Abstract
Background:
Patient identification is an important step for advance care planning (ACP) discussions.
Objectives:
We conducted a scoping review to identify prognostic indices potentially useful for initiating ACP.
Eligibility criteria:
We included studies that developed and/or validated a multivariable prognostic index for all-cause mortality between 6 months and 5 years in community-dwelling adults.
Sources of evidence:
PubMed was searched in October 2018 for articles meeting our search criteria. If a systematic review was identified from the search, we checked for additional eligible articles in its references.
Data abstraction:
We abstracted data on population studied, discrimination, calibration, where to find the index, and variables included. Each index was further assessed for clinical usability.
Results:
We identified 18 articles with a total of 17 unique prognostic indices after screening 9,154 titles. The majority of indices (88%) had c-statistics greater than or equal to 0.70. Only 1 index was externally validated. Ten indices, 8 developed in the U.S. and 2 in the U.K., were considered clinically usable.
Conclusion:
Of the 17 unique prognostic indices, 10 may be useful for implementation in the primary care setting to identify patients who may benefit from ACP discussions. An index classified as ‘clinically usable’ may not be easy-to-use because of a large number of variables that are not routinely collected and the need to program the index into the electronic medical record.
Introduction
In the United States (U.S.) and worldwide, there is recognition that advance care planning (ACP) is important in patient care.1–3 A group of experts using the Delphi process defined ACP as follows: “ACP is a process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals, and preferences regarding future medical care. The goal of ACP is to help ensure that people receive medical care that is consistent with their values, goals and preferences during serious and chronic illness.”4 ACP is a process that allows physicians and other health care professionals to provide care concordant with patient-defined goals and values.5 ACP is not limited to ensuring the designation of a proxy for healthcare decision-making or documentation of code status although these aspects are usually part of the discussion.
While ACP can reduce anxiety and depression in patients and families and increase the likelihood for patients to receive medical care concordant with their goals and values,6–8 only about a third of the population in the U.S. participate in some form of ACP.9 Current research efforts focus on expanding implementation of ACP and measuring its quality and clinical impact.10,11
Although ACP is potentially appropriate for nearly all adult patients, given the realities of a busy practice, it would be useful to have a system for identifying patients with a more limited prognosis. Family medicine physicians are well situated to engage in ACP due to the continuity of care that they provide.12 However, they are often uncertain about which patients to involve in ACP conversations and when to have the discussion.13 Prognosis is often used for referral to hospice or palliative care.14–16 Prognosis is a possible trigger for primary care physicians to initiate ACP with patients.17
We conducted a scoping review and summarized prognostic indices that predict all-cause mortality in community-dwelling adults. The purpose was to identify prognostic indices potentially useful for supporting implementation of ACP in primary care. The key question was: “What studies developed and/or validated a prognostic index for 6-month to 5-year all-cause mortality in community-dwelling adults?” Our objective was to identify indices that might assist family physicians and others with identifying patients who may be appropriate for ACP discussions well before the final weeks of life.
Methods
This review was informed by the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist.18
Eligibility criteria
We adapted the following criteria from the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) checklist19: the study developed and/or validated a multivariable prediction model in community-dwelling adults and the outcome was all-cause mortality in the range of 6-months to 5-years. The time frame was chosen to aid clinicians in initiating ACP before a patient is eligible for hospice, but when a serious illness conversation would be appropriate.8,14 Individuals with a life-expectancy of less than 6 months ideally should undergo a series of ACP conversations, but the focus of this paper was to identify algorithms that could help predict life expectancy for longer intervals and thus promote ACP earlier than it might otherwise be done. All types of articles meeting our eligibility criteria were included.
Information sources and search strategy
PubMed was searched on October 27, 2018 for articles using the strategy indicated in Figure 1. PubMed includes all articles from 1966 or the first year a given journal was published. Search terms were built using concepts such as advance care planning, prognostic index, serious illness, and mortality. Filters such as ‘humans,’ ‘English-language,’ and ‘adult: 19+ years’ were applied. References of a systematic review that met the eligibility criteria were checked to identify additional studies.
Figure 1.
Final Search Query as displayed on PubMed.
Selection of sources of evidence
The literature search and screening of titles were completed by one author (PK). Articles with titles indicating development and/or validation of a prognostic index in community-dwelling adults were chosen. Then the abstracts of these articles were reviewed by two independent investigators (PK and JD/BTL/MBS) for possible inclusion. Differences were resolved by discussion among all reviewers. Full-text articles of abstracts that met our inclusion criteria were assessed for eligibility by one researcher (PK). During full-text review, studies were excluded if: 1) a prognostic index was not internally validated, 2) it identified individual predictors of mortality were identified, but did not develop a usable index, or 3) over half of the cohort used to develop the index was hospitalized or nursing home patients. Eighteen articles met inclusion criteria.20–37
Data abstraction
Two investigators (PK, BTL) reviewed each article and tabulated each prognostic index according to broad categories of usability (clinically usable vs. not usable) and summarized key information regarding each index in Tables 1 to 3. Final tables were agreed on by all authors. An index was considered ‘clinically usable’ if the instrument scoring and interpretation were available either in the article or online and ‘not usable’ otherwise. A website link to each index, if available, was included in Table 3.
Table 1.
Descriptive statistics of the 17 unique indices*
| Time frame for mortality | N | (%) | |
| 6-month20,21 | 1 | (5.9) | |
| 1-year22-26 | 5 | (29.4) | |
| 15-month27 | 1 | (5.9) | |
| 2-year28 | 1 | (5.9) | |
| 3-year29,30 | 2 | (11.7) | |
| 4-year31 | 1 | (5.9) | |
| 5-year32-37 | 6 | (35.3) | |
| Country | |||
| USA20,21,25,26,28,30,31,34–37 | 10 | (58.8) | |
| UK22,23,33 | 3 | (17.6) | |
| Italy24,27 | 2 | (11.8) | |
| Russia29 | 1 | (5.9) | |
| South Korea32 | 1 | (5.9) | |
| C-statistics | |||
| 0.50 – 0.59 (poor) | 0 | (0) | |
| 0.60 – 0.69 (moderate)29,30 | 2 | (11.7) | |
| 0.70 – 0.79 (good)22,23,25,31–34,38 | 8 | (47.1) | |
| 0.80 – 0.89 (very good)20,21,24,26–28,36,37 | 7 | (41.2) | |
| 0.90 – 1.00 (excellent) | 0 | (0) | |
| Calibration | |||
| Well calibrated | 13 | (76.5) | |
| <10% difference22,24,26–28,30,31,34,35,37 | 10 | (58.8) | |
| Hosmer-Lemeshow p>0.0533,36 | 2 | (11.8) | |
| Cox calibration regression25 (perfect calibration: α = 0, β = 1) | 1 | (5.9) | |
| Poorly calibrated (>10% difference)32 | 1 | (5.9) | |
| Calibration curve only20,21 | 1 | (5.9) | |
| Not reported23,29 | 2 | (11.8) | |
| Usability† | |||
| Clinically usable22,26–28,30,31,33,34,36,37 | 10 | (58.8) | |
| Not usable20,21,23–25,29,32,35 | 7 | (41.2) | |
Table 3.
Evaluation of prognostic indices according to usability and time frame of mortality outcome.
| Author (Year) | Population (Country) | Outcome | Where to find Risk Tool and Scoring | Variables included in the prognostic index† |
|---|---|---|---|---|
| Clinically Usable‡ | ||||
| 1. Hippisley-Cox & Coupland (2017)22 | Primary care patients aged 65 or older (England) | 1-year all-cause mortality | Instrument and scoring information not found in article. QMortality®−2017 risk calculator (https://qmortality.org/, accessed April 30, 2019) |
Demographics: age, ethnic group Medications: antipsychotics, corticosteroids Social history: alcohol intake, smoking status, living in a care home, Vital signs/labs: abnormal liver function test result, anemia, body mass index, high platelet count Medical diagnosis: asthma or chronic obstructive pulmonary disease, atrial fibrillation, cancer, cardiovascular disease, chronic kidney disease, chronic liver disease or pancreatitis, congestive heart failure, dementia, diabetes type 1, diabetes type 2, epilepsy, learning disability, leg ulcer, Parkinson’s disease, rheumatoid arthritis, venous thromboembolism Functional measures: Townsend deprivation score, poor mobility Other: unplanned hospital admissions in the past 12 months, visits to a general practitioner in the past 12 months with either appetite loss, unexplained weight loss, or dyspnea |
| 2. Gagne et al. (2011)26 | Medicare enrollees aged 65 years or older (USA) | 1-year all-cause mortality | Instrument is presented in Table 3 and how to interpret score in bottom panel Figure 2.26
Gagne Index (https://eprognosis.ucsf.edu/gagne.php, accessed April 30, 2019) |
Medical diagnosis: alcohol abuse, deficiency anemia, any tumor, cardiac arrhythmias, chronic pulmonary disease, coagulopathy, complicated diabetes, congestive heart failure, dementia, fluid and electrolyte disorders, hemiplegia, HIV/AIDS, hypertension, liver disease, metastatic cancer, peripheral vascular disorder, psychosis, pulmonary circulation disorders, renal failure, weight loss |
| 3. Mazzaglia et al. (2007)27 | Community-dwelling adults aged 65 years and older (Italy) | 15-month all-cause mortality | Instrument and how to interpret score are partially available in the article (Table 2 and Figure 1A).27 Authors do not state how to score the 7-item screening test (p. 1956). Mazzaglia Index (https://eprognosis.ucsf.edu/mazzaglia.php, accessed April 30, 2019) |
Demographics: age, sex Medications: ≥5 prescription medications Functional measures: positive responses to a screening test45 (need help in performing basic ADL, need help in performing IADL, poor vision, poor hearing, weight loss, use of homecare services, and self-perceived inadequacy of income) Other: hospitalization in the previous 6 months |
| 4. Carey et al. (2004)28 | Frail community-dwelling adults aged 70 years and older (USA) | 2-year all-cause mortality | Instrument in Table 3 and interpretation of scoring in Table 4.28 Carey 2 Year Index (https://eprognosis.ucsf.edu/carey2.php, accessed April 30, 2019) |
Demographics: age, sex Functional measures: dependence in bathing, dependence in shopping, difficulty walking several blocks, difficulty pulling/pushing heavy objects |
| 5. Carey et al. (2008)30 | Community-living patients aged 75 years and older enrolled in the Program of All-Inclusive Care for the Elderly (USA) | 1-, 2-, and 3-year all-cause mortality | Instrument in Table 3 and interpretation of scoring in Table 4.30 Carey 3 index available online (https://eprognosis.ucsf.edu/carey3.php, accessed April 30, 2019) |
Demographics: age, male sex Medical diagnosis: congestive heart failure, COPD, malignant neoplasm, renal failure or insufficiency Functional measures: dependence in toileting, dependence in dressing |
| 6. Lee et al. (2006)31 | Community-dwelling adults aged 50 years and older (USA) | 4-year all-cause mortality | Instrument available in the article (Box on p. 807), score interpretation Table 4.31 Calculator online (https://eprognosis.ucsf.edu/lee.php, accessed April 30, 2019) |
Demographics: age, male sex Social history: current smoker Vital signs/labs: BMI < 25 Medical diagnosis: diabetes mellitus, cancer, lung disease, heart failure, Functional measures: bathing, managing finances, walking several blocks, pushing/pulling heavy objects |
| 7. Ganna & Ingelsson (2015)33 | Community-based participants aged 40 to 70 years (UK) | 5-year all-cause mortality | Instrument available online, but not in the article. (https://www.ubble.co.uk/risk-calculator/, accessed April 30, 2019) |
Women Demographics: age, gender Social history: financial assistance, smoking history Medical diagnosis: cancer Functional measures: disability or infirmity, usual walking pace, Other: number of live births, presence of long-standing illness, self-rated overall health, serious life events in the last 2 years, visit with a general practitioner for nerves, anxiety, tension or depression Men Demographics: age, gender Social history: financial assistance, number of vehicles owned in a household, number of people living in house, relatedness of people living in house, smoking history Medical diagnosis: diabetes, cancer, history of heart attack, angina, stroke, or high blood pressure Functional measures: usual walking pace Others: self-rated overall health, serious life events in the last 2 years |
| 8. Mathias et al. (2013)34 | Outpatients aged 50 years and older (USA) | 5-year all-cause mortality | Instrument available online, but not in the article. (http://info.eecs.northwestern.edu/FiveYearLifeExpectancyCalculator, accessed September 2019) |
Demographics: age, sex Medications: digoxin prescription, loop diuretic prescription Vital signs/labs: mean diastolic blood pressure, albumin – mean, median, standard deviation for the prior year, creatinine – mean, median, standard deviation for the prior year Medical diagnosis: any vascular disease, heart failure, hypertension, chronic kidney disease, diabetes mellitus, dementia, HIV, anemia, any cancer, any liver disease Functional measures: number of visits to primary care provider in the year prior to the index visit, number of hospitalizations 0–1 years prior, number of hospitalizations 1–2 years prior |
| 9. Zhang et al. (2012)36 | Community-dwelling elderly population aged 70 years and older (USA) | 1- and 5-year all-cause mortality | Instrument and scoring available in the article (Figures 1 and 2).36 |
1-year Demographics: age, gender Medical diagnosis: coronary artery disease Functional measures: IADL stage 5-year Demographics: age, gender Medical diagnosis: cancer, coronary artery disease, diabetes, other heart disease Functional measures: IADL stage Other: self-rated health status |
| 10. Schonberg et al. (2009)37 | Community-dwelling adults aged 65 and older (USA) | 5-year all-cause mortality | Instrument available in the article (Table 2) and scoring in Table 3.37 Available online (https://eprognosis.ucsf.edu/leeschonberg.php, accessed April 30, 2019) |
Demographics: age, male sex Social history: smoking status Vital signs/labs: body mass index < 25 Medical diagnosis: cancer, emphysema/chronic bronchitis, diabetes mellitus Functional measures: needs help of other persons handling routine needs, difficulty walking Others: overnight hospitalizations in past year, perceived health |
| Not usable‡ | ||||
| 11. Duarte et al. (2015)21 | Primary and tertiary care patients aged 65 years and older (USA) | 6-month all-cause mortality | Instrument available in the Appendix (self-reported patient questionnaire)21; scoring not shown. |
Demographics: age, sex Social history: proxy status, smoking status Medical diagnosis: any cancer, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD) Functional measures: activities of daily living (ADLs), health-related quality of life (HRQOL) |
| 12. Han et al. (2012)20 | Medicare Health Outcomes Survey respondents aged 65 years or older (USA) | 6-month all-cause mortality | Not in article or online. | See Duarte et. al (2015)21 |
| 13. Crooks et al. (2016)23 | Primary and secondary care patients aged 20 years and older (England) | 1-year all-cause mortality | Not in article or online. |
Social history: alcohol or illegal drug use Medical diagnosis: burns, chromosomal anomalies, cerebrovascular disease, chronic obstructive pulmonary disease (COPD), cirrhosis, dementia, diabetes, epilepsy, esophageal, heart conduction disorders, heart failure, interstitial lung disease, liver disease, lung disease due to external agents, malignancy of respiratory tract and intrathoracic organs, malignancy of lymphatic and hematopoietic tissue, metastases, neoplasm histology, nephritis, nephrosis and nephrotic syndrome, non-deficiency and non-hemolytic anemias, non-malignant white cell, non-organic psychoses, other central nervous system disorders, platelet and splenic disorders, paralysis, Parkinson’s disease, spinal disease, peripheral vascular disease, pleural disease, stomach and duodenal diseases |
| 14. Pilotto et al. (2013)24 | Community-dwelling adults aged 65 years and older (Italy) | 1-year all-cause mortality | A link to download free software program in Italian available in the article but must know Italian. (http://www.operapadrepio.it/impi/svamasetup.exe, accessed April 2019) |
Demographics: age, sex Social history: nursing care needs, social support network Functional measures: Barthel Index (ADL and mobility) Others: Short Portable Mental Status Questionnaire, Exton-Smith Scale for pressure ulcer |
| 15. Wang et al. (2013)25 | Primary care patients of the Veterans Health Administration aged 18–110 years (USA) | 1-year all-cause mortality | Not in article or online. |
Demographics: age, sex Medications: alpha-blockers, Antidepressants, antiplatelet, angiotensin converting enzyme inhibitor/angiotensin receptor blocker, anticholinergics, antipsychotics, benzodiazepines, beta-agonists, beta-blockers, bumetanide, calcium channel blockers, digoxin, furosemide, HMG-CoA inhibitors, other hypertension drugs, insulin, metformin, metolazone, nitrate long lasting, nitrate short lasting, opioid narcotics, non-steroidal anti-inflammatory drug, non-statin lipid lowering agents, thiazolidinediones, potassium-sparing diuretic, oral steroids, sulfonylureas, diuretic combinations, torsemide, warfarin Social history: substance abuse Vital signs/labs: albumin, blood pressure (diastolic), blood pressure (systolic), blood urea nitrogen, BMI, creatinine, diastolic blood pressure, systolic blood pressure, heart rate, respiration, potassium, white blood cell count Medical diagnosis: acute myocardial infarction, old myocardial infarction, unstable angina, stroke, hemiplegia, Atherosclerosis, depression, heart failure, respiratory failure, valvular heart disease, diabetes, hypertension, COPD, pneumonia, peripheral vascular disease, metastatic cancer, psychotic disorder, liver disease, atrial fibrillation, post-traumatic stress disorder, mental disorder Other: malnutrition, function disease, trauma, coronary artery bypass graft surgery, enrollment priority group 1–8, Deyo-Charlson index, emergency room visits in the past year, cardiology visit in the past year, service connection ≥50%, number of providers, primary care visits in the past year, phone visits in the past year, other non-face visits in the past year, outpatient visits in the past year, mental health hospitalization in the past year, all hospitalization, number of medication refills |
| 16. Turusheva et al. (2017)29 | Community-dwelling adults aged 65 years and older (Russia) | 3-year all-cause mortality | Instrument in Box 1.29 Interpretation unclear. Instrument in Box 1.29 Interpretation unclear. |
Model 1 Demographics: age, male sex Vital signs/labs: anemia, forced expiratory volume in 1 second (FEV1)/[Height(Ht)3], mid-arm muscle area (MAMA) Functional measures: Short physical performance battery (SPPB) Model 2 Demographics: age, male sex Vital signs/lab: brain natriuretic peptide (hBNP), anemia, C-Reactive Protein (CRP), MAMA, FEV1/Ht3 Functional measures: SPPB |
| 17. Jung et al. (2016)32 | Community-dwelling adults aged 65 years and older (South Korea) | 3- and 5-year all-cause mortality | Application for mobile devices is available for download in Korean (personal communication with authors). |
Demographics: age, gender Functional measures: activities of daily living (ADLs), instrumental activities of daily living (IADLs) Other: Charlson Comorbidity Index or Cumulative Illness Rating Scale for Geriatrics, Korean version of the Geriatric Depression Scale, Korean Mini-Mental State Examination, Mini Nutritional Assessment or Nutrition Screening Initiative |
| 18. Tan et al. (2013)35 | Medicare beneficiaries aged 66–90 years (USA) | 1- and 5-year all-cause mortality | Not in article or online. |
Demographics: age Social history: alcohol abuse, drug abuse Medical diagnosis: acquired immunodeficiency syndrome, cardiac arrhythmia, chronic pulmonary disease, chronic blood loss anemia, coagulopathy, congestive heart failure, deficiency anemia, depression, diabetes without chronic complications, diabetes with chronic complications, fluid and electrolyte disorders, hypertension (uncomplicated), hypertension (complicated), hypothyroidism, liver disease, lymphoma, metastatic cancer, neurological disorders other than paralysis, obesity, paralysis, peptic ulcer disease excluding bleeding, peripheral vascular disease, psychoses, pulmonary circulation disease, renal failure, rheumatoid arthritis/collagen vascular disease, solid tumor without metastasis, valvular disease, weight loss |
Not shown if the prognostic index did not have variables in one of the major categories (demographics, medications, social history, vital signs/labs, medical diagnosis, functional measures, other).
Clinically usable if the mortality risk can be calculated using the instrument and interpreted using tables and/or figures in the paper without referring to the main text in the article, and ‘Not usable’ otherwise or if risk calculator is in a language other than English.
Discrimination of a prognostic index, as measured by the c-statistic in the cut-point analyses of the index, was categorized as poor (< 0.60), moderate (0.60 – 0.69), good (0.70 – 0.79), very good (0.80 – 0.89), or excellent (≥ 0.90).38,39 Tools were considered well calibrated if the percentage difference between predicted and observed mortality in a given risk group was less than 10, and poor if greater than or equal to 10 percent.38 Other calibration and fitting methods such as Hosmer-Lemeshow statistics, a test where statistical significance indicates poor calibration, and Cox calibration regression, where an α intercept of 0 and β slope of 1 indicate perfect calibration, were included if reported.40 If the index predicted mortality at more than one time-point, it was categorized under the longest mortality estimate that did not exceed 5 years, but information regarding the authors’ other cut points was included in Table 2.
Table 2.
Prognostic indices for community-dwelling adults, by the most recent published year and authors in alphabetical order, according to the increasing order of the time frame of mortality index.
| Author | Index | Population | Accuracy* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Calibration | |||||||||
| Development | Validation | ||||||||
| Discrimination (95% CI) | |||||||||
| Development | Validation | Development | Validation | ||||||
| 6-month all-cause mortality | |||||||||
| Duarte et al. (2015)21 | 6-mo in inpatient and outpatient age ≥65 y | See Han et al. (2012) 20 | n = 467 Mean age 80 y 56% female 7% 6-mo mortality |
Graph only (Figure 2 of paper)21; used Hosmer-Lemeshow statistic, p = 0.66, indicates the updated version of the original model is well calibrated | |||||
| C = 0.75; same cohort used by Han et al. (2012) | C = 0.73 (0.63–0.82) | ||||||||
| Han et al. (2012)20 | 6-mo in Medicare beneficiaries age ≥65 y | n = 21,870 Mean age 78 y 59% female 15% 6-mo mortality |
Used 10-fold cross validation of development cohort | Graph only (Figure 3 of paper)20 | |||||
| C = 0.75 | C-statistic NR | ||||||||
| 1-year all-cause mortality | |||||||||
| Hippisley-Cox & Coupland (2017)22 | 1-y primary care patients age ≥65 y | n = 1,466,598 Mean age 75 y 45% male 95% white or not recorded 12% 1-y mortality |
n = 499,478 Mean age 75 y 45% male 95% white or not recorded 12% 1-y mortality |
NR | Women | ||||
| Risk percentile | Predicted, 1-y (%) |
Observed, 1-y (%) |
|||||||
| ≥ 50th | 13.5 | 13.1 | |||||||
| ≥ 90th | 36.1 | 35.4 | |||||||
| ≥ 98th | 59.4 | 50.6 | |||||||
| Men | |||||||||
| Risk percentile | Predicted, 1-y (%) |
Observed, 1-y (%) |
|||||||
| ≥ 50th | 13.8 | 13.6 | |||||||
| ≥ 90th | 36.8 | 37.7 | |||||||
| ≥ 98th | 64.6 | 56.9 | |||||||
|
C-statistic NR |
C = 0.853 (0.850–0.856), women C = 0.844 (0.841–0.847), men |
||||||||
| Crooks et al. (2016)23 | 1-y in primary care patients age 20–100 y | n = 328,628 Mean age NR % gender NR 3% 1-y mortality |
n = 328,636 Mean age NR % gender NR 3% 1-y mortality |
NR; used Akaike’s information criterion (AIC) and likelihood ratio test (p<0.0001) to compare relative goodness of fit | |||||
| C = 0.88 | C = 0.88 | ||||||||
| Pilotto et al. (2013)24 | 1-y in community-dwelling age ≥65 y | n = 7,876 Mean age 82 y 63% female 43% 1-y mortality |
n = 4,144 Mean age 82 y 63% female 44% 1-y mortality |
Quintile of predicted risk | Predicted, 1-y (%) |
Observed, 1-y (%) |
Quintile of predicted risk | Predicted, 1-y (%) |
Observed, 1-y (%) |
| 1 | 14.6 | 11.9 | 1 | 14.6 | 9.6 | ||||
| 2 | 25.0 | 21.2 | 2 | 25.2 | 23.0 | ||||
| 3 | 37.7 | 36.4 | 3 | 38.4 | 40.4 | ||||
| 4 | 58.2 | 66.4 | 4 | 59.4 | 64.4 | ||||
| 5 | 91.9 | 90.2 | 5 | 92.8 | 91.5 | ||||
| C = 0.79 (0.78–0.80) | C = 0.79 (0.78–0.80) | ||||||||
| Wang et al. (2013)25 |
1-y in primary care patients within the Veterans Health Administration age 18–110 y | n = 2,761,392 Mean age 64 y 94% male 2.6 % 1-y mortality |
n = 1,837,016 Random splitting; can assume similar characteristics to development cohort |
NR | See Figure 125 (lower middle panel) Used Cox calibration regression, which indicated model was extremely well-calibrated; slope (95% CI): 1.002 (0.995 – 1.009), intercept (95% CI): 0.001 (−0.021 – 0.023) |
||||
| C-statistic NR | C = 0.851 (0.850–0.853) | ||||||||
| Gagne et al. (2011)26 | 1-y in Medicare enrollees age ≥65 y | n = 120,679 Mean age 80 y 83% female 9% 1-y mortality |
n = 123,855 Mean age 79 y 77% female 7% 1-y mortality |
NR | Predicted, 1-y (%) | Observed, 1-y (%) | |||
| <7 | 3.1 | ||||||||
| 7 - <17 | 11.8 | ||||||||
| ≥17 | 29.2 | ||||||||
| C-statistic NR | C = 0.788 (0.786–0.791) | ||||||||
| 15-month all-cause mortality | |||||||||
| Mazzaglia et al. (2007)27 | 15-mo in community-dwelling age ≥65 y | n = 2,470 Mean age 75 y 56% female 5% 15-mo mortality |
N = 2,926 Mean age 75 y 59% female 4% 15-mo mortality |
Risk Score | Observed, 15-mo (%) (95% CI) | Risk Score | Observed, 15-mo (%) (95% CI) | ||
| 0 | 0.2 (0.04–1.1) | 0 | 0.3 (0.03–1.1) | ||||||
| 1 | 1.4 (0.4–3.6) | 1 | 0.9 (0.1–2.1) | ||||||
| 2 | 1.1 (0.4–2.3) | 2 | 0.7 (0.2–1.1) | ||||||
| ≥ 3 | 9.6 (7.9–11.5) | ≥ 3 | 8.2 (6.7–9.8) | ||||||
| C = 0.75 (0.72–0.78) | C = 0.75 (0.73–0.78) | ||||||||
| 2-year all-cause mortality | |||||||||
| Carey et al. (2004)28 | 2-y in community- dwelling age ≥70 y | n = 4,516 Mean age 78 y 61% female 84% White 10% 2-y mortality |
n = 2,877 Mean age 78 y 61% female 73% White 12% 2-y mortality |
Risk Score | Observed, 3-y (%) | Risk Score | Observed, 3-y (%) | ||
| 0–2 | 3 | 0–2 | 5 | ||||||
| 3–6 | 11 | 3–6 | 12 | ||||||
| 7–10 | 34 | 7–10 | 36 | ||||||
| C = 0.76 | C = 0.74 | ||||||||
| 3-year all-cause mortality | |||||||||
| Turusheva et al. (2017)29 | 3-y in community-dwelling adults age ≥65 y | n = 379 Mean age 77 y 75% female 13% 2.5-y mortality |
n = 567 Mean age 85 y 63% female 23% 3-y mortality |
NR | NR (external validation in adults ≥80); they show a net reclassification index (NRI) of 0.0011 (95% CI - 0.1742–0.1884) |
||||
|
C = 0.72 (0.70–0.74), model 1 C = 0.73 (0.71–0.75), model 2 |
C = 0.59 (0.54–0.64), model 1 C = 0.60 (0.55–0.64), model 2 |
||||||||
| Carey et al. (2008)30 | 1-, 2-, & 3-y in community-dwelling age ≥55 y | n = 2,232 Mean age 79 y 68% female 40% white 13% 1-y mortality NR 2-y mortality 37% 3-y mortality |
n = 1,667 Mean age 79 y 76% female 65% white 13% 1-y mortality NR 2-y mortality 36% 3-y mortality |
Risk Score | Observed (%) | Risk Score | Observed (%) | ||
| 1-y | 0–3 | 6.4 | 1-y | 0–3 | 6.8 | ||||
| 4–5 | 12.1 | 4–5 | 10.8 | ||||||
| ≥5 | 20.6 | ≥5 | 22.2 | ||||||
| 2-y | 0–3 | 13.8 | 2-y | 0–3 | 14.5 | ||||
| 4–5 | 24.3 | 4–5 | 24.8 | ||||||
| ≥5 | 39.7 | ≥5 | 40.5 | ||||||
| 3-y | 0–3 | 20.9 | 3-y | 0–3 | 18.1 | ||||
| 4–5 | 36.2 | 4–5 | 35.7 | ||||||
| ≥5 | 54.1 | ≥5 | 55.1 | ||||||
| C = 0.66 | C = 0.69 | ||||||||
| 4-year all-cause mortality | |||||||||
| Lee et al. (2006)31 | 4-y in community-dwelling adults age ≥50 y | n = 11,701 Mean age 67 y 57% female 81% white 10% black 12% 4-y mortality |
n = 8,009 Mean age 67 y 56% female 71% white 19% black 13% 4-y mortality |
Point Score | Observed, 4-y (%) | Point Score | Observed, 4-y (%) | ||
| 0–5 | 3 | 0–5 | 4% | ||||||
| 6–9 | 15 | 6–9 | 15% | ||||||
| 10–13 | 40 | 10–13 | 42% | ||||||
| ≥14 | 67 | ≥14 | 64% | ||||||
| C = 0.84 | C = 0.82 | ||||||||
| 5-year all-cause mortality | |||||||||
| Jung et al. (2016)32 | 3- and 5-y in community-dwelling adults age ≥65 y | n = 988 Mean age 76 y 56% female 9% 3-y mortality 18% 5-y mortality |
n = 1,109 Mean age 77 y 64 % female 20% 3-y mortality 31% 5-y mortality |
Geriatric Prognosis Index (GPI) Score | Predicted, 3-y (%) |
Observed, 3-y (%) |
Geriatric Prognosis Index (GPI) Score | Observed, 3-y (%) | |
| 0 | 0.7 | 0 | 0 | 11.8 | |||||
| 2 | 3.1 | 4.3 | 2 | 13.5 | |||||
| 4 | 11.9 | 14.4 | 4 | 22.5 | |||||
| 6 | 36.6 | 34.5 | 6 | 36.5 | |||||
| 8 | NR | NR | 8 | 66.7 | |||||
| Predicted, 5-y (%) |
Observed, 5-y (%) |
Observed, 5-y (%) | |||||||
| 0 | 14.7 | 0 | 0 | 11.8 | |||||
| 2 | 12.7 | 8.7 | 2 | 18.8 | |||||
| 4 | 35.1 | 31.1 | 4 | 31.3 | |||||
| 6 | 78.8 | 58.6 | 6 | 60.8 | |||||
| 8 | NR | NR | 8 | 83.3 | |||||
|
C = 0.78 (0.74–0.82), 3-y C = 0.80 (0.76–0.83), 5-y |
C = 0.73 (0.69–0.72), 3-y C = 0.80 (0.77–0.82), 5-y |
||||||||
| Ganna & Ingelsson (2015)33 | 5-y in community-dwelling patients age 40–70 y | n = 498,103 Mean age 57 y, male Mean age 56 y, female 54% female 2% 5-y mortality |
n = 35,810 Mean age NR % gender NR 2% 5-y mortality |
NR | Used Hosmer-Lemeshow statistics Women, p = 0.28; well-calibrated Men, p = 0.0402; not well-calibrated |
||||
| C-statistic NR |
C = 0.79 (0.76–0.83), women C = 0.80 (0.77–0.83), men |
||||||||
| Mathias et al. (2013)34 |
5-y in adults age ≥50 y | n = 7,463 Mean age 62 y 40% male 51% white 11% 5-y mortality |
Used 10-fold cross-validation of development cohort | NR | 5-y mortality risk decile | Predicted, 5-y (%) | Observed, 5-y (%) | ||
| <10% | 3.6 | 3.6 | |||||||
| 20 to <30% | 24.4 | 24.2 | |||||||
| 50 to <60% | 54.8 | 55.5 | |||||||
| 70 to 80% | 74.7 | 68.8 | |||||||
| ≥90% | 92.5 | 85.7 | |||||||
| Hosmer Lemeshow, p = 0.20 | |||||||||
| C-statistic NR | C = 0.86 (0.85–0.87) | ||||||||
| Tan et al. (2013)35 | 1- & 5-y in Medicare beneficiaries age 66–90 y | n = 568,656 Mean age 76 y, female Mean age 75 y, male 60% female Mortality NR for either 1-y or 5-y, Graph only for survival (See Figure 1)35 |
n = 568,655 Mean age NR 60% female Mortality NR for either 1-y or 5-y, Graph only for survival (See Figure 1)35 |
NR | Predicted, 1-y (%) | Observed, 1-y (%) | |||
| < 25% | 4.3 | ||||||||
| 25% – 49% | 33.4 | ||||||||
| 50% – 74% | 46.6 | ||||||||
| ≥75% | 52.4 | ||||||||
| Predicted, 5-y (%) | Observed, 5-y (%) | ||||||||
| < 25% | 12.1 | ||||||||
| 25% – 49% | 38.1 | ||||||||
| 50% – 74% | 63.5 | ||||||||
| ≥75% | 80.4 | ||||||||
| Intercept | Slope | ||||||||
| Female | 1-y | 0.312 | 1.119 | ||||||
| 5-y | 0.075 | 1.065 | |||||||
| Male | 1-y | 0.238 | 1.102 | ||||||
| 5-y | 0.064 | 0.065 | |||||||
| Intercept values close to 0 and slope close to 1 indicate good calibration | |||||||||
| Female C = 0.81, 1-y C = 0.79, 5-y |
Male C = 0.79, 1-y C = 0.76, 5-y |
Female C = 0.79, 1-y C = 0.78, 5-y |
Male C = 0.77, 1-y C = 0.76, 5-y |
||||||
| Zhang et al. (2012)36 | 1- & 5-y in community-dwelling adults age ≥70 y | n = 4,434 49.7% age ≥70-<75 y 63.3% female 89.2% white 3.3% 1-y mortality 22.8% 5-y mortality |
n = 2,939 49.3% age ≥70-<75 y 62.4% female 88.3% white 4.1% 1-y mortality 24.0% 5-y mortality |
Risk Groups (Sum Scores) | Observed, 1-y (%) | Risk Groups (Sum Scores) |
Observed, 1-y (%) | ||
| Very Low (0–2) | 1.8 | Very Low (0–2) | 2.0 | ||||||
| Low (3–4) | 4.7 | Low (3–4) | 6.5 | ||||||
| Moderate (5–6) | 7.6 | Moderate (5–6) | 11.5 | ||||||
| High (7–8) | 21.9 | High (7–8) | 18.8 | ||||||
| Very High (≥9) | 47.1 | Very High (≥9) | 31.6 | ||||||
| Observed, 5-y (%) | Observed, 5-y (%) | ||||||||
| Very Low (0–2) | 6.9 | Very Low (0–2) | 8.7 | ||||||
| Low (3–6) | 12.8 | Low (3–6) | 14.5 | ||||||
| Moderate (7–14) | 30.0 | Moderate (7–14) | 30.3 | ||||||
| High (15–17) | 55.0 | High (15–17) | 57.6 | ||||||
| Very High (≥18) | 79.9 | Very High (≥18) | 66.1 | ||||||
| Hosmer-Lemeshow statistics p > 0.1 for 1-y and 5-y, which indicates good overall calibration | |||||||||
|
C = 0.72, 1-y C = 0.74, 5-y |
C = 0.72, 1-y C = 0.72, 5-y |
||||||||
| Schonberg et al. (2009)37 | 5-y in community-dwelling adults age >65 y | n = 16,077 27% age ≥80 y 62% female 85% non-Hispanic white 17% 5-y mortality |
n = 8,038 Random splitting; can assume similar characteristics to development cohort |
Point Score | Observed, 5-y (%), (95% CI) |
Point Score | Observed, 5-y (%), (95% CI) |
||
| 0–1 | 2 (1–4) | 0–1 | 3 (1–6) | ||||||
| 6–7 | 11 (10–14) | 6–7 | 12 (10–15) | ||||||
| 10–11 | 25 (23–28) | 10–11 | 29 (25–33) | ||||||
| 14–15 | 47 (32–42) | 14–15 | 49 (43–55) | ||||||
| ≥18 | 71 (65–77) | ≥18 | 62 (54–70) | ||||||
| C-statistic NR | C = 0.75 | ||||||||
Data for 6-month to 5-year mortality only, with 95% confidence interval if reported
Predicted and observed mortality rate, or ranges of mortality rate, from low to high risk groups if reported (e.g., percentiles or classes (low, middle, high))
CI = confidence interval, -mo = n-month all-cause mortality, NA = not available, NR = Not Reported, y = years, -y = n-year all-cause mortality
Results
Search results
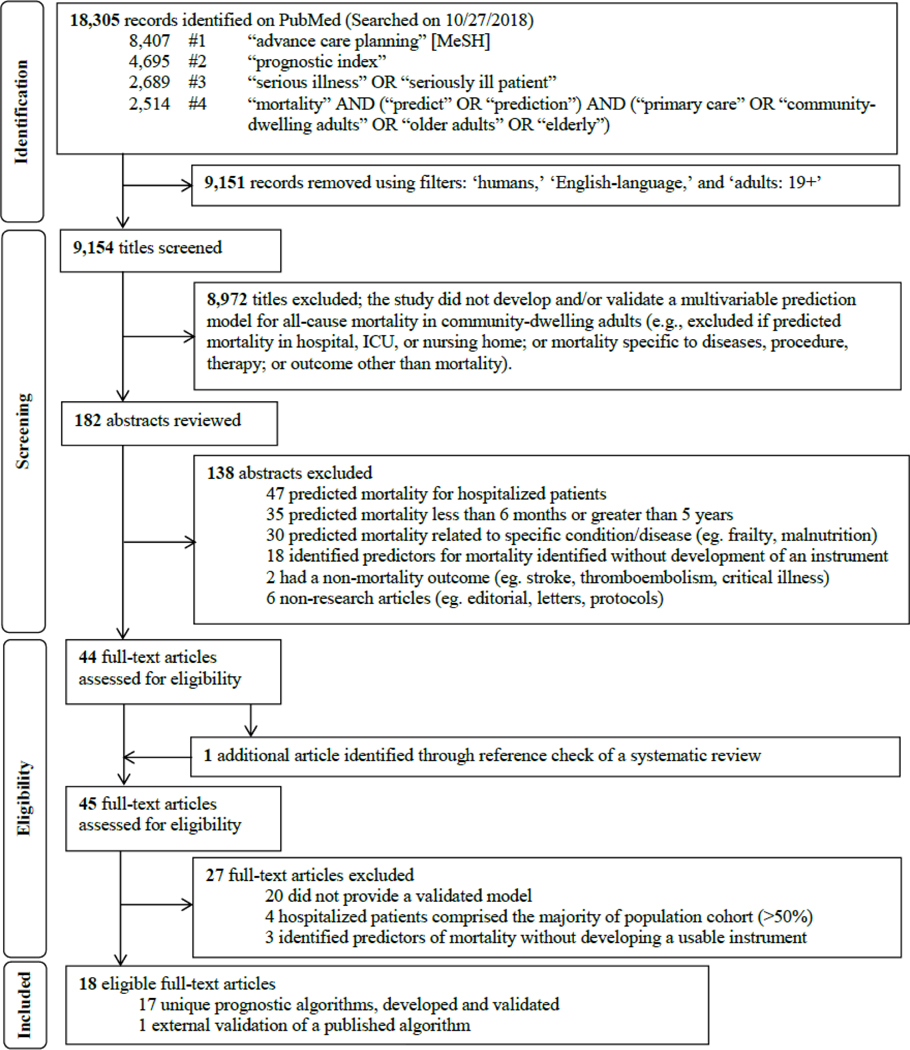
Figure 2 shows the flow diagram of the study selection process, adapted from the PRSIMA statement.41 Using our pre-defined search terms, 18,305 records were identified; applying filters on PubMed excluded 9,151 records and 9,154 titles were screened. After the title screening process, 182 abstracts were reviewed for potential eligibility. Forty-four full-text articles were assessed, and 1 additional unique article was found in the references of a systematic review identified through the search,38 leading to 45 articles that were considered for full-text review. Of these, 18 articles met our inclusion criteria and were summarized in Tables 1 to 3.20–37 Of these, 1 study externally validated a published index,21 yielding a total of 17 unique indices. Table 1 summarizes the 17 indices. The majority of indices were developed in the U.S. (n = 10), followed by Europe (n = 6) and Asia (n = 1).
Figure 2.
Flow diagram of study selection process to identify potentially useful prognostic indices in the primary care setting to help initiate advance care planning, adapted from the PRISMA statement.24
The systematic review conducted by Yourman et al. identified 16 unique prognostic indices that were developed in the community, nursing home, and hospital settings.38 Five out of 6 prognostic indices in the community setting for predicting 1- to 5-year mortality were captured using the search terms in this scoping review.27,28,30,31,37 The combined comorbidity score to predict 1-year mortality by Gagne et al. was not captured, possibly because it is listed under the medical subjects heading term ‘hospital mortality’ on PubMed.26 It met our inclusion criteria and was included in the final list for full-text review. The remaining 10 of 16 studies in Yourman et al. were excluded based on our eligibility criteria.
Characteristics of identified indices
Table 2 summarizes the 18 articles meeting criteria for full review, including the population studied, information on the development and/or validation cohort, calibration statistics, and discrimination as assessed by the c-statistic. Calibration and discrimination should be described for clinical prediction models.42 There was heterogeneity in reporting calibration of prognostic indices, but the majority of indices were well calibrated, as indicated by less than 10% difference in the predicted and observed mortality rates.22,24,26–28,30,31,34,35,37 Two studies did not report calibration.23,29 No prognostic indices had excellent discrimination (c-statistic ≥ 0.90). Eight indices had very good discrimination (c-statistic 0.80–0.89),22,23,25,31–35 7 had good discrimination (c-statistic 0.70–0.79),21,24,26–28,36,37 and 2 had moderate discrimination (c-statistic 0.60–0.69).29,30
Table 3 groups the indices by clinical usability. For each index we report authors and year published, population and country, mortality time frame, where to find, and all variables included in the instrument. Ten articles presented their prognostic indices either in the article or online, and were classified as ‘clinically usable.’22,26–28,30,31,33,34,36,37 There was 100% agreement between the 2 reviewers (PK, BTL) on clinical usability. Although Pilotto et al.24 included a link to downloadable software, we classified it as ‘not usable’ because it requires knowledge of Italian. The modified Geriatric Prognostic Index by Jung et al.32 is available as a free downloadable application on mobile devices, but we classified it as ‘not usable’ because it requires knowledge of Korean and uses scales not commonly used in the U.S.
Assessment of individual indices
Summarized below are 17 published indices identified from 18 articles according to the time frame of the mortality index.
6-month mortality
Duarte et al.21 externally validated the Patient-Reported Outcome Mortality Prediction Tool (PROMPT) in patients age 65 and older in Maine, U.S. The development cohort was that used by Han et al.20 The PROMPT questionnaire shown in their Appendix is a patient self-reported questionnaire that takes 15 minutes. The calibration curve was shown, but no information was provided on predicted or observed mortality rates for the different risk groups. The index had good discrimination.
Han et al.20 developed PROMPT, which estimates 6-month mortality risk using cohorts from the 1998–2003 Medicare Health Outcomes Survey of community-dwelling adults aged 65 years and older in the U.S. The calibration curve was shown, but no actual data was provided for the predicted or observed mortality rates for the different risk groups. The index had good discrimination.
1-year mortality
The QMortality® risk prediction equation developed by Hippisley-Cox and Coupland is a 1-year mortality index for primary care patients aged 65 and older.22 Using a large, validated medical research database in England, the algorithms for both men and women were well calibrated and had very good discrimination.
Crooks et al. developed a co-morbidity score to predict 1-year mortality using 3 national administrative databases in England.23 All people older than 20 years registered to a primary care practice were followed for a year and were randomly divided into two halves for development and validation. Characteristics of each cohort were not reported separately. The relative goodness of fit was statistically significant when compared to the Charlson and the Elixhauser indices (likelihood ratio test, p < 0.0001), indicating improvement in model fitting for the score developed by Crooks et al.23 It also had better discriminatory performance than Charlson43 (c-statistic, 0.87, 95% CI 0.87–0.87) or Elixhauser comorbidity measures44 (c-statistic, 0.87, 95% CI 0.87–0.87). Charlson43 and Elixhauser44 are well known prognostic indices.
Pilotto et al. developed an index based on assessment of community-dwelling adults older than 65 years living at home in Italy.24 The index was well calibrated across all risk groups and had good discrimination.
Wang et al. developed a model to predict 1-year mortality in patients aged 18 to 100 years who were assigned to a Veterans Health Administration primary care provider.25 It was well calibrated (Cox Intercept, α = 0.001 (95% CI, −0.001–0.023); Cox Slope, β = 1.002 (95% CI, 0.998 – 1.008)) and had very good discrimination. However, a narrow range of mortality was observed (0.1%−9.1%) for patients categorized between 5th and 90th predicted risk percentiles. Coefficients included in the model to predict death are available in a supplemental table with 95% confidence intervals.
Gagne et al. used low-income Medicare enrollees from Pennsylvania and New Jersey. The index was well-calibrated and had good discrimination.26 Mortality ranged from 3 to 29%.
15-month mortality
Mazzaglia et al. developed and validated a 15-month mortality index for community-dwelling older adults using data from a screening survey of patients answered by primary care physicians from two regions of Florence, Italy.27 The final model includes a number of positive responses to another screening test, which is not available in the article.45 The index was well calibrated and showed good discrimination. The reported mortality was narrow, ranging from 0 to10%.
2-year mortality
Carey et al. developed a functional morbidity index to predict 2-year mortality in community-dwelling older adults aged 70 and older using data from the Asset and Health Dynamics Among the Oldest Old (AHEAD) study in the U.S.28 The index was well calibrated across the risk groups and demonstrated good discrimination.
3-year mortality
Turusheva et al. developed 2 models of mortality risk score to predict 3-year mortality.29 The derivation cohort (n=379) was randomly sampled using data from a prospective cohort study of community-dwelling older adults aged 65–91 years in Saint Petersburg, Russia. The authors validated the 2 models using a cohort from an external cohort study of people aged 80 years or older in Belgium (n = 567). Both models had good discrimination in the development cohort, but poor to moderate performance in validation. Calibration was not reported in the study.
The other 3-year mortality index for community-dwelling elderly was developed by Carey et al.30 This index allows for prediction of 1-, 2-, and 3-year mortality. Its data source was patients enrolled in the Program of All-inclusive Care for the Elderly (PACE) in the Western, Midwestern, and Eastern regions of the U.S. The index was developed using the cohort from the Western region and validated in the other two regions. The index had moderate discrimination and was well calibrated across all risk groups for 1-, 2-, and 3-year mortality.
4-year mortality
A 4-year mortality index was developed by Lee et al. in community-dwelling adults aged 50 years and older in the U.S. who answered the Health and Retirement Survey from 1992–1998.31 The development and validation cohorts were chosen based on geographic location in the U.S. The index was well calibrated across all risk groups and had very good discrimination.
5-year mortality
Jung et al. developed a geriatric prognosis index to predict 3- and 5-year mortality.32 Its data source for development was the Korean Longitudinal Study on Health and Aging cohort, which included people aged 65 years and older living in a suburban city of South Korea. A retrospective review of medical records of people aged 60 years and older who had a geriatric assessment in the outpatient geriatric clinic or inpatient ward was used for validation. The proportion of inpatients used for the validation cohort was not reported. The index requires the use of a number of other scores such as the Charlson Comorbidity Index and multiple geriatric scales. Three-year mortality was well calibrated for all risk groups. Calibration for 5-year mortality was poor for higher risk groups, but well calibrated for lower and middle risk groups. For both 3- and 5-year mortality, the 95% confidence interval for mortality was wide for all risk groups. The index had good discrimination for 3-year mortality and very good discrimination for 5-year mortality.
Ganna and Ingelsson developed a 5-year mortality prediction score using U.K. Biobank participant data from England and Wales, and validated it using participants from Scotland.33 Prediction models were developed separately for men (13-items) and women (11-items). These had very good discrimination for men and good discrimination for women. The score for men was poorly calibrated (Hosmer-Lemeshow, p = 0.0402), but the score for women was well calibrated (Hosmer-Lemeshow, p = 0.28, women). For the Hosmer-Lemeshow test, statistical significance (p < 0.05) means poor calibration.
The Ensemble Index developed by Mathias et al. to predict 5-year mortality was developed using predictive data mining and analysis of electronic health records data from Epic (Verona, WI) and Cerner (Kansas City, MO).34 The random forest ensemble technique with alternating decision tree was used to develop the model, and tenfold cross validation was used. Its discrimination was very good, showing a higher c-statistic when compared to the Walter life expectancy method and Charlson Comorbidity Index,46,47 and it was well calibrated across all risk groups.
Tan et al. developed a life expectancy model that adapts the Elixhauser comorbidity measure to predict 1- and 5-year mortality in the Medicare population in the U.S.35,44 Randomly sampling 5% of Medicare claims data between 1999 and 2009, the dataset was randomly split for development and validation. The model was well calibrated for all risk groups for 5-year mortality, and low to middle-risk groups for 1-year mortality in both males and females. Calibration in the high-risk groups for 1-year mortality in both males and females was poor. Discrimination was very good for 1-year mortality in females and good for 5-year mortality in females and 1- and 5-year mortality in males.
Zhang et al. developed a 1- and 5-year mortality index using data collected alongside a national health survey of non-institutionalized adults in the U.S.36 The development cohort came from randomly selecting 60% and using the remaining 40% for validation. The models were well-calibrated according to the Hosmer-Lemeshow statistics. Both the 1- and 5-year mortality index had good discrimination and predicted a wide range of mortality between low- and high-risk groups (2 to 42% for 1-y and 7 to 81% for 5-y).
Schonberg et al. developed a 5-year mortality index for adults aged 65 years and older with good discrimination.37 Linking data from the National Health Interview Survey and the National Death Index, two thirds were randomly selected for development and the remainder for validation. The index was well calibrated across all risk groups and predicted a wide range of mortality between the lowest to highest risk groups.
Discussion
This review summarizes 17 unique prognostic indices from 18 articles that predict all-cause mortality between 6 months to 5 years in community-dwelling adults. Our review summarizes the performance of prognostic indices and assesses their potential for clinical use aimed at supporting implementation of ACP in the primary care setting. Ten papers included algorithms that were usable in the setting of primary care office.22,26–28,30,31,33,34,36,37 Our search criteria included adults 18 years and older. However, only 3 of the 10 usable indices were developed and validated in a population cohort that included patients less than 65 years.31,33,34 Three systematic reviews have identified prognostic indices that predict mortality in community-dwelling adults,38,48,49 but none of these made recommendations on which tool to prioritize for clinical implementation. Even a prognostic index that is accurate, externally validated, well calibrated, and with a low risk of bias may still have limited clinical use and impact if it is difficult to use and if the physician does not have access to all variables necessary for a specific prognostic algorithm. Several of the indices we identified as clinically usable require knowledge of multiple variables and are impractical without systematic collection of these variables or additional programming in an electronic medical record.
Implications for future research
Currently available approaches to prognostication include clinical intuition and algorithms. A validated approach using clinical intuition to trigger palliative care is to ask the Surprise Question (SQ): “Would I be surprised if this patient died in the next 12 months?”17 Because the SQ was not originally developed to predict mortality, more research is needed to test how the SQ can aid in the patient identification process for physicians to initiate ACP.16 Combining the SQ with another prognostic tool has the potential to enhance accuracy in determining a patient’s prognosis.50
The indices we classified as ‘clinically usable’ may not be easy-to-use. They often require knowledge of many variables that may not be easily accessible to the practicing physician. Future research should compare the clinically usable indices we identified for time spent per patient and resources required to program them into their existing electronic medical records to see which ones are most feasible in busy practices, given the large number of variables that many of them have.22,26–28,30,31,33,34,36,37 It is possible that these algorithms could be programmed into the electronic medical record to prompt physicians to discuss ACP with appropriate patients, the same way many other best practice alerts are now. It is currently unclear which if any of the indices we identified might work best for initiating ACP discussions. With a growing interest in the use of machine learning and artificial intelligence in medical care, our results can guide researchers who wish to test multiple algorithms simultaneously.51,52
Our work has implications for practice-based research networks that wish to expand the implementation of ACP in the primary care setting. For example, the Patient-Centered Outcomes Research Institute (PCORI) recently funded 7 studies to encourage expansion of ACP and palliative care.53,54 The Meta-network Learning And Research Center (Meta-LARC) ACP trial is one of these studies.55 Meta-LARC is a consortium of 7 practice-based research networks (PBRNs) in the U.S. and Canada including over 900 primary care practices and approximately 4,000 clinicians who care for over 3 million patients. Meta-LARC is dedicated to increasing the quality, effectiveness, and safety of primary care through accelerated research and collaborative learning (https://www.ohsu.edu/xd/outreach/oregon-rural-practice-based-research-network/meta-larc/index.cfm/). The ACP trial will use the infrastructure of Meta-LARC to conduct a cluster randomized trial in 42 primarily family physician practices in the U.S. and Canada to compare the efficacy of clinician-led vs. team-based approaches to implement ACP in primary care.
Limitations
Risk of bias in individual indices was not assessed, as it was not applicable for our review. Publication bias may exist because we searched only on PubMed, which may miss some articles. Given the heterogeneity in the way studies reported their calibration, straightforward comparisons were impossible. Studies that included administrative data may have included hospitalized and nursing home patients. While we attempted to exclude indices developed on cohorts where more than 50% were hospitalized or in nursing homes, not all papers provided this information. For this study, we abstracted the calibration statistics as reported by the authors of each prognostic index. Currently, methods to assess model performance are not standardized and are reported in a variety of ways. Future studies of prognostic indices should report calibration using standard means.42 Clinicians and researchers can choose to implement the prognostic algorithms we classified as usable, and test whether appropriate patients for ACP conversations are identified in the primary care setting.
Conclusion
Our review identified 18 studies with 17 published prognostic indices that are potentially useful for patient identification for ACP conversations. Eight prognostic indices from the U.S. and 2 from the U.K. were identified as ‘clinically’ usable.22,26–28,30,31,33,34,36,37 An index classified as ‘clinically usable’ may not be easy-to-use because of a large number of variables that are not routinely collected and the need for programming the index into the electronic medical record. Future research should validate these indices in other populations, compare across indices to determine time spent per patient, and program them into electronic medical records to see which ones are most feasible in busy practices.
Acknowledgements:
We would like to thank Sharon Straus, MD, University of Toronto, Toronto, Ontario, and Annette Totten, PhD, Oregon Health and Science University, Portland, Oregon for their feedback on this manuscript.
Funding statement:
This work was partially supported by the Iowa Academy of Family Physicians Endowed Chair for Rural Medicine Fund, Department of Family Medicine, University of Iowa Carver College of Medicine and the Patient-Centered Outcomes Research Institute® (PCORI®) Award (PLC-1609-36277). The funders had no role in any part of the study including its conception and design, acquisition, or analysis and interpretation of data, and drafting or revision of the manuscript. The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
Footnotes
Conflicting and Competing Interests:
None.
References
- 1.Jimenez G, Tan WS, Virk AK, Low CK, Car J, Ho AHY. Overview of systematic reviews of advance care planning: summary of evidence and global lessons. J Pain Symptom Manage . 2018;56(3):436–459. [DOI] [PubMed] [Google Scholar]
- 2.United States Government Accountability Office. Advance Care Planning: Selected States’ Efforts to Educate and Address Access Challenges. 2019. Accessed March 6, 2019. [Google Scholar]
- 3.Rietjens JAC, Sudore RL, Connolly M, et al. Definition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care. Lancet Oncol . 2017;18(9):e543–e551. [DOI] [PubMed] [Google Scholar]
- 4.Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage. 2017;53(5):821–832.e821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med. 2014;28(8):1000–1025. [DOI] [PubMed] [Google Scholar]
- 6.Houben CHM, Spruit MA, Groenen MTJ, Wouters EFM, Janssen DJA. Efficacy of advance care planning: a systematic review and meta-analysis. J Am Med Dir Assoc. 2014;15(7):477–489. [DOI] [PubMed] [Google Scholar]
- 7.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff KE, Sudore R, Miao Y, Boscardin WJ, Smith AK. Advance care planning and the quality of end-of-life care in older adults. J Am Geriatr Soc. 2013;61(2):209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav KN, Gabler NB, Cooney E, et al. Approximately one in three US adults completes any type of advance directive for end-of-life care. Health Aff (Millwood). 2017;36(7):1244–1251. [DOI] [PubMed] [Google Scholar]
- 10.Lakin JR, Koritsanszky LA, Cunningham R, et al. A systematic intervention to improve serious illness communication in primary care. Health Aff (Millwood). 2017;36(7):1258–1264. [DOI] [PubMed] [Google Scholar]
- 11.Sudore RL, Heyland DK, Lum HD, et al. Outcomes that define successful advance care planning: a Delphi panel consensus. J Pain Symptom Manage. 2018;55(2):245–255.e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Committee on Approaching Death: Addressing Key End of Life Issues; Institute of Medicine Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington (DC): National Academies Press (US); 2015. [PubMed] [Google Scholar]
- 13.De Vleminck A, Houttekier D, Pardon K, et al. Barriers and facilitators for general practitioners to engage in advance care planning: a systematic review. Scand J Pri Health Care. 2013;31(4):215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuart B The NHO Medical Guidelines for Non-Cancer Disease and local medical review policy: hospice access for patients with diseases other than cancer. Hosp J. 1999;14(3–4):139–154. [PubMed] [Google Scholar]
- 15.Thomas K, Noble B. Improving the delivery of palliative care in general practice: an evaluation of the first phase of the Gold Standards Framework. Palliat Med. 2007;21(1):49–53. [DOI] [PubMed] [Google Scholar]
- 16.Thomas K Improving end of life care: a matter of life and death. London J Prim Care (Abingdon). 2009;2(2):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billings JA, Bernacki R. Strategic targeting of advance care planning interventions: the Goldilocks phenomenon. JAMA Intern Med. 2014;174(4):620–624. [DOI] [PubMed] [Google Scholar]
- 18.Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. [DOI] [PubMed] [Google Scholar]
- 19.Moons KG, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–73. [DOI] [PubMed] [Google Scholar]
- 20.Han PK, Lee M, Reeve BB, et al. Development of a prognostic model for six-month mortality in older adults with declining health. J Pain Symptom Manage. 2012;43(3):527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duarte CW, Black AW, Murray K, et al. Validation of the patient-reported outcome mortality Prediction tool (PROMPT). J Pain Symptom Manage. 2015;50(2):241–247.e246. [DOI] [PubMed] [Google Scholar]
- 22.Hippisley-Cox J, Coupland C. Development and validation of QMortality risk prediction algorithm to estimate short term risk of death and assess frailty: cohort study. BMJ. 2017;358:j4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crooks CJ, Card TR, West J. The use of a Bayesian hierarchy to develop and validate a co-morbidity score to predict mortality for linked primary and secondary care data from the NHS in England. PloS One. 2016;11(10):e0165507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilotto A, Gallina P, Fontana A, et al. Development and validation of a Multidimensional Prognostic Index for mortality based on a standardized Multidimensional Assessment Schedule (MPI-SVaMA) in community-dwelling older subjects. J Am Med Dir Assoc. 2013;14(4):287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368–373. [DOI] [PubMed] [Google Scholar]
- 26.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzaglia G, Roti L, Corsini G, et al. Screening of older community-dwelling people at risk for death and hospitalization: the Assistenza Socio-Sanitaria in Italia project. J Am Geriatr Soc. 2007;55(12):1955–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey EC, Walter LC, Lindquist K, Covinsky KE. Development and validation of a functional morbidity index to predict mortality in community-dwelling elders. J Gen Intern Med. 2004;19(10):1027–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turusheva A, Frolova E, Bert V, Hegendoerfer E, Degryse JM. Validation of a new mortality risk prediction model for people 65 years and older in northwest Russia: the Crystal risk score. Arch Gerontol Geriatr. 2017;71:105–114. [DOI] [PubMed] [Google Scholar]
- 30.Carey EC, Covinsky KE, Lui LY, Eng C, Sands LP, Walter LC. Prediction of mortality in community-living frail elderly people with long-term care needs. J Am Geriatr Soc. 2008;56(1):68–75. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295(7):801–808. [DOI] [PubMed] [Google Scholar]
- 32.Jung HW, Kim JW, Han JW, et al. Multidimensional Geriatric Prognostic Index, based on a geriatric assessment, for long-term survival in older adults in Korea. PLoS One. 2016;11(1):e0147032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganna A, Ingelsson E. 5 year mortality predictors in 498,103 UK Biobank participants: a prospective population-based study. Lancet. 2015;386(9993):533–540. [DOI] [PubMed] [Google Scholar]
- 34.Mathias JS, Agrawal A, Feinglass J, Cooper AJ, Baker DW, Choudhary A. Development of a 5 year life expectancy index in older adults using predictive mining of electronic health record data. J Am Med Inform Assoc. 2013;20(e1):e118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan A, Kuo YF, Goodwin JS. Predicting life expectancy for community-dwelling older adults from Medicare claims data. Am J Epidemiol. 2013;178(6):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Xie D, Kurichi JE, Streim J, Zhang G, Stineman MG. Mortality predictive indexes for the community-dwelling elderly US population. J Gen Intern Med. 2012;27(8):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schonberg MA, Davis RB, McCarthy EP, Marcantonio ER. Index to predict 5-year mortality of community-dwelling adults aged 65 and older using data from the National Health Interview Survey. J Gen Intern Med. 2009;24(10):1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. [DOI] [PubMed] [Google Scholar]
- 40.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alba AC, Agoritsas T, Walsh M, et al. Discrimination and calibration of clinical prediction models: users’ guides to the medical literature. JAMA. 2017;318(14):1377–1384. [DOI] [PubMed] [Google Scholar]
- 43.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 44.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 45.Roti L, Corsini G, Colombini A, Mazzaglia G, Maciocco G, Marchionni N, Di Bari M, Ferrucci L A screening instrument to identify older community-dwellers at risk for death and hospitalization in Tuscany, Italy. The “Assistenza Socio-Sanitaria in Italia” project. J Am Geriatr Soc. 2006;54(4):S90–S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. JAMA. 2001;285(21):2750–2756. [DOI] [PubMed] [Google Scholar]
- 47.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 48.O’Caoimh R, Cornally N, Weathers E, et al. Risk prediction in the community: a systematic review of case-finding instruments that predict adverse healthcare outcomes in community-dwelling older adults. Maturitas. 2015;82(1):3–21. [DOI] [PubMed] [Google Scholar]
- 49.National Institute for Health and Care Excellence. Multimorbidity: Assessment, Prioritisation and Management of Care for People with Commonly Occurring Multimorbidity. London: National Institute for Health and Care Excellence (UK) 2016. Accessed March 6, 2019. [PubMed] [Google Scholar]
- 50.Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Intern Med. 2016;176(12):1863–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rose S Mortality risk score prediction in an elderly population using machine learning. Am J Epidemiol. 2013;177(5):443–452. [DOI] [PubMed] [Google Scholar]
- 52.Udelsman B, Chien I, Ouchi K, Brizzi K, Tulsky JA, Lindvall C. Needle in a haystack: natural language processing to identify serious illness. J Palliat Med. 2019;22(2):179–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patient-Centered Outcomes Research Institute (PCORI). Cycle 3 2016 Funding Cycle PCORI Funding Annoucement: Community-based Palliative Care Delivery for Adult Patients with Advanced Illnesses and their Caregivers. PCORI website. https://www.pcori.org/sites/default/files/PCORI-PFA-2016-Cycle-3-Palliative-Care.pdf. Updated May 19, 2017. Accessed April 10, 2019. [Google Scholar]
- 54.National US Library of Medicine. Team-based Versus Primary Care Clinician-led Advance Care Planning in Practice-based Research Networks website. https://ClinicalTrials.gov/show/NCT03577002. Updated December 3, 2018. Accessed March 6, 2019. [Google Scholar]
- 55.Totten AM, Fagnan LJ, Dorr D, et al. Protocol for a cluster randomized trial comparing team-based to clinician-focused implementation of advance care planning in primary care. J Palliat Med. 2019;22(S1):82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]