Intranasal corticosteroids can be used as primary or adjunct therapy for many inflammatory conditions within the nasal cavity
About 5% of Canadians are affected by sinonasal inflammation.1 There is broad expert consensus supporting the effectiveness of intranasal corticosteroids for treating allergic and nonallergic rhinitis, acute rhinosinusitis and chronic rhinosinusitis with and without nasal polyposis.2,3
There are 9 intranasal corticosteroids approved in Canada, all with similar efficacy
Intranasal corticosteroids affect both early and late inflammatory responses by inhibiting the production of proinflammatory cytokines, inflammatory enzymes, lymphocyte proliferation and delayed hypersensitivity. 2,3 Although efficacy is similar across different corticosteroids, 2 certain types have specific indications (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.201266/tab-related-content).
Intranasal corticosteroids are safe, but local adverse effects are common
One in 10 patients have local adverse effects, including burning or stinging, dryness and epistaxis.2 Systemic adverse effects are very uncommon, and there is no correlation with use and hypothalamic–pituitary–adrenal suppression or increased intraocular pressure.4 One study linked beclomethasone dipropionate to decreased growth velocity in children,4 but no association has been found for other intranasal corticosteroids. For patients on concurrent oral and inhaled steroids, there is a theoretical increased risk for systemic side effects; therefore, a second-generation steroid spray with low bioavailability is suggested (Appendix 1).5
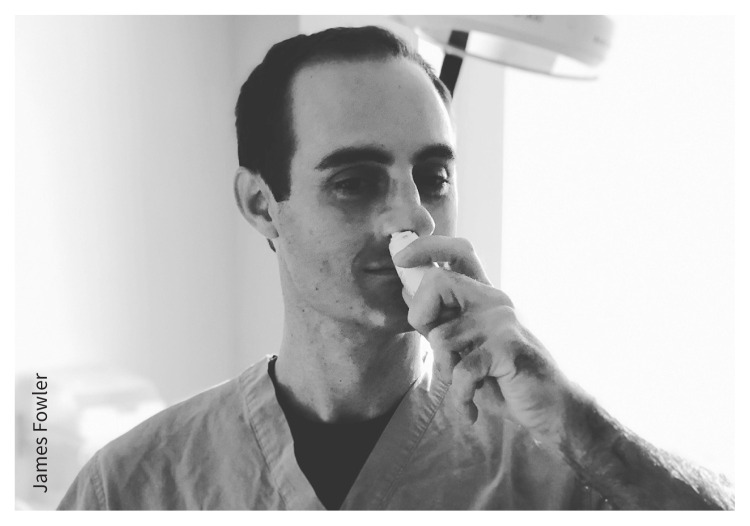
Correct administration of intranasal corticosteroids is crucial for desired therapeutic effect
Providers should show patients how to direct the intranasal applicator away from the nasal septum. This can be achieved by using the contralateral hand for steroid application (Figure 1). Proper technique will reduce local adverse effects and increase adherence. Daily use for 8–12 weeks is often required for full therapeutic benefit, but adherence can be affected by cost (Appendix 1).
Figure 1:
Photograph of the author (L.J.S.) showing the contralateral hand technique for applying intranasal corticosteroid.
James Fowler
Fewer intranasal corticosteroids are available for children and pregnant women
For children, mometasone furoate (≥ 3 yr), fluticasone propionate (≥ 4 yr), triamcinolone acetonide (≥ 4 yr) and ciclesonide (≥ 6 yr) are currently approved.2 Budesonide is the only intranasal corticosteroid with established safety in pregnancy.6
Footnotes
Competing interests: Leigh Sowerby reports receiving personal fees from Mylan, GSK and Sanofi, and grants from GSK, Roche and AstraZeneca, outside of the submitted work. No other competing interests were declared.
This article has been peer reviewed.
References
- 1.Kaplan A. Canadian guidelines for chronic rhinosinusitis: clinical summary. Can Fam Physician 2013;59:1275–81. [PMC free article] [PubMed] [Google Scholar]
- 2.Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol 2018;8:108–352. [DOI] [PubMed] [Google Scholar]
- 3.Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol 2016;6:S22–209. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Fonacier LS. Adverse effects of nonsystemic steroids (inhaled, intranasal, and cutaneous): a review of the literature and suggested monitoring tool. Curr Allergy Asthma Rep 2016;16:44. [DOI] [PubMed] [Google Scholar]
- 5.Juniper EF, Ståhl E, Doty RL, et al. Clinical outcomes and adverse effect monitoring in allergic rhinitis. J Allergy Clin Immunol 2005;115(Suppl 1):S390–413. [DOI] [PubMed] [Google Scholar]
- 6.Alhussien AH, Alhedaithy RA, Alsaleh SA. Safety of intranasal corticosteroid sprays during pregnancy: an updated review. Eur Arch Otorhinolaryngol 2018;275:325–33. [DOI] [PubMed] [Google Scholar]