Abstract
Treatment options are limited for patients with advanced forms of myeloproliferative neoplasms (MPN), including blast phase disease (MPN-BP). Decitabine has frequently been employed but its efficacy and safety profile are not well described in this population. We retrospectively reviewed 42 patients treated with decitabine either alone or in combination with ruxolitinib at our institution: 16 MPN-BP, 14 MPN accelerated phase (MPN-AP), and 12 myelofibrosis with high risk features (MF-HR). The median overall survival (OS) for MPN-BP patients was 2.6 months, and in those who received 2 or more cycles of decitabine therapy it was 6.7 months [3.8–29.8]. MPN-BP patients with a poor performance status and required hospitalized at time of initiation of decitabine had a dismal survival. After a median follow up of 12.4 months in MPN-AP patients, and 38.7 months in MF-HR patients, the median OS was not reached for either cohort, with one and two patients alive at 60 months, respectively. The probability of spleen length reduction or transfusion independence within 12 months of initiating decitabine was 28.6% or 23.5%, respectively. The combination of decitabine and ruxolitinib appeared to improve overall survival over single agent decitabine (21 versus 12.9 months, respectively). Decitabine alone or in combination with ruxolitinib appears to have clinical benefit in patients with advanced phase MPN when initiated early in disease course prior to evolution to MPN-BP.
Keywords: Myeloproliferative neoplasms, Myelofibrosis, Blast phase, Accelerated phase, Acute myeloid leukemia, Decitabine
1. INTRODUCTION
The BCR-ABL1-negative myeloproliferative neoplasms (MPN) are a heterogeneous group of clonal hematopoietic stem cell malignancies whose pathogenesis are linked to hyperactivity of the JAK-STAT pathway [1]. Amongst the many sequelae affecting morbidity and mortality is transformation to acute myeloid leukemia (AML), defined by the presence of 20% or greater myeloblasts in the peripheral blood or bone marrow. Chronic phase (CP) primary myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET) are associated with a 10–20%, 4%, and 1% risk, respectively, of transformation to blast phase (BP) disease, predominantly of a myeloid phenotype [2, 3].
Retrospective studies have identified prognostic factors associated with development of MPN-BP. Noted patient-specific risk factors are advanced age and exposure to cytoreductive agents such as radioactive P-32 and alkylating agents [4, 5]. Disease-specific risk factors include anemia, red blood cell transfusion dependence, platelet count < 100 × 109/L, and peripheral blood blasts ≥ 3% [6–9]. Furthermore, certain disease genotypes in MF including the absence of a driver mutation (JAK2, CALR, MPL), and presence of a of certain non-driver mutations including ASXL1, TET2, SRSF2, RUNX1, and TP53 have been shown to confer an increased risk of leukemic transformation [10–14]. Cytogenetic abnormalities involving chromosomes 5, 7, or 17p have also been associated with a six-fold increased risk of evolution to MPN-BP, in addition to +1q [10, 3, 15].
MPN-BP is generally associated with poor outcomes, with a median overall survival (OS) of approximately 3–5 months [16–18]. Currently, there is no standard approach to treatment of this patient population. Intensive chemotherapy alone provides minimal benefit, most studies showing an OS similar to supportive care [16, 19, 17, 20]. Hematopoietic stem cell transplantation (HSCT) is the only treatment modality shown to alter the course of the disease, but has historically been limited in practice as most patients are not candidates due to advanced age and/or significant competing comorbidities [21, 22]. Therefore, the lack of effective management options for advanced phases of MPN represents an urgent unmet clinical need.
Decitabine (deoxyazanucleoside 5-aza-2’-deoxycytidine) (DEC) is an S-phase specific therapeutic activated by deoxycytidine kinase resulting in a pyrimidine analogue that is incorporated into DNA causing irreversible inhibition of DNA methyltransferase [23]. In 2010, Mascarenhas et al. reported the clinical benefit of DEC in reducing spleen size and RBC transfusion requirements, and an associated median OS that was not reached at 9 months in a small cohort of MPN-BP patients [24]. Bader et al. later confirmed this mortality benefit in a single institutional retrospective study at MD Anderson, with a median OS of 6.9 months in MPN-BP patients, 9.7 months in MPN-AP patients (10–19% blasts), and 27 months in MF-high-risk patients (<10% blasts) [25].
In this current study, we aim to further characterize the patient population with advanced phases of MPN treated with DEC alone or in combination with ruxolitinib, delineate the clinical and survival outcomes, and examine the safety profile of DEC in this setting.
2. METHODS
2.1. Patient Population
We retrospectively reviewed the electronic medical records of all patients seen in the Myeloproliferative Disorders Program at Mount Sinai Hospital that were treated with DEC from 2012–2018. Patients with MPN-BP seen in consultation at our institution but treated by a local physician were not included in this analysis. Cohorts were assigned a particular disease status at DEC initiation. MPN-BP was defined by the presence of ≥20% blasts in the bone marrow or peripheral blood, while accelerated phase (AP) was defined as 10 to 19% blasts [26]. The MF-high risk (HR) cohort was defined as intermediate-2 or high risk disease by the Dynamic International Prognostic Scoring System (DIPSS) and was determined to be at a heightened risk for leukemic transformation by the presence of circulating blasts of 4–9% in the peripheral blood, 5–9% blasts in the bone marrow, or MPN-MDS (myelodysplastic syndrome) overlap. [9]. Patients were included in this study if they were receiving single agent DEC or combination therapy of DEC and ruxolitinib (Jakafi, Incyte).
2.2. Mutational Profiles
Mutational profiles were determined from PCR detection of JAK2V617F, or when available, a next-generation sequencing (NGS) panel of 44 genes associated with myeloid malignancies (Genoptix, Carlsbad, CA, USA) from cells harvested from either the peripheral blood or a bone marrow aspirate when obtained [27].
2.3. Cytogenetic Information
Cytogenetic analyses were performed by the Tumor CytoGenomics Laboratory at Mount Sinai. As previously reported, an unfavorable karyotype was defined as the presence of +1q, inv(3)/t(3;3), −5/del(5q),−7/del7(7q),+8,11q23 rearrangements, and del(12p) [28–30].
2.4. Clinical Responses
Patients were assessed for clinical response on at a monthly basis for up to 24 months on DEC therapy. Spleen length was categorized as minimal (0–5cm), moderate (6–10cm) or severe (11+cm) by palpation and a spleen response was defined as a downgrade in spleen length category. Red blood cell transfusion (RBC) dependence was defined by Gale Criteria (2+ units/month over a 3 month period), and a response in transfusion dependence was improvement from dependence to independence [31]. A response in ECOG performance status was any decrease along the scale. The absence of blasts or a 50% reduction in peripheral blast numbers by manual review of the peripheral blood smear was considered a blast response. Complication rates in the same follow-up period were also assessed. Complications of interest included infections (bacterial, viral, or fungal), thromboses (arterial, venous) documented by radiological studies, and hemorrhage requiring hospitalization and/or transfusional support. Time frames for all events were documented.
2.5. Statistical Analysis
Continuous patient-related, disease-related, and treatment-related variables were summarized by the median and interquartile range (IQR), while categorical variables were summarized by frequency (N) and percentage (%). Cumulative incidence functions (CIF) were used to estimate the cumulative probabilities of spleen reduction, transfusion independence, ECOG score <3, and blast percentage reduction over time in a competing risk setting with death from any cause as the competing event. The Aalen estimator method, based on the theory of counting processes, was used to estimate the standard error of the CIF [32]. The method of Kaplan-Meier was used to estimate the OS distribution with patients censored at the last date known to be alive. The reverse Kaplan-Meier method introduced by Schemper and Smith was used to estimate the median follow-up time, treating censored observations as ‘events’ and patients that were deceased as ‘censored’ [33]. Statistical analyses were performed with the SAS Version 9.4 (SAS Institute Inc., Cary, North Carolina) software package.
3. RESULTS
3.1. Baseline Demographics
Overall, a total of 42 patients treated with DEC were identified including 16 MPN-BP, 14 MPN-AP, and 12 MF-HR patients (Table 1). The median age for the MPN-BP and AP cohorts at the time of disease evolution were 66.5 and 67.3 years, respectively, while MF-HR patients were older with a median age of 72.3 years. There was no gender preference and the Charlson Comorbidity Index (CCI) scores varied widely. 42.9% (18/42) of the merged cohort had an initial MPN diagnosis of MF, 19.0% (8/42) post-PV MF, 28.6% (12/42) post-ET MF, and 9.5% (4/42) myelodysplastic syndrome (MDS)/MPN overlap syndrome. None of the MPN-BP patients had received more than three prior therapies, and only 2 (15.4%) MPN-AP patients had received more than three prior therapies. Of those who had received prior MPN therapy, hydroxyurea (47.6%) was the most common treatment prior to the current disease state requiring DEC therapy.
Table 1:
Baseline demographics by disease phase
| Patient Baseline Characteristics | |||
|---|---|---|---|
| AP (N=14) | HR (N=12) | Total (N=26) | |
| Age, Median (Range) | 67.3 (48.7–76.2) | 72.3 (58.0–82.8) | 70.3 (48.7–82.8) |
| Female Gender, N (%) | 4 (28.6%) | 6 (50.0%) | 10 (38.5%) |
| CCI | |||
| 0–1 | 8 (57.1%) | 3 (25%) | 11 (42.3%) |
| 2–4 | 4 (28.6%) | 4 (33.3%) | 8 (30.8%) |
| 4+ | 2 (14.3%) | 5 (41.7%) | 7 (26.9%) |
| Initial MPN | |||
| MF | 5 (35.7%) | 7 (58.3%) | 12 (46.2%) |
| PV | 1 (7.1%) | 3 (25.0%) | 4 (15.4%) |
| ET | 7 (50.0%) | 0 (0.0%) | 7 (26.9%) |
| MDS-MPN | 1 (7.1%) | 2 (16.7%) | 3 (11.5%) |
| Number of Prior Therapies | |||
| 0 | 3 (21.4%) | 1 (8.3%) | 4 (15.4%) |
| 1 | 7 (50.0%) | 5 (41.7%) | 12 (46.2%) |
| 2 | 2 (14.3%) | 6 (50.0%) | 8 (30.8%) |
| 3+ | 2 (14.3%) | 0 (0.0%) | 2 (7.7%) |
| Type of Prior Therapy | |||
| Cytoreductive | 11 (78.6%) | 7 (58.3%) | 18 (69.2%) |
| Hydroxyurea | 8 (57.1%) | 3 (25.0%) | 11 (42.3%) |
| IFN-alpha | 1 (7.1%) | 2 (16.7%) | 3 (11.5%) |
| JAK2 Inhibitor | 4 (28.6%) | 4 (33.3%) | 8 (30.8%) |
| Hypomethylating Agent | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Immunomodulating | 1 (7.1%) | 1 (8.3%) | 2 (7.7%) |
| Busulfan | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Erythropoietic/Danazol | 1 (7.1%) | 4 (33.3%) | 5 (19.2%) |
| Splenectomy | 1 (7.1%) | 2 (16.7%) | 3 (11.5%) |
| Genetics | |||
| JAK2 | 6 (42.9%) | 5 (71.4%) | 11 (42.3%) |
| Missing | N=0 | N=5 | N=5 |
| Myeloid Molecular Panel | |||
| MPL | 3 (50.0%) | 0 (0.0%) | 3 (11.5%) |
| CALR | 1 (16.7%) | 1 (25.0%) | 2 (7.7%) |
| Triple Negative | 0 (0.0%) | 1 (25.0%) | 1 (3.8%) |
| High Molecular Risk | 3 (50.0%) | 4 (100.%) | 7 (26.9%) |
| Missing | N=8 | N=8 | N=16 |
| Cytogenetic | |||
| Normal | 6 (41.9%) | 5 (41.7%) | 11 (42.3%) |
| Abnormal | 8 (57.1%) | 7 (58.3%) | 15 (57.7%) |
| Adverse | 5 (35.7%) | 2 (16.7%) | 7 (26.9%) |
| Baseline Blood Counts, Median (Range) | |||
| WBC, × 109/L | 6.4 (2.5–122.0) | 15.9 (1.7–218.1) | 13.1 (1.7–218.1) |
| Hemoglobin, g/dL | 8.9 (7.1–12.4) | 9.0 (6.2–13.5) | 9.0 (6.2–13.5) |
| Platelet, × 109/L | 180.0 (9.0–482.0) | 28.0 (8.0–602.0) | 100.0 (8.0–602.0) |
| Peripheral Blasts, % | 10.0 (1.0–26.0) | 3.5 (0.0–8.0) | 5.5 (0.0–26.0) |
| Tranfusion Dependence | 4 (28.6%) | 8 (66.7%) | 12 (46.2%) |
| ECOG | |||
| 0 | 4 (28.6%) | 1 (8.3%) | 5 (19.2%) |
| 1 | 5 (35.7%) | 7 (58.3%) | 12 (46.2%) |
| 2 | 5 (35.7%) | 4 (33.3%) | 9 (34.6%) |
| 3 | 0 (0.0%) | 0 (0.0%) | 0 (0%) |
BP = blast phase, AP = accelerated phase; HR = MF-”high risk”; CCI = Charlson Comorbidity Index; MPN = myeloproliferative neoplasm; MF = myelofibrosis; PV = polycythemia vera; ET = essential thrombocytopenia; MDS-MPN = myelodysplastic syndrome-myeloproliferative neoplasm
Overall, 83.3% (35/42) of patients included in this study had JAK2 mutational testing available and 38.1% (16/42) of patients had NGS results available at time of MF-HR, MPN-AP, or MPN-BP diagnosis. Of those who had JAK2 mutational testing, 60.0% harbored JAK2V617F with a median variant allele fraction (VAF) of 56.7% (range, 2.4 to 94.4). Additional available genomic data included 18.8% (3/16) MPL mutations, 25.0% (4/16) CALR mutations, and 12.5% (2/16) triple negative (JAK2, MPL, CALR). 68.8% (11/16) of available patients had a high molecular risk mutation (HMR), including 18.8% (3/16) with a TP53 mutation. Mutated JAK2 and ASXL1 were the most commonly co-occurring mutations (Figure 1). Additionally, 64% (27/42) of patients tested had abnormal karyotype and 38% (16/42) were unfavorable. Unfavorable karyotype was most common in MPN-BP patients with 56.3% (9/16) compared to 25.0% (5/14) in MPN-AP patients and 16.7% (2/12) in MF-HR patients. The most common karyotypic abnormality was −7/7q, which was present in 15.5% (7/42) of patients (1 MPN-BP, 5 MPN-AP, and 1 MF-HR) followed by 1q abnormality present in 7.1% (3/42) of patients (all MPN-BP). Co-occurrence of +1q and del(5q) occurred in one MPN-BP patient and with +8 in another patient. Co-occurrence of t(3;3) and monosomy 7 was identified in 1 MPN-BP patient and del(12p) and monosomy 7 in another MPN-BP patient.
Figure 1. Chord diagram by gene appearance.
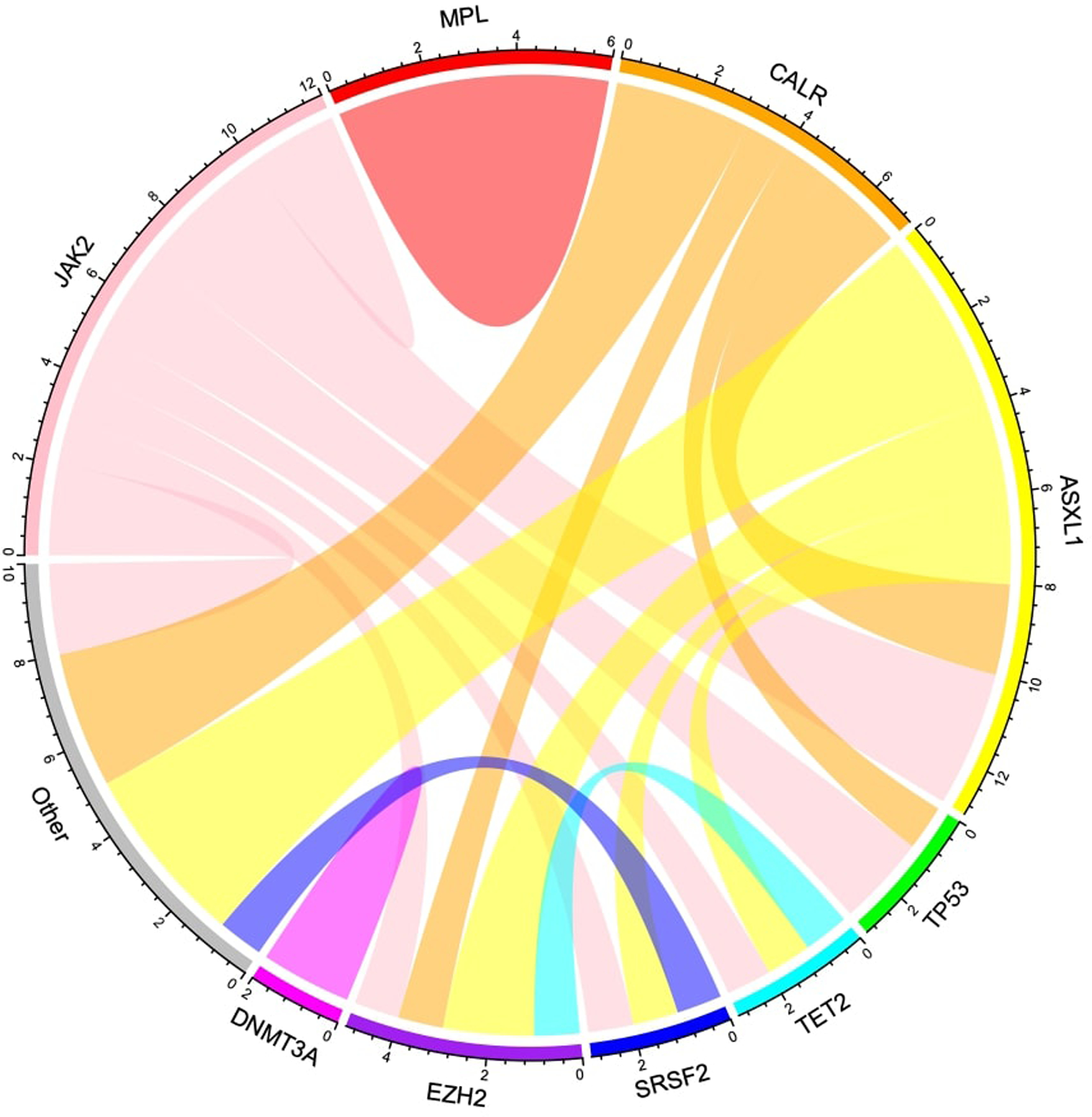
Mutated JAK2 and ASXL1 were the most commonly co-occurring mutations. MPL did not co-occur with other non-driver mutations while CALR and ASXL1 did not occur in isolation.
Over half of the patients were RBC transfusion dependent (54.8%, 23/42), most frequently in the MPN-BP cohort (68.8%, 11/16). ECOG score was also highest in MPN-BP patients with 17.6% (3/17) of patients noted to have an ECOG score of 3.
3.2. Decitabine
All patients received DEC 20 mg/m2 intravenously for five consecutive days every 4 weeks. The median number of cycles of DEC was 1.5 in MPN-BP, 4.5 in MPN-AP, and 6.5 in MF-HR patients (Table 2). Overall, 35.7% (15/42 – 6 BP, 6 AP, 3 HR) of patients received concurrent ruxolitinib therapy. Four patients with MPN-BP were enrolled in the Myeloproliferative Disorder Research Consortium (MPD-RC) 109 prospective trial of combination DEC and ruxolitinib (NCT02076191).
Table 2:
Decitabine administration by disease phase
| Decitabine Data | |||
|---|---|---|---|
| AP (N=14) | HR (N=12) | Total (N=26) | |
| Cycles of Decitabine | |||
| Median (Range) | 4.5 (1–56) | 6.5 (0–54) | 5.5 (1–56) |
| Time to Decitabine, months | |||
| Median (Range) | 0.5 (0–3) | 0 (0–0) | 0.2 (0–3) |
| Concurrent Therapy | |||
| Ruxolitinib | 6 (42.9%) | 3 (25.0%) | 9 (34.6%) |
| Other | 1 (7.1%) | 0 (0.0%) | 1 (3.8%) |
BP = blast phase; AP = accelerated phase; HR = MF-”high risk”
3.3. Outcomes
The median follow-up duration was 23.6 [2.1-NE] months in MPN-BP, 12.4 [2.1–48.8] months in MPN-AP, and 38.7 [1.8–61.5] months in MF-HR patient cohort. The median OS for the MPN-BP patients was 2.6 months, with the longest living patient alive at 24 months. The discrepancy between median follow-up and OS in the MPN-BP is due to the method used to estimate median follow-up (reverse Kaplan-Meier estimator), which does not consider deaths as events when calculating follow-up. Thus, early deaths in the MPN-BP group do not influence the median follow-up. Patients who received at least 2 cycles of DEC in the MPN-BP group had an OS of 6.7 months with a median duration of follow-up of 23.6 months. [3.8–29.8]. Median OS was not reached for MPN-AP and MF-HR patients, with one and two patients alive at 60 months, respectively (Figure 2, Figure 3). Across the integrated cohort of patients, DEC monotherapy (27 patients – 11 BP, 7 AP, 9 HR) was associated with a median OS of 12.9 months while DEC combination therapy with ruxolitinib (15 patients – 6 BP, 6 AP, 3 HR) was associated with a median OS of 21.0 months (Supplemental Figure 1).
Figure 2.
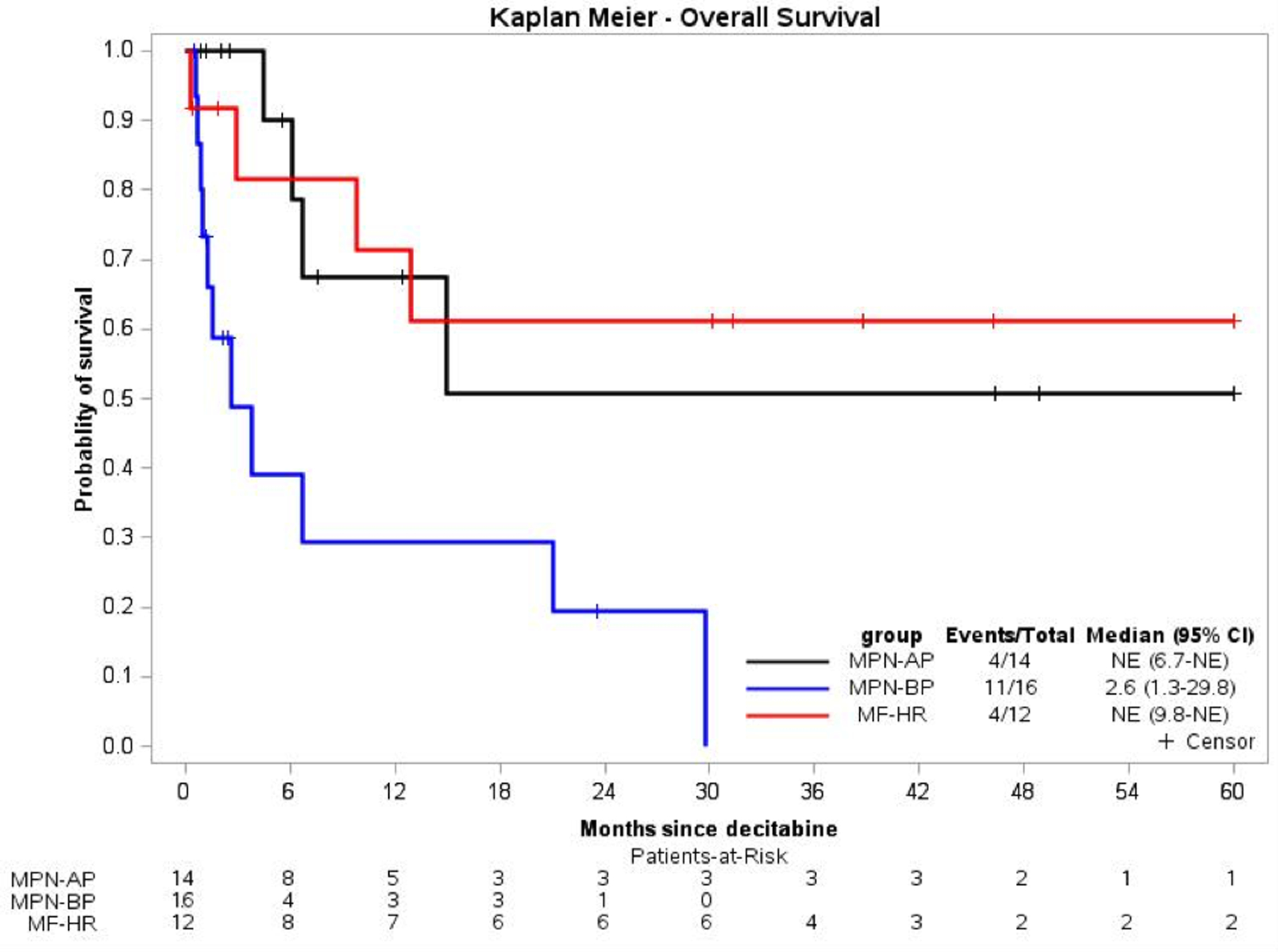
Kaplan-Meier plot of survival probability from decitabine initiation by disease phase.
Figure 3: Swimmers plots by disease phase for time receiving decitabine therapy.
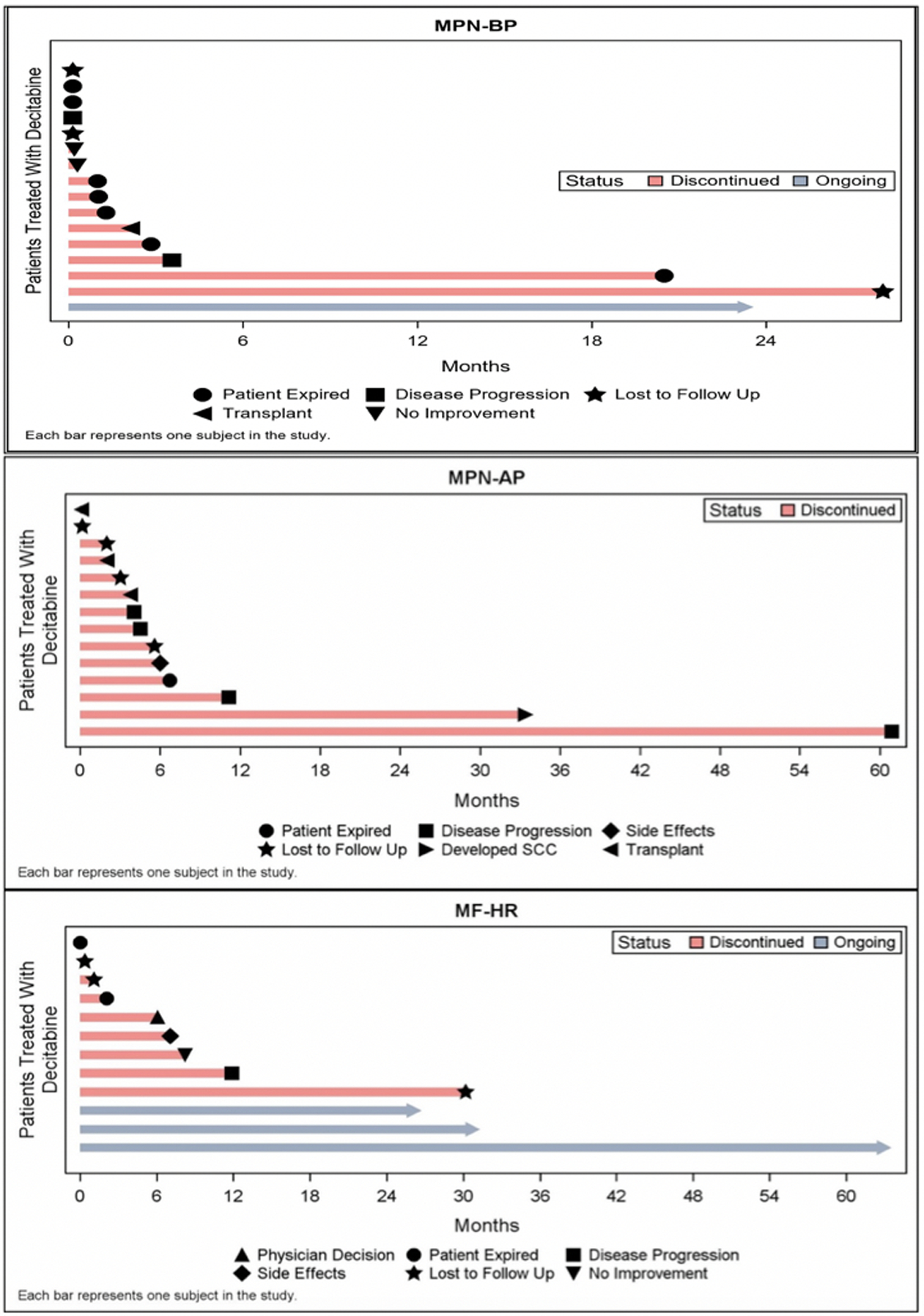
The most common reason for decitabine discontinuation in the MPN-BP and MF-HR cohorts is death, while in the MPN-AP cohort is disease progression.
Overall, the probability of spleen length reduction from moderate or massive splenomegaly to minimal splenomegaly within 12 months of initiating DEC therapy was 28.6% [95% CI: 9.5%−51.3%]. The 12-month probability of transfusion independence among patients transfusion dependent at the start of DEC, was 23.5% [95% CI: 9.0%−41.8%] and probability of improvement of ECOG score was 28% [95% CI: 11.7%−47%]. Lastly, probability of attaining a complete or 50% reduction in peripheral blast count within 12 months of DEC treatment was 54.6% [95% CI: 35.7%−70%] (Supplemental Table 1). Patients who achieved at least a 50% reduction in blast count within 24 months of initiating DEC were more likely to be spleen responders (p=0.05). No such association existed between blast reduction and transfusion independence (p=0.22) or OS (p=0.62). Follow up cytogenetics were not sufficiently available to assess for karyotypic response.
DEC was well tolerated with minimal clinically significant adverse events noted (Supplemental Table 2, Supplemental Table 3)). Bacterial infections were the most common complication, with the highest risk in the first month, and the prevalence was not significantly different between the patients who received concomitant ruxolitinib and those who did not. Viral infections (n=2), however, occurred only in patients receiving concurrent ruxolitinib. No cases of arterial thrombosis were observed, and all venous thrombotic events (n=3) were deep venous thromboses that occurred within the first 6 months of DEC therapy. Similarly, all hemorrhagic events (n=2: uncontrolled epistaxis requiring hospitalization, uncontrolled epistaxis requiring cauterization) occurred within the first three months of DEC therapy and were associated with extreme thrombocytopenia. Additionally, only two patients in the cohort had to discontinue treatment due to treatment related adverse events. The most common reasons for discontinuation were: death (n=10), progression of disease (n=7), transplantation (n=3), and no improvement while receiving DEC therapy (n=3).
4. DISCUSSION
MPN-BP has historically been associated with a dismal outcome with HSCT as the only therapeutic approach offering a potential for cure and long term survival [21, 22, 34]. Supportive care only is associated with a median OS of 3–5 months, and AML-like induction chemotherapy regimens do not meaningfully improve progression-free or OS in the absence of HSCT consolidation [16, 19, 17, 20]. DEC is Food and Drug Administration-approved for the treatment of MDS and frequently used off-label for the treatment of AML. DEC has also been shown to have clinical activity in MPN-BP with a favorable OS of 6–9 months and has emerged as a promising outpatient treatment option for this secondary AML population [24, 25]. The objective of this single-center, retrospective study, was to clarify the clinical benefit and tolerability of DEC across a group of advanced-phase MPN patients including “high risk” MF, MPN-AP, and MPN-BP.
Surprisingly, the MPN-BP cohort within this study had an associated median OS of only 2.6 months with DEC therapy. This was lower than reported from prospective studies and is comparable to historical cohorts of supportive care only. There are several potential explanations for this discrepancy. Importantly, patients treated by their local physician were not included in this study. Because these patients are likely to have a good performance status and be eligible for outpatient DEC therapy, the MPN-BP cohort included in our study is enriched with higher risk, sicker patients with a poor performance status, many of whom may require rapid initiation of DEC. This is reflected in the poor baseline performance status of our MPN-BP patients (50% ECOG 2/3) and the fact that 56% required hospitalization at time of DEC initiation. Secondly, half of the MPN-BP cohort (8 patients) had only 1 cycle of DEC before death. In a large phase III study of DEC in patients with AML, the median time to best response was 4.3 months [35]. Therefore, it is reasonable to conclude that a single cycle of DEC did not have sufficient time to achieve therapeutic effect and that these deaths were secondary to rapidly progressive disease. In fact, this poor OS of patients with MPN-BP suggests that many patients do not live long enough to garner benefit from DEC.
The MPN-AP and MF-HR cohorts did not reach a median OS. While these patients are anticipated to have better outcomes than MPN-BP, this difference in OS may also suggest optimal benefit of DEC when initiated early in the course of disease evolution. For comparison, in one single center study the OS of patients with MPN-AP and MF-HR (as defined in our study) was 24 [52–66] months and 28 [18–38] months, respectively[36]. Masarova et al. showed that patients with bone marrow blasts >5% or peripheral blood blasts 4–9% had a survival comparable to MPN-AP and should, therefore, be considered candidates for cytoreductive therapy with DEC or HSCT [36]. Furthermore, blast count influence on OS was independent of DIPSS score and driver mutation status. In agreement with Masarova and colleagues, MPN-AP and MF-HR patients treated with DEC in our study had similar survivals (p=0.85). Clinical benefit with DEC treatment was seen in terms of reduction in spleen size, transfusion burden, peripheral blood blast count, and improvement in performance status. DEC was well tolerated throughout the combined cohorts. Of note, patients in our cohort received DEC for 5 days, and not a 10-day regimen as is common in many European centers. This dosing schedule is standard at our institution and has recently been confirmed to be equivalent to 10 days in a head-to-head comparison in older patients with newly diagnosed AML [37].
It is important to highlight that 36% (15/42) of the cohort also received concurrent ruxolitinib therapy with a median OS of 21 months compared to 12.9 in the DEC monotherapy group, however, this difference was not statistically significant (p=0.77). This observation is notable given the recent final results of the Myeloproliferative Disorders Research Consortium (MPD-RC) 109 multicenter, phase 1/2 trial of combination DEC and ruxolitinib therapy in MPN-AP/BP patients. The median OS of the combined dose escalation cohorts was 7.9 months in the phase 1 study, and at the recommended phase 2 dose of 25 mg twice daily of ruxolitinib in cycle 1 and then 10 mg twice daily in subsequent cycles, a median OS of 9.7 months in the MPN-BP group was demonstrated [38, 39].
This current study is not without its limitations. Given the retrospective nature, details related to certain outcome measures may not be captured. For example, the cause of death was unknown in 10 patients. Additionally, a number of patients were lost to follow-up limiting survival analyses. Furthermore, the small sample size precluded multivariate analysis for OS. Finally, we have incomplete mutational data on the majority of our patients, reducing our ability to fully characterize this cohort of patients.
5. CONCLUSION
DEC either alone or in combination with ruxolitinib appears to have clinical benefit and the potential to extend survival in patients with advanced phase MPN when initiated early in disease course evolution to MPN-BP. For hospitalized patients or those with poor performance status, DEC is unlikely to significantly alter the natural course of disease. Bacterial infection was the most common treatment emergent adverse event. The combination of DEC and ruxolitinib may confer additional survival benefit over DEC alone and warrants further prospective evaluation. Overall, DEC is a viable treatment option with existing preclinical rationale and MPN murine modeling to support its use in patients with disease in evolution with a goal of survival extension and may be optimally employed prior to development of MPN-BP.
Supplementary Material
Supplemental Figure 1. Kaplan-Meier plot of survival probability from decitabine initiation with concurrent ruxolitinib. The median overall survival (OS) for the decitabine monotherapy cohort was 12.9 months while median OS for the decitabine combination ruxolitinib cohort was 21.0 months.
6. ACKNOWLEDGEMENTS
The authors wish to acknowledge Ami Patel for reviewing the manuscript.
9. FUNDING SOURCES
The authors wish to acknowledge the support of the Biostatistics Shared Resource Facility, Icahn School of Medicine at Mount Sinai, and NCI Cancer Center Support Grant P30 CA196521-01.
Footnotes
STATEMENT OF ETHICS
This study was approved by the Program for the Protection of Human Subjects at the Icahn School of Medicine at Mount Sinai. The authors state that this research complies with all internationally-accepted standards for research practice and is in compliance with the Helsinki Declaration.
DISCLOSURE STATEMET
JM received clinical research funding paid to the institution from Incyte, Novartis, Roche, Promedior, Merck, CTI Biopharma, Janssen, PharmaEssentia, Celgene, Merus, and Arog. Clinical trial steering committee member for Roche, Incyte, Celgene, and CTI Biopharma. MK has received research funding from Incyte, Celgene, Constellation, and Blueprint Medicines, and consulting fees from La Jolla Pharmaceutical. RH serves on the advisory board for Novartis and La Jolla Pharmaceuticals. The remaining authors have no conflicts of interest to disclose.
11. REFERENCES
- 1.Rampal R, Al-Shahrour F, Abdel-Wahab O, Patel JP, Brunel JP, Mermel CH, et al. Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014. May 29;123(22):e123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cervantes F, Tassies D, Salgado C, Rovira M, Pereira A, Rozman C. Acute transformation in nonleukemic chronic myeloproliferative disorders: actuarial probability and main characteristics in a series of 218 patients. Acta haematologica. 1991;85(3):124–7. [DOI] [PubMed] [Google Scholar]
- 3.Tefferi A, Guglielmelli P, Larson DR, Finke C, Wassie EA, Pieri L, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014. October 16;124(16):2507–13; quiz 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finazzi G, Caruso V, Marchioli R, Capnist G, Chisesi T, Finelli C, et al. Acute leukemia in polycythemia vera: an analysis of 1638 patients enrolled in a prospective observational study. Blood. 2005. April 1;105(7):2664–70. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkholm M, Derolf AR, Hultcrantz M, Kristinsson SY, Ekstrand C, Goldin LR, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011. June 10;29(17):2410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangat N, Wolanskyj AP, McClure RF, Li CY, Schwager S, Wu W, et al. Risk stratification for survival and leukemic transformation in essential thrombocythemia: a single institutional study of 605 patients. Leukemia. 2007. February;21(2):270–6. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Li CY, Mesa RA, Wu W, Hanson CA, Pardanani A, et al. Risk factors for leukemic transformation in patients with primary myelofibrosis. Cancer. 2008. June 15;112(12):2726–32. [DOI] [PubMed] [Google Scholar]
- 8.Passamonti F, Rumi E, Elena C, Arcaini L, Merli M, Pascutto C, et al. Incidence of leukaemia in patients with primary myelofibrosis and RBC-transfusion-dependence. British journal of haematology. 2010. September;150(6):719–21. [DOI] [PubMed] [Google Scholar]
- 9.Gangat N, Caramazza D, Vaidya R, George G, Begna K, Schwager S, et al. DIPSS plus: a refined Dynamic International Prognostic Scoring System for primary myelofibrosis that incorporates prognostic information from karyotype, platelet count, and transfusion status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011. February 1;29(4):392–7. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Wahab O, Manshouri T, Patel J, Harris K, Yao J, Hedvat C, et al. Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer research. 2010. January 15;70(2):447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang SJ, Rampal R, Manshouri T, Patel J, Mensah N, Kayserian A, et al. Genetic analysis of patients with leukemic transformation of myeloproliferative neoplasms shows recurrent SRSF2 mutations that are associated with adverse outcome. Blood. 2012. May 10;119(19):4480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vannucchi AM, Lasho TL, Guglielmelli P, Biamonte F, Pardanani A, Pereira A, et al. Mutations and prognosis in primary myelofibrosis. Leukemia. 2013. September;27(9):1861–9. [DOI] [PubMed] [Google Scholar]
- 13.Tefferi A, Lasho TL, Finke CM, Knudson RA, Ketterling R, Hanson CH, et al. CALR vs JAK2 vs MPL-mutated or triple-negative myelofibrosis: clinical, cytogenetic and molecular comparisons. Leukemia. 2014. July;28(7):1472–7. [DOI] [PubMed] [Google Scholar]
- 14.McNamara CJ, Panzarella T, Kennedy JA, Arruda A, Claudio JO, Daher-Reyes G, et al. The mutational landscape of accelerated- and blast-phase myeloproliferative neoplasms impacts patient outcomes. Blood advances. 2018. October 23;2(20):2658–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcellino BK, Hoffman R, Tripodi J, Lu M, Kosiorek H, Mascarenhas J, et al. Advanced forms of MPNs are accompanied by chromosomal abnormalities that lead to dysregulation of TP53. Blood Adv. 2018. December 26;2(24):3581–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: a single-institution experience with 91 cases. Blood. 2005. February 1;105(3):973–7. [DOI] [PubMed] [Google Scholar]
- 17.Tam CS, Nussenzveig RM, Popat U, Bueso-Ramos CE, Thomas DA, Cortes JA, et al. The natural history and treatment outcome of blast phase BCR-ABL- myeloproliferative neoplasms. Blood. 2008. September 1;112(5):1628–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tefferi A, Mudireddy M, Mannelli F, Begna KH, Patnaik MM, Hanson CA, et al. Blast phase myeloproliferative neoplasm: Mayo-AGIMM study of 410 patients from two separate cohorts. Leukemia. 2018. May;32(5):1200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Passamonti F, Rumi E, Arcaini L, Castagnola C, Lunghi M, Bernasconi P, et al. Leukemic transformation of polycythemia vera: a single center study of 23 patients. Cancer. 2005. September 1;104(5):1032–6. [DOI] [PubMed] [Google Scholar]
- 20.Noor SJ, Tan W, Wilding GE, Ford LA, Barcos M, Sait SN, et al. Myeloid blastic transformation of myeloproliferative neoplasms--a review of 112 cases. Leukemia research. 2011. May;35(5):608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy JA, Atenafu EG, Messner HA, Craddock KJ, Brandwein JM, Lipton JH, et al. Treatment outcomes following leukemic transformation in Philadelphia-negative myeloproliferative neoplasms. Blood. 2013. April 4;121(14):2725–33. [DOI] [PubMed] [Google Scholar]
- 22.Keyzner A, Han S, Shapiro S, Moshier E, Schorr E, Petersen B, et al. Outcome of Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Chronic and Advanced Phase Myelofibrosis. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2016. December;22(12):2180–86. [DOI] [PubMed] [Google Scholar]
- 23.Issa JP, Kantarjian HM. Targeting DNA methylation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009. June 15;15(12):3938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mascarenhas J, Navada S, Malone A, Rodriguez A, Najfeld V, Hoffman R. Therapeutic options for patients with myelofibrosis in blast phase. Leukemia research. 2010. September;34(9):1246–9. [DOI] [PubMed] [Google Scholar]
- 25.Badar T, Kantarjian HM, Ravandi F, Jabbour E, Borthakur G, Cortes JE, et al. Therapeutic benefit of decitabine, a hypomethylating agent, in patients with high-risk primary myelofibrosis and myeloproliferative neoplasm in accelerated or blastic/acute myeloid leukemia phase. Leukemia research. 2015. September;39(9):950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tam CS, Kantarjian H, Cortes J, Lynn A, Pierce S, Zhou L, et al. Dynamic model for predicting death within 12 months in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009. November 20;27(33):5587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genoptix. Myeloid Molecular Panel. Carlsbad, CA. [Google Scholar]
- 28.Hussein K, Pardanani AD, Van Dyke DL, Hanson CA, Tefferi A. International Prognostic Scoring System-independent cytogenetic risk categorization in primary myelofibrosis. Blood. 2010. January 21;115(3):496–9. [DOI] [PubMed] [Google Scholar]
- 29.Najfeld V, Tripodi J, Scalise A, Silverman LR, Silver RT, Fruchtman S, et al. Jumping translocations of the long arms of chromosome 1 in myeloid malignancies is associated with a high risk of transformation to acute myeloid leukaemia. British journal of haematology. 2010. November;151(3):288–91. [DOI] [PubMed] [Google Scholar]
- 30.Caramazza D, Begna KH, Gangat N, Vaidya R, Siragusa S, Van Dyke DL, et al. Refined cytogenetic-risk categorization for overall and leukemia-free survival in primary myelofibrosis: a single center study of 433 patients. Leukemia. 2011. January;25(1):82–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gale RP, Barosi G, Barbui T, Cervantes F, Dohner K, Dupriez B, et al. What are RBC-transfusion-dependence and -independence? Leukemia research. 2011. January;35(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aalen O Nonparametric Inference for a Family of Counting Processes. [Google Scholar]
- 33.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Controlled clinical trials. 1996. August;17(4):343–6. [DOI] [PubMed] [Google Scholar]
- 34.Lancman G, Brunner A, Hoffman R, Mascarenhas J, Hobbs G. Outcomes and predictors of survival in blast phase myeloproliferative neoplasms. Leukemia research. 2018. July;70:49–55. [DOI] [PubMed] [Google Scholar]
- 35.Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012. July 20;30(21):2670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masarova L, Bose P, Newberry K, Abou Zahr A, Cortes JE, Kantarjian HM, et al. Correlation between blast percentage in myelofibrosis and outcomes: a single-center experience. Journal of Clinical Oncology. 2017; 35(15_suppl):e18562–e62 [Google Scholar]
- 37.Short NJ, Kantarjian HM, Loghavi S, Huang X, Qiao W, Borthakur G, et al. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: a randomised phase 2 trial. Lancet Haematol. 2019. January;6(1):e29–e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rampal RK, Mascarenhas J, Kosiorek HE, Bhave R, Hexner EO, Wang ES, et al. Efficacy of Combined Ruxolitinib and Decitabine in Patients with Accelerated and Blast-Phase Myeloproliferative Neoplasms: Results of a Phase II Study. Blood. 2018;132(Suppl 1):3027. [Google Scholar]
- 39.Rampal RK, Mascarenhas JO, Kosiorek HE, Price L, Berenzon D, Hexner E, et al. Safety and efficacy of combined ruxolitinib and decitabine in accelerated and blast-phase myeloproliferative neoplasms. Blood advances. 2018. December 26;2(24):3572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Kaplan-Meier plot of survival probability from decitabine initiation with concurrent ruxolitinib. The median overall survival (OS) for the decitabine monotherapy cohort was 12.9 months while median OS for the decitabine combination ruxolitinib cohort was 21.0 months.


