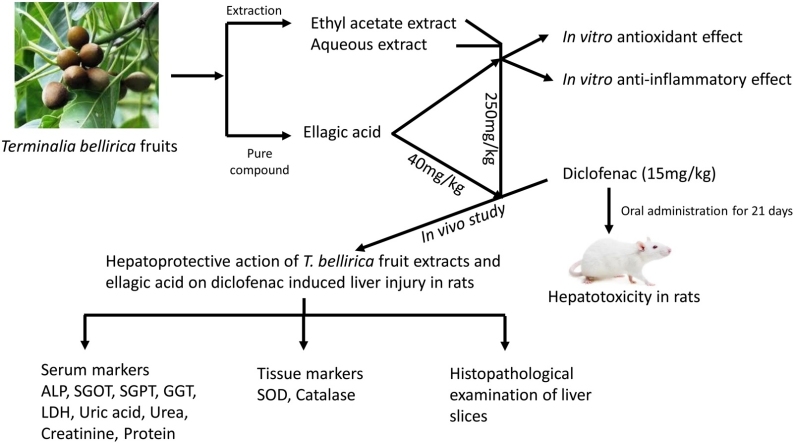
Graphical abstract
Keywords: Terminalia bellirica, Ellagic acid, Diclofenac, Antioxidant, Anti-inflammatory, Hepatotoxicity
Abstract
Long term usage and overdose of diclofenac (DCF), an anti-inflammatory drug is known to cause oxidative stress and liver injury. The present study reports the antioxidant, anti-inflammatory and hepatoprotective activities of Terminalia bellirica (Tb) fruit aqueous and ethyl acetate extracts and its bioactive compound ellagic acid (EA) against DCF-induced toxicity. in vitro antioxidant activities were measured by ABTS and FRAP assays while anti‐inflammatory activity was assessed by the albumin denaturation method. The adverse effects of DCF and hepatoprotective potential of Tb extracts and EA were assessed in serum and liver tissue of rats after oral administration for 21 days. Silymarin was used as standard hepatoprptective agent for comparison. Hepatic markers analyzed in serum included ALP, GPT, GOT, LDH, γ-glutamyl transferase, total protein, creatinine, and uric acid while superoxide dismutase (SOD) and catalase (CAT) were analyzed in liver tissue. The EA exhibited superior ABTS radical scavenging, FRAP, and anti-inflammatory activities as compared to fruit extracts. DCF treatment led to rise in the levels of most of the serum hepatic markers with decline in total serum protein as well as SOD and CAT in liver tissue. The supplementation of extracts, EA and silymarin in DCF treated rats significantly reduced the adverse effects of DCF on serum and tissue markers. Histopathology of the liver indicated that extracts and EA significantly decreased the degree of liver fibrosis. The hepatoprotective ability of EA was comparable to the silymarin but activity of Tb fruit extracts was little lower. Among fruit extracts ethyl acetate extract exhibited better activity than aqueous extract. The results revealed that ellagic acid and T. bellirica fruit extracts have potential to mitigate oxidative stress and hepatotoxicity produced by long term use of diclofenac.
1. Introduction
Diclofenac (DCF) is a derivative of phenylacetic acid and an active member of non-steroidal anti-inflammatory drugs (NSAIDs) group which is frequently prescribed drug during fever, inflammation and moderate pain conditions. It also acts as an antibacterial agent by inhibiting DNA synthesis [1]. The mechanism of DCF action is believed to be associated with the inhibition of prostaglandin-endoperoxide synthase, also known as cyclooxygenase (COX) [2]. The liver metabolizes DCF to 4-hydroxydiclofenac and other hydroxylated derivatives. These metabolites undergo the process of sulfation and glucuronidation before they are finally excreted in the bile (35 %) and urine (65 %) [3]. Besides, prolonged medication with DCF has been associated with a higher incidence of liver injury, ranging from the mild, asymptomatic, reversible rise in serum hepatic biomarkers leading to hepatitis and jaundice, including fatal hepatitis [4]. Studies on DCF induced hepatotoxicity revealed that it causes excessive free radicals formation and an unusual rise in the level of liver function markers in serum [5]. A wide range of studies revealed that the free radicals such as reactive oxygen/nitrogen species (ROS/RNS) exert oxidative stress which is a major cause of hepatic abnormalities like degeneration, necrosis apoptosis, swelling, etc [6]. In the absence of targeted hepatoprotective drugs in allopathic practices, plants and their bioactive compounds have played a major role in the treatment of various hepatic disorders.
During various inflammatory conditions, an excessive formation of phagocytes, production of O2֯, OH֯ radicals and non‐free radical species (H2O2) takes place [7] which can damage the surrounding tissue either by direct oxidation or indirectly with hydrogen peroxide (H2O2) and OH radicals. Tissue damage then provokes an inflammatory response by the production of mediators and chemotactic factors [8]. The ROS is also known to activate matrix metalloproteinase (e.g., collagenase) causing increased destruction of tissues e.g. collagen damage is seen in various arthritic reactions [9]. Therefore, the natural agents that can mitigate these ROS can be useful in the treatment of inflammatory disorders.
Terminalia bellirica (Gaertn.) Roxb. (Family Combretaceae), popularly known as ‘Belleric myrobalan’, is a large deciduous tree widely occurring throughout the Indian subcontinent, Nepal, Sri Lanka, and south-east Asia [10]. T. bellirica fruit is one of the main components or integral part of the traditional laxative formulation, ‘Triphala’ which is used in a variety of ailments such as leucorrhoea, common cold, constipation, headache, pharyngitis, liver diseases, gastrointestinal complaints and hair fall [11]. Phytochemical investigation of T. bellirica (Tb) fruits have revealed the presence of ellagic acid, corilagin, arjunolic acid, ethyl gallate, galloyl glucose, lignans (termilignan), chebulaginic acid, phenyllemblin, β-sitosterol, glucoside (bellericanin), gallo-tannic acid, coloring matter, resins and greenish yellow oil as a major ingredients [12]. Earlier studies explained that Tb fruit displays numerous pharmacological properties comprising antidiabetic [13], antioxidant [14], anti-bacterial [15,16], anti-HIV [17], anti-malarial, anti-fungal [18], and hepatoprotective property [19].
Ellagic acid (EA) is a polyphenolic compound present in Tb fruit in a considerable amount (13−23 mg/g) [20,21] and has been studied widely for its medicinal attributes. However, concentration of overall EA derivatives in fruit is four times higher than that of pure EA [22]. The antioxidant effects of EA are mediated by its metal chelating capacity, free radical scavenging ability, and induction of cellular antioxidant enzymes activity. EA has also been shown to protect cells from DNA damage caused by free radicals [23,24]. Very few studies have investigated the free radical inhibitory activities of EA in vitro and in vivo [25]. Literature is silent about anti-inflammatory effects of EA. Similarly little or no information is available regarding efficacy of EA and Tb as hepatoprotectant in DCF induced toxicity. Silymarin is a natural compound having hepatoprotective and antioxidant action and hence it is used for prevention and treatment of various liver injuries/diseases including chronic hepatitis and liver cirrhosis [[26], [27], [28]]. Moreover, it is routinely used as a standard hepatoprotective compound in research activities related with xenobiotic induced hepatotoxicity. The present study reports antioxidant, anti-inflammatory and hepatoprotective activities of Tb fruit ethyl acetate (Eth) and aqueous (AQ) extracts and EA against diclofenac induced oxidative stress. Silymarine has been used as standard hepatoprotective agent for comparison. To the best of our knowledge, it is the first report on the evaluation of Tb extracts (Eth and AQ) and EA for in vitro anti-inflammatory and hepatoprotective effects against DCF induced liver injury in albino Wistar rats.
2. Materials and methods
2.1. Chemicals
Silymarin (Sigma), ellagic acid (Himedia laboratories Pvt Ltd.), diclofenac sodium (Novartis, India), ABTS (Sisco Research Laboratory Pvt. Ltd.) were procured from standard suppliers. Biochemical kits for estimation of creatinine, uric acid, total protein, alkaline phosphatase (ALP), serum glutamic-oxaloacetic transaminase (SGOT), Serum glutamic pyruvic transaminase (SGPT), γ-glutamyl transferase (GGT) and lactate dehydrogenase (LDH) were purchased from Erba Transasia Bio-medicals Ltd. All other laboratory chemicals such as 2,4,6-tripyridyl-s-triazine (TPTZ), potassium persulfate, bovine serum albumin, sodium hydroxide, hydrogen peroxide etc. were purchased from Sisco research laboratory Pvt. Ltd. New Delhi, India.
2.2. Collection of plant fruits, their identification and extraction
T. bellirica fruits were purchased from local market of Allahabad and identification was confirmed by Prof. D. K. Chauhan (Department of Botany, University of Allahabad). The fruits were shade dried at room temperature for 10–15 days, ground into a fine powder and extracted sequentially with ethyl acetate and water using the Soxhlet apparatus [29]. The excess amount of solvent in extracts was removed under reduced pressure. The total yield in Eth and AQ extracts was 4.5 % and 23 %, respectively. The extracts were further dissolved in respective solvents for the determination of antioxidant, anti-inflammatory and hepatoprotective activities.
2.3. Experimental animals
Healthy albino Wistar rats of either sex, approximately of the same age (weight 170−230 g), were procured from the Indian Institute of Toxicological Research (Lucknow, Uttar Pradesh, India). They were acclimatized at the departmental animal house (25 ± 2 °C), humidity 40 ± 5% with 12-h light and dark cycles for 1 week before and during experiments. Animals were provided with a standard pellet diet (Paramount Techno-Chem, Varanasi, Uttar Pradesh, India) and water was given ad libitum. The in vivo study was performed following the Guidelines of Institutional Animal Ethics Committee as approved by the National Committee for Control and Supervision of Experiments on Animals.
2.4. Experimental design and treatment schedule
The rats were divided into six groups of five each. Group-I included normal rats; Group-II DCF treated rats (15 mg/kg); Group-III DCF treated rats co-administered with the standard drug (Silymarin 40 mg/kg); Group-IV DCF treated rats co-administered with EA (40 mg/kg); Group-V DCF treated rats co-administered with AQ extract (200 mg/kg) and Group-VI DCF treated rats co-administered with Eth extract (200 mg/kg). During experimental period all the rats were administered orally a single dose of the drugs or extracts or combinations thereof for 21 days.
2.5. Measurement of body weight and relative weight
The body weight of the rats was analyzed before the treatment period and also after 6 h of the administration of drugs on each day until sacrifice. The weight of the liver was also analyzed after the sacrifice. The relative liver weight was determined by the following formula:
2.6. Collection of blood
Approximately 4 mL blood was drawn by puncturing rat heart out of which 2.5 mL blood was allowed to clot and serum was separated after centrifugation at 2500 rpm for 10 min. The remaining blood was collected into anticoagulant vials and kept on ice for less than 1 h before processing. The sample was centrifuged at 3000 rpm for 10 min and plasma was collected. Both the serum and plasma and were transferred into separate vials and stored at −70 °C for further analysis.
2.7. Estimation of in vitro antioxidant assays
2.7.1. ABTS radical scavenging assay
The ABTS [2,2-azino-bis(3-ethylbenzothiazolin)-6-sulfonic acid] radical scavenging ability of fruit extracts and EA was determined by decolorization assay as described by Re et al. [30]. ABTS radical was generated by reacting 7 mM stock solution of ABTS·+ with 2.45 mM K2S2O8 and leave the mixture in the dark at room temperature for 12−16 h before use. The ABTS·+ solution was diluted with ethanol to give an absorbance of 0.70 ± 0.05 at 734 nm, reagent blank reading was taken. After addition of 1.9 mL of diluted ABTS+ solution to 50 μL of each of the different concentrations of the extracts, reaction mixture was incubated at room temperature in the dark. The absorbance was measured at 734 nm after 6 min of initial mixing by using a UV/VIS spectrophotometer. Ascorbic acid was used as a standard. All determinations were carried out in triplicate. The percentage ABTS radical scavenging ability of extracts at 734 nm was calculated using the formula:
Where Ac and As is the absorbance of the control and sample, respectively.
2.7.2. Ferric reducing antioxidant power (FRAP) assay
The reducing ability of fruit extracts was measured by the method of Benzie and Strain [31]. To 100 μL of extracts (2 mg/mL) and EA (1 mg/10 mL), 4.5 mL FRAP reagent (containing acetate buffer pH 3.6, 10 mM 2,4,6-tripyridyl-s-triazine (TPTZ) in 40 mM HCl, and 20 mM FeCl3·6H2O in the ratio of 10:1:1) was added and after 5 min absorbance was measured at 593 nm. All determinations were performed in triplicate. Calibration curve of ferrous sulfate (100−1000 μmol/l) was used, and results were expressed in μmol Fe2+/mg dry weight of extract and calculated as mean ± standard deviation (SD).
2.8. Assessment of in vitro anti-inflammatory activity
Inhibition of albumin denaturation was assayed using the method of Mizushima and Kobayashi [32] followed with minor modifications. The reaction mixture was containing 50 μL of different concentrations of fruit extracts (3 mg/mL) and EA (1 mg/mL) and 450 μL of 1% aqueous solution of bovine serum albumin. The pH of the reaction mixture was adjusted using 1 N HCl. The samples were incubated at 37 °C for 20 min at room temperature followed by at 57 °C for 10 min in the water bath. After cooling, 2.5 mL of phosphate buffer solution was added in each test tube. The turbidity in the sample was measured spectrophotometrically at 660 nm. DCF (1 mg/mL) was used as a standard anti-inflammatory drug. The experiment was performed in triplicate. Percent inhibition of protein denaturation was calculated as follows:
Where Ac and As is the absorbance of the control and sample, respectively. The control was prepared by adding 50 μl of phosphate buffer in place of the sample.
2.9. Biochemical analysis
2.9.1. Assessment of Liver marker enzymes in serum
The biochemical parameters viz., SGPT, SGOT, GGT, LDH, ALP, total protein, uric acid, and creatinine were assayed using commercially available kits (Erba Diagnostics Kits).
2.9.2. Preparation of liver tissue homogenate
The 10 % (w/v) homogenates of liver were prepared in phosphate buffer solution (0.1 M, pH-7.4 having 0.15 M KCl) and centrifuged at 1000×g for 30 min at 4−6 °C. The supernatant was separated by gentle decantation and used for assay of antioxidant enzymes.
2.9.3. Assessment of antioxidant enzymes in liver tissue homogenate
The activity of superoxide dismutase (SOD, E.C. 1.15.1.1) was measured by the method of Marklund and Marklund [33]. The absorbance of a colored complex involving pyrogallol auto-oxidation at 412 nm was measured for 3 min at the interval of 30 s with or without the enzyme protein. One unit of the enzyme activity was expressed as a 50 % inhibition of auto-oxidation of pyrogallol per minute. The catalase (CAT, E.C. 1.11.1.6) activity was measured according to the method of Beers and Sizer [34] by measuring the decrease in the absorbance for H2O2 consumption at 240 nm at the interval of 30 s for 3 min. One unit of CAT activity was defined as micromoles of H2O2 decomposed per min using molar absorbance of H2O2 (43.6 M−1 cm−1).
2.10. Determination of total protein in liver tissue homogenate
The protein content in the liver tissue was measured by the method of Lowry et al. [35] and BSA was used standard.
2.11. Histological analysis of liver
The liver biopsies of the rats were fixed in 10 % formalin, dehydrated in graded alcohol, and then embedded in paraffin wax. The paraffin-embedded tissue was sectioned (5 μm) serially using SPINCON rotary microtome (model number SP‐1120/SP‐1120A). The sections were distributed on albumin coated sterilized glass slides, stretched and stained with hematoxylin and eosin (H&E) dyes [36]. The slides containing tissue sections stained with H&E were observed under a light microscope at ×40 magnification after being mounted using dibutylphthalate polystyrene xylene (DPX).
2.12. Statistical analysis
The results obtained were expressed as a mean ± standard deviation (SD) by the use of one way ANOVA followed by Student Newman-Keul's test. The values were considered significant at p < 0.05.
3. Results
3.1. In vitro antioxidant activity
Tb fruit extracts (Eth and AQ) and EA were found to be significant scavengers (p < 0.05) of the ABTS radical and this activity was comparable to standard solution ascorbic acid (IC50 value 1.46 μg/mL). EA (IC50 1.71 μg/mL) showed higher antioxidant activity as compared to Eth (IC50 11.78 μg/mL) and AQ extracts (IC50 14.44 μg/mL) in ABTS radical scavenging assay. The activities of test extracts were comparatively lower than the activity shown by ascorbic acid.
During FRAP assay, EA showed considerably higher reducing ability (99,708\±63.08 μmol FeSO4.7H2O equivalent/mg) in comparison to Eth extract (6527.05±87.18μmol FeSO4.7H2O equivalent/mg). The result was statistically significant (p < 0.05).
3.2. In vitro anti-inflammatory activity
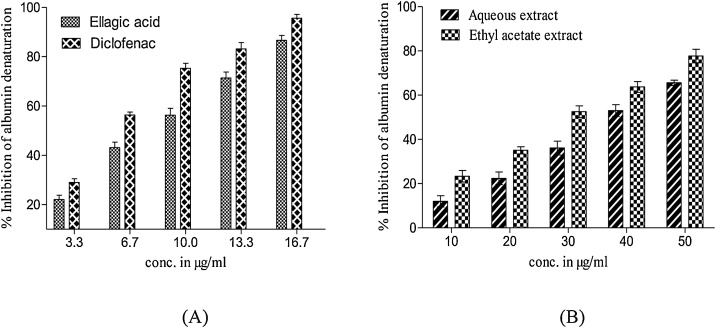
Antiinflammatory activity of extracts and EA was investigated by inhibition of heat-induced albumin denaturation. Both the extracts and EA exhibited concentration-dependent anti-inflammatory activity. EA exhibited superior activity (IC50 7.64 μg/mL) as compared to AQ (IC50 36.22 μg/mL) and Eth (IC50 28.03 μg/mL) extracts by inhibiting the heat-induced denaturation of albumin more effectively as shown in Fig. 1. Maximum inhibition of albumin denaturation by EA (86.62 ± 0.65 %) was observed at the concentration of 16.7 μg/mL while AQ and Eth extract produced 67.57 % and 77.67 % inhibition, respectively at the concentration of 50 μg/mL. DCF, a standard anti‐inflammatory drug, showed comparatively higher anti-inflammatory activity (95.56 ± 0.56 %) with lower IC50 value (5.66 μg/mL).
Fig. 1.
Anti-inflammatory activity of diclofenac, ellagic acid, and T. bellirica fruit extracts in heat-induced albumin denaturation assay. (A) Percentage albumin denaturation inhibiton by diclofenac and ellagic acid; (B) Percentage albumin denaturation inhibition by Tb fruit aqueous and ethyl acetate extracts. Results were expressed in mean ± SD.
3.3. Change in body weight and relative liver weight
DCF treatment led to a remarkable decline (18.54 %) in rat body weight over three weeks duration (Table 1). However, control rats showed about 17.82 % increase in body weight during the same period. The percentage weight loss of group II rats was significantly higher than those of group I (p < 0.0001) and groups III-V (p < 0.005). Co-administration of EA and Tb fruit extracts (AQ and Eth) with DCF exhibited restorative effect (7–11.5 % recovery) on body weight. Standard drug silymarin showed maximum recovery potential (11.5 %) followed by EA (9.68 %), Eth (9.5 %) and AQ (7%).
Table 1.
Effect of administration of T. bellirica fruit extracts and ellagic acid on the body weight and relative liver weight of diclofenac treated rats for 21 days. Group-I: control rats; Group-II: DCF treated rats; Group-III: DCF + SLM treated rats; Group-IV: DCF + EA treated rats; Group-V: DCF + AQ treated rats; Group-VI: DCF + Eth treated rats. Each value is expressed in mean ± SD (n = 5). Abbreviations: DCF- diclofenac, SLM- silymarin, EA- ellagic acid, AQ- aqueous extract, Eth- ethyl acetate extract. (*) represents a significant difference from control (p < 0.0001). (#) represents a significant difference from group II (p < 0.005).
| Groups | Bodyweight Change (%) |
Absolute liver Weight (g) |
Relative liver Weight (%) |
|---|---|---|---|
| Group-I | 17.82 ± 3.03 | 4.24 ± 0.06 | 2.35 ± 0.09 |
| Group-II | −18.54 ± 1.78* | 7.44 ± 0.28* | 4.31 ± 0.07* |
| Group-III | −6.99 ± 1.57# | 4.79 ± 0.10# | 3.06 ± 0.07# |
| Group-IV | −8.86 ± 0.78# | 5.39 ± 1.21# | 3.26 ± 0.35# |
| Group-V | −11.59 ± 1.06# | 5.89 ± 0.21# | 3.56 ± 0.08# |
| Group-VI | −9.05 ± 4.70# | 6.40 ± 0.22# | 2.99 ± 0.01# |
An inverse correlation was observed between body weight and relative liver weight. Relative liver weight enhanced from 2.35 % in control to 4.31 % in DCF treated rats (p < 0.0001) while treatment with EA and Tb fruit extracts (AQ and Eth) showed a restorative effect on the liver weight of rats. In comparison to Group II rats, the recovery with the administration of EA and Tb fruit extracts was statistically significant (p < 0.005).
3.4. Serum biochemical analysis
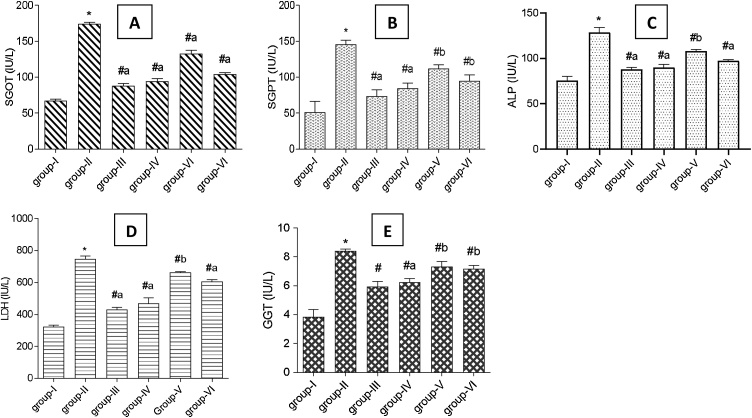
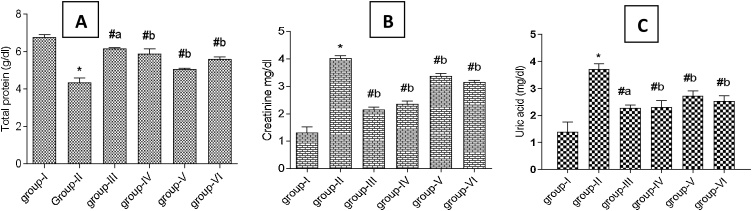
The hepatoprotective potential of Tb fruit extracts and EA was assessed by determining the levels of serum hepatic function markers after 21 days of DCF intoxication in rats (Fig. 2, Fig. 3). In DCF treated rats (Group II) the level of SGOT, SGPT, ALP, LDH, GGT, creatinine, and uric acid increased considerably in blood with a reduction in total protein as compared to control (Group I). However, supplementation of EA, Tb fruit Eth and AQ extracts and SLM caused a significant reduction in the level of SGOT, SGPT, ALP, LDH, GGT, uric acid and creatinine towards normalcy as compared to DCF treated group II rats (p < 0.005). The highest recovery in the level of serum liver function markers was observed in the standard drug SLM treated group III rats (p < 0.0005). However, the results produced by EA treatment was comparable to SLM treatment. A noteworthy increase in serum total protein was observed with the treatment (Fig. 3A).
Fig. 2.
A-E: Effect of T. bellirica fruit extracts (aqueous and ethyl acetate) and ellagic acid on the serum enzyme markers of liver function (A) Serum glutamic-oxaloacetic transaminase (SGOT), (B) Serum glutamic pyruvic transaminase (SGPT), (C) Alkaline phosphatase (ALP), (D) Lactate dehydrogenase (LDH), (E) γ-glutamyl transferase (GGT) in diclofenac treated rats. Group-I: control rats; Group-II: DCF treated rats; Group-III: DCF + SLM treated rats; Group-IV: DCF + EA treated rats; Group-V: DCF + AQ treated rats; Group-VI: DCF + Eth treated rats. Values represent mean ± SD (n = 5). Abbreviations: DCF- diclofenac, SLM- silymarin, EA- ellagic acid, AQ- aqueous extract, Eth- ethyl acetate extract. (*) represents a significant difference from control (p < 0.0001). (#) represents significant difference from group II (a- p < 0.0005, b- p < 0.005).
Fig. 3.
A-C: Effect of T. bellirica fruit extracts (aqueous and ethyl acetate) and ellagic acid on non-enzymic markers of the liver function in serum (A)Total protein, (B) Creatinine, (C) Uric acid in diclofenac (DCF) treated rats for 21 days. Group-I: control rats; Group-II: DCF treated rats; Group-III: DCF + SLM treated rats; Group-IV: DCF + EA treated rats; Group-V: DCF + AQ treated rats; Group-VI: DCF + Eth treated rats. Values represent mean ± SD (n = 5). Abbreviations: DCF- diclofenac, SLM- silymarin, EA- ellagic acid, AQ- aqueous extract, Eth- ethyl acetate extract. (*) represents a significant difference from control (p < 0.0001). (#) represents significant difference from group II (a- p < 0.0005, b- p < 0.005).
3.5. Assessment of antioxidant enzymes in liver tissue homogenate
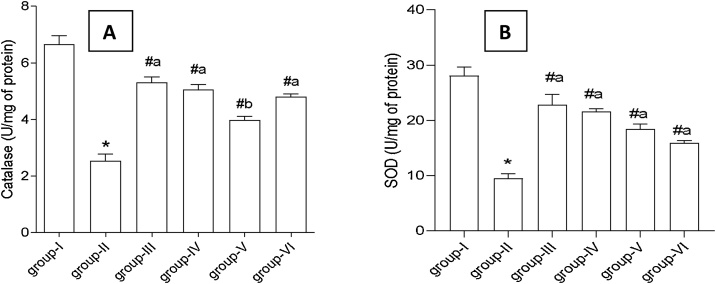
Antioxidant status of liver tissue was monitored by evaluating the activities of CAT and SOD in rats administered with DCF and test compounds. Activities of both the antioxidant enzymes declined substantially after three weeks of DCF treatment in Group II rats indicating toxicity (Fig. 4A-B). In quantitative term activities of catalase and SOD were found to be 6.66 U/mg and 28.11U/mg, respectively in control rats which reduced to 2.54 U/mg and 9.48 U/mg, respectively under the influence of DCF (Group II) showing 34–38 % of the original activity. SLM treatment augmented the activities of catalase and SOD up to 80 % of the normal values. Almost similar enhancement in enzyme activities was obtained with EA treatment (76 % of normal values). Appreciable recovery (66–72 %) in the activity of both the antioxidant enzymes was observed with the treatment of Eth extract (Group VI). AQ extract accounted for comparatively lower recovery (56–60 %) at the same dose (Fig. 4).
Fig. 4.
A-B: Effect of T. bellirica fruit extracts and ellagic acid on the liver tissue antioxidant enzymes (A) Catalase (CAT), (B) Superoxide dismutase (SOD) in diclofenac treated rats for 21 days. Group-I: control rats; Group-II: DCF treated rats; Group-III: DCF + SLM treated rats; Group-IV: DCF + EA treated rats; Group-V: DCF + AQ treated rats; Group-VI: DCF + Eth treated rats. Values represent mean ± SD (n = 5). Abbreviations: DCF- diclofenac, SLM- silymarin, EA- ellagic acid, AQ- aqueous extract, Eth- ethyl acetate extract. (*) represents a significant difference from control (p < 0.0001). (#) represents significant difference from group II (a- p < 0.0005, b- p < 0.005).
3.6. Histopathological changes in liver
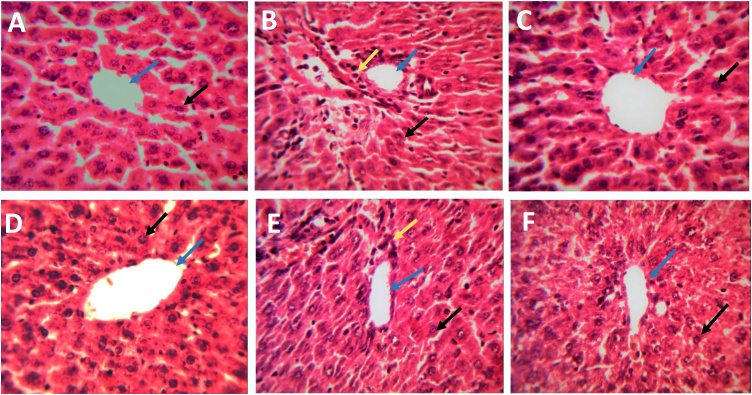
Histological sections of the normal rat liver slices revealed a normal liver lobular architecture with central vein and radiating hepatic cords accompanied by intact hepatocytes with sinusoidal spaces and evenly distributed cytoplasm (Fig. 5A). DCF treated group exhibited noticeable morphological alterations and fibrosis, as shown by disruption of the tissue architecture, extension of fibers, large fibrous septa formation, pseudo-lobe separation, and fibers accumulation. This is evidenced by massive hepatocellular degeneration, necrosis, sinusoidal dilatation, infiltration of inflammatory cell and cytoplasmic vacuolation (Fig. 5B). However, concurrent administration of EA, Tb fruit AQ and Eth extracts with DCF accounted for the reasonable improvement in the hepatic architecture as observed by reduction in liver damage and associated hepatic injuries by suppressing hepatocellular degeneration and necrosis and thereby significantly improving liver structure and function (Fig. 5D-F). Administration of SLM, the standard drug, showed comparatively better hepatoprotective potential (Fig. 5C). The histopathological improvement observed in liver slices administered with EA, Tb extracts and SLM in DCF treated rats finds direct correlation with the liver weight, body weight and serum liver function markers along with tissue antioxidants.
Fig. 5.
Histopathological analysis of experimental liver tissues following administration of T. bellirica fruit extracts and ellagic acid (EA) in diclofenac (DCF) treated rats. A- Control rats; B- DCF treated rats; C- DCF + silymarin treated rats; D- DCF + EA treated rats; E- DCF + aqueous extract-treated rats; F- DCF + ethyl acetate extract treated rats. The blue color arrow represents central vain; black arrow represents the nucleus, and the yellow arrow represents inflammatory cells.
4. Discussion
The adverse effects of drugs and synthetic antioxidants have drawn the attention of researchers to explore new sources of natural antioxidants and hepatoprotectants which are more potent to control oxidative stress and avert the initiation of disease propagation [37,38]. Antioxidants are those compounds that inhibit the oxidation of critical biomolecules by preventing the propagation of oxidizing chain reactions [39]. Interestingly, few studies on the antioxidant properties of Tb fruit have been done earlier [13,19,40]. However, this is the first study that assessed the antioxidant, anti-inflammatory and hepatoprotective attributes of Tb fruit aqueous and ethyl acetate extracts and EA against DCF induced liver injury in albino Wistar rats.
Antioxidant evaluation of Tb fruit Eth and AQ extracts and EA showed significant radical scavenging activities during in vitro ABTS radical scavenging and FRAP assays. The radical ABTS+ is a chemically stable chromophore over a wide pH range that shows maximum absorbance in the wavelength range of 600−750 nm. ABTS+ radical is soluble in aqueous and organic media; hence it is useful for determining antioxidant activity of water-soluble and fat-soluble samples [41]. Test samples exhibited radical quenching ability in a concentration-dependent manner and blue colored chromophore was reduced to colorless ABTS. Therefore, the degree of discoloration made it possible to evaluate the percentage of inhibition of the ABTS cation radical by test compounds, which is determined as a function of the antioxidant concentration and the reaction time [42]. IC50 values for EA, Eth, and AQ were found to be 1.71 μg/mL, 11.78 μg/mL and 14.44 μg/mL, respectively. The radical scavenging activity of EA was comparable to the activity shown by standard antioxidant ascorbic acid (IC50 value 1.46 μg/mL) [43]. However, the activity of Eth and AQ extracts of Tb fruit was a little lower. A comparatively higher amount of Tb extracts were required to enhance the ABTS radical quenching efficacy comparable to EA and ascorbic acid.
FRAP assay was used to determine the potential of extracts and EA to reduce ferric ions. The blue-colored Fe3+ tripyridyltriazine complex gets reduced to colorless Fe2+ form because of the electron-donating ability of extracts and EA [44]. During in vitro antioxidant analysis, EA showed higher antioxidant efficacy followed by Eth and AQ extracts. Activity of EA was comparable to the standard antioxidant. Comperatively lower activity in fruit extracts could be correlated with lower amount of ellagic acid in extracts as EA constitutes about 2.3 % of the Tb fruit [21]. Therefore, Eth and AQ extracts exhibited relatively lower antioxidant activity as compared to pure EA. Moreover, several other phytochemicals present in Tb fruit such as gallic acid, chebulic acid, corilagin, and ferulic acid, etc. might also be responsible for showing this activity [21].
Inflammation is a common symptom of many chronic diseases. It is a normal protective response to tissue injury caused by physical trauma, noxious chemical or microbial agents [45]. The inflammatory drugs (salicylic acid, phenylbutazone, aceclofenac, etc.) have shown dose-dependent ability to prevent heat-induced protein denaturation but these are related to several adverse effects like gastric irritation, ulcer, etc. [32,46]. In the present study Tb fruit, Eth and AQ extracts and EA showed concentration-dependent in vitro anti-inflammatory activity in heat-induced denaturation of albumin (Fig. 1). Percentage inhibition of albumin denaturation concerning control is referred to as a degree of protein stabilization [47]. At lower concentrations, the activity of EA (IC50 7.64 μg/mL) on protein denaturation inhibition was comparable to the activity of DCF (IC50 5.66 μg/mL). However, Eth and AQ extracts also exhibited noteworthy anti-inflammatory activity at higher concentrations. This could be attributed to the increase in the amount of ellagic acid content in extracts at higher concentration as EA constitutes about 2.3 % of Tb fruit [21]. Several studies suggested that tissue protein denaturation is related to inflammatory and arthritic diseases because of the production of autoantigens [48]. Therefore, the current study on Tb extracts exhibiting protein denaturation preventive action in vitro would provide a new direction towards research and development of anti-inflammatory drugs.
The liver performs a critical role in inhibiting the accumulation of compounds by converting them into a suitable form for excretion. All compounds undergo xenobiotic metabolism, through several rounds of biochemical transformations. During this process, some of the intermediates show adverse responses [49]. Usually, the liver is highly prone to injury by intermediate products of drug metabolism. Therefore, a better quantitative understanding of the balance between the xenobiotic detoxification process and hepatic damage could provide better direction for safe levels in both pharmaceutical and toxicological situations [50]. Particularly, the capability to predict the toxicity profile of lead candidates is necessary to rationalize pharmaceutical drug development [51,52]. Hepatic injuries modify the transport action and membrane permeability of hepatocytes leading to oozing of enzymes from the cells and subsequently level of various liver function markers in the blood increases [53]. Therefore, the abnormal rise of hepatic serum enzymatic and non-enzymatic markers coupled with reduction in serum proteins into the circulation suggested severe impairment to hepatic tissue during DCF intoxication.
During the study period, daily administration of DCF for continuous 21 days led to 18.5 % decrease in body weight along with a significant rise (4.3 %) in relative liver weight signifying the adverse effect of DCF intoxication in rats (Table 1). It has been reported that DCF causes gastric ulceration due to which the animals find it difficult to have food thereby resulting in malnutrition [54]. The rise in liver weight was however observed to be related to a concomitant increase in the level of serum markers such as SGOT, SGPT, GGT, ALP, LDH, creatinine, and uric acid (Figs. 2, 3) Other studies on NSAIDs-induced toxicity have also reported an elevated levels of hepatic markers [55,56]. However, treatment with Tb fruit extracts and EA accounted for considerable recovery in body weight and liver weight indicating the hepatoprotective effect of Tb phytoconstituents against DCF induced hepatotoxicity (Table 1). A decline in total protein (Fig. 3A) was an indicator of massive necrosis of the hepatocyte as well as altered hepatic functions [57,58]. The level of total protein in serum also increased significantly coupled with a reduction in SGOT, SGPT, GGT, ALP, creatinine, uric acid, and LDH levels under influence of test compounds which substantiated the hepatoprotective action of EA and Tb fruit Eth and AQ extracts (Figs. 2, 3). SLM treatment produced best hepatoprotective effect against DCF mediated toxicity as indicated by recovery of the pathophysiological parameters towards normal level in group III rats. Hepatoprotective activities of EA was comparable to the activities shown by SLM. This fact further provided support to the little lower hepatoprotective and antioxidant activities of Tb AQ and Eth extracts as they possessed relatively lower amount of bioactive compounds as compared to EA [59,21,25]. The concentration of total EA derivatives in fruit is reported to be four times higher than that of pure EA [22]. Thus, amount of total EA derivatives in test extracts (200 mg/kg) was computed to be 18.4 mg/kg which is about half of the amount of EA (40 mg/kg) fed to rats. Therefore, comparatively lower activity observed in the fruit extracts could be correlated with the lesser amount of EA content in the extracts. Moreover, solubility of EA is greater in ethyl acetate as compared to water. This fact further corroborates the higher biological activity observed in the Eth extract. Furthermore, the antioxidant activity of EA might be associated with the direct scavenging action on DCF generated ROS and indirectly through the induction of antioxidant enzymes and thereby protecting hepatocytes against ROS mediated liver injury [60,61].
The endogenous antioxidant markers (SOD and CAT) in DFC administered rats were found to be decreased in hepatic tissue. SOD catalyzes the dismutation of superoxide radicals into O2 to H2O2 which has an important role in scavenging ROS [62]. Decreased SOD activity in group II rats might be associated with a reduction in ROS scavenging action, whereas administration of EA and Tb extracts (Eth and AQ) in DFC treated rats caused enhancement in the level of SOD (group IV—VI). CAT also plays an essential role in protecting cells from oxidative damages [37]. Decreased level of CAT in DFC treated rats diminished the ability of hepatic tissue to defend against oxidative insult. EA and Tb extracts improved the level of CAT in hepatocytes (Fig. 4). Toxicity produced by continued DCF treatment for three weeks and its amelioration by EA and Tb extracts was also reflected in histological sections of the liver slices. Toxicity leads to morphological alterations in hepatic tissue and degree of toxicity directly depicts the degree of liver injury [63]. The histopathological examination of DCF treated rat liver exhibited typical alterations in liver slices such as ballooning, degeneration of hepatocytes, hepatocellular necrosis, and inflammatory cell infiltration (Fig. 5B) which was consistent with the assay results for biochemical parameters associated with hepatotoxicity in the serum and tissue. Further SLM, EA and Tb extract treatment led to an improvement in morphological features (Fig. 5C-F). Hence changes observed in histological sections substantiated the biochemical results.
Since active extract has numerous classes of phytochemical moieties and interaction between them have been postulated for producing synergistic effect to elicit their antioxidant, wound healing, haematopoetic, erythropoetic and other biological activities [59,64]. It is likely that a synergistic interplay berween several Terminalia extract components such as tannin and pseudotannins, gallic acid and its esters, chebulic, chebulagic, chebulinic acids and non-chebulic acid, ellagitannins, corilagin, ellagic acid, flavanols, triterpenes etc. takes place for producing diverse biological activities [21,22]. Hence observed biological activities in extracts could also be attributed to synergism between various phytoconstituents.
The present study suggested that ellagic acid possesses significant protective potential against oxidative stress and liver injury resulting from long term intake of diclofenac as compared to T. bellirica fruit aqueous and ethyl acetate extracts. The study further put forward that plants and their compounds have enormous potential in mitigating hepatotoxicity and oxidative stress resulting from adverse drug effects.
5. Conclusion
Ethyl acetate and aqueous extracts of T. bellirica fruit and one of its bioactive compounds ellagic acid have shown antioxidant and anti-inflammatory activities in vitro. It was observed that long term intake of diclofenac, an anti-inflammatory agent, was liable for causing hepatotoxicity in rats which was characterized by alterations in biomarkers of oxidative stress and liver function in blood and tissue as well as in liver sections. Administration of T. bellirica fruit aqueous and ethyl acetate extracts and ellagic acid demonstrated substantial hepatoprotective efficacy against oxidative stress and hepatic damage in rats. Hepatoprotective efficacy of ellagic acid was comparable to silymarin. The study suggested that T. bellirica fruit constituents have enormous medicinal efficacy as an antioxidant, anti-inflammatory, and hepatoprotective agents. To best of our knowledge, this is the first report showing beneficial effects of T. bellirica fruit extracts and ellagic acid against diclofenac mediated hepatotoxicity. However, further studies are required to understand the exact mechanism of action of various constituents present in T. bellirica which might be helpful in developing the new therapeutic agent of natural origin.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
A.G. and H.K.R. acknowledge UGC New Delhi for financial assistance in the form of CRET Research Fellowship. R.K., A.K.S. and R.G. acknowledge CSIR-UGC New Delhi for financial assistance in the form of Senior Research Fellowships. All the authors gratefully acknowledge UGC-SAP and DST-FIST facilities of the Department of Biochemistry, University of Allahabad, Allahabad.
Edited by Dr. A.M Tsatsaka
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.12.010.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Alqahtani F.Y., Aleanizy F.S., Tahir E.E., Alquadeib B.T., Alsarra I.A., Alanazi J.S., Abdelhady H.G. Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles. Saudi Pharm. J. 2019;27:82–87. doi: 10.1016/j.jsps.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alabi Q.K., Akomolafea R.O., Olukirana O.S., Adeyemi W.J., Nafiua A.O., Adefisayoa O.S. The Garcinia kola biflavonoid kolaviron attenuates experimental hepatotoxicity induced by diclofenac. Pathophysiology. 2017;24:281–290. doi: 10.1016/j.pathophys.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Vane J.R., Botting R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998;47:78–87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 4.Hamza A.A. Curcuma longa, Glycyrrhiza glabra and Moringa oleifera ameliorate diclofenac-induced hepatotoxicity in rats. Am. J. Pharmacol. Toxicol. 2007;2:80–88. [Google Scholar]
- 5.Banks A.T., Zimmerman H.J., Ishak K.G., Harter J.G. Diclofenac-associated hepatotoxicity: analysis of 180 cases reported to the Food and Drug Administration as adverse reactions. Hepatology. 1995;22:820–827. [PubMed] [Google Scholar]
- 6.Kumar R., Singh A.K., Gupta A., Bishayee A., Pandey A.K. Therapeutic potential of Aloe vera- A miracle gift of nature. Phytomed. 2019;60:152996. doi: 10.1016/j.phymed.2019.152996. [DOI] [PubMed] [Google Scholar]
- 7.Gilham B., Papachristodoulou K., Thomas J.H. Butterworth‐Heinemenn.; Oxford: 1997. Wills’ Biochemical Basis of Medicine. [Google Scholar]
- 8.Lewis D.A. In: anti‐inflammatory drugs from plants and marine sources. Agent Actions Suppl. 1989;27:3–373. [PubMed] [Google Scholar]
- 9.Cotran R.S., Kumar V., Robbins S.L. vol. 12. W.B. Saunders Company; Philadelphia: 1994. p. 377. (Robbins, Pathologic Basis of Disease). [Google Scholar]
- 10.Gupta A., Kumar R., Bhattacharya P., Bishayee A., Pandey A.K. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: a systematic and comprehensive review. Phytomedicine. 2020;77:153278. doi: 10.1016/j.phymed.2020.153278. [DOI] [PubMed] [Google Scholar]
- 11.Amrithpal S.S. Science Publishers; 2011. Herbalism Phytochemistry and Ethanopharmacology; pp. 357–361. [Google Scholar]
- 12.Rastogi R.P., Mehrotra B.N. Lucknow and National Institute of Communication and Information Resources; New Delhi: 2004. Compendium of Medicinal Plants, Vol.1. Central Drug Research Institute (CDRI) pp. 406. [Google Scholar]
- 13.Gupta A., Kumar R., Pandey A.K. Antioxidant and antidiabetic activities of Terminalia bellirica fruit extracts in alloxan induced diabetic rats. S. Afr. J. Bot. 2020;130:308–315. [Google Scholar]
- 14.Hazra B., Sarkar R., Biswas S., Mandal N. Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement. Altern. Med. 2010;10:20. doi: 10.1186/1472-6882-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elizabeth K.M. Antimicrobial activity of Terminalia bellerica. Indian J. Clin. Biochem. 2005;20:150–153. doi: 10.1007/BF02867416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aqil F., Ahmad I. Antibacterial properties of traditionally used Indian medicinal plants. Methods Find. Exp. Clin. Pharmacol. 2007;29:79–92. doi: 10.1358/mf.2007.29.2.1075347. [DOI] [PubMed] [Google Scholar]
- 17.Ayoob F.A., Awad H.M., El-Kousy S.M., Rashed K.N., Al-sayed N.H. Phytochemical and biological investigations of Terminalia bellerica Roxb. Leaves. J. Pharm. Res. 2014;8:500–510. [Google Scholar]
- 18.Valsaraj R., Pushpangadan P., Smitt U.W., Adsersen A., Christensen S.B., Sittie A., Nyman U., Nielsen C., Olsen C.E. New Anti-HIV-1, antimalarial, and antifungal compounds from Terminalia bellerica. J. Nat. Prod. 1997;60:739–742. doi: 10.1021/np970010m. [DOI] [PubMed] [Google Scholar]
- 19.Rashed K., Potocnjak I., Giacometti J., Skoda M., Domitrovic R. Terminalia bellerica aerial parts ethyl acetate extract exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. J. Funct. Foods. 2014;8:319–330. [Google Scholar]
- 20.Dhanani T., Shah S., Kumar S. A validated high-performance liquid chromatography method for determination of tannin-related marker constituents gallic acid, Corilagin, chebulagic acid, ellagic acid and chebulinic acid in four Terminalia species from India. J. Chromatogr. Sci. 2015;53:625–632. doi: 10.1093/chromsci/bmu096. [DOI] [PubMed] [Google Scholar]
- 21.Singh A., Bajpai V., Kumar S., Kumar B., Srivastava M., Kumar K.B.R. Comparative profiling of phenolic compounds from different plant parts of six Terminalia species by liquid chromatography-tandem mass spectrometry with chemometric analysis. Ind. Crop. Prod. 2016;87:236–246. [Google Scholar]
- 22.Pfundstein B., El-Desouky S.K., Hull W.E., Haubner R., Erben G., Owen R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia belerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry. 2010;71:1132–1148. doi: 10.1016/j.phytochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Nino W.R., Zazueta C. Ellagic acid: pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015;97:84–103. doi: 10.1016/j.phrs.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A., Pandey A.K. Plant secondary metabolites with hepatoprotective efficacy. In: Galanakis C.M., editor. Nutraceuticals and Natural Product Pharmaceuticals. Elsevier academic press; London, U.K: 2019. pp. 71–104. [Google Scholar]
- 25.Priydarsini K.I., Khopde S.M., Kumar S.S., Mohan H. Free radical studies of ellagic acid, a natural phenolic antioxidant. J. Agric. Food Chem. 2002;50:2200–2206. doi: 10.1021/jf011275g. [DOI] [PubMed] [Google Scholar]
- 26.Hamza R.Z., Al-Harbi M.S. Amelioration of paracetamol hepatotoxicity and oxidative stress on mice liver with Silymarin and Nigella sativa extract supplements. Asian Pac. J. Trop. Biomed. 2015;5:521–531. [Google Scholar]
- 27.Freitag A.F., Cardia G.F.E., da-Rocha B.A., Aguiar R.P., Silva-Comar F.M.S., Spironello R.A., Grespan R., Caparroz-Assef S.M., Bersani-Amado C.A., Cuman R.K.N. Hepatoprotective effect of silymarin (Silybum marianum) on hepatotoxicity induced by acetaminophen in spontaneously hypertensive rats. Evid.-Based Complementary Altern. Med. 2015;2015:538317. doi: 10.1155/2015/538317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pradhan S., Girish S. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. Med. Res. 2006;124:491–504. [PubMed] [Google Scholar]
- 29.Mishra A.K., Mishra A., Kehri H.K., Sharma B., Pandey A.K. Inhibitory activity of Indian spice plant Cinnamomum zeylanicum extracts against Alternaria solani and Curvularia lunata, the pathogenic dematiaceous moulds. Ann. Clin. Microb. Anti. 2009;8:9. doi: 10.1186/1476-0711-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Re R., Pellegrini N., Proteggente A., pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 31.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 32.Mizushima Y., Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J. Pharm. Pharmacol. 1968;20:169–173. doi: 10.1111/j.2042-7158.1968.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 33.Marklund S., Marklund G. Involvement of superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 34.Beers R.F., Jr., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 35.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 36.Cardiff R.D., Miller H.C., Munn R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014:655–658. doi: 10.1101/pdb.prot073411. [DOI] [PubMed] [Google Scholar]
- 37.Sharma U.K., Kumar R., Ganguly R., Gupta A., Sharma A.K., Pandey A.K. Cinnamaldehyde, An active Component of Cinnamon provides Protection against Food colour induced Oxidative stress and Hepatotoxicity in albino Wistar rats. Vegetos. 2018;31:123–129. [Google Scholar]
- 38.Sharma U.K., Sharma A.K., Kumar R., Gupta A., Pandey A., Pandey A.K. Pharmacological activities of cinnamaldehyde and eugenol: antioxidant, cytotoxic and anti-leishmanial studies. Cell. Mol. Biol. 2017;63:73–78. doi: 10.14715/cmb/2017.63.6.15. [DOI] [PubMed] [Google Scholar]
- 39.Kumar R., Gupta A., Singh A., Bishayee A., Pandey A.K. Antioxidant and antihyperglycemic activities of bottlebrush plant (Callistemon lanceolatus) stem extracts. Medicines. 2020;7:11. doi: 10.3390/medicines7030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nampoothiri S.V., Prathapan A., Cherian O.L., Raghu K.G., Venugopalan V.V., Sundaresan A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011;49:125–131. doi: 10.1016/j.fct.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Stagos D., Portesis N., Spanou C. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem. Toxicol. 2012;50:4115–4124. doi: 10.1016/j.fct.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 42.Kuskoski E.M., Asuero A.G., Troncoso A.M., Mancini-Filho J., Fett R. Application of various chemical methods to determine antioxidant activity in fruit pulp. Food Sci. Technol (Campinas) 2005;25:726–732. [Google Scholar]
- 43.Kilic I., Yesiloglu Y., Bayrak Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2014;15:447–452. doi: 10.1016/j.saa.2014.04.052. [DOI] [PubMed] [Google Scholar]
- 44.Kumar S., Kumar R., Dwivedi A., Pandey A.K. In vitro antioxidant, antibacterial and cytotoxic activity and in vivo effect of Syngonium podophyllum and Eichhornia crassipes leaf extracts on isoniazid induced oxidative stress and hepatic markers. Biomed Res. Int. 2014;2014:459452. doi: 10.1155/2014/459452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashley N.T., Weil Z.M., Nelson R.J. Inflammation: mechanisms, costs, and natural variation. Annu. Rev. Ecol. Evol. Syst. 2012;43:385–406. [Google Scholar]
- 46.Voravuthikunchai S., Lortheeranuwat A., Jeeju W., Sririrak T., Phongpaichit S., Supawita T. Effective medicinal plants against enterohaemorrhagic Escherichia coli 0157:47. J. Ethnopharmacol. 2004;94:49–54. doi: 10.1016/j.jep.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Sakat S., Juvekar A.R., Gambhire M.N. In vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata Linn. Int. J. Pharma. Pharm. Sci. 2010;2:146–155. [Google Scholar]
- 48.Liu F., Zhng Y., Yu Q., Shen Y., Zheng Z., Cheng J., Zhang W., Ye Y. Exploration of the binding between ellagic acid, a potentially risky food additive, and bovine serum albumin. Food Chem. Toxicol. 2019;134:1108672. doi: 10.1016/j.fct.2019.110867. [DOI] [PubMed] [Google Scholar]
- 49.Kumar S., Sharma U.K., Sharma A.K., Pandey A.K. Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: phytochemical analysis and antioxidant effect. Cell. Mol. Biol. 2012;58:174–181. [PubMed] [Google Scholar]
- 50.Higuchi S., Wu R., Zhou M., Ravikumar T.S., Wang P. Downregulation of hepatic cytochrome P-450 isoforms and PPAR-γ: Their role in hepatic injury and proinflammatory responses in a double-hit model of hemorrhage and sepsis. J. Surg. Res. 2007;137:46–52. doi: 10.1016/j.jss.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 51.Dorne J.L., Skinner L., Frampton G.K., Spurgeon D.J., Ragas A.M. Human and environmental risk assessment of pharmaceuticals: differences, similarities, lessons from toxicology. Anal. Bioanal. Chem. 2007;387:1259–1268. doi: 10.1007/s00216-006-0963-7. [DOI] [PubMed] [Google Scholar]
- 52.Kumar S., Gupta A., Pandey A.K. Calotropis procera root extract has the capability to combat free radical mediated damage. ISRN Pharmacol. 2013;2013 doi: 10.1155/2013/691372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta A., Singh A.K., Kumar R., Ganguly R., Rana H.K., Pandey P.K. Corilagin in cancer: a critical evaluation of anticancer activities and molecular mechanisms. Molecules. 2019;24:3399. doi: 10.3390/molecules24183399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piao S., Sitch S., Ciais P., Friedlingstein P., Peylin P., Wang X. Evaluation of terrestrial carbon cycle models for their response to climate variability and to CO2 trends. Glob. Change Biol. Bioenergy. 2013;9:2117–2132. doi: 10.1111/gcb.12187. [DOI] [PubMed] [Google Scholar]
- 55.Simon J.P., Evan P.S. Aqueous leaves extract of Madhuca longifolia attenuate diclofenac-induced hepatotoxicity: impact on oxidative stress, inflammation, and cytokines. J. Cell. Biochem. 2018;119:6125–6135. doi: 10.1002/jcb.26812. [DOI] [PubMed] [Google Scholar]
- 56.Gupta A., Pandey A.K. Aceclofenac-induced hepatotoxicity: an ameliorative effect of Terminalia bellerica fruit and ellagic acid. World J. Hepatol. 2020;12:949–964. doi: 10.4254/wjh.v12.i11.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ezzat I.E., Salem O.A.I., Shousha M.A., Abd el-Moneim A.E. The influence of gamma-irradiation and protein deficiency on survival body weight and some blood component in rats. Egypt. J. Biochem. Mol. Biol. 1989;7:125–152. [Google Scholar]
- 58.Rao R.H. Fasting glucose homeostasis in the adaptation to chronic nutritional deprivation in rats. Am. J. Physiol. 1995;268:873–879. doi: 10.1152/ajpendo.1995.268.5.E873. [DOI] [PubMed] [Google Scholar]
- 59.Cock I.E. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae) Inflammopharmacology. 2015;23(5):203–229. doi: 10.1007/s10787-015-0246-z. [DOI] [PubMed] [Google Scholar]
- 60.Lino T., Nakahara K., Miki W. Less damaging effect of whisky in rat stomachs in comparison with pure ethanol. Digestion. 2001;64:214–221. doi: 10.1159/000048864. [DOI] [PubMed] [Google Scholar]
- 61.Palani S., Santhakumari D., Balachandar S., Ambika S. Protective role of Ellagic acid in modulating Iron induced Nephrotoxicity in rats. Int. J. Adv. Res. Biol. Sci. 2015;2(8):35–42. 62. [Google Scholar]
- 62.Sharma U.K., Kumar R., Gupta A., Ganguly R., Pandey A.K. Renoprotective effect of cinnamaldehyde in food color induced toxicity. 3Biotech. 2018;8:212. doi: 10.1007/s13205-018-1241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma U.K., Kumar R., Gupta A., Ganguly R., Singh A.K., Ojha A.K., Pandey A.K. Ameliorating efficacy of eugenol against metanil yellow induced toxicity in albino Wistar rats. Food Chem. Toxicol. 2019;126:34–40. doi: 10.1016/j.fct.2019.01.032. [DOI] [PubMed] [Google Scholar]
- 64.Fahmy N.M., Al-Sayed E., Singab A.N. Genus Terminalia: a phytochemicals and biological review. Med. Aromat. Plants. 2015;4:218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.