Abstract
Paclitaxel, a chemotherapeutic agent, is routinely administered for the treatment of various solid organ malignancies. In rare instances, patients receiving infusions of paclitaxel may present with signs of an acute abdomen. Ischemia and necrosis of the bowel wall from chemotherapy-induced neutropenia and direct toxic effects of the drug have been implicated as the cause. We present a case of necrotizing small and large bowel perforation in a patient with breast cancer, 2 weeks after paclitaxel administration.
Keywords: Neutropenic enterocolitis, Bowel perforation, Acute abdomen, Chemotherapy, Paclitaxel, Abdominal pain
Introduction
Chemotherapeutic agents are used to treat various malignancies. As these agents do not differentiate between cancer cells and rapidly dividing normal human cells, systemic toxicity is a frequent side effect [1]. A spectrum of gastrointestinal tract adverse events ranging from relatively benign nausea and vomiting to fatal fulminant enterocolitis and intestinal perforation have been reported secondary to chemotherapeutic drugs [2]. Cytotoxic chemotherapeutic agents such as cytosine arabinoside, vinca alkaloids, doxorubicin [3], paclitaxel, docetaxel [1, 4, 5], 5-fluorouracil, carboplatin, cisplatin [6], gemcitabine, and PEGylated interferon in combination with ribavirin, amongst others, have been implicated in causing gastrointestinal toxicity [7].
Gastrointestinal toxicities due to chemotherapy include secretory/osmotic diarrhea [2, 8], altered intestinal motility [9], gastrointestinal perforation especially with angiogenesis inhibitors [10], direct mucosal cell toxicity with microtubule inhibitors such as taxanes [11], and decreased host defenses such as with immune check point modulators [12]. Neutropenic enterocolitis, ischemic colitis, and Clostridium difficile colitis have all been reported after high-dose chemotherapy [13].
Neutropenic or necrotizing enterocolitis (NEC), known as typhlitis when the ileocecal region is involved, is the breakdown of gut mucosal integrity seen in patients with severe myelosuppression, causing transmural inflammation of the colon [14]. It is known to complicate the treatment of various solid and hematological malignancies. Its pathogenesis involves impaired host immune defenses due to neutropenia and/or chemotherapy-induced direct mucosal injury, predisposing the gut to pathogenic organisms [8].
Taxane group chemotherapeutic agents such as paclitaxel and docetaxel are known to cause gastrointestinal toxicity, including NEC. Docetaxel is reported to be the more toxic of the two [1]. Paclitaxel is a plant-based biosynthetic form of taxane that has been used in the treatment of various solid organ malignancies including ovarian, breast, lung, and bladder [11]. Paclitaxel promotes microtubule assembly by enhancing the action of tubulin dimers, stabilizing existing microtubules, and inhibiting their disassembly, thereby interfering with the late G2 mitotic phase of the cell cycle. It distorts mitotic spindles, resulting in the breakage of chromosomes, which prevents the cell from entering into further phases of the cell cycle and finally leads to apoptosis [15]. A few cases of paclitaxel-induced bowel perforation have been reported in the literature [11, 16, 17, 18, 19].
We present a case of necrotizing small and large bowel perforation in a patient with breast cancer 2 weeks after paclitaxel administration.
Case Report
A 79-year-old female with the medical comorbidities of hypertension, diabetes mellitus, asthma, gastroesophageal reflux disease, osteoporosis, and depression was sent to the emergency department from the oncology clinic for the evaluation of fever and hypotension. She had been diagnosed with stage 2, triple-marker (estrogen/progesterone/HER2)-negative, invasive ductal carcinoma of the right breast a year prior to presentation, and had undergone a modified radical mastectomy with lymph node dissection 6 months earlier. Subsequently, she had been receiving adjuvant chemotherapy with a DAC (doxifluridine/Adriamycin/cyclophosphamide) regimen and weekly paclitaxel. She had already completed 11 cycles of the regimen, with her last dose of paclitaxel administered 2 days prior to presentation.
Her cancer treatment course had been complicated by nausea, lethargy, loss of appetite, and intermittent episodes of non-bloody diarrhea. Infectious etiologies such as C. difficile had been ruled out, and the diarrhea along with the other symptoms were thought to be secondary to the chemotherapeutic agents. She was taking loperamide and ondansetron in order to control these symptoms. A few months ago, she had also received filgrastim for neutropenia (absolute neutrophil count 1,100/μL), after which her white cell counts improved and the neutropenia resolved (absolute neutrophil count 6,200/μL).
She was a lifelong smoker with a 20-pack-year smoking history and denied any alcohol or illicit substance use. Her family history was remarkable for a daughter with breast cancer, also on treatment. She had undergone a screening colonoscopy 2 years previously, revealing a tubular adenoma and colonic diverticulosis.
On initial evaluation, she was febrile (temperature 100.5°F) and hypotensive (blood pressure 86/55 mm Hg) with sinus tachycardia (heart rate 114 bpm) and tachypnea (respiratory rate 22 breaths/min), necessitating management with intravenous fluids and broad-spectrum antibiotics (intravenous vancomycin and piperacillin/tazobactam). Her initial physical examination was grossly normal, including normal mentation, a benign abdomen, and clear lungs on auscultation. However, she had noticeable cachexia due to her underlying malignancy.
Her laboratory results revealed anemia (hemoglobin 8.9 g/dL) and neutropenia (absolute neutrophil count 1,300/μL). She was stabilized and admitted to the oncology unit for further management. She continued to receive chemotherapy as an inpatient, and her initial workup to identify any source of infection, including cultures (blood and urine) and a chest X-ray, was unrevealing. Two days later, the antibiotics were discontinued, and the patient was started on treatment for asthma exacerbation with intravenous steroids. Over the next few days, she remained in a stable condition and her steroids were transitioned to an oral tapered regimen.
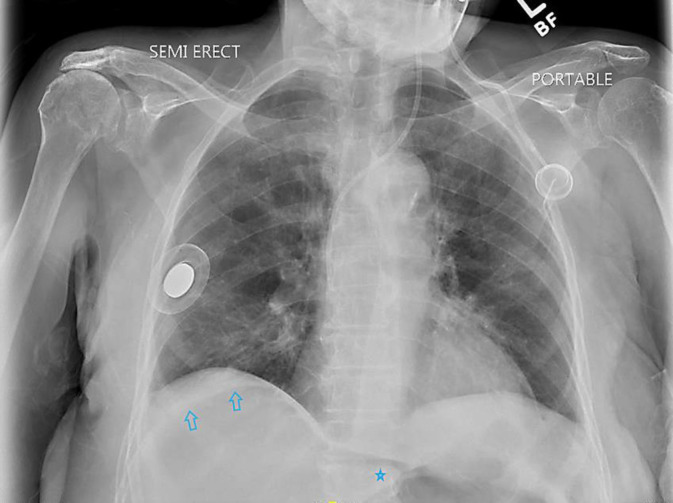
On the 12th day of admission, she was transferred to the critical care unit as her general medical condition suddenly deteriorated. She complained of a dry cough and diffuse abdominal pain. On examination, she had a pale complexion and diffuse abdominal tenderness to palpation, with guarding. A stat X-ray of the chest (Fig. 1) was obtained, on which she was noted to have free intraperitoneal air under the right diaphragm. Subsequently, she underwent computed tomography (CT) of the abdomen (Fig. 2), which demonstrated a prominent amount of free intraperitoneal gas with an abnormal right colonic area of ill-defined soft tissue morphology that was opined to be the site of visceral perforation. She was evaluated by the surgical team and underwent emergent exploratory laparotomy.
Fig. 1.
Chest X-ray demonstrating subdiaphragmatic areas of lucency over the right upper quadrant (arrows), with apparent outlining of the left hepatic margin (asterisk) concerning for free intraperitoneal gas.
Fig. 2.
a CT of the abdomen and pelvis without contrast demonstrating free intraperitoneal air (arrows) and an abnormal right colonic morphology with thickening in the region of the hepatic flexure (asterisk), possibly the site of the perforation with adjacent free fluid. Also seen is a large left renal cyst (circle). b CT of the abdomen and pelvis without contrast demonstrating a large air-fluid level in the left mid-abdomen between bowel loops (asterisk). This may also be a site of perforation with a loculated collection. It is surrounded by small bowel loops anteriorly, and the colon along its posterior margin, from which it appears inseparable.
Intraoperatively, a large perforation in the proximal transverse colon and hepatic flexure, with nonviable tissue in the sigmoid colon, was noted. Other intraoperative findings included multiple areas of patchy necrosis of the small intestine with perforation, and contamination of the abdominal cavity with bile and feces. Her intraoperative course was complicated by profound hypotension (systolic blood pressure 70 mm Hg) and blood loss. She required blood and albumin transfusions, along with vasopressors and fluid support during the surgery. Due to the extent of her disease and hemodynamic instability, resection was not attempted, and eventually the abdominal wound was closed.
Her postoperative course saw a continued state of shock and multiorgan failure requiring vasopressors and mechanical ventilator support. Further resuscitative measures were unsuccessful, eventually leading to the patient's demise less than a day after surgery.
Discussion
A novel form of paclitaxel, the nanoparticle-albumin bound, is a water-soluble, negatively charged, stable variant that is said to be twice as potent [20]. However, albumin-bound paclitaxel has also been reported to cause gastrointestinal complications, including intestinal perforation, ischemic colitis, and neutropenic colitis [20]. Common toxicities of paclitaxel therapy include alopecia, neutropenia, hypotension, fever, hepatotoxicity, myalgia, and peripheral neuropathy [21]. NEC leading to bowel perforation as a toxicity has rarely been reported [11, 22, 23, 24, 25].
The etiology of NEC can be multifactorial, and includes drugs, infections, intramural hemorrhage, ischemia, and altered immune function. The pathogenesis of chemotherapy-induced NEC involves direct mucosal injury and/or impaired host immune defenses due to neutropenia, predisposing the gut to pathogenic organisms [26]. Conditions impacting the immune system, such as acquired immunodeficiency syndrome (AIDS), myelodysplastic syndromes, solid organ and bone marrow transplantations, solid malignant tumors, and lymphomas, amongst others, can all predispose patients to developing NEC [27, 28]. Signs and symptoms compatible with NEC include abdominal pain, fever, diarrhea, abdominal distension, and blood or mucus in the stool [27].
When secondary to chemotherapy, NEC is said to occur around 2 weeks after initiation [14], corresponding to the neutrophil count nadir. It most commonly occurs in the right colon, although it can manifest anywhere in the small or large bowel. CT scan findings include nonspecific wall thickening, edema, and pericolic fat stranding. Transmural necrosis and perforation are known to occur in severe cases [27]. Various bacterial and/or fungal organisms are often seen infiltrating the bowel wall. Polymicrobial infection is frequent [29].
Structural abnormalities of the bowel, such as diverticulitis, previous surgeries, or infiltrative tumors, heighten the risk of NEC following chemotherapy [14]. Administration of dexamethasone after paclitaxel therapy [30], tumor lysis syndrome [31], and neutropenia with an absolute neutrophil count less than 1,000/mm3 have all been implicated as risk factors for NEC with subsequent bowel perforation [32]. Prior episodes of neutropenic enterocolitis also appear to increase the risk of chemotherapy-induced NEC [33].
Endoscopy is relatively contraindicated in NEC; yet histological examination remains the gold standard for its diagnosis. Endoscopic characteristics of NEC, obtained via colonoscopy or sigmoidoscopy, include mucosal ulceration, edema, erosions, erythema, pseudomembrane, nodularity, friability, and loss of vascular pattern [13]. Conservative treatment with antibiotics, bland diet, hydration, and an effective pain treatment is favored for the treatment of neutropenic enterocolitis [34], whereas surgical intervention is necessary in cases of fulminant colitis and perforation [35].
Another proposed mechanism for bowel injury due to paclitaxel is its direct effect on the gastrointestinal mucosa without observable neutropenia [1]. It is said to arrest cellular division and promote intestinal cell death, which eventually leads to perforation. This was suggested by Hruban et al. [32], who showed the presence of gastrointestinal mitotic arrest in autopsy-derived specimens of the esophagus, stomach, small intestine, colon, liver, skin, bone marrow, and testes following paclitaxel treatment.
Bowel perforation associated with chemotherapeutic agents is an uncommon presentation, but one with a high mortality rate of 48% when treated medically and of 21% when treated surgically [36]. The incidence of paclitaxel-induced intestinal perforation is reported as 2.5% and is associated with a high mortality rate of 57% [30]. Only a handful of cases have been described in the literature. Seewaldt et al. [37] reported gastrointestinal necrosis with an incidence of 2.3% amongst patients treated with paclitaxel for ovarian cancer. Rose and Piver [18] alluded to colonic perforation in 3 patients undergoing paclitaxel-based chemotherapy.
Patients with bowel perforation can be asymptomatic or present with severe abdominal pain with hemodynamic instability. An upright radiograph can detect free intraperitoneal air under the diaphragm. CT scanning is the imaging modality of choice to detect the free air and the site of perforation [38]. Immediate hemodynamic resuscitation should be initiated with intravenous fluids and prophylactic antibiotics, and prompt surgical consultation should be obtained.
Statement of Ethics
The patient provided written informed consent (including the publication of images).
Conflict of Interest Statement
The authors declare no conflicts of interest.
Funding Sources
No funding was received.
Author Contributions
D.H. Shaikh and A. Baiomi searched the literature and wrote and revised the manuscript. H. Abbas, S. Mehershahi, and S. Gongati edited and revised the manuscript. S.K. Nayudu revised and approved the final version, and is the article's guarantor. All authors certify that they contributed sufficiently to the intellectual content and data analysis. Each author has reviewed the final version of the manuscript and approves it for publication.
Data Availability Statement
Should the editors request the data upon which the work is based, the authors shall produce it.
References
- 1.Boussios S, Pentheroudakis G, Katsanos K, Pavlidis N. Systemic treatment-induced gastrointestinal toxicity: incidence, clinical presentation and management. Ann Gastroenterol. 2012;25((2)):106–18. [PMC free article] [PubMed] [Google Scholar]
- 2.Davila M, Bresalier RS. Gastrointestinal complications of oncologic therapy. Nat Clin Pract Gastroenterol Hepatol. 2008 Dec;5((12)):682–96. doi: 10.1038/ncpgasthep1277. [DOI] [PubMed] [Google Scholar]
- 3.Montoya JE, Luna HG, Morelos AB, Catedral MM, Lava AL, Amparo JR, et al. Association of creatinine clearance with neutropenia in breast cancer patients undergoing chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide (FAC) Med J Malaysia. 2013 Apr;68((2)):153–6. [PubMed] [Google Scholar]
- 4.Ibrahim NK, Sahin AA, Dubrow RA, Lynch PM, Boehnke-Michaud L, Valero V, et al. Colitis associated with docetaxel-based chemotherapy in patients with metastatic breast cancer. Lancet. 2000 Jan;355((9200)):281–3. doi: 10.1016/S0140-6736(99)06195-4. [DOI] [PubMed] [Google Scholar]
- 5.D'Amato G, Rocha Lima C, Mahany JJ, Muro-Cacho C, Haura EB. Neutropenic enterocolitis (typhilitis) associated with docetaxel therapy in a patient with non-small-cell lung cancer: case report and review of literature. Lung Cancer. 2004 Jun;44((3)):381–90. doi: 10.1016/j.lungcan.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Stojanovska V, Sakkal S, Nurgali K. Platinum-based chemotherapy: gastrointestinal immunomodulation and enteric nervous system toxicity. Am J Physiol Gastrointest Liver Physiol. 2015 Feb;308((4)):G223–32. doi: 10.1152/ajpgi.00212.2014. [DOI] [PubMed] [Google Scholar]
- 7.Lee CS, Ryan EJ, Doherty GA. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: the role of inflammation. World J Gastroenterol. 2014 Apr;20((14)):3751–61. doi: 10.3748/wjg.v20.i14.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milles SS, Muggia AL, Spiro HM. Colonic Histologic Changes Induced by 5-Fluorouracil. Gastroenterology. 1962;43((4)):391–9. [Google Scholar]
- 9.Osterlund P, Ruotsalainen T, Peuhkuri K, Korpela R, Ollus A, Ikonen M, et al. Lactose intolerance associated with adjuvant 5-fluorouracil-based chemotherapy for colorectal cancer. Clin Gastroenterol Hepatol. 2004 Aug;2((8)):696–703. doi: 10.1016/s1542-3565(04)00293-9. [DOI] [PubMed] [Google Scholar]
- 10.Ropert S, Vignaux O, Mir O, Goldwasser F. VEGF pathway inhibition by anticancer agent sunitinib and susceptibility to atherosclerosis plaque disruption. Invest New Drugs. 2011 Dec;29((6)):1497–9. doi: 10.1007/s10637-010-9500-9. [DOI] [PubMed] [Google Scholar]
- 11.Jayakody S, Wright DB, Chiong C, Liu M, Bouffler C, El-Khoury T. Rectal perforation following paclitaxel and carboplatin chemotherapy for advanced ovarian cancer: a case report and review of the literature. J Med Case Reports. 2018 Aug;12((1)):221. doi: 10.1186/s13256-018-1759-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, Marthey L, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018 Nov;67((11)):2056–67. doi: 10.1136/gutjnl-2018-316948. [DOI] [PubMed] [Google Scholar]
- 13.Xia R, Zhang X. Neutropenic enterocolitis: A clinico-pathological review. World J Gastrointest Pathophysiol. 2019 Oct;10((3)):36–41. doi: 10.4291/wjgp.v10.i3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesher L, Rolston KV. Neutropenic enterocolitis, a growing concern in the era of widespread use of aggressive chemotherapy. Clin Infect Dis. 2013 Mar;56((5)):711–7. doi: 10.1093/cid/cis998. [DOI] [PubMed] [Google Scholar]
- 15.Mukhtar E, Adhami VM, Mukhtar H. Targeting microtubules by natural agents for cancer therapy. Mol Cancer Ther. 2014 Feb;13((2)):275–84. doi: 10.1158/1535-7163.MCT-13-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu ZC, Wu D, Lan SJ, Wang DD. [A case of multiple intestinal perforation secondary to paclitaxel and carboplatin combined chemotherapy for lung cancer] Zhonghua Zhong Liu Za Zhi. 2019 May;41((5)):399–400. doi: 10.3760/cma.j.issn.0253-3766.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Mandai K, Shirakawa A, Uno K, Yasuda K. Endoscopic Ultrasound-Guided Drainage of Intra-Abdominal Abscess after Gastric Perforation in a Patient Receiving Ramucirumab and Paclitaxel for Advanced Gastric Cancer. Case Rep Oncol. 2017 Jan;10((1)):15–20. doi: 10.1159/000455226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose PG, Piver MS. Intestinal perforation secondary to paclitaxel. Gynecol Oncol. 1995 May;57((2)):270–2. doi: 10.1006/gyno.1995.1140. [DOI] [PubMed] [Google Scholar]
- 19.Samejima J, Adachi H, Kawamoto M, Saeki H, Kato N, Fujisawa J, et al. [Rectal perforation in a patient treated with combination chemotherapy for lung cancer] Gan To Kagaku Ryoho. 2009 Feb;36((2)):301–4. [PubMed] [Google Scholar]
- 20.Chen E, Abu-Sbeih H, Thirumurthi S, Mallepally N, Khurana S, Wei D, et al. Clinical characteristics of colitis induced by taxane-based chemotherapy. Ann Gastroenterol. 2020 Jan-Feb;33((1)):59–67. doi: 10.20524/aog.2019.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guchelaar HJ, ten Napel CH, de Vries EG, Mulder NH. Clinical, toxicological and pharmaceutical aspects of the antineoplastic drug taxol: a review. Clin Oncol (R Coll Radiol) 1994;6((1)):40–8. doi: 10.1016/s0936-6555(05)80367-x. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Hu P, Yue Y, Duan Z. A case of occult bowel perforation after a cycle of chemotherapy for advanced epithelial ovarian carcinoma. Eur J Gynaecol Oncol. 2012;33((5)):540–2. [PubMed] [Google Scholar]
- 23.Seewaldt V, Cain JM, Greer BE, Tamimi H, Figge DC. Bowel complications with taxol therapy. J Clin Oncol. 1993 Jun;11((6)):1198. [PubMed] [Google Scholar]
- 24.Schorge JO. Laparoscopic diverting loop ileostomy for spontaneous colon perforation in advanced ovarian cancer. Gynecol Oncol Rep. 2019 Mar;28:84–5. doi: 10.1016/j.gore.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendivil AA, Micha JP, Brown JV, 3rd, Rettenmaier MA, Abaid LN, Lopez KL, et al. Increased incidence of severe gastrointestinal events with first-line paclitaxel, carboplatin, and vorinostat chemotherapy for advanced-stage epithelial ovarian, primary peritoneal, and fallopian tube cancer. Int J Gynecol Cancer. 2013 Mar;23((3)):533–9. doi: 10.1097/IGC.0b013e31828566f1. [DOI] [PubMed] [Google Scholar]
- 26.Wade DS, Nava HR, Douglass HO., Jr Neutropenic enterocolitis. Clinical diagnosis and treatment. Cancer. 1992 Jan;69((1)):17–23. doi: 10.1002/1097-0142(19920101)69:1<17::aid-cncr2820690106>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues FG, Dasilva G, Wexner SD. Neutropenic enterocolitis. World J Gastroenterol. 2017 Jan;23((1)):42–7. doi: 10.3748/wjg.v23.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davila ML. Neutropenic enterocolitis. Curr Opin Gastroenterol. 2006 Jan;22((1)):44–7. doi: 10.1097/01.mog.0000198073.14169.3b. [DOI] [PubMed] [Google Scholar]
- 29.Rolston KV, Bodey GP, Safdar A. Polymicrobial infection in patients with cancer: an underappreciated and underreported entity. Clin Infect Dis. 2007 Jul;45((2)):228–33. doi: 10.1086/518873. [DOI] [PubMed] [Google Scholar]
- 30.de Haan D, van den Berg M. Colonic perforation secondary to taxol therapy: an unusual presentation. Onkologie. 2006 Nov;29((11)):541–2. doi: 10.1159/000096150. [DOI] [PubMed] [Google Scholar]
- 31.Carter J, Durfee J. A case of bowel perforation after neoadjuvant chemotherapy for advanced epithelial ovarian cancer. Gynecol Oncol. 2007 Dec;107((3)):586–9. doi: 10.1016/j.ygyno.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Hruban RH, Yardley JH, Donehower RC, Boitnott JK. Taxol toxicity. Epithelial necrosis in the gastrointestinal tract associated with polymerized microtubule accumulation and mitotic arrest. Cancer. 1989 May;63((10)):1944–50. doi: 10.1002/1097-0142(19890515)63:10<1944::aid-cncr2820631013>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 33.Keidan RD, Fanning J, Gatenby RA, Weese JL. Recurrent typhlitis. A disease resulting from aggressive chemotherapy. Dis Colon Rectum. 1989 Mar;32((3)):206–9. doi: 10.1007/BF02554529. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor K, Dijkstra B, Kelly L, McDermott EW, Hill AD, O'Higgins N. Successful conservative management of neutropenic enterocolitis: a report of two cases and review of the literature. ANZ J Surg. 2003 Jun;73((6)):463–5. doi: 10.1046/j.1445-2197.2003.02660.x. [DOI] [PubMed] [Google Scholar]
- 35.Radulović S, Golubović Z, Jovanović B, Pejanović J. [Surgery for neutropenic enterocolitis, a complication in acute lymphoblastic leukemia] Srp Arh Celok Lek. 2004 Oct;132(Suppl 1):119–21. doi: 10.2298/sarh04s1119r. [DOI] [PubMed] [Google Scholar]
- 36.Ettinghausen SE. Collagenous colitis, eosinophilic colitis, and neutropenic colitis. Surg Clin North Am. 1993 Oct;73((5)):993–1016. doi: 10.1016/s0039-6109(16)46137-2. [DOI] [PubMed] [Google Scholar]
- 37.Seewaldt VL, Cain JM, Goff BA, Tamimi H, Greer B, Figge D. A retrospective review of paclitaxel-associated gastrointestinal necrosis in patients with epithelial ovarian cancer. Gynecol Oncol. 1997 Nov;67((2)):137–40. doi: 10.1006/gyno.1997.4842. [DOI] [PubMed] [Google Scholar]
- 38.Torrisi JM, Schwartz LH, Gollub MJ, Ginsberg MS, Bosl GJ, Hricak H. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology. 2011 Jan;258((1)):41–56. doi: 10.1148/radiol.10092129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Should the editors request the data upon which the work is based, the authors shall produce it.