Abstract
Background:
Iron-deficiency anemia in pregnancy is a major public health problem despite the efforts taken by the Ministry of Health and Family Welfare for the past five decades. Adherence to iron and folic acid supplementation (IFAS) is the key factor for the prevention and management of nutrition anemia.
Aim:
The aim of this study was to assess the adherence to its associated factors and to explore the reasons for the non-adherenc among pregnant women attending a tertiary care center.
Materials and Methods:
It is an explanatory mixed-methods design (quantitative cross-sectional analytical design and qualitative descriptive design).
Statistical Analysis Used:
Results presented as proportion with 95% confidence interval (CI). Chi-square test was done to assess the association of the factors to adherence. Qualitative data were transcribed verbatim, translated to English, and analyzed by manual content analysis.
Results:
A total of 340 pregnant women were included, and the adherence to IFAS among the antenatal mothers was 63.8 (95% CI [58.61–68.6]). The factors associated with adherence to IFAS (prevalence ratio with 95% CI) were primigravida status [1.22 [1.02–1.45]), nonanemic in the first trimester (1.27 [1.09–1.49]), and absence of side effects (3.16 [1.95–5.12]). Conceptual framework was constructed using the emerging themes: (i) knowledge-related factors, (ii) behavior-related factors, and (iii) facilitating factors.
Conclusion:
About three-fourth of the participants were adherent to IFAS. Compliance is directly influenced by the gravida status, anemic status, and absence of side effects. Based on qualitative results, measures to improve palatability and the quality of IFAS are recommended.
Keywords: Antenatal care, compliance, iron and folic acid tablets, pregnancy
Introduction
Anemia due to nutritional deficiency is a major public health problem worldwide, especially in developing countries. The most common cause of anemia in pregnancy is iron deficiency, which is responsible for 95% of all anemia during pregnancy.[1,2] Physiological changes that happen during pregnancy, fetal growth, and development increase the need for iron and folic acid (IFA).[3] Anemia during pregnancy has adverse effects on both the mother and fetus.[2] The maternal outcomes of iron deficiency are reduced immunity, cardiac arrest, premature rupture of membrane, preterm labor, hemorrhage, and reduced work capacity. Similarly, fetal and neonatal complications include increased susceptibility to prematurity, fetal distress, and low birth weight, amounting to high perinatal morbidity and mortality. The infants born to anemic mothers have higher chances of being anemic.[1]
According to the World Health Organization (WHO), the global prevalence of anemia among antenatal mothers was 38.2% and among women of reproductive age group was 29.4%.[4] Compared to the global scenario, the fourth National Family Health Survey (NFHS-4) in India showed a higher prevalence of anemia among antenatal women (50%) and among women of reproductive age group (53%).[5]
The United Nations Secretary-General declared the years 2015–2025 as the Decade of Nutrition.[6] The Government of India also launched the Anemia Mukt Bharat (AMB) under the umbrella program POSHAN Abhiyaan. This program aims to bring down three percentage points, the prevalence of anemia every year, between the years 2018 and 2020.[7]
In 2018, the WHO recommends daily iron and folic acid supplementation (IFAS) to prevent maternal anemia and weekly supplementation in areas where maternal anemia prevalence is <20%.[8] Daily intake of IFAS during pregnancy helps to reduce the overall lifetime risk of any anemia by 70% and iron-deficiency anemia by 57%, respectively.[9] Consistent with the WHO dosage recommendations, AMB recommends a daily dose of IFAS starting from the second trimester and continued throughout the pregnancy (minimum of 180 days during pregnancy). Each tablet contains 60 mg elemental iron plus 500 mcg folic acid.[7]
Although the Ministry of Health and Family Welfare has implemented various anemia control programs over the past five decades, only 30.3% of the pregnant women had consumed IFAS for at least 100 days as per NFHS-4.[5] Adherence to IFAS might be hindered by several factors such as poor antenatal service utilization, irregular supply of tablets, lack of counseling on compliance, lack of knowledge about the benefits of IFAS, and other probable side effects to IFAS.[9]
There are only limited studies undertaken to explore the real reasons for adherence to IFAS among pregnant women in India. This study will enable the primary care physicians to have better approach in managing the antenatal mothers by understanding their facilitators and barriers to IFAS adherence. Hence, this study assessed the adherence to IFAS and its associated factors and also explored the reasons for nonadherence to IFAS.
Materials and Methods
Study design
We used an explanatory mixed-methods design constituting Phase 1 (quantitative cross-sectional analytical design) followed by Phase 2 (qualitative descriptive design).[10]
Study setting
The study was conducted among the pregnant mothers attending the outpatient department (OPD), Women and Child Hospital (WCH) of a tertiary center, Puducherry, India. All maternal and child health services were available at the health center, and IFAS was given free of cost to all the antenatal mothers on a monthly basis. The average OPD attendance in the Obstetrics and Gynecology Department was around 5000 per month. According to NFHS-4, 87.7% and 66% of the pregnant women in Puducherry had at least four antenatal visits and consumed IFAS for 100 days, respectively.[5]
Study population and duration
All women in the WCH OPD with gestational age more than 27 weeks and who were given IFA tablets for at least 1 month before the data collection period were included. The total duration of the data collection was 2 months (September 1 to October 31, 2019).
Sample size
Phase 1 (quantitative)
Assuming an alpha error of 5%, confidence interval (CI) of 95%, absolute precision of 5%, and the expected proportion of adherence among antenatal mothers as 67%,[5,11] the required sample size was calculated to be 340 using an online software OpenEpi version 3.01 (AG Dean, KM Sullivian & MM Soe). We enrolled all consecutive eligible mothers into the study.
Phase 2 (qualitative)
A total of eight in-depth interviews (IDIs) among the mothers and four key informant interviews (KIIs) among the health-care providers were conducted. Antenatal mothers who were vocal and the health-care providers (consultant, public health nurse (PHN), senior resident, and staff nurse) who provided antenatal services were interviewed. Saturation of findings was used to guide the sample size.
Data variables, sources of data, and collection methods
Phase 1 (quantitative)
We collected the data using a pretested, semi-structured questionnaire which included sociodemographic and obstetric details. The information on the antenatal services received and the status of anemia (WHO classification <11 g/dl)[8] during the first and third trimesters were obtained from their records. Antenatal women (including both who are receiving prophylactic dose and treatment dose), who reported to have consumed IFA tablets for at least 4 days a week, were considered adherent.[8] A four item Morisky scale was used to assess adherence to IFAS. Questions like forgetfulness, problem in remembering to take medication, doesn't take medication when feeling better and when feeling worse were asked. A Score 1 was given for “yes” and 0 was given for “no”. Participants with higher score were considered to have low adherence to IFAS for better clarity.[12]
Phase 2 (qualitative)
Descriptive approach was used for qualitative data collection. Before the interviews, the interview guide was pretested among a few members to assess its appropriateness; changes were made wherever necessary. The principal investigator was female, fluent in local language, and had training in qualitative interviews. Written consent was obtained briefing the purpose of the study. The participants were interviewed for 15–20 min in their local language, Tamil. Face-to-face interviews were conducted in a separate room in the tertiary care center to ensure privacy and convenience for the participants. All interviews were audio-recorded, and field notes were taken during the interview to capture all important information.
Data entry and analysis
Quantitative data
We entered data using Epicollect5 version 1.1.8 (developed by Imperial college, London),[12] and analysis was done using SPSS Version 21.0. (Armonk, NY: IBM Corp).[13] Continuous variables were summarized as mean and standard deviation (SD) or median and interquartile range (IQR) based on the normality. Categorical variables were expressed as frequency and proportions. Adherence was expressed as proportion with 95% CI. The association of sociodemographic and obstetric variables with adherence to IFAS was assessed using Chi-square test presented as prevalence ratio (PR) with 95% CI. P < 0.05 was considered as statistically significant.
Qualitative data
Audio-recorded interviews were transcribed verbatim into Tamil and translated to English on the same day of interview. By content analysis, manual coding was carried out to generate categories or themes. After completion, both qualitative and quantitative data were compared to ensure triangulation.
Ethics approval
Ethics approval was obtained from the Institute Research Monitoring Committee (N0.JIP/PSM/MPH/19) on May 31, 2019, and Institute Ethics Committee (JIP/IEC/2019/305) on August 27, 2019. Informed written consent was obtained from all the participants.
Results
Quantitative (Phase 1)
A total of 340 third-trimester antenatal mothers were approached for the quantitative part of the study, and everyone agreed to participate. The mean (SD) age of the participants was 25.02 (3.83) years. The median (IQR) monthly family income in INR of the participants was Rs. 8000 (2000-14750). Three-fourth of the participants were from Tamil Nadu and one-fourth (25.9%) were from Puducherry [Table 1].
Table 1.
Sociodemographic factors associated with adherence to iron and folic acid supplementation among antenatal mothers attending a tertiary care center, Puducherry 2019 (n=340)
| Characteristics | Total | Adherence to IFA tablets*, n (%) Yes (%) | Prevalence ratio (95% CI) |
|---|---|---|---|
| Total | 340 | 217 (63.82) | - |
| Age (years) | |||
| 17-20 | 31 (9.1) | 18 (58.1) | 1 |
| 21-25 | 175 (51.5) | 119 (68) | 0.76 (0.47-1.21) |
| 26-30 | 100 (29.4) | 60 (60) | 0.95 (0.59-1.54) |
| >30 | 34 (10) | 20 (58.8) | 0.98 (0.55-1.74) |
| Education (years) | |||
| 0-8 | 30 (8.8) | 22 (73.3) | 1.19 (0.73-1.92) |
| 9-12 | 170 (50) | 109 (64.1) | 1.04 (0.66-1.62) |
| 13 | 127 (37.4) | 78 (61.4) | 0.99 (0.63-1.56) |
| >14 | 13 (8.8) | 8 (61.5) | 1 |
| Occupation | |||
| Homemaker | 317 (93.2) | 204 (64.4) | 0.87 (0.60-1.26) |
| Employed | 23 (6.8) | 13 (56.5) | 1 |
| Religion | |||
| Hindu | 317 (93.2) | 198 (62.5) | 0.93 (0.52-1.66) |
| Christian | 6 (1.8) | 4 (66.7) | 1 |
| Muslim | 17 (5) | 15 (88.2) | 1.32 (0.73-2.39) |
*Antenatal women, who reported to have taken IFA tablets for ≥4 days a week. IFA=Iron and folic acid
Around 71.2% of the population had at least 4–8 antenatal care (ANC) visits. Sixty-two percent of the study participants had received more than 180 tablets and 65% had received health education from the health-care providers. Among the total participants, 16.8% of the pregnant women experienced some side effects to IFA tablets, among whom 55% reported their side effects to their health-care provider (data not provided in tables).
The adherence to IFAS among the antenatal mothers was 63.8% (95% CI: 58.61–68.6). The factors associated with adherence to IFAS were primigravida status (PR: 1.22 [95% CI: 1.02–1.45]), nonanemic in the first trimester (PR: 1.27 [95% CI: 1.09–1.49]), received health education (PR: 1.23, [95% CI: 1.03–1.47]), and did not experience side effects (PR: 3.16 [95% CI: 1.95–5.12]) [Table 2].
Table 2.
Obstetric and health service-related factors associated with adherence to iron and folic acid supplementation among antenatal mothers attending a tertiary care center, Puducherry 2019 (n=340)
| Characteristics | Total | Adherence to IFA tablets, n (%) Yes (%) | Prevalence ratio with CI |
|---|---|---|---|
| Total | 340 | 217 (63.82) | - |
| Gravida status | |||
| Primi | 200 (58.8) | 138 (69) | 1.22 (1.02-1.45) |
| Multi | 140 (41.2) | 79 (56.4) | 1 |
| Number of abortions | |||
| Nil | 307 (90.3) | 198 (64.5) | 1 |
| One | 26 (07.6) | 15 (57.7) | 0.89 (0.63-1.25) |
| Two or more | 7 (02.1) | 4 (57.1) | 088 (0.46-1.69) |
| Received health education | |||
| Yes | 222 (65.3) | 150 (67.6) | 1.23 (1.03-1.47) |
| No | 118 (34.7) | 67 (56.8) | 1 |
| Anemia status in the first trimester | |||
| Anemic | 242 (71.2) | 136 (58.6) | 1 |
| Nonanemic | 98 (28.8) | 81 (75) | 1.27 (1.09-1.49) |
| Anemia status in the third trimester | |||
| Anemic | 232 (68.2) | 152 (62.8) | 1 |
| Nonanemic | 108 (31.8) | 65 (66.3) | 1.05 (0.88-1.25) |
| Side effects to IFAS | |||
| Present | 232 (68.2) | 13 (22.8) | 1 |
| Absent | 108 (31.8) | 204 (72.1) | 3.16 (1.95-5.12) |
CI=Confidence interval, IFA=Iron and folic acid, IFAS=IFA supplementation
The most common reasons stated for adherence were to improve their health (40%) and their baby's health (46.5%). About 7.7% of the participants reported that adherence was due to doctor's advice, whereas 6.9% mentioned as emphasis from the family members. Reasons given for nonadherence were forgetfulness (32%) and side effects to IFA tablets (27.5%).
Morisky scale was used to capture the behavior-related factors of medication adherence, nearly seventy percent (68.2%) of the participants reported that they had forgotten to take tablets at some point of time, and 43.8% reported that they avoided IFAS when they felt worse by taking the tablets. Around 37.4% ignored to take tablets even after remembering, and 12.1% did not take tablets whenever they felt that they were good health [Table 3].
Table 3.
Adherence to iron and folic acid tablets according to 4-item Morisky Medication Adherence Scale among antenatal mothers attending a tertiary care center, Puducherry 2019 (n=340)
| Adherence status‡ | Score | Frequency (%) |
|---|---|---|
| Low | 3-4 | 80 (23.5) |
| Medium | 1-2 | 187 (55) |
| High | 0 | 73 (21.5) |
‡An answer yes is given a score of 1 and no is given score of 0
Qualitative (Phase 2)
A total of 8 IDIs and 4 KIIs were conducted among the antenatal mothers and health-care providers, respectively. Themes and codes were generated to build a conceptual framework [Flowchart 1].
Flowchart 1.
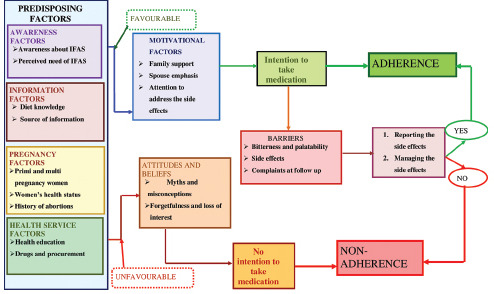
Conceptual framework regarding the factors influencing the adherence to iron and folic acid tablets among the antenatal mothers
Knowledge-related factors
Awareness on the importance and strategies for the prevention of anemia in antenatal period was moderate among the participants. The participants knew that iron was essential to improve blood count (hemoglobin), and anemia could affect their health as well as their baby's health. Many mothers stated that they took iron tablets in response to the emphasis by the health education provided by the health workers.
“My Hb level is 8 points or something…, earlier it was 11.5 points. I have to take IFAS to increase the Hb level” – A 23-year-old mother with anemia.
“Most of the mothers are aware of the importance of taking IFA tablets, because when they register their pregnancy in the PHC for receiving maternity benefits they are given enough health education” – A public health nurse.
Distorted perception reinforced by negative experiences
The common reasons given for nonadherence to IFAS was the myths and misconceptions. The antenatal mothers have received various false information from their family members or from their neighborhood.
“Elders in my house advised me not to take tablets, they think that the baby's brain development might get affected or baby might get some disability because of these tablets” – A mother from rural area.
“One person told me that if I eat this tablet, my baby might become big and I will not have normal delivery. Baby will also become dark because of these tablets” – A 27-year-old mother.
Irregular consumption and avoidance
The interview revealed that forgetfulness was an important reason for nonadherence. Many mothers reported that they forget to take tablets, at times, as they were given multiple tablets. Furthermore, some mothers stated the household chores, food timings, and traveling as various other reasons for nonadherence to IFAS.
“A mother from urban area”
“I eat very late if I am engaged in household work. So I forgot to take the tablets. Similarly, if there were attending any family function I forgot to take the tablet” – A 27-year-old mother.
Attitude toward facing the side effects
The participants reported that factors such as palatability and side effects hinder them from consuming the medications. Factors such as family support and appropriate management of side effects enhance their adherence to IFAS.
“I vomit if I take these tablets after having food, so I stopped taking it” – An educated mother
While going bathroom, stools were black and smelly. I had indigestion while taking these medicine – A 25-year-old mother.
“The main problem for bad adherence is their side effects. If we manage the side effects properly then they will have better compliance” – An obstetrician.
Health service-related factors and social factors
The trust on health-care providers and family support influence the adherence to IFAS. Furthermore, few mothers felt that the quality of the IFAS given in the government sector is not as good as the IFAS from the private pharmacies.
I don't have any fear because doctors are only giving this medicine, wherever I go for checkup they are telling to take this. Doctor told that my blood level is low, so I must take tablet regularly – A 26-year-old mother with anemia.
Tablets purchased from pharmacy do not have that iron smell. It has a coating and it is easy to swallow. Government tablets do not have such coating – A mother from rural area.
Needs expressed to enable adherence
The antenatal mothers also stated that their adherence will be better if the quality of IFAS is improved in terms of its taste, smell, and its size.
“If they remove the bitter taste and iron smell from the tablets, I will not have vomiting and will take tablets properly” – A mother from rural area.
“This tablet is very big if the size is reduced it will be easy to swallow” – A 21-year-old mother.
Discussion
This mixed-methods study was conducted among the antenatal mothers attending a tertiary care center in Puducherry to identify challenges and opportunities for revitalizing the existing anemia control programs. Three-fourth of the mothers had at least 4 ANC visits. More than half of the study participants had received more than 180 tablets and health education. The NFHS-4 also reports that around 94.5% of the women in Puducherry have received IFA tablets[7] suggesting better health-care delivery in the study setting.
The adherence to IFAS was 63.8% (95% CI [58.61–68.6]), which was in consistent with the findings of the other studies conducted in various parts of India.[2,14,15] A study conducted in Pakistan[16] also showed similar results to our study. The prevalence of adherence found in this study is higher than the studies done in other African nations.[4,17,18,19,20] This difference could be explained by the sociocultural differences of the study population, setting, quality and delivery of health-care services, and the definition of adherence to IFAS.
Our study observed that women with anemia in the first trimester, primigravida, without any side effects were more adherent comparatively. Similar findings were observed from other studies done in India[2,14,19] and Ethiopia.[4,21] The observed higher adherence among primigravida mothers than multigravida mothers might be due to perceived risks by the new mothers compared to multigravida. Similarly, the statement given by the health-care workers and an antenatal mother of two children also endorsed that during their first pregnancy, mothers had better adherence to IFAS. A few studies done in India[2,14,19,22] and a meta-analysis of 15 studies done in Ethiopia[21] also show the association between the knowledge toward IFAS and adherence status. Better the knowledge, better the perception about the benefits of consuming iron tablets.
The prevalence of adherence was more among women without any side effect which was also supported by qualitative findings. Furthermore, the fear related to the side effects of IFA tablets (perceived or experienced), inadequate counseling, and underreporting of side effects influence their adherence to IFAS. This can be overcome by encouraging mothers to report IFA side effects and managing it appropriately. Mothers' interest for their own health and their baby's' heath could be taken as a medium to further educate them on the importance of adherence. Further research needs to be done with repeated reminders to mothers using digital applications to rectify reasons such as forgetfulness or ignorance due to busy household chores.
Even though the government has been providing tablets free of cost to reduce the economic burden of the poor, it fails to independently improve adherence to IFAS until supplemented by these abovementioned efforts. AMB should emphasize addressing the quality of the tablets in terms of size, smell, and palatability as expressed by the mothers. Therefore, research on identifying exact cause of anemia and trial of newer pharmacological combination of IFAS to improve palatability should be conducted.
This study has several strengths. First, we employed a mixed-methods design to capture the quantitative and qualitative picture of the issue. Second, a validated 4-item Morisky Medication Adherence Scale was used to capture information on the behavior-related factors for adherence status. Finally, we adhered to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines and the Consolidated Criteria for Reporting Qualitative Research guidelines for reporting the quantitative and qualitative components, respectively. However, the limitation of the study such as social desirability bias and quality of self-reported adherence status cannot be fully ruled out.
Conclusion
About three-fourth of the participants were adherent to IFAS. The adherence status is directly influenced by the order of pregnancy, anemic status, and absence of side effects. Reporting of the tolerance to the tablets needs more emphasis. Improving the palatability of the tablets by flavor coating them and reducing the size and number of doses might have a positive impact on the adherence to IFAS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I would like to thank the health-care providers and the security staff of the Obstetrics and Gynecology Department for helping me during the data collection period.
References
- 1.Shewasinad S, Negash S. Adherence and associated factors of prenatal iron folic acid supplementation among pregnant women who attend ante natal care in health facility at Mizan-Aman Town, Bench Maji Zone, Ethiopia, 2015. J Preg Child Health. 2017;4:335. [Google Scholar]
- 2.Gebremariam AD, Tiruneh SA, Abate BA, Engidaw MT, Asnakew DT. Adherence to iron with folic acid supplementation and its associated factors among pregnant women attending antenatal care follow up at Debre Tabor General Hospital, Ethiopia, 2017. PLoS One. 2019;14:e0210086. doi: 10.1371/journal.pone.0210086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assefa H, Abebe SM, Sisay M. Magnitude and factors associated with adherence to Iron and folic acid supplementation among pregnant women in Aykel town, Northwest Ethiopia. BMC Pregnancy Childbirth. 2019;19:296. doi: 10.1186/s12884-019-2422-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 5.International Institution for Population Sciences (IIPS) and ICF. NHFS-4, 201-16 India. Mumbai: IIPS; 2017. [Google Scholar]
- 6.Kassa ZY, Awraris T, Daba AK, Tenaw Z. Compliance with iron folic acid and associated factors among pregnant women through pill count in Hawassa city, South Ethiopia: A community based cross-sectional study. Reprod Health. 2019;16:14. doi: 10.1186/s12978-019-0679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anemia Mukt Bharat — Vikaspedia. [Last accessed on 2019 Dec 31]. Available from: http://vikaspediain/health/health-campaigns/anemia-mukt-bharat .
- 8.World Health Organization. Guideline. 2012. [Last accessed on 2019 Dec 05]. Available from: http://wwwncbinlmnihgov/books/NBK132263/
- 9.Museka-Saidi TM, Mlambo TT, Aburto N, Keith RS. Strengthen iron folate supplementation of pregnant women in Ntchisi District, Malawi. World Nutr. 2018;9:254–60. [Google Scholar]
- 10.Doyle L, Brady AM, Byrne G. An overview of mixed methods research – Revisited. J Res Nurs. 2016;21:623–35. [Google Scholar]
- 11.Kumar PS, Priya BY. Compliance to iron folic acid supplementation among antenatal mothers attending a primary health centre. Int J Adv Res Dev. 2018;3:223–32. [Google Scholar]
- 12.Epicollect5 – Free and Easy-To-Use Mobile Data-Gathering Platform. [Last accessed on 2020 Mar 15]. Available from: https://fiveepicollectnet/
- 13.SPSS Statistics 20 Available for Download. 2014. [Last accessed on 2020 Mar 15]. Available from: https://wwwibmcom/support/pages/spss-statistics-20-available-download .
- 14.Mithra P, Unnikrishnan B, Rekha T, Nithin K, Mohan K, Kulkarni V, et al. Compliance with iron-folic acid (IFA) therapy among pregnant women in an urban area of south India. Afr Health Sci. 2013;13:880–5. doi: 10.4314/ahs.v13i4.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangwan K, Kumar N, Bhatt BS. Socio demographic determinants of IFA intake during pregnancy among mothers in rural area of Rohtak, Haryana, India. Int J Basic Appl Med Sci. 2014;4:49–56. [Google Scholar]
- 16.Kessani L, Kumar R, Rathore A, Khalid R. Associated factors and compliance with iron-folic acid therapy among pregnant women of Karachi, Pakistan. RMJ. 2018;43:319–23. [Google Scholar]
- 17.Ibrahim ZM, El-Hamid SA, Mikhail H, Khattab MS. Assessment of adherence to Iron and folic acid supplementation and prevalence of anemia in pregnant women. Med J Cairo Univer. 2011;79:115–21. [Google Scholar]
- 18.Kamau MW, Mirie W, Kimani S. Compliance with Iron and folic acid supplementation (IFAS) and associated factors among pregnant women: Results from a cross-sectional study in Kiambu County, Kenya. BMC Public Health. 2018;18:580. doi: 10.1186/s12889-018-5437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manasa K, Chandrakumar S G, Prashantha B. Assessment of compliance with iron-folic acid therapy during pregnancy among postnatal mothers in tertiary care centre, Mysuru. Int J Community Med Public Health. 2019;6:1665–9. [Google Scholar]
- 20.Lyoba WB, Mwakatoga JD, Festo C, Mrema J, Elisaria E. Adherence to iron-folic acid supplementation and associated factors among pregnant women in Kasulu communities in North-Western Tanzania. Int J Reprod Med. 2020;2020:3127245. doi: 10.1155/2020/3127245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sendeku FW, Azeze GG, Fenta SL. Adherence to iron-folic acid supplementation among pregnant women in Ethiopia: A systematic review and meta-analysis. BMC Pregnancy Childbirth. 2020;20:138. doi: 10.1186/s12884-020-2835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dutta AJ, Patel P, Bansal RK. Compliance to iron supplementation among pregnant women: A cross sectional study in Urban Slum. Natl J Community Med. 2014;5:457–62. [Google Scholar]


