Abstract
The success of vaccination programs is contingent upon irrefutable scientific safety data combined with high rates of public acceptance and population coverage. Vaccine hesitancy, characterized by lack of confidence in vaccination and/or complacency about vaccination that may lead to delay or refusal of vaccination despite the availability of services, threatens to undermine the success of coronavirus disease 2019 (COVID-19) vaccination programs. The rapid pace of vaccine development, misinformation in popular and social media, the polarized sociopolitical environment, and the inherent complexities of large-scale vaccination efforts may undermine vaccination confidence and increase complacency about COVID-19 vaccination. Although the experience of recent lethal surges of COVID-19 infections has underscored the value of COVID-19 vaccines, ensuring population uptake of COVID-19 vaccination will require application of multilevel, evidence-based strategies to influence behavior change and address vaccine hesitancy. Recent survey research evaluating public attitudes in the United States toward the COVID-19 vaccine reveals substantial vaccine hesitancy. Building upon efforts at the policy and community level to ensure population access to COVID-19 vaccination, a strong health care system response is critical to address vaccine hesitancy. Drawing on the evidence base in social, behavioral, communication, and implementation science, we review, summarize, and encourage use of interpersonal, individual-level, and organizational interventions within clinical organizations to address this critical gap and improve population adoption of COVID-19 vaccination.
Abbreviations and Acronyms: COVID-19, coronavirus disease 2019
The success of vaccination programs is contingent upon irrefutable scientific safety data combined with high rates of public acceptance and population coverage. Vaccine hesitancy threatens to undermine the success of coronavirus disease 2019 (COVID-19) vaccination programs. In this article, we review, summarize, and encourage use of interpersonal, individual-level, and organizational interventions within clinical organizations to address this critical gap and improve population adoption of COVID-19 vaccination.
Vaccine Hesitancy as a Global Health Threat
Successful immunization programs require high rates of acceptance and population coverage.1 , 2
Thus, the availability of safe and effective vaccines is insufficient; vaccines have to be widely accepted by the public and by the health care community to confer population benefit.2 , 3 Mounting evidence teaches that segments of the US public experience some degree of hesitancy about accepting vaccination.3 Indeed, vaccination hesitancy, lack of confidence in vaccination, and/or complacency about vaccination that may lead to delay in acceptance or refusal of vaccination despite access to vaccination services4 , 5 was deemed a top 10 threat to global health by the World Health Organization in 2019.6 Although this designation preceded the COVID-19 pandemic, the sociopolitical response to the pandemic in the United States and other countries provides a timely example of this threat.
Vaccination confidence is influenced by trust in the safety and effectiveness of vaccines, trust in health care professionals and public health and health care delivery systems, and trust in the policymakers who develop vaccination requirements.5 Experts have noted a decline in public confidence in vaccination.7 Vaccination complacency is influenced by individuals’ health beliefs (eg, perceived risk of vaccination, perceived risk and severity of disease, perceived need for the vaccine, and self-efficacy of vaccination) and their assessment of the risks and benefits of vaccination.5 Ironically, the success of vaccination has contributed to such complacency by reducing perceived risk and severity of disease.7
COVID-19 Vaccine Hesitancy
A convergence of critical uncertainties and social trends will likely exacerbate vaccine hesitancy specific to COVID-19.3 , 8, 9, 10, 11, 12, 13, 14, 15 The COVID-19 vaccine is offered to a public suffering from pandemic fatigue,3 while misinformation in popular and social media and conspiracy beliefs about COVID-19 pandemic and COVID-19 vaccination are perpetuated.16, 17, 18, 19 Research conducted during the COVID-19 pandemic to ascertain public willingness to accept COVID-19 vaccination and to assess vaccination confidence and complacency reveal a general decline in public acceptance of a potential vaccine coupled with increasing vaccine hesitancy.16, 17, 18, 19, 20 Factors associated with COVID-19 vaccine hesitancy generally mirror factors known to influence vaccine hesitancy for other vaccines. These factors include vaccine-related attributes, political factors, and vaccine-related attitudes and beliefs.4
Vaccine Attributes
Survey data reveal public hesitancy about COVID-19 vaccine effectiveness, uncertainty regarding the protection duration, and apprehension about safety or adverse effects.17 , 19 Critical information about vaccine attributes, such as duration of immunity and immunogenicity, is gradually accumulating and will vary by vaccine manufacturer and/or among populations.11 , 12 This emerging understanding of COVID-19 immunology and virology,8, 9, 10 coupled with the unprecedented speed of vaccine development, threaten to undermine public confidence.
Political Factors
Efforts to rapidly develop with aggressive federal funding and deploy vaccines with emergency use authorization from the US Food and Drug Administration may worsen concerns about vaccine safety and effectiveness.21 , 22 Data from public surveys in the United States also reveal the influence of political factors on hesitancy wherein lack of trust in those endorsing vaccination, country of vaccine origin, and concerns about profit or political motives increase public mistrust.13 , 17 , 19
Individual Attitudes and Characteristics
General mistrust of vaccination, misperceptions about the severity of COVID-19 infection, and a preference for natural immunity have also been found to be associated with greater hesitancy.13 , 23 Survey data also revealed greater hesitancy among those with lower education, unemployed individuals, younger populations, and certain ethnic and racial minority groups including Hispanics and African Americans who have been disproportionately affected by COVID-19.17 , 18 , 20 , 23 , 24
Evidence-Based Strategies for Addressing COVID-19 Vaccine Hesitancy
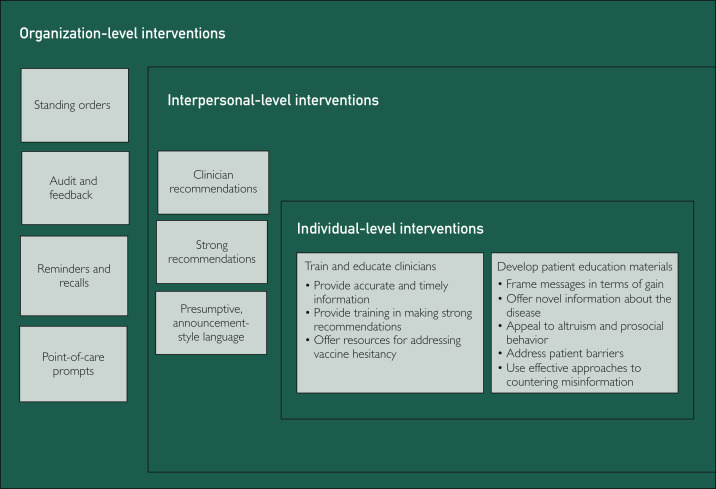
The present review is offered to summarize effective strategies to address vaccine hesitancy for use by health care professionals and clinical organizations in the care of their patients and employees. To improve population adoption of COVID-19 vaccination, it is imperative to draw on implementation of multilevel, evidence-based strategies to increase vaccine uptake and address vaccine hesitancy. Equally critical is the widespread adoption of evidence-based best practices that have been developed and refined with previous vaccines. These factors include evidence-based efforts from social, behavioral, communication, and implementation science that can inform clinical efforts at the interpersonal, individual, and organization levels to address COVID-19 vaccine hesitancy and support public health efforts (Figure ).
Figure.
Evidence-based strategies for clinical organizations to address coronavirus disease 2019 vaccine hesitancy.
Policy- and Community-Level Interventions
Policy-level interventions, such as reducing out-of-pocket expense for patients and requiring vaccination for child care and school and college attendance, and community-level interventions that address access barriers by offering vaccination programs in schools and child care centers and providing vaccination in Women, Infants, and Children programs are very effective strategies for increasing vaccination rates.25 Although interventions at the policy and community level do not specifically address vaccine hesitancy, they do set the stage for interventions to address vaccine hesitancy within clinical organizations. Adoption of best practices in clinical organizations at the interpersonal, individual, and organizational levels from the evidence base in social, behavioral, communication, and implementation science can improve clinical efforts to address COVID-19 vaccine hesitancy and support public health efforts to increase vaccination rates (Figure).
Interpersonal-Level Interventions
Interpersonal-level interventions address the interactions between clinicians and patients (or parents of patients, as is most often the case with pediatric populations). Clinicians have been consistently documented to result in higher vaccination rates across a variety of preventive behaviors including vaccines.26, 27, 28 Clinicians remain the most trusted source of information, in general29 and specifically in regard to COVID-19.30 The quality and strength of clinician recommendations have also been reported to influence vaccination rates.28 , 31, 32, 33 Indeed, in a recently conducted survey, respondents indicated a greater likelihood of accepting the COVID-19 vaccine if recommended by their clinician.20 Observational studies33 , 34 and clinical trials35 , 36 with childhood vaccines indicate that use of presumptive, announcement-style language (“Today you’ll be getting your vaccine”) rather than conversational, participatory-style language (“How would you feel about getting a vaccine today?”) results in higher vaccination uptake.34 , 35 Strong recommendations from trusted clinicians may improve vaccine confidence, reduce concerns about safety, and improve uptake of the COVID-19 vaccine. Effective interpersonal strategies to address vaccine hesitancy with specific examples are summarized in Table 1 .
Table 1.
Examples of Evidence-Based Strategies for Clinical Organizations to Address COVID-19 Vaccine Hesitancy at the Interpersonal and Individual Level
| Interpersonal strategies | |
|---|---|
| Strategy | Example |
| Make recommendations | “Your clinician recommends that you get the COVID-19 vaccine.” |
| Make strong recommendations. | “COVID-19 vaccination is safe and effective, and I strongly recommend that you to get your COVID-19 vaccine today.” |
| Use presumptive-style language. | “After this visit, the nurse will give you your COVID-19 vaccine.” |
| Individual strategies | |
|---|---|
| Strategy | Example |
| Frame messages in terms of gain | “Getting the COVID-19 vaccine will protect you and your family.” |
| Offer novel information about the disease | “We are learning that COVID-19 infections can result in longer-lasting and debilitating health problems such as fatigue, headaches, joint pain, and sleep difficulty.” |
| Appeal to altruism and prosocial behavior | “Getting the COVID-19 vaccine not only protects you, it will also protect the people you care about including your family and friends.” |
| Address patient barriers and use effective approaches to countering misperceptions | Affirm values: “I know you care deeply about [protecting the health of your loved ones; doing what is right for your community; taking good care of your health; protecting your family], and getting the COVID-19 vaccine can help you do so.” |
| Explain motivation for misinformation: “Your concern(s) about [vaccine safety; adverse effects; vaccine need; vaccine timing] is a common misperception that has been sensationalized in popular media.” | |
| Repeat factual information: “Substantial evidence exists regarding [the safety of the COVID-19 vaccine that has been documented in tens of thousands patients; the temporary and mild nature of adverse effects; the need for the COVID-19 vaccine to protect individuals and to end the pandemic; the need for individuals to get vaccinated as soon as the vaccine becomes available to them].” | |
COVID-19, coronavirus disease 2019.
Individual-Level Interventions
Individual-level interventions, in this context, target members of the health care team and patients. Although research teaches that education of clinicians and patients offered in the absence of other strategies is largely ineffective,32 when offered in combination with interventions at the organization and interpersonal level, individual-level educational interventions can empower health care teams to promote vaccination and optimize efforts to address hesitancy among patients (see Table 1 for examples).25 , 28 , 32 , 33 , 37, 38, 39, 40, 41
Clinicians
To ensure readiness to offer strong recommendations to their patients, clinicians must have adequate training and education regarding the evidence supporting COVID-19 vaccination, such as information about vaccine efficacy, safety, and reactogenicity. Clinicians must also be well equipped with information regarding the need for the vaccine and its critical role in the strategic prevention of COVID-19 in individuals and among populations. Evidence-based interventions for care teams include education and training offered to provide clinicians with readily accessible information about different COVID-19 vaccines, and additional resources (Table 2 ) should also be offered to clinicians to address the vaccine-related attributes, political factors, and attitudes and beliefs that contribute to vaccine hesitancy.28 , 32 , 33 , 36, 37, 38
Table 2.
COVID-19 Vaccination Training and Education Resources for Clinicians
| Source | Brief description |
|---|---|
| Centers for Disease Control and Prevention www.cdc.gov/vaccines | COVID-19 vaccination training and education tools |
| • COVID-19 Vaccine Training Module For Healthcare Professionals | |
| • Preparing to Provide COVID-19 Vaccines | |
| • Talking to Patients about COVID-19 Vaccines | |
| • Making a Strong Recommendation for COVID-19 Vaccination | |
| • Answering Patients’ Questions | |
| • What Healthcare Professionals Need to Know | |
| • COVID-19 Vaccination Training Programs and Reference Materials for Healthcare Professionals | |
| The Immunization Action Coalition www.immunize.org | Provides practical solutions and resources to support health care professionals and to educate patients |
| Vaccinate Your Family https://vaccinateyourfamily.org/vaccines-diseases/covid19/ | Offers information and resources on COVID-19 and COVID-19 vaccines for patients and health care professionals |
| Mayo Clinic https://www.mayoclinic.org/coronavirus-covid-19 | COVID-19 vaccine information |
| • COVID-19 vaccine: Get the facts | |
| • Mayo Clinic answers questions about COVID-19 vaccine | |
| • COVID-19 vaccine myths debunked | |
| • COVID-19 Vaccine (PDF) |
COVID-19, coronavirus disease 2019.
The emergence of several vaccines with varying dosing schedules and storing requirements will intensify logistic and communication challenges, which may, in turn, render addressing vaccine hesitancy more challenging.13 Clinicians should be provided with resources (Table 2) and training in making strong recommendations and addressing vaccine hesitancy.28 , 32 , 33 , 36, 37, 38 Capacity-building strategies, including training, technical assistance, and other support, have been found to increase clinicians adoption and implementation of evidence-based interventions.39 , 40
Training and education in addressing patient concerns should be offered to clinicians in an easy format so that they, in turn, can make their strongest recommendations to patients. The training must also foster communication that is honest, culturally appropriate, and consistent. Furthermore, training and education are necessary to empower all health care staff, and not just clinicians, who interact with patients. These personnel include nurses, receptionists, scheduling and appointment staff, and other administrative assistants.2
Patients
Developing and offering patient education materials in combination with other evidence-based strategies can improve vaccination rates.25 This approach can be strengthened by drawing upon behavior and communication science evidence that has consistently shown that positive framing or gain-framing of messages is effective for promoting prevention.41 Specifically, a positive frame involves emphasizing the benefits gained by participating in vaccination (eg, “Getting the COVID-19 vaccine will protect you and your family”) vs a negative frame, which would emphasize the risks of failing to vaccinate (eg, “If you choose not to get the COVID-19 vaccine, you are putting yourself and your family at risk”). Appeals to altruism and prosocial behavior (eg, protecting one’s family) have also been found to be effective communication strategies for promotion of vaccination.42
Tailoring patient reminders to vaccinate to address common patient barriers and concerns may improve uptake.25 This process may additionally involve the offer of novel information about the disease rather than an explicit attempt to counter common misperceptions about the vaccine to be optimally effective.43 Research has revealed that direct efforts to counter misperceptions may backfire, resulting in an increase in misperceptions or a decrease in intention to be vaccinated.32 , 43, 44, 45 If correcting misperceptions about the vaccine is needed, it is important to (1) frame the messages in ways that affirm the audience’s worldview or personal values (eg, protection of family, social responsibility, patriotic duty) to increase receptivity, (2) explain the motivation behind the misinformation about the vaccine and provide a factual alternative narrative so that people are not left with a gap in their mental representation of the vaccine, and (3) repeat factual information to strengthen its efficacy and avoid repeating the misinformation.46
Individual-level interventions targeting care teams and patients, offered in the context of organization-level and interpersonal-level interventions drawing upon effective and evidence-based communication strategies, can optimize efforts to address hesitancy in clinical settings. Table 1 summarizes evidence-based strategies and offers specific examples.
Organization-Level Interventions
Drawing upon implementation science, a variety of organization-level interventions have been found to increase vaccination rates by supporting the work of clinicians or removing barriers to vaccination for patients. These interventions include availability of standing orders for nurse visits, audit and feedback, reminder/recall systems, point-of-care prompts, and home visits.25 , 32 , 47 As with other routine and at-risk vaccinations, clinicians should seek to approach every patient visit as an opportunity to assess vaccination status and deliver needed vaccines; organization-level interventions support this approach.48 Standing orders support nurse visits without clinical examinations and allow nurses to vaccinate patients without individual, patient-specific orders written by the clinicians. These orders are sometimes referred to as nurse protocols rather than standing orders. These orders increase vaccination, in general, and improve access for patients.25 , 32 , 47 Standing orders expand access to vaccination and streamline the vaccination process for patients.25 , 32 Audit and feedback interventions, which involve regular presentation of vaccination performance metrics to clinicians, have also shown effectiveness for vaccination in general.25 , 32 , 47 Regular clinician appraisal of vaccination rates in their patient panel has been found to improve coverage rates.48 Inclusion of peer performance or benchmark performance metrics in audit and feedback interventions can also promote a sense of normative behavior among clinicians. Reminders and recalls, which involve directly contacting patients to inform them that a vaccination is due, coming due, or past due, have consistently had effectiveness in improving vaccination rates.47 , 49 Point-of-care prompts, which flag recommended vaccinations during clinical encounters either through review of current vaccination records or through electronic clinical decision support, are effective for increasing vaccination rates.25 , 32 , 47 Home visits can also improve vaccination rates and may be particularly useful in reaching underserved populations.25 Lastly, organization-level interventions implemented in combination involving the use of 2 or more coordinated interventions to increase vaccination rates have also been effective.25 In general, bundled approaches that target multiple levels of influence (eg, organizational, interpersonal, individual) and engage stakeholders (eg, patients, clinicians) are most effective.
Practical constraints require organizations to select from among available evidence-based strategies to identify approaches that are feasible and acceptable within their specific contexts. Strategies to increase uptake of evidence-based interventions, such as COVID-19 vaccination, must be selected and tailored to conform to the unique needs and resources of clinical settings and to address known barriers to adoption.39 It is therefore critical to conduct local context assessments to understand relevant barriers and resources.50 Furthermore, couching efforts within iterative evaluation approaches can assist with efficiently finding and adapting the right interventions over time.51
Conclusion
Vaccine hesitancy threatens to compromise the success of COVID-19 vaccination programs. Ensuring adequate population adoption of COVID-19 vaccination will entail addressing increasing vaccine hesitancy among a pandemic-weary public. Fortunately, the use of evidence-based strategies to increase vaccination uptake provides health care systems with a road map to navigate vaccine hesitancy. Implementation of evidence-based strategies at the organizational, interpersonal, and individual levels in clinical organizations to increase uptake of COVID-19 vaccination is the crucial last leg of the arduous race to end the COVID-19 pandemic.
Footnotes
Grant Support: This work was made possible in part with support from the Mayo Clinic Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery and the National Institutes of Health grant 5R01CA21788903.
Potential Competing Interests: Dr Virk has received royalties from Travel Health and Wellness LLC. Dr Jacobson has served on safety review committees for Merck & Co for 2 postlicensure studies of human papillomavirus vaccine safety and on an external data monitoring committee for Merck & Co for a series of prelicensure trials of a novel pneumococcal vaccine. The other authors report no competing interests.
Supplemental Online Material
References
- 1.Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. High contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1470–1477. doi: 10.3201/eid2607.200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaffer DeRoo S., Pudalov N.J., Fu L.Y. Planning for a COVID-19 vaccination program. JAMA. 2020;323(24):2458–2459. doi: 10.1001/jama.2020.8711. [DOI] [PubMed] [Google Scholar]
- 3.Brunson E.K., Schoch-Spana M. A social and behavioral research agenda to facilitate COVID-19 vaccine uptake in the United States. Health Secur. 2020;18(4):338–344. doi: 10.1089/hs.2020.0106. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson R.M., St Sauver J.L., Finney Rutten L.J. Vaccine hesitancy. Mayo Clin Proc. 2015;90(11):1562–1568. doi: 10.1016/j.mayocp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald N.E., SAGE Working Group on Vaccine Hesitancy Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Ten threats to global health in 2019. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
- 7.Larson H.J., Cooper L.Z., Eskola J., Katz S.L., Ratzan S. Addressing the vaccine confidence gap. Lancet. 2011;378(9790):526–535. doi: 10.1016/S0140-6736(11)60678-8. [DOI] [PubMed] [Google Scholar]
- 8.Kirkcaldy R.D., King B.A., Brooks J.T. COVID-19 and postinfection immunity: limited evidence, many remaining questions. JAMA. 2020;323(22):2245–2246. doi: 10.1001/jama.2020.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi Y., Lagniton P.N.P., Ye S., Li E.Q., Xu R.-H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng W., Zong W., Wang F., Ju S. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a review. Mol Cancer. 2020;19(1):100. doi: 10.1186/s12943-020-01218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lurie N., Saville M., Hatchett R., Halton J. Developing Covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 12.Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368(6494):948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- 13.Khamsi R. If a coronavirus vaccine arrives, can the world make enough? Nature. 2020;580(7805):578–580. doi: 10.1038/d41586-020-01063-8. [DOI] [PubMed] [Google Scholar]
- 14.Jolley D., Douglas K.M. The effects of anti-vaccine conspiracy theories on vaccination intentions. PLoS One. 2014;9(2):e89177. doi: 10.1371/journal.pone.0089177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karafillakis E., Larson H.J., ADVANCE Consortium The benefit of the doubt or doubts over benefits? a systematic literature review of perceived risks of vaccines in European populations. Vaccine. 2017;35(37):4840–4850. doi: 10.1016/j.vaccine.2017.07.061. [DOI] [PubMed] [Google Scholar]
- 16.Lewis J.R. What is driving the decline in people’s willingness to take the COVID-19 vaccine in the United States. https://doi.org/10.1001/jamahealthforum.2020.1393 [published online November 18, 2020]? JAMA Health Forum. [DOI] [PubMed]
- 17.Kreps S., Prasad S., Brownstein J.S. Factors associated with US adults' likelihood of accepting COVID-19 vaccination. JAMA Netw Open. 2020;3(10):e2025594. doi: 10.1001/jamanetworkopen.2020.25594. [published correction appears in JAMA Netw Open. 2020;3(11):e2030649] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malik A.A., McFadden S.M., Elharake J., Omer S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26:100495. doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogue K., Jensen J.L., Stancil C.K. Influences on attitudes regarding potential COVID-19 vaccination in the United States. Vaccines (Basel) 2020;8(4):582. doi: 10.3390/vaccines8040582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiter P.L., Pennell M.L., Katz M.L. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine. 2020;38(42):6500–6507. doi: 10.1016/j.vaccine.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen A. Why the push for a quick coronavirus vaccine could backfire. Politico LLC. Politico website. https://www.politico.com/news/2020/03/20/why-the-push-for-a-quick-coronavirus-vaccine-could-backfire-139854 Published March 20,2020. Accessed November 22, 2020.
- 22.Trogen B., Oshinsky D., Caplan A. Adverse consequences of rushing a SARS-CoV-2 vaccine: implications for public trust. JAMA. 2020;323(24):2460–2461. doi: 10.1001/jama.2020.8917. [DOI] [PubMed] [Google Scholar]
- 23.Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann Intern Med. 2020;173(12):964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor S., Landry C.A., Paluszek M.M., Groenewoud R., Rachor G.S., Asmundson G.J.G. A proactive approach for managing COVID-19: the importance of understanding the motivational roots of vaccination hesitancy for SARS-CoV2. Front Psychol. 2020;11:575950. doi: 10.3389/fpsyg.2020.575950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Community Preventive Services Task Force The guide to community preventive services. Centers for Disease Control and Prevention website. https://www.thecommunityguide.org/
- 26.Ylitalo K.R., Lee H., Mehta N.K. Health care provider recommendation, human papillomavirus vaccination, and race/ethnicity in the US National Immunization Survey. Am J Public Health. 2013;103(1):164–169. doi: 10.2105/AJPH.2011.300600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darden P.M., Thompson D.M., Roberts J.R. Reasons for not vaccinating adolescents: National Immunization Survey of Teens, 2008-2010. Pediatrics. 2013;131(4):645–651. doi: 10.1542/peds.2012-2384. [DOI] [PubMed] [Google Scholar]
- 28.Darden P.M., Jacobson R.M. Impact of a physician recommendation. Hum Vaccin Immunother. 2014;10(9):2632–2635. doi: 10.4161/hv.29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jackson D.N., Peterson E.B., Blake K.D., Coa K., Chou W.-Y.S. Americans' trust in health information sources: trends and sociodemographic predictors. Am J Health Promot. 2019;33(8):1187–1193. doi: 10.1177/0890117119861280. [DOI] [PubMed] [Google Scholar]
- 30.Bogart L.M., Ojikutu B.O., Tyagi K. COVID-19 related medical mistrust, health impacts, and potential vaccine hesitancy among black Americans living with HIV. J Acquir Immune Defic Syndr. 2021;86(2):200–207. doi: 10.1097/QAI.0000000000002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenthal S.L., Weiss T.W., Zimet G.D., Ma L., Good M.B., Vichnin M.D. Predictors of HPV vaccine uptake among women aged 19-26: importance of a physician's recommendation. Vaccine. 2011;29(5):890–895. doi: 10.1016/j.vaccine.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 32.Jacobson R.M., Agunwamba A.A., St Sauver J.L., Finney Rutten L.J. The most effective and promising population health strategies to advance human papillomavirus vaccination. Expert Rev Vaccines. 2016;15(2):257–269. doi: 10.1586/14760584.2016.1116947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson R.M., St Sauver J.L., Griffin J.M., MacLaughlin K.L., Finney Rutten L.J. How health care providers should address vaccine hesitancy in the clinical setting: evidence for presumptive language in making a strong recommendation. Hum Vaccin Immunother. 2020;16(9):2131–2135. doi: 10.1080/21645515.2020.1735226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brewer N.T., Hall M.E., Malo T.L., Gilkey M.B., Quinn B., Lathren C. Announcements versus conversations to improve HPV vaccination coverage: a randomized trial. Pediatrics. 2017;139(1):e20161764. doi: 10.1542/peds.2016-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malo T.L., Hall M.E., Brewer N.T., Lathren C.R., Gilkey M.B. Why is announcement training more effective than conversation training for introducing HPV vaccination? a theory-based investigation. Implement Sci. 2018;13(1):57. doi: 10.1186/s13012-018-0743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubé E., MacDonald N.E. How can a global pandemic affect vaccine hesitancy [editorial]? Expert Rev Vaccines. 2020;19(10):899–901. doi: 10.1080/14760584.2020.1825944. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson R.M. Making the C.A.S.E. for the human papillomavirus vaccine: how to talk to parents and adolescents. Minn Med. 2014;97(2):38–42. [PubMed] [Google Scholar]
- 38.Jacobson R.M., Van Etta L., Bahta L. The C.A.S.E. approach: guidance for talking to vaccine-hesitant parents. Minn Med. 2013;96(4):49–50. [PubMed] [Google Scholar]
- 39.Leeman J., Birken S.A., Powell B.J., Rohweder C., Shea C.M. Beyond "implementation strategies": classifying the full range of strategies used in implementation science and practice. Implement Sci. 2017;12(1):125. doi: 10.1186/s13012-017-0657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leeman J., Calancie L., Hartman M.A. What strategies are used to build practitioners' capacity to implement community-based interventions and are they effective? a systematic review. Implement Sci. 2015;10:80. doi: 10.1186/s13012-015-0272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallagher K.M., Updegraff J.A. Health message framing effects on attitudes, intentions, and behavior: a meta-analytic review. Ann Behav Med. 2012;43(1):101–116. doi: 10.1007/s12160-011-9308-7. [published correction appears in Ann Behav Med. 2013;46(1):127] [DOI] [PubMed] [Google Scholar]
- 42.Chou W.-Y.S., Budenz A. Considering emotion in COVID-19 vaccine communication: addressing vaccine hesitancy and fostering vaccine confidence. Health Commun. 2020;35(14):1718–1722. doi: 10.1080/10410236.2020.1838096. [DOI] [PubMed] [Google Scholar]
- 43.Horne Z., Powell D., Hummel J.E., Holyoak K.J. Countering antivaccination attitudes. Proc Natl Acad Sci U S A. 2015;112(33):10321–10324. doi: 10.1073/pnas.1504019112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nyhan B., Reifler J., Richey S., Freed G.L. Effective messages in vaccine promotion: a randomized trial. Pediatrics. 2014;133(4):e835–e842. doi: 10.1542/peds.2013-2365. [DOI] [PubMed] [Google Scholar]
- 45.Nyhan B., Reifler J. Does correcting myths about the flu vaccine work? an experimental evaluation of the effects of corrective information. Vaccine. 2015;33(3):459–464. doi: 10.1016/j.vaccine.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Lewandowsky S., Ecker U.K.H., Seifert C.M., Schwarz N., Cook J. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106–131. doi: 10.1177/1529100612451018. [DOI] [PubMed] [Google Scholar]
- 47.Briss P.A., Rodewald L.E., Hinman A.R., Task Force on Community Preventive Services Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(1, suppl):97–140. doi: 10.1016/s0749-3797(99)00118-x. [DOI] [PubMed] [Google Scholar]
- 48.Elam-Evans L.D., Yankey D., Jeyarajah J. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years—United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(29):625–633. [PMC free article] [PubMed] [Google Scholar]
- 49.Szilagyi P., Vann J., Bordley C. Interventions aimed at improving immunization rates. Cochrane Database Syst Rev. 2002;4:CD003941. doi: 10.1002/14651858.CD003941. [DOI] [PubMed] [Google Scholar]
- 50.Pfadenhauer L.M., Gerhardus A., Mozygemba K. Making sense of complexity in context and implementation: the Context and Implementation of Complex Interventions (CICI) framework. Implement Sci. 2017;12(1):21. doi: 10.1186/s13012-017-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keith R.E., Crosson J.C., O'Malley A.S., Cromp D., Taylor E.F. Using the Consolidated Framework for Implementation Research (CFIR) to produce actionable findings: a rapid-cycle evaluation approach to improving implementation. Implement Sci. 2017;12(1):15. doi: 10.1186/s13012-017-0550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.