Abstract
Background
The dynamics of urinary trace elements in patients with COVID-19 still remains to be investigated.
Methods
A retrospective study was performed on a cohort of 138 confirmed COVID-19 patients for their urinary levels of essential and/or toxic metals including chromium, manganese, copper, arsenic, selenium, cadmium, mercury, thallium and lead according to the different disease severity (severe or non-severe) and outcome (recovered or deceased).
Results
Urinary concentrations of chromium, manganese, copper, selenium, cadmium, mercury and lead after creatinine adjustment were found to be higher in severe patients than the non-severe cases with COVID-19. And among the severe cases, these elements were also higher in the deceased group than the recovered group. When the weeks of the post-symptom onset were taken in account, the changes of these urinary elements were existed across the clinical course since the disease onset. These urinary elements were found to be mostly positively inter-correlated, and further positively correlated with other laboratory inflammatory parameters including serum cytokines (IL-1B, IL2R, IL6, IL8, IL10, TNFα), ferritin, and neutrophil count and white blood cell count. As a independently predictive factor, urinary creatinine-adjusted copper of ≥25.57 μg/g and ≥99.32 μg/g were associated with significantly increased risk of severe illness and fatal outcome in COVID-19, respectively.
Conclusions
These results suggest abnormities in urinary levels of the trace metals were tightly associated with the severe illness and fatal outcome of COVID-19.
Keywords: COVID-19, Trace elements, Urine, Severity, Outcome
1. Introduction
The outbreak of coronavirus disease 2019 (COVID-19) that caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is evolving rapidly worldwide (Zhu et al., 2020). Since December 2019, there were more than 7.7 million confirmed cases and more than 420 000 deaths reported globally, as of June 13, 2020. WHO declared this outbreak a pandemic on March 11, 2020.
It is now undeniable that the managed care of patients with COVID-19 encompasses the identification of clinical and laboratory parameters, enabling accurate risk stratification of progressing toward severe or critical disease (Bonetti, 2020). Urinary tract involvement is commonplace in patients with COVID-19, and that progressive deterioration of renal function shall be considered an unfavorable prognostic factor (Henry and Lippi, 2020). The clinical significance of urinalysis for predicting the severity of coronavirus disease 2019 (COVID-19) has been commonly reported recently (Bonetti, 2020; Liu, 2020). However, the urinary levels of trace metals in patients with COVID-19 still remains to be investigated.
The main trace elements of general concerns are the most toxic ones such as arsenic (As), cadmium (Cd), lead (Pb), mercury (Hg), thallium (Tl) and chromium (Cr) (toxicity depends on the Cr species), and the essential elements like copper (Cu), manganese (Mn) copper and selenium (Se), which are required in certain amounts as they have important biological functions (Callan et al., 2015). Many toxic trace elements are excreted via kidneys, and long term exposure to those heavy metals may lead to their accumulation in the kidney, induce a tubular impairment and renal injury (Zeng et al., 2019). Even low dose of these exposures could resulted in a potential renal toxicity and reduced glomerular filtration rate (GFR) (Wu et al., 2018).
In the present study, we are aimed to perform a retrospective analysis of patients with COVID-19 for their urinary levels of essential and/or toxic metals including chromium, manganese, copper, arsenic, selenium, cadmium, mercury, thallium and lead according to the severity of the disease (severe or severe) and outcomes (recovered or deceased), respectively. This study of urinary trace elements would be helpful in the evaluation of the dynamic changes in patients with COVID-19.
2. Materials and methods
2.1. Ethnics statements
This study was approved by the Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology in Wuhan, China (No.:TJ-IRB20200201). All the procedures involving human samples conformed to the principles outlined in the Declaration of Helsinki. Participation was voluntary and informed consent was obtained in all cases. All the analysis were performed on existing samples collected during standard diagnostic tests, posing no extra burden to patients.
2.2. Patients
The retrospective cohort study was performed in a designated hospital for COVID-19 treatment in Tongji Hospital of Huazhong University of Science and Technology, Wuhan, Hubei Province. A total of 138 patients with confirmed COVID-19 admitted to the hospital were enrolled into the study. All the patients with COVID-19 was diagnosed and categorized into severe and non-severe cases according to the Guidelines of the Diagnosis and Treatment of New Coronavirus Pneumonia published by the National Health Commission of China (National Health Commissio, 2020). Patients who had epidemiology history, clinical manifestations that mimic COVID-19 were diagnosed after examination of SARS-CoV-2 RNA by real time polymerase chain reaction (RT-PCR) and chest computed tomography (CT) scanning.
The clinical classification were briefly described as below: (1) Mild cases: Mild symptoms without sign of pneumonia on imaging. (2) Moderate cases: Fever and respiratory symptoms with radiological findings of pneumonia. (3) Severe cases: Respiratory distress (≧30 breaths/min), or Oxygen saturation≤93% at rest, or Arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≦ 300 mmHg, or Lesion progression within 24–48 h > 50% by chest imaging. (4) Critical cases: Respiratory failure and requiring mechanical ventilation, or Shock, or With other organ failure that requires ICU care. In the present study, The mild and moderate cases were collectively referred to as non-severe cases, while the severe and critical cases were collectively referred to as severe cases.
All the recovered patients with COVID-19 had completely resolved symptoms and signs, had significant improvement in pulmonary and extrapulmonary organ dysfunction, and no longer needed supportive care, with confirmed viral clearance by repeated tests for SARS-COV-2 before hospital discharge. All the patients had determined outcomes, and the characteristics were summarized in Table 1 .
Table 1.
Demographic and clinical characteristics of the patients with COVID-19.
| Elements | All patients | Non-severe | Severe | p |
|---|---|---|---|---|
| Age, median [IQR], y | 61.5 (55–68) | 60 (52–65) | 65 (56–72) | <0.001 |
| Sex | 0.4075 | |||
| Male | 79 (57.2%) | 38 (54.3%) | 41 (60.3%) | |
| Female | 59 (42.8%) | 32 (45.7%) | 27 (39.7%) | |
| Outcome | <0.001 | |||
| Recovered | 117 (84.8%) | 70 (100.0%) | 47 (69.1%) | |
| Deceased | 21 (15.2%) | 0 (0.0%) | 21 (30.9%) | |
| Length of stay (IQR), d | 14.5 (8–32) | 8 (6–11) | 31 (18–52) | <0.001 |
| Onset to admission (IQR), d | 26 (11–41) | 40 (28–44) | 13 (8–21) | <0.001 |
| Initial symptoms | ||||
| Fever | 99 (71.7%) | 52 (74.3%) | 47 (69.1%) | 0.500 |
| Cough | 84 (60.9%) | 42 (60.0%) | 42 (61.8%) | 0.832 |
| Feeble | 26 (18.8%) | 12 (17.1%) | 14 (20.6%) | 0.605 |
| Chest tightness | 19 (13.8%) | 11 (15.7%) | 8 (11.8%) | 0.501 |
| Shortness of breath | 37 (26.8%) | 10 (14.3%) | 27 (39.7%) | <0.001 |
| Diarrhea | 27 (19.6%) | 12 (17.1%) | 15 (22.1%) | 0.467 |
| Comorbidities | ||||
| Hypertension | 50 (36.2%) | 19 (27.1%) | 31 (45.6%) | 0.024 |
| Cardiovascular disease | 12 (8.7%) | 4 (5.7%) | 8 (11.8%) | 0.207 |
| Diabetes | 24 (17.4) | 9 (12.9%) | 15 (22.1%) | 0.154 |
| Malignancy | 4 (2.9%) | 1 (1.4%) | 3 (4.4%) | 0.296 |
| Cerebrovascular disease | 10 (7.2) | 3 (4.3%) | 7 (10.3%) | 0.173 |
| None | 46 (33.3%) | 29 (41.4%) | 13 (19.1%) | 0.004 |
| Laboratory parameters | ||||
| Creatinine, plasma, μmol/L | 64.5 (50–83.25) | 70 (58.75–80) | 59 (47–87) | 0.045 |
| eGFR, ml/min/1.73 m2 | 93.7 (77–103.1) | 94.25 (84.53–101.43) | 93.1 (65.4–103.4) | 0.272 |
| Ferritin, plasma, μg/L | 592.7 (261.7–1372.98) | 314.7 (192.8–480.1) | 1263.8 (616.8–2103.2) | <0.001 |
| Cytokines, serum | ||||
| IL1B, pg/ml | <5.0 (<5.0–6.55) | 2.5 (2.5–2.5) | 2.5 (2.5–7.93) | 0.023 |
| IL2R, U/ml | 423 (294.75–877.25) | 339.5 (207.5–440.25) | 659.5 (349.75–1103.75) | <0.001 |
| IL6, pg/ml | 8.87 (2.18–46.89) | 2.34 (0.75–6.05) | 32.09 (7.00–146.43) | <0.001 |
| IL8, pg/ml | 15.85 (7.43–30.78) | 10.15 (6.68–19.58) | 18.45 (9.43–47.05) | <0.001 |
| IL10, pg/ml | <5.0 (<5.0–7.8) | <5.0 (<5.0 - <5.0) | 6.2 (<5.0–11.7) | <0.001 |
| TNFα, pg/ml | 8.15 (5.85–12.6) | 7.3 (5.1–9.4) | 10.3 (6.05–16.2) | 0.001 |
| Whole blood cell counts | ||||
| Lymphocyte, × 10⁹/L | 1.07 (0.63–1.72) | 1.64 (1.32–2.18) | 0.78 (0.45–1.13) | <0.001 |
| Monocyte, × 10⁹/L | 0.5 (0.39–0.69) | 0.52 (0.45–0.66) | 0.48 (0.32–0.7) | 0.076 |
| Neutrophil, × 10⁹/L | 4.81 (3.25–8.67) | 3.25 (2.39–4.45) | 7.43 (4.32–11.12) | <0.001 |
| WBC, × 10⁹/L | 7.33 (5.19–10.22) | 5.64 (4.62–7.63) | 9.17 (5.78–12.83) | <0.001 |
2.3. Data collection
A medical record review was performed to collect the information on demographic characteristics (age, gender, preexisting comorbidities, the date of symptoms onset), clinical symptoms and signs, laboratory test results (lymphocyte, monocyte, neutrophil and white blood cell count (WBC), serum cytokines (IL-1B, IL2R, IL6, IL8, IL10, TNFα), plasma ferritin, plasma creatinine, eGFR, urinary analysis) that were measured on the same day or adjacent day that the urine specimen for trace elemental analysis was collected. The outcomes were obtained from electronic medical records for the final analysis, which included recovered/discharged and deceased.
2.4. Sample preparation and instrumental analysis
For trace elemental analysis, a total of 210 early morning urine (most concentrated) were collected from the 138 COVID-19 patients. Among them, 36 patients had at least two urine specimens at different time points. Specimens were collected in the 15 ml polystyrene urine collection bottles (sterile). After collection, specimens were taken to the laboratory within 2 h. All measurements were carried out in the Department of laboratory medicine, Tongji hospital of Tongji Medical College in Huazhong University of Science & Technology, with a quadruple inductively coupled plasma mass spectrometer (ICP-MS) equipped with a concentric glass nebulizer and a cyclonic spray chamber ((7700x ICP-MS system, Agilent Technologies, USA). Analyses were performed in standard mode and dynamic collision cell mode. For Chromium (Cr), Manganese (Mn), Copper (Cu), Arsenic (As) and Selenium (Se), the assays were run in dynamic collision cell mode (collision cell gas: helium, >99.995%). For Cadmium (Cd), Mercury (Hg), Thallium (Tl) and Lead (Pb), the assays were run in standard mode.
The preparations of calibration standards, the detailed ICP-MS operating conditions and the limit of quantification (LoQ) were described in our previous report (Zeng et al., 2019). The LoQ was determined as 0.24 (Cr), 0.53 (Mn), 0.42 (Cu), 0.13 (As), 1.20 (Se), 0.04 (Cd), 0.72 (Hg), 0.06 (Tl) and 0.05 (Pb) μg/L, respectively. Urine samples (400 μl) were diluted 1:10 (v/v) with an dilution solution containing 0.1% (v/v) Triton X-100 (Sigma-Aldrich, France) and 0.1% nitric acid (≥69%, Merck, Germany), and then mixed sufficiently using a table-top vortexes for 1 min. The samples were then subjected to ICP-MS analysis.
2.5. Quality control and quality assurance
The department of laboratory medicine of Tongji hospital is a ISO15189 accredited laboratory, and also certified by College of American Pathologist (CAP). Internal quality assessment (IQA) was carried out during analysis by using the traceable ClinChek urine materials (ref.:8847–8849, RECIPE, Germany). Urine controls were tested following every twenty specimens to ensure quality throughout screening. The Z-score was calculated, and the values within the +2 to −2 range were satisfactory and indicated analytical trueness. External quality assessment (EQA) was carried out by participation in the College of American Pathologists (CAP) proficiency program (https://www.cap.org/laboratory-improvement/proficiency-testing).
2.6. Data analysis
Descriptive statistics were performed with continuous variables estimated as median and interquartile range (IQR), and categorical variables summarized as frequencies and proportions. The normal range of each trace elements were described as we recently reported (Zeng et al., 2019). Considering the distributions of the elements tested by the Kolmogorov-Smirov test, were mostly not normal, The nonparametric tests were used in our data analysis. The Mann-Whitney U test was used to compare the results between different disease severities and different outcomes, respectively. The spearman correlation test and its statistical significance were used to assess the correlation between the different elements. The urinary trace elements were tested for their discriminative power in determining the predictive criteria for severe illness and fatal outcome by calculating the area under the receiver operating characteristic (ROC) curve. Sensitivity and specificity were calculated according to ROC curves for each parameter. Multivariate analyses were performed using a logistic regression model to adjust for the effects from age and sex. For data analysis, individual results below the limit of quantification (LoQ) were replaced by the (LoQ/2) value. All analyses were performed using SPSS version 22.0 (SPSS, Chicago, USA) and p < 0.05 was considered statistically significant.
3. Results
The present study included a total of 138 hospitalized patients with confirmed COVID-19. The median age was 61.5 years old (IQR: 55–68), and 59 (42.8%) were female and all were of Wuhan residents. The patients were classified on admission according to severity of the disease, including 70 (50.7%) non-severe cases and 68 (49.3%) severe cases (Table 1). All the patients had determined outcomes, of which all the non-severe cases (70, 100.0%) and 47 (69.1%) severe cases recovered, and the remained 21 (30.9%) severe cases deceased. Altogether, a total of 117 (84.8%) patients recovered and 21 (15.2%) deceased. The median duration of hospital stay was 14.5 days (IQR: 8–32). The median length from disease onset to admission was 26 days (IQR: 11–41). The frequently seen symptoms at hospital admission were fever, cough, feeble, shortness of breath, chest tightness, diarrhea. Of the 138 patients, 92 (66.7%) had underlying conditions on admission, including hypertension (50, 36.2%), diabetes (24, 17.4), cardiovascular disease (12, 8.7%), malignancy (4, 2.9%), cerebrovascular disease (10, 7.2%) (Table 1).
In comparison with non-severe patients, the severe cases were older (Median: 65 vs 60 years), had shorter intervals between symptoms onset and admission to hospital (13 vs 40 days), and longer hospital stay (31 vs 8 days). The severe cases also showed a higher frequency of shortness of breath (39.7% vs 14.3%) and an underlying disorder, especially hypertension (45.6% vs 27.1%), and had a higher frequency of fatal outcome (30.9% vs 0%) (Table 1). Laboratory parameters including plasma creatinine, ferritin, serum cytokines and hemocytes were obtained (Table 1). Compared with non-severe patients, the severe cases showed higher levels of ferritin (Median: 1263.8 vs 314.7 μg/L) and cytokine IL2R (659.5 vs 339.5 U/ml), IL6 (32.09 vs 2.34 pg/ml), IL8 (18.45 vs 10.15 pg/ml), IL10 (6.2 vs < 5.0 pg/ml) and TNFα (10.3 vs 7.3 pg/ml), and neutrophil (7.43 vs 3.25, × 109/L) and WBC (9.17 vs 5.64, × 109/L), and showed lower levels of urinary creatinine (4241 vs 9299 μmol/L) and lymphocyte (0.78 vs 1.64, × 109/L).
A total of 210 urine specimens were collected from the 138 COVID-19 patients. Among them, 36 patients had at least two urine specimens at different time points. The urinary analysis including urinary protein qualification and urinary creatinine determination were performed for all these samples. In comparison with non-severe group, the severe group showed higher percentage of proteinuria incidence (62.4% vs 12.0%), altogether both group collectively showed 43.8% of proteinuria incidence in all the COVID-19 patients (Table 2 ). Urinary creatinine was found to be much lower in the severe group than the non-severe group (Median: 4241 vs 9299 μmol/L).
Table 2.
Levels of urinary trace elements in 210 urinary specimens from the 138 severe and non-severe patients with COVID-19.
| Varables | Disease status, median (IQR) |
|||
|---|---|---|---|---|
| All | Non-severe | Severe | p | |
| Proteinuria, N (%) | 92 (43.8%) | 9 (12.0%) | 83 (62.4%) | <0.001 |
| Creatinine, urine, μmol/L | 5234.5 (2929.5–9299) | 9299 (5238–12451) | 4241 (2144.75–6107.75) | <0.001 |
| Urinary elements [normal range], μg/L | ||||
| Cr [< 0.24–0.5] | 1.3 (0.79–2.57) | 0.88 (0.62–1.2) | 1.96 (1–3.62) | <0.001 |
| Mn [< 0.53–1.92] | 0.65 (<0.53–1.17) | <0.53 (<0.53–0.66) | 0.83 (<0.53–1.53) | <0.001 |
| Cu [4.00–21.42] | 21.95 (14.23–44.57) | 15.84 (10.48–20.68) | 32.14 (17.22–75.43) | <0.001 |
| As [10.54–174.53] | 11.34 (4.38–26.75) | 27.61 (17.64–42.2) | 6.01 (2.31–12.2) | <0.001 |
| Se [10.46–82.71] | 22.95 (14.44–36.08) | 25.55 (19.04–37.64) | 20.27 (13.53–35.34) | 0.024 |
| Cd [0.34–3.39] | 1.25 (0.66–2.58) | 0.95 (0.65–1.47) | 1.49 (0.75–3.52) | <0.001 |
| Hg [< 0.72–2.16] | <0.72 (<0.72–1.4) | 0.75 (<0.72–1.52) | <0.72 (<0.72–1.31) | 0.697 |
| Tl [0.11–0.89] | 0.13 (<0.06–0.26) | 0.35 (0.18–0.46) | 0.08 (<0.06–0.14) | <0.001 |
| Pb [0.24–2.29] | 0.82 (0.53–1.43) | 0.82 (0.59–1.31) | 0.83 (0.5–1.64) | 0.939 |
| CR adjusted Urinary elements [normal range], μg/g | ||||
| Cr [0.05–0.43] | 2.33 (0.93–6.02) | 0.87 (0.61–1.58) | 4.01 (1.81–11.21) | <0.001 |
| Mn [0.09–1.99] | 1.09 (0.44–3.03) | 0.32 (0.24–0.94) | 1.82 (0.85–4.59) | <0.001 |
| Cu [4.39–13.37] | 32.34 (16.68–108.63) | 15.55 (12.41–20.45) | 77.71 (32.04–248.28) | <0.001 |
| As [14.23–103.04] | 21.22 (10.79–31.74) | 29.44 (23.13–36.5) | 14.00 (8.31–23.55) | <0.001 |
| Se [15.86–38.13] | 37.37 (26.74–58.75) | 27.65 (22.03–35.49) | 45.63 (32.19–72.04) | <0.001 |
| Cd [0.27–2.23] | 2.11 (0.95–5.53) | 1.01 (0.7–1.43) | 2.96 (1.71–9.53) | <0.001 |
| Hg [0.15–1.62] | 1.3 (0.68–2.22) | 0.81 (0.49–1.39) | 1.54 (0.83–2.92) | <0.001 |
| Tl [0.11–0.53] | 0.22 (0.12–0.39) | 0.31 (0.21–0.44) | 0.16 (0.09–0.31) | <0.001 |
| Pb [0.26–1.91] | 1.3 (0.75–2.51) | 0.86 (0.58–1.36) | 1.85 (0.99–4.55) | <0.001 |
Abbreviations: CR, creatinine; Cr, chromium; Mn, manganese; Cu, copper; As, arsenic; Se, selenium; Cd, cadmium; Hg, mercury; Tl, thallium; Pb, lead. Notes: normal range were derived from previous report (Zeng et al., 2019).
For urinary trace element analysis, tested values of Cr, Cu, As, Se, Cd and Pb were all above the LoQ, while Mn, Hg and Tl showed low test levels, as 39.0% of Mn, 50.0% of Hg, 31.4% of Tl measurements were below the LoQ. For all these urine specimens, most of the urinary trace elements including Cr, Mn, Cu, As, Se, Cd, and Tl displayed significant differences between non-severe and severe cases (Table 2). In comparison with non-severe patients, the severe cases had a higher levels of Cr (Median: 1.96 vs 0.88 μg/L), Mn (0.83 vs < 0.53 μg/L), Cu (32.14 vs 15.84 μg/L) and Cd (1.49 vs 0.95 μg/L), and had a lower levels of As (6.01 vs 27.61 μg/L), Se (20.27 vs 25.55 μg/L) and Tl (0.08 vs 0.35 μg/L). When the urinary trace elemental levels were adjusted by the urinary creatinine, all the trace elements displayed differences (Table 2, Figure S1). The higher levels in the severe cases than non-severe cases remains for the urinary Cr (Median: 4.01 vs 0.87 μg/g), Mn (1.82 vs 0.32 μg/g), Cu (77.71 vs 15.55 μg/g) and Cd (2.92 vs 1.01 μg/g), and additionally for Se (45.63 vs 27.65 μg/g), Hg (1.54 vs 0.81 μg/g) and Pb (1.85 vs 0.86 μg/g). The lower levels in the severe cases than non-severe cases remains for the urinary As (Median: 14.00 vs 29.44 μg/g) and Tl (0.16 vs 0.31 μg/g).
Of the severe patients, between the recovered and deceased group, urinary Cr, Mn, Cu, As, Cd, Hg and Pb displayed significant differences (Table 3 ), among which Cr, Mn, Cu, Cr, Hg and Pb were higher while As were lower in the deceased group than the recovered group. When adjusted by the urinary creatinine, all the eight elements, expect for Tl, displayed significant differences, among which, Cr, Mn, Cu, Cd, Hg, Pb and additionally Se were higher, and As remains lower in the deceased group than the recovered group (Table 3, Figure S2).
Table 3.
Levels of urinary trace elements in the severe patients with COVID-19 according to different outcomes (recovered versus deceased).
| Elements | Outcome |
||
|---|---|---|---|
| Recovered | Deceased | p | |
| Urinary elements [normal range], μg/L | |||
| Cr [< 0.24–0.5] | 1.78 (0.83–3.22) | 2.74 (1.5–4.82) | 0.010 |
| Mn [< 0.53–1.92] | 0.68 (0.27–1.07) | 1.26 (0.84–2.44) | <0.001 |
| Cu [4.00–21.42] | 25.32 (14.02–39.26) | 82.89 (46.71–143.89) | <0.001 |
| As [0.54–174.53] | 7.92 (3.97–15) | 3.28 (1.84–7.11) | <0.001 |
| Se [10.46–82.71] | 19.95 (12.22–34.36) | 25.5 (14.02–38.81) | 0.543 |
| Cd [0.34–3.39] | 1.14 (0.61–2.47) | 3.64 (1.68–7.9) | <0.001 |
| Hg [< 0.72–2.16] | 0.36 (0.36–1.21) | 0.94 (0.36–1.71) | 0.012 |
| Tl [0.11–0.89] | 0.08 (0.03–0.15) | 0.03 (0.03–0.13) | 0.153 |
| Pb [0.24–2.29] | 0.74 (0.48–1.16) | 1.07 (0.57–4.72) | 0.010 |
| CR adjusted Urinary elements [normal range], μg/g | |||
| Cr [0.05–0.43] | 3.17 (1.71–7.54) | 7.9 (3.55–22.17) | <0.001 |
| Mn [0.09–1.99] | 1.28 (0.62–3.3) | 3.69 (1.82–14.24) | <0.001 |
| Cu [4.39–13.37] | 45.95 (24.84–96.88) | 311.53 (119.31–456.7) | <0.001 |
| As [14.23–103.04] | 17.63 (9.98–24.5) | 8.93 (6.04–14.61) | 0.001 |
| Se [15.86–38.13] | 40.56 (30.27–61.18) | 66.75 (43.39–120.13) | <0.001 |
| Cd [0.27–2.23] | 2.35 (1.26–4.24) | 11.25 (3.94–24.72) | <0.001 |
| Hg [0.15–1.62] | 1.43 (0.76–2.24) | 2.49 (1.18–10.82) | <0.001 |
| Tl [0.11–0.53] | 0.16 (0.08–0.28) | 0.18 (0.1–0.64) | 0.247 |
| Pb [0.26–1.91] | 1.47 (0.9–3.01) | 4.04 (1.63–20.62) | <0.001 |
Abbreviations: Cr, chromium; Mn, manganese; Cu, copper; As, arsenic; Se, selenium; Cd, cadmium; Hg, mercury; Tl, thallium; Pb, lead. Notes: normal range were derived from previous report (Zeng et al., 2019).
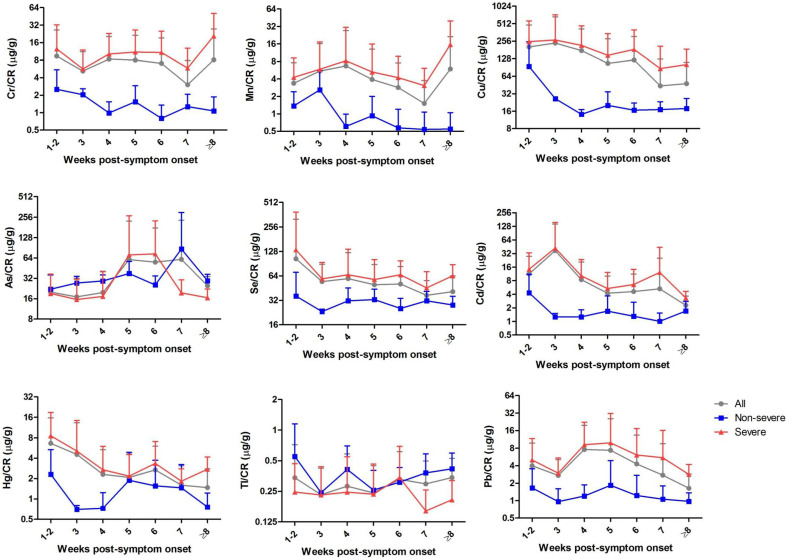
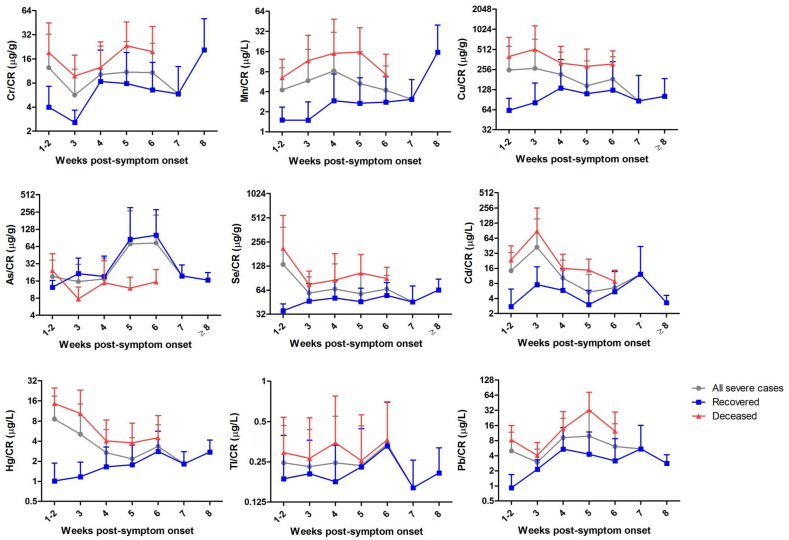
The longitudinal analysis was performed by using the results from the 210 urine specimens according to the weeks of post-symptoms onset to specimen collection. As shown in Fig. 1 , in comparison with the non-severe patients, the severe cases showed higher median levels of Cr, Mn, Cu, Se, Cd, Hg and Pb after creatinine correction, which consistently existed across the clinical course since the disease onset. Among these elements, Cr and Mn seems to show similar increasing trends while Cu and Cd showed similar decreasing trends with the weeks post-symptom onset. Furthermore, specifically for the severe cases with different outcomes, urinary creatinine-adjusted Cr, Mn, Cu, Se, Cd, Hg, Tl and Pb were found to be higher in the deceased group than the recovered group during the whole disease courses from symptom onset, while urinary As were found to be lower in the deceased group than the recovered group (Fig. 2 ).
Fig. 1.
Longitudinal dynamics of the urinary trace elements in severe and non-severe COVID-19 patients. Urinary creatinine-adjusted levels of the nine trace elements were estimated longitudinally by using the results from the 210 urine specimens according to the weeks of post-symptoms onset to specimen collection. The mean values were delineated on weeks of disease onset. Data are shown as mean ± SD. Abbreviations: Cr, chromium; Mn, manganese; Cu, copper; As, arsenic; Se, selenium; Cd, cadmium; Hg, mercury; Tl, thallium; Pb, lead.
Fig. 2.
Longitudinal dynamics of the urinary trace elements in severe COVID-19 patients according to the different disease outcome (deceased or recovered). Urinary creatinine-adjusted levels of the nine trace elements were estimated longitudinally by using the results only from severe COVID-19 patients according to the weeks of post-symptoms onset to specimen collection. The mean values were delineated on weeks of disease onset. Data are shown as mean ± SD. Abbreviations: Cr, chromium; Mn, manganese; Cu, copper; As, arsenic; Se, selenium; Cd, cadmium; Hg, mercury; Tl, thallium; Pb, lead.
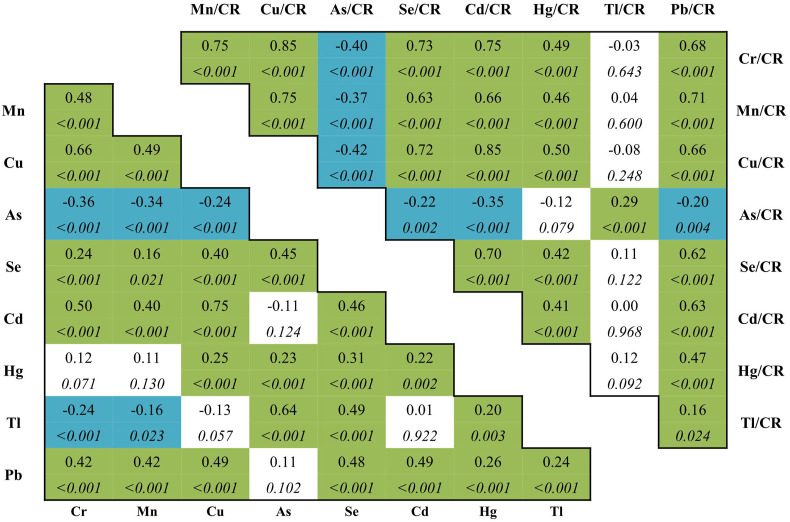
The correlation matrix of all the nine urinary trace elements with or without creatinine adjustment were summarized in Fig. 3 . Urinary Cr, Mn, Cu, Cd showed positively inter-correlated, with the highest coefficient of 0.752 (p < 0.05) for Cd–Cu. While urinary As and Tl belonged to another cluster, which were almost negatively correlated with the four trace element above, with the lowest coefficient of −0.356 (p < 0.05) for As–Cr. Creatinine adjusted levels of these elements mostly retained their inter-correlations. The highest coefficients were 0.853 for Cr–Cu, 0.848 for Cd–Cu, and 0.751 for both Cr–Cd and Cr–Mn. The lowest coefficients were −0.420 for As–Cu, −0.399 for As-cCr, and −0.371 for As–Mn, and −0.350 for As–Cd.
Fig. 3.
Spearman correlations between the urinary elements. Correlation coefficients (CC, above) and p values (below, italic) for the spearman correlation between urinary elements unadjusted and adjusted by creatinine. Green and blue color represent positively and negatively correlations, respectively. Abbreviations: CR, creatinine; Cr, chromium; Mn, manganese; Cu, copper; As, arsenic; Se, selenium; Cd, cadmium; Hg, mercury; Tl, thallium; Pb, lead.
Given increasing knowledge on the importance of inflammation and cytokine processes for COVID-19 evolution, we further assessed the correlations of the urinary creatinine-adjusted trace elements with blood cytokines (IL-1B, IL2R, IL6, IL8, IL10, TNFα), ferritin and hemocytes (lymphocyte, monocyte, neutrophil and WBC) (Figure S3), respectively. Urinary creatinine-adjusted levels of Cr, Mn, Cu, Se, Cd, Hg and Pb were almost all positively correlated with the cytokine IL-1B, IL2R, IL6, IL8, IL10, TNFα, and ferritin, and neutrophil and WBC, but were negatively correlated with lymphocyte. Conversely, the element As were negatively correlated with most of the cytokines, ferritin, and neutrophil and WBC, but positively correlated with lymphocyte (Figure S3).
As mentioned above that the urinary trace elements displayed differences at univariate analysis between different disease severities and outcomes, we further calculated the optimum diagnostic cut-off points for the severe versus non-severe patients, and the recovered versus deceased severe patients, respectively, by using the ROC curve method. The specified cut-off points and the sensitivity, specificity and the area underneath the ROC curve (AUC) belonging to those cut-off points are shown in Table S1. Considering the correlations existed among the urinary trace elements, and these trace elements were further mostly correlated with the laboratory inflammatory parameters, thus we selected the urinary trace element with maximum AUC and applied the binary Logistic regression to the predetermined cut-off values. For the severe illness of COVID-19, the urinary creatinine-adjusted Cu with maximum AUC of 0.913, were determined to be independently predictive of severe illness (p < 0.05). The cut-off point was calculated as 25.57 μg/g. The risk increased 36.51 (95%CI: 15.57–85.63) times in the presence of Cu/CR ≥ 25.57 μg/g (Table 4 ), which could discriminate 84.2% of the samples from severe illness (data not shown). When we used this same cut-off for estimation of the fatal outcome among the severe patients, the OR was 13.37 (95% CI: 1.74–102.67), which could discriminate 70.1% of the samples of fatal outcome from those of recovery (data not shown). For the fatal outcome of COVID-19, the urinary creatinine-adjusted Cu with maximum AUC of 0.843, were determined to be independently predictive of fatal outcome (p < 0.05). The cut-off point was calculated as 99.32 μg/g. The risk increased 15.43 (95%CI: 6.00–39.70) in the presence of Cu/CR ≥ 99.32 μg/g (Table 4), which could discriminate 78.4% of the samples from fatal outcome (data not shown).
Table 4.
The risk factors for severe illness and fatal outcome in COVID-19 patients by using cut-off points of creatine-adjusted urinary copper.
| Severe illness | ||||
|---|---|---|---|---|
| Non-severe | Severe | OR (95% CI) | p | |
| Cu/CR, μg/g | ||||
| ≥ 25.57 | 8 | 109 | 36.51 (15.57–85.63) | <0.001 |
| < 25.57 |
67 |
25 |
||
| Fatal outcome | ||||
| Recovered |
Deceased |
OR (95% CI) |
p |
|
| Cu/CR, μg/g | ||||
| ≥ 99.32 | 22 | 33 | 15.43 (6.00–39.70) | <0.001 |
| < 99.32 | 72 | 7 | ||
Abbreviations: CR, creatinine; Cu, copper.
4. Discussion
To our knowledge, this is the first study focusing on the associations of the urinary trace elements with severe illness and fatal outcome of COVID-19. We performed a retrospective analysis of the nine urinary trace elements including chromium, manganese, copper, arsenic, selenium, cadmium, mercury, thallium and lead in a cohort of 138 patients with COVID-19 according to the disease severity (non-severe or severe) and outcome (recovered or deceased), respectively. In summary of our results, the urinary creatinine-adjusted concentrations of chromium, manganese, copper, selenium, cadmium, mercury and lead were found to be higher in severe patients than the non-severe cases. And among the severe cases, these elements were also higher in the deceased group than the recovered group. These urinary elements were found to be positively inter-correlated (p < 0.05), and further positively correlated with other laboratory inflammatory parameters including serum cytokines (IL-1B, IL2R, IL6, IL8, IL10, TNFα), ferritin, and neutrophil counts and WBC. When the weeks of the post-symptom onset were taken in account, the changes of these urinary elements were found to be existed across the clinical course since the disease onset.
Above all, abnormal urine analysis and kidney dysfunction have been commonly reported in COVID-19 patients (Yang et al., 2020). Acute kidney injury (AKI) occurred in 46% of the patients hospitalized with COVID-19 (Chan, 2020). Of the patients with AKI and urine studies, 84% had proteinuria, 81% had hematuria, and 60% had leukocyturia (Chan, 2020). As expected, our data suggested 43.8% of the analyzed urine samples had proteinuria, and the percentage were much higher in the severe group than the non-severe group (62.4% vs 12.0%). Given the evidence that urinary heavy metals circulate mainly bound to low-molecular-weight (LMW) proteins (Chaumont et al., 2012), the increased levels of urinary heavy metals observed in severe COVID-19 patients might simply reflect the impairment in the renal uptake of proteins sharing the same affinity for tubular binding sites. Furthermore, the positive inter-elemental correlations among most of the increased urinary elements observed in our study implied that urinary co-excretion may existed, to some extent, as a support of the hypothesis.
Among the increased urinary elements mentioned above, chromium increased in urine has been reported to be associated with impairment of renal function in the hypertensive population (Wu et al., 2018). Chromium is thought as a “hypoglycemic metal element”, which could improving glucose tolerance through insulin resistance (Zhou et al., 2019; Lewicki et al., 2014). Drastic increase of urinary chromium in patients with diabetes (Zhou et al., 2019), and also in non-diabetic, normotensive subjects with increased insulin resistance has been confirmed (Bahijri and Alissa, 2011). The increased has been shown to actually stem from increased absorption of chromium (Rhodes et al., 2010), which was resulted from the increases of blood insulin concentrations, including increases stemming from increases in blood glucose concentration (Vincent, 2017). Considering hyperglycemia, not only in people with diabetes, is a bad prognostic factor of sever COVID-19 (Ceriello, 2020), we suspected the glucose homeostasis alterations would probably be a related factor with the higher level of urinary chromium loss in severe and deceased COVID-19 cases.
For manganese in human body, most of excess is conjugated to bile by the liver and get eliminated via fecal excretion, while the excretion through urine is very limited in amount (Chen et al., 2018). Hepatic problems may resulted in manganese accumulation in the human body (Chen et al., 2018), which would probably lead to the higher urinary manganese in severe COVID-19 patients, as 14–53% of patients with COVID-19 had hepatic dysfunction, particularly in those with severe disease (Jothimani, 2020). Moreover, urinary manganese and copper were reported to positively correlate with plasma interleukins and increased biomarker signals of inflammation in pregnant women (Aung et al., 2020), suggesting the associations may existed of urinary manganese with immune perturbations in COVID-19.
Copper plays a pivotal role in the regulation of inflammatory processes, which is associated with inflammation, oxidative stress and metabolic abnormalities (Bo et al., 2008). It has been proved that copper could stimulate the synthesis and release of interleukin-8 in human endothelial cells (Bar-Or et al., 2003). Urinary copper increased monotonically with plasma C-reactive protein (CRP) elevation, and plasma CRP was positively associated with the prevalence of metabolic syndrome (Ma et al., 2020). We supposed that the higher level of urinary copper was associated with enhanced inflammatory processes in severe COVID-19 patients. In addition, similarly with manganese, body copper homeostasis is mainly regulated by the liver, which removes excess copper via bile. However, as liver dysfunction were commonly reported in COVID-19 patients, the impairment of biliary excretion are associated with excess urinary copper excretion (Ritland et al., 1977).
For selenium, the urinary concentration is used as an indicator of selenium status (Pedrosa et al., 2012). Liver is the central organ for selenium regulation and produces excretory selenium forms to regulate whole-body selenium (Burk and Hill, 2015). Therefore, liver dysfunction in severe COVID-19 were suspected to be associated with excess urinary selenium excretion, to some degree with a similar way with copper and manganese.
Cadmium, along with mercury and lead are among the most toxic metals (Vacchi-Suzzi et al., 2016). Kidney is the main accumulation and excretion organ of cadmium and mercury (Li et al., 2010; Yang and Shu, 2015). Associations between urinary lead levels and reduced kidney function have been reported (Jain, 2019). It has been well established that cadmium circulates in plasma as a LMW cadmium-metallothionein complex that follows the same glomerular filtration-tubular reabsorption pathway as other LMW proteins (Chaumont et al., 2012). Lead is reabsorbed by proximal tubule cells as a complex with LMW proteins comparable to cadmium-metallothionein complex (Gonick, 2011). Mercury binding metallothionein was also observed in cases of methylmercury poisoning (Li et al., 2016). We suspected the higher levels of urinary cadmium, mercury and lead in severe COVID-19 patients were probably stemmed from the kidney tubular damage, resulting in proteinuria.
Additionally, various studies have observed that urinary cadmium was closely related to many human chronic diseases, such as cardiovascular diseases, diabetes (Larsson and Wolk, 2016; Tinkov et al., 2017), which were common comorbidities of COVID-19. Exposure to cadmium and lead could cause adverse effect on human health on the respiratory system with lung function impairment (Rehman et al., 2018), by inducing oxidative damage to lung epithelial cells and activating inflammatory signaling cascades. The oxidative damage in turn could lead to impairment in lung function and mediate the pathogenesis of pulmonary diseases (Wei et al., 2020).
Compared with the increased trace elements, urinary arsenic and thallium showed inverse change in the severe COVID-19 patients, which is unexpected. This potential explanation would be related to reverse causality, where impairment of renal function would decrease the total filtration of the toxicant, thus leading to the decreased levels in urine (Wu et al., 2018). Besides, dimethylarsenate (DMA) is most frequently detected in urine samples (Weidemann et al., 2015), thus we suspected that urinary excretion of arsenic and thallium might be mainly as non-protein binding form, showing different processes of renal elimination, compared with other heavy metals. However, the underlying mechanisms needed to be clarified in future studies. Additionally, urinary arsenic levels were seen to be negatively associated with peripheral blood mononuclear cell formation of fractalkine and IL-7, and positively associated with that for IL-13, IL-17 and MIP-1 α in children (Parvez et al., 2019), suggesting the associations may existed for urinary arsenic with perturbations of inflammatory processes in COVID-19.
There are some limitations should be noted, as this study was based on only 138 severe or non-severe patients with COVID-19 in Wuhan of China, thus future multi-center studies on a larger cohort were needed to verified the findings. Moreover, the reverse causality bias cannot be definitely ruled out, as the present study mainly focused on the associations of urinary trace elements with the disease severity and outcome during the infection, more longitudinal study including the stages of pre-infections were needed in the future. The lack of data on essential element zinc, and data on diet and occupation is also a limitation of the study.
In conclusion, we provided a comprehensive analysis of the abnormalities of nine urinary trace elements for the COVID-19 disease, and their associations with different disease severities and outcomes. We identified the urinary chromium, manganese, copper, cadmium, mercury, arsenic and thallium as associated factors with the prognosis of severe COVID-19, which should be persistently monitored, not only useful for the identification of COVID-19, but are also helpful in the evaluation of the dynamic changes in patients with COVID-19.
CRediT author statement
Hao-Long Zeng: Conceptualization, Data curation, Methodology, Writing – original draft, Bo Zhang: Data curation, Methodology, Xu Wang: Methodology, Qing Yang: Conceptualization, Supervision, Review & editing, Liming Cheng: Supervision, Review & editing.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
The authors thank all the medical care workers who participated in the sample collection. The work was supported by National Natural Science Foundation of China (31600666).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2020.110670.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Aung M.T., et al. Manganese is associated with increased plasma interleukin-1beta during pregnancy, within a mixtures analysis framework of urinary trace metals. Reprod. Toxicol. 2020;93:43–53. doi: 10.1016/j.reprotox.2019.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahijri S.M., Alissa E.M. Increased insulin resistance is associated with increased urinary excretion of chromium in non-diabetic, normotensive Saudi adults. J. Clin. Biochem. Nutr. 2011;49(3):164–168. doi: 10.3164/jcbn.10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Or D., et al. Copper stimulates the synthesis and release of interleukin-8 in human endothelial cells: a possible early role in systemic inflammatory responses. Shock. 2003;20(2):154–158. doi: 10.1097/01.shk.0000068318.49350.3a. [DOI] [PubMed] [Google Scholar]
- Bo S., et al. Associations of dietary and serum copper with inflammation, oxidative stress, and metabolic variables in adults. J. Nutr. 2008;138(2):305–310. doi: 10.1093/jn/138.2.305. [DOI] [PubMed] [Google Scholar]
- Bonetti G., et al. Urinalysis parameters for predicting severity in coronavirus disease 2019 (COVID-19) Clin. Chem. Lab. Med. 2020;58(9):e163–e165. doi: 10.1515/cclm-2020-0576. [DOI] [PubMed] [Google Scholar]
- Burk R.F., Hill K.E. Regulation of selenium metabolism and transport. Annu. Rev. Nutr. 2015;35:109–134. doi: 10.1146/annurev-nutr-071714-034250. [DOI] [PubMed] [Google Scholar]
- Callan A.C., et al. Investigation of the relationship between low environmental exposure to metals and bone mineral density, bone resorption and renal function. Int. J. Hyg Environ. Health. 2015;218(5):444–451. doi: 10.1016/j.ijheh.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Ceriello A. Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res. Clin. Pract. 2020;163:108186. doi: 10.1016/j.diabres.2020.108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L., et al. AKI in hospitalized patients with COVID-19. J. Am. Soc. Nephrol. 2020;32(1):151–160. doi: 10.1681/asn.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont A., et al. Associations between proteins and heavy metals in urine at low environmental exposures: evidence of reverse causality. Toxicol. Lett. 2012;210(3):345–352. doi: 10.1016/j.toxlet.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Chen P., Bornhorst J., Aschner M. Manganese metabolism in humans. Front Biosci (Landmark Ed) 2018;23:1655–1679. doi: 10.2741/4665. [DOI] [PubMed] [Google Scholar]
- Gonick H.C. Lead-binding proteins: a review. J. Toxicol. 2011;2011:686050. doi: 10.1155/2011/686050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int. Urol. Nephrol. 2020;52(6):1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R.B. Lead and kidney: concentrations, variabilities, and associations across the various stages of glomerular function. J. Trace Elem. Med. Biol. 2019;54:36–43. doi: 10.1016/j.jtemb.2019.03.007. [DOI] [PubMed] [Google Scholar]
- Jothimani D., et al. COVID-19 and the liver. J. Hepatol. 2020;73(5):1231–1240. doi: 10.1016/j.jhep.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S.C., Wolk A. Urinary cadmium and mortality from all causes, cancer and cardiovascular disease in the general population: systematic review and meta-analysis of cohort studies. Int. J. Epidemiol. 2016;45(3):782–791. doi: 10.1093/ije/dyv086. [DOI] [PubMed] [Google Scholar]
- Lewicki S., et al. The role of Chromium III in the organism and its possible use in diabetes and obesity treatment. Ann. Agric. Environ. Med. 2014;21(2):331–335. doi: 10.5604/1232-1966.1108599. [DOI] [PubMed] [Google Scholar]
- Li S.J., et al. Mercury-induced membranous nephropathy: clinical and pathological features. Clin. J. Am. Soc. Nephrol. 2010;5(3):439–444. doi: 10.2215/CJN.07571009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., et al. Elevated mercury bound to serum proteins in methylmercury poisoned rats after selenium treatment. Biometals. 2016;29(5):893–903. doi: 10.1007/s10534-016-9961-1. [DOI] [PubMed] [Google Scholar]
- Liu R., et al. The value of urine biochemical parameters in the prediction of the severity of coronavirus disease 2019. Clin. Chem. Lab. Med. 2020;58(7):1121–1124. doi: 10.1515/cclm-2020-0220. [DOI] [PubMed] [Google Scholar]
- Ma J., et al. Associations between essential metals exposure and metabolic syndrome (MetS): exploring the mediating role of systemic inflammation in a general Chinese population. Environ. Int. 2020;140:105802. doi: 10.1016/j.envint.2020.105802. [DOI] [PubMed] [Google Scholar]
- National Health Commission of the People’s Republic of China . 2020. Diagnosis and Treatment Scheme of New Coronavirus Infected Pneumonia. [Google Scholar]
- Parvez F., et al. Exposure to low-dose arsenic in early life alters innate immune function in children. J. Immunot. 2019;16(1):201–209. doi: 10.1080/1547691X.2019.1657993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrosa L.F., et al. Fecal selenium excretion is regulated by dietary selenium intake. Biol. Trace Elem. Res. 2012;149(3):377–381. doi: 10.1007/s12011-012-9430-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman K., et al. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018;119(1):157–184. doi: 10.1002/jcb.26234. [DOI] [PubMed] [Google Scholar]
- Rhodes N.R., et al. Urinary chromium loss associated with diabetes is offset by increases in absorption. J. Inorg. Biochem. 2010;104(7):790–797. doi: 10.1016/j.jinorgbio.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Ritland S., Steinnes E., Skrede S. Hepatic copper content, urinary copper excretion, and serum ceruloplasmin in liver disease. Scand. J. Gastroenterol. 1977;12(1):81–88. [PubMed] [Google Scholar]
- Tinkov A.A., et al. The role of cadmium in obesity and diabetes. Sci. Total Environ. 2017;601–602:741–755. doi: 10.1016/j.scitotenv.2017.05.224. [DOI] [PubMed] [Google Scholar]
- Vacchi-Suzzi C., et al. Is urinary cadmium a biomarker of long-term exposure in humans? A review. Curr Environ Health Rep. 2016;3(4):450–458. doi: 10.1007/s40572-016-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent J.B. New evidence against chromium as an essential trace element. J. Nutr. 2017;147(12):2212–2219. doi: 10.3945/jn.117.255901. [DOI] [PubMed] [Google Scholar]
- Wei W., et al. Lead exposure and its interactions with oxidative stress polymorphisms on lung function impairment: results from a longitudinal population-based study. Environ. Res. 2020;187:109645. doi: 10.1016/j.envres.2020.109645. [DOI] [PubMed] [Google Scholar]
- Weidemann D., et al. Association of arsenic with kidney function in adolescents and young adults: results from the national health and nutrition examination survey 2009-2012. Environ. Res. 2015;140:317–324. doi: 10.1016/j.envres.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., et al. Association of co-exposure to heavy metals with renal function in a hypertensive population. Environ. Int. 2018;112:198–206. doi: 10.1016/j.envint.2017.12.023. [DOI] [PubMed] [Google Scholar]
- Yang H., Shu Y. Cadmium transporters in the kidney and cadmium-induced nephrotoxicity. Int. J. Mol. Sci. 2015;16(1):1484–1494. doi: 10.3390/ijms16011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., et al. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit. Care. 2020;24(1):356. doi: 10.1186/s13054-020-03065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H.L., et al. Analysis of urinary trace element levels in general population of Wuhan in central China. Environ. Sci. Pollut. Res. Int. 2019;26(27):27823–27831. doi: 10.1007/s11356-019-05973-7. [DOI] [PubMed] [Google Scholar]
- Zhou Q., et al. Comparison of chromium and iron distribution in serum and urine among healthy people and prediabetes and diabetes patients. BioMed Res. Int. 2019;2019:1–8. doi: 10.1155/2019/3801639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.