Highlights
-
•
Drosophila larva enables combining comprehensive synapse resolution connectivity maps with cellular-resolution activity maps and behavior maps.
-
•
This approach provides a way to elucidate neural implementation of universal brain computations such as multisensory integration, learning, and action-selection.
-
•
Early multisensory convergence recruits specific sensorimotor loops for specific actions.
-
•
Higher-order brain areas integrate modalities in a more centralized way and produce high-order, valence-like signals for goal-oriented behavior.
Abstract
The larva of Drosophila melanogaster is emerging as a powerful model system for comprehensive brain-wide understanding of the circuit implementation of neural computations. With an unprecedented amount of tools in hand, including synaptic-resolution connectomics, whole-brain imaging, and genetic tools for selective targeting of single neuron types, it is possible to dissect which circuits and computations are at work behind behaviors that have an interesting level of complexity. Here we present some of the recent advances regarding multisensory integration, learning, and action selection in Drosophila larva.
Current Opinion in Neurobiology 2020, 65:129–137
This review comes from a themed issue on Whole-brain interactions between neural circuits
Edited by Larry Abbott and Karel Svoboda
For a complete overview see the Issue and the Editorial
Available online 23rd November 2020
https://doi.org/10.1016/j.conb.2020.09.008
0959-4388/© 2020 The Author(s). Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
Ability to sense, act, remember, or anticipate emerges from the way the nervous system is organized into networks that allow signals to flow, interact, and change. Nerve cell types and numbers vary across different organisms, but many neuronal computations and behaviors appear to be done in a similar way across mammals and insects. For example, odor signals are processed via two parallel high-order pathways differing in representation and plasticity [1,2]. Feeding circuit is formed of multilayered loops linking external/enteric sensory inputs to motor/secretory outputs [3,4]. Dopaminergic neurons encoding reinforcement of different valences project onto spatially distinct associative regions [5].
Given the phylogenetic distance between insects and mammals (half a billion year), the fact that similar circuit solutions for complex problems have been conserved or reinvented through evolution points towards some fundamental principles linking structure and function in the central nervous system (CNS).
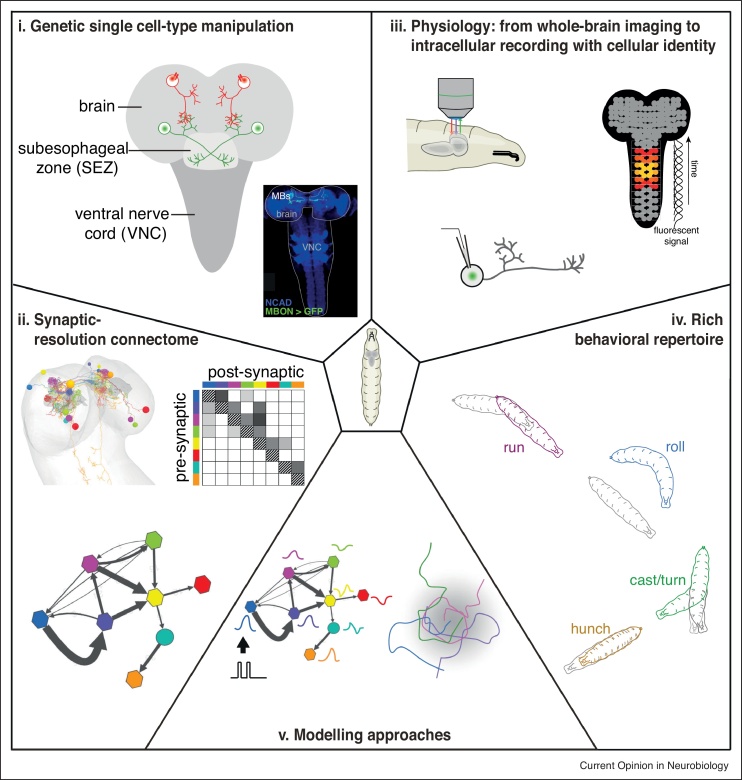
Here we review recently described circuits in the CNS of the larva of Drosophila melanogaster. The insect CNS comprises a brain, a subesophageal zone (SEZ), and a ventral nerve cord (VNC, Figure 1i). This tripartite organization is similar to the forebrain/cerebellum, brainstem, and spinal cord of vertebrates. The relatively small CNS of the Drosophila larva as a model offers a number of advantages for studying the circuit implementation of neural computations in a comprehensive way (Figure 1).
Figure 1.
Approaches for studying neural circuit in Drosophila larva.
i. Drosophila larva beneficiates from a comprehensive genetic toolkit for selective targeting of uniquely identified neuron types, often a single pair of left-right homologous neurons [21,22]. Left, schematic of the tripartite organization of the larval CNS: brain, SEZ and VNC. Right, confocal image of a CNS immunostained against n-cadherin (blue) and GFP expressed in a single pair of MB output neurons (green).
ii. Synaptic resolution connectome. A full CNS imaged with EM has been reconstructed; the connectivity of many neural circuits at synaptic resolution is now known. Quantitative studies have shown that strong connections are symmetrical between left and right side [7] and conserved across individuals and larval stages [19].
iii. Accessibility to physiological activity: in vivo imaging [26, 27, 28, 29, 30, 31], ex vivo whole-brain imaging [23], intracellular recordings of uniquely identified neurons that can be selectively labelled by GFP [8,9,18,77].
iv. Substantial behavioral repertoire: discrete actions such as run, stop, head cast, turn, hunch, backup, roll can be automatically tracked and categorized.
v. Modelling approaches. Thanks to the few number of larval neurons (ca. 15,000 including 2500 brain neurons), reconstructed connectivity can be implemented in an artificial neural network [8,16,18]. Behavioral hallmarks can also be reproduced by an agent-based model [52,56,67].
Studying neural circuits in Drosophila larva
The early larval central nervous system contains fewer (ca. 15,000) and smaller neurons compared to the adult Drosophila making it amenable to relatively rapid electron microscopy imaging and circuit reconstruction with synaptic resolution [6] (Figure 1ii). So far, circuits for somatosensory processing [6, 7, 8] and motor programming [9, 10, 11] in the VNC, feeding [3] and neuromodulation [12] in the SEZ, as well as first-order sensory [14,15], and higher-order associative centers [16, 17, 18] in the brain have been reconstructed with synaptic resolution in the same EM volume of a first-instar (i.e. early larval stage) nervous system, and comprehensive reconstruction of the CNS is within reach. The reproducibility of this type of data has been tested by Gerhard et al. [19], who compared portions of nociceptive circuits in an early (first-instar) and a later (third-instar) stage larvae, and found that the fraction of total synaptic input associated with defined pre-synaptic partner is maintained despite a five-fold change in size. In many cases, the knowledge about connectivity could be augmented with immunohistology against neurotransmitters. This has allowed the identification of various types of circuit motifs [8,14, 15, 16,18].
The connectome alone is not sufficient for understanding circuit mechanisms [20], but it provides a necessary roadmap for mechanistic studies. In complement, Drosophila larva is amenable to a large variety of functional studies (Figure 1i,iii). With multiple genetic tools for selective targeting and manipulating individual cell types [21,22], functional connectivity between neurons can be tested by combining optogenetic activation of presynaptic neurons with e.g. electrophysiological recording [8,9,18] or calcium imaging of postsynaptic neurons [6,10,13,18]. Imaging the activity of motoneurons can also be used as a proxy for behavior and allows the visualization of fictive actions [23, 24, 25]. Due to the transparency of its body wall, imaging in living animals has recently been developed in immobilized [26,27] as well as in moving animals [27, 28, 29, 30, 31].
In addition, the larva has a rich behavioral repertoire, exhibiting a range of distinct actions and sequences [6,8,32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42] (Figure 1iv) and is capable of robust associative learning [16, 17, 18,43, 44, 45, 46, 47, 48, 49]. Automatic tracking of individual larvae and behavioral categorization greatly facilitates relating the structure of larval circuits to their function [6,8,13,31, 32, 33, 34, 35, 36, 37,39,49,50]. High-throughput behavioral inactivation and activation screens allow identifying neurons promoting or repressing specific actions or computations [8,33,36,41,50,51].
Finally, these approaches are often combined with modelling that can generate testable predictions about the possible roles of specific circuit motifs in behavior [6,8,11,16,18,37,52,56] (Figure 1v).
Circuits for multisensory integration, learning, and action-selection
As described in other organisms [57], specific sensory modalities act jointly early on in signal processing, at the first or second-order neuron, to build a meaningful representation of a stimulus. Later in processing, at higher-order brain regions, more sensory modalities can be combined to keep track of environmental variability. Circuit analyses in Drosophila larva are starting to elucidate the way in which all dimensions of multisensory experiences are integrated for appropriate action selection.
Multisensory integration at early stages
In the VNC, local sensorimotor loops influence crucial aspects of locomotion and distinct types of responses to various somatosensory stimuli [6,8,27,30,34,38,58, 59, 60, 61]. Among them, nocifensive behaviors rely on multidendritic nociceptive neurons [6,38,60,61]. Depending on the type of threat, larvae can respond to nociceptive stimulation by accelerating forward [34,39,62], crawling backward [34], or rolling sideways [6,38,60,61], the latter being the most vigorous nocifensive response (Figures 1iv, 2 ). Neural activation and inactivation screens revealed neurons necessary and/or sufficient for nocifensive behaviors [6,33,34,38,39,61,63]. Connectome reconstruction unraveled how sensory inputs target these bottleneck neurons [6, 7, 8,19,34,61].
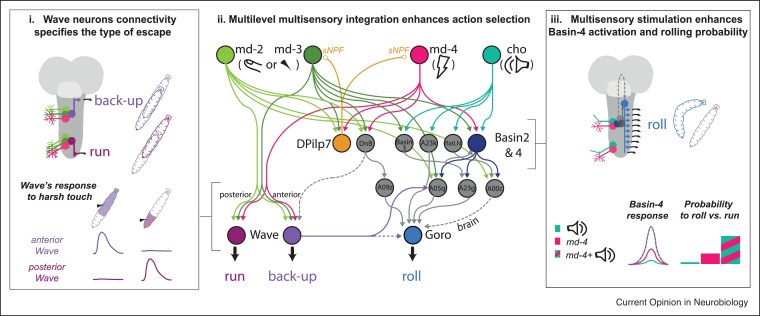
Figure 2.
Circuits for nocifensive behavior in larva.
Sensorimotor circuits for escape response selection to a nociceptive stimulus have been particularly well studied in Drosophila larva. The particular nocifensive behavior depends on the type of threat: predator attack, noxious heat or harsh touch can evoke rolling response [6,38,60,61], instead weaker and less threatening stimuli can evoke fast forward crawling or back-up [34,39,62]. The nature of the response relies on the type of somatosensory neurons co-activated with the polymodal larval nociceptors (multidendritic class IV ‘md-4’, magenta). Components of the circuits reconstructed downstream of the nociceptive neurons [6,61] and of the main somatosensory neurons (chordotonal ‘cho’, ‘md-2’ and ‘md-3’, greens) [7,8,34] are depicted. Circles indicate pairs or class of neurons, plain lines are direct connections, dotted lines indirect ones, all reconstructed in the same EM volume.
i. ‘Wave’ neurons repeatedly tiling the VNC integrate spatially defined multimodal inputs to promote either run or back-up in response to, respectively, posterior or anterior harsh touch stimulation [34]. These neurons also indirectly contact the roll-promoting neuron ‘Goro’ [6,34].
ii. Multiple levels of multisensory integration occur during the selection of rolling: both early in sensory processing, and at more downstream nodes in the network [6, 7, 8,19,34,60,61]. Modelling shows that multiple levels of multisensory integration enhances action selection [6].
iii. ‘Basin 4’ super-additively integrates ‘cho’ and md-4’ inputs. Increased Basin-4 activation by multisensory cues, in turn increases the likelihood of rolling behavior, through Goro activation [6].
Forward or backward escapes have been shown to rely on the homologous segmentally repeated ‘Wave’ neurons [34] that integrate two types of somatosensory inputs: touch sensors and nociceptors. Importantly, Wave neurons in posterior segments induce forward escape, whereas the ‘Wave’ neurons located in the anterior segments induce backward escape (Figure 2i). EM reconstruction revealed Wave neurons in all segments receive synaptic input from nociceptive and touch-sensing neurons, but they have different output targets in different segments: the anterior Wave neurons synapse onto circuits in anterior segments that promote backward crawling, whereas the Wave neurons target posterior segments and promote forward crawling. Combining EM reconstruction with functional studies therefore revealed the way in which homologous neurons integrate and target different partners in different regions of the nervous system to mediate opposite behaviors.
The most vigorous and energetically costly rolling escape is elicited in response to predator attack [38]. Presenting mechanosensory cues with nociceptive ones facilitates rolling [6,61], likely because it better approximates predator attack which also stimulates multiple senses. Rolling is mediated by a command-like ‘Goro’ neuron that receives indirect functional inputs from both mechanosensory and nociceptive sensory neurons [6,61]. EM reconstructions of circuits downstream of mechanosensory and nociceptive neurons and upstream of the Goro neurons elucidated precisely where and how the information from these distinct sensory modalities is integrated. This revealed that mechanosensory and nociceptive information converges early on in the sensory processing hierarchy onto first-order multisensory interneurons that integrate the information superadditively [6] (Figure 2ii). Multiple interneurons, gathering slightly different somatosensory modalities, relay multisensory nociceptive inputs to Goro and are sufficient to evoke rolling [6,61]. Additionally, later stages of multisensory integration at higher-order nodes enhance action selection [6]. Furthermore, when activated in combination with nociceptive neurons, touch-sensing neurons integrate the multiple mechanosensory inputs through sNPF feedback release and facilitate rolling [60]. Thus, knowing both how the different nodes of circuits are connected and their functional properties provided a mechanistic insight into the way in which nocifensive behaviors are selected (Figure 2).
Higher-order integration: learning and value coding
Larval behavior is plastic and adapts to experience (review in [47]). Mushroom Bodies (MB) in the larval brain are necessary to form associative memory [43]. This memory is expressed by changing navigation towards a cue (e.g. an odor) that has been associated with a reward (e.g. sucrose) or a punishment (e.g. quinine).
MBs in insects translate rich sensory representation into a low dimension signal relevant for behavior and encoded by the population of MB output neurons (MBONs; [64]) (Figure 3). EM reconstruction of the comprehensive set of 223 intrinsic MB neurons, called Kenyon cells (KCs), in a first-instar larva and all of their pre- and postsynaptic partners revealed the detailed synaptic-resolution architecture of the learning circuit and uncovered a number of unexpected circuit motifs. Most Kenyon cells were found to integrate random combinations of inputs from olfactory projection neurons, but a subset received stereotyped inputs from single projection neurons [16]. Combining the connectome with modelling revealed that this distribution results in enhanced contrast between cues encoded by the KCs, improving performance of a model output neuron on a discrimination task [16]. In addition, as in many other organisms [5], larval dopaminergic neurons (DANs) carry reinforcing signal which lead to appetitive or aversive memory formation (reviewed in [47]). DANs target different regions of the KC axons and gate synaptic plasticity [65] between a subset of KCs activated by a cue and the output neurons extending their dendrites in this region of the MB. The minimal microcircuit composed of KCs-to-MBONs synapses modulated by DANs is sufficient to explain how cues-elicited behavior changes upon associative trials [16,44]. EM revealed additionally that KCs reciprocally synapse onto DANs [16]. Consistent with this, activation of KCs alone (i.e. by sensory cues without reinforcement) can lead to plasticity [48]. Whether the KC-to-DAN synapse is subject to plasticity is an exciting open question.
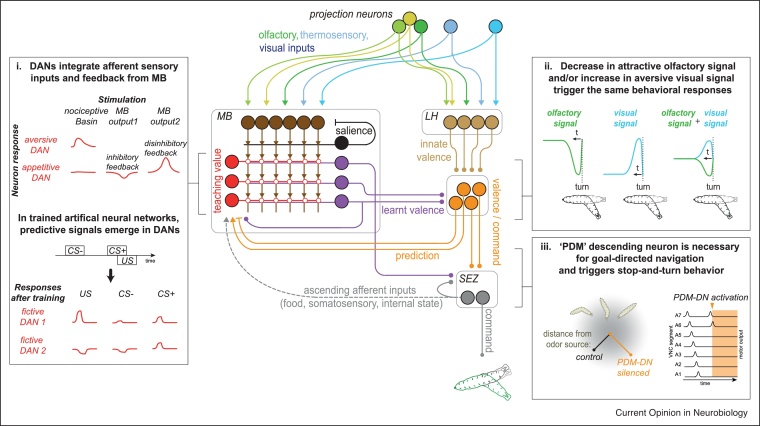
Figure 3.
Circuits for high-order integration and value computation.
In the larval brain the MB and the LH receive convergent sensory inputs from olfactory, thermosensory and visual projection neurons (green and light blue) [16,14,15]. The LH neurons (light brown) are assumed to parse the information according to innate valence. The MB Kenyon cells (dark brown) are thought to decorrelate sensory signals via highly divergent connections, expose them to reinforcement-gated plasticity (red open circles), and generate learnt valence signals (purple). MB is characterized by a recurrent architecture: a GABAergic neuron (black) gathers signals at the KCs axons and feeds back onto the KCs dendrites [16,17]; in addition, KCs signal back to the teaching neurons (mostly DANs, red) [16,49] and so do the MB output neurons (purple and orange feedbacks) [16,18]. These multilayered paths provide neural substrates for integrating prediction to the teaching signals, in addition to other inputs from the SEZ, for adaptive memory update (See Box i [18]). Further down the circuit, MB and LH are likely integrated for valence signals that can be used as instructive signal for navigation (orange, See Box ii [54] and iii [35]). The SEZ (grey) transforms more [16,35] or less [3] integrated signals into behavior.
i.Top panel, the experimental exploration of the responses of some teaching DANs, combining calcium transient and optogenetic stimulations, confirms that DANs integrate external (multisensory nociceptive neurons Basin) and memory-related (MB outputs neurons) inputs combining the recording of cell activity and optogenetic stimulations. Bottom panel, In an artificial neural network incorporating the connectivity of the MB and the response tuning of the DANs, discriminative signal for conditioned stimuli that predict (CS+) or not (CS-) the unconditioned stimulus (US) emerges in the DANs after associative training [18].
ii. Reverse-correlation experiments [13,53, 54, 55] link olfactory and visual inputs to navigation [54]. Under fictive odor stimulation (the optogenetic activation of receptors for attractive odors continuously varying in intensity), redirecting turns are initiated by a decrease in the signal, while under real visual stimulation (aversive blue light varying in intensity) turns are initiated when the signal increases. The sensorimotor transformation estimated for the combination of inputs suggests a linear integration of olfactory and visual inputs, probably readable at the level of LH output.
iii. PDM-DN, a neuron reconstructed in EM, downstream of LH neurons and descending to the SEZ, is necessary for navigating towards an odor source [35]. The optogenetic activation of this neuron triggers stop and redirection in crawling animals by stopping the wave of body contraction that goes through the body and shutting off the activity of the corresponding motoneurons.
What do DANs encode? Of the 8 DANs projecting onto the MB, 4 are necessary and/or sufficient to form sugar memory [17,46]. Three others are sufficient to form an aversive memory and we found they respond to somatosensory stimulations [18] (Figure 3i) Thus, food-related signal from the SEZ and somatosensory signal from the VNC seem translated into dopaminergic signal onto the MB, reminiscent to appetitive and aversive reinforcement encoded by different DANs in the striatum of rodents [5]. EM reconstruction of the circuits upstream of DANs is starting to provide insights into the way in which teaching signals that drive learning are computed. A comprehensive reconstruction of all neurons (109) presynaptic to the DANs (or other modulatory neurons, [16]) [18], revealed that 7 are MBONs [16] and 60 are directly or indirectly postsynaptic to MBONs [18]. In total, the mono- and polysynaptic feedback from MB represent more than 50% of the synaptic inputs received by DANs. Artificial network constrained by the reconstructed larval MB connectivity revealed that these recurrent signals improve performance and flexibility of learning tasks [18]. Strikingly, the responses of the artificial DANs to the different associative cues changed over the course of training, consistent with prediction signals formulated in theories of reinforcement learning and with findings in mammalian DANs (reviewed in [66]). Whether such adaptive responses also naturally emerge during learning in behaving larvae is still an open question.
The many layers of recurrence in the MBs [16, 17, 18,48] and the fact that KCs receive inputs from multiple modalities - olfactory, visual, thermosensory, gustatory [16] (Figure 3)- raise the question of the nature of the signal after MB processing. Many MBONs interact [16] and a few of them integrate inputs from multiple MBONs from compartments of opposite valence [16, 17, 18]. These MBONs are well poised to collect and compute the overall result of MB processing. The way in which the MBON signals are combined with LH outputs that signal innate valence and used to guide action selection is still an open question. Agent-based modelling suggests that a fully centralized system combining all value inputs into a unified value code would reproduce larval navigation accurately [52,56,67] On the other hand, the artificial neural network constrained by the connectome found the coding of predicted value can be represented within the numerous feedback neurons in a distributed way [18], although how this signal is built and used for economic decisions is not yet fully understood. With its few neurons and low redundancy, the brain of Drosophila larva is a model of choice to combine more experimental and theoretical approaches and deepen our understanding of these mechanisms.
Action selection
Drosophila larvae can generate many exclusive actions. Studies are beginning to elucidate the way in which multisensory inputs, higher-order valence signals, and context are used for action selection. In recent years progress has been made in understanding the circuits that mediate the selection of distinct types of escape responses in response to threatening somatosensory stimuli: roll, fast crawl, turn, back-up, and hunch. The selection of the most vigorous escape, roll, is enhanced by integrating nociceptive with mechanosensory inputs [6,61] (Figure 2). So far, many circuit motifs involved in the selection of these actions and their organization into sequences have been identified in the VNC. For example, reciprocally connected inhibitory interneurons mediate behavioral choice between hunch and turn in response to an air-puff [8], lateral disinhibition promotes sequence transitions between these actions, and specialized local feedback disinhibition provides positive feedback that stabilizes a behavior and prevents reversals to the preceding one. The combination of these interconnected circuit motifs can implement both behavior selection and the organization of behaviors into a sequence. Interestingly the connectome reveals that descending neurons from the brain and SEZ synapse onto many of the VNC interneurons involved in somatosensory responses [6,8]. The way in which brain circuits bias somatosensory choices is an exciting open question for the future.
A second major action selection paradigm in the larva is the choice whether to crawl or turn and which way to turn when navigating gradients of aversive or repulsive cues. The alternation between runs (forward crawl events), stops, and turns can be done without brain inputs [68] but their transitions based on sensory inputs rely on the brain (besides likely modalities detected by VNC sensory neurons, [8,68]). These choices require the computation of the value of the cue, which is done based on both innate and learnt valences. Ongoing reconstruction of neurons downstream MB and LH will inform about the way in which innate and learnt values are integrated. In addition, different modalities converge onto the same pattern of navigation responses [13,37,40,41,53]. Different sensory inputs have been shown to be translated into turn action following the same signal transformation function [54] (Figure 3ii). It is therefore possible that all modalities converge onto a common center involved in computing an overall integrated value of a cue and guiding navigation. This interpretation also fits the convergence of projection neurons of various modalities onto the two brain structures MB [16] and LH [14,15] (Figure 3). To comprehensively characterize the circuits that underlie navigational decisions several screens have been conducted for neurons that contribute to these behaviors [50,69, 70, 71]. Possible bottleneck targets of navigational circuits are the descending neurons, such as the ‘PDM-DN’ which can trigger stop in a deterministic way [35] (Figure 3iii). This command neuron has been located in the SEZ [35] while other command neurons are located in the VNC (for roll [6]; for backup [34]). Likely, the VNC and SEZ regions are thus the last steps where conflict for different types of actions may be resolved. Reconstructing the full pathways for different behavioral drives all the way from a comprehensive set of sensory inputs to a comprehensive set of command neurons will allow identifying their sites of interactions and potential conflict resolution; this may result in refining our views of the processes defined as action selection or decision [8,36].
Conclusion
Providing a precise roadmap with the connectome, Drosophila larva helps formulate and test new hypotheses about the way in which neural circuits implement fundamental computations such as multisensory integration, learning, value computation, and action-selection.
Future research will include richer behavioral situations (e.g. [42,72,73]), in vivo recording of whole-brain activity [23, 24, 25], modelling approaches (e.g. [8,11,16,18,52]). In parallel, whole-brain RNAseq reveals genes expressed in individual neurons [74,75] that might be essential for these computations. The comparatively small size enables rapid reconstruction of connectomes from multiple individuals (e.g. [76]) and opens doors to an exciting new area of experimental connectomics to address questions about the structural correlates of specific memory traces, individual differences in circuits that underlie distinct personality traces, and discovering the effects of various mutants on the circuit architecture.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank Elise Croteau-Chonka for the drawings of the larvae, Albert Cardona for help with Figure 2, Nadine Randel and Marc Corrales for comments on the manuscript. The authors also wish to thank all the neuroscientists involved in research in Drosophila larva. M.Z. and C.E. were supported by the ERC consolidator grant LeaRNN - 819650; M.Z. was also supported by the Wellcome Trust Investigator Award: 205050/B/16/Z.
Contributor Information
Claire Eschbach, Email: ce394@cam.ac.uk.
Marta Zlatic, Email: mz209@cam.ac.uk.
References
- 1.Vosshall L.B., Stocker R.F. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 2.Sosulski D.L., Lissitsyna Bloom M., Cutforth T., Axel R., Datta S.R. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miroschnikow A. Convergence of monosynaptic and polysynaptic sensory paths onto common motor outputs in a drosophila feeding connectome. Elife. 2018;7 doi: 10.7554/eLife.40247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swanson L.W. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- 5.Watabe-Uchida M., Uchida N. Multiple dopamine systems: weal and woe of dopamine. Cold Spring Harb Symp Quant Biol. 2018;83:83–95. doi: 10.1101/sqb.2018.83.037648. [DOI] [PubMed] [Google Scholar]
- 6.Ohyama T. A multilevel multimodal circuit enhances action selection in Drosophila. Nature. 2015;520:633–639. doi: 10.1038/nature14297. [DOI] [PubMed] [Google Scholar]; EM reconstruction of the circuit processing convergent nociceptive and mechanosensory inputs. Functional characterization at behavior and physiology level of this multisensory integration shows superadditive integration, later translated to the command neuron ‘Goro’ for rolling behavior.
- 7.Schneider-Mizell C.M. Quantitative neuroanatomy for connectomics in Drosophila. Elife. 2016;5 doi: 10.7554/eLife.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jovanic T. Competitive disinhibition mediates behavioral choice and sequences in Drosophila. Cell. 2016;167:858–870. doi: 10.1016/j.cell.2016.09.009. [DOI] [PubMed] [Google Scholar]; The authors combine genetic screening, EM reconstruction, electrophysiology and modelling to identify the neural basis of the choice of larvae between different actions in response to an air puff. They describe interconnected circuit motives in the VNC where mechanosensory inputs lift reciprocal disinhibition, biasing larval response towards one or the other action.
- 9.Zwart M.F. Selective inhibition mediates the sequential recruitment of motor pools. Neuron. 2016;91:615–628. doi: 10.1016/j.neuron.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fushiki A. A circuit mechanism for the propagation of waves of muscle contraction in Drosophila. Elife. 2016;5 doi: 10.7554/eLife.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarin A.A., Mark B., Cardona A., Litwin-Kumar A., Doe C.Q. A multilayer circuit architecture for the generation of distinct locomotor behaviors in Drosophila. Elife. 2019;8 doi: 10.7554/eLife.51781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlegel P. Synaptic transmission parallels neuromodulation in a central food-intake circuit. Elife. 2016;5 doi: 10.7554/eLife.16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein M. Sensory determinants of behavioral dynamics in Drosophila thermotaxis. Proc Natl Acad Sci U S A. 2015;112:220–229. doi: 10.1073/pnas.1416212112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berck M.E. The wiring diagram of a glomerular olfactory system. Elife. 2016;5 doi: 10.7554/eLife.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larderet I. Organization of the drosophila larval visual circuit. Elife. 2017;6 doi: 10.7554/eLife.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eichler K. The complete connectome of a learning and memory centre in an insect brain. Nature. 2017;548:175–182. doi: 10.1038/nature23455. [DOI] [PMC free article] [PubMed] [Google Scholar]; EM reconstruction of the larval MB circuit. First, this showed different kinds of larval KCs: first-born single-claw KCs receiving a single olfactory input, and later-born multi-claw KCs receiving random input from olfactory and other modalities. Second, it revealed new canonical motives at each MB compartment: feedback from KC onto segregated modulatory inputs, and direct projection of modulatory neurons onto the MB output neurons.
- 17.Saumweber T. Functional architecture of reward learning in mushroom body extrinsic neurons of larval Drosophila. Nat Commun. 2018;9 doi: 10.1038/s41467-018-03130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eschbach C. Recurrent architecture for adaptive regulation of learning in the insect brain. Nat Neurosci. 2020;23:544–555. doi: 10.1038/s41593-020-0607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; EM reconstruction of the larval MB second-order circuit, combined with functional study shows that dopaminergic neurons with similar functions receive similar inputs. It also found that 50% of the inputs received by MB modulatory neurons are feedbacks from MB compartments of same and/or of opposite valence. Artificial neural networks constrained with the reconstructed connectivity address the role of these feedbacks.
- 19.Gerhard S., Andrade I., Fetter R.D., Cardona A., Schneider-Mizell C.M. Conserved neural circuit structure across drosophila larval development revealed by comparative connectomics. Elife. 2017;6 doi: 10.7554/eLife.29089. [DOI] [PMC free article] [PubMed] [Google Scholar]; EM reconstruction of segments of the VNC allows comparison of nociceptive circuits across larval individuals and stages showing remarkable conservation of circuits despite five-fold difference in size. In particular, the authors point that the fraction of total synaptic input associated with defined pre-synaptic partner is maintained.
- 20.Bargmann C.I., Marder E. From the connectome to brain function. Nat Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- 21.Li H.H. A GAL4 driver resource for developmental and behavioral studies on the larval CNS of Drosophila. Cell Rep. 2014;8:897–908. doi: 10.1016/j.celrep.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 22.Owald D., Lin S., Waddell S. Light, heat, action: neural control of fruit fly behaviour. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemon W.C. Whole-central nervous system functional imaging in larval Drosophila. Nat Commun. 2015;6 doi: 10.1038/ncomms8924. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors adapt multi-view light-sheet microscope to allow fast, subsecond scale, functional imaging of the whole extracted CNS of Drosophila larva at a spatial resolution of up to 1 μm. They demonstrate fictive spontaneous forward and backward crawling through waves of motoneurons calcium activity and are able to correlate them with local activity in the brain and SEZ.
- 24.Pulver S.R. Imaging fictive locomotor patterns in larval Drosophila. J Neurophysiol. 2015;114:2564–2577. doi: 10.1152/jn.00731.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chhetri R.K. Whole-animal functional and developmental imaging with isotropic spatial resolution. Nat Methods. 2015;12:1171–1178. doi: 10.1038/nmeth.3632. [DOI] [PubMed] [Google Scholar]
- 26.Si G. Structured odorant response patterns across a complete olfactory receptor neuron population. Neuron. 2019;101:950–962. doi: 10.1016/j.neuron.2018.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; To characterize the dose-response profiles of each of the 21 olfactory receptor neurons to 34 different odors and rules governing olfactory transduction, the authors notably develop a microfluidic device to trap living larvae, expose them to water-borne odorants in a highly controlled manner, while imaging neural activity through the transparent cuticle.
- 27.He L. Direction selectivity in Drosophila proprioceptors requires the Mechanosensory Channel Tmc. Curr Biol. 2019;29:945–956. doi: 10.1016/j.cub.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bouchard M.B. Swept confocally-aligned planar excitation (SCAPE) microscopy for high speed volumetric imaging of behaving organisms. Nat Photonics. 2015;9:113–119. doi: 10.1038/nphoton.2014.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marescotti M., Lagogiannis K., Webb B., Davies R.W., Armstrong J.D. Monitoring brain activity and behaviour in freely moving Drosophila larvae using bioluminescence. Sci Rep. 2018;8:9246. doi: 10.1038/s41598-018-27043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaadia R.D. Characterization of proprioceptive system dynamics in behaving Drosophila larvae using high-speed volumetric microscopy. Curr Biol. 2019;29:935–944. doi: 10.1016/j.cub.2019.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karagyozov D., Mihovilovic Skanata M., Lesar A., Gershow M. Recording neural activity in unrestrained animals with three-dimensional tracking two-photon microscopy. Cell Rep. 2018;25:1371–1383.e10. doi: 10.1016/j.celrep.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report a two-photon microscope capable of recording neural activity at the submillisecond and submicron resolution in translucent animals moving in 3D. They notably demonstrate cellular recordings of calcium transients in motoneurons and premotor interneurons in larvae crawling forward and backward or even head sweeping.
- 32.Gershow M. Controlling airborne cues to study small animal navigation. Nat Methods. 2012;9:290–296. doi: 10.1038/nmeth.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogelstein J.T. Discovery of brainwide neural-behavioral maps via multiscale unsupervised structure learning. Science. 2014;344:386–392. doi: 10.1126/science.1250298. [DOI] [PubMed] [Google Scholar]
- 34.Takagi S. Divergent connectivity of homologous command-like neurons mediates segment-specific touch responses in Drosophila. Neuron. 2017;96:1373–1387.e6. doi: 10.1016/j.neuron.2017.10.030. [DOI] [PubMed] [Google Scholar]; From the experimental observation that a harsh touch at the front or the back of the larva elicit different escape responses, respectively back-up crawling or forward acceleration, the authors identify homologous neurons located in different segment of the VNC that are necessary and sufficient for the two types of response. Then, EM reconstruction allows to find that the pathways linking sensory inputs and motor outputs differ for back and front body location.
- 35.Tastekin I. Sensorimotor pathway controlling stopping behavior during chemotaxis in the drosophila melanogaster larva. Elife. 2018;7 doi: 10.7554/eLife.38740. [DOI] [PMC free article] [PubMed] [Google Scholar]; Combining neuronal manipulation, calcium imaging, and EM reconstruction the authors describe a brain neuron descending to the SEZ responsible for stop behavior and allowing proper navigation in an odor gradient. The role of its direct postsynaptic neuron descending from the SEZ to the VNC is also explored.
- 36.Masson J.B. Identifying neural substrates of competitive interactions and sequence transitions during mechanosensory responses in Drosophila. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez-Marin A., Stephens G.J., Louis M. Active sampling and decision making in Drosophila chemotaxis. Nat Commun. 2011;2:441. doi: 10.1038/ncomms1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson J.L., Tsubouchi A., Tracey W.D. Larval defense against attack from parasitoid wasps requires nociceptive neurons. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohyama T. High-throughput analysis of stimulus-evoked behaviors in Drosophila larva reveals multiple modality-specific escape strategies. PLoS One. 2013;8 doi: 10.1371/journal.pone.0071706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kane E.A. Sensorimotor structure of Drosophila larva phototaxis. Proc Natl Acad Sci U S A. 2013;110:e3868–e3877. doi: 10.1073/pnas.1215295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Humberg T.H. Dedicated photoreceptor pathways in Drosophila larvae mediate navigation by processing either spatial or temporal cues. Nat Commun. 2018;9:1260. doi: 10.1038/s41467-018-03520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D., Alvarez M., Lechuga L.M., Louis M. Species-specific modulation of food-search behavior by respiration and chemosensation in Drosophila larvae. Elife. 2017;6 doi: 10.7554/eLife.27057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pauls D., Selcho M., Gendre N., Stocker R.F., Thum A.S. Drosophila larvae establish appetitive olfactory memories via mushroom body neurons of embryonic origin. J Neurosci. 2010;30:10655–10666. doi: 10.1523/JNEUROSCI.1281-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schleyer M. A behavior-based circuit model of how outcome expectations organize learned behavior in larval Drosophila. Learn Mem. 2011;18:639–653. doi: 10.1101/lm.2163411. [DOI] [PubMed] [Google Scholar]
- 45.von Essen A.M.H.J., Pauls D., Thum A.S., Sprecher S.G. Capacity of visual classical conditioning in Drosophila larvae. Behav Neurosci. 2011;125:921–929. doi: 10.1037/a0025758. [DOI] [PubMed] [Google Scholar]
- 46.Rohwedder A. Four individually identified paired dopamine neurons signal reward in larval Drosophila. Curr Biol. 2016;26:661–669. doi: 10.1016/j.cub.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Thum A.S., Gerber B. Connectomics and function of a memory network: the mushroom body of larval Drosophila. Curr Opin Neurobiol. 2019;54:146–154. doi: 10.1016/j.conb.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Lyutova R. Reward signaling in a recurrent circuit of dopaminergic neurons and peptidergic Kenyon cells. Nat Commun. 2019;10:3097. doi: 10.1038/s41467-019-11092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paisios E., Rjosk A., Pamir E., Schleyer M. Common microbehavioral ‘footprint’ of two distinct classes of conditioned aversion. Learn Memory. 2017;24:191–198. doi: 10.1101/lm.045062.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tastekin I. Role of the subesophageal zone in sensorimotor control of orientation in drosophila larva. Curr Biol. 2015;25:1448–1460. doi: 10.1016/j.cub.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 51.Clark M.Q., McCumsey S.J., Lopez-Darwin S., Heckscher E.S., Doe C.Q. Functional genetic screen to identify interneurons governing behaviorally distinct aspects of Drosophila larval motor programs. G3: Genes Genomes Genet. 2016;6:2023–2031. doi: 10.1534/g3.116.028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies A., Louis M., Webb B. A model of Drosophila larva chemotaxis. PLoS Comput Biol. 2015;11 doi: 10.1371/journal.pcbi.1004606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schulze A. Dynamical feature extraction at the sensory periphery guides chemotaxis. Elife. 2015;4 doi: 10.7554/eLife.06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gepner R., Skanata M.M., Bernat N.M., Kaplow M., Gershow M. Computations underlying Drosophila photo-taxis, odor-taxis, and multi-sensory integration. Elife. 2015;4 doi: 10.7554/eLife.06229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gepner R., Wolk J., Wadekar D.S., Dvali S., Gershow M. Variance adaptation in navigational decision making. Elife. 2018;7 doi: 10.7554/eLife.37945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schleyer M., Reid S.F., Pamir E., Saumweber T., Paisios E., Davies A., Gerber B., Louis M. The impact of odor-reward memory on chemotaxis in larval Drosophila. Learn Memory. 2015;22:267–277. doi: 10.1101/lm.037978.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang W.-H., Chen A., Rasch M.J., Wu S. Decentralized multisensory information integration in neural systems. J Neurosci. 2016;36:532–547. doi: 10.1523/JNEUROSCI.0578-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hughes C.L., Thomas J.B. A sensory feedback circuit coordinates muscle activity in Drosophila. Mol Cell Neurosci. 2007;35:383–396. doi: 10.1016/j.mcn.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song W., Onishi M., Jan L.Y., Jan Y.N. Peripheral multidendritic sensory neurons are necessary for rhythmic locomotion behavior in Drosophila larvae. Proc Natl Acad Sci U S A. 2007;104:5199–5204. doi: 10.1073/pnas.0700895104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu C. Modality-specific sensory integration and neuropeptide-mediated feedback facilitate mechano-nociceptive behavior in Drosophila. Nat Neurosci. 2017;20:1085–1095. doi: 10.1038/nn.4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burgos A. Nociceptive interneurons control modular motor pathways to promote escape behavior in Drosophila. Elife. 2018;7 doi: 10.7554/eLife.26016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang Y. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan Z. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221–225. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menzel R. The insect mushroom body, an experience-dependent recoding device. J Physiol Paris. 2014;108:84–95. doi: 10.1016/j.jphysparis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 65.Hige T., Aso Y., Modi M.N., Rubin G.M., Turner G.C. Heterosynaptic plasticity underlies aversive olfactory learning in Drosophila. Neuron. 2015;88:985–998. doi: 10.1016/j.neuron.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schultz W. Neuronal reward and decision signals: from theories to data. Physiol Rev. 2015;95:853–951. doi: 10.1152/physrev.00023.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wystrach A., Lagogiannis K., Webb B. Continuous lateral oscillations as a core mechanism for taxis in drosophila larvae. Elife. 2016;5 doi: 10.7554/eLife.15504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berni J., Pulver S.R., Griffith L.C., Bate M. Autonomous circuitry for substrate exploration in freely moving Drosophila larvae. Curr Biol. 2012;22:1861–1870. doi: 10.1016/j.cub.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao W. A disinhibitory mechanism biases Drosophila innate light preference. Nat Commun. 2019;10:124. doi: 10.1038/s41467-018-07929-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jovanic T. Neural substrates of Drosophila larval anemotaxis. Curr Biol. 2019;29:554–566. doi: 10.1016/j.cub.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eschbach C. Circuits for integrating learnt and innate valences in the fly brain. bioRxiv. 2020 doi: 10.1101/2020.04.23.058339. [DOI] [Google Scholar]
- 72.Mancini N. Reversal learning in drosophila larvae. Learn Memory. 2019;26:424–435. doi: 10.1101/lm.049510.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vogt K. Internal state configures olfactory behavior and early sensory processing in Drosophila larvae. bioRxiv. 2020 doi: 10.1101/2020.03.02.973941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brunet Avalos C., Maier G.L., Bruggmann R., Sprecher S.G. Single cell transcriptome atlas of the Drosophila larval brain. Elife. 2019;8 doi: 10.7554/eLife.50354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cocanougher B.T., Wittenbach J.D., Long X., Kohn A.B. Comparative single-cell transcriptomics of complete insect nervous systems. bioRxiv. 2020 doi: 10.1101/785931. [DOI] [Google Scholar]
- 76.Valdes-Aleman J. Synaptic specificity is collectively determined by partner identity, location and activity. bioRxiv. 2019 doi: 10.1101/697763. [DOI] [Google Scholar]
- 77.Marley R., Baines R.A. Whole-cell patch recording from Drosophila larval neurons. Cold Spring Harb Protoc. 2011;2011 doi: 10.1101/pdb.prot065664. [DOI] [PubMed] [Google Scholar]