Abstract
The coronavirus disease 2019 (COVID-19) emerges as current outbreak cause by Novel Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2). This infection affects respiratory system and provides uncontrolled systemic inflammatory response as cytokine storm. The main concern about SARS-CoV-2 pandemic is high viral pathogenicity with no specific drugs. MicroRNAs (miRs) as small non-coding RNAs (21–25 nt) regulate gene expression. The SARS-CoV-2 encoded-miRs affect human genes that involved in transcription, translation, apoptosis, immune response and inflammation. Also, they alter self-gene regulation and hijacked host miRs that provide protective environment to maintain its latency. On the other hand, Host miRs play critical role in viral gene expression to restrict infection. Over expression/inhibition of miRs might result in cell cycle irregularity, impaired immune response or cancer. In this manner, exact role of each miR should be specified. Mimic encoded-miRs like antagomirs showed successful result in phases of clinical trial prevent from negative effects of viral encoded-miRs. Products of mimic miRs are inexpensive corresponds to synthesis of primer; they are short and nanoscale in size. Although SARS-CoV-2 genome is undergoing evaluation, detection of exact molecular pathogenesis open up opportunities to for vaccine development. Salivaomics can evaluate SARS-CoV-2 genome, transcriptome, proteome and biomarkers like miRs in oral related and cancer disease. In this review, we studied the challenge and opportunities of miRs in therapeutic approach for SARS-CoV-2 infection, then overviewed the role of miRs in saliva droplet during SARS-CoV-2 infection and related cancer.
Keywords: COVID-19, SARS-CoV-2, microRNA, Therapeutic approach, Saliva biomarker
1. Introduction
Novel Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2) that emerges as current viral outbreak is caused by beta coronavirus, a member of Coronaviridae family. The current disease caused by SARS-CoV-2 was termed coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO).1
The SARS-CoV-2 spread globally since December 2019 following animal-to-human cross-species transmission. It was identified by next generation sequencing in January 2020 as seventh human coronavirus after 229E, OC43, NL63, HKU1, SARS-CoV and Middle East respiratory syndrome (MERS) types. SARS-CoV (in 2002–2003, China), MERS (in 2012, Saudi Arabia) and SARS-CoV-2 (2019, Wuhan city, China) are caused by beta coronaviruses that affects respiratory system by binding to the angiotensin converting enzyme 2 (ACE2) receptor in lung. For this reason, symptoms of upper respiratory tract reveals.2
Clinical symptoms are various following SARS-CoV-2 infection, most of the patient present fever, dry cough and tiredness. In addition, dyspnea, lung invasive lesions in chest radiographs, loss of smell or taste senses, ache and pains and diarrhea are observed.3
Based on the first genome sequencing information, SARS-CoV-2 is a positive single strand RNA (+ssRNA) with 29891 nt length genome and low GC content that encoding 9860 amino acid.4 The genome of SARS-CoV-2 be made up 11 open reading frames (ORFs) encode 16 nonstructural proteins and structural protein including spike glycoprotein (S), membrane (M), small envelope (E), nucleocapsid (N) and six accessory proteins (3a, 6, 7a, 7b, 8, and 10).5 The spike protein play crucial role in viral infection because its receptor binding domains (RBDs) connected to the ACE2 receptor in mucosa cell and entry into the host cell and result in pneumonia.6
Release a large number of systemic pro-inflammatory cytokine and chemokine by immune host cell following stimulated secretion of viral invasion provide deadly uncontrolled systemic inflammatory response. This cytokine storm including IL-6, TNFα, IL-1β, IL-8 and IL2R result in Acute Respiratory Distress Syndrome (ARDS) that is the main cause of death in COVID-19 patients.7
Current evidence approved SARS-CoV-2 genome got mutations that almost are point mutation. Position of mutation affect RNA secondary structure and cryptic splice site that help to virus avoided to target by host microRNAs and provide viral fitness.8
MicroRNAs (miRs) are non-coding RNAs, small endogenous molecules (21–25 nt length), that regulate gene expression by inhibition of translation process or mRNA degradation depending upon their complementarity. They play a key role post-transcriptionally by binding to 3ʹ UTR of specific target mRNAs.9 It is demonstrated that more than half human mRNA transcripts are regulated by miRs and a single mRNA can target by multiple miRs and controversy one miR can bind to several target mRNAs.10 More than 2500 miRs are involved in human gene regulation that involve in various biological pathway like proliferation, differentiation, cancer, development, cell death and metabolism. In addition, previous studies declared host miRs play critical role in viral infection by modulate virus pathogenesis. To induce this antiviral effects, miRs can bind to both 3ʹ UTR and protein coding region of virus transcripts and alter viral tissue tropism or replication, transcription and translation.11 On the other hand, viral-encoded miRs modulate self-gene regulation and also affect host gene expression. This can provide protective environment for life cycle of virus and maintain its latency. In this manner, some viruses able to escape from host immune response and can survive.
Viral and host miRs interaction provide a cross talk between two regulatory systems result in infection. So, understand of this novel miR regulatory system can evident therapeutic approach.
The main concern about SARS-CoV-2 pandemic is high viral pathogenicity with no specific drugs that increased mortality rate and threat human health. Moreover, none of previous infection exhibited pandemic health crisis like SARS-CoV-2.12 Although there is a complicated interaction between host cell and viral infection but detection of molecular SARS-CoV-2 pathogenesis help us to find out more new ways of therapeutic approaches. In this review, we investigate the molecular pathogenesis of SARS-CoV-2 that provide by miRs regulatory system as small non-coding RNAs that produce in virus and host cells and then overview the role of miRs in saliva droplet during SARS-CoV-2 infection and related cancers.
The effects of SARS-CoV-2 encoded-miRs on human and viral target genes:
So far, more than 300 viral miRs are detected which affect host and self-gene expression. The first viral encoded-miRs identified in Epstein - Barr virus (EBV) infected cells that regulate gene expression involved in immune response.13
Previous studies demonstrated some viruses encoded miRs that downregulate crucial genes involve in cell process and induce lung infection.14 It was forecasted among 90 mature SARS-CoV-2 encoded miRs that bind to 3′ UTR region of human genes, 40 miRs banded to 73 human genes. Result of gene ontology (GO) analysis showed these genes play role in Notch signaling, DNA endodeoxyribonuclease and deoxyribonuclease activities, cellular response to peptide hormone stimulus and modulation of fatty acid metabolism.15 The mentioned pathways participate in apoptosis process that helps to development of viral infection. The virus applies some strategy to defeat apoptotic defense. For example, cation transport regulator-like protein 1 (CHAC1) gene as proapoptotic enzyme that involve in Notch signaling is target by SARS-CoV-2 encoded miR named as MD2-5p. Another gene, RAD9A -as a checkpoint in cell arrest during DNA damage and repair requirement-interact with BAX and Bcl-2 and targeted by MR147–3p.16 It was demonstrated SARS-CoV-2 miRs silence P53, as an apoptosis inducer, and BMPR2 genes that involve in pathogenesis of respiratory infection.17 Other study predicted 26 mature SARS-CoV-2 miRs affect human genes in apoptosis and also EGF, FGF receptor signaling, angiogenesis and VEGF pathway.18 Likewise, it was shown 30 viral miRs can target 1367 human genes in transcription, metabolism, immune system and some pathway like Wnt and EGFR.19 Some SARS-CoV-2 encoded-miRs affect human genes are listed in Table 1.
Table 1.
List of selected SARS-CoV-2-encoded miRs target host gene.
| miR | Target gene | Up/down Regulation | Tissue | Effect | Ref. |
|---|---|---|---|---|---|
| nCoV-MD3 -3P | p53 | Down | insilico | Subvert the key role of p53 in suppress of viral replication, pulmonary vascular homeostasis and upregulation of IFN I result in respiratory infection | 17 |
| nCoV-MD241–3P | BMPR2 | Down | insilico | Reduce expression of innate immune response genes like IFN I and result in respiratory infection | 17 |
| MD2-5p | CHAC1 | Down | insilico | It interfered with pro-apoptotic enzyme activity and apoptosis process | 15 |
| MR147–3p | RAD9A | Down | insilico | As a checkpoint, it interfered to cell arrest when DNA damaged and need to repaired | 15 |
| MR147–3p | TMPRSS2 | Up | Gut | Promote infection | 24 |
| MR385–3p | TGFBR3 | Up | Innate and adaptive immune cells | It boost Th1 differentiation and modulate regulatory T-cell activation and survival | 46 |
| MR359–5p | MYH9 and ITGB5 | Up | All tissue except heart for MYH9 gene | Cytoskeleton proteins are critical for viral replication and life cycle and help virus for surfing, internalization, and migration within the cell |
15 |
| MR66–3p | TNF-α | Up | Spleen | TNF-α as a key cytokines provides “cytokine storm” during inflammation | 15 |
| MR147–3p | TMPRSS2 | Up | Gut | As a biological membrane interfered with maintain of intestine equilibrium i.e., transporting ions, small molecules, and macromolecules and present gastrointestinal symptoms | 15 |
| MR198–3p | ADAR | Up | Liver | It suppress IFN system responses in viral infections and manifest signs of liver damage | 15 |
| MR328–5p | RXRA | Up | Lung, spleen, gut, liver | It reduced host antiviral effect following IFN-I suppression | 15 |
It was reported SARS-CoV-2 encoded miRs can target 5ʹ UTR of human genes like promoter and enhancer regions and activated gene expression. More upregulation of gene expression is observed in immune response specially chemokine signaling and cytoskeleton related protein. Also, among various tissues that infected by SARS-CoV-2 lung is more gene targeted by viral miRs and spleen and gut go after lung.15 In addition, alignment of SARS-CoV-2 encoded-miRs and human miRs with high similarity showed they are involved in same pathway that affects pathophysiology, inflammation and clinical features.14
Overall, the host gene expression can alter in SARS-CoV-2-infected cells in compare to normal. For instance, among 124 selected genes, upregulation of 104 genes and downregulation of 20 genes were observed and analysis of different pathway detected target genes are involved in inflammation response and manifestation of clinical pathogenesis.14 To prevent negative effects of viral encoded-miRs on host genes, antagomirs showed successful result in phases of clinical trial for viral infection like miR-122 in hepatitis C infection.20
2. The effect of human encoded-miRs on SARS-CoV-2 pathogenesis and infection
Host miRs play pivotal role in progression of viral infection. It was shown cellular miRs act as anti-viral host defense and modulated viral infection. The interaction of host miRs and viral genome result in inhibition of translation or stabilization of viral RNA that prevent from replication. Previous studies suggested human miRs play anti-viral role against viral infection such as HIV-1, HCV, CMV, influenza and dengue.21
One of the challenges is viral escape from host inhibition following several ways: 1) block host miRs function, 2) avoidance to be target by host miRs following own 3′ UTR sequence modification with mutation or length shortness that cannot bind complementary, 3) provide secondary structure with too long 3′ UTR sequence. Another challenge is hijacked host miRs that help virus to modulate cell biological process, also function of target genes affect by hijacked miRs can alter similarly. It was assumed 28 human miRs hijacked by SARS-CoV-2 and affects more than 800 genes. Most of this hijacked human miRs predicted significantly interfering with immune response in COVID-19 patients.15
CDC reported 80% of COVID-19 related death is observed among adult individuals ≥65 years with severe outcome in United States.22 It was predicted one of the probable reason that aged adult present sever COVID-19 or more mortality is miR expression levels go down. It was assumed SARS-CoV-2 can replicate and produce essential particles parallels low overall miR expression in aged individuals similar to previous reports.23
It should be consider some illness like cardiovascular and lung related disease can alter expression of human miR repertoires significantly, so affect SARS-CoV-2 and host cell interaction.24 This is one of the reasons that some people are classified as high risk groups that should be more careful about their self. Some problems with COVID-19 patients like oxygen dependent, ventilation needs and Shortness of breath increase pulmonary hypertension and chronic lung diseases that linked to miR-1307–3p involved in TGF-β signaling. It has been proposed miR-1307 as a therapeutic target in SARS-CoV-2 infection because TGF-β play crucial role in lung development and mentioned diseases.25
It was suggested 3 critical human miRs can apply for COVID-19 therapeutic strategies because they bind complete complementary to SARS-CoV-2 gRNA without any side effect on host genes, they are miRs 5197–3p, 4778–3p and 6864–5p. The miRs 5197–5p was identified especially as more effective on viral infection such as SARS-CoV, MERS-CoV and COVID-19 and also propose for hepatitis B and herpes simplex virus (HSV-1) infections.24 In Table 2 we listed some altered human encoded-miRs expression and related genes affected in SARS-CoV-2 infection in lung.24,25
Table 2.
Human top ranked miRs expression in lung during COVID-19 progression.
| miR | Target gene | Up/down Regulation | Effect |
|---|---|---|---|
| miR-8066 |
-PRLR, CXCL6, IL6, IL17, IL10 and ACVR1 -TGF-β pathway, mucin type O-Glycan and cytokine-cytokine receptor interaction -chemokine binding receptors - FGFR pathway |
Up | -Activate NfKB to target pro-inflammatory cytokines and induce cytokine storm -Increased NfKB mediated TLR-8 expression -Change N-glycosylation patterns -Upregulation of FGFR pathways |
| miR-5197–3p | TGF-β, mucin type O-Glycan and cytokine-cytokine receptor interaction | Up | -Similar to miR-8066 -And also GABAergic synapse, morphine addiction and metabolism of xenobiotics by cytochrome P450 |
| miR-3611 | GABAergic synapse, morphine addiction and metabolism of xenobiotics by cytochrome P450 | Down | -Probably promote viral replication |
| miR-1468–5p | TGF-1 and MAPKs signalling | Up | -Cardiac fibrosis |
| miR-1307–3p | TGF-β and semaphorin signalling | Up | -Promote inflammatory responses |
| miR-3691–3p | TGF-signalling, FGF2 and VCAM1 | Down | -Lung pathogenesis |
| miR-3934–3p | TGFBR1 and SMAD3 | Down | -Affect glycosaminoglycan biosynthesis-heparan sulfate/heparin, other types of O-glycan biosynthesis and vitamin digestion-absorption mechanisms |
3. The role of miRs as saliva biomarker in pathogenesis of viral infection and cancer
Microdroplets are tiny particles (10 μm) carrying the SARS-CoV-2 and can transmit to others via exhale, loud conversation and sneezing.26 As mention above, expression of miRs can alter in different condition like viral infection or cancer. Moreover, miRs are present in liquid body such as saliva, tear, urine and plasma. One of the reasons that is used the plasma from recovered COVID-19 patients is presence of antiviral miRs in addition to antibodies following previous SARS-CoV-2 infection.27 In viral infection, like SARS-CoV-2, profile of miR expression change and investigation of this alternation can obtain by liquid body such as saliva. Saliva collection provides advantage including rapid, non-invasive, stable diagnosis with reliable indication and early diagnosis before signs appear. In addition, an advanced technique named “salivaomics” can evaluate genome, transcriptome, proteome and biomarkers such as miRs in oral related and cancer disease.28 The human miRs can use as biomarkers for viral infection and cancer diagnosis, because they are regulator and impact gene related expression. In Table 3 we mentioned some human miRs target SARS-CoV-2 genome and involve in cancer. In this manner, they can be utilized for prognostic approach. Up to date, some studies approved implement of miRs as biomarkers in head and neck squamous cell carcinomas (HNSCCs) including Oral, hypopharyngeal, esophageal and tongue squamous cell carcinomas.29 We previous reported the validity of saliva biomarkers presence in HNSCC in compare to blood and other tissues.30, 31, 32 The salivary miRs are extricated from normal or tumor cells and also during apoptosis or emitted of exosome or microvesicles.33 They demonstrated high potential utility in disease monitoring. For example, profile of salivary miR expression identified significant decreasing of miR-139 in tongue SCC in compare to control group. Furthermore, the levels of miR-139 expression return to normal after surgery and indicated miR-139 play tumor suppressor role with biomarker potential.34 Interestingly, outcome of a diagnostic validity study demonstrated 93% overall agreement in saliva specimen in compare to nasopharyngeal aspirate in detection of viral respiratory infection like SARS-CoV-2.35
Table 3.
List of selected human miRs target SARS-CoV-2 genome and involve in cancer progression.
| miR | Effect | Ref. |
|---|---|---|
| miR-197–5p | Cardiovascular disease | 47 |
| miR-338–3p | Liver, lung and gastric cancers | 48, 49, 50 |
| miR-4778–3p | Cervical cancer radioresistance | 51,52 |
| miR-6864–5p | Urothelial Carcinoma of the Bladder | 51,53 |
| miR-5197–3p | Squamous cell lung carcinoma | 51,54 |
| miR-15b-5p | Coronary Artery Disease | 55 |
| miR-15a-5p | Kidney disease | 56 |
| miR-548c-5p | Colorectal Cancer | 57 |
| miR-548d-3p | Osteosarcoma | 58 |
| miR-409–3p | Osteosarcoma | 59 |
| miR-30b-5p | Esophageal squamous cell carcinoma Diabetic retinopathy |
60,61 |
| miR-505–3p | Prostate cancer | 62 |
| miR-520c-3p | Obesity/diabetes | 63 |
| miR-30e-3p | Myocardial Injury | 64 |
| miR-23c | Hepatocellular carcinoma | 65 |
| miR-30d-5p | Non-small cell lung cancer | 66 |
| miR-4684–3p | Colorectal cancer | 67 |
| miR-518a-5p | Gastrointestinal tumors | 68 |
| miR-5197 | Non-small cell lung cancer (NSCLC), HTLV-1, HIV-1, Ebola | 69, 70, 71, 72 |
| miR-3611 | Chronic obstructive pulmonary disease (COPD) HIV-1 infection |
73 |
| miR-3934 | Colon cancer, lung cancer, NSCLC, rectal carcinoma mucosa | 74, 75, 76 |
| miR-1307 | Severity of pulmonary hypertension in systemic scleroderma | 77 |
| miR-3691–3p | Chronic obstructive pulmonary disease | 78 |
| miR1468–5p | Glioma, hepatocellular carcinoma, Alzheimer’s disease | 79, 80, 81 |
4. Discussion
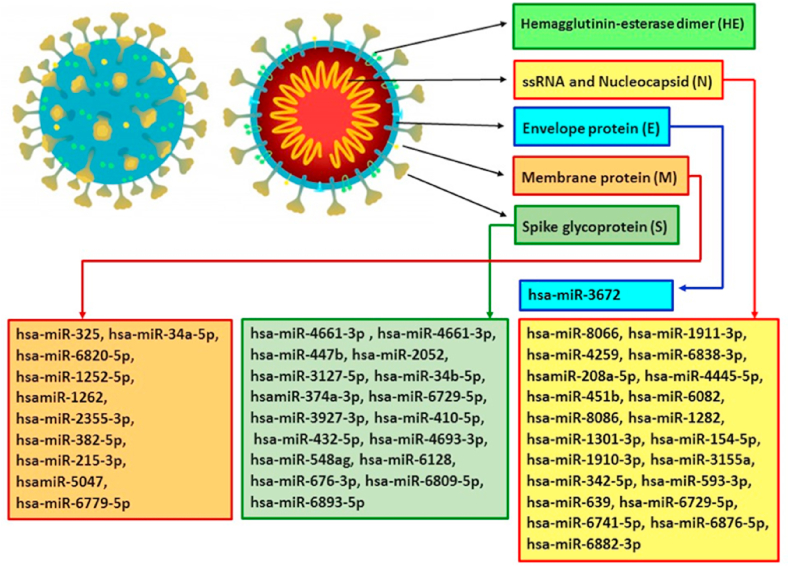
Current evidence approved microRNAs affect regulation of viral gene expression to restrict infection. In this review, we studied the challenge and opportunities of miRs as smallest non-coding RNAs in SARS-CoV-2 infection. The dual role of host miRs cannot be declined, they can play role as antiviral regulatory factors and also interact with viral miRs that benefice for virus replication and propagation. Based on insilico analysis seems first function is more possible, but in vitro and in vivo studies are needed to validate obtained data.23 It was detected difference of gene expression between healthy and SARS-CoV-2 infected lung cells associated with regulatory role of miRs in some crucial pathways, like as TGF-β. Alternation in TGF-β pathway stimulates host response as cytokine storm and affect immune defense, and also cause clinical features.36 Increasing of host miRs to target SARS-CoV-2 genes like S, M, N, E and ORF1ab will be obstruct viral entry and replication. In Fig. 1 we mentioned some human miRs target SARS-CoV-2 genome that product structural protein. As well, reduction of host miRs provides environment for more viral replication and accessibility for the host immune system. So, modification of host-miR levels alters specific cellular processes that are crucial for control of infection.19 Therapeutic approach based on replacement of mimic miRs can apply to increase low host miR levels to suppress viral infection or take modified miRs products that cannot be degraded and uptake from the cells effectively.37 Previous studies indicated intranasal inhalation of mimic miRs protect animal from viral respiratory infection.38,39 Target delivery with least toxicity is crucial in miR-based therapeutic strategies. It should be considered over expression/inhibition of miRs might result in cell cycle irregularity, impaired immune response or cancer. In this manner, exact role of each miR should be specified.40
Fig. 1.
Selected human miRs target SARS-CoV-2 genome that product structural protein. S protein: bind to host cell receptor, consist of two subunits; S1 define virus-host range and cellular tropism, S2 modulates virus-cell membrane fusion. E protein: form hydrophilic pores on host membranes in addition to viral recognition and pathogenesis. M protein: Three transmembrane proteins bind to nucleucapsid. N protein: Produce two domains that binding to viral RNA genome.
Although result of genome analysis estimated ~78.7% sequence homology between SARS-CoV and COVID-19,41 but target of host miRs for both viral genomes can significantly be difference. Recent study demonstrated 848 and 873 human miRs target SARS and COVID-19 genomes respectively that only 588 common human miRs target both viral sequences. Also, they estimated only 315 miRs were unique for isolated COVID-19 that obtained from different geographical region.23 Recent studies evaluated SARS-CoV-2 strain mutations in different geographical region and find out viral genome get more mutated. Mutations mediate host immune response and affect virus survival with more pathogenicity.24 Furthermore, evolution of SARS-CoV-2 genome helps virus to overcome host antiviral response special miRs as essential regulatory molecular pathway. On the other hand, virus can get advantage of host miRs to suppress own replication to escape from immune response but promote its transmission stronger. After successful transmission, the virus can rapidly mutate own strains to elevate host specificity to avoid host miRs interaction following RNA polymerase activity without proofreading.21 Altogether 810 mutations are discovered in SARS-CoV-2 genome consists of 646 SNPs, 19 indels, 145 deletions and also 33 sequence of pre-miRs overlapped with these mutations. And also, more than half mutation was predicated synonym and most of them were substitution of C/G to U. Outcome of these variations might attenuate phenotype of SARS-CoV-2 with reducing viral miRs repertories and affect miR-binding site and their regulatory role in defense mechanism.15 Detection of new SARS-CoV-2 mutations should be considered because genetic variation in SARS-CoV-2 genome prevent from vaccine development.42 Similarly, this virus is undergoing evolution, natural selection effect and purifying selection,43 so detection and evaluation of exact molecular pathogenesis open up opportunities to apply best therapeutic approaches. There are challenges in modification of host miR levels or replacement of mimic miRs that should be considered their side effects. Selected miRs should be effective, safe and well tolerated by patients. Moreover, design and products of mimic miRs are inexpensive corresponds to synthesis of primer sequence, they are short and nanoscale in size that can deliver by exosomes.44 The protein-protein interaction network between human and SARS-CoV-2 was identified by affinity-purification mass spectrometry, the outcome of this study can help us to focus on miRs that target translation inhibitors and sigma 1, 2 receptors.45
5. Conclusion
The effects of miR based therapeutic approaches approved in viral infection. Alternation in human miRs expression including overexpression or mimic replacement, inhibition or suppression helps to block viral entry or replication in host cells. Target SARS-CoV-2 genes such as S, M, N, E and ORF1ab by increasing host miRs attenuate viral latency. In contrast, decreasing in human miRs against SARS-CoV-2 infection provide more viral replication and accessibility for immune system. The crucial challenge for selection of candidate human miRs against SARS-CoV-2 infection is the side effects following alternation of gene. More investigation assists to least the side effects. On the other hand, the impact of SARS-CoV-2 encoded-miRs on host genes should not be ignored. As a solution, potential therapeutic of antagomirs as the small molecular inhibitors can be evaluated for SARS-CoV-2 infection. The stability, effective delivery, higher binding affinity and uptake with less degradation by nuclease are the common problem in target miRs therapy process. The SARS-CoV-2 genome sequence discovery and its alignment with the other correlated Coronaviridae family help us to help from previous therapeutic approaches. Salivaomics present high potential utility in detection of biomarker such as miRs; it can help in vaccine development for SARS-CoV-2.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
References
- 1.Xia X. Extreme genomic CpG deficiency in SARS-CoV-2 and evasion of host antiviral defense. Mol. Biol. Evol. 2020;37(9):2699–2705. doi: 10.1093/molbev/msaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou M., Zhang X., Qu J. Coronavirus disease 2019 (COVID-19): a clinical update. Front Med. 2020;14:126–135. doi: 10.1007/s11684-020-0767-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tort F.L., Castells M., Cristina J. A comprehensive analysis of genome composition and codon usage patterns of emerging coronaviruses. Virus Res. 2020;283:197976. doi: 10.1016/j.virusres.2020.197976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim D., Lee J.-Y., Yang J.-S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181(4):914–921. doi: 10.1016/j.cell.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khrustalev V.V., Giri R., Khrustaleva T.A., Kapuganti S.K., Stojarov A.N., Poboinev V.V. bioRxiv; 2020. Translation-associated Mutational U-Pressure in the First ORF of SARS-CoV-2 and Other Coronaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He L., Hannon G.J. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W., Zhao Y., Zhang F. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girardi E., López P., Pfeffer S. On the importance of host MicroRNAs during viral infection. Front Genet. 2018;9:439. doi: 10.3389/fgene.2018.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Rohaimi A.H., Al Otaibi F. Genes & Diseases; 2020. Novel SARS-CoV-2 Outbreak and COVID19 Disease; a Systemic Review on the Global Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang M., Yu F., Wu W., Wang Y., Ding H., Qian L. Epstein-Barr virus-encoded microRNAs as regulators in host immune responses. Int J Biol Sci. 2018;14:565–576. doi: 10.7150/ijbs.24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen B.R. MicroRNAs as mediators of viral evasion of the immune system. Nat Immunol. 2013;14:205–210. doi: 10.1038/ni.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Wang J, Xu Y, et al. Implications of the Virus-Encoded miRNA and Host miRNA in the Pathogenicity of SARS-CoV-2. arXiv preprint arXiv:200404874 2020.
- 16.Guo X., Huang Y., Qi Y. Human cytomegalovirus miR-UL36-5p inhibits apoptosis via downregulation of adenine nucleotide translocator 3 in cultured cells. Arch Virol. 2015;160:2483–2490. doi: 10.1007/s00705-015-2498-8. [DOI] [PubMed] [Google Scholar]
- 17.Saini S., Saini A., Thakur C.J., Kumar V., Gupta R.D., Sharma J. Genome-wide computational prediction of miRNAs in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) revealed target genes involved in pulmonary vasculature and antiviral innate immunity. Mol Biol Res Commun. 2020;9:83–91. doi: 10.22099/mbrc.2020.36507.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andruska A., Spiekerkoetter E. Consequences of BMPR2 deficiency in the pulmonary vasculature and beyond: contributions to pulmonary arterial hypertension. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morales L., Oliveros J.C., Fernandez-Delgado R., tenOever B.R., Enjuanes L., Sola I. SARS-CoV-Encoded small RNAs contribute to infection-associated lung pathology. Cell Host Microbe. 2017;21:344–355. doi: 10.1016/j.chom.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Ree M.H., de Vree J.M., Stelma F. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: a phase 1B, double-blind, randomised controlled trial. Lancet. 2017;389:709–717. doi: 10.1016/S0140-6736(16)31715-9. [DOI] [PubMed] [Google Scholar]
- 21.Trobaugh D.W., Klimstra W.B. MicroRNA regulation of RNA virus replication and pathogenesis. Trends Mol Med. 2017;23:80–93. doi: 10.1016/j.molmed.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Team C.C.-R. Morbidity and Mortality Weekly Report; 2020. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) — United States, February 12–March 16, 2020; pp. 343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fulzele S., Sahay B., Yusufu I. COVID-19 virulence in aged patients might Be impacted by the host cellular MicroRNAs abundance/profile. Aging Dis. 2020;11:509–522. doi: 10.14336/AD.2020.0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arisan E.D., Dart A., Grant G.H. The prediction of miRNAs in SARS-CoV-2 genomes: hsa-miR databases identify 7 key miRs linked to host responses and virus pathogenicity-related KEGG pathways significant for comorbidities. Viruses. 2020;12 doi: 10.3390/v12060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao B., Zhou M.X., Zhou F.K. Exosome-Derived MiRNAs as biomarkers of the development and progression of intracranial aneurysms. J Atherosclerosis Thromb. 2020;27:545–610. doi: 10.5551/jat.51102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ningthoujam R. COVID 19 can spread through breathing, talking, study estimates. Curr Med Res Pract. 2020;10:132–133. doi: 10.1016/j.cmrp.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szelenberger R., Kacprzak M., Saluk-Bijak J., Zielinska M., Bijak M. Plasma MicroRNA as a novel diagnostic. Clin Chim Acta. 2019;499:98–107. doi: 10.1016/j.cca.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Sri Santosh T., Parmar R., Anand H., Srikanth K., Saritha M. A review of salivary diagnostics and its potential implication in detection of covid-19. Cureus. 2020;12 doi: 10.7759/cureus.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koshizuka K., Hanazawa T., Fukumoto I., Kikkawa N., Okamoto Y., Seki N. The microRNA signatures: aberrantly expressed microRNAs in head and neck squamous cell carcinoma. J Hum Genet. 2017;62:3–13. doi: 10.1038/jhg.2016.105. [DOI] [PubMed] [Google Scholar]
- 30.Mohajertehran F., Ayatollahi H., Khazaeni K., Shakeri M.T., Mohtasham N. Overexpression of high-mobility motor box 1 in the blood and tissues of patients with head and neck squamous cell carcinoma. Iran J Otorhinolaryngol. 2018;30:261–271. [PMC free article] [PubMed] [Google Scholar]
- 31.Mohajertehran F., Ayatollahi H., Jafarian A.H. Overexpression of lactate dehydrogenase in the saliva and tissues of patients with head and neck squamous cell carcinoma. Rep Biochem Mol Biol. 2019;7:142–149. [PMC free article] [PubMed] [Google Scholar]
- 32.Mohajertehran F., Sahebkar A., Zare R., Mohtasham N. The promise of stem cell markers in the diagnosis and therapy of epithelial dysplasia and oral squamous cell carcinoma. J Cell Physiol. 2018;233:8499–8507. doi: 10.1002/jcp.26789. [DOI] [PubMed] [Google Scholar]
- 33.Rapado-González Ó. Majem B., Muinelo-Romay L. Human salivary microRNAs in Cancer. J Canc. 2018;9:638–649. doi: 10.7150/jca.21180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu R., Yang M., Meng Y. Tumor-suppressive function of miR-139-5p in esophageal squamous cell carcinoma. PloS One. 2013;8 doi: 10.1371/journal.pone.0077068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.To K.K.W., Yip C.C.Y., Lai C.Y.W. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect. 2019;25:372–378. doi: 10.1016/j.cmi.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Zhao X., Nicholls J.M., Chen Y.G. Severe acute respiratory syndrome-associated coronavirus nucleocapsid protein interacts with Smad3 and modulates transforming growth factor-beta signaling. J Biol Chem. 2008;283:3272–3280. doi: 10.1074/jbc.M708033200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls. Treasure Island FL: © 2020. StatPearls Publishing LLC.; 2020. Features, evaluation and treatment coronavirus (COVID-19) [PubMed] [Google Scholar]
- 38.Peng S., Wang J., Wei S. Endogenous cellular MicroRNAs mediate antiviral defense against influenza A virus. Mol Ther Nucleic Acids. 2018;10:361–375. doi: 10.1016/j.omtn.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L., Gao F., Jiang Y. Cellular miR-130b inhibits replication of porcine reproductive and respiratory syndrome virus in vitro and in vivo. Sci Rep. 2015;5:17010. doi: 10.1038/srep17010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tahamtan A., Inchley C.S., Marzban M. The role of microRNAs in respiratory viral infection: friend or foe? Rev Med Virol. 2016;26:389–407. doi: 10.1002/rmv.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang C., Liu Z., Chen Z. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J Med Virol. 2020;92:667–674. doi: 10.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang S. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature. 2020;579:321. doi: 10.1038/d41586-020-00751-9. [DOI] [PubMed] [Google Scholar]
- 43.Li X., Giorgi E.E., Marichannegowda M.H. Emergence of SARS-CoV-2 through recombination and strong purifying selection. Sci Adv. 2020 doi: 10.1126/sciadv.abb9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z., Dombroski J.A., King M.R. Engineering of exosomes to target cancer metastasis. Cell Mol Bioeng. 2020;13:1–16. doi: 10.1007/s12195-019-00607-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gordon D.E., Jang G.M., Bouhaddou M. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Panhuys N. TCR signal strength alters T–DC activation and interaction times and directs the outcome of differentiation. Front Immunol. 2016;7 doi: 10.3389/fimmu.2016.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosseini Rad Sm A., McLellan A.D. Implications of SARS-CoV-2 mutations for genomic RNA structure and host microRNA targeting. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21134807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu X., Tan D., Hou Z. The effect of miR-338-3p on HBx deletion-mutant (HBx-d382) mediated liver-cell proliferation through CyclinD1 regulation. PloS One. 2012;7 doi: 10.1371/journal.pone.0043204. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Liang Y., Xu X., Wang T. The EGFR/miR-338-3p/EYA2 axis controls breast tumor growth and lung metastasis. Cell Death Dis. 2017;8:e2928. doi: 10.1038/cddis.2017.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li P., Chen X., Su L. Epigenetic silencing of miR-338-3p contributes to tumorigenicity in gastric cancer by targeting SSX2IP. PloS One. 2013;8 doi: 10.1371/journal.pone.0066782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ivashchenko A., Rakhmetullina A., Aisina D. 2020. How miRNAs Can Protect Humans from Coronaviruses COVID-19, SARS-CoV, and MERS-CoV. [Google Scholar]
- 52.Zhang Y., Li P., Hu J. Role and mechanism of miR-4778-3p and its targets NR2C2 and Med19 in cervical cancer radioresistance. Biochem Biophys Res Commun. 2019;508:210–216. doi: 10.1016/j.bbrc.2018.11.110. [DOI] [PubMed] [Google Scholar]
- 53.Inamoto T., Uehara H., Akao Y. A panel of MicroRNA signature as a tool for predicting survival of patients with urothelial carcinoma of the bladder. Dis Markers. 2018 doi: 10.1155/2018/5468672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu J., Shu Y., Xu T. Microarray expression profiling and bioinformatics analysis of circular RNA expression in lung squamous cell carcinoma. Am J Transl Res. 2018;10:771–783. [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu L.P., Zhou J.P., Zhang J.X. MiR-15b-5p regulates collateral artery formation by targeting AKT3 (protein kinase B-3) Arterioscler Thromb Vasc Biol. 2017;37:957–968. doi: 10.1161/ATVBAHA.116.308905. [DOI] [PubMed] [Google Scholar]
- 56.Shang J., He Q., Chen Y. miR-15a-5p suppresses inflammation and fibrosis of peritoneal mesothelial cells induced by peritoneal dialysis via targeting VEGFA. J Cell Physiol. 2019;234:9746–9755. doi: 10.1002/jcp.27660. [DOI] [PubMed] [Google Scholar]
- 57.Peng Z.Y., Gu R.H., Yan B. Downregulation of exosome-encapsulated miR-548c-5p is associated with poor prognosis in colorectal cancer. J Cell Biochem. 2018;120(2):1457–1463. doi: 10.1002/jcb.27291. [DOI] [PubMed] [Google Scholar]
- 58.Chen J., Yan C., Yu H., Zhen S., Yuan Q. miR-548d-3p inhibits osteosarcoma by downregulating KRAS. Aging (N Y) 2019;11:5058–5069. doi: 10.18632/aging.102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu L., Zhang Y., Huang Z. MiR-409-3p inhibits cell proliferation and invasion of osteosarcoma by targeting zinc-finger E-box-binding homeobox-1. Front Pharmacol. 2019;10:137. doi: 10.3389/fphar.2019.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J., Lv H., Zhang B. miR-30b-5p acts as a tumor suppressor microRNA in esophageal squamous cell carcinoma. J Thorac Dis. 2019;11:3015–3029. doi: 10.21037/jtd.2019.07.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazzeo A., Lopatina T., Gai C., Trento M., Porta M., Beltramo E. Functional analysis of miR-21-3p, miR-30b-5p and miR-150-5p shuttled by extracellular vesicles from diabetic subjects reveals their association with diabetic retinopathy. Exp Eye Res. 2019;184:56–63. doi: 10.1016/j.exer.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 62.Tang Y., Wu B., Huang S. Downregulation of miR-505-3p predicts poor bone metastasis-free survival in prostate cancer. Oncol Rep. 2019;41:57–66. doi: 10.3892/or.2018.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ortega F.J., Mercader J.M., Catalán V. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59:781–792. doi: 10.1373/clinchem.2012.195776. [DOI] [PubMed] [Google Scholar]
- 64.Wang X.T., Wu X.D., Lu Y.X. Potential involvement of MiR-30e-3p in myocardial injury induced by coronary microembolization via autophagy activation. Cell Physiol Biochem. 2017;44:1995–2004. doi: 10.1159/000485905. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L., Wang Y., Wang L. miR-23c suppresses tumor growth of human hepatocellular carcinoma by attenuating ERBB2IP. Biomed Pharmacother. 2018;107:424–432. doi: 10.1016/j.biopha.2018.07.155. [DOI] [PubMed] [Google Scholar]
- 66.Gao L., He R.Q., Wu H.Y. Expression signature and role of miR-30d-5p in non-small cell lung cancer: a comprehensive study based on in silico analysis of public databases and in vitro experiments. Cell Physiol Biochem. 2018;50:1964–1987. doi: 10.1159/000494875. [DOI] [PubMed] [Google Scholar]
- 67.Wu X., Li S., Xu X. The potential value of miR-1 and miR-374b as biomarkers for colorectal cancer. Int J Clin Exp Pathol. 2015;8:2840–2851. [PMC free article] [PubMed] [Google Scholar]
- 68.Shi Y., Gao X., Hu Q. PIK3C2A is a gene-specific target of microRNA-518a-5p in imatinib mesylate-resistant gastrointestinal stromal tumor. Lab Invest. 2016;96:652–660. doi: 10.1038/labinvest.2015.157. [DOI] [PubMed] [Google Scholar]
- 69.Iwuchukwu I., Nguyen D., Beavers M. MicroRNA regulatory network as biomarkers of late seizure in patients with spontaneous intracerebral hemorrhage. Mol Neurobiol. 2020:1–12. doi: 10.1007/s12035-020-01872-y. [DOI] [PubMed] [Google Scholar]
- 70.Michlewski G., Cáceres J.F. Post-transcriptional control of miRNA biogenesis. RNA. 2019;25:1–16. doi: 10.1261/rna.068692.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazurov D., Ilinskaya A., Heidecker G., Filatov A. Role of O-glycosylation and expression of CD43 and CD45 on the surfaces of effector T cells in human T cell leukemia virus type 1 cell-to-cell infection. J Virol. 2012;86:2447–2458. doi: 10.1128/JVI.06993-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon E.J., Linstedt A.D. Site-specific glycosylation of Ebola virus glycoprotein by human polypeptide GalNAc-transferase 1 induces cell adhesion defects. J Biol Chem. 2018;293:19866–19873. doi: 10.1074/jbc.RA118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yerukala Sathipati S., Ho S.Y. Identifying the miRNA signature associated with survival time in patients with lung adenocarcinoma using miRNA expression profiles. Sci Rep. 2017;7:7507. doi: 10.1038/s41598-017-07739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hiew M.S.Y., Cheng H.P., Huang C.-J. Incomplete cellular reprogramming of colorectal cancer cells elicits an epithelial/mesenchymal hybrid phenotype. J Biomed Sci. 2018;25:57. doi: 10.1186/s12929-018-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chung K.F. Drugs to suppress cough. Expet Opin Invest Drugs. 2005;14:19–27. doi: 10.1517/13543784.14.1.19. [DOI] [PubMed] [Google Scholar]
- 77.Ruan D.T., Gao S., Shelat H., King B., Geng Y.-J. Differential expression of microRNA and arachidonic acid metabolism in aspirin-treated human cardiac and peri-cardiac fat-derived mesenchymal stem cells. Vasc Pharmacol. 2020:106651. doi: 10.1016/j.vph.2020.106651. [DOI] [PubMed] [Google Scholar]
- 78.Morty R.E., Königshoff M., Eickelberg O. Transforming growth factor-beta signaling across ages: from distorted lung development to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:607–613. doi: 10.1513/pats.200908-087RM. [DOI] [PubMed] [Google Scholar]
- 79.Erener S., Marwaha A., Tan R., Panagiotopoulos C., Kieffer T.J. Profiling of circulating microRNAs in children with recent onset of type 1 diabetes. JCI Insight. 2017;2 doi: 10.1172/jci.insight.89656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang K., Zhi T., Xu W. MicroRNA-1468-5p inhibits glioma cell proliferation and induces cell cycle arrest by targeting RRM1. Am J Cancer Res. 2017;7:784–800. [PMC free article] [PubMed] [Google Scholar]
- 81.Liu F., Zhao H., Gong L., Yao L., Li Y., Zhang W. MicroRNA-129-3p functions as a tumor suppressor in serous ovarian cancer by targeting BZW1. Int J Clin Exp Pathol. 2018;11:5901–5908. [PMC free article] [PubMed] [Google Scholar]