economic cost [4].
Current techniques for evaluating the left atrium such as echocardiography are well suited to providing an estimate of area and volume but lack the spatial resolution to evaluate other characteristics of the left atrium that may be important. Recently coronary computed tomographic angiography (CCTA) has been used to assess left atrial wall thickness (LAWT). Changes in LAWT have been identified in various pathological conditions, including atrial fibrillation (AF) [5], and cyanotic congenital heart disease [6], as well as with normal aging [7], and may be an important aspect of atrial remodelling. Heterogeneity in LAWT throughout the chamber has been demonstrated in pathological [5], and imaging studies [8]. Despite this, reference in-vivo values for the healthy population have not been reported to provide a baseline for interpreting pathological changes.
The aim of the current study was to provide reference ranges for indexed LA cavity volume (LACVi), LA surface area (LASAi), LAWT and LA tissue volume (LATVi) in an asymptomatic population without previously documented cardiovascular disease. Using data from the BUDAPEST GLOBAL (Burden of atherosclerotic plaques study in twins - Genetic Loci and the Burden of Atherosclerotic Lesions) Study [9], the LA was characterised using CT imaging data. A previously validated strategy [10], [11], [40] was used to generate wall thickness maps of the LA to provide a comprehensive description of tissue thickness in three dimensions. These data were subsequently compared against clinical indices to consider whether LA remodelling could be identified through changes in atrial volume, atrial surface area or tissue thickness in an asymptomatic population.
2. Methods
2.1. Study design and study population
The BUDAPEST GLOBAL Study, a single-centre prospective trial, enrolled twin subjects from the Hungarian Twin Registry [12], [13], [40], with self-reported Caucasian ethnicity who had been co-enrolled into the international, multicentre Genetic Loci and the Burden of Atherosclerotic Lesions (GLOBAL) clinical study (NCT01738828) [9,14]. Altogether 202 twin participants, 122 monozygotic and 80, same-gender dizygotic twin siblings were included. Medical history and anthropomorphic measurements were collected as previously described [9]. All enrolled subjects provided written informed consent and the study was approved by the National Scientific and Ethics Committee (institutional review board number: ETT TUKEB 58401/2012/EKU [828/PI/12], Amendment-1: 12292/2013/EKU [165/2013]) and was carried out according to the principles stated in the Declaration of Helsinki. As per the study protocol, coronary CT angiography (CCTA) was carried out for research purposes.
Into this current substudy of the BUDAPEST GLOBAL study, 198 subjects were included (198 twin subjects comprising 59 monozygotic (MZ) and 40 same-sex dizygotic (DZ) twin pairs; 63% female). Subjects with non-diagnostic image quality or insufficient CT coverage of left atrium were excluded.
2.2. CT data acquisition
Every subject underwent prospectively ECG triggered CCTA evaluation using a 256-slice multidetector-row CT (Brilliance iCT, Philips HealthTech, Best, the Netherlands). The detailed scan protocol has been described previously [9]. Briefly, image acquisition was carried out during a single breath hold inspiration, in cranio-caudal direction in axial mode. The scanner settings were the followings: 128 mm × 0.625 mm collimation, 270 ms gantry rotation time, 100–120 kV tube voltage and 200–300 mAs tube current, depending on the study subjects’ BMI. Images were reconstructed with a slice thickness of 0.8 mm with 0.4 mm of increment, which resulted in an approximately 0.6 mm isotropic resolution. Imaging was acquired during a single inspiratory breath hold at 78% of the R-R interval, prior to atrial contraction.
2.3. Data analysis
2.3.1. 3D wall thickness maps
CCTA data were analysed as previously described to generate left atrial segmentations and 3D LAWT maps10. Regions with uncertain delineation of the epicardial border, preventing accurate tissue thickness assessment, most commonly occurring in the region of the left superior pulmonary vein and the left atrial appendage, were manually identified and excluded from analysis. Further details are provided in the supplementary materials.
Left atrial surface area and left atrial volume were automatically calculated within the CEMRG application from the left atrial blood pool segmentation, in order to estimate the endocardial left atrial surface area, following cropping of the pulmonary veins (PV) and left atrial appendage (LAA) using the CEMRG open source image processing toolkit (www.cemrg.co.uk), as shown in example in Fig. 1, which is based on the MITK library (www.mitk.org). Further details are provided in the supplementary materials. Left atrial tissue volume, defined as the total volume of atrial tissue comprising the wall of the left atrium, was estimated by multiplying the LA surface area (LASA) by the mean calculated tissue thickness. Further details are given in the supplementary materials.
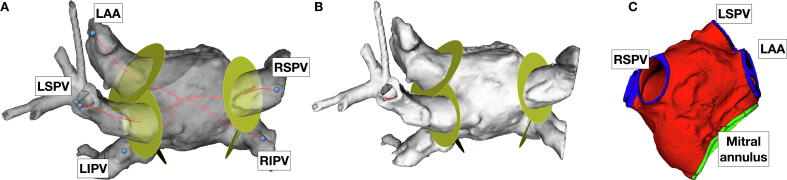
Fig. 1.
Standardization of identification of pulmonary vein ostia prior to removal from atrial wall mesh. Panel A: Posterior-anterior view of semi-transparent segmentation of left atrium with vessels and appendage attached. Seeds dropped at the left atrial appendage (LAA), left superior pulmonary vein (LSPV), left inferior pulmonary vein (LIPV), right superior pulmonary vein (RSPV) and right inferior pulmonary vein (RIPV) are used to calculate centre line to centre of mass of segmentation (red lines). Discs (yellow) identify position along centre line corresponding to criteria used to identify PV/LAA ostia. Panel B: Same segmentation as panel A, yellow discs indicate position along centre line identified as ostia of vessels. Panel C: Anterior-posterior view of mesh generated from segmentation after standardized cropping of pulmonary veins and LAA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.3.2. Atlas creation
Data from all of the processed CCTA scans were combined into an atlas based on a standard atrial geometry. This is shown in Fig. 2. From the atlas regional tissue thickness was assessed in five locations, defined manually as the anterior aspect, the septum, the roof and the superior and inferior posterior wall.
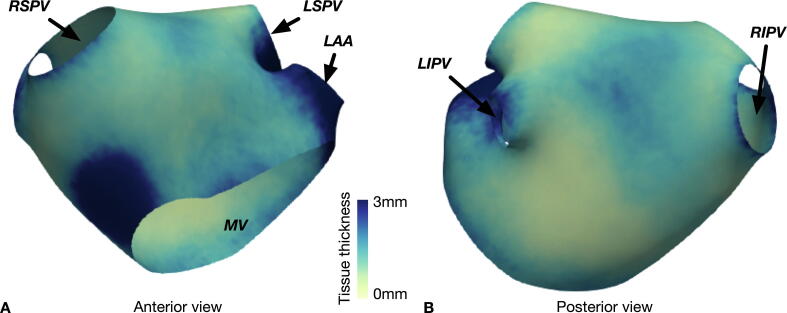
Fig. 2.
Atlas generated from atrial wall thickness measurements. Panel A: Anterior view of left atrium showing increased tissue thickness measurements in the septum. Panel B: Posterior view of the left atrium, in which increased tissue thickness measurements are seen in the superior aspect compared to the inferior aspect.
2.4. Statistical analysis
All data were assessed for normality using the Shapiro-Wilk’s test. Median and range are reported for non-normally distributed data, in addition 5 – 95% range is reported for tissue thickness data. Left atrial parameters between different groups were compared using the non-parametric Mann-Whitney U test or the Kruskal-Wallis 1-way ANOVA with subsequent Bonferroni correction for multiple comparisons in the event of a statistically significant result. Multivariate linear regression analysis was carried out using the clinical characteristics considered as predictor variables of indexed left atrial tissue parameters. Body surface area was calculated according to the Mostella formula [15], and BSA normalised LASA, LACV and LATV were calculated by dividing the relevant parameter by the calculated BSA. A result was considered statistically significant at the 5% significance level (p < 0.05).
3. Results
All participants provided written, informed consent prior to enrolment. 5 subjects were excluded due to imaging artefact or incomplete LA coverage. The final analysed cohort included 56 MZ and 38 same-sex DZ twin pairs and 5 unpaired subjects (2 from a DZ twin pair and 3 from a MZ twin pair). Baseline demographic and clinical variables are described in the supplementary materialsl (Table 1SM). It is noted that the median age of the male subjects (53, range 37 – 74) was lower than the female subjects (58, range 36 – 73) included in the analysis. For multivariate linear regression indexed left atrial tissue parameters did not require transformation for normality. Results from multivariate regression analysis are presented in Table 2, Table 3.
Table 2.
Multivariate linear regression incorporating clinical characteristics as predictors of median left atrial wall thickness assessed from CCTA.
| Median LAWT |
|||
|---|---|---|---|
| Predictors | Estimates | CI | p |
| (Intercept) | 0.44 | 0.31 – 0.56 | <0.001 |
| Female | −0.13 | −0.25 – -0.01 | 0.031 |
| Smoker | −0.15 | −0.27 – -0.03 | 0.015 |
| Diabetes | −0.09 | −0.29 – 0.11 | 0.386 |
| CAD | −0.13 | −0.27 – 0.01 | 0.065 |
| Hypertension | 0.09 | −0.03 – 0.21 | 0.148 |
| Observations | 193 | ||
| R2 / R2 adjusted | 0.070 / 0.040 | ||
Table 3.
Multivariate linear regression incorporating clinical characteristics as predictors of indexed left atrial parameters assessed from CCTA.
| LASAi |
LACVi |
LATVi |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | Estimates | CI | p | Estimates | CI | p | Estimates | CI | p |
| (Intercept) | 4527.59 | 4300.58 – 4754.60 | <0.001 | 29707.08 | 27391.87 – 32022.30 | <0.001 | 8.38 | 7.53 – 9.23 | <0.001 |
| Female | 282.48 | 73.12 – 491.84 | 0.008 | 372.19 | −1763.03 – 2507.42 | 0.731 | −0.13 | −0.92 – 0.66 | 0.741 |
| Smoker | 128.76 | −80.35 – 337.87 | 0.226 | 1362.02 | −770.64 – 3494.69 | 0.209 | −0.80 | −1.59 – -0.02 | 0.045 |
| Diabetes | −471.77 | −830.23 – -113.30 | 0.010 | −2295.41 | −5951.37 – 1360.54 | 0.217 | −1.29 | −2.64 – 0.05 | 0.060 |
| CAD | −44.92 | −286.34 – 196.49 | 0.714 | −943.88 | −3406.03 – 1518.27 | 0.450 | −0.52 | −1.43 – 0.38 | 0.257 |
| Hypertension | 194.40 | −15.51 – 404.32 | 0.069 | 3075.00 | 934.08 – 5215.91 | 0.005 | 0.77 | −0.02 – 1.55 | 0.057 |
| Observations | 193 | 193 | 193 | ||||||
| R2 / R2 adjusted | 0.079 / 0.049 | 0.055 / 0.025 | 0.059 / 0.029 | ||||||
3.1. CT derived values
Median left atrial surface area (LASA) was 88 cm2 (range 55 – 143 cm2). After correcting for body surface area (BSA) median LASAi was 47 cm2/m2 (range 27 – 67 cm2/m2). Overall left atrial cavity volume (LACV) was 58 ml (range 26 – 124 ml) and, after correcting for BSA, LACVi was 30 ml/m2 (range 12 – 50 ml/m2). Median left atrial tissue volume (LATV) was 13.9 ml (range = 5.4 – 38.5 ml) and, after correcting for BSA, LATVi was 7.5 ml/m2 (range = 2.5 – 16.1 ml/m2). These values are shown in Table 1.
Table 1.
Left atrial parameters assessed by CCTA in study participants.
| Left atrial surface area | Left atrial cavity volume | Left atrial tissue mass | |
|---|---|---|---|
| Median | 88 cm2 | 58 ml | 13.9 ml |
| Range | 55 – 143 cm2 | 26 – 124 ml | 5.4 – 38.5 ml |
| BSA normalised median | 47 cm2/m2 | 30 ml/m2 | 7.5 ml/m2 |
| BSA normalised range | 27 – 67 cm2/m2 | 12 – 50 ml/m2 | 2.5 – 16.1 ml/m2 |
Overall median tissue thickness in the left atrium calculated from the registered meshes was 1.45 mm. LAWT was dependent on the location: LAWT was lowest at the inferior portion of the posterior LA wall (median = 1.14 mm, range 0.57 – 5.99 mm, 5 – 95% range 0.72 – 2.71 mm), then the roof (median = 1.55 m, range 0.56 – 15.19 mm, 5 – 95% range 0.70 – 3.72), anterior aspect (median = 1.65 mm, 0.71 – 5.76 mm, 5 – 95% range 0.83 – 2.99 mm), superior portion of the posterior wall (median = 1.73 mm, 0.58 – 11.79, 5 – 95% range 0.84 – 2.89 mm) and greatest in the septum (median = 2.0 mm, 0.83 – 6.49 mm, 5 – 95% range 1.07 – 3.44 mm) (p < 0.001). Pairwise comparisons were performed with a Bonferroni correction for multiple comparisons and demonstrated that measurements were significantly different between all regions except between the roof and anterior wall (p = 0.612) and between anterior aspect and superior portion of the posterior wall (p > 0.999).
3.2. Group differences
3.2.1. Sex
Median height and weight were lower in female than in male subjects (weight = 68.7 kg v 86.9 kg, p < 0.001; height = 161 cm v 177 cm, p < 0.001). LACV was greater in male than female subjects (61 ml v 53 ml, p = 0.001), however, LACVi was not significantly different between the sexes (31 ml v 30 ml, p = 0.02). LASA was greater in male than female subjects (93 cm2 v 85 cm2, p < 0.001, however LASAi was greater in female than in male subjects (female = 47.8 ml/m2 v 45.8 ml/m2 male, p = 0.031). Sex maintained statistical significance as a predictor of LASAi in multivariate regression analysis (p = 0.008, Table 3).
Median LAWT showed a trend towards being lower in female than in male subjects that did not reach statistical significance in univariate analysis (1.22 mm v 1.31 mm, p = 0.055). When adjusted for the impact of other predictors in multivariate regression analysis, female sex was a significant predictor of median LAWT (p = 0.031, Table 2). The anterior aspect of the left atrial wall was thinner in female (median = 1.60 mm, range 0.71 – 5.76 mm) than male (median = 1.85 mm, range 0.81 – 5.76 mm). There were no other differences between sexes in regional tissue thickness. Overall LATV was greater in male than female subjects (15.6 ml v 13.5 ml, p = 0.006), however there was no difference in BSA normalised LATVi (female = 7.5 ml/m2 v 7.4 ml/m2 male, p = 0.852). These data are shown in the supplementary materials (Table 2SM) and illustrated in Fig. 3.
Fig. 3.
Comparison of CT derived left atrial parameters between female and male subjects.
3.2.2. Hypertension
LASA and LACV were greater in hypertensive (median LASA = 90.5 cm2 range 57.3 – 143.5 cm2; median LACV = 60.9 ml, range 28.6 – 124.3 ml) than normotensive (median LASA = 83.8 cm2, range 55.5 – 122.0 cm2; median LAV = 51.3 ml, range 25.6 -= 100.9 ml) subjects (p = 0.007 and p = 0.002 respectively). After normalising for BSA, LACVi remained significantly higher in hypertensive (33 ml/m2) than normotensive (29 ml/m2, p = 0.009) subjects, a difference which persisted in multivariable analysis (p = 0.005), while LASAi was not significantly different (hypertensive = 48 cm2/m2 v 47 cm2/m2 normotensive, p = 0.158) and tissue thickness was not significantly different between the groups (hypertensive 1.21 mm v 1.32 mm normotensive, p = 0.306). There was no statistically significant difference in LATV or LATVi between hypertensive (median LATV = 14.3 ml, range 6.3 – 38.5 ml; median LATVi = 7.9 ml/m2, range 3.0 – 16.1 ml/m2) and normotensive (median LATV = 13.7 ml, range 5.4 – 31.7 ml; median LATVi = 76.25 ml/m2, range 2.5 – 15.3 ml/m2) subjects (p = 0.067 and p = 0.204 respectively).
3.2.3. Smoking and other clinical variables
LASA and LATV were not significantly different between smoking (median LASA = 91 cm2, range 55 – 143 m2; median LACV = 60 ml, range = 28 – 124 ml) and non-smoking (median LASA = 87 cm2, range 59 – 124 cm2; median LACV = 55 ml, range = 26 – 101 ml) subjects (p = 0.121 and 0.125 respectively) and neither were BSA normalised LASAi (non-smokers 46.7 cm2/m2 v 47.8 cm2/m2 smokers, p = 0.178) or LACVi (non-smoking 29 ml/m2 v 32 ml/m2, p = 0.098). Median LAWT was significantly lower in smoking (median thickness = 1.19 mm, range = 0.63 – 2.89 mm) than non-smoking (median LAWT = 1.32 mm, range = 0.56 – 2.92 mm) subjects (p = 0.020). LATV was not significantly different between smoking (median = 13.4 ml, range = 5.4 – 38.5 ml) and non-smoking (median = 14.5 ml, range = 5.6 – 31.7 ml) subjects (p = 0.062), but, when normalised for BSA, LATVi was lower in smoking (median = 6.7 ml, range = 2.5 – 16.1 ml) than non-smoking (median = 7.2 ml, range = 3.8 – 11.7 ml) subjects (p = 0.028), a difference which persisted in the multivariate analysis (p = 0.045, Table 2). In the univariate analysis, there were no differences in atrial parameters between diabetic and non-diabetic subject or between subjects with and without identified coronary artery disease. In the multivariate analysis, diabetes was associated with significantly lower LASAi (p = 0.010) (Table 3).
4. Discussion
In the current study we report normal values for traditional and novel atrial parameters and identify that these may change as a result of hypertension and smoking. Our study provides left atrial tissue parameter ranges for patients with the described clinical characteristics through a standardised atrial assessment using CCTA.
In the absence of AF and mitral valve disease, LACV reflects left ventricular filling pressure3 and is a powerful predictor of mortality [16,17]. Indexing LACV to BSA is considered mandatory, given the dependence of LACV on body size [18], a phenomenon demonstrated by the disappearance of apparent sex-dependent differences in LACV when indexed against BSA [2,3]. Historically LACV has been assessed with 2-dimensional and 3-dimensional transthoracic echocardiography, which under-estimates LACV by approximately 25% when compared with CCTA [19], and cardiac magnetic resonance (CMR) imaging [20,21]. The results presented here are consistent with these previous observations, with the median LACVi of 30 ml/m2 above the range reported in TTE studies [19,22,23]. In contrast, LACVi observed in this study is lower than reported for a healthy cohort assessed with CMR [24]. In the CMR study, LACVi was 40 ml/m2, compared to 30 ml/m2 in the current study. While CCTA and CMR estimates of LACVi demonstrate excellent correlation, the measurements obtained using CMR tend to be lower than CCTA [25]. The lower estimate of LACVi from the current CCTA study likely reflects the inclusion of the LAA in the LACVi calculated in the previous CMR study and may also reflect intrinsic differences in volume estimation due the imaging technique, as well as the standardised approach to PV removal that was employed in the current study. There is a clear benefit to standardising the strategy with which the PVs (and LAA, if required) are removed from segmentations as subjective identification of the PV ostia is likely to be a source of significant variability. When compared with previous CT based studies, the median LACVi in the current study is lower than reported in a previous CT study [26], in healthy participants (LACVi = 54 ml/m2) in which the LAA was also included in the volume estimates, and from a further CT based study in which absolute volumes only were reported [27]. The issues of inclusion of the LAA and standardised identification of the PV ostia are most likely to explain these differences and it is proposed that future studies will benefit from adopting a standardised approach to identification and cropping of the PVs in order to minimize the effect of this variable on variability of LACVi estimates.
LAWT has been measured in previous imaging studies, most of which have been performed using cardiac CT. Previous studies have considered overall and regional tissue thickness assessed from CT imaging, and in general report LAWT of a similar range to those reported in the current study [7], [28], [29], [30], [31], with a clear tendency for lower tissue thickness measurements in-vivo when compared with histological studies [32].
Although neither LACV nor LATV were significantly different between the sexes once corrected for BSA, LASA was greater in males (93 cm2) than females (85 cm2). After correction for BSA, LASAi was in fact greater in females (47.8 cm2/m2) than males (45.8 cm2/m2, p = 0.031). While the magnitude of this difference is small (~5%), this result appears to indicate a difference in atrial shape between male and female participants that is not captured by absolute or indexed LACV or LATV assessment. The sample is not large enough to exclude all potential confounders that may also contribute to such a difference, but this preliminary observation may be relevant to the recognised differences in incidence and response to atrial pathology that exists between males and females [33].
Participants who smoked had a significantly lower LAWT than participants who did not smoke. Smoking is known to cause structural and functional changes in left ventricular tissue, and is associated with diastolic left ventricular dysfunction, both of which occur in a dose dependent manner [34]. This is the first demonstration of morphological changes in the left atrium in the context of smoking. It may be speculated that the observed change may be the result of known impact of tobacco on systemic blood pressure [35], an effect of increased LVEDP on atrial structure, a direct toxic effect of tobacco on myocardium [34], or a combination of these mechanisms, however further studies would be required to clarify the mechanistic basis for this observation.
A number of imaging [36], [37], [38], [39] and pathological [5], studies have reported differences in atrial wall thickness between participants with and without AF or other cardiovascular pathologies [40]. While there was a small reduction in median LAWT among smokers in the current study, there was a significant overlap between both the groups and these results are observed in the absence of any difference in LASAi or LACVi. Furthermore, there was no difference in LAWT or LATV/LATVi between hypertensive and normotensive subjects. Overall, this study does not indicate that LAWT or LATV assessed using the current methodology is increased in the presence of conditions known to predispose to the subsequent development of AF.
4.1. Limitations
A small number of very high values were calculated for tissue thickness despite exclusion of clearly unreliable sections of the segmentation and we believe these values are spurious. This may reflect errors in the growing algorithm that were not identified manually or be the result of automatic tracking of field lines not reflecting an appropriate route between the endocardium and epicardium. To maintain objectivity these results were included in the statistical analysis, but the 5 – 95th percentile ranges more likely represent an accurate range of atrial tissue thickness estimates. This limitation would be an important consideration if localized wall thickness measurements were used to titrate therapy. Group comparisons did not account for covariates to maximize simplicity and transparency of analyses. The sample was not balanced by design on all relevant covariates so substantive differences could still remain. All analyses assumed independence of observation which, strictly speaking, the siblings in the sample do not meet. Straightforward corrections for clustering are not available for smaller samples and non-parametric tests, so the tests at hand were selected for simplicity and because authors consider the potential bias negligible. Furthermore, the impact of the genetic similarity between the MZ siblings further reduces the independence of the observations. This represents a limitation in the data set for the current analysis which the authors acknowledge. The relatively high prevalence of hypertension and asymptomatic coronary disease in this cohort limit the generalisability of the tissue parameters derived, however they may still be interpreted as reference values for cohorts with similar cardiovascular risk profiles. No rhythm monitoring was carried out during this study and so the prevalence of AF in this cohort cannot be established.
5. Conclusion
In this group with the described prevalence of cardiovascular risk factors, ranges for left atrial parameters including LACVi, LASAi, LATVi and global and regional tissue thickness derived from CCTA are presented and may serve as reference values for future studies. Consistent with previous studies, LACVi is similar between sexes and increased in hypertension. LASAi is greater in females than males in the absence of differences in LACVi or LATVi, which may indicate differential shape changes between the sexes. AF predisposing conditions, other than sex, were not associated with differences in tissue thickness.
Financial disclosures: This data was collected as part of the BUDAPEST-GLOBAL study. This study was funded by a grant from the EFSD New Horizons Program and Global Genomics Group, LLC.
This work was supported by the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z) and the National Institute for Health Research Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London. The views expressed here are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Declaration of Competing Interest
The authors report no relationships that could be construed as a conflict of interest.
Acknowledgments
Acknowledgements
This data was collected as part of the BUDAPEST-GLOBAL study. This study was funded by a grant from the EFSD New Horizons Program and Global Genomics Group, LLC.
This work was supported by the Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z) and the National Institute for Health Research Biomedical Research Centre at Guy’s and St. Thomas’ NHS Foundation Trust and King’s College London. The views expressed here are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
John Whitaker is funded by a Medical Research Council UK Clinical Research Training Fellowship (grant code MR/N001877/1).
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100694.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Klabunde RE. CV Physiology. 2nd ed. Lippincott Williams and Wilkins; 2012. https://www.cvphysiology.com/Heart Failure/HF008. Accessed October 21, 2018.
- 2.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–271. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 3.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 4.Camm A.J., Kirchhof P., Lip G.Y.H. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 5.Platonov P.G., Ivanov V., Ho S.Y., Mitrofanova L. Left atrial posterior wall thickness in patients with and without atrial fibrillation: data from 298 consecutive autopsies. J Cardiovasc Electrophysiol. 2008;19(7):689–692. doi: 10.1111/j.1540-8167.2008.01102.x. [DOI] [PubMed] [Google Scholar]
- 6.Wolf C., Seslar S., Boer K. Atrial Remodelling After the Fontan Operation. Am J Cardiol. 2009;104(12):1737–1742. doi: 10.1016/j.amjcard.2009.07.061.Atrial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan N.-H., Tsao H.-M., Chang N.-C., Chen Y.-J., Chen S.-A. Aging dilates atrium and pulmonary veins: implications for the genesis of atrial fibrillation. Chest. 2008;133(1):190–196. doi: 10.1378/chest.07-1769. [DOI] [PubMed] [Google Scholar]
- 8.Whitaker J., Panikker S., Fastl T. Cardiac CT assessment of tissue thickness at the ostium of the left atrial appendage predicts acute success of radiofrequency ablation. PACE - Pacing Clin Electrophysiol. 2017;40(11):1218–1226. doi: 10.1111/pace.13203. [DOI] [PubMed] [Google Scholar]
- 9.Maurovich-Horvat P., Tárnoki D.L., Tárnoki Á.D. Rationale, design, and methodological aspects of the BUDAPEST-GLOBAL study (Burden of Atherosclerotic Plaques Study in Twins - Genetic Loci and the Burden of Atherosclerotic Lesions) Clin Cardiol. 2015;38(12):699–707. doi: 10.1002/clc.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bishop M., Rajani R., Plank G. Three-dimensional atrial wall thickness maps to inform catheter ablation procedures for atrial fibrillation. Europace. 2016;18(3):376–383. doi: 10.1093/europace/euv073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitaker J, Fish J, Harrison J, et al. Lesion IndexTM guided ablation facilitates continuous, transmural and durable lesions in a porcine recovery model. Circ Arrhythm Electrophysiol. [DOI] [PubMed]
- 12.Whitaker J. Lesion index-guided ablation facilitates continuous, transmural, and durable lesions in a porcine recovery model. Circ Arrhythm Electrophysiol. 2018;11(4) doi: 10.1161/CIRCEP.117.005892. e005892. [DOI] [PubMed] [Google Scholar]
- 13.Littvay L., Métneki T.ÁD., The T.DL., Registry H.T. Twin Res Hum Genet. 2013;16(1):185–189. doi: 10.1017/thg.2012.76. [DOI] [PubMed] [Google Scholar]
- 14.Voros S., Maurovich-Horvat P., Marvasty I.B. Precision phenotyping, panomics, and system-level bioinformatics to delineate complex biologies of atherosclerosis: Rationale and design of the “Genetic Loci and the Burden of Atherosclerotic Lesions” study. J Cardiovasc Comput Tomogr. 2014;8(6):442–451. doi: 10.1016/j.jcct.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Mosteller R. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 16.Blume G.G., Mcleod C.J., Barnes M.E. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr. 2011;12(6):421–430. doi: 10.1093/ejechocard/jeq175. [DOI] [PubMed] [Google Scholar]
- 17.Fatema K., Bailey K.R., Petty G.W. Increased Left Atrial Volume Index: Potent Biomarker for First-Ever Ischemic Stroke. Mayo Clin Proc. 2008;83(10):1107–1114. doi: 10.4065/83.10.1107. [DOI] [PubMed] [Google Scholar]
- 18.Galderisi M., Cosyns B., Edvardsen T. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imag. Eur Heart J Cardiovasc Imaging. 2017;18(12):1301–1310. doi: 10.1093/ehjci/jex244. [DOI] [PubMed] [Google Scholar]
- 19.Kircher B., Abbott J.A., Pau S. Left atrial volume determination by biplane two-dimensional echocardiography: Validation by cine computed tomography. Am Heart J. 1991;121(3):864–871. doi: 10.1016/0002-8703(91)90200-2. [DOI] [PubMed] [Google Scholar]
- 20.Vandenberg B.F., Weiss R.M., Kinzey J. Comparison off loft atrial volume by two-dimensional echocardiography and cine-computed tomography. Am J Cardiol. 1995;75(10):754–757. doi: 10.1016/S0002-9149(99)80676-6. [DOI] [PubMed] [Google Scholar]
- 21.Rodevan O, Bjornerheim R, Ljosland M, Maehle J, Smith HJ, Ihlen H. Left atrial volumes assessed by three- and two-dimensional echocardiography compared to MRI estimates. Int J Card Imaging. 1999;15(5):397-410. http://www.ncbi.nlm.nih.gov/pubmed/10595406. Accessed October 20, 2018. [DOI] [PubMed]
- 22.Pritchett A.M., Jacobsen S.J., Mahoney D.W., Rodeheffer R.J., Bailey K.R., Redfield M.M. Left atrial volume as an index of left atrial size: A population-based study. J Am Coll Cardiol. 2003;41(6):1036–1043. doi: 10.1016/S0735-1097(02)02981-9. [DOI] [PubMed] [Google Scholar]
- 23.Triposkiadis F., Tentolouris K., Androulakis A. Left atrial mechanical function in the healthy elderly: New insights from a combined assessment of changes in atrial volume and transmitral flow velocity. J Am Soc Echocardiogr. 1995;8(6):801–809. doi: 10.1016/S0894-7317(05)80004-5. [DOI] [PubMed] [Google Scholar]
- 24.Maceira A.M., Cosín-Sales J., Roughton M., Prasad S.K., Pennell D.J. Reference left atrial dimensions and volume estimation by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12(65):1–10. doi: 10.1186/1532-429X-15-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agner B.F.R., Kühl J.T., Linde J.J. Assessment of left atrial volume and function in patients with permanent atrial fibrillation: Comparison of cardiac magnetic resonance imaging, 320-slice multi-detector computed tomography, and transthoracic echocardiography. Eur Heart J Cardiovasc Imaging. 2014;15(5):532–540. doi: 10.1093/ehjci/jet239. [DOI] [PubMed] [Google Scholar]
- 26.Lin F.Y., Devereux R.B., Roman M.J. Cardiac chamber volumes, function and mass as determined by 64-multidetector row computed tomography. J Am Coll Cardiol Cardiovasc Imaging. 2008;1(6):782–786. doi: 10.1016/j.jcmg.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Mahabadi A.A., Samy B., Seneviratne S.K. Quantitative assessment of left atrial volume by electrocardiographic-gated contrast-enhanced multidetector computed tomography. J Cardiovasc Comput Tomogr. 2009;3(2):80–87. doi: 10.1016/j.jcct.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemola K., Sneider M., Desjardins B. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation. 2004;110(24):3655–3660. doi: 10.1161/01.CIR.0000149714.31471.FD. [DOI] [PubMed] [Google Scholar]
- 29.Imada M., Funabashi N., Asano M., Uehara M., Ueda M., Komuro I. Anatomical remodeling of left atria in subjects with chronic and paroxysmal atrial fibrillation evaluated by multislice computed tomography. Int J Cardiol. 2007;119(3):384–388. doi: 10.1016/j.ijcard.2006.07.162. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmeister P.S., Chaudhry G.M., Mendel J. Evaluation of left atrial and posterior mediastinal anatomy by multidetector helical computed tomography imaging: relevance to ablation. J Interv Card Electrophysiol. 2007;18(3):217–223. doi: 10.1007/s10840-007-9096-y. [DOI] [PubMed] [Google Scholar]
- 31.Beinart R., Abbara S., Blum A. Left atrial wall thickness variability measured by CT scans in patients undergoing pulmonary vein isolation. J Cardiovasc Electrophysiol. 2011;22(11):1232–1236. doi: 10.1111/j.1540-8167.2011.02100.x. [DOI] [PubMed] [Google Scholar]
- 32.Whitaker J., Rajani R., Chubb H. The role of myocardial wall thickness in atrial arrhythmogenesis. Europace. 2016;18(12) doi: 10.1093/europace/euw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michelena H.I., Powell B.D., Brady P.A., Friedman P.A., Ezekowitz M.D. Gender in atrial fibrillation: Ten years later. Gend Med. 2010;7(3):206–217. doi: 10.1016/J.GENM.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Nadruz W., Claggett B., Gonçalves A. Smoking and Cardiac Structure and Function in the Elderly: The ARIC Study (Atherosclerosis Risk in Communities) Circ Cardiovasc Imaging. 2016;9(9):1–7. doi: 10.1161/CIRCIMAGING.116.004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thuy A.B., Blizzard L., Schmidt M.D., Luc P.H., Granger R.H., Dwyer T. The association between smoking and hypertension in a population-based sample of Vietnamese men. J Hypertens. 2010;28(2):245–250. doi: 10.1097/HJH.0b013e32833310e0. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K., Funabashi N., Uehara M. Left atrial wall thickness in paroxysmal atrial fibrillation by multislice-CT is initial marker of structural remodeling and predictor of transition from paroxysmal to chronic form. Int J Cardiol. 2011;148(2):139–147. doi: 10.1016/j.ijcard.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 37.Gupta D.K., Shah A.M., Giugliano R.P. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. 2014;35(22):1457–1465. doi: 10.1093/eurheartj/eht500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dewland T.A., Wintermark M., Vaysman A. Use of Computed Tomography to Identify Atrial Fibrillation Associated Differences in Left Atrial Wall Thickness and Density. Pacing Clin Electrophsyiology. 2014;36(1):55–62. doi: 10.1111/pace.12028.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi K, Okumura Y, Watanabe I, et al. Relation Between Left Atrial Wall Thickness in Patients with Atrial Fibrillation and Intracardiac Electrogram Characteristics and ATP-Provoked Dormant Pulmonary Vein Conduction. J Cardiovasc Electrophysiol. 2015:n/a-n/a. doi:10.1111/jce.12660 [DOI] [PubMed]
- 40.Hayashi H., Hayashi M., Miyauchi Y. Left atrial wall thickness and outcomes of catheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Interv Card Electrophysiol. 2014;40(2):153–160. doi: 10.1007/s10840-014-9894-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.