Introduction
Over the past several decades, it has become clear that type 2 diabetes (T2DM) is no longer limited to adults. In fact, T2DM in pediatrics poses a major challenge to population health with a rising incidence (highest in patients of racial minorities and of lower socioeconomic status) and a high rate of complications.1–5 Childhood-onset T2DM also demonstrates more rapid progression to beta-cell failure with high treatment failure rates as evidenced by the TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) trial, the largest multi-center randomized controlled trial on treatment options in pediatric T2DM in which children with T2DM were randomized to one of three treatment arms (metformin monotherapy, metformin + lifestyle intervention, and metformin + rosiglitazone) and the primary outcome was treatment failure, defined by persistently elevated hemoglobin A1c (HbA1c) >8% over a 6-month period or inability to wean from temporary insulin therapy within 3 months.6–8 Thus, recognizing and treating T2DM early in its course is imperative. Unfortunately, few pharmacologic therapies are FDA-approved for pediatric T2DM.
Etiology and Genetics of Pediatric Type 2 Diabetes
The pathophysiology of T2DM starts with declines in skeletal muscle, adipose tissue, and hepatic insulin sensitivity, which necessitates increased pancreatic beta-cell insulin secretion to maintain glucose homeostasis.9,10 Hyperglycemia develops when compensatory insulin secretion fails to counter worsening insulin sensitivity and manifests as pre-diabetes and, ultimately, T2DM.9–11 Hyperglycemic clamp testing has shown that compared to obese controls without T2DM, youth who develop pediatric T2DM have 50% reduced insulin sensitivity, increased fasting hepatic glucose production, and an 86% lower disposition index, a composite measure describing the relationship between insulin sensitivity and beta-cell function.12 In addition, insulin secretion can be sufficiently deficient to precipitate diabetes ketoacidosis (DKA), which occurs in ~6% of cases of pediatric T2DM but is an uncommon presentation in adult T2DM.6,13 Similar to adult T2DM, pediatric T2DM can present with hyperosmolar hyperglycemic non-ketoacidosis (HHNK), a condition which should be considered if blood glucose ≥ 600 mg/dL.14
Obesity is a primary risk factor for reduced insulin sensitivity in children and adults.13 The Metabolic syndrome comprises the clustering of disorders related to central obesity including insulin resistance, hypertension, and dyslipidemia.15 & 16 Other risk factors in adults and children include conditions associated with insulin resistance (such as polycystic ovary syndrome (PCOS)), first-degree relative with T2DM, and high risk racial minority groups (i.e. African American, Latino, Native American, Asian American, Pacific Islander) (Figure 1).13 First- or second-degree relative with T2DM is present in 74–100% of youth with T2DM.17 Morevoer, Magge et al. demonstrated that siblings of adolescents with T2DM had a four-times greater odds of abnormal glucose tolerance compared with overweight controls.17 Several adult T2DM-related genetic variants such as the TCF7L2 locus have also been identified in African American youth with T2DM and HNF1A G319S in Oji-Cree Native Canadian youth.9,10
Figure 1.
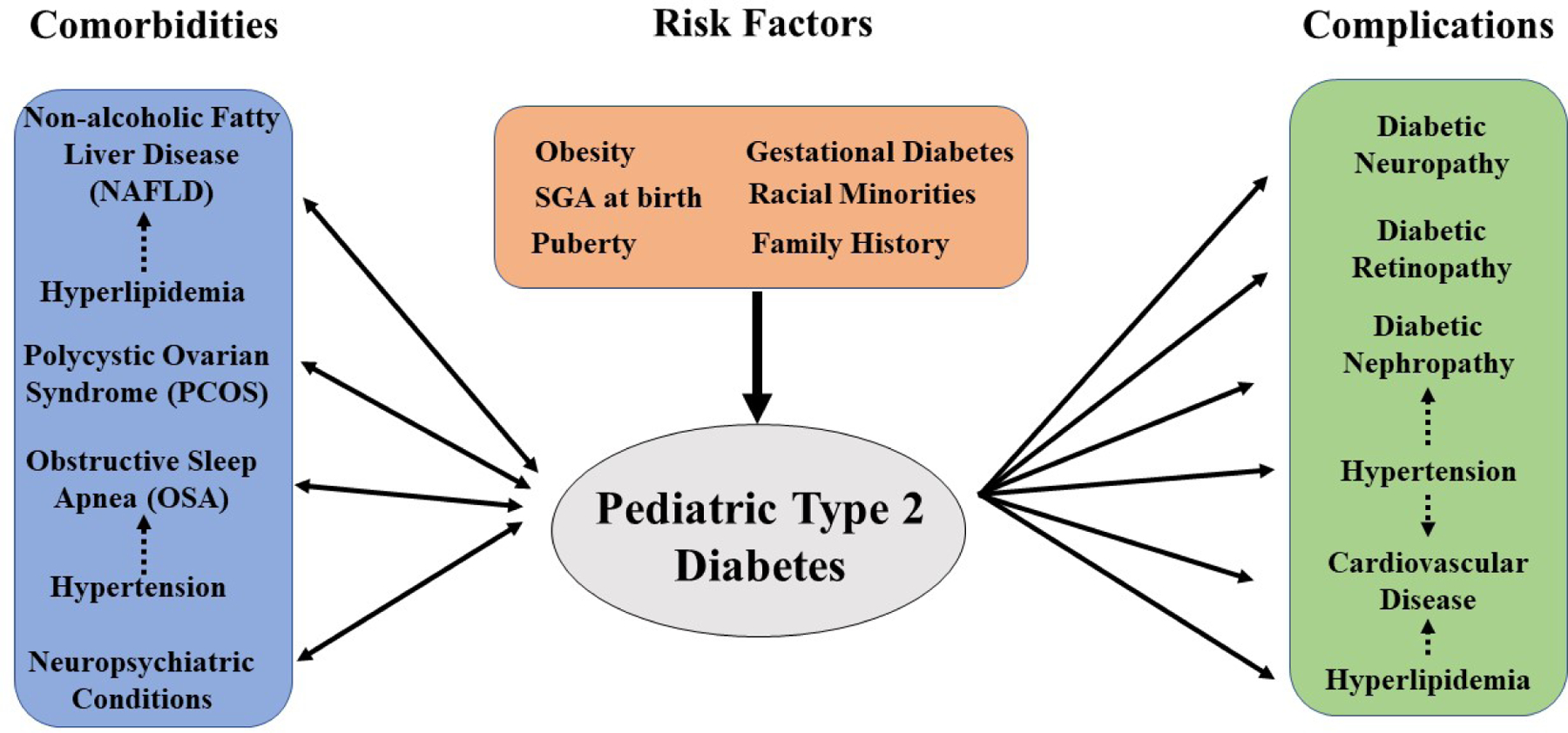
Common Comorbidities, Risk Factors and Complications in Pediatric T2DM13
For youth with genetic or other risk factors, obesity and the onset of puberty can precipitate the development of T2DM. Puberty in adolescents leads to an approximately 30% reduction in insulin sensitivity, and the majority of pediatric T2DM new diagnoses occur during puberty.18,19 Among peri-natal risk factors, infants born to mothers with gestational diabetes and children born SGA (small for gestational age) are at a higher risk for development of metabolic syndrome, insulin resistance and T2DM in childhood.16,20 (Figure 1)
Prevalence, Incidence, and Demographics of Pediatric Type 2 Diabetes
The incidence of pediatric T2DM has been rising since it was first described in the 1980s and corresponds with the increasing childhood obesity rates.1–3,21 The SEARCH for Diabetes in Youth database, a population-based registry of diabetes with surveillance of 69,457,475 youths aged <20 years, has demonstrated that the prevalence of T2DM in youth in the United States was 0.34 per 1000 in 2001 and rose by ~30% to 0.46 per 1000 (0.046%) in 2009.2 SEARCH also found that from 2002 to 2015, the pediatric T2DM incidence in the United States increased by 4.8% annually (from 9.0 cases per 100,000 youths per year in 2002–2003 to 13.8 cases per 100,000 youths per year in 2014–2015).1,3 In comparison, the CDC estimated that 8.6% of all U.S. adults, about 21 million individuals, were diagnosed with T2DM in 2016.22
In 2003, about half or more of new diabetes diagnoses in minority children aged 10–19 years were T2DM compared to 15% in non-Hispanic White patients.23 Additionally, the highest annual percent change increase in T2DM incidence in the SEARCH database from 2002–2015 was among Asians and Pacific Islanders (7.7% per year), followed by Hispanics (6.5% per year), non-Hispanic African Americans (6.0% per year), and Native Americans (3.7% per year).3 In the TODAY study, the baseline characteristics of the 704 participants demonstrated that youth with T2DM are also disproportionately of lower socioeconomic status: 41.5% with household income <$25,000, 16.8% had parents with highest education level of a bachelor’s degree or higher, and 52.1% live in single-parent households.4,8 Similar demographics are seen in adults in whom the T2DM prevalence is highest among non-Hispanic African Americans (11.5%), followed by Hispanics (9.1%), non-Hispanic Whites (8.0%), and non-Hispanic Asians (6.9%) and decreases with higher levels of educational attainment.22
Screening and Diagnosis of Pediatric T2DM
The American Diabetes Association (ADA) currently recommends screening for T2DM with HbA1c in youth “after the onset of puberty or ≥ 10 years of age, whichever occurs earlier, who are overweight (BMI ≥ 85th percentile) or obese (BMI ≥ 95th%-ile), and who have one or more additional risk factors for diabetes.” 13 If HbA1c is normal, the ADA recommends repeat testing at a minimum of 3-year intervals.13 The ADA recommends testing for T2DM and pre-diabetes in obese youth more frequently, such as every 2 years as suggested by the Expert Committee Obesity Guidelines, if BMI continues to increase.13,24 Children identified with pre-diabetes should have at least annual repeat testing.13
Currently, the ADA recommends HbA1c for pediatric T2DM screening based on limited data on diabetes screening methods in pediatrics, the known association of elevated HbA1c with long-term diabetes outcomes, and ease of use as patients do not need to fast.13,25 Other measures of average glycemia, such as fructosamine, can be used in diseases such as iron deficiency anemia, sickle cell disease, thalassemia, and other hemoglobinopathies.13,25 Glycemic screening includes fasting blood glucose and oral glucose tolerance tests (OGTT), although how these methods compare to HbA1c in pediatrics is unclear.25
The diagnostic criteria for diabetes and pre-diabetes are summarized in Table 1.
Table 1.
Diagnosis of Pre-diabetes and Diabetes. Data from Editor: Riddle MC. American Diabetes Association: Standards of Medical Care in Diabetes −2019. Diabetes Care. 2019;42:S1–S193.
| Diagnosis Definition | |
|---|---|
| Pre-diabetes | Any of the following: |
| 1. HbA1c: 5.7–6.4% | |
| 2. Fasting plasma glucose: 100–125 mg/dL | |
| 3. Impaired glucose tolerance (IGT): 75-g OGTT 2-hour glucose 140–199 mg/dL | |
| Diabetes | Two of the following: |
| 1. HbA1c ≥ 6.5% | |
| 2. Fasting blood glucose ≥ 126 mg/dL | |
| 3. 75-g OGTT 2-hour glucose >200 mg/dL | |
| 4. Random plasma glucose ≥ 200 mg/dL in setting of hyperglycemia symptoms | |
Differentiating among pediatric T2DM, Type 1 Diabetes (T1DM), and monogenic diabetes (MODY) can be difficult as overweight, obesity, and acanthosis nigricans can be present in children with T1DM and MODY.26,27 In addition, T2DM can present with ketosis or DKA.28 Testing for diabetes-associated pancreatic islet autoantibodies can be useful to distinguish between T1DM and T2DM. However, these autoantibodies can be positive in patients who appear to have a clinical course more consistent with T2DM.26,28 In the TODAY study, 9.8% (118) of the 1206 youth with T2DM screened had positive pancreatic autoantibodies: 71 (5.9%) were positive for a single antibody (29 anti-GAD-65 antibody, 42 anti-IA-2 antibody), and 47(3.9%) were positive for both antibodies.28 The number of positive antibodies can assist in distinguishing between T2DM and T1DM as the risk of developing T1DM is high in children with more than one positive pancreatic islet autoantibody.13,29
MODY has also been noted in up to 8% of youth with features suggestive of T2DM.26 Thus, genetic testing should be considered in youth with multiple family members with diabetes without typical features of T1DM or T2DM, such as negative diabetes auto-antibodies, non-obese and lacking other features of metabolic syndrome.13,26 Markers of beta-cell insulin secretion including a fasting c-peptide and urinary c-peptide creatinine ratio (UCPCR) may be useful in distinguishing among T2DM, T1DM, and MODY.26 Katz et al. demonstrated that fasting C-peptide >0.85 ng/ml at diagnosis can differentiate T1DM from T2DM with a sensitivity of 83% and a specificity of 89%, and UCPCR >0.7 nmol/mmol has been shown by Besser et al. to have 100% sensitivity and 81% specificity to distinguish between T1DM and non-T1DM (MODY or T2DM).26,27,30
Comorbidities in Pediatric T2DM
Comorbid conditions associated with obesity and metabolic syndrome can occur concurrently with prediabetes and T2DM in both youth and adults (Figure 1).9 Among adults with T2DM, the prevalence of concurrent non-alcoholic fatty liver disease (NAFLD) ranges from 40–70%.31 In a cohort study of 675 children with NAFLD, 30% had comorbid T2DM or pre-diabetes.32 A case-control study of 150 overweight children with biopsy-proven NAFLD compared to 150 overweight children without NAFLD demonstrated that the risk factors for NAFLD in pediatrics are aspects associated with metabolic syndrome including central obesity (waist circumference >102 cm in boys and 88cm in girls), higher triglycerides (> 150 mg/dL), high total cholesterol (> 200 mg/dL), higher LDL cholesterol (> 130 mg/dL), higher systolic (> 130 mm Hg) and diastolic blood pressure (> 85 mm Hg) and lower HDL cholesterol levels (< 40 mg/dL).32,33 Signs of NAFLD include elevated liver enzymes or signs of fatty liver on ultrasound.34 Children with NAFLD and comorbid pediatric T2DM have 3.1 times higher odds of developing nonalcoholic steatohepatitis (NASH), a progressive form of NAFLD, which has a greater risk of progressing to cirrhosis.32 In adults with NAFLD, T2DM is associated with higher rates of the development of NASH, liver cirrhosis, and hepatocellular carcinoma.32
Obstructive sleep apnea (OSA) is also associated with central obesity and insulin resistance and is an additional independent risk factor for cardiovascular complications, including elevated blood pressure, in children and adults.35,36 Pediatric patients with obesity should be screened for symptoms of OSA including habitual snoring, apneic pauses while sleeping, or excessive daytime sleepiness; however, history and physical examination have low positive predictive value (65%) in identifying OSA in youth.37 If symptoms are present, patients should be referred for a diagnostic evaluation for OSA with polysomnography.38 In addition, sleep disruption and OSA are associated with reduced insulin sensitivity in adults with T2DM, and treatment of OSA may improve glycemic control in patients with pediatric T2DM.39–41
PCOS characterized by hyperandrogenism and chronic anovulation, has a higher prevalence in youth with obesity, is associated with insulin resistance with or without obesity, and predisposes youth and adults to the development of diabetes.42,43 Insulin resistance is present in 30–40% of adults with PCOS, including both obese and non-obese.42 In 27 youth with PCOS and BMIs ranging from 20.4–54.4 kg/m2, OGTT identified IGT in 30% and T2DM in 4%.42 No relationship between BMI and 2-hour OGTT plasma glucose was found, and the subject with lowest BMI had IGT.42
Psychiatric comorbidities are also common with pediatric T2DM. In a retrospective chart review of 237 patients with pediatric T2DM, Katz et al. demonstrated neuropsychiatric disease was present in 19% of patients at diabetes presentation; diagnoses included depression, attention-deficit hyperactivity disorder (ADHD), schizophrenia, bipolar disorder, and neurodevelopmental disorders.44 In the TODAY trial, 14.8% screened positive for clinical depression and 30% screened positive for binge eating.45 Some of these psychiatric comorbidities in pediatric T2DM may be secondary to the use of second-generation antipsychotics, which is associated with weight gain and worsened insulin resistance in pediatric cohort studies.46,47
Treatment for Pediatric T2DM
At this time, although several pharmacologic medication categories are available for treatment of adult T2DM, only three pharmacologic medications are currently FDA-approved for pediatric T2DM patients: metformin, insulin, and the GLP-1 receptor agonist, liraglutide.13,48 ADA guidelines recommend monitoring of HbA1c every 3 months to inform treatment.13 The ADA suggests a target HbA1c in pediatric T2DM of < 7% but also states that goal HbA1c < 6.5% could be used.13 The latter is supported by the TODAY study, in which hypoglycemia was rare even for patients who progressed to insulin therapy and 5-year diabetic complication rates were high.8,45 (Figure 2)
Figure 2.
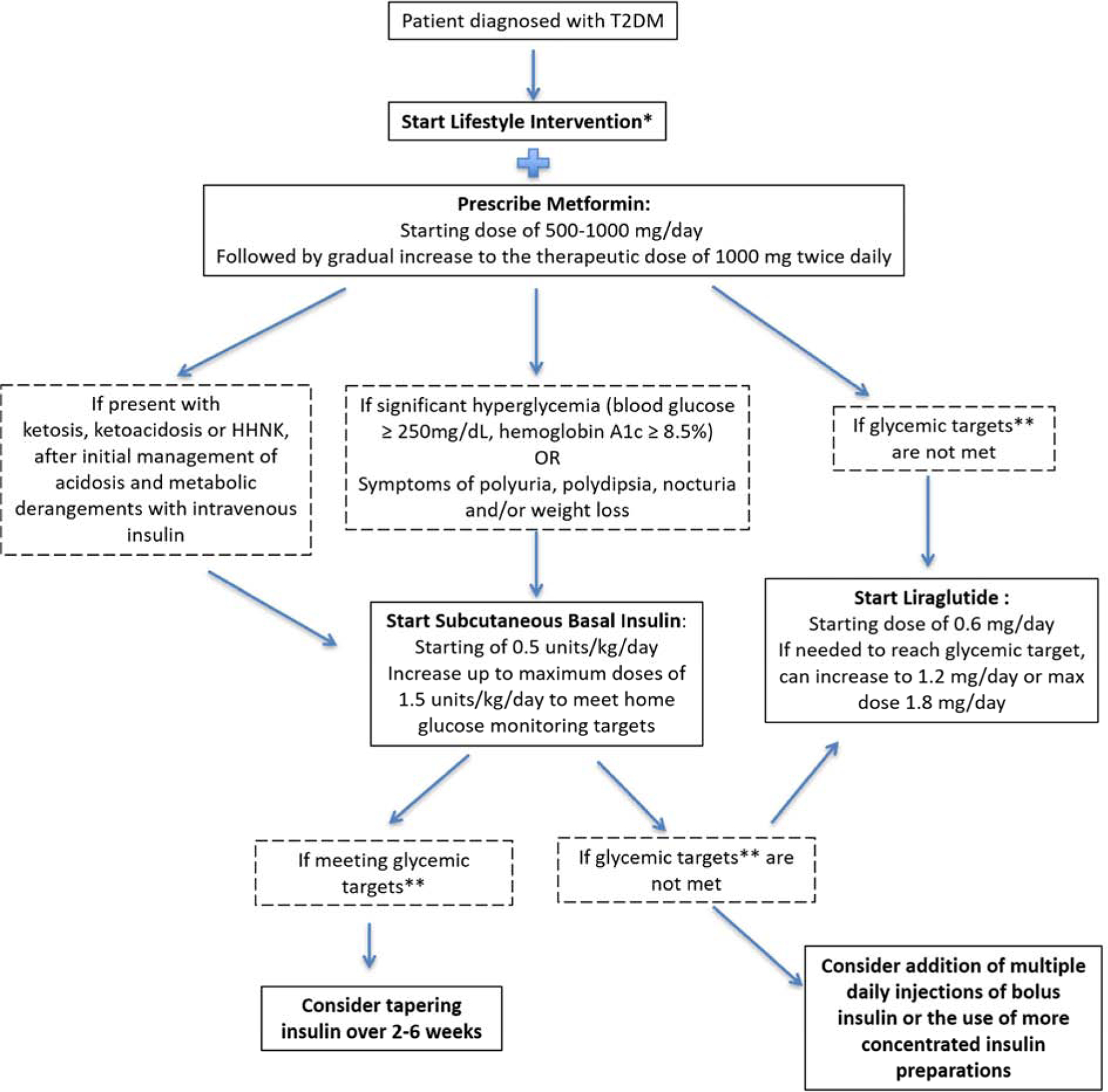
Treatment Algorithm for Pediatric T2DM. Data from References 9, 13, and 48.
- At least 30–60 minutes of moderate to vigorous physical activity at least 5 days per week and reduction of sedentary behavior
- Healthy eating patterns and reduction of calorie-dense, nutrition-poor foods, especially sugar-sweetened beverages
- Sleep 8–10 hours per night
**Glycemic Target as recommended by the ADA is hemoglobin A1c < 7.0% OR hemoglobin A1c < 6.5% for patients with shorter duration of diabetes or lesser degrees of beta-cell dysfunction as long as can be achieved without significant risk factors, including severe hypoglycemia
Metformin is recommended as the first line therapy for T2DM in adults and youth with normal renal function (Figure 2).13 The UK Prospective Diabetes Study Group has shown that in adults, metformin monotherapy can lead to risk reduction in all diabetes-related clinical endpoints, diabetes-related deaths and all-cause mortality compared to insulin therapy.49 Metformin also has a weight loss effect: in the pediatric MOCA trial in which 151 obese children with pre-diabetes or IGT were randomized to metformin or placebo, the metformin group had a statistically significant reduction in BMI (- 0.1 SD) and weight (−2.6 kg) after 6 months of treatment.50
Basal insulin should be initiated in patients with significant hyperglycemia, ketosis, symptoms of polyuria, polydipsia, nocturia and/or weight loss, or are failing to reach glycemic targets on other pharmacologic therapies, but should be tapered once meeting glycemic target (Figure 2).9,13 In the TODAY study, for those that had treatment failure and progression to add-on insulin therapy in any arm, the response 1-year post-insulin initiation was variable with 33% with HbA1c decrease of ≥ 0.5%, 46% with < 0.5% change in HbA1c, and 21% with an increase in HbA1c ≥ 0.5%.51
Liraglutide, a daily injectable GLP-1 agonist, was approved for use in pediatric T2DM in Spring 2019. Liraglutide is indicated in patients ≥ 10 years of age on metformin +/− basal insulin therapy and who have not met glycemic targets (i.e. HbA1c < 7 % or < 6.5% based on provider preference) (Figure 2).13 In the clinical trial by Tamborlane and colleagues, 134 pediatric patients with T2DM on metformin with or without basal insulin were randomized to liraglutide or placebo and monitored for 52 weeks: 64% were able to attain HbA1c < 7% with liraglutide compared to 36% with placebo.48 In the LEAD trial, in which 1091 adults were randomized to liraglutide or placebo added to metformin monotherapy, liraglutide was associated with 1.0% lower HbA1c compared to a 0.1% HbA1c increase with placebo.52 Although the adult LEAD trial demonstrated 1.8–2.8 kg weight loss in all liraglutide groups, weight and BMI reductions in the pediatric trial were not statistically different in the liraglutide and placebo groups.48,52 Common side effects of liraglutide include nausea and mild gastrointestinal disturbance that improve over time.48,52,53 Minor hypoglycemia was more common with liraglutide in youth (0.386 events/patient/year) compared to in adults in LEAD (0.14 events/patient/year).48,52 Liraglutide is contraindicated in people with personal or family history of medullary thyroid carcinoma or personal history of pancreatitis.13
Barriers to use of each of these medications are well-recognized. Both insulin and liraglutide are only offered in injectable forms for pediatric T2DM at this time. Metformin has a strong track record of safety, but side effects include abdominal pain and nausea, which can reduce its acceptability.4,6 Children may also have difficulties with pill swallowing. While severe insulin résistance in pediatric T2DM makes hypoglycemia less likely, hypoglycemia remains a concern with insulin use.8 In addition, although liraglutide and metformin contribute to weight loss, if progression to insulin is required, insulin has a weight-positive effect.13,48,50,52 The TODAY trial also revealed that overall medication adherence 1) declined over time to 56% by 48 months and 2) was correlated with baseline depressive symptoms.54
Because these pharmacologic options currently face barriers to use in pediatric T2DM and adherence is challenging, lifestyle intervention is a therapy mainstay (Figure 2). Weight loss can improve insulin sensitivity in pediatric T2DM. In a randomized controlled behavioral weight loss trial, an 8% reduction in BMI was associated with improved insulin sensitivity in obese adolescents.55 In addition, the TODAY trial demonstrated that weight loss was associated with improved cardiometabolic outcomes including decreases in systolic blood pressure, LDL cholesterol, triglycerides, total cholesterol and increases in HDL cholesterol over 24 months.56 The use of meal replacements in lifestyle modification programs can also be considered as their use has demonstrated short-term weight loss in obese adolescents and improved diet composition in adults.57,58 However, the 595 participants randomized to the lifestyle intervention combined with metformin therapy arm in the TODAY trial (consisting of a family-based behavioral approach with intensive weekly intervention sessions for the first 6 months followed by bi-weekly visits alternating with bi-weekly telephone calls the second 6 months followed by monthly visits and telephone calls from months 12–24) had a 5-year treatment failure rate of 46.6% .8,56 In addition, TODAY study participants who lost weight by month 6 in the weight-management intervention arm did not sustain weight loss in months 12 and 24.56 Adherence to the lifestyle program in was only 60% and self-monitoring was low for all participants.59 This low adherence may be partly attributable to the TODAY study design in which T2DM participants were randomized to one of three treatment groups (1/3 were in the lifestyle intervention), compared to other successful intensive family-based weight loss trials in pediatrics such as the Bright Bodies program, which directly recruited participants to participate in a weight loss trial, an approach that could contribute to higher baseline patient motivation.56,60
For youth with T2DM who fail lifestyle modifications and continue to have morbid obesity, bariatric surgery could be considered as a potential treatment option. Qualifications for bariatric surgery per American Society for Metabolic and Bariatric Surgery guidelines include: class III obesity (BMI > 140% of the 95th percentile or BMI ≥40 kg/m2 BMI) or class II obesity (120% of the 95th percentile or BMI ≥35 to ≤39 kg/m2) with a comorbidity. Of the 30 participants (baseline BMI 54.4kg/m2) in the Teen-LABS study with baseline T2DM who underwent bariatric surgery, mean HbA1c decreased from 6.8% to 5.5%.61
Progression of Pediatric T2DM
Pediatric T2DM has been shown to have higher rates of treatment failure compared to adult T2DM. In the TODAY trial, youth with T2DM demonstrated a 45.6% overall treatment failure defined by persistently elevated HbA1c >8% or inability to wean off insulin therapy.8 Treatment failure at 5-years with metformin-monotherapy is higher in pediatric (51.7%) vs adult (21%) T2DM.8,62 TODAY trial participants on rosiglitazone and metformin combined therapy had treatment failure rate of 38.6%, higher than seen in an adult retrospective cohort study in which patients on metformin/ thiazolidinedione combination therapy had treatment failure of 14.3%.8,63 These rates of treatment failure in the TODAY trial are particularly striking with a short mean baseline diabetes duration of only 7.8 months.8 In addition, youth in the TODAY study on metformin for 3 months with HbA1c > 6.3% or increasing HbA1c had higher risk for worsening glycemic control.64
In addition to high treatment failure rates, pediatric T2DM also has faster progression to beta-cell failure than adult T2DM. Adult T2DM have gradual beta-cell function decline occuring over 10 to 12 years at average rate of 7% per year, whereas youth with T2DM demonstrate declines as high as 35% per year.6,7,10,65,66 Indeed, worsening diabetes control on metformin monotherapy occurring as early as 1.5 to 2 years after diagnosis is a manifestation of this rapid beta-cell function decline.6,10,12,65–67 Higher frequency of DKA in youth with new-onset T2DM also attests to these higher rates of beta-cell failure in pediatric T2DM.6,13 Baseline predictors of treatment failure and beta-cell decline in pediatric patients are higher fasting glucose, higher HbA1c, and DKA.6,7 These findings suggest earlier diagnosis and treatment may be protective against beta-cell decline.
The Restoring Insulin Secretion (RISE) study compared insulin sensitivity and beta-cell response in 66 youth and 355 adults with IGT or recently diagnosed T2DM and demonstrated that youth with IGT or T2DM have 1) 50% lower insulin sensitivity as measured by hyperglycemic clamp or OGTT and 2) hyper-responsive beta-cells with higher OGTT-stimulated C-peptide and insulin.68 In addition, insulin clearance is lower in pediatric vs adult T2DM and more insulin is required to achieve the same fasting blood glucose.69,70 The RISE trial also attempted to evaluate methods to reduce beta-cell decline in pediatric T2DM and randomized 91 youth with IGT or T2DM to 1) metformin for 1 year or 2) intensive insulin treatment with insulin glargine for 3 months followed by metformin for 9 months, but did not show any improvement or slowing of beta-cell decline in either arm.70 Given these findings, early diagnosis and prevention of pediatric T2DM is key prior to significant beta-cell failure.
Complications of Pediatric Type 2 Diabetes
Diabetes can lead to microvascular complications (i.e. nephropathy, retinopathy, and neuropathy) and macrovascular complications (i.e. cardiac, cerebrovascular, and peripheral vascular diseases) (Figure 1).5 These complications have been described in pediatric T1DM and adult-onset T2DM and generally occur about 15–20 years following diagnosis.5 Pediatric T2DM, however, is associated with higher risk of complications than pediatric T1DM.5 By the end of the TODAY trial’s 5-year study increases in rates of hypertension from 11.6% to 33.8%, micro-albuminuria from 6.3% to 16.6%, and low-density lipoprotein cholesterol (LDL) from 4.5% to 10.7%.45 In addition, at the end of the TODAY trial, 14% of participants had retinopathy.45 The albuminuria progression rate in youth based on the TODAY study is 2.6% annual rate, similar to the adult UKPDS study in adults of 2.0%.45,71,72 From the UKPDS study in adults, diabetic patients without microalbuminuria at diagnosis were predicted to develop nephropathy at a median of 19 years after diagnosis and those with micro-albuminuria at diagnosis were predicted to worsen to macroalbuminuria or worse at a median of 11 years after diagnosis.71 In a cohort study of 342 youth with T2DM, major diabetic complications (e.g. dialysis, blindness, and amputations) were noted in 1.1% at 10 years, 26% at 15 years and 47.9% at 20 years after diagnosis.5
In a retrospective analysis with >20 years of data from 354 patients with T2DM and 470 patients with pediatric T1DM of similar age of onset, pediatric T2DM was also noted to have a significantly higher mortality rate at shorter disease duration than pediatric T1DM, likely driven by this study’s demonstrated higher rate of cardiovascular deaths in T2DM.73 Cardiovascular changes in pediatric T2DM are prevalent as shown by echocardiography performed on 455 participants with cardiovascular risk factors (hypertension and obesity) in the TODAY cohort in which increased mean left ventricular (LV) mass and mean left atrial (LA) size and abnormal LV geometry were noted.74 Carotid intima media thickness, a strong predictor of cardiovascular events in adults, is also high in youth with obesity and T2DM.75 In addition, cardiac autonomic instability, as measured by heart rate variability, was present in 8% of the TODAY cohort and associated with increased arterial stiffness, as measured by carotid–femoral pulse-wave velocity.76 Participants with pediatric T2DM in the TODAY trial also had significant dyslipidemia and inflammation, contributors to premature atherosclerosis, with rises in LDL cholesterol and use of statins (8.6% to 22%), triglycerides, plasma non-esterified fatty acids, and high-sensitivity C-reactive protein.77 Some studies have also demonstrated higher endothelial dysfunction, measured by lower brachial flow mediated dilation, in T2DM youth compared to youth with T1DM who have comparable HbA1c but longer diabetes duration.75,78
Pregnancy complications are also seen in women who have had pediatric-onset T2DM. Pregnancies occurred in 10% of the TODAY study cohort: 26% of which had loss or stillbirth, and of the live births 15.4% were preterm and 20.5% had congenital anomalies (50% cardiac, 50% other).79 These statistics demonstrate the significant complications in pediatric T2DM, particularly in comparison to pediatric T1DM, and underscore the need for a clear understanding of the etiology and progression of pediatric T2DM.
Monitoring for Comorbidities and Complications
American Diabetes Association recommendations for monitoring for comorbid conditions and complications are summarized in Table 2.
Table 2.
Recommendations for Monitoring for Comorbidities and Complications in T2D** Data from Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: A position statement by the American diabetes association. Diabetes Care. 2018;41(12):2648–2668. and Editor: Riddle MC. American Diabetes Association: Standards of Medical Care in Diabetes −2019. Diabetes Care. 2019;42:S1–S193.
| Recommended Monitoring | |
|---|---|
| Comorbidity | |
| NAFLD | 1. Measurement of AST and ALT should be done at diagnosis and annually |
| 2. Refer to gastroenterology if persistent elevation or worsening of AST and ALT levels | |
| OSA | 1. Screen for symptoms of OSA at each visit |
| 2. If symptoms of OSA present, a polysomnogram should be performed and referral to a pediatric sleep specialist if PSG is abnormal | |
| PCOS | 1. Females with T2DM should be evaluated for symptoms of PCOS. Metformin therapy may improve symptoms of hyperandrogenism. |
| Psychosocial Factors | 1. Diabetes distress, mental health and disordered eating behaviors should be assessed regularly |
| 2. Social barriers including food insecurity, housing instability, and financial barriers should be assessed and social work consultation should be made if present | |
| Complications | |
| Nephropathy | 1. Blood pressure should be measured at every visit |
| 2. If blood pressure > 95th%-ile for age, sex and height, lifestyle management should be initiated. If continued elevation, consider anti-hypertensive therapy (ACE inhibitors or angiotensin receptor blocker) after 6 months of lifestyle management and referral to nephrology. | |
| 3. Urine albumin/creatinine ratio should be obtained at diagnosis and annually. If elevated (>30mg/g creatinine), referral to nephrology is indicated. | |
| Neuropathy | 1. Foot exam should be performed annually with assessment of foot pulses, pinprick, and 10-g monofilament sensation tests, testing of vibration sensation using a 128-Hz tuning fork, and ankle reflexes. |
| Retinopathy | 1. Dilated fundoscopy or retinal photography should be done at diagnosis and annually |
| Cardiovascular | 1. Lipid testing at diagnosis and annually |
| 2. Consider therapy with a statin if LDL remains > 130 mg/dL after 6 months of lifestyle intervention | |
| 3. Consider therapy with a fibrate if triglycerides are elevated (fasting > 400 mg/dL) to reduce risk of pancreatitis | |
Summary and future directions
Pediatric T2DM is an increasing public health concern with a rising incidence corresponding to the rise in pediatric obesity. Despite a similar pathophysiology to adult T2DM, pediatric T2DM demonstrates faster pancreatic beta-cell decline and increased treatment failure rates compared to adult T2DM. Youth with T2DM will be entering adulthood with significant risks for morbidity and mortality at younger ages than the adult T2DM population. The available treatment options have not been effective in reducing beta-cell decline and are complicated by high non-adherence. Early diagnosis and treatment of pediatric T2DM and development of new pharmacologic therapies, trials of approved adult T2DM therapies, such as the recently approved liraglutide, and effective behavioral interventions are critically needed to stem the continued rise in pediatric T2DM and its sequelae.
KEY POINTS:
Major risk factors for the development of pediatric Type 2 Diabetes include obesity, puberty and Type 2 Diabetes in a first- or second-degree relative.
The incidence of pediatric Type 2 Diabetes has been rising with the increase in pediatric obesity, disproportionately affecting minorities and those of lower socioeconomic status.
Recommended screening for T2DM includes hemoglobin A1c for children ≥ 10 years of age or pubertal and overweight with at least one risk factor for Type 2 Diabetes.
Current treatment options for pediatric Type 2 Diabetes include lifestyle changes (weight reduction, physical activity and improved sleep), metformin, insulin, and liraglutide.
Pediatric Type 2 Diabetes has faster beta cell decline and early progression to complications compared to adult Type 2 Diabetes.
SYNOPSIS:
Pediatric Type 2 Diabetes (T2DM) is rising in incidence with risk factors including obesity, puberty, family history of T2DM in a first or second-degree relative, history of small-for-gestational age at birth, child of a gestational diabetes pregnancy, minority racial group, and lower socioeconomic status. The pathophysiology of T2DM consists of insulin resistance and progression to pancreatic beta cell failure, which has been noted to be more rapid in pediatric T2DM compared to adult T2DM. Treatment options are limited and include lifestyle modification and approved pharmacologic therapies of metformin, insulin, and liraglutide, a glucagon like peptide 1 agonist. Treatment failure and nonadherence rates are high in pediatric T2DM; therefore, early diagnosis and treatment and the development of new pharmacologic options and/or effective behavioral interventions are needed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT
The authors have nothing to disclose.
References
- 1.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;376(15):1419–1429. doi: 10.1056/NEJMoa1610187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA - J Am Med Assoc. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Divers J, Mayer-Davis EJ, Lawrence JM, et al. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths - Selected Counties and Indian Reservations, United States, 2002–2015. MMWR Morb Mortal Wkly Rep. 2020;69(6):161–165. doi: 10.15585/mmwr.mm6906a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: The TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96(1):159–167. doi: 10.1210/jc.2010-1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care. 2014;37(2):436–443. doi: 10.2337/dc13-0954 [DOI] [PubMed] [Google Scholar]
- 6.Levitt Katz LE, Magge SN, Hernandez ML, Murphy KM, McKnight HM, Lipman T. Glycemic control in youth with type 2 diabetes declines as early as two years after diagnosis. J Pediatr. 2011. doi: 10.1016/j.jpeds.2010.07.011 [DOI] [PubMed]
- 7.Arslanian S, Pyle L, Payan M, et al. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and -βcell function in TODAY. Diabetes Care. 2013;36(6):1749–1757. doi: 10.2337/dc12-2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;366(24):2247–2256. doi: 10.1056/NEJMoa1109333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: A position statement by the American diabetes association. Diabetes Care. 2018;41(12):2648–2668. doi: 10.2337/dci18-0052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet. 2011;378(9786):169–181. doi: 10.1016/S0140-6736(11)60614-4 [DOI] [PubMed] [Google Scholar]
- 11.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV., Bonadonna R. Β-Cell Function Across the Spectrum of Glucose Tolerance in Obese Youth. Diabetes. 2005;54(6):1735–1743. doi: 10.2337/diabetes.54.6.1735 [DOI] [PubMed] [Google Scholar]
- 12.Gungor N, Bacha F, Saad R, Janosky J, Arslania S. Youth Type 2 Diabetes. Diabetes Care. 2005;28(3):638–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Editor: Riddle MC. American Diabetes Association: Standards of Medical Care in Diabetes −2019. Diabetes Care. 2019;42:S1–S193. doi: 10.2337/dc19-SINT01 [DOI] [PubMed] [Google Scholar]
- 14.Fourtner SH, Weinzimer SA, Levitt Katz LE. Hyperglycemic hyperosmolar non-ketotic syndrome in children with type 2 diabetes. Pediatr Diabetes. 2005;6(3):129–135. doi: 10.1111/j.1399-543X.2005.00113.x [DOI] [PubMed] [Google Scholar]
- 15.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. 2018;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3). doi: 10.1542/peds.2004-1808 [DOI] [PubMed] [Google Scholar]
- 17.Magge SN, Stettler N, Jawad AF, Levitt Katz LE. Increased Prevalence of Abnormal Glucose Tolerance among Obese Siblings of Children with Type 2 Diabetes. J Pediatr. 2009;154(4). doi: 10.1016/j.jpeds.2008.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caprio S, Plewe G, Diamond MP, et al. Increased insulin secretion in puberty: A compensatory response to reductions in insulin sensitivity. J Pediatr. 1989;114(6):963–967. doi: 10.1016/S0022-3476(89)80438-X [DOI] [PubMed] [Google Scholar]
- 19.Goran MI, Gower BA. Longitudinal Study on Pubertal Insulin Resistance. Diabetes. 2001;50:2444–2450. [DOI] [PubMed] [Google Scholar]
- 20.Nam HK, Lee KH. Small for gestational age and obesity: Epidemiology and general risks. Ann Pediatr Endocrinol Metab. 2018;23(1):9–13. doi: 10.6065/apem.2018.23.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC. Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. 2018;141(3). doi: 10.1542/peds.2017-3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bullard KM, Cowie CC, Lessem SE, Saydah SH, Menke A, Geiss LS. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type — United States, 2016. 2018;67(12):2016–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dabelea D, Bell RA, D’Agostino RBJ, Johansen JM. Incidence of Diabetes in Youth in the United States. JAMA. 2007;297(24):2716–2724. [DOI] [PubMed] [Google Scholar]
- 24.Barlow SE. Expert Committee Recommendations Regarding the Prevention , Assessment , and Treatment of Child and Adolescent Overweight and Obesity : Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 25.Kapadia C, Zeitler P. Hemoglobin A1c measurement for the diagnosis of Type 2 diabetes in children. Int J Pediatr Endocrinol. 2012;2012(1):2–5. doi: 10.1186/1687-9856-2012-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitt Katz LE. C-peptide and 24-hour urinary c-peptide as markers to help classify types of childhood diabetes. Horm Res Paediatr. 2015;84(1):62–64. doi: 10.1159/000430094 [DOI] [PubMed] [Google Scholar]
- 27.Levitt Katz LE, Jawad AF, Ganesh J, Abraham M, Murphy K, Lipman TH. Fasting c-peptide and insulin-like growth factor-binding protein-1 levels help to distinguish childhood type 1 and type 2 diabetes at diagnosis. Pediatr Diabetes. 2007;8(2):53–59. doi: 10.1111/j.1399-5448.2007.00236.x [DOI] [PubMed] [Google Scholar]
- 28.Klingensmith GJ, Pyle L, Arslanian S, et al. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: Results from the TODAY study. Diabetes Care. 2010;33(9):1970–1975. doi: 10.2337/dc10-0373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J. Seroconversion to Multiple Islet Autoantibodies and Risk of Progression to Diabetes in Children. JAMA. 2013;309(23):2473–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Besser RE, Shields BM, Hammersley SE, et al. Home urine C-peptide creatinine ratio (UCPCR) testing can identify type 2 and MODY in pediatric diabetes. Pediatr Diabetes. 2013;14(3):181–188. doi: 10.1111/pedi.12008 [DOI] [PubMed] [Google Scholar]
- 31.Anstee QM, Mcpherson S, Day CP. How big a problem is non-alcoholic fatty liver disease? BMJ. 2011;343:1–5. doi: 10.1136/bmj.d3897 [DOI] [PubMed] [Google Scholar]
- 32.Newton KP, Hou J, Crimmins NA, et al. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr. 2016;170(10):1–8. doi: 10.1001/jamapediatrics.2016.1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwimmer JB, Pardee PB, Lavine JE, Blumkin AK, Cook S. Cardiovascular Risk Factors and the Metabolic Syndrome in Pediatric Nonalcoholic Fatty Liver Disease. Circulation. 2008;118(3):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Middleton JP, Wiener RC, Barnes BH, Gurka MJ, Deboer MD. Clinical Features of Pediatric Nonalcoholic Fatty Liver Disease : A Need for Increased Awareness and a Consensus for Screening. Clin Pediatr (Phila). 2014;53(14):1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jean-Louis G, Zizi F, Clark LT, Brown CD, McFarlane SI. Obstructive Sleep Apnea and Cardiovascular Disease: Role of the Metabolic Syndrome and Its Components. J Clin Sleep Med. 2008;4(3):261–272. [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly A, Dougherty S, Cucchiara A, Marcus CL, Brooks LJ. Catecholamines , Adiponectin , and Insulin Resistance as Measured by HOMA in Children with Obstructive Sleep Apnea. Sleep. 2010;33(9):1185–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcus CL, Brooks LJ, Davidson Ward S, et al. Diagnosis and Management of Childhood Obstructive Sleep Apnea Syndrome. Pediatrics. 2012;130(3):e714–e735. [DOI] [PubMed] [Google Scholar]
- 38.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for obstructive sleep apnea in adults us preventive services task force recommendation statement. JAMA. 2017;317(4):407–414. [DOI] [PubMed] [Google Scholar]
- 39.Koren D, Katz LEL, Brar PC, Gallagher PR, Berkowitz RI, Brooks LJ. Sleep architecture and glucose and insulin homeostasis in obese adolescents. Diabetes Care. 2011. doi: 10.2337/dc11-1093 [DOI] [PMC free article] [PubMed]
- 40.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008. doi: 10.1093/sleep/31.5.619 [DOI] [PMC free article] [PubMed]
- 41.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010. doi: 10.2337/db09-0699 [DOI] [PMC free article] [PubMed]
- 42.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans JJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1017–1023. doi: 10.1210/jcem.87.3.8305 [DOI] [PubMed] [Google Scholar]
- 43.Kelsey MM, Braffett BH, Geffner ME, et al. Menstrual Dysfunction in Girls from the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) Study. J Clin Endocrinol Metab. 2018;103(6):2309–2318. doi: 10.1210/jc.2018-00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levitt Katz LE, Swami S, Abraham M, et al. Neuropsychiatric disorders at the presentation of type 2 diabetes mellitus in children. Pediatr Diabetes. 2005;6(2):84–89. doi: 10.1111/j.1399-543X.2005.00105.x [DOI] [PubMed] [Google Scholar]
- 45.Tryggestad JB, Willi SM. Complications and comorbidities of T2DM in adolescents: Findings from the TODAY clinical trial. J Diabetes Complications. 2015;29(2):307–312. doi: 10.1016/j.jdiacomp.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coughlin M, Goldie CL, Tregunno D, Tranmer J. Enhancing metabolic monitoring for children and adolescents using second-generation antipsychotics. 2018;2009:1188–1198. doi: 10.1111/inm.12417 [DOI] [PubMed] [Google Scholar]
- 47.Baeza I, Vigo L, De E, et al. The effects of antipsychotics on weight gain , weight ‑ related hormones and homocysteine in children and adolescents : a 1 ‑ year follow ‑ up study. Eur Child Adolesc Psychiatry. 2017;26(1):35–46. doi: 10.1007/s00787-016-0866-x [DOI] [PubMed] [Google Scholar]
- 48.Tamborlane WV, Barrientos-Pérez M, Fainberg U, et al. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med. 2019;381(7):637–646. doi: 10.1056/NEJMoa1903822 [DOI] [PubMed] [Google Scholar]
- 49.Turner R Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131):854–865. doi: 10.1016/S0140-6736(98)07037-8 [DOI] [PubMed] [Google Scholar]
- 50.Kendall D, Vail A, Amin R, et al. Metformin in Obese Children and Adolescents : The MOCA Trial. J Clin Endocinol Metab. 2013;98(January):322–329. [DOI] [PubMed] [Google Scholar]
- 51.Bacha F, El ghormli L, Arslanian S, et al. Predictors of response to insulin therapy in youth with poorly-controlled type 2 diabetes in the TODAY trial. Pediatr Diabetes. 2019;20(7):871–879. doi: 10.1111/pedi.12906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nauck M, Frid A, Hermansen K, et al. Efficacy and Safety Comparison of Liraglutide, Glimepiride, and Placebo, All in Combination With Metformin, in Type 2 Diabetes: The LEAD (Liraglutide Effect and Action in Diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. doi: 10.2337/dc08-1355.Clinical [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaag A, Schmitz O, Sethi BK, Lalic N. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus ( LEAD-5 met + SU ): a randomised controlled trial Plasma glucose. 2009:2046–2055. doi: 10.1007/s00125-009-1472-y [DOI] [PMC free article] [PubMed]
- 54.Katz LL, Anderson BJ, McKay SV., et al. Correlates of medication adherence in the TODAY cohort of youth with type 2 diabetes. Diabetes Care. 2016;39(11):1956–1962. doi: 10.2337/dc15-2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abrams P, Levitt Katz LE, Moore RH, et al. Threshold for improvement in insulin sensitivity with adolescent weight loss. J Pediatr. 2013;163(3):785–790. doi: 10.1016/j.jpeds.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marcus MD, Wilfley DE, El ghormli L, et al. Weight change in the management of youth-onset type 2 diabetes: the TODAY clinical trial experience. Pediatr Obes. 2017;12(4):337–345. doi: 10.1111/ijpo.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berkowitz RI, Wadden TA, Gehrman CA, et al. Meal Replacements in the Treatment of Adolescent Obesity. 2011;19(6):1193–1199. doi: 10.1038/oby.2010.288.Meal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raynor HA, Anderson AM, Miller GD, et al. Partial Meal Replacement Plan and Quality of the Diet at 1 Year: Action for Health in Diabetes (Look AHEAD) Trial. J Acad Nutr Diet. 2015;115(5):731–742. doi: 10.1016/j.jand.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berkowitz RI, Marcus MD, Anderson BJ, et al. Adherence to a lifestyle program for youth with type 2 diabetes and its association with treatment outcome in the TODAY clinical trial. Pediatr Diabetes. 2018;19(2):191–198. doi: 10.1111/pedi.12555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savoye M, Shaw M, Dziura J, et al. Effects of a Weight Management Program on Body Composition and Metabolic Parameters in Overweight Children. 2020;297(24):2697–2704. [DOI] [PubMed] [Google Scholar]
- 61.Inge TH, Laffel LM, Jenkins TM, et al. Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr. 2018;172(5):452–460. doi: 10.1001/jamapediatrics.2017.5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224 [DOI] [PubMed] [Google Scholar]
- 63.Rascati K, Richards K, Lopez D, Cheng LI, Wilson J. Progression to insulin for patients with diabetes mellitus on dual oral antidiabetic therapy using the US department of defense database. Diabetes, Obes Metab. 2013;15(10):901–905. doi: 10.1111/dom.12103 [DOI] [PubMed] [Google Scholar]
- 64.Zeitler P, Hirst K, Copeland KC, et al. HbA1c after a short period of monotherapy with metformin identifies durable glycemic control among adolescents with type 2 diabetes. Diabetes Care. 2015;38(12):2285–2292. doi: 10.2337/dc15-0848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kahn SE. Clinical, review 135: The importance of β-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86(9):4047–4058. doi: 10.1210/jc.86.9.4047 [DOI] [PubMed] [Google Scholar]
- 66.Gungor N, Arslanian S. Diabetes Mellitus of Youth. J Pediatr. 2004:656–659. doi: 10.1016/j.jpeds.2003.12.045 [DOI] [PubMed]
- 67.Bacha F, Gungor N, Lee S, Arslanian SA. In Vivo Insulin Sensitivity and Secretion in Obese Youth: What are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care. 2009;32(1):100–105. doi: 10.2337/dc08-1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edelstein SL, Kahn SE, Arslanian SA, et al. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic Clamp. Diabetes Care. 2018;41(8):1696–1706. doi: 10.2337/dc18-0244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edelstein SL. Restoring Insulin Secretion (RISE): Design of studies of β-Cell preservation in prediabetes and early Type 2 diabetes across the life span. Diabetes Care. 2014;37(3):780–788. doi: 10.2337/dc13-1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nadeau KJ, Hannon TS, Edelstein SL, et al. Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care. 2018;41(8):1717–1725. doi: 10.2337/dc18-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CC, Holman RR. Development and progression of nephropathy in type 2 diabetes : The United Kingdom Prospective Diabetes Study. 2003;63:225–232. doi: 10.1046/j.1523-1755.2003.00712.x [DOI] [PubMed] [Google Scholar]
- 72.Chiang JL, Boer IH De, Goldstein-fuchs J. Diabetic Kidney Disease: A Report From an ADA Consensus Conference. Am J Kidney Dis. 2014;64(4):510–533. doi: 10.1053/j.ajkd.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 73.Constantino MI, Molyneaux L, Limacher-Gisler F, et al. Long-term complications and mortality in young-onset diabetes: Type 2 diabetes is more hazardous and lethal than type 1 diabetes. Diabetes Care. 2013;36(12):3863–3869. doi: 10.2337/dc12-2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katz LL, Gidding SS, Bacha F, et al. Alterations in left ventricular, left atrial, and right ventricular structure and function to cardiovascular risk factors in adolescents with type 2 diabetes participating in the TODAY clinical trial. Pediatr Diabetes. 2015. doi: 10.1111/pedi.12119 [DOI] [PMC free article] [PubMed]
- 75.Shah AS, Urbina EM. Vascular and Endothelial Function in Youth with Type 2 Diabetes Mellitus. Curr Diab Rep. 2017;17(6):1–7. doi: 10.1007/s11892-017-0869-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah AS, El Ghormli L, Vajravelu ME, et al. Heart rate variability and cardiac autonomic dysfunction: Prevalence, risk factors, and relationship to arterial stiffness in the treatment options for type 2 diabetes in adolescents and youth (TODAY) study. Diabetes Care. 2019;42(11):2143–2150. doi: 10.2337/dc19-0993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levitt Katz LE, Bacha F, Gidding SS, et al. Lipid Profiles, Inflammatory Markers, and Insulin Therapy in Youth with Type 2 Diabetes. J Pediatr. 2018. doi: 10.1016/j.jpeds.2017.12.052 [DOI] [PMC free article] [PubMed]
- 78.Ohsugi K, Sugawara H, Ebina K, et al. Comparison of brachial artery flow-mediated dilation in youth with type 1 and type 2 diabetes mellitus. J Diabetes Investig. 2014;5(5):615–620. doi: 10.1111/jdi.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klingensmith GJ, Pyle L, Nadeau KJ, et al. Pregnancy Outcomes in Youth with Type 2 Diabetes: The TODAY Study Experience. Diabetes Care. 2016;39(1):122–129. doi: 10.2337/dc15-1206 [DOI] [PMC free article] [PubMed] [Google Scholar]


