Abstract
Background
Bone retains regenerative potential into adulthood, and surgeons harness this plasticity during distraction osteogenesis (DO). The underlying biology governing bone development, repair, and regeneration are divergent between the craniofacial and appendicular skeleton. Each type of bone formation is characterized by unique molecular signaling and cellular behavior. Recent discoveries have elucidated the cellular and genetic processes underlying skeletal development and regeneration, providing an opportunity to couple biological and clinical knowledge in order to improve patient care.
Method
A comprehensive literature review of basic and clinical literature regarding craniofacial and long bone development, regeneration, and DO was performed.
Results
The current understanding in craniofacial and long bone development and regeneration are discussed, and clinical considerations for the respective DO procedures are presented.
Conclusions
DO is a powerful tool to regenerate bone and thus address a number of craniofacial and appendicular skeletal deficiencies. The molecular mechanisms underlying bone regeneration, however, remain elusive. Recent work has determined that embryological morphogen gradients constitute important signals during regeneration. Additionally, striking discoveries have illuminated the cellular processes underlying mandibular regeneration during DO, showing that skeletal stem cells reactivate embryological neural crest transcriptomic processes to carry out bone formation during regeneration. Furthermore, innovative adjuvant therapies to complement DO utilize biological processes active in embryogenesis and regeneration. Additional research is needed to further characterize the underlying cellular mechanisms responsible for improved bone formation through adjuvant therapies and the role skeletal stem cells play during regeneration.
INTRODUCTION
The skeleton possesses unprecedented biomedical research potential, and translational research in skeletal biology may significantly benefit patients. The incidence of skeletal dysplasia is 1:5,000 live births1,2 while traumatic fracture, osteoporosis, and arthritis are the predominant skeletal disorders during aging3–5. The annual healthcare cost of fracture repair resulting from osteoporosis is approximately $17 billion in the United States alone3,6. With the large burden of skeletal pathologies permeating all stages of life, uncovering the fundamental biological processes driving skeletal development and regeneration is essential in the development of novel targeted therapies.
The skeleton consists of specialized connective tissues including ossified and non-ossified elements, bone marrow stroma, and supportive tissues7. Numerous cell types make up these tissues, including osteocytes, chondrocytes, hematologic, and stromal cells. The common progenitor cell that gives rise to the bone, cartilage, and stromal elements during development, repair, and regeneration is the skeletal stem cell (SSC)8,9. Recent discoveries highlight the significant role of the SSC as the enactor of mandibular regeneration during distraction osteogenesis (DO) – the process of lengthening bone through endogenous tissue engineering using a guided mechanical environment10. Additionally, morphogens expression is tightly regulated to provide signaling gradients necessary for skeletal growth. We review the current knowledge of the developmental and regenerative biology of the craniofacial and appendicular skeleton.
DEVELOPMENT OF CRANIAL BONES
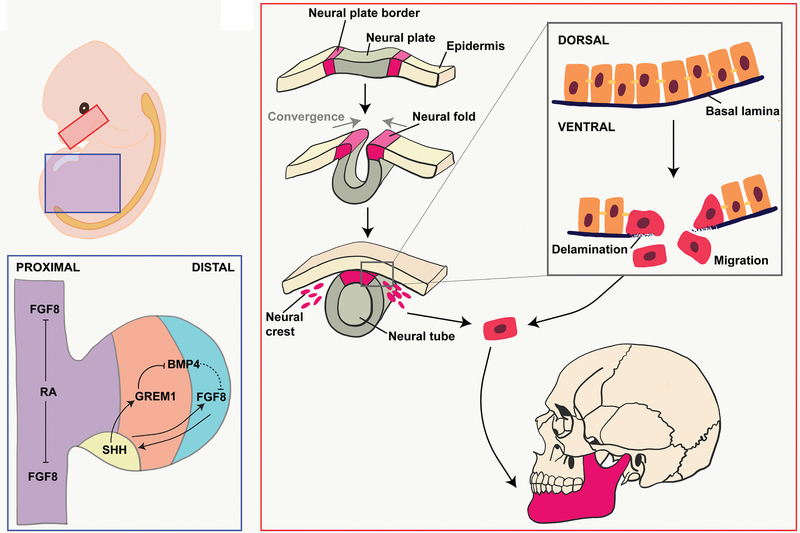
Most cranial bones arise from ectodermal neural crest cells (NCCs), which originate from the dorsal margins of the closing neural tube (Figure 1). During neurulation, the borders of the neural plate converge at the dorsal midline to form the neural tube. At this point, the NCCs from the roof plate undergo epithelial-to-mesenchymal transition during nueralation12–14, including delamination and migration events (Figure 1). Delamination begins with the dorsal expression of bone morphogenetic proteins (BMP), which lead to decreased expression of occludins and cadherins resulting in reduced cellular adhesion13,15. Occludins are an integral component of tight junctions, while cadherins are important in the formation of adherens junctions16,17. Both occludins and cadherins are important in maintaining cell-to-cell adhesion. Concurrently, NCCs secrete matrix metalloproteinases, which break down the overlying basal lamina13,18,19. The permeable basal lamina and decreased cellular attachments enable NCCs to migrate throughout the embryo.
Figure 1. Embryonic View of Bone Development.
(A) Developing mandible (red box) and lower limb (blue box). (B) Endothelial-to-mesenchymal transition of cranial neural crest cells. (C) Signaling throughout the limb bud trunk (purple), zone of polarizing activity (yellow), progress zone (orange), and apical endodermal ridge (blue).
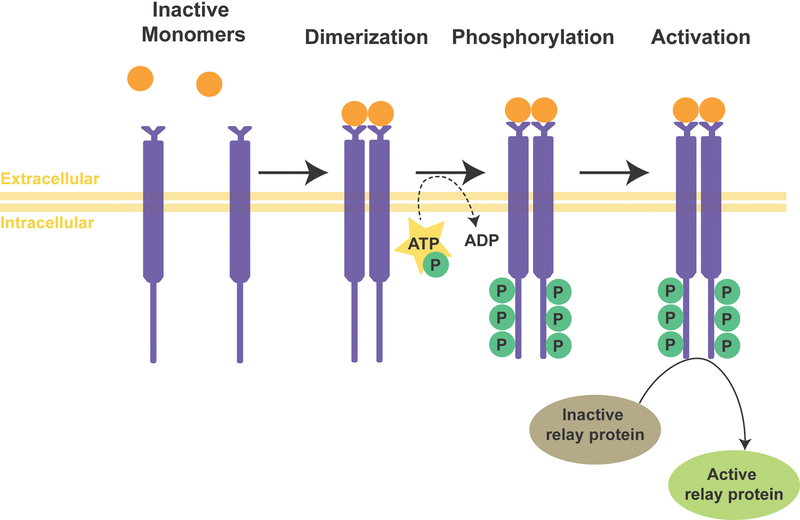
NCCs migrate from rostral to caudal due to repulsive guidance between molecular signals, extracellular matrix interactions, and cellular contact inhibition20,21. Expression of the Eph receptor tyrosine kinase by NCCs allows them to bind to the ephrin transmembrane ligand, leading to cytoskeletal rearrangement and cellular repulsion22. In general, tyrosine kinases catalyze the phosphorylation of tyrosine residues, which cause a functional change in the protein23 (Figure 2). Both Eph and ephrin ligands are membrane-bound proteins which require direct cell-cell interactions for activation. NCCs, additionally, express integrin α5β1 which guides migration by binding to ligands such as collagen, laminin, and fibronectin on the extracellular matrix24,25. Integrins are transmembrane receptors that activate signal transduction pathways mediating cellular-extracellular matrix interactions and intracellular cytoskeleton rearrangements26.
Figure 2. Tyrosine Kinase Pathway.
The single pass, type I receptor tyrosine kinase resides in the plasma membrane. The receptor tyrosine kinase is activated through the binding of a ligand leading to a ligand-induced dimerization with the cytoplasmic tyrosine kinase domain. The dimerization results in autophosphorylation of the tyrosine residues inducing conformational changes which stabilize the active site of the kinase. The phosphotyrosine residues act as recruitment sites for downstream signaling proteins.
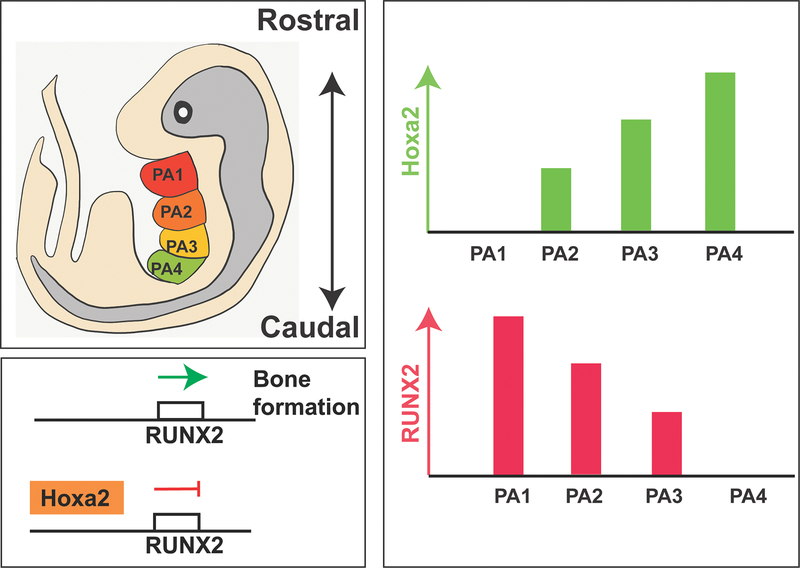
After cranial NCCs colonize the facial prominences, the cells aggregate, condense, and differentiate in response to signals from the surrounding niche27. NCCs that colonize the first arch form the maxilla and mandible21. The transcriptional profile of NCC populations uniquely corelate with their origination on the neural tube anterior-posterior axis. For example, Hox gene expression in the pharyngeal arch cell populations increases in the posterior direction28,29. Hox genes are homeobox genes that specify body plan regions along the head-tail axis30. The Hox proteins ensure the correct structures form in the correct places of the body. Overexpression of Hoxa2 in the first pharyngeal arch limits mandible formation31–33 (Figure 3A). Furthermore, Hoxa2 has been shown to suppress the expression of Runx2, which is important in skeletogenesis. Runx2 promotes bone differentiation, and inhibition of Runx2 through Hoxa2 expression limits bone formation34–36 (Figure 3B). As craniofacial bone development proceeds through intramembranous ossification, absence of Hoxa2 expression is critical for bone formation in the first pharyngeal arch (Figure 3C)35.
Figure 3. Hoxa2/Runx2 Pathway.
(A) Mouse embryo with developing pharyngeal arches (PA). PA1 (red) gives rise to the muscles of mastication and mandible. PA2 (orange) gives rise to the muscles of facial expression and hyoid bone. PA3 (yellow) gives rise to the greater horn and lower body of the hyoid bone. PA4 (green) gives rise to the thyroid and cricoid cartilage. (B) In the absence of Hoxa2, Runx2 activation will occur leading to bone formation. In the presence of Hoxa2, Runx2 will be suppressed limiting bone formation. (C) The expression of Hoxa2 increases in the caudal direction of the pharyngeal arches with PA1 not having expression of Hoxa2, while PA4 possesses a high level of Hoxa2 expression. Analogously, the expression of Runx2 decreases in the caudal direction of the pharyngeal arches with PA1 having the greatest expression of Runx2, while PA4 possesses a low level of Runx2 expression.
Intramembranous ossification is a process distinct to bone development of the mandible, clavicle, and most bones of the skull. Intramembranous ossification initiates during fetal development in utero, and the skull and clavicles are not fully ossified at birth37. These bones fully ossify at different post-natal time points and follow a similar ossification paradigm: (1) formation of ossification center, (2) matrix formation, (3) periosteum weaving, and (4) compact bone formation37. A concentration of mesenchymal cells differentiate into bone-depositing osteoblasts that cluster to form an ossification center38. Next, the osteoblasts secrete collagenous matrix proteins, or osteoids, which calcify and confine the osteoblasts. Once the osteoblasts are embedded onto the osteoid, the osteoblasts develop into osteocytes. Synchronously, osteogenic cells from adjacent connective tissue differentiate into osteoblasts on the periphery of the growing bone. Ongoing bone deposition allows collections of osteoids to congregate near capillaries, forming the trabecular matrix of spongy bone. Osteoblasts on the periphery of the spongy bone develop into the periosteum. This newly formed periosteum produces compact bone around the spongy bone, while the spongy bone surrounding nearby blood vessels condenses into bone marrow37. Intramembranous ossification thus results in formation of the bone without an intermediate cartilaginous anlage.
DEVELOPMENT OF LONG BONES
Long bone development begins with outgrowth of the limb buds from the trunk (Figure 1) in the presumptive forelimb and hindlimb locations. Cells from the lateral plate mesoderm migrate to create a mass of proliferative bone progenitor cells known as the limb field. Three areas of significance form to pattern the growing limb bud: apical ectodermal ridge (AER), progress zone, and zone of polarizing activity (ZPA) (Figure 1). The AER is a structurally distinct ridge of epithelium located at the distalmost extent of the limb bud. The AER bisects the dorsal and ventral aspects of the growing limb bud and is necessary for limb outgrowth39. Second, the progress zone is a mass of cells found underneath the AER40,41. The progress zone is necessary for limb type specification, with cells in this zone harboring intrinsic properties to determine limb type. The third structure is the ZPA, which is restricted to the posterior aspect of the bud and provides signals directing limb bud growth along the anterior-posterior axis42.
Morphogenetic signaling gradients are central to direct limb length and patterning during development. For example, proximal-distal specification relies upon the antagonistic relationship between retinoic acid (RA) and fibroblast growth factor (FGF) 843,44. Cell fates are influenced by a proximal source of RA originating from the embryonic trunk and a distal source of FGF-8 originating from the AER45. Anterior-posterior specification occurs through Sonic hedgehog, BMP, and Gremlin signaling, which originates in the ZPA domain42,46–48, while dorsal-ventral specification relies upon a gradient of WNT and BMP49. Long bone development further continues through endochondral ossification.
Endochondral ossification is responsible for bone formation of all skeletal elements other than the craniofacial bones and clavicle. This begins when progenitor cells differentiate into chondrocytes and synthesize extracellular matrix abundant in Type II collagen37,50–54. This cartilaginous model prefigures the shape of ossified bone and enlarges through chondrocyte proliferation. The chondrocytes are divided into three zones during this process. First is the zone of proliferation, located in the center and contains rapidly dividing chondrocytes. These cells stop proliferating in the zone of maturation. The outermost, hypertrophic zone is composed of chondrocytes that secrete a distinct matrix containing Type X collagen37,50,54–59. Concomitantly, the hypertrophic chondrocytes direct the cells in the perichondrium to differentiate into osteoblasts60–62. Moreover, angiogenesis of the hypertrophic zone and perichondrium allow ossification of the cartilage matrix by the invading osteoblasts11,63–67.
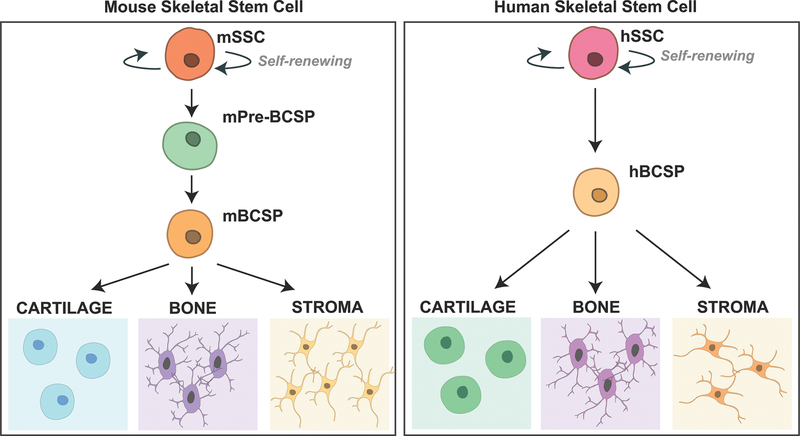
Our understanding of the cellular basis in limb development has greatly advanced with the recent discovery of the SSC. These cells were first identified in the femoral growth plates of mice, and possess the ability to self-renew and differentiate into bone, cartilage, and stromal subtypes8. Further evidence supporting the intrinsic ability of the mouse SSC (mSSC) and its downstream progenitor cells to generate these tissue types included production of ossicle, cartilage, and marrow after transplantation of purified cells into a kidney capsule niche8 (Figure 4A). The corresponding human SCC was subsequently isolated from the femoral growth plate, exhibiting similar properties of self-renewal and differentiation into each skeletal tissue type9 (Figure 4B).
Figure 4. Skeletal Stem Cell Hierarchy.
(A) The skeletal stem cell hierarchy in mice beginning with a self-renewing mouse Skeletal Stem Cell (mSSC) differentiating to lineage-restricted bone, cartilage, and stromal cells through a Bone, Cartilage, Stromal Progenitor (BCSP) cell8. (B) The skeletal stem cell hierarchy in humans beginning with a self-renewing human Skeletal Stem Cell (hSSC) differentiating to lineage-restricted bone, cartilage, and stromal cells through a Bone, Cartilage, Stromal Progenitor (BCSP) cell9.
The identification of the SSC has illuminated important skeletal biology. For example, downstream progenitor subsets that are derived from the mSSC have been shown to execute long bone fracture repair68. Following femoral fracture, these cells exhibited increased cell frequency, viability, and enhanced osteogenic function. Intriguingly, the injury-responsive mSSC transcriptional profile showed upregulation of the same genes and signaling morphogens important in long bone embryogenesis, such as BMP and Hedgehog68. These data highlight the molecular overlap between long bone embryogenesis and regeneration.
With regards to the role of the SSC in regenerative contexts, a recent study explored the behavior of mSSCs during mandibular distraction osteogenesis (MDO). This work revealed the bone regenerate was clonally derived from mSSCs10. The pathway by which mSSCs respond to the mechanical force of MDO involves upregulation of focal adhesion kinase (FAK) signaling10 (Figure 5). The underlying genetic programs responding to FAK signal transduction in mSSCs included activation of transcriptional elements characteristic of primitive NCCs10,69. This genetic reversion of mandibular mSSCs back to primitive NCCs characteristics underscores the importance of understanding bone embryogenesis as it applies to bone regeneration biology. Furthermore, the fact that MDO can proceed in patients with Type II collagenopathy harken back to intramembranous ossification, which occurs without a cartilage intermediate70. In fact, mSSCs are capable of forming new mandibular bone through intramembranous ossification during MDO, thereby recapitulating developmental processes during regeneration10.
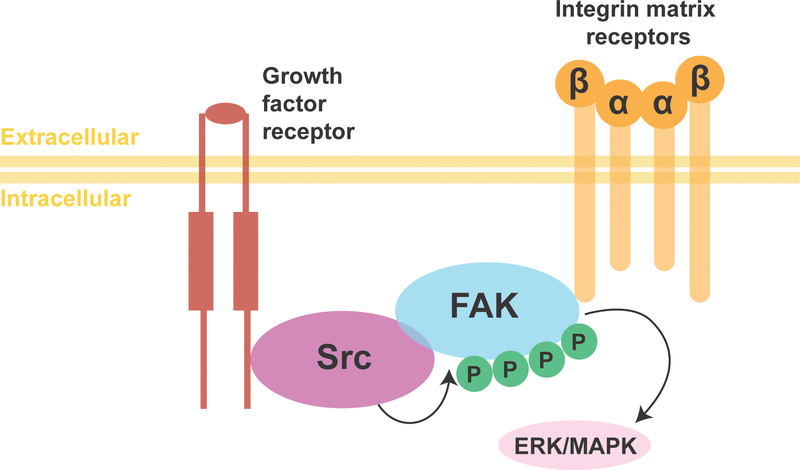
Figure 5. Focal Adhesion Kinase Signaling Pathway.
Cytoplasmic tyrosine kinase FAK becomes activated after interacting with transmembrane integrin proteins allowing FAK to form a complex with Src family kinase. The complex initiates downstream signaling pathways through the phosphorylation of other proteins such as ERK/MAPK.
ADJUVANT THERAPIES FOR MANDIBULAR DISTRACTION OSTEOGENESIS
MDO is an advantageous strategy to treat mandibular hypoplasia, which is a feature of multiple clinical problems related to mandibular deficiencies. While generally performed in children, MDO may be applied to adult populations, as well71,72. MDO is a dynamic process rather than a single intervention. Common corticotomies used include the oblique body/angle cut, vertical ramus cut, and inverted-L ramus cut, which vary in use based upon age and mandibular anatomy71,73. Postoperatively, the distraction protocol begins with a variable period of 0–5 days. After latency, distraction occurs with a total rate of 1 to 2 mm of distraction per day divided over a frequency of 1 to 4 times per day. The final phase of MDO involves consolidation of the regenerate over a time period of 6 to 12 weeks71,74. The overall complication rate ranges from 20–40%, and a technical learning curve may exist with increased complication rates noted earlier in a surgeon’s experience75. Major postoperative compilations of MDO include malunion/nonunion, premature consolidation, and relapse75.
The ability of adjuvant therapies to enhance the MDO surgeries has been studied using various animal models. Deferoxamine (DFO) accelerates bone consolidation in rats undergoing MDO76 by chelating iron, which result in the stimulation of the hypoxia inducible factor 1-α (HIF-1α) pathway. HIF-1α is a subunit of the heterodimeric transcription factor HIF-1, which is considered the master transcriptional regulator for cellular and developmental response towards hypoxia77–79. With regards to regeneration, the upregulation of HIF-1α led to improved wound healing of damaged tissue in mice, while down-regulation of HIF-1α resulted in diminished wound closure80. Specifically, in terms of bone regeneration, mSSC chromatin architecture sequencing revealed that the HIF-1α transcriptional network plays a substantial role during MDO10. Additionally, a recent case report showed improved pterygomaxillary area and density in a patient receiving DFO during MDO after irradiation, highlighting the potential clinical relevance of DFO as an adjuvant therapy during MDO81.
Another prominent molecule that supports MDO bone regeneration is BMP, with high levels of expression confirmed during distraction and subsequent decline during consolidation82. BMP interacts with cell surface receptors known as BMP receptors. The interaction leads to signal transduction resulting in the mobilization of members from the SMAD family of proteins, which are essential for fracture repair and bone growth83. Recombinant human BMP-2 (rhBMP-2) has been approved for patient administration to improve fracture repair in the tibia and for specific spinal indications. Contraindications for use include any type of anterior cervical spine fusion and soft tissue swelling near the esophagus and trachea, given the propensity of rhBMP to cause swelling in this region. Other reports have also highlighted problems with ectopic bone growth and variability in dosage delivered with current carrier systems84. Aside from BMP, vascular endothelial growth factor and FGF-2 expression have also been found to increase during consildation85,86. Exogenous growth factor administration along similar timelines holds potential to promote improved bone formation during MDO87.
Alternatively, mesenchymal stem cells (MSC) have been studied as a cellular therapeutic to enhance bone consolidation in the setting of MDO. Sheep hemi-mandibles treated with MSCs on the first day of consolidation had greater total and compact bone ratio in the regenerate zone88. Another study examined endogenous recruitment of MSCs to the site of bone formation using a rat model of MDO; the stromal cell-derived factor-1/chemokine receptor-4 pathway activation was found to promote migration of MSCs to the distraction site89. However, these studies were not able to determine contribution efficiency of the recruited MSCs to the distraction regenerate.
Non-invasive therapies that aid in bone formation during MDO include low-level laser (LLL) therapy and low-intensity pulsed ultrasound (LIPUS). LLL therapy consists of an 800 nm wavelength range daily laser treatments during distraction and/or consolidation showing improved bone regeneration in rabbits90. How LLL promotes bone formation from a mechanistic standpoint, however, remains poorly understood. Compared to LLL therapy, there is more insight into the cellular mechanism of LIPUS in promoting of bone formation. The technology utilizes low intensity and pulsed mechanical waves in order to induce regenerative and anti-inflammatory effect on bone, cartilage, and tendon91. The mechanism by which LIPUS induces regenerative effects is unknown; however, one theory is the non-thermal phenomena, where the mechanical waves cause changes in cellular physiology92–94. As such, FAK, which was shown to be upregulated in mSSCs during mouse MDO, may have an important role as mechanical signals are transduced into cellular signals10.
ADJUVANT THERAPIES FOR LIMB DISTRACTION OSTEOGENESIS
Just as MDO may be used to address a wide range of underlying pathology, limb distraction osteogenesis (LDO) is applied to various bone and soft tissue deficits of the appendicular skeleton. These include limb length discrepancies, oncologic resection, traumatic deformity correction, and treatment of ankle osteoarthritis95–98. Clinical procedures for LDO are similar to MDO, and preoperative imaging and planning are the first steps. Careful evaluation of bone vascular health and surrounding soft tissue is important for LDO99. Postoperative protocols also mirror MDO distraction strategies, with 5–7 days of latency and subsequent distraction carried out at 0.75–1.0 mm per day, paired with ongoing physical therapy into the consolidation phase100,101. The complication rate of appendicular distraction appears to be higher than mandibular distraction. The most frequent complications are frame-related followed by nonunion99. The data are heterogenous, with some retrospective studies noting total number of complications exceeding the number of patients enrolled in the study102,103.
Similar to MDO, bone marrow MSCs have been studied to determine their therapeutic role in reducing the treatment time of LDO. One rabbit study utilized autogenous bone marrow MSCs from the tibia, with transplantation of one million cells into the distraction gap after four to six ex vivo passages104. Another study in dogs used allogenic bone marrow MSCs from the tibia and transplanted one million cells into the distraction gap after three passages105. While MSC administration led to faster bone formation, heterogenous cell culture and administration methodology precludes clinical application at this time.
LIPUS has also been investigated in the setting of tibial DO. In the study, twenty minutes of therapy at a frequency of 1.5 MHz and impulse length of 200 μsec daily throughout the distraction period demonstrated an increased radiologic callus density by 33%106. Another study applied the same parameters of LIPUS therapy during both distraction and consolidation, which led to faster healing107. While the use of LIPUS as a potential adjuvant therapy in LDO is promising, quantification of bone formation is inconsistent impairing direct comparisons.
Overall, data surrounding the use of adjuvant therapies to augment LDO are not robust, and many strategies are extrapolated from their use in MDO. For example, LLL therapy has not been studied in the context of LDO. However, given its promising results in MDO, LLL therapy holds potential to enhance bone formation during LDO. Overall, understanding the biological basis of DO will continue to illuminate potential translational therapies to improve bone regeneration and thus clinical outcomes. Furthermore, interpreting these strategies from the perspective of resident SSCs in long bone, from which they were first described, will be key for appreciating how these approaches may be clinically translated.
CONCLUSION
Although progress has been achieved in skeletal biology research, the fundamental understanding of regulatory mechanisms governing bone development and regeneration remain elusive. Studies have uncovered the morphogens and transcription factors necessary for skeletal growth; however, the role of these factors in bone regeneration has yet to be been determined. Discovery of the SSC has improved our understanding of the cellular underpinnings of bone regeneration, highlighting that recapitulation of developmental processes in various postnatal processes. Harnessing these cells as therapeutic targets may prove a powerful tool in addressing the clinical challenges of both MDO and LDO, as well as other skeletal disorders. Further research should seek to better understand the molecular biology of SSCs, along with their precise behavior during tissue production, maintenance, and repair. Developmental mapping of skeletal tissue, including characterization of cellular, molecular, and genetic patterns giving rise to craniofacial and long bones are crucial in understanding bone regenerative processes and the role of adjuvant therapies during treatment. Current data examining adjuvant therapies to enhance bone formation during DO are preliminary; continued work to determine the biological basis of DO will inform the development of innovative surgical techniques and adjunctive treatments. Furthermore, the biological understanding of MDO is more advanced than LDO in regard to development, regeneration, and adjuvant therapy outcomes, which presents a key opportunity to progress the scientific knowledge surrounding LDO. Advances in our grasp of skeletal regenerative and developmental biology hold potential for translation of clinical interventions to provide patients with improved solutions for skeletal defects and injury.
Table 1.
Table of abbreviations used throughout the review with adjoining definitions.
| Abbreviation | Full Name | Definition |
|---|---|---|
| DO | distraction osteogenesis | the process of lengthening bone through endogenous tissue engineering using a guided mechanical environment |
| SSC | skeletal stem cell | reside in the postnatal bone marrow and give rise to cartilage, bone, hematopoiesis-supportive stroma |
| NCC | neural crest cell | temporary group of cells arising from the embryonic ectoderm cell layer, and giving rise to a diverse cell lineage |
| BMP | bone morphogenic protein | growth factors consisting of pivotal morphogenetic signals determining tissue architecture throughout the body |
| AER | apical ectodermal ridge | structure that forms at the distal end of the limb bud acting as a major signaling center to ensure proper development of a limb |
| ZPA | zone of polarizing activity | area of the developing limb that contains signals which instruct the developing limb bud to form along the anterior/posterior axis |
| RA | retinoic acid | a metabolite for vitamin A1 that is required for growth and development |
| FGF | fibroblast growth factor | Cell signaling proteins that are crucial in normal development |
| mSSC | mouse skeletal stem cell | skeletal stem cells isolated from mouse bones |
| MDO | mandibular distraction osteogenesis | a surgical procedure that lengthens the lower jaw |
| FAK | focal adhesion kinase | protein tyrosine kinase concentrated in focal adhesions forming amongst cells attaching to extracellular matrix |
| DFO | deferoxamine | a medication that binds iron and aluminum |
| HIF-1α | hypoxia inducible factor 1-α | transcription factor that respond to hypoxia |
| rhBMP-2 | recombinant human BMP-2 | BMPs generated using recombinant DNA technology for clinical use |
| MSC | mesenchymal stem cell | multipotent stromal cells that can differentiate into a variety of cell types |
| LLL | low-level laser | application of red and near infra-red light over injuries or lesions to improve wound and soft tissue healing and reduce inflammation |
| LIPUS | low-intensity pulsed ultrasound | low intensity and pulsed mechanical waves used to induce regenerative and anti-inflammatory effects on bone, cartilage, and tendon |
| LDO | limb distraction osteogenesis | A surgical procedure that lengthens long bones of the appendicular skeleton including the tibia and femur |
Acknowledgments
FINANCIAL DISCLOSURE STATEMENT:
This work was supported by the National Institutes of Health (NIH) R01DE026730 (M.T.L.), R01DE027323 (M.T.L.), R01DE027346 (D.C.W.), and 5T32GM119995-02 (H.N.S.); Hagey Lab for Pediatric Regenerative Medicine (M.T.L.); and a generous gift from Carmelita Ko and Keith Tsu (D.C.W.). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this manuscript.
REFERENCES
- 1.Orioli IM, Castilla EE, Barbosa-Neto JG. The birth prevalence rates for the skeletal dysplasias. J Med Genet. 1986;23(4):328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krakow D Skeletal dysplasias. Clin Perinatol. 2015;42(2):301–319, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis JA, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44(5):734–743. [DOI] [PubMed] [Google Scholar]
- 5.Unnanuntana A, Gladnick BP, Donnelly E, Lane JM. The assessment of fracture risk. J Bone Joint Surg Am. 2010;92(3):743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray NF, Chan JK, Thamer M, Melton LJ 3rd. Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):24–35. [DOI] [PubMed] [Google Scholar]
- 7.Karsenty G The complexities of skeletal biology. Nature. 2003;423(6937):316–318. [DOI] [PubMed] [Google Scholar]
- 8.Chan CK, Seo EY, Chen JY, et al. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160(1–2):285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan CKF, Gulati GS, Sinha R, et al. Identification of the Human Skeletal Stem Cell. Cell. 2018;175(1):43–56 e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ransom RC, Carter AC, Salhotra A, et al. Mechanoresponsive stem cells acquire neural crest fate in jaw regeneration. Nature. 2018;563(7732):514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Runyan CM, Gabrick KS. Biology of Bone Formation, Fracture Healing, and Distraction Osteogenesis. J Craniofac Surg. 2017;28(5):1380–1389. [DOI] [PubMed] [Google Scholar]
- 12.Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol. 2004;275(1):1–11. [DOI] [PubMed] [Google Scholar]
- 13.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theveneau E, Mayor R. Neural crest delamination and migration: from epithelium-to-mesenchyme transition to collective cell migration. Dev Biol. 2012;366(1):34–54. [DOI] [PubMed] [Google Scholar]
- 15.Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J Cell Sci. 2003;116(Pt 3):499–511. [DOI] [PubMed] [Google Scholar]
- 16.Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11(12):4131–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulpiau P, van Roy F. Molecular evolution of the cadherin superfamily. The international journal of biochemistry & cell biology. 2009;41(2):349–369. [DOI] [PubMed] [Google Scholar]
- 18.Nistico P, Bissell MJ, Radisky DC. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harb Perspect Biol. 2012;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9(7):557–568. [DOI] [PubMed] [Google Scholar]
- 20.Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development (Cambridge, England). 1996;122(10):3229–3242. [DOI] [PubMed] [Google Scholar]
- 21.Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development (Cambridge, England). 2000;127(6):1161–1172. [DOI] [PubMed] [Google Scholar]
- 22.Smith A, Robinson V, Patel K, Wilkinson DG. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr Biol. 1997;7(8):561–570. [DOI] [PubMed] [Google Scholar]
- 23.Cox MN, D.R. Lehninger: Principles of Biochemistry. Fifth Edition ed: W.H. Freeman; 2008. [Google Scholar]
- 24.Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, DeSimone DW. Integrin alpha5beta1 supports the migration of Xenopus cranial neural crest on fibronectin. Dev Biol. 2003;260(2):449–464. [DOI] [PubMed] [Google Scholar]
- 25.McLennan R, Kulesa PM. In vivo analysis reveals a critical role for neuropilin-1 in cranial neural crest cell migration in chick. Dev Biol. 2007;301(1):227–239. [DOI] [PubMed] [Google Scholar]
- 26.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268(5208):233–239. [DOI] [PubMed] [Google Scholar]
- 27.Minoux M, Holwerda S, Vitobello A, et al. Gene bivalency at Polycomb domains regulates cranial neural crest positional identity. Science. 2017;355(6332). [DOI] [PubMed] [Google Scholar]
- 28.Couly G, Grapin-Botton A, Coltey P, Ruhin B, Le Douarin NM. Determination of the identity of the derivatives of the cephalic neural crest: incompatibility between Hox gene expression and lower jaw development. Development (Cambridge, England). 1998;125(17):3445–3459. [DOI] [PubMed] [Google Scholar]
- 29.Gendron-Maguire M, Mallo M, Zhang M, Gridley T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993;75(7):1317–1331. [DOI] [PubMed] [Google Scholar]
- 30.Holland PW, Booth HA, Bruford EA. Classification and nomenclature of all human homeobox genes. BMC biology. 2007;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rijli FM, Mark M, Lakkaraju S, Dierich A, Dolle P, Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75(7):1333–1349. [DOI] [PubMed] [Google Scholar]
- 32.Gavalas A, Studer M, Lumsden A, Rijli FM, Krumlauf R, Chambon P. Hoxa1 and Hoxb1 synergize in patterning the hindbrain, cranial nerves and second pharyngeal arch. Development (Cambridge, England). 1998;125(6):1123–1136. [DOI] [PubMed] [Google Scholar]
- 33.Kanzler B, Kuschert SJ, Liu YH, Mallo M. Hoxa-2 restricts the chondrogenic domain and inhibits bone formation during development of the branchial area. Development (Cambridge, England). 1998;125(14):2587–2597. [DOI] [PubMed] [Google Scholar]
- 34.Dobreva G, Chahrour M, Dautzenberg M, et al. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125(5):971–986. [DOI] [PubMed] [Google Scholar]
- 35.Grammatopoulos GA, Bell E, Toole L, Lumsden A, Tucker AS. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development (Cambridge, England). 2000;127(24):5355–5365. [DOI] [PubMed] [Google Scholar]
- 36.Pasqualetti M, Ori M, Nardi I, Rijli FM. Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development (Cambridge, England). 2000;127(24):5367–5378. [DOI] [PubMed] [Google Scholar]
- 37.Betts JG, Desaix P, Johnson E, Johnson JE, Korol O, Kruse D, Poe B, Wise JA, Womble M, Young KA Bone Formation and Development In: OpenStax, ed. Anatomy and Physiology. 1st ed.: OpenStax; 2017. [Google Scholar]
- 38.Stricker S, Mundlos S. FGF and ROR2 receptor tyrosine kinase signaling in human skeletal development. Curr Top Dev Biol. 2011;97:179–206. [DOI] [PubMed] [Google Scholar]
- 39.Pizette S, Abate-Shen C, Niswander L. BMP controls proximodistal outgrowth, via induction of the apical ectodermal ridge, and dorsoventral patterning in the vertebrate limb. Development (Cambridge, England). 2001;128(22):4463–4474. [DOI] [PubMed] [Google Scholar]
- 40.Tickle C, Wolpert L. The progress zone -- alive or dead? Nature cell biology. 2002;4(9):E216–217. [DOI] [PubMed] [Google Scholar]
- 41.Tickle C How the embryo makes a limb: determination, polarity and identity. J Anat. 2015;227(4):418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75(7):1401–1416. [DOI] [PubMed] [Google Scholar]
- 43.Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453(7193):401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thaller C, Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987;327(6123):625–628. [DOI] [PubMed] [Google Scholar]
- 45.Cornell RA, Kimelman D. Activin-mediated mesoderm induction requires FGF. Development (Cambridge, England). 1994;120(2):453–462. [DOI] [PubMed] [Google Scholar]
- 46.Buscher D, Bosse B, Heymer J, Ruther U. Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mechanisms of development. 1997;62(2):175–182. [DOI] [PubMed] [Google Scholar]
- 47.Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development (Cambridge, England). 1995;121(10):3163–3174. [DOI] [PubMed] [Google Scholar]
- 48.Sagai T, Masuya H, Tamura M, et al. Phylogenetic conservation of a limb-specific, cis-acting regulator of Sonic hedgehog ( Shh). Mamm Genome. 2004;15(1):23–34. [DOI] [PubMed] [Google Scholar]
- 49.Parr BA, McMahon AP. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995;374(6520):350–353. [DOI] [PubMed] [Google Scholar]
- 50.Berendsen AD, Olsen BR. Bone development. Bone. 2015;80:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nusspaumer G, Jaiswal S, Barbero A, et al. Ontogenic Identification and Analysis of Mesenchymal Stromal Cell Populations during Mouse Limb and Long Bone Development. Stem cell reports. 2017;9(4):1124–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gentili C, Cancedda R. Cartilage and bone extracellular matrix. Curr Pharm Des. 2009;15(12):1334–1348. [DOI] [PubMed] [Google Scholar]
- 53.Heinegard D Fell-Muir Lecture: Proteoglycans and more--from molecules to biology. International journal of experimental pathology. 2009;90(6):575–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aszodi A, Bateman JF, Gustafsson E, Boot-Handford R, Fassler R. Mammalian skeletogenesis and extracellular matrix: what can we learn from knockout mice? Cell structure and function. 2000;25(2):73–84. [DOI] [PubMed] [Google Scholar]
- 55.Egawa S, Miura S, Yokoyama H, Endo T, Tamura K. Growth and differentiation of a long bone in limb development, repair and regeneration. Dev Growth Differ. 2014;56(5):410–424. [DOI] [PubMed] [Google Scholar]
- 56.Mariani FV, Martin GR. Deciphering skeletal patterning: clues from the limb. Nature. 2003;423(6937):319–325. [DOI] [PubMed] [Google Scholar]
- 57.Grabowski P Physiology of Bone. Endocr Dev. 2015;28:33–55. [DOI] [PubMed] [Google Scholar]
- 58.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2(4):389–406. [DOI] [PubMed] [Google Scholar]
- 59.Kronenberg HM. The role of the perichondrium in fetal bone development. Ann N Y Acad Sci. 2007;1116:59–64. [DOI] [PubMed] [Google Scholar]
- 60.Caplan AI. Bone development and repair. BioEssays : news and reviews in molecular, cellular and developmental biology. 1987;6(4):171–175. [DOI] [PubMed] [Google Scholar]
- 61.Pazzaglia UE, Congiu T, Sibilia V, Pagani F, Benetti A, Zarattini G. Relationship between the chondrocyte maturation cycle and the endochondral ossification in the diaphyseal and epiphyseal ossification centers. Journal of morphology. 2016;277(9):1187–1198. [DOI] [PubMed] [Google Scholar]
- 62.Shapiro IM, Adams CS, Freeman T, Srinivas V. Fate of the hypertrophic chondrocyte: microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate. Birth Defects Res C Embryo Today. 2005;75(4):330–339. [DOI] [PubMed] [Google Scholar]
- 63.White A, Wallis G. Endochondral ossification: a delicate balance between growth and mineralisation. Curr Biol. 2001;11(15):R589–591. [DOI] [PubMed] [Google Scholar]
- 64.Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys. 2008;473(2):98–105. [DOI] [PubMed] [Google Scholar]
- 66.Karelina TV, Goldberg GI, Eisen AZ. Matrix metalloproteinases in blood vessel development in human fetal skin and in cutaneous tumors. J Invest Dermatol. 1995;105(3):411–417. [DOI] [PubMed] [Google Scholar]
- 67.Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS, Mirams M. Endochondral ossification: how cartilage is converted into bone in the developing skeleton. The international journal of biochemistry & cell biology. 2008;40(1):46–62. [DOI] [PubMed] [Google Scholar]
- 68.Marecic O, Tevlin R, McArdle A, et al. Identification and characterization of an injury-induced skeletal progenitor. Proc Natl Acad Sci U S A. 2015;112(32):9920–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bell S, Terentjev EM. Focal Adhesion Kinase: The Reversible Molecular Mechanosensor. Biophys J. 2017;112(11):2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garza RM, Alyono JC, Dorfman DW, Wan DC. Mandibular Distraction in a Patient With Type II Collagenopathy. J Craniofac Surg. 2017;28(8):2073–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ow AT, Cheung LK. Meta-analysis of mandibular distraction osteogenesis: clinical applications and functional outcomes. Plastic and reconstructive surgery. 2008;121(3):54e–69e. [DOI] [PubMed] [Google Scholar]
- 72.McCarthy JGF, R.L. Distraction of the Mandible. New York City, New York: Springer Nature; 2017. [Google Scholar]
- 73.McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plastic and reconstructive surgery. 1992;89(1):1–8; discussion 9–10. [PubMed] [Google Scholar]
- 74.Guerrero CABWH, Contasti-Bocco GI, Rodriguez AM, Contasti RV Intraoral Mandibualr Distraction. New York City, New York: Springer Nature; 2017. [Google Scholar]
- 75.Master DL, Hanson PR, Gosain AK. Complications of mandibular distraction osteogenesis. J Craniofac Surg. 2010;21(5):1565–1570. [DOI] [PubMed] [Google Scholar]
- 76.Donneys A, Deshpande SS, Tchanque-Fossuo CN, et al. Deferoxamine expedites consolidation during mandibular distraction osteogenesis. Bone. 2013;55(2):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes & development. 1998;12(2):149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith TG, Robbins PA, Ratcliffe PJ. The human side of hypoxia-inducible factor. British journal of haematology. 2008;141(3):325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, Strehin I, Bedelbaeva K, et al. Drug-induced regeneration in adult mice. Sci Transl Med. 2015;7(290):290ra292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Momeni A, Rapp S, Donneys A, Buchman SR, Wan DC. Clinical Use of Deferoxamine in Distraction Osteogenesis of Irradiated Bone. J Craniofac Surg. 2016;27(4):880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rauch F, Lauzier D, Croteau S, Travers R, Glorieux FH, Hamdy R. Temporal and spatial expression of bone morphogenetic protein-2, −4, and −7 during distraction osteogenesis in rabbits. Bone. 2000;27(3):453–459. [DOI] [PubMed] [Google Scholar]
- 83.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–241. [DOI] [PubMed] [Google Scholar]
- 84.Agrawal V, Sinha M. A review on carrier systems for bone morphogenetic protein-2. J Biomed Mater Res B Appl Biomater. 2017;105(4):904–925. [DOI] [PubMed] [Google Scholar]
- 85.Hu J, Zou S, Li J, Chen Y, Wang D, Gao Z. Temporospatial expression of vascular endothelial growth factor and basic fibroblast growth factor during mandibular distraction osteogenesis. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2003;31(4):238–243. [DOI] [PubMed] [Google Scholar]
- 86.Warren SM, Mehrara BJ, Steinbrech DS, et al. Rat mandibular distraction osteogenesis: part III. Gradual distraction versus acute lengthening. Plastic and reconstructive surgery. 2001;107(2):441–453. [DOI] [PubMed] [Google Scholar]
- 87.Makhdom AM, Hamdy RC. The role of growth factors on acceleration of bone regeneration during distraction osteogenesis. Tissue Eng Part B Rev. 2013;19(5):442–453. [DOI] [PubMed] [Google Scholar]
- 88.Aykan A, Ozturk S, Sahin I, et al. Biomechanical analysis of the effect of mesenchymal stem cells on mandibular distraction osteogenesis. J Craniofac Surg. 2013;24(2):e169–175. [DOI] [PubMed] [Google Scholar]
- 89.Cao J, Wang L, Du ZJ, et al. Recruitment of exogenous mesenchymal stem cells in mandibular distraction osteogenesis by the stromal cell-derived factor-1/chemokine receptor-4 pathway in rats. Br J Oral Maxillofac Surg. 2013;51(8):937–941. [DOI] [PubMed] [Google Scholar]
- 90.Kan B, Tasar F, Korkusuz P, et al. Histomorphometrical and radiological comparison of low-level laser therapy effects on distraction osteogenesis: experimental study. Lasers Med Sci. 2014;29(1):213–220. [DOI] [PubMed] [Google Scholar]
- 91.El-Bialy. Therapeutic Ultrasound in Dentistry:Applications for Dentofacial Repair, Regeneration, and Tissue Engineering. Springer; 2018. [Google Scholar]
- 92.Hagiwara T, Bell WH. Effect of electrical stimulation on mandibular distraction osteogenesis. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2000;28(1):12–19. [DOI] [PubMed] [Google Scholar]
- 93.Andrade Gomes do Nascimento LE, Sant’anna EF, Carlos de Oliveira Ruellas A, Issamu Nojima L, Goncalves Filho AC, Antonio Pereira Freitas S. Laser versus ultrasound on bone density recuperation after distraction osteogenesis-a cone-beam computer tomographic analysis. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2013;71(5):921–928. [DOI] [PubMed] [Google Scholar]
- 94.Khanna A, Nelmes RT, Gougoulias N, Maffulli N, Gray J. The effects of LIPUS on soft-tissue healing: a review of literature. Br Med Bull. 2009;89:169–182. [DOI] [PubMed] [Google Scholar]
- 95.Chim H, Sontich JK, Kaufman BR. Free tissue transfer with distraction osteogenesis is effective for limb salvage of the infected traumatized lower extremity. Plastic and reconstructive surgery. 2011;127(6):2364–2372. [DOI] [PubMed] [Google Scholar]
- 96.de Baat P, de Baat C, Bessems JH. [Distraction osteogenesis in orthopaedics]. Ned Tijdschr Tandheelkd. 2008;115(6):306–313. [PubMed] [Google Scholar]
- 97.Papakostidis C, Bhandari M, Giannoudis PV. Distraction osteogenesis in the treatment of long bone defects of the lower limbs: effectiveness, complications and clinical results; a systematic review and meta-analysis. The bone & joint journal. 2013;95-b(12):1673–1680. [DOI] [PubMed] [Google Scholar]
- 98.Sabharwal S, Nelson SC, Sontich JK. What’s New in Limb Lengthening and Deformity Correction. J Bone Joint Surg Am. 2015;97(16):1375–1384. [DOI] [PubMed] [Google Scholar]
- 99.Watson JT. Distraction osteogenesis. The Journal of the American Academy of Orthopaedic Surgeons. 2006;14(10 Spec No):S168–174. [DOI] [PubMed] [Google Scholar]
- 100.Herzenberg JaS S Tibial Lengthening with Circular External Fixation In: Wiesel SW, ed. Operative Techniques in Pediatric Orthopedic Surgery. 2 ed. China: Wolters Kluwer; 2016:558–566. [Google Scholar]
- 101.Herzenberg JaS S Femoral Lengthening with External Fixatio In: Wiesel SW, ed. Operative Techniques in Pediatric Orthopedic Surgery. 2 ed. China: Wolters Kluwer; 2016:538–549. [Google Scholar]
- 102.Liantis P, Mavrogenis AF, Stavropoulos NA, et al. Risk factors for and complications of distraction osteogenesis. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2014;24(5):693–698. [DOI] [PubMed] [Google Scholar]
- 103.Vargas Barreto B, Caton J, Merabet Z, Panisset JC, Pracros JP. Complications of Ilizarov leg lengthening: a comparative study between patients with leg length discrepancy and short stature. International orthopaedics. 2007;31(5):587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harada Y, Nakasa T, Mahmoud EE, et al. Combination therapy with intra-articular injection of mesenchymal stem cells and articulated joint distraction for repair of a chronic osteochondral defect in the rabbit. J Orthop Res. 2015;33(10):1466–1473. [DOI] [PubMed] [Google Scholar]
- 105.Zeng JJ, Guo P, Zhou N, Xie QT, Liao FC. Treatment of large bone defects with a novel biological transport disc in non-vascular transport distraction osteogenesis. Int J Oral Maxillofac Surg. 2016;45(5):670–677. [DOI] [PubMed] [Google Scholar]
- 106.Salem KH, Schmelz A. Low-intensity pulsed ultrasound shortens the treatment time in tibial distraction osteogenesis. International orthopaedics. 2014;38(7):1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song MH, Kim TJ, Kang SH, Song HR. Low-intensity pulsed ultrasound enhances callus consolidation in distraction osteogenesis of the tibia by the technique of lengthening over the nail procedure. BMC Musculoskelet Disord. 2019;20(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]