Abstract
This study aimed to determine whether pig responses to heat stress (HS) were directly due to heat exposure (regardless of feeding level and pattern) or were indirectly due to the reduction of feed intake (FI) and to determine if increasing feeding frequency (splitting heat increments) can improve pig response to HS. A total of 48 pigs (66.1 ± 1.7 kg) were allocated to four groups in three replicates. After 7 d in thermoneutral (TN) conditions (22 °C; period 1 [P1; day −7 to −1]), pigs were placed in either TN or HS (32 °C) conditions for 20 d (period 2 [P2; day 0 to 19]). The diet was provided either ad libitum (AL; 2 distributions/d) or pair-fed (PF8; 8 distributions/d) using HS–AL pigs as the reference group. Thus, the four experimental groups were TN–AL, HS–AL, TN–PF8, and HS–PF8. The daily ration of PF8 pigs was distributed at every 90-min intervals from 0900 to 1930 hours. Data were analyzed using the PROC MIXED procedure with replicate (n = 3), experimental group (n = 4), and their interactions as fixed effects, and the REPEATED statement was used for repeated measures data. Pigs had a similar average daily feed intake (ADFI) during P1 (P > 0.05). In P2, HS–AL and PF8 pigs had lower ADFI (−19%), average daily gain (−25%), and final body weight (−6.1 kg) than TN–AL pigs (P < 0.01). TN–AL pigs had thicker backfat than TN–PF8 pigs (P < 0.05), while the HS pigs had intermediate results. HS pigs had a higher perirenal fat percentage based on the contrast analysis between PF8 pigs (P < 0.05). Thermoregulatory responses of pigs increased with HS exposure but did not differ between HS or between TN groups (P > 0.05). For TN pigs, variation in muscle temperature (Tmuscle) depended on feeding and physical activity, while for HS pigs, Tmuscle gradually increased throughout the day. The Tmuscle of PF8 pigs increased with each additional meal but plateaued earlier for HS–PF8 than TN–PF8 pigs; an increase in Tmuscle per meal was also lower in HS–PF8 than TN–PF8 (P < 0.05). Exposure to HS decreased plasma T3 and T4 (P < 0.05) and increased plasma creatinine (P < 0.05). Between the PF8 groups, HS pigs also had a transient increase in plasma insulin on day 8 (P < 0.05). The effect of HS on FI decreased the growth rate of pigs but there are heat-induced effects, such as altered physiological responses, which might explain the direct HS effects seen in other literature especially in terms of increased adiposity. The increased feed provision frequency in the present study did not improve the HS response of pigs.
Keywords: feeding behavior, heat stress, meal frequency, pair-feeding, pig, thermoregulation
Introduction
With increasing global temperature and more frequent summer heat waves (Perkins-Kirkpatrick and Gibson, 2017), the climatic environment remains to be a major limiting factor in swine production in many countries. Reduction of pig performance during heat stress (HS) is mainly related to the reduced feeding level (Collin et al., 2001a) as a way to reduce the thermic effect of feeding (TEF) and thereby of heat production (HP) (Quiniou et al., 2001). However, pair-feeding (PF) models demonstrate that heat-induced reduction of feed intake (FI) does not fully explain the negative consequences of HS (Baumgard and Rhoads, 2013; Ross et al., 2017). Using PF studies, HS exposure has been shown to limit lipid mobilization or increase body adiposity in ruminants (Wheelock et al., 2010), rats (Katsumata et al., 1990) poultry (Zeferino et al., 2016), and pigs (Kouba et al., 2001; Pearce et al., 2013; Sanz Fernandez et al., 2015a; Kellner et al., 2016). These reported changes are theorized to be adaptive responses to help the animals to better cope with HS.
Using PF as a nutrient intake control is a widely accepted experimental method but has its limitations, since it imposes a different type of stress on animals (e.g., prolonged fasting and alteration of feeding behavior), which can increase stress responses and plasma nonesterified fatty acids (NEFA) release (Russell et al., 2008). PF pigs may also be subjected to the additional stress of an abrupt shift from being fed ad libitum (AL) to suddenly receive 2 to 3 joule-deficit meals per day. Furthermore, being trained to a fixed meal pattern may alter ghrelin responses (Drazen et al., 2006), which could then have consequences on other metabolic responses (Pradhan et al., 2013). It is thus difficult to isolate and determine the direct effects of HS especially if confounded with the effects of changing the feeding pattern and of the feeding pattern itself. Therefore, in designing the PF model to evaluate direct HS effects, the present study aimed to remove these confounding variables by having a PF group in both TN and HS conditions with the same feed distribution frequency and size and adapting these two groups to the set feeding schedule even before the experimental period.
Modifying feeding behavior via feeding frequency has already been shown to alter growth performance and metabolic response even in pigs housed in the same thermoneutral (TN) conditions (Schneider et al., 2011; Le Naou et al., 2014). Meanwhile, exposure to high ambient temperature itself alters feeding behavior in pigs such as decreased meal size and/or frequency in lactating sows (Quiniou et al., 2000) and in younger pigs (Collin et al., 2001b). Increasing meal frequency has also been linked to improved performance during HS exposure in dairy cows (West, 1999) and in pigs (Labussière et al., 2016a), which can be linked to the lower TEF per meal and thus lower instantaneous heat load for the animals. This study, therefore, aims to evaluate and to confirm indirect HS effects (caused by nutrient intake reduction) and direct HS effects (regardless of nutrient intake and meal patterns) on finishing pig performance and to determine if increasing meal frequency can help in mitigating these effects.
Materials and Methods
The experiment was conducted in accordance with the French legislation on animal experimentation and was approved by a Committee for Consideration of Ethics in Animal Experimentation (authorization: APAFIS#18973-2019020622003043).
Animals and treatments
The experiment was conducted from June to October 2019 in the INRAE experimental facilities at the Unité Expérimentale Physiologie et Phénotypage des Porcs (UE 3P) located in Saint-Gilles, France. The experimental design consists of two environmental treatments (TN vs. HS) and, within each environment, two feeding management treatments in which the diet (Table 1) was provided either AL (2 distributions/d) or PF (PF8; 8 distributions/day) with AL-fed HS pigs as the reference group. Thus, there were four experimental groups: TN–AL, TN–PF8, HS–AL, and HS–PF8.
Table 1.
Composition of the experimental diet1
| Ingredients, % as-fed | Finisher diet |
|---|---|
| Wheat | 34.00 |
| Barley | 25.00 |
| Corn | 15.00 |
| Soybean meal | 5.06 |
| Rapeseed meal | 9.00 |
| Soft wheat bran | 8.00 |
| Rapeseed oil | 1.00 |
| l-Lysine HCI | 0.65 |
| l-Threonine | 0.09 |
| l-Tryptophan | 0.05 |
| dl-Methionine | 0.04 |
| Calcium carbonate | 1.10 |
| Vitamin–mineral premix2 | 0.50 |
| Salt | 0.40 |
| Acidifier | 0.10 |
| Phytase | 0.01 |
| Analyzed chemical composition, % dry matter basis | |
| Dry matter | 88.72 |
| Crude protein | 14.80 |
| Crude ash | 4.53 |
| Crude fat | 3.10 |
| Neutral detergent fiber | 16.46 |
| Acid detergent fiber | 5.68 |
| Acid detergent lignin | 1.73 |
| Starch | 45.8 |
| Calculated values, % dry matter basis | |
| Net energy, MJ/kg | 10.97 |
| SID lysine, g/ kg | 9.18 |
1Diet fed in pellet form.
2Provided per kilogram of complete diet: vitamin A, 1,000,000 IU; vitamin D3, 200,000 IU; vitamin E, 4,000 mg; vitamin B1, 400 mg; vitamin B2, 800 mg; calcium pantothenate, 2,170 mg; niacin, 3,000 mg; vitamin B12, 4 mg; vitamin B6, 200 mg; vitamin K3, 400 mg; folic acid, 200 mg; biotin, 40 mg; choline chloride, 100,000 mg; iron (sulfate), 11,200 mg; iron (carbonate), 4,800 mg; copper (sulfate), 2,000 mg; zinc (oxide), 20,000 mg; manganese (oxide), 8,000 mg; iodine (iodate), 40 mg; cobalt (carbonate), 20 mg; and selenium (selenite), 30 mg.
A total of 48 Pietrain × (Large White × Landrace) female finishing pigs (66.1 ± 1.7 kg live body weight [BW]) in three successive replicates were used in the experiment and placed in individual pens (1 × 2 m on iron-slatted floor). Within each replicate, pigs were allotted to the four experimental groups according to live BW and litter origin (same sire). Lights were switched on from 0730 to 1930 hours in both rooms. All pigs were maintained at a constant temperature of 22 °C (actual: 22.1 ± 1.0 °C) during 11 d of adaptation and then for another 7-d period (period 1 [P1]: day −7 to −1). Pigs of the TN group were then maintained at 22 °C (actual: 21.41 ± 0.25 °C) until the end of the experiment (period 2 [P2]: day 0 to 19). For pigs of the HS group, the ambient temperature was gradually changed at a rate of 2 °C/h from 1200 to 1800 hours on day 0 and was thereafter maintained at a constant temperature of 32 °C (actual: 32.6 ± 1.8 °C) until day 21. The decision to use 22 °C as the TN room temperature and 32 °C as room temperature to induce HS was based on previous literature (Brown-Brandl et al., 2001; Renaudeau et al., 2011).
For the PF8 pigs, PF was based on the pig’s predicted BW and on the 3-d average FI per kilogram BW of the HS–AL group. The HS–AL group was exceptionally given feed allowance of −20% and −10% of their pre-challenge average daily feed intake (ADFI) on days 1 and 2 of P2, respectively, instead of feeding them AL to remove the risk of inaccurate PF on the first days of the thermal challenge. The values used (−20% and −10% ADFI reduction on the first 2 days) were based on the results from a previously conducted experiment in HS pigs (Serviento et al., 2020). In AL groups, feed was distributed at 0900 and at 1530 hours, while for PF8 groups, the calculated daily ration was divided into eight feed provisions (around 300 to 400 g each depending on feed allowance), which were distributed at every 90-min interval from 0900 to 1930 hours. Pigs had free access to water during the whole experimental period. The day prior to slaughter (i.e., day 20), feed was removed from the feeders at 1800 hours. In order to standardize the digestive tract content, each pig was given a 500-g feed ration 2 h before slaughter.
Measurements
Growth, slaughter, and feeding behavior
The pigs were individually weighed on days −7, 0, and 20 of the experimental period and on the day of slaughter. The individual daily FI of the pigs was measured as the difference between offered and refused feed. Refusals and spillages were collected daily at 0800 hours before the first feed distribution of the day. In case of wet refusals, dry matter was determined (in a ventilated oven at 103 °C for 24 h) and used to correct FI on an as-fed basis. An infrared barrier system was installed in each of the individual feed trough to record time, duration, and number of feeding bouts. A visit was recorded each time the infrared barrier was interrupted by the presence of the pig; the duration of each visit was the elapsed time between the recorded time the pig was detected by the infrared barrier and the time the pig left.
Pigs were slaughtered in the experimental slaughterhouse of INRAE-UE3P by electrical stunning and exsanguination and in compliance with the current EU regulations (Commission Delegated Regulation, 2017). Hot carcass, perirenal fat, and visceral organs (liver, kidneys, and heart) were weighed just after slaughter. Backfat (G2) and muscle (M2) depths were measured on one dorsal spot between the third and fourth last ribs at 6 cm of spinal canal axis, using a Capteur Gras Maigre (Lean Fat Sensor) (CGM) device (Fives Syleps, Lorient, France). Backfat thickness (BFT) was measured directly on the carcass using a caliper square at three different locations: at the level of the first and last ribs and of Gluteus medius (minimum fat thickness). The day after slaughter, cold carcass weight was measured and recorded.
Physiological parameters
Thermoregulation parameters were measured on the pigs at 1000 hours on days −4, 0, 1, 2, 3, 6, 10, 13, 17, and 20. To evaluate the acute thermoregulatory response, additional measurements were done on day 0 (transition day of the HS room to high ambient temperature) at 1200, 1400, 1600, and 1800 hours. Rectal temperature (Trectal) was measured using a digital thermometer (Microlife Corporation, Paris, France; accuracy ±0.1 °C) and skin temperature (Tskin) by a Type K thermocouple probe (HH-21 model, Omega, Stamford, CT, USA; accuracy ±0.1 °C). Respiratory rate (RR) was determined by counting flank movements for 30 s and was expressed in breath per min (bpm) only on resting animals (total observations n = 619). At the beginning of the adaptation period, a measurement probe for measuring internal body temperature (Anipill, Body Cap, Hérouville Saint-Clair, France) was implanted on eight pigs per experimental group (in two pigs per group for replicates 1 and 2 and in four pigs for replicate 3). The capsule was implanted 2 to 3 cm deep into the brachiocephalic muscle at the neck of the animal similar to the method described by Renaudeau (2016). The capsules recorded the body temperature at the muscular level (Tmuscle) of the pigs every 2 min.
Blood was collected on restrained animals via the jugular vein in two heparin tubes (one 8 mL and one 2 mL; Vacutest Kima, Arzegrande, Italy) on days −1, 1, 8, and 20 at 1330 hours. Blood carbon dioxide (partial pressure or pCO2 and concentration or [CO2]), bicarbonate, and pH were measured in the 2-mL tubes using a blood gas analyzer (ABL80 FLEX, Radiometer SAS, France) within 30 min following the sampling. The blood samples in the 8-mL tubes were centrifuged (3,000 × g; 10 min; 4 °C), and plasma was stored at −20 °C until analysis. Commercially available kits were used to measure plasma levels of NEFA (FUJIFILM Wako Chemicals Europe GmbH, Neuss, Germany), creatinine (Creatinine [Jaffe], Thermo Fisher Scientific Oy, Vantaa, Finland), glucose (Glucose [HK], Thermo Fisher Scientific Oy, Vantaa, Finland), and urea (Urea, Thermo Fisher Scientific Oy, Vantaa, Finland). Intra-assay coefficient of variation (CV) of NEFA, creatinine, glucose, and urea was 0.4%, 3.7%, 2.0%, and 1.5%, respectively; inter-assay CV was 9.8%, 4.8%, 8.7%, and 7.0%, respectively. Plasma levels of insulin (ST AIA-PACK IRI, Tosoh Corporation, Tokyo, Japan), total triiodothyronine or T3 (ST AIA-PACK T3, Tosoh Corporation, Tokyo, Japan), and total thyroxin or T4 (ST AIA-PACK T4, Tosoh Corporation, Tokyo, Japan) were also determined, and their intra-assay CV was 2.3%, 3.8%, and 3.9%, respectively.
Calculations and statistical analysis
There were four experimental groups based on the climatic environment (TN vs. HS) and based on the feeding management (AL vs. PF8): TN–AL, TN–PF8, HS–AL, and HS–PF8. For each of the three replicates, there were four pigs per experimental group. Three pigs (one TN–AL, one TN–PF8, and one HS–AL) were removed from the final calculation and statistical analysis because of sickness or causes unrelated to feeding management and/or environmental treatments. For growth, feeding behavior, slaughter, and blood parameters, the pig (n = 45) was considered as the experimental unit. In looking at the relationship between the Tmuscle and the corresponding feeding behavior (presence at feeder), only data from pigs implanted with an internal temperature probe that worked properly were included (n = 26 wherein TN–AL, n = 6; TN–PF8, n = 8; HS–AL, n = 5; and HS–PF8, n = 7).
Growth, slaughter, and physiological parameters
For growth and feeding behavior data, the trial was divided into two periods: P1 (day −7 to −1) and P2 (day 0 to 19). Growth parameters such as average daily gain (ADG; g/d), ADFI (g/d or g·kg BW–1·d–1), and feed conversion ratio (FCR) were calculated per period. For the feeding behavior data, visits to the feeder that lasted for <5 s were removed from the dataset. Two consecutive visits separated by a time interval not longer than a given meal criterion were considered to belong to one meal. The calculated meal criterion of the present study was 15 min. These data were based on the first derivative of the polynomial function estimating the number of meals based on different meal criteria (Renaudeau et al., 2002). This adopted meal criterion was used for further calculation of feeding behavior parameters per period such as meal frequency (number of meals/day) and meal duration (min∙meal−1·d−1 and min·meal−1). Growth and feeding behavior data were analyzed using the REPEATED measure of the PROC MIXED procedure (SAS Inst. Inc., Cary, NC) with replicate (n = 3), experimental group (n = 4), period (n = 2) or day for live BW (n = 3), and their interactions as fixed effects, and pen and sire as random effects.
For carcass traits, carcass dressing was calculated as the percentage of hot carcass to slaughter BW (sBW), while carcass lean meat content was calculated using the CGM measurements (G2 and M2) according to the equation published by Daumas et al. (2010): Lean meat content (%) = 62.19 − 0.729 × G2 + 0.144 × M2. Slaughter data were analyzed using a PROC MIXED model with replicate (n = 3), experimental group (n = 4), and their interactions as fixed effects, and pen and sire as random effects.
Thermoregulation traits measured on day 0 were used for the acute HS responses. Daily means of Tmuscle and thermoregulatory responses measured at 1000 hours were used to assess chronic HS responses. Physiological parameters were analyzed using the PROC MIXED procedure with replicate (n = 3), experimental group (n = 4), day (n = 4 for blood and n = 10 for chronic thermoregulation responses) or time of measurement (n = 6 for acute thermoregulation and n = 13 for acute Tmuscle responses), and their interactions as fixed effects. Pen and sire were included as random effects, and the REPEATED statement was used to specify covariance structures for repeated measures data.
Orthogonal contrast statements were used to evaluate the overall effect of feeding management (TN–AL and HS–AL) vs. (TN–PF8 and HS–PF8), of climatic environment in AL-fed groups: (TN–AL) vs. (HS–AL), and of climatic environment in PF8-fed groups: (TN–PF8) vs. (HS–PF8). P < 0.05 was considered as significant, and P < 0.10 was considered as a trend.
Pattern of body temperature and presence at the feeder
Data from day 6 (the day in which Trectal of HS groups stabilized) until day 19 were considered, and data from day 8 (blood sampling day) were removed. Data averaged every 10 min were used to look at the nychthemeral pattern of the pigs. Data (n = 144 for every 10 min) were analyzed using the REPEATED statement of the PROC MIXED procedure with replicate (n = 3), experimental group (n = 4), and their interactions as fixed effects, and pen and sire as random effects.
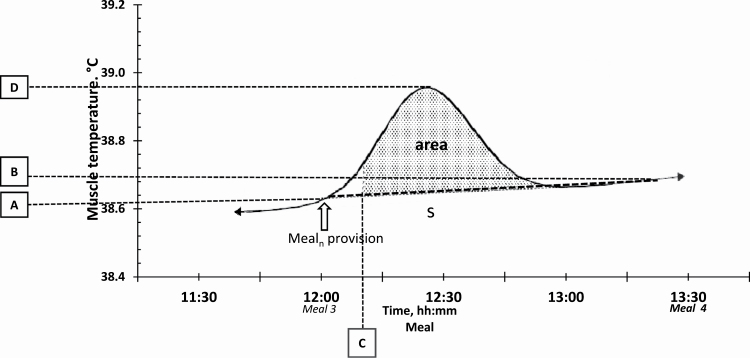
For the AL-fed groups, data of Tmuscle and presence at the feeder were averaged per hour, and a reference value was considered from the hour at which feeder activity is the lowest for each respective group: hour 11 for TN–AL and hour 0 for HS–AL. The description of the kinetics was then based on the contrast comparison of hourly values to the reference. As meal provision was similar for both PF8 groups, the corresponding data were used to evaluate the effect of ambient temperature on the relationship between body temperature and feeding behavior traits. For each meal, each pig, and each day, the median Tmuscle value measured 10 min before the meal (mealn) distribution was determined (baseline Tmuscle or parameter A in Figure 1) and the same was done for the following meal (mealn+1) (B parameter). The slope (S) between points A and B was calculated; for the eighth meal, the slope was calculated using its baseline Tmuscle (i.e., median Tmuscle from 1920 to 1930 hours) and the median Tmuscle after 90 min (2050 to 2100 hours) to have the similar time range with the first seven meals. The threshold point at which Tmuscle started to increase after the meal distribution (C) corresponded to the time at which Tmuscle was significantly different from A, and, finally, parameter D is the maximum Tmuscle after the meal provision. The effect of meal distribution and temperature on the Tmuscle was evaluated by calculating the surface under the curve as defined in Figure 1. The described parameters were analyzed using the repeated measures of the PROC MIXED model with replicate (n = 3), experimental group (n = 2), meal (n = 8), and their interactions as fixed effects, and with pen and sire as random effects. The SLICE option was used to determine contrasts between the two PF8 groups per meal and among the meals per experimental group.
Figure 1.
Graphical illustration of how the area under the curve was calculated for a specific meal using the Tmuscle (°C) of the PF8 pigs and the time passed (min) after each meal provision. A = baseline Tmuscle of mealn; B = baseline Tmuscle of mealn+1; C = starting point of peak in Tmuscle (P < 0.05 vs. point A); D = maximum Tmuscle of mealn; S = slope between points A and B; area = area under the Tmuscle curve.
Results
Growth and slaughter performance
Table 2 provides the summary of the growth performance and feeding behavior traits of the finishing pigs according to the experimental treatment. There was no significant difference among all groups in any of the parameters during P1. In P2, the reference group HS–AL and both PF8 groups had lower ADFI (−547 g·d–1 or −5.1 g·kg BW–1·d–1 on average; P < 0.01) and ADG (−279 g·d–1 on average; P < 0.01) compared with the TN–AL group. FCR of HS–PF8 pigs was higher than the value measured for TN–AL pigs (+0.37; P = 0.04) and tended to be higher than FCR of HS–AL (+0.29; P = 0.09) but did not differ from FCR of TN–PF8 pigs (P = 0.38). Based on the contrast analysis between the environmental treatments on P2, exposure to HS decreased total meal duration in both the AL and the PF8 groups (−22 min on average; P < 0.03). Regarding the contrast between the two feeding managements, pigs fed AL had fewer meals per day than the PF8 pigs in P2 (−2.9 meals·d−1 on average; P < 0.01) but their meals had a longer duration (+1.3 min·meal−1 on average; P = 0.04).
Table 2.
Effect of HS and feeding management on growth performance and feeding behavior of finishing pigs1
| Contrasts P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | TN–AL | TN–PF8 | HS–AL | HS–PF8 | RSD2 | Statistics3 | AL vs. PF84 | AL: TN vs. HS5 | PF8: TN vs. HS6 |
| Live BW, kg | |||||||||
| Day −7 | 66.2 | 66.0 | 65.8 | 67.0 | 1.3 | R**, D**, G × D** | 0.65 | 0.81 | 0.52 |
| Day 0 | 74.6 | 73.8 | 73.5 | 74.6 | 0.89 | 0.51 | 0.60 | ||
| Day 20 | 97.7a | 91.7b | 91.6b | 90.9b | <0.01 | <0.01 | 0.61 | ||
| ADFI, g·d−1 | |||||||||
| P1 | 2,605 | 2,527 | 2,655 | 2,649 | 135 | G**, P**, G × P** | 0.45 | 0.54 | 0.15 |
| P2 | 2,929a | 2,415b | 2,388b | 2,343b | <0.01 | <0.01 | 0.40 | ||
| ADFI per BW, g·kg BW–1·d–1 | |||||||||
| P1 | 37.1 | 36.2 | 38.1 | 37.7 | 1.9 | R**, G**, P**, G × P** | 0.29 | 0.30 | 0.13 |
| P2 | 34.1a | 29.2b | 28.9b | 28.6b | <0.01 | <0.01 | 0.54 | ||
| ADG, g·d−1 | |||||||||
| P1 | 1,195 | 1,122 | 1,100 | 1,085 | 134 | G**, P**, G × P* | 0.18 | 0.14 | 0.56 |
| P2 | 1,154a | 903b | 911b | 811b | <0.01 | <0.01 | 0.14 | ||
| FCR | |||||||||
| P1 | 2.19 | 2.29 | 2.45 | 2.49 | 0.29 | R*, G*, P** | 0.43 | 0.06 | 0.14 |
| P2 | 2.55b | 2.70ab | 2.63ab | 2.92a | <0.01 | 0.53 | 0.11 | ||
| Total meal duration/day, min·d–1 | |||||||||
| P1 | 62 | 63 | 69 | 60 | 17 | G × P* | 0.60 | 0.45 | 0.74 |
| P2 | 73 | 72 | 51 | 50 | 0.87 | 0.03 | 0.03 | ||
| No. of meals/day, n·d−1 | |||||||||
| P1 | 12.2 | 10.9 | 11.3 | 13.0 | 1.6 | P**, G × P**, R × G × P* | 0.76 | 0.41 | 0.08 |
| P2 | 11.8b | 15.1a | 11.8b | 14.2ab | <0.01 | 0.95 | 0.44 | ||
| Meal duration, min·meal−1 | |||||||||
| P1 | 5.2 | 6.2 | 6.4 | 4.8 | 1.5 | G × P* | 0.59 | 0.17 | 0.17 |
| P2 | 6.5a | 4.9ab | 4.8ab | 3.9b | 0.04 | 0.05 | 0.33 | ||
1A total of 48 pigs were allocated to four experimental groups in three replicates. All pigs were housed at thermoneutrality (P1: day −7 to −1) before being subjected to either thermoneutral (TN) or heat stress (HS) conditions (P2: day 0 to 19). In each environment, diet was provided either ad libitum (AL) (2 feed provisions/day) or pair fed (PF8) (8 feed provisions/day) with the HS pigs fed AL as the reference group.
2Residual standard deviation.
3The pig was considered as the experimental unit. Data were analyzed using PROC MIXED model with replicate (R), experimental group (G), day (D), or period (P), and their interactions as fixed effects.
4Contrast statement: [TN–AL, HS–AL] vs. [TN–PF8, HS–PF8].
5Contrast statement: [TN–AL] vs. [HS–AL].
6Contrast statement: [TN–PF8] vs. [HS–PF8].
a–cLSmeans within a row with different superscripts letters differ (P < 0.05) according to the experimental group.
*P < 0.05; **P < 0.01.
Looking at the slaughter performance (Table 3), the HS–AL, TN–PF8, and HS–PF8 pigs had lower sBW (−6.6 kg on average; P < 0.01) and hot carcass weight (−4.3 kg on average; P = 0.02) than the TN–AL pigs, while lean meat content was similar among all groups (62.6% on average; P > 0.10). The feeding management significantly affected kidneys and liver proportions relative to sBW (higher in AL than in PF8 pigs; P = 0.03 and P =0.02, respectively). The TN–PF8 pigs had a lower average BFT than TN–AL pigs (P < 0.05), while the HS groups had intermediate results. Meanwhile, based on the contrast analysis between the two PF8 groups, average BFT did not significantly differ (P = 0.27) but HS increased the perirenal fat percentage of PF8 pigs (P < 0.04).
Table 3.
Effect of HS and feeding management on slaughter traits of finishing pigs1
| Contrasts P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | TN–AL | TN–PF8 | HS–AL | HS–PF8 | RSD2 | Statistics3 | AL vs. PF85 | AL: TN vs. HS6 | PF8: TN vs. HS7 |
| Slaughter BW, kg | 96.7a | 90.6b | 90.2b | 89.6b | 3.8 | R*, G** | 0.02 | <0.01 | 0.57 |
| Hot carcass wt., kg | 75.0a | 70.8b | 70.4b | 70.8b | 3.5 | R**, G* | 0.10 | <0.01 | 0.98 |
| Carcass dressing, % | 77.6 | 78.2 | 78.1 | 78.9 | 1.3 | R* | 0.13 | 0.42 | 0.28 |
| Lean meat, % | 62.8 | 62.5 | 62.1 | 62.8 | 1.6 | 0.71 | 0.35 | 0.73 | |
| Ave BFT, mm4 | |||||||||
| BFT first rib | 34.0a | 31.1ab | 30.0b | 32.5ab | 3.2 | G*, R × L* | 0.85 | <0.01 | 0.34 |
| BFT last rib | 19.3 | 16.9 | 17.9 | 18.5 | 0.38 | 0.32 | 0.26 | ||
| BFT gluteus | 12.6 | 11.0 | 11.4 | 11.3 | 0.39 | 0.40 | 0.88 | ||
| Average | 22.0a | 19.7b | 19.8ab | 20.8ab | 0.29 | 0.02 | 0.27 | ||
| Total viscera, g∙kg-1 sBW8 | 224 | 218 | 219 | 211 | 13 | R* | 0.13 | 0.42 | 0.28 |
| Kidneys | 3.8a | 3.3b | 3.4ab | 3.3b | 0.4 | G* | 0.03 | 0.05 | 0.90 |
| Liver | 20.3a | 18.5b | 19.3ab | 18.6b | 1.4 | R**, G* | 0.02 | 0.15 | 0.90 |
| Perirenal fat | 8.0 | 7.8 | 8.4 | 9.4 | 1.6 | R* | 0.37 | 0.61 | 0.04 |
| Heart | 3.7 | 3.7 | 3.5 | 3.6 | 0.4 | R* | 0.37 | 0.25 | 0.45 |
| Others | 188 | 184 | 184 | 176 | 13 | R* | 0.17 | 0.50 | 0.16 |
1A total of 48 pigs were allocated to four experimental groups in three replicates. All pigs were housed at thermoneutrality for 7 d (P1) before being subjected to either thermoneutral (TN) or heat stress (HS) conditions for 20 d (P2). In each environment, diet was provided either ad libitum (AL) (2 feed provisions/day) or fed (PF8) (8 feed provisions/day) with the HS pigs fed AL as the reference group.
2Residual standard deviation.
3The pig was considered as the experimental unit. Data were analyzed using PROC MIXED model with replicate (R), experimental group (G), and their interactions as fixed effects.
4The pig was considered as the experimental unit. Data were analyzed using PROC MIXED model with replicate (R), experimental group (G), measurement location (L), and their interactions as fixed effects.
5Contrast statement: [TN–AL, HS–AL] vs. [TN–PF8, HS–PF8].
6Contrast statement: [TN–AL] vs. [HS–AL].
7Contrast statement: [TN–PF8] vs. [HS–PF8].
8Not emptied: including total weight of heart and lungs, digestive tract, kidneys, and liver.
a–cLSmeans within a row with different superscripts letters differ (P < 0.05) according to the experimental group.
*P < 0.05; **P < 0.01.
Thermoregulatory responses
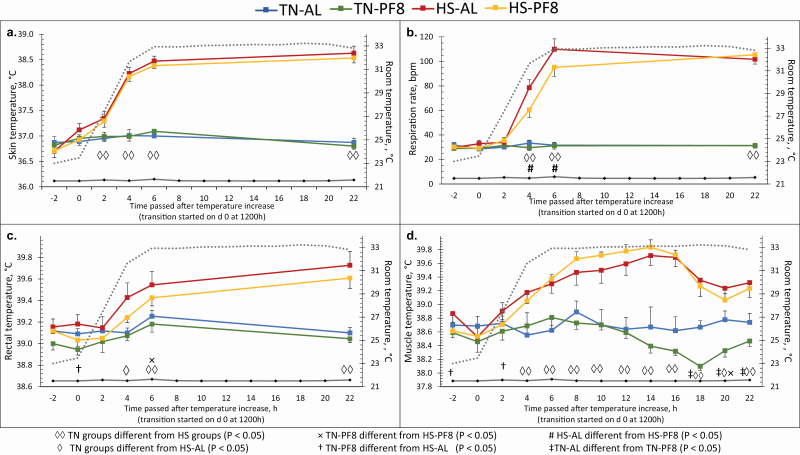
Figure 2 shows the acute thermoregulatory responses for the HS groups during the transition period. The two HS groups had similar Tskin throughout all the experiment (P > 0.10) and started to be significantly higher than the two TN groups just 2 h after the temperature rise (P < 0.01). RR of the HS groups also increased steadily with the room temperature and started to be significantly different from the one of TN groups after 4 h of temperature rise (P < 0.01). The TN groups had similar RR throughout the transition period (P > 0.10). However, HS–AL pigs had faster RR than the HS–PF8 pigs after 6 and 8 h of room temperature increase (P < 0.01). During the transition of the HS room temperature, HS–AL pigs started to have significantly higher Trectal than the TN pigs just after 4 h (P < 0.02), while it was later for HS–PF8 (higher Trectal than TN–PF8 and TN–AL from 6 and 22 h, respectively; P < 0.01). The two HS groups had higher Tmuscle than the TN–AL pigs starting at 4 h after the transition (P < 0.01). Compared with the TN–PF8 pigs, Tmuscle started to be significantly higher in HS–AL and HS–PF8 pigs after 2 and 4 h, respectively (P < 0.05).
Figure 2.
Effect of the climatic environment and feeding management on acute response of finishing pigs in terms of Tskin (a), respiration rate (b), Trectal (c), and Tmuscle (d) (means ± SE). From day −7 to −1, pigs were housed at thermoneutrality (TN; 22 °C). On day 0, the ambient temperature of HS room (•••••••) was increased at a rate of 2 °C/h from 1200 to 1800 hours and was maintained at a constant temperature of 32 °C thereafter, while the TN room ( ) remained at 22 °C. In each environment, the diet was provided either AL (2 distributions/d) or PF8 (8 distributions/day) with HS pigs fed AL as the reference group.
) remained at 22 °C. In each environment, the diet was provided either AL (2 distributions/d) or PF8 (8 distributions/day) with HS pigs fed AL as the reference group.
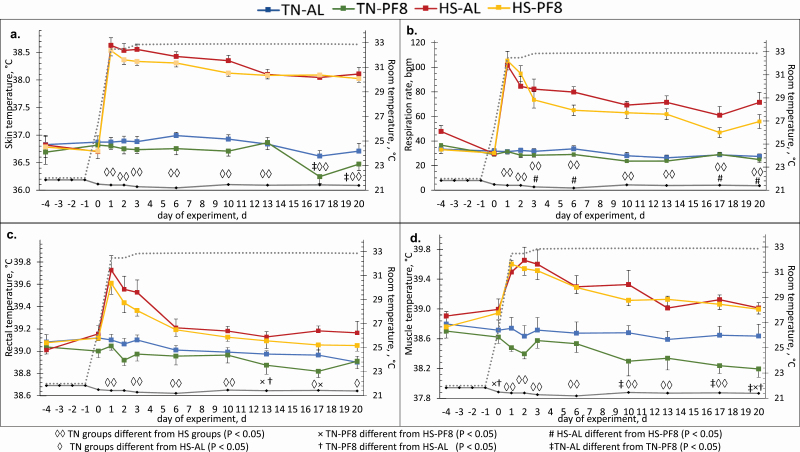
Chronic thermoregulatory responses of the pigs are shown in Figure 3. All parameters were similar among the four experimental groups on days −4 and 0 (P > 0.10) but, upon exposure to high ambient temperature, were elevated in the two HS groups starting on day 1 (P < 0.01). Pigs under HS had constantly higher Tskin and RR than TN pigs from the onset until the end of HS exposure (P < 0.01). Between the two TN-raised groups, Tskin of pigs fed AL was significantly higher on day 17 (P < 0.01) and day 20 (P = 0.01) than the PF8 pigs. Meanwhile, between the HS-raised groups, the Tskin of AL-fed pigs tended to be higher on day 3 (P = 0.06) and day 10 (P = 0.08) than the Tskin of PF8 pigs. RR of HS–AL pigs was also higher than RR of HS–PF8 pigs on day 3 (P = 0.04), day 6 (P < 0.01), day 10 (trend at P = 0.05), day 17 (P = 0.01), and day 20 (P < 0.01). Rectal temperature of the HS groups slowly declined and seemed to stabilize 6 d after the onset of HS but was still generally higher than that of TN groups. These differences were significant on days 10, 17, and 20, when Trectal of HS–AL pigs was higher than Trectal of the two TN groups (P < 0.04). Meanwhile, Trectal of HS–PF8 pigs was higher than TN–PF8 pigs only on day 17 (P < 0.01). The HS groups had higher daily Tmuscle than TN–PF8 pigs from day 0 until day 20 (P < 0.03) and TN–AL pigs from day 1 until 17 (P < 0.05), while this was only a tendency on day 20 (P ≤ 0.08). In TN conditions, pigs fed AL also had higher Tmuscle than PF8 pigs on days 10, 17, and 20 (P ≤ 0.04).
Figure 3.
Effect of the climatic environment and feeding management on the Tskin (a), respiration rate (b), Trectal (c), and Tmuscle (d) of finishing pigs (means ± SE). From day −7 to −1, pigs were housed at thermoneutrality and then from day 0 to 21, either in TN ( ; 22 °C) or in HS (•••••••; 32 °C) conditions. In each environment, the diet was provided either AL (2 distributions/day) or PF8 (8 distributions/day) with HS pigs fed AL as the reference group.
; 22 °C) or in HS (•••••••; 32 °C) conditions. In each environment, the diet was provided either AL (2 distributions/day) or PF8 (8 distributions/day) with HS pigs fed AL as the reference group.
Plasma and blood gas parameters
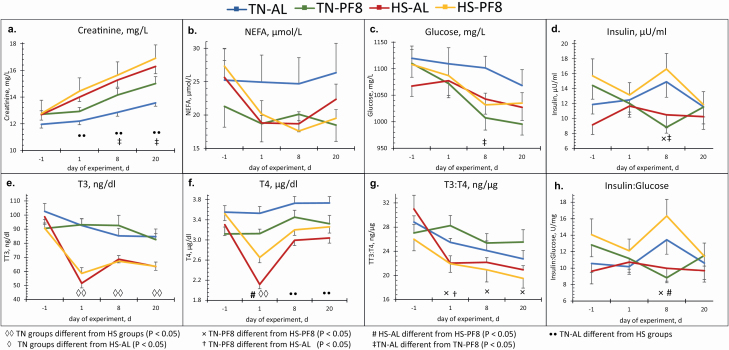
The results on the plasma parameters are shown in Figure 4. On day −1 of the experiment, plasma concentrations of metabolites measured at 1300 hours were similar among the four groups (P > 0.05). Plasma creatinine was lower in the TN–AL pigs groups compared with the two HS groups (P < 0.01 on days 1, 8, and 20) and TN–PF8 (P < 0.01 on days 8 and 20). The level of plasma NEFA was not significantly different among all groups regardless of the day, while that of glucose was only different on day 8 between TN–AL and TN–PF8 (P = 0.04). On day 8, TN–PF8 pigs had lower plasma insulin than the HS–PF8 (P < 0.01) and TN–AL pigs (P = 0.04). Meanwhile, plasma insulin:glucose ratio was higher in the HS–PF8 pigs than both TN–PF8 (P < 0.01) and HS–AL pigs (P < 0.02) on day 8. In hot conditions, increasing feeding frequency tended to increase plasma insulin (P = 0.07) and significantly increased the plasma insulin:glucose ratio (P = 0.02) with higher values for HS–PF8 than for HS–AL pigs. Exposure to HS decreased plasma T3 whatever the feeding management treatment (P < 0.01 on days 1 and 8; P < 0.03 on day 20). Plasma T4 concentration was lower in HS–AL pigs compared with HS–PF8 pigs on day 1 (P < 0.02), but both HS groups had lower levels than TN–AL pigs on days 1, 8, and 20 (P < 0.03) and TN–PF8 pigs but only on day 1 (P ≤ 0.03). Plasma T3:T4 ratio of TN–PF8 pigs was higher than HS–PF8 on days 1, 8, and 20 (P < 0.02) and HS–AL pigs only on day 1 of HS (P < 0.01).
Figure 4.
Effect of the climatic environment and feeding management on plasma metabolites [creatinine (a), NEFA (b), glucose (c)] and plasma hormones [insulin (d), T3 (e), T4 (f), T3:T4 (g), and insulin:glucose (h)] of finishing pigs: (means ± SE). From day −7 to d−1, pigs were housed at thermoneutrality and then from day 0 to 21, either in TN (22 °C) or in HS (32 °C) conditions. In each environment, the diet was provided either AL (2 distributions/day) or PF8 (8 distributions/day) with HS pigs fed AL as the reference group.
Still, in Figure 4, the contrast analysis of the environmental treatment within each feeding management (TN–AL vs. HS–AL or TN–PF8 vs. HS–PF8) shows that HS exposure decreased plasma T3 (P < 0.01 on days 1, 8, and 20) and increased plasma creatinine levels (contrast at P < 0.01 on days 1, 8, and 20 between AL groups; P < 0.03 on days 8 and 20 between PF8 groups). Plasma T4 levels were also decreased by HS exposure on days 1, 8, and 20 within AL pigs (P < 0.01) but only on day 1 within PF8 pigs (P < 0.01). HS also decreased the ratio of plasma T3:T4 between the PF8 groups (P < 0.01 on days 1, 8, and 20) but not within the AL groups (P > 0.05). On day 8 of the experiment, HS tended to decrease plasma insulin and insulin:glucose ratio of HS pigs when comparing the two AL-fed groups (P = 0.07 and P = 0.09, respectively), but these parameters were increased in HS pigs when comparing the two PF8 groups (P < 0.01 for both parameters).
Looking at the blood gas parameters in Table 4, blood pCO2 levels were higher in TN groups than HS–PF8 on day 8 (P < 0.01) and in TN–PF8 than HS–PF8 on day 20 (P < 0.01). Compared with TN pigs, blood (CO2) was lower in HS–PF8 pigs on day 1 (P = 0.01) and in both HS groups on day 8 (P < 0.04). Blood pH of HS–PF8 pigs was higher than HS–AL pigs and TN–PF8 pigs on days 1 and 8 (P < 0.05) and on days 8 and 20 (P < 0.01), respectively. The blood pH of the two AL groups did not significantly differ regardless of the environment (P > 0.10). From the contrast analysis, HS exposure decreased blood (CO2) and bicarbonate on days 1 and 8 (P < 0.04) in AL-fed groups and significantly decreased blood pCO2 on days 1, 8, and 20 (P < 0.03) and (CO2) levels on days 1 and 8 (P < 0.01) in PF8 pigs.
Table 4.
Effect of HS and feeding management on the blood gas parameters of finishing pigs1
| Contrasts P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items | TN–AL | TN–PF8 | HS–AL | HS–PF8 | RSD2 | Statistics3 | AL vs. PF84 | AL: TN vs. HS5 | PF8: TN vs. HS6 |
| pCO2, mmHg | |||||||||
| Day −1 | 62.3 | 73.4 | 72.4 | 68.7 | 9.6 | G**, D*, G × D* | 0.21 | 0.02 | 0.26 |
| Day 1 | 64.8 | 67.1 | 66.8 | 58.1 | 0.28 | 0.63 | 0.03 | ||
| Day 8 | 73.8a | 76.1a | 66.7ab | 59.5b | 0.41 | 0.09 | <0.01 | ||
| Day −20 | 68.5ab | 77.0a | 69.8ab | 62.8b | 0.82 | 0.77 | <0.01 | ||
| [CO2], mmol/L | |||||||||
| Day −1 | 28.8b | 32.1a | 30.1ab | 30.5ab | 2.3 | R**,G**, G × D** | <0.01 | 0.19 | 0.13 |
| Day 1 | 32.0a | 31.9a | 29.5ab | 28.6b | 0.51 | 0.03 | <0.01 | ||
| Day 8 | 32.6a | 32.5a | 29.6b | 29.4b | 0.80 | <0.01 | <0.01 | ||
| Day −20 | 31.1 | 31.8 | 29.7 | 30.0 | 0.46 | 0.19 | 0.09 | ||
| pH | |||||||||
| Day −1 | 7.33 | 7.31 | 7.29 | 7.31 | 0.05 | G** | 0.79 | 0.06 | 0.89 |
| Day 1 | 7.33ab | 7.33ab | 7.31b | 7.36a | 0.08 | 0.31 | 0.08 | ||
| Day 8 | 7.31ab | 7.29b | 7.30b | 7.36a | 0.19 | 0.69 | <0.01 | ||
| Day −20 | 7.32ab | 7.28b | 7.31ab | 7.34b | 0.72 | 0.62 | <0.01 | ||
| HCO3−, mmol/L | |||||||||
| Day −1 | 31.3b | 34.6a | 32.7ab | 33.1ab | R**, G*, D* | 0.02 | 0.24 | 0.22 | |
| Day 1 | 34.9 a | 35.0 a | 31.9ab | 31.6b | 0.85 | 0.02 | 0.01 | ||
| Day 8 | 34.5 | 35.0 | 32.0 | 32.3 | 0.64 | 0.04 | 0.02 | ||
| Day −20 | 34.2 | 34.8 | 33.8 | 32.8 | 0.80 | 0.74 | 0.09 | ||
1A total of 48 pigs were allocated to 4 experimental groups in 3 replicates. All pigs were housed at thermoneutrality (P1: day −7 to −1) before being subjected to either thermoneutral (TN) or heat stress (HS) conditions (P2: day 0 to 19). In each environment, diet was provided either ad libitum (AL) (2 feed provisions/day) or pair fed 8 (PF8) (8 feed provisions/day) with the HS pigs fed AL as the reference group.
2Residual standard deviation.
3The pig was considered as the experimental unit. Data were analyzed using PROC MIXED model with replicate (R), experimental group (G), day (D), and their interactions as fixed effects.
4Contrast statement: [TN–AL, HS–AL] vs. [TN–PF8, HS–PF8].
5Contrast statement: [TN–AL] vs. [HS–AL].
6Contrast statement: [TN–PF8] vs. [HS–PF8].
a–cLSmeans within a row with different superscripts letters differ (P < 0.05) according to the experimental group.
*P < 0.05, **P < 0.01.
Pattern of body temperature and feeding behavior
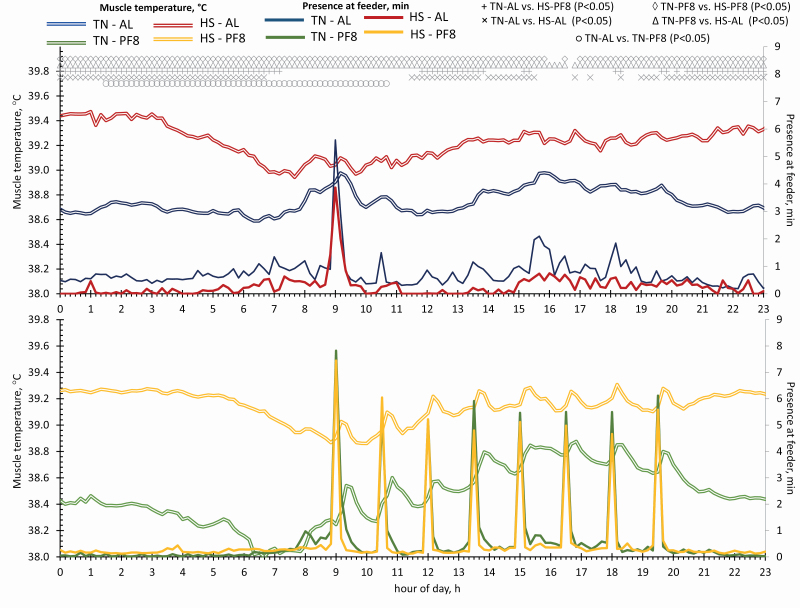
Figure 5 shows the nycthemeral variations of feeding activity and Tmuscle of the finishing pigs. Looking at the Tmuscle every 10 min, HS groups had higher Tmuscle than TN–PF8 pigs throughout the day (P < 0.05) except from 1600 to 1700 hours (trend at P < 0.10). Compared with TN–AL pigs, HS pigs also had generally higher Tmuscle (P < 0.05). Regardless of feeding management, pigs raised in HS conditions had similar Tmuscle throughout the day (P > 0.10), while TN–PF8 pigs had significantly lower Tmuscle than TN–AL pigs from 0130 to 1040 hours (P < 0.05).
Figure 5.
Effect of the environmental treatment and feeding management on the nycthemeral pattern of Tmuscle (°C) and presence at the feeder (min) of finishing pigs. From day −7 to −1, pigs were housed at thermoneutrality and then from day 0 to 21, either in TN (22 °C) or in HS (32 °C) conditions. In each environment, the diet was provided either AL (2 distributions/day) or PF8 (8 distributions/day) with HS pigs fed AL as the reference group. Data included are from day 6 to 19 of the experimental period with day 0 as the transition period of HS groups from TN to HS conditions.
To describe feeding activity and Tmuscle of each AL-fed group, averaged hourly data were compared with the reference value or to the data at the hour at which feeder activity was lowest. In the TN–AL group and in comparison to the reference value, feeder activity was significantly higher at 0900, 1500, 1600, and 1800 hours (P < 0.03) and tended to be higher at 1700 hours (P = 0.06) than the reference value. The pattern of Tmuscle was similar and was significantly higher from 0800 to 1000 hours, 1400 to 1900 hours (P < 0.02), and tended to be higher at 1300 hours (P = 0.06) than the Tmuscle than the reference value. For the HS–AL group, feeder activity was higher at 0900, 1500, 1600, 1800, and 2000 hours (P < 0.02) and tended to be higher at 0600, 0800, and 1900 hours (P < 0.10) than the reference value. In contrast, Tmuscle of the HS–AL group at the hour of lowest feeder activity (at 0000 hours) was higher than Tmuscle from 0400 to 2100 hours (P < 0.05).
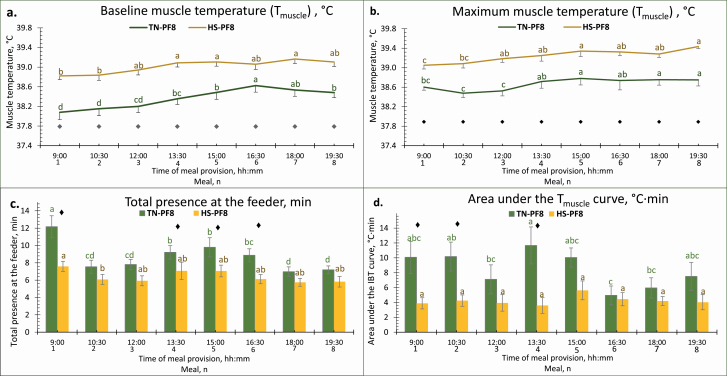
The results regarding the effect of environmental temperature on Tmuscle patterns in PF8 groups are shown in Figure 6. Baseline Tmuscle seemed to increase with each meal with the fourth meal having higher values than the first two meals in HS–PF8 pigs (P < 0.05) and with the sixth meal having higher values than the previous meals in TN–PF8 (P < 0.05). For each PF8 group, maximum Tmuscle also increased with each meal until the fifth meal, which had higher values than the first to second meals and the first to third meals for HS–PF8 and TN–PF8 pigs, respectively (P < 0.05). Feeder presence was the highest in the first meal compared with the other seven meals for the TN–PF8 pigs (P < 0.05), while for the HS–PF8 pigs, it was only higher compared with the second (P = 0.03) and not for the other meals (P ≥ 0.08). In terms of the body temperature increase, HS pigs had overall less area under the Tmuscle curve regardless of the meal (−4.7 °C·min on average; P < 0.01), but this difference was more apparent in the first four meals (P < 0.02 in meals 1, 2, and 4; P = 0.10 in meal 3). The time (or order) of the meal tended to affect the area under the curve for the TN–PF8 pigs (P = 0.07) but not for the HS–PF8 pigs (P = 0.99). In both PF8 groups, Tmuscle increased after each meal provision with a delay of around 11 min whatever the meal order (P = 0.77) or the ambient temperature (P = 0.50) (data not shown).
Figure 6.
Effect of the environmental treatment and order of meal in terms of Tmuscle [baseline (a) and maximum (b)], total presence at the feeder (c), and area under the Tmuscle curve (calculated based on Figure 1) of PF8 finishing pigs. From day −7 to −1, pigs were housed at thermoneutrality and then from day 0 to 21, either in TN (22 °C) or in HS (32 °C) environment. Pigs were PF to HS pigs fed AL, and the diet was provided in 8 distributions/day. Data included are from day 6 to 19 of the experimental period with day 0 as the transition period of HS groups from TN to HS conditions. a–cWithin each feeding management, means with different superscript letters differ according to the meal (P <0.05). ♦Groups are different within each meal (P <0.05).
Discussion
The study aimed to confirm direct HS effects as described in the literature and to determine if, with the same feeding level, increasing feed provision frequency in hot conditions could help pigs to better adapt to HS.
Body temperature pattern and feed restriction in TN conditions
In order to facilitate discussion on pig responses of HS, this subsection briefly establishes first the normal body temperature pattern and the effects of feed restriction in pigs under TN conditions. For both TN groups, Tmuscle peaks coincided with peaks of feeder activity. This close relationship between body core temperature and feeding behavior could be related to the HP from the TEF and from the concomitant variation in physical activity from standing up and walking to the feeder (van Milgen et al., 1998). This observation is similar to those described in the literature (Ingram and Dauncey, 1985; Morales et al., 2018) wherein body temperature pattern depends largely on feeding (level and behavior) and on the related physical activity. In the TN groups, the total duration of daily presence at the feeder was not different despite dissimilar ADFI. This result is probably linked with a feed anticipation behavior phenomenon in TN–PF8 pigs similar to observations by Collin et al. (2001c) in restricted-fed pigs. During feed restriction, a higher proportion of the total energy intake goes to maintenance, and protein deposition is prioritized instead of lipid (Quiniou et al., 1996), which explains why restricted-fed TN pigs in this study were lighter and thinner. Restricted feeding also decreased visceral organ weights, which may have reduced maintenance energy (Koong et al., 1982), but PF8 pigs were also more active due to more feeding distributions, thus energy costs for physical activity may explain the numerical increase of FCR and the accentuated effect on ADG.
Effect of exposure to high ambient temperature
In the present study, pigs exposed to hot conditions tried to maintain their body temperature first by dissipating heat using sensible pathways as indicated by their increased Tskin (Collin et al., 2001a) and then through the evaporative pathway as indicated by faster RR. Rise in Trectal and in Tmuscle indicates that, at 32 °C, pigs are no longer able to lose more body heat to maintain their homoeothermic status. There was afterward a gradual decrease in the thermoregulation responses as the pigs adapted to HS, comparable to the biphasic acclimation HS response previously reported by Renaudeau et al. (2010). Similar to the results of Liu et al. (2016), panting of HS–AL pigs decreased their blood (CO2) and, due to reduced carbonic acid formation, also their blood bicarbonate. This was a transient response in HS–AL pigs as levels returned to normal by day 20, which may be due to the slower RR once the pigs have acclimated to HS.
According to Collin et al. (2001c), adaptation to HS is mainly related to HP reduction caused by a lower FI (lower TEF) and decreased maintenance requirements. Between the two AL-fed groups (TN–AL vs. HS–AL), HS exposure decreased ADFI by 541 g·d–1 close to an estimated value of −550 g·d–1 for 75-kg pigs (Renaudeau et al., 2011). This 18% decrease in ADFI consequently reduced BW gain by 21% and was linked to a shorter time spent at the feeder, which is comparable to previous reports of lower consumption time in hot conditions (Quiniou et al., 2000; Collin et al., 2001a). It can be assumed that the meal size of the AL-fed pigs in the present study decreased with heat exposure since the number of visits to the feeder was not affected but ADFI was reduced. The observed shorter duration of standing at the feeder and the smaller meal sizes may be considered as adaptation responses to HS (Collin et al., 2001b) as they reduce energy from HP associated with physical activity and TEF. Reduction in ADFI in HS animals is usually linked with a decrease in fasting HP (Labussière et al., 2016b) and, though this was not measured in our study, it may explain the lower levels of plasma thyroid hormones in HS–AL pigs as they are also indicative of lower metabolic rate (Yavuz et al., 2019).
In this study, exposure to a high ambient temperature not only altered the pattern of feeding behavior of the AL pigs but also of their body temperature. In TN pigs, the Tmuscle pattern increased with feeding and physical activity but in HS–AL pigs, Tmuscle increased progressively during the day (which corresponds with the HP associated with TEF) but remained high until the early morning of the following day (until 0300 hours in the present study). The Tmuscle of HS–AL pigs at the hour of lowest feeder activity (hour 0) was even higher than the Tmuscle during the day in which hours of highest feeder presence were observed. This observation is similar to the results of Cervantes et al. (2018) on ileal temperatures of HS pigs even though the pigs in their study were subjected to a natural diurnal pattern of high ambient temperature compared with the constant, controlled temperature of the current study. This suggests that the body temperature pattern of HS pigs does not generally depend on the type of HS challenge pattern they are subjected to. As reviewed by Gonzalez-Rivas et al. (2020), the saturated heat loss pathways of the HS pigs during the day could have slowed down gastric emptying and motility rates as observed in animals under HS since these processes are known to contribute to HP. This somewhat explains higher Tmuscle during the night despite the low feeder activity and suggests that body temperature differences of TN and HS pigs are more apparent at night, thus only considering measurements during the day may underestimate thermoregulatory responses of HS pigs (Renaudeau, 2020).
Exposure to hot conditions resulted in lighter pigs with slightly lower proportions of kidneys and liver, which could be due to the feed reduction effect of HS as this was also observed in TN–PF8 pigs. However, FI reduction does not seem to fully explain other physiological responses of HS pigs. Comparing HS–AL and TN–PF8 responses, HS–AL pigs had a higher increase in plasma creatinine and greater reduction in plasma T4 than TN–PF8 pigs despite similar ADFI. Furthermore, plasma T3 responses were maintained in TN–PF8 but were greatly reduced in HS–AL pigs. It can be argued that HS–AL pigs should have been fatter than the TN–PF8 pigs since they had higher creatinine levels (higher protein catabolism) and lower plasma thyroid hormones (lower lipolysis and metabolic rate). Indeed, reduced feeding level significantly reduced BFT in TN–PF8 pigs but not in HS pigs, and, compared with TN–AL, there was a numerical increase in the perirenal fat in HS–AL pigs not in TN–PF8 pigs. Along with the observed physiological responses, it could also be possible that slower gastric emptying in HS pigs “shortened” their fasting period during the night and thus decreased the need to break down fat for energy.
The present study thus confirms that when pigs can no longer maintain a homeothermic status in hot conditions, one of the ways that they reduce HP or heat load indirectly is by decreasing ADFI consequentially altering feeding behavior. Exposure to high ambient temperature also changes nychthemeral pattern and other physiological responses of pigs, which is mostly related to feeding and the HP associated with it. However, HS effects do not seem to be explained by merely feed reduction, and these heat-induced effects unrelated to ADFI are tackled more in detail in the next subsection.
Direct and indirect effects of HS at the animal level
In distinguishing the direct effects of HS, the present study aimed to remove or at least minimize the confounding effects of different feeding levels and pattern (indirect HS effect) and of pair-feeding itself including feed restriction and imposed meal patterns. Hence, the TN–PF8 and HS–PF8 pigs in this study were not only PF to the HS–AL pigs, but both groups were also adapted to the same feeding schedule even before the experimental period. Regardless of feeding management, exposure to high ambient temperature increased the thermoregulatory responses of the pigs as described in the previous section. Comparing the Tmuscle pattern of the two PF8 groups, the ambient temperature did not affect the average lag time between meal distribution and the rise in Tmuscle, which is around 11 min, similar to the delay of <15 min reported by Morales et al. (2018). In both PF8 groups, Tmuscle increased with each additional meal provision, which agrees with the pattern of HP related to TEF. However, baseline and maximum Tmuscle plateaued earlier for the HS–PF8 pigs (both at the third meal) than for the TN–PF8 (at the sixth and fourth meal, respectively) although both groups had similar intake per kg BW and thus theoretically should have had the same HP due to TEF (de Lange et al., 2006). Less heat was also dissipated per meal by the HS–PF8 than the TN–PF8 pigs as indicated by the smaller area under Tmuscle curve, suggesting that the heat loss capacity of HS pigs may have been maximized or compromised. This is consistent with the fact that body temperature increase depends on the heat exchange between the animal and the environment (Renaudeau et al., 2012). It is also in line with observations of Cervantes et al. (2018) who indicated that the body temperature of pigs in hot conditions depends on both ambient temperature and FI, and that heat load of the pigs before a meal limits their capacity to dissipate TEF-related heat.
The reduction in ADFI in TN–PF8 pigs resulted in an ADG reduction of 22% similar to HS–AL pigs (21% for HS–AL) but slightly lower than the reduction in HS–PF8 pigs (30%). Comparing the ADG of the two PF8 groups, those exposed to HS seemed to have an additional 8% BW loss, which is not explained by merely feed restriction. In dairy cows, Baumgard and Rhoads (2013) reported 35% to 50% production losses, which are unrelated to FI. Our results are in contrast to those of Collin et al. (2001c) and Pearce et al. (2013), wherein the HS pigs in their studies gained more weight than PF TN counterparts despite the ADFI reduction (around 9 and 19 g/kg BW, respectively, vs. 5 g/kg BW in our study). Differences in results may be due to implemented PF design (e.g., PF TN counterparts were compared with AL-fed HS pigs in their studies) or to their shorter experimental challenge duration (7 d) which may not have been enough for their PF TN pigs to adapt to the sudden meal size reduction.
Between the PF8 groups, those exposed to HS had a greater proportion of perirenal fat (internal level) similar to the results of Kouba et al. (2001) but only numerically higher BFT. Seibert et al. (2018) also reported higher abdominal adipocyte size in HS pigs than their TN-PF counterpart. According to Le Bellego et al. (2002), maximal protein deposition is considered to be limited in hot conditions due to its high energetic cost. From that, it can be assumed that the extra energy was redirected for lipid deposition. The redirection of fat storage internally (perirenal fat) rather than externally (backfat) could be an adaptation response, which is hypothesized to reduce body insulation and facilitate heat dissipation during long-term heat exposure (Le Dividich et al., 1998). The higher fat deposition of HS–PF8 than TN–PF8 pigs might also be related to HS pigs possibly having a “shortened” fasting period as discussed previously or to their increased insulin and insulin:glucose responses, similar to the findings of other PF studies in pigs (Pearce et al., 2013; Sanz Fernandez et al., 2015b). Baumgard and Rhoads (2013) reviewed in detail possible theories on the increased insulin response of HS pigs. In our study, this insulin response appears to be a direct effect of HS as it was only observed in the contrast between PF8 pigs in response to the environmental conditions. Higher insulin levels should have theoretically increased protein deposition since it is also an anabolic hormone for protein synthesis (Davis et al., 2001). However, it was only a transient response in HS–PF8 pigs (only on day 8), while their plasma creatinine, an indicator of muscle protein catabolism (Muller et al., 2017), was constantly higher than in TN–PF8 pigs, which perhaps negated the insulin effect on protein deposition and thus on lean meat content. In our study, plasma creatinine seemed to be a function of both FI reduction (since levels in TN–PF8 decreased compared with TN–AL) and also a direct effect of heat exposure (since HS pigs had higher levels than TN–PF8 pigs despite similar feeding level).
High ambient temperature also reduced the levels of plasma thyroid hormones and T3:T4 ratio (i.e., conversion rate of T4 to T3) in PF8 pigs, which are known indicators of lipolysis and increased metabolic rate (Silva, 2006). This is similar to the results of Sanz Fernandez et al. (2015a) and supports previously discussed claims that HS increases fatness and decreases maintenance energy requirements. Other studies also reported that increased fatness could be related to a decreased plasma NEFA response of HS pigs (Pearce et al., 2013; Sanz Fernandez et al., 2015a) but this was not the case in our study since TN–PF8 and HS–PF8 pigs had similar levels. The higher plasma NEFA exhibited by TN-raised PF compared with AL-fed HS pigs in the aforementioned studies may be unrelated to direct HS effects but could be an effect of PF itself and the prolonged fasting period (Russell et al., 2008). Nutritional status of the pigs during blood sampling could also explain the differences of results across studies since, according to Kouba et al. (2001), HS pigs have higher plasma NEFA than PF TN pigs in an unfed state but not in a fed state which was the case in our study. Nevertheless, our study agrees with previous pig studies on the direct HS effects such as lower thyroid activity and increased (transient) insulin response, which may have contributed to their altered body composition. Other effects of heat exposure demonstrated in this study also include increased plasma creatinine and the inability to export heat efficiently to the environment with a consequence on the body temperature pattern.
Increasing feed provision frequency in HS pigs
This subsection focuses on the two HS groups (HS–AL and HS–PF8) with the same feeding levels but different feed provision frequencies. Aside from fasting HP, heat from the utilization of nutrients contributes significantly to heat load of pigs under HS so the idea was to avoid overwhelming their heat loss pathways by splitting HP into smaller increments via the provision of feed into smaller portions. Between the two HS groups, the meal frequency of the HS–PF8 pigs was effectively increased by design consequently decreasing their meal sizes and time they spent per meal. Although only numerical, the HS–PF8 pigs had a slightly lower BW gain despite the similar ADFI with HS–AL. It is possible that the feeding strategy for the HS–PF8 pigs did not allow them as many opportunities as the HS–AL pigs to adjust their feeding behavior which is, in fact, also an adaptation response to heat exposure. Increasing feeding frequency seemed to improve RR in the present study as HS–PF8 pigs had generally slower breathing than the HS–AL pigs. However, this does not indicate an overall better HS response since the measurement was done only in the morning when HS–PF8 pigs have only eaten one meal and thermoregulatory responses of HS pigs increase throughout the day as previously demonstrated. In the present study, thermoregulation response did not improve with increasing feeding frequency, and even the nychthemeral pattern of the Tmuscle during chronic HS was similar between the two HS groups regardless of the time of day. These results might be related to the discussed HS effect of slowing down gastric emptying and/or digestion rate, which also somewhat explains why body temperature progressively increased during the day and is maintained during the night despite the low feeder activity for both HS groups.
Hence, increasing feeding frequency during hot conditions did not improve pig performance or thermoregulatory responses in the present study. It is possible, though, that it is not the increased feeding frequency per se that had no effect on pig response to HS but the specific way in which it was implemented in our study. In poultry studies, the timing of feeding such as the provision of 80% to 100% of diets in the afternoon improved turkey performance during HS (Farghly et al., 2018), and feed withdrawal in times of high heat load help mitigate HS effects in meat-type poultry (Syafwan et al., 2011). It might be interesting to evaluate other feeding management strategies (e.g., longer feeding intervals, timing of feed provision later during the day, or frequent feeding but during the night) to improve the ability of pigs to better cope with HS.
Conclusions
In the present study, HS pigs reduce FI, which changes their normal feeding pattern and reduces their growth performance. Using a pair-feeding design that removes the effects of the feeding level and pattern, HS pigs are shown to export heat less efficiently than their TN counterpart. Thus, the need to lower heat increment may help explain changes in their body temperature pattern (possibly linked to a slowed-down digestion process) and altered body composition (could be related to limited protein deposition). Other direct effects of heat exposure in pigs also include decreased thyroid hormone activity, increased plasma creatinine, and transient insulin responses. With the same feeding level, increasing feeding frequency did not improve pig response to HS and this may be related to the compromised heat dissipation ability of pigs and its consequence on the digestion process.
Acknowledgments
The authors want to thank F. Le-Gouevec, A. Chauvin, M. Genissel, J. Georges, J. Delamarre, and H. Demay from UE3P, INRAE, 35590 Saint-Gilles, France for animal care and sample collection, A. Constentin, P. Ganier, A. Marchais, C. Mustière, C. Perrier, G. Robin, and R. Comte for lab analyses. This study and the PhD grant of A.M. Serviento were funded by Lallemand Animal Nutrition (Blagnac, France) and French Technology Research National Agency.
Glossary
Abbreviations
- ADG
average daily gain
- ADFI
average daily feed intake
- AL
ad libitum
- BFT
backfat thickness
- BW
body weight
- FCR
feed conversion ratio
- FI
feed intake
- HP
heat production
- HS
heat stress or heat-stressed
- NEFA
nonesterified fatty acids
- pCO2
partial pressure of carbon dioxide
- RR
respiratory rate
- sBW
slaughter body weight
- TEF
thermic effect of feeding
- Tmuscle
muscle temperature
- TN
thermoneutral
- Trectal
rectal temperature
- Tskin
skin temperature
Conflict of interest statement
The authors declare that there is no conflict of interest
Literature Cited
- Baumgard L. H., and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Brown-Brandl T. M., Eigenberg R. A., Nienaber J. A., and Kachman S. D.. . 2001. Thermoregulatory profile of a newer genetic line of pigs. Livest. Prod. Sci. 71:253–260. doi: 10.1016/S0301-6226(01)00184-1 [DOI] [Google Scholar]
- Cervantes M., Antoine D., Valle J. A., Vásquez N., Camacho R. L., Bernal H., and Morales A.. . 2018. Effect of feed intake level on the body temperature of pigs exposed to heat stress conditions. J. Therm. Biol. 76:1–7. doi: 10.1016/j.jtherbio.2018.06.010 [DOI] [PubMed] [Google Scholar]
- Collin A., Lebreton Y., Fillaut M., Vincent A., Thomas F., and Herpin P.. . 2001a. Effects of exposure to high temperature and feeding level on regional blood flow and oxidative capacity of tissues in piglets. Exp. Physiol. 86:83–91. doi: 10.1113/eph8602102 [DOI] [PubMed] [Google Scholar]
- Collin A., van Milgen J., Dubois S., and Noblet J.. . 2001b. Effect of high temperature on feeding behaviour and heat production in group-housed young pigs. Br. J. Nutr. 86:63–70. doi: 10.1079/BJN2001356 [DOI] [PubMed] [Google Scholar]
- Collin A., van Milgen J., Dubois S., and Noblet J.. . 2001c. Effect of high temperature and feeding level on energy utilization in piglets1. J. Anim. Sci. 79:1849–1857. doi: 10.2527/2001.7971849x [DOI] [PubMed] [Google Scholar]
- Commission Delegated Regulation (EU) 2017/1182 of 20 April. 2017 2017 Supplementing Regulation (EU) No 1308/2013 of the European Parliament and of the Council. Available from https://eur-lex.europa.eu/eli/reg_del/2017/1182/oj. Accessed 24 November 2020.
- Daumas G., Causeur D., and Predin J.. . 2010. Validation de l’équation française de prédiction du taux de muscle des pièces (TMP) des carcasses de porc par la méthode CGM. Conference paper for the 42nd Journées de la Recherche Porcine in Paris (pp. 229–230), France from 2–3 February 2010. http://www.journees-recherche-porcine.com/texte/2010/qual/PQ8.pdf [Google Scholar]
- Davis T. A., Fiorotto M. L., Beckett P. R., Burrin D. G., Reeds P. J., Wray-Cahen D., and Nguyen H. V.. . 2001. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 280:E770–E779. doi: 10.1152/ajpendo.2001.280.5.E770 [DOI] [PubMed] [Google Scholar]
- Drazen D. L., Vahl T. P., D’Alessio D. A., Seeley R. J., and Woods S. C.. . 2006. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 147:23–30. doi: 10.1210/en.2005-0973 [DOI] [PubMed] [Google Scholar]
- Farghly M. F. A., Alagawany M., and Abd El-Hack M. E.. . 2018. Feeding time can alleviate negative effects of heat stress on performance, meat quality and health status of turkey. Br. Poult. Sci. 59:205–210. doi: 10.1080/00071668.2017.1413233 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rivas P. A., Chauhan S. S., Ha M., Fegan N., Dunshea F. R., and Warner R. D.. . 2020. Effects of heat stress on animal physiology, metabolism, and meat quality: a review. Meat Sci. 162:108025. doi: 10.1016/j.meatsci.2019.108025 [DOI] [PubMed] [Google Scholar]
- Ingram D. L., and Dauncey M. J.. . 1985. Circadian rhythms in the pig. Comp. Biochem. Physiol. A. Comp. Physiol. 82:1–5. doi: 10.1016/0300-9629(85)90695-4 [DOI] [PubMed] [Google Scholar]
- Katsumata M., Yano H., Ishida N., and Miyazaki A.. . 1990. Influence of a high ambient temperature and administration of clenbuterol on body composition in rats. J. Nutr. Sci. Vitaminol. (Tokyo). 36:569–578. doi: 10.3177/jnsv.36.569 [DOI] [PubMed] [Google Scholar]
- Kellner T. A., Baumgard L. H., Prusa K. J., Gabler N. K., and Patience J. F.. . 2016. Does heat stress alter the pig’s response to dietary fat? J. Anim. Sci. 94:4688–4703. doi: 10.2527/jas.2016-0756 [DOI] [PubMed] [Google Scholar]
- Koong L. J., Nienaber J. A., Pekas J. C., and Yen J. T.. . 1982. Effects of plane of nutrition on organ size and fasting heat production in pigs. J. Nutr. 112:1638–1642. doi: 10.1093/jn/112.8.1638 [DOI] [PubMed] [Google Scholar]
- Kouba M., Hermier D., and Le Dividich J.. . 2001. Influence of a high ambient temperature on lipid metabolism in the growing pig. J. Anim. Sci. 79:81–87. doi: 10.2527/2001.79181x [DOI] [PubMed] [Google Scholar]
- Labussière E., Dubois S., Castex M., and Renaudeau D.. . 2016a. Effect of dietary live yeast supplementation on thermal heat acclimatization in finishing male pigs. In: Skomiał, J., and H. Lapierre, editors. Proceedings of the 5th International Symposium on Energy and Protein Metabolism and Nutrition (Isep); 12–15 September 2016; Cracovie, Poland https://books.google.fr/books/about/Energy_and_Protein_Metabolism_and_Nutrit.html?id=bLQ3MQAACAAJ&redir_esc=y [Google Scholar]
- Labussière E., Dubois S., van Milgen J., and Noblet J.. . 2016b. Fasting heat production and metabolic body weight in growing animals. In: Skomiał, J., and H. Lapierre, editors. Proceedings of the 5th International Symposium on Energy and Protein Metabolism and Nutrition (Isep);12–15 September 2016;Cracovie, Poland https://books.google.fr/books/about/Energy_and_Protein_Metabolism_and_Nutrit.html?id=bLQ3MQAACAAJ&redir_esc=y [Google Scholar]
- de Lange K., van Milgen J., Noblet J., Dubois S., and Birkett S.. . 2006. Previous feeding level influences plateau heat production following a 24 h fast in growing pigs. Br. J. Nutr. 95:1082–1087. doi: 10.1079/bjn20061748 [DOI] [PubMed] [Google Scholar]
- Le Bellego L., Van Milgen J., and Noblet J.. . 2002. Effect of high ambient temperature on protein and lipid deposition and energy utilization in growing pigs. Anim. Sci. 75:85–96. doi: 10.1017/s1357729800052863 [DOI] [Google Scholar]
- Le Dividich J., Noblet J., Herpin P., van Milgen J., and Quiniou N.. . 1998. Thermoregulation. In: Wiseman, J., M. A. Varley, and J. P. Chadwick, editors. Progress in Pig Science. (ed.). Nottingham: (UK: ): Nottingham University Press; p. 229−263. https://search.library.ucdavis.edu/primo-explore/fulldisplay?docid=01UCD_ALMA21217176180003126&context=L&vid=01UCD_V1&lang=en_US&search_scope=alma_scope&adaptor=Local%20Search%20Engine&tab=catalog_tab&query=sub,exact,%20Swine%20--%20Congresses,AND&mode=advanced [Google Scholar]
- Le Naou T., Le Floc′h N., Louveau I., van Milgen J., and Gondret F.. . 2014. Meal frequency changes the basal and time-course profiles of plasma nutrient concentrations and affects feed efficiency in young growing pigs1. J. Anim. Sci. 92:2008–2016. doi: 10.2527/jas.2013-7505 [DOI] [PubMed] [Google Scholar]
- Liu F., Cottrell J. J., Furness J. B., Rivera L. R., Kelly F. W., Wijesiriwardana U., Pustovit R. V., Fothergill L. J., Bravo D. M., Celi P., . et al. 2016. Selenium and vitamin E together improve intestinal epithelial barrier function and alleviate oxidative stress in heat-stressed pigs. Exp. Physiol. 101:801–810. doi: 10.1113/EP085746 [DOI] [PubMed] [Google Scholar]
- van Milgen J., Bernier J. F., Lecozler Y., Dubois S., and Noblet J.. . 1998. Major determinants of fasting heat production and energetic cost of activity in growing pigs of different body weight and breed/castration combination. Br. J. Nutr. 79:509–517. doi: 10.1079/bjn19980089 [DOI] [PubMed] [Google Scholar]
- Morales A., Ibarra N., Chávez M., Gómez T., Suárez A., Valle J. A., Camacho R. L., and Cervantes M.. . 2018. Effect of feed intake level and dietary protein content on the body temperature of pigs housed under thermo neutral conditions. J. Anim. Physiol. Anim. Nutr. (Berl). 102:e718–e725. doi: 10.1111/jpn.12824 [DOI] [PubMed] [Google Scholar]
- Muller T. L., Hewitt R. J. E., D′Souza D. N., and van Barneveld R. J.. . 2017. Factors influencing the measure of creatinine in non-reproductive pigs. Anim. Prod. Sci. 57:2418. doi: 10.1071/ANv57n12Ab062 [DOI] [Google Scholar]
- Pearce S. C., Gabler N. K., Ross J. W., Escobar J., Patience J. F., Rhoads R. P., and Baumgard L. H.. . 2013. The effects of heat stress and plane of nutrition on metabolism in growing pigs1. J. Anim. Sci. 91:2108–2118. doi: 10.2527/jas.2012-5738 [DOI] [PubMed] [Google Scholar]
- Perkins-Kirkpatrick S. E., and Gibson P. B.. . 2017. Changes in regional heatwave characteristics as a function of increasing global temperature. Sci. Rep. 7:12256. doi: 10.1038/s41598-017-12520-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan G., Samson S. L., and Sun Y.. . 2013. Ghrelin: much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 16:619–624. doi: 10.1097/MCO.0b013e328365b9be [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiniou N., Noblet J., and Dourmad J.-Y.. . 1996. Effect of energy intake on the performance of different types of pig from 45 to 100 kg body weight. 2. Tissue gain. Anim. Sci. 63:289–296. doi: 10.1017/S1357729800014843 [DOI] [Google Scholar]
- Quiniou N., Noblet J., van Milgen J., and Dubois S.. . 2001. Modelling heat production and energy balance in group-housed growing pigs exposed to low or high ambient temperatures. Br. J. Nutr. 85:97–106. doi: 10.1079/bjn2000217 [DOI] [PubMed] [Google Scholar]
- Quiniou N., Renaudeau D., Dubois S., and Noblet J.. . 2000. Influence of high ambient temperatures on food intake and feeding behaviour of multiparous lactating sows. Anim. Sci. 70:471–479. doi: 10.1017/S1357729800051821 [DOI] [Google Scholar]
- Renaudeau D. 2016. Evaluation of a telemetry system for measuring core body temperature in the pig. Journées la Rech. Porc. en Fr. 48:249–250. https://www.cabdirect.org/cabdirect/abstract/20173094659 [Google Scholar]
- Renaudeau D. 2020. Impact of single or repeated short-term heat challenges mimicking summer heat waves on thermoregulatory responses and performances in finishing pigs. Transl. Anim. Sci. 4. doi: 10.1093/tas/txaa192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaudeau D., Anais C., Tel L., and Gourdine J. L.. . 2010. Effect of temperature on thermal acclimation in growing pigs estimated using a nonlinear function1. J. Anim. Sci. 88:3715–3724. doi: 10.2527/jas.2009-2169 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J.. . 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi: 10.1017/S1751731111002448 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Gourdine J. L., and St-Pierre N. R.. . 2011. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 89:2220–2230. doi: 10.2527/jas.2010-3329 [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Quiniou N., Dubois S., and Noblet J.. . 2002. Effects of high ambient temperature and dietary protein level on feeding behavior of multiparous lactating sows. Anim. Res. 51:227–243. doi: 10.1051/animres:2002020 [DOI] [PubMed] [Google Scholar]
- Ross J. W., Hale B. J., Seibert J. T., Romoser M. R., Adur M. K., Keating A. F., and Baumgard L. H.. . 2017. Physiological mechanisms through which heat stress compromises reproduction in pigs. Mol. Reprod. Dev. 84:934–945. doi: 10.1002/mrd.22859 [DOI] [PubMed] [Google Scholar]
- Russell J. C., Proctor S. D., Kelly S. E., and Brindley D. N.. . 2008. Pair feeding-mediated changes in metabolism: stress response and pathophysiology in insulin-resistant, atherosclerosis-prone JCR:LA-cp rats. Am. J. Physiol. Endocrinol. Metab. 294:E1078–E1087. doi: 10.1152/ajpendo.90257.2008 [DOI] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Johnson J. S., Abuajamieh M., Stoakes S. K., Seibert J. T., Cox L., Kahl S., Elsasser T. H., Ross J. W., Clay Isom S., . et al. 2015a. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol. Rep. 3:e12315. doi: 10.14814/phy2.12315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Fernandez M. V., Stoakes S. K., Abuajamieh M., Seibert J. T., Johnson J. S., Horst E. A., Rhoads R. P., and Baumgard L. H.. . 2015b. Heat stress increases insulin sensitivity in pigs. Physiol. Rep. 3:e12478. doi: 10.14814/phy2.12478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. D., Tokach M. D., Goodband R. D., Nelssen J. L., Dritz S. S., Derouchey J. M., and Sulabo R. C.. . 2011. Effects of restricted feed intake on finishing pigs weighing between 68 and 114 kilograms fed twice or 6 times daily. J. Anim. Sci. 89:3326–3333. doi: 10.2527/jas.2010-3154 [DOI] [PubMed] [Google Scholar]
- Seibert J. T., Abuajamieh M., Sanz Fernandez M. V., Johnson J. S., Kvidera S. K., Horst E. A., Mayorga E. J., Lei S., Patience J. F., Ross J. W., . et al. 2018. Effects of heat stress and insulin sensitizers on pig adipose tissue. J. Anim. Sci. 96:510–520. doi: 10.1093/jas/skx067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serviento A. M., Lebret B., and Renaudeau D.. . 2020. Chronic prenatal heat stress alters growth, carcass composition, and physiological response of growing pigs subjected to postnatal heat stress. J. Anim. Sci. 98. doi: 10.1093/jas/skaa161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E. 2006. Thermogenic mechanisms and their hormonal regulation. Physiol. Rev. 86:435–464. doi: 10.1152/physrev.00009.2005 [DOI] [PubMed] [Google Scholar]
- Syafwan S., Kwakkel R. P., and Verstegen M. W. A.. . 2011. Heat stress and feeding strategies in meat-type chickens. Worlds. Poult. Sci. J. 67:653–674. doi: 10.1017/S0043933911000742 [DOI] [Google Scholar]
- West J. W. 1999. Nutritional strategies for managing the heat-stressed dairy cow1. J. Anim. Sci. 77:21–35. doi: 10.2527/1997.77suppl_221x [DOI] [PubMed] [Google Scholar]
- Wheelock J. B., Rhoads R. P., VanBaale M. J., Sanders S. R., and Baumgard L. H.. . 2010. Effects of heat stress on energetic metabolism in lactating Holstein cows1. J. Dairy Sci. 93:644–655. doi: 10.3168/jds.2009-2295 [DOI] [PubMed] [Google Scholar]
- Yavuz S., Salgado Nunez Del Prado S., and Celi F. S.. . 2019. Thyroid hormone action and energy expenditure. J. Endocr. Soc. 3:1345–1356. doi: 10.1210/js.2018-00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeferino C. P., Komiyama C. M., Pelícia V. C., Fascina V. B., Aoyagi M. M., Coutinho L. L., Sartori J. R., and Moura A. S.. . 2016. Carcass and meat quality traits of chickens fed diets concurrently supplemented with vitamins C and E under constant heat stress. Animal 10:163–171. doi: 10.1017/S1751731115001998 [DOI] [PubMed] [Google Scholar]