Abstract
Purpose:
The objectives of this study were to evaluate the feasibility and efficacy of fractional CO2 laser therapy in gynecologic cancer survivors.
Methods:
This was a pilot, multi-institutional randomized sham-controlled trial of women with gynecologic cancers with dyspareunia and/or vaginal dryness. Participants were randomized to fractional CO2 laser treatment or sham laser treatment. The primary aim was to estimate the proportion of patients who had improvement in symptoms based on the Vaginal Assessment Scale (VAS). Secondary aims included changes in sexual function assessed using the Female Sexual Functioning Index (FSFI) and urinary symptoms assessed using the the Urinary Distress Inventory (UDI-6).
Results:
Eighteen women participated in the study, ten in the treatment arm and eight in the sham arm. The majority of participants had stage I (n=11, 61.1%) or II (n=3, 16.7%) endometrial cancer with adenocarcinoma histology (n=9, 50%). In total, 15 (83.3%) of the participants completed all treatments and follow-up visit. There was no difference in the change in the median VAS score from baseline to follow-up. However, there was an improvement in change in the median total FSFI score with treatment compared with sham (Δ 6.5 vs −0.3, p=0.02). The change in the median UDI-6 score was lower in the treatment arm (Δ −14.6 vs −2.1, p=0.17), but this was not statistically significant. There were no reported serious adverse events.
Conclusions:
Fractional CO2 laser therapy is feasible in gynecologic cancer survivors, with preliminary evidence of safety. In addition, there was preliminary evidence of improvement in sexual function compared with sham treatment.
Clinicaltrial.gov Identifier:
NCT03372720 (OSU-17261; NCI-2017-02051)
Keywords: Cancer survivors, dyspareunia, genitourinary syndrome of menopause, vaginal atrophy, vaginal dryness, sexual function, atrophic vaginitis, laser therapy
Introduction
Genitourinary syndrome of menopause (GSM) is a constellation of symptoms including vaginal dryness, burning, itching, bleeding, dyspareunia, and dysuria that results from decreased estrogen. Up to 50% of postmenopausal women are affected by symptoms that result from thinning of the vaginal epithelium, alterations in collagen and elastin in the vaginal tissue, a decrease in blood flow to the vagina, a decrease in vaginal secretions, and an increase in vaginal pH that lead to decreased lubrication and elasticity of the vagina [1-3]. GSM can result from natural, surgical, or oncologic menopause and is known to affect survivors of gynecologic cancers, although the exact incidence has not been well studied [4, 5].
Women with gynecologic cancers, particularly those who have been treated with radiation, have a high incidence of vaginal dyspareunia, vaginal dryness, and sexual dysfunction [6-8]. Vaginal moisturizers and lubricants are typically recommended for symptom management, but unfortunately these topical agents do not treat the underlying problem of GSM [4, 5, 9, 7]. While low dose vaginal estrogen can help mitigate signs and symptoms, compliance with treatment is low, symptoms can return upon discontinuation, and insufficient improvement has been reported with both systemic and vaginal estrogens [10-13]. Additionally, vaginal estrogen use is controversial for some women with gynecologic cancers [14] and clinicians are often hesitant to prescribe vaginal estrogens in this population[15]. Fractional CO2 laser therapy is a potentially effective treatment for GSM that remodels vaginal tissue by activation of fibroblasts, collagen production, and neovascularization through direct controlled thermal damage to the vaginal mucosa [16, 17].
Prospective non-randomized observational trials have supported that laser treatments are effective for relief of symptoms of GSM in postmenopausal women [17-22]. Retrospective and single arm prospective studies of the use of fractional CO2 in breast cancer survivors have demonstrated improvement of symptoms of GSM, including vaginal dryness and dyspareunia but are limited by lack of placebo or sham arm to demonstrate true treatment effectiveness [23-25]. A recently published prospective, randomized controlled trial that compared fractional CO2 laser therapy to vaginal estrogen treatment showed that both improved symptoms in postmenopausal women with no significant difference between the two treatments [26]. There is currently no published data of the benefits of this treatment for women with gynecologic cancers with GSM. The purpose of the current pilot study was to study the feasibility, tolerability and preliminary efficacy of fractional CO2 laser therapy to reduce vaginal dryness and dyspareunia in gynecologic cancer survivors.
Materials and Methods:
Sample and eligibility
This study was a multi-institutional, randomized, pilot, sham-controlled, single-blind trial of women with gynecologic cancers who had one or more persistent symptoms of GSM. Participants with cervical, endometrial, vaginal, vulvar or ovarian cancer were eligible if they had reported dyspareunia and/or vaginal dryness with a severity of four or greater on a scale from 0 (none) to 10 (most severe) that was persistent over four or more weeks and/or they were unable to be sexually active due to pain. Patients were required to have completed all cancer-related treatment six months or more prior to enrollment. Exclusion criteria included patients with recurrent or metastatic cancer, pelvic organ prolapse stage II or higher, prior reconstructive pelvic surgery involving mesh, and hormone therapy or local vaginal hormone therapy within 6 weeks prior to enrollment.
The study was approved by the Institutional Review Board (IRB) for each participating institution. Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines.
Procedures:
Demographics, clinical data, and inclusion/exclusion criteria were obtained and recorded for all patients. Participants were randomized in a 1:1 fashion to fractional CO2 laser treatment or sham laser treatment at three time points, approximately 30 days apart. The provider performing the laser or sham treatment was not blinded to the study arm for each participant; however the participants and study coordinators were blinded. Subjective and objective symptoms of GSM were assessed prior to the first treatment or sham treatment (T1), prior to the second (T2) and third treatment or sham treatment (T3), and at follow-up four weeks after T3 (T4). Additional questionnaires assessing sexual function, urinary function, and patient satisfaction scores were obtained at the study time points. To reduce bias, participants were given the questionnaires at each time point by an individual that was blinded to the study arm and was different than the physician performing the laser or sham treatments.
The treatment consisted of fractional microablative CO2 laser (MonaLisa Touch ™, DEKA Florence, Italy) treatment at three time points, according to a standard protocol [24]. Prior to treatment, a vaginal exam was performed. EMLA cream was applied to the introitus and removed after 15 minutes. The laser was set to 30 W power with a dwell time of 1000 microseconds, a dermal optical thermolysis (DOT) spacing of 1000 micrometers, and a smart stack parameter of one for the first treatment and three for remaining treatments. The standard internal probe was inserted to the vaginal apex and a burst of laser pulses were delivered to treat the entire circumference and length of the vagina from the apex to the introitus [17]. The vestibule and posterior fourchette were then treated separately using an external vulvar probe. For the external treatment, the laser was set to 26 W power with a dwell time of 800 microseconds, a dermal optical thermolysis (DOT) spacing of 800 micrometers, and a smart stack parameter of one. Sham laser treatments were performed at the same three time points. The laser probe was inserted into the vagina and rotated, identical to an actual treatment, however, no pulse was delivered. The vulvar probe was also placed over the introitus and vestibule without delivering a treatment.
Participants were asked to abstain from intercourse and use of vaginal creams and lubricants for 48 hours prior to and following the procedure. Participants were asked to return for follow-up at T4, at which time a gynecologic exam was done and patient questionnaires were completed.
Measures:
Self-reported measures
Subjective vaginal symptoms:
The Female Sexual Medicine and Women's Health Program form developed at Memorial Sloan Kettering Cancer Center was used to assess both subjective and objective findings related to GSM [27]. This form consists of the Vaginal and Vulvar Assessment Scales (VAS and VuAS), which are validated clinical measurement tools to assess vulvovaginal symptoms, including vaginal dryness, soreness, irritation, and dyspareunia over the preceding 4 weeks. Symptoms are rated as mild, moderate, or severe, and each item is scored from 0 (none) to 3 (severe) with a composite score calculated by taking the average of the items answered. A lower score indicates better function. In cancer patients and survivors, the two scales were found to have high internal consistency and had a strong correlation with the Female Sexual Function Index FSFI [28].
Sexual function:
Sexual function was measured using the FSFI, a 19-item self -reported instrument that has been previously validated to measure sexual functioning in women in clinical trials. It measures 6 domains of sexual functioning. Overall test-retest reliability coefficients are high for each domain and the internal consistency is high with a Cronbach’s alpha of 0.82 and higher. The total maximum score is 36, with a higher score indicating better functioning. A score of <26.55 supports sexual dysfunction [29]. The FSFI has been validated in gynecologic cancer survivors [30].
Urinary function:
Urinary symptoms were assessed using the Urogenital Distress Inventory-6 (UDI-6) short form, a validated 6 item questionnaire with three subscales: irritative symptoms, obstructive/discomfort, and stress symptoms. The subscale scores are added for a total score, with a higher score indicating higher disability [31].
Patient satisfaction:
Patients were asked to rate their satisfaction with the vaginal laser procedure on a scale of 0-10 with 0 being completely dissatisfied and 10 being extremely satisfied. Participants were asked to rate their satisfaction on this scale at T2, T3 and T4.
Adverse Events (AE):
AE were self-reported prior to T2 and T3 and at T4. They were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0 (CTCAE).
Clinician assessment
Objective vaginal symptoms:
All participants had a gynecologic evaluation for objective symptoms related to GSM prior to each treatment using the Vaginal Health Assessment (VHA), a physical exam tool that accompanies the VAS and VuAS [28]. Exam findings included vaginal pH, moisture, elasticity, rugosity, epithelial thickness, vascularity, and integrity. Clinicians conducting these examinations were not blinded to whether patients had received laser treatment or sham laser treatment.
Statistics
The primary objective of this pilot trial was to estimate the effect of vaginal laser therapy for relieving vaginal symptoms in patients with a history of a gynecologic cancer to inform future design of a randomized phase III clinical trial. The primary endpoint was the change in the GSM symptoms from baseline to the end of treatment using the VAS. Other endpoints included the change from baseline to end of treatment for the VuAS, the FSFI total and subscale scores, the UDI total score, changes in vaginal atrophy as measured by the VHA, patient satisfaction, and adverse events as measured by the CTCAE v. 4.0. Descriptive statistics and statistical plots formed the foundation of statistical analysis for this trial. In addition, Wilcoxon rank sum tests were used to compare the primary and continuous secondary endpoints between the fractional CO2 laser and sham laser arms, in an exploratory manner. Due to the small sample size, categorical endpoints were analyzed descriptively.
Results
The initial accrual goal for the study was 30 patients. However, during the conduct of this trial, the U.S. Food and Drug Administration (FDA) issued a warning regarding the use of vaginal laser therapy for patients with the GSM [32]. Pursuant to this, the protocol was closed to further accrual in September 2018.
Twenty-three participants were registered for the study between May 2018 and September 2018 (Figure 1). Eighteen participants completed all three sessions (10 treatment, 8 sham) and were evaluable for the primary endpoint. Of the five participants who were not evaluable for the primary endpoint, one randomized to the sham arm only completed baseline surveys and did not start sham laser treatments, due to the FDA action, one was not eligible based on oral hormone replacement therapy, and three others who received prior radiation were initially consented but not randomized due to the FDA warning and were ultimately deemed ineligible before the study was officially closed. Baseline characteristics for the eighteen evaluable participants are summarized in Table 1. The majority of participants had stage I (n=6, 33.3%) or II (n=1, 5.6%) endometrial cancer with adenocarcinoma histology (n=6, 33%). Eight (44%) participants had received prior radiation, including three who received laser treatment and five who received sham laser. Of the three patients who received prior radiation on the treatment arm, two were treated with chemoradiation involving brachytherapy for cervical cancer and one was treated with intracavitary brachytherapy alone for a uterine carcinosarcoma. Demographics and cancer characteristics appeared balanced between the treatment or sham groups.
Figure 1:
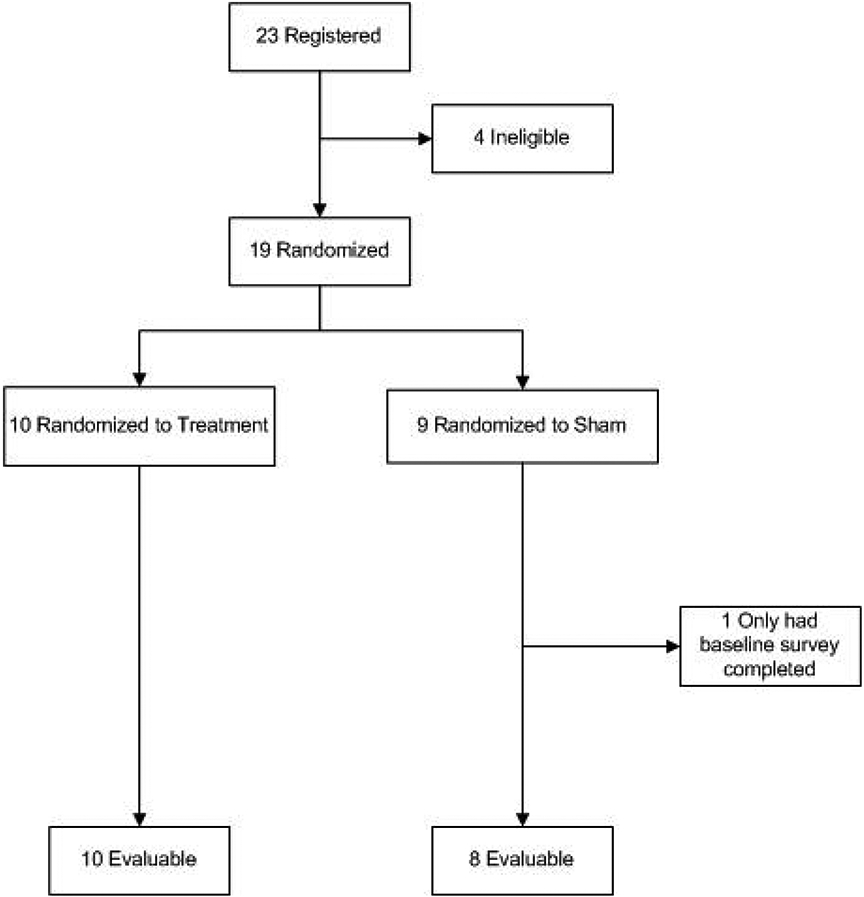
Patient consort diagram
Table 1.
Patient demographics and clinical characteristics
| Arm | ||||
|---|---|---|---|---|
| Treatment (N=10) |
Sham (N=8) |
Total (N=18) |
P-value | |
| Age at On Study | 0.92901 | |||
| N | 10 | 8 | 18 | |
| Mean (SD) | 56.0 (11.17) | 56.8 (5.95) | 56.3 (8.98) | |
| Median | 59.5 | 56.5 | 57.5 | |
| Range | 35.0, 72.0 | 45.0, 63.0 | 35.0, 72.0 | |
| Prior Radiation, n (%) | 0.34162 | |||
| Prior Radiation | 3 (30.0%) | 5 (62.5%) | 8 (44.4%) | |
| No Prior Radiation | 7 (70.0%) | 3 (37.5%) | 10 (55.6%) | |
| Type of cancer, n (%) | 1.00002 | |||
| Cervical | 2 (20.0%) | 1 (12.5%) | 3 (16.7%) | |
| Endometrial | 4 (40.0%) | 3 (37.5%) | 7 (38.9%) | |
| Ovarian | 3 (30.0%) | 3 (37.5%) | 6 (33.3%) | |
| Vaginal | 1 (10.0%) | 0 (0.0%) | 1 (5.6%) | |
| Vulvar | 0 (0.0%) | 1 (12.5%) | 1 (5.6%) | |
| Histology, n (%) | 0.17532 | |||
| Adenocarcinoma | 6 (60.0%) | 3 (42.9%) | 9 (52.9%) | |
| Other | 3 (30.0%) | 0 (0.0%) | 3 (17.6%) | |
| Serous Carcinoma | 1 (10.0%) | 2 (28.6%) | 3 (17.6%) | |
| Squamous Cell Carcinoma | 0 (0.0%) | 2 (28.6%) | 2 (11.8%) | |
| Missing | 0 | 1 | 1 | |
| Stage, n (%) | 0.71902 | |||
| I | 0 (0.0%) | 1 (12.5%) | 1 (5.9%) | |
| IA | 5 (55.6%) | 3 (37.5%) | 8 (47.1%) | |
| IB | 1 (11.1%) | 1 (12.5%) | 2 (11.8%) | |
| II | 1 (11.1%) | 1 (12.5%) | 2 (11.8%) | |
| IIB | 1 (11.1%) | 0 (0.0%) | 1 (5.9%) | |
| III | 0 (0.0%) | 2 (25.0%) | 2 (11.8%) | |
| IIIB | 1 (11.1%) | 0 (0.0%) | 1 (5.9%) | |
| Missing | 1 | 0 | 1 | |
| Chemotherapy, n (%) | 0.58462 | |||
| No | 3 (33.3%) | 1 (14.3%) | 4 (25.0%) | |
| Yes | 6 (66.7%) | 6 (85.7%) | 12 (75.0%) | |
| Missing | 1 | 1 | 2 | |
| Surgery, n (%) | 1.00002 | |||
| No | 1 (10.0%) | 0 (0.0%) | 1 (5.6%) | |
| Yes | 9 (90.0%) | 8 (100.0%) | 17 (94.4%) | |
| Vaginal Lubricant Use, n (%) | 0.15152 | |||
| No | 7 (87.5%) | 1 (33.3%) | 8 (72.7%) | |
| Unknown | 0 (0.0%) | 1 (33.3%) | 1 (9.1%) | |
| Yes | 1 (12.5%) | 1 (33.3%) | 2 (18.2%) | |
| Missing | 2 | 5 | 7 | |
| Vaginal Moisturizer Use, n (%) | 0.09092 | |||
| No | 8 (100.0%) | 2 (50.0%) | 10 (83.3%) | |
| Unknown | 0 (0.0%) | 2 (50.0%) | 2 (16.7%) | |
| Missing | 2 | 4 | 6 | |
| Vaginal Estrogen Use, n (%) | 0.05172 | |||
| No | 8 (88.9%) | 1 (25.0%) | 9 (69.2%) | |
| Unknown | 0 (0.0%) | 2 (50.0%) | 2 (15.4%) | |
| Yes | 1 (11.1%) | 1 (25.0%) | 2 (15.4%) | |
| Missing | 1 | 4 | 5 | |
Kruskal-Wallis p-value
Fisher Exact p-value
Of the 18 participants, 15 (83.3%) completed treatment and follow-up (T4) per protocol criteria. One participant who was randomized to the treatment arm did not complete the VAS questionnaire at baseline, but completed all other questionnaires. For this participant, T2 was used as the first data point. Three participants, one on the treatment arm and two on the sham arm, did not complete T4 due to the FDA-related early study closure. T3 was used as the final data point for these three participants. Except for these three, all of the other participants were able to complete treatment and sham sessions, as well as follow-up, as scheduled.
Primary endpoint based on VAS
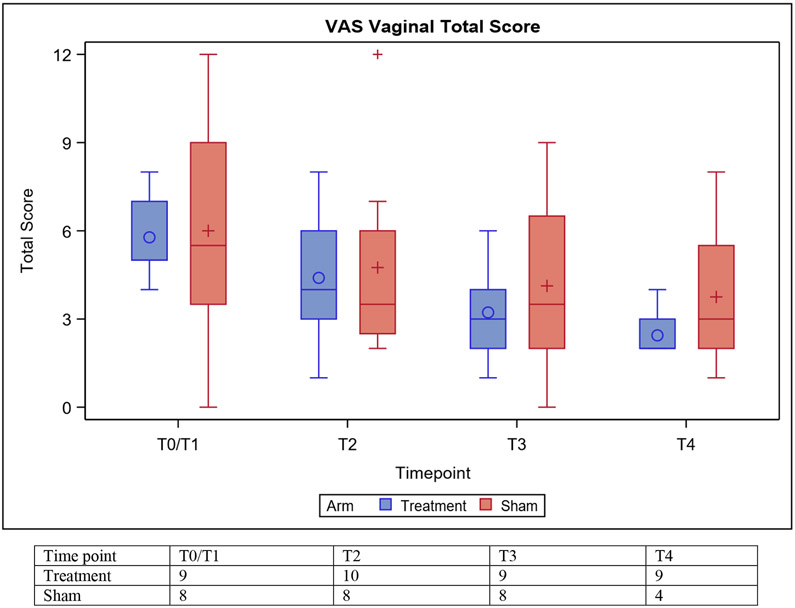
There was no difference in the change in total vaginal score on the VAS from baseline to follow-up between treatment arms (median Δ −3.0 vs −1.0, p=0.30) (Table 2). However, at baseline 7 (70%) participants on the treatment arm reported moderate (n=5, 50%) to severe (n=2, 20%) vaginal dryness. At the end of the three laser treatments, 4 (40%) participants reported moderate dryness and no participant reported severe dryness. In the sham arm, 6 (75%) participants reported moderate (n=2, 25%) to severe (n=4, 50%) vaginal dryness at baseline. At the completion of the three sham sessions, 5 (62.5%) participants still reported moderate (n=2, 25%) to severe (n=3, 37.5%) vaginal dryness. Overall, 6 (60%) participants on the treatment arm, compared to 37.5% (n=3) on the sham arm, had a lower than baseline vaginal dryness score on the VAS. There were no reports of worsening vaginal dryness compared to baseline in the treatment arm; however, three participants on the sham arm reported worse vaginal dryness at follow-up compared to baseline (Supplementary material 1).
Table 2.
Change in scores from baseline to follow-up for the VAS, VuAS, UDI-6 and FSFI
| Arm | |||
|---|---|---|---|
| Treatment (N=10) |
Sham (N=8) |
P-value | |
| Total vaginal score on the VAS* | 0.301 | ||
| Median | −3.0 | −1.0 | |
| Range | −6.0, 0.0 | −9.0, 3.0 | |
| Total vulvar score on VuAS* | 0.561 | ||
| Median | −1.0 | −2.0 | |
| Range | −3.0, 1.0 | −7.0, 1.0 | |
| Overall UDI-6 Score* | 0.171 | ||
| Median | −14.6 | −2.1 | |
| Range | −83.3, 12.5 | −29.2, 16.7 | |
| Total FSFI score ** | 0.021 | ||
| Median | 6.5 | −0.3 | |
| Range | −2.0, 17.1 | −8.8, 2.7 | |
Kruskal-Wallis p-value
Higher score indicates worse symptoms
Lower score indicates worse symptoms; not all patients answered all questions
VAS = Vaginal Assessment Scale; VuAS = Vulvar Assessment Scale; UDI-6 = Urinary Distress Inventory-6; FSFI= Female Sexual Function Index
Seven (70%) participants in the treatment arm also reported moderate (n=4, 40%) to severe (n=3, 30%) dyspareunia prior to treatment. By the final time point, 3 (30%) participants reported moderate dyspareunia. One participant on the treatment arm did not attempt intercourse throughout the study and could not be evaluated for dyspareunia. In the sham arm, 6 (75%) participants reported moderate (n=3, 37.5%) to severe (n=3, 37.5%) dyspareunia prior to sham treatment. At the time of the final treatment, 5 (62.5%) participants had not attempted intercourse during treatment and 2 (25%) participants reported severe dyspareunia. Overall, 6 (60%) participants on the treatment arm and 4 (50%) participants on the sham arm had a lower than baseline dyspareunia on the VAS. One participant in each group reported greater than baseline dyspareunia (Supplementary material 1). The patient on the treatment arm who reported mild dyspareunia at T0 but severe dyspareunia following treatment had endometrial cancer without adjuvant radiation and was eligible for the study due to dyspareunia rated as 8/10 on initial screening.
Figure 2 shows the median VAS score at baseline, prior to the second and third treatments, and at follow-up for both arms.
Figure 2:
Distribution of Vaginal Assessment Scale (VAS) scores at baseline (T0/1), prior to T2, T3, and T4. Mean (○, +), median (horizontal lines), and the 1st and 3rd quartiles (Q1: bottom and Q3: top of box) are displayed. The whiskers are the distance equal to 1.5 times the interquartile range (IQR) from Q1 and Q3. The diagram also shows outliers (+) which are values that above or below the whisker ends.
Secondary endpoints (VuAS, UDI-6, FSFI, Patient satisfaction, AE)
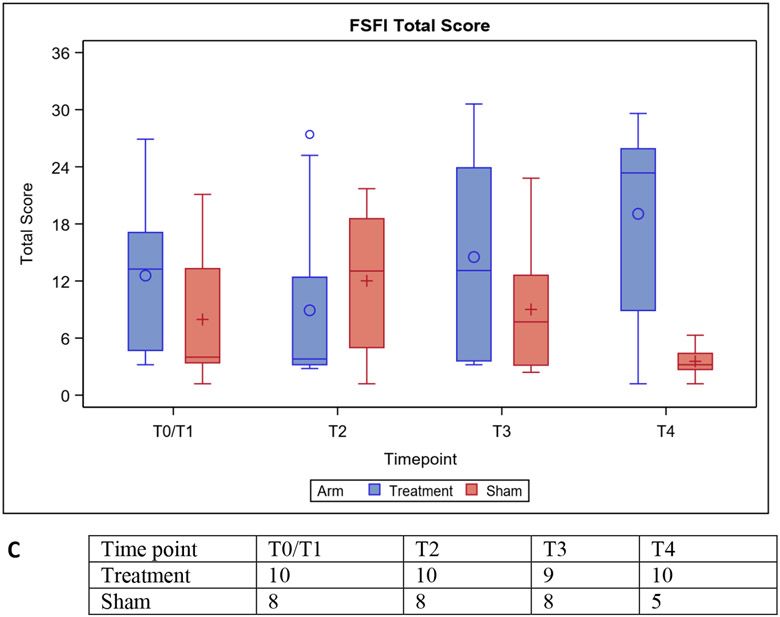
There was no difference in the change in the total vulvar score on the VuAS between treatment arms (Table 2). There was a decrease in the change in the overall UDI-6 score with treatment compared to sham but this was not statistically significant (Table 2). There was improvement in the total FSFI domain score with treatment compared to sham (p=0.0209) (Table 2). These improvements appeared in FSFI domains related to desire (p=0.0013)) and orgasm (p=0.0176). Figures 3 shows the VuAS, FSFI, and UDI-6 scores at baseline, prior to the second and third treatments, and at follow-up for both arms.
Figure 3:
Distribution of Vulvar Assessment Scale (VuAS) (A), Urinary Distress Inventory-6 (UDI-6) (B), and Female Sexual Function Index (FSFI) (C) scores at baseline (T0/1), T2, T3, and T4. Mean (○, +), median (horizontal lines), and the 1st and 3rd quartiles (Q1: bottom and Q3: top of box) are displayed. The whiskers are the distance equal to 1.5 times the interquartile range (IQR) from Q1 and Q3. The diagram also shows outliers (+) which are values that above or below the whisker ends.
Overall, participants were satisfied with the vaginal laser procedures. For those who completed T4, 6 (100%) participants on the treatment arm rated their satisfaction as eight or greater as compared to 1 (16.7%) of patients in the sham arm. Those in the sham arm reported lower satisfaction scores, with 4 participants (66.7%) rating it as two or less (Supplementary material 2).
The majority of AEs were grade 1, which included vaginal discharge (n=3), vaginal dryness (n=3), and vaginal pain (n=1) in the treatment arm, and vaginal pain (n=5) in the sham arm. Grade 2 AEs included vaginal inflammation in the treatment arm (n=2) and sham arm (n=1). One grade 3 AE of flank pain was reported in the treatment arm, which was deemed unrelated to treatment (Supplementary material 3).
Objective physical exam findings
At baseline, the majority of participants in the study had a vaginal pH > 6.5 (n=10; 55.6%) (scoring: <5, 5-6.5. >6.5), minimal vaginal moisture (n=11, 61.1%) (scoring: normal, minimal, none), minimal vaginal rugosity (n=13, 72.2%) (scoring: normal, minimal, none), and fair vaginal elasticity (n=13, 72.2%) (scoring: excellent, fair, poor) according to the VHA. At the time of follow-up, one participant in the treatment arm had normalization of the vaginal pH to < 5, compared to no patients on the sham arm. Two participants who initially reported minimal moisture in the treatment arm had normal moisture at the end of treatment, but no participant on the sham arm reported an improvement to normal moisture. Other physical exam improvements in the treatment arm included improvement in rugosity in two participants and vaginal elasticity in three participants. Two participants on the sham arm also had improvements in vaginal elasticity.
Discussion
This pilot study showed that fractional CO2 laser therapy is feasible in gynecologic cancer survivors, with 83.3% of participants completing all three treatments per protocol criteria, including a four-week follow-up. All participants completed treatments and sham treatments without serious AE. Although our primary endpoint did not show a statistically significant difference, likely due to small sample size and loss of statistical power from early study closure resulting in lower than planned accrual, this study did show some changes in vaginal symptoms of GSM with treatment. While not conclusive of efficacy given the small sample size and limited follow-up, a higher percentage of participants in the treatment arm reported lower than baseline vaginal symptoms, predominantly vaginal dryness, compared to the sham arm. Additionally, there was apparent improvement in FSFI and UDI-6 scores with treatment compared to sham. While the change in the UDI-6 score was not statistically significant, the observed decrease in the score in the treatment group may be clinically meaningful [33] and important for future studies.
The findings of this study are consistent with other studies that have shown improvement in sexual function with fractional CO2 laser therapy [12, 21, 25, 34]. A prospective study of 77 postmenopausal women with vaginal atrophy treated with vaginal laser therapy showed an improvement in sexual function at 12-week post-treatment follow-up [34]. Also, a recently published prospective study of 64 breast cancer survivors reported a statistically significant improvement from baseline in vaginal symptoms, sexual function, and urinary symptoms following fractional CO2 laser treatment, with no serious adverse events reported [25]. Other studies have demonstrated improvement in vaginal symptoms of GSM [18-22, 35], including a large multicenter retrospective study of 645 postmenopausal women with vaginal atrophy treated with three or four laser treatments who had improvement in dyspareunia and vaginal dryness, itching, and burning with treatment [22]. In addition, studies investigating other energy-based vaginal therapies including erbium laser [36], Er:YAG laser [37], and radiofrequency treatments have reported results suggesting efficacy for GSM [38].
While fractional CO2 laser therapy has been studied in postmenopausal women and breast cancer survivors, this is the first study to investigate the use of this treatment in women with gynecologic cancers. This population of cancer survivors has a high incidence of GSM and sexual dysfunction with few effective treatment options [6, 7]. In contrast to patients with breast cancer, many gynecologic cancer survivors may be candidates for local hormonal therapies, the gold standard treatments for GSM in postmenopausal women [39]. However, due to patient reported issues that affect compliance with topical agents, such as “messiness and unpleasantness” of application and side effects including vaginal discharge [40], laser therapy may be preferable over topical treatments that require continual use to prevent the return of symptoms [10-13]. Local estrogen therapy and laser treatment were also found to be equivalent in improving GSM symptoms based on the recently reported VeLVET study, which randomized 69 postmenopausal women participants with GSM to fractional CO2 laser therapy or to low dose vaginal estrogen. Another prospective study of fractional CO2 laser therapy alone, topical estriol alone, or combination therapy with laser treatment and estriol, showed that combined therapy with laser and topical estriol improved vaginal dryness and dyspareunia, while monotherapy with estriol improved only vaginal dryness, suggesting a possible role for dual therapy [41]. Fractional CO2 laser therapy was also recently found to be more effective at improving vaginal symptoms than other topical agents, including promestriene cream or lubricants, in postmenopausal women [42] and may provide an alternative treatment option to topical agents, including vaginal estrogens, which are only effective if the patient remains compliant with regular use [43].
The strength of the current study is that it is the first study of fractional CO2 laser therapy in gynecologic cancer survivors, including women who have had prior pelvic or vaginal radiation, and provides preliminary evidence of safety. While few in number, no patient who received radiation suffered an AE. Additionally, not only was this multicenter study conducted prospectively but it also utilized a sham arm in order to preliminarily evaluate true treatment response from a placebo response.
A limitation of this study was the early study closure following the FDA warning against the use of energy-based devices for vaginal cosmetic procedures such as “vaginal rejuvenation” [32]. The early study closure resulted in lower than planned accrual, small sample size, and incomplete data for some participants, which likely affected efforts to understand efficacy and toxicity differences between the study arms. Therefore, the findings of the study are hypothesis generating for future studies. Although long-term safety and efficacy data are lacking, a few cases of substantial toxicity have been reported [44]. While there were no reported SAEs on this study, very few women had prior pelvic or vaginal radiation, which has typically been a contraindication for laser therapy and was an exclusion criteria on the VeLVET study [26]. Since radiation-related vaginal changes are different than those caused by estrogen deficiency alone [45], the safety of laser treatments in this population cannot be extrapolated from the currently published literature in postmenopausal women and breast cancer survivors, and larger prospective studies are needed. Another limitation was the lack of information on partner status, including relationship duration and quality, as well as detailed information on frequency and type of sexual activity at each time point. This information will be considered in future studies. Additional limitations of the study include the lack of long-term follow-up, with the last follow-up at only four weeks post-treatment.
Conclusion
Fractional CO2 laser therapy is feasible in gynecologic cancer survivors with preliminary evidence of safety in this pilot study which will need confirmation in larger future studies. Treatment resulted in improvement in sexual function compared to sham treatment. Effectiveness of treatment was limited due to study sample size but based on the preliminary evidence of safety and improvement in sexual function, fractional CO2 laser therapy warrants further investigation in a larger randomized controlled trial with longer term follow-up and inclusion of more patients with a history of radiation therapy, to determine true safety and efficacy.
Supplementary Material
Highlights.
This study was conducted to evaluate the efficacy of fractional CO2 laser treatments in gynecologic cancer survivors suffering from the genitourinary syndrome of menopause (GSM).
The key finding of this study was that there were no serious adverse events as a result of fractional CO2 laser treatment or sham laser in gynecologic cancer survivors suffering from vaginal dryness or dyspareunia. Fractional CO2 laser treatments appeared to improve sexual function, when compared to sham laser
Fractional CO2 laser treatments have been studied in postmenopausal women and breast cancer survivors with GSM. However, this study is the first study of fractional CO2 laser treatment in gynecologic cancer survivors.
Acknowledgment
We acknowledge Eric Wolfe for his assistance with statistical analysis for this study.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), UG1CA233196, UG1CA233320, UG1CA233331, UG1CA232760, and UG1CA189825. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
Charles Loprinzi reports personal fees from PledPharma, Disarm Therapeutics, Asahi Kasei, Metys Pharmaceuticals, OnQuality, and Mitsubishi Tanabe, which are all outside the submitted work. The other authors have no relevant conflicts of interest or financial disclosures. No financial support was provided by Hologic inc. The laser device was provided by loan to OSU from Hologic/Cynosure. None of the investigators received financial support from Hologic/Cynosure. The company was not involved in study data analysis or manuscript preparation.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The study was approved by the Institutional Review Board (IRB) for each participating institution. Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines.
Provenance and peer review
This article was not commissioned and was externally peer reviewed.
Research data (data sharing and collaboration)
There are no linked research data sets for this paper. Data will be made available on request.
References
- 1.Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric : the journal of the International Menopause Society. 2014;17(1):3–9. doi: 10.3109/13697137.2013.871696. [DOI] [PubMed] [Google Scholar]
- 2.Krychman M, Graham S, Bernick B, Mirkin S, Kingsberg SA. The Women's EMPOWER Survey: Women's Knowledge and Awareness of Treatment Options for Vulvar and Vaginal Atrophy Remains Inadequate. The journal of sexual medicine. 2017;14(3):425–33. doi: 10.1016/j.jsxm.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Maturitas. 2014;79(3):349–54. doi: 10.1016/j.maturitas.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor E, Benrubi D, Faubion SS. Menopausal Hormone Therapy in Gynecologic Cancer Survivors: A Review of the Evidence and Practice Recommendations. Clinical obstetrics and gynecology. 2018;61(3):488–95. doi: 10.1097/grf.0000000000000381. [DOI] [PubMed] [Google Scholar]
- 5.Del Carmen MG, Rice LW. Management of menopausal symptoms in women with gynecologic cancers. Gynecologic oncology. 2017;146(2):427–35. doi: 10.1016/j.ygyno.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Jensen PT, Froeding LP. Pelvic radiotherapy and sexual function in women. Translational andrology and urology. 2015;4(2):186–205. doi: 10.3978/j.issn.2223-4683.2015.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amsterdam A, Krychman ML. Sexual dysfunction in patients with gynecologic neoplasms: a retrospective pilot study. The journal of sexual medicine. 2006;3(4):646–9. doi: 10.1111/j.1743-6109.2006.00204.x. [DOI] [PubMed] [Google Scholar]
- 8.Noronha AF, Mello de Figueiredo E, Rossi de Figueiredo Franco TM, Candido EB, Silva-Filho AL. Treatments for invasive carcinoma of the cervix: what are their impacts on the pelvic floor functions? International braz j urol : official journal of the Brazilian Society of Urology. 2013;39(1):46–54. doi: 10.1590/s1677-5538.ibju.2013.01.07. [DOI] [PubMed] [Google Scholar]
- 9.The 2017 hormone therapy position statement of The North American Menopause Society. Menopause (New York, NY). 2017;24(7):728–53. doi: 10.1097/gme.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 10.Friedman LC, Abdallah R, Schluchter M, Panneerselvam A, Kunos CA. Adherence to vaginal dilation following high dose rate brachytherapy for endometrial cancer. International journal of radiation oncology, biology, physics. 2011;80(3):751–7. doi: 10.1016/j.ijrobp.2010.02.058. [DOI] [PubMed] [Google Scholar]
- 11.De Rosa N, Lavitola G, Giampaolino P, Morra I, Nappi C, Bifulco G. Impact of Ospemifene on Quality of Life and Sexual Function in Young Survivors of Cervical Cancer: A Prospective Study. BioMed research international. 2017;2017:7513610. doi: 10.1155/2017/7513610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefano S, Stavros A, Massimo C. The use of pulsed CO2 lasers for the treatment of vulvovaginal atrophy. Current opinion in obstetrics & gynecology. 2015;27(6):504–8. doi: 10.1097/gco.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 13.Portman D, Palacios S, Nappi RE, Mueck AO. Ospemifene, a non-oestrogen selective oestrogen receptor modulator for the treatment of vaginal dryness associated with postmenopausal vulvar and vaginal atrophy: a randomised, placebo-controlled, phase III trial. Maturitas. 2014;78(2):91–8. doi: 10.1016/j.maturitas.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Sinno AK, Pinkerton J, Febbraro T, Jones N, Khanna N, Temkin S et al. Hormone therapy (HT) in women with gynecologic cancers and in women at high risk for developing a gynecologic cancer: A Society of Gynecologic Oncology (SGO) clinical practice statement: This practice statement has been endorsed by The North American Menopause Society. Gynecologic oncology. 2020;157(2):303–6. doi: 10.1016/j.ygyno.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Biglia N, Bounous VE, D'Alonzo M, Ottino L, Tuninetti V, Robba E et al. Vaginal Atrophy in Breast Cancer Survivors: Attitude and Approaches Among Oncologists. Clinical breast cancer. 2017. doi: 10.1016/j.clbc.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Athanasiou S, Pitsouni E, Falagas ME, Salvatore S, Grigoriadis T. CO2-laser for the genitourinary syndrome of menopause. How many laser sessions? Maturitas. 2017;104:24–8. doi: 10.1016/j.maturitas.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Sokol ER, Karram MM. Use of a novel fractional CO2 laser for the treatment of genitourinary syndrome of menopause: 1-year outcomes. Menopause (New York, NY). 2017. doi: 10.1097/gme.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 18.Perino A, Calligaro A, Forlani F, Tiberio C, Cucinella G, Svelato A et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015;80(3):296–301. doi: 10.1016/j.maturitas.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Salvatore S, Nappi RE, Zerbinati N, Calligaro A, Ferrero S, Origoni M et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric : the journal of the International Menopause Society. 2014;17(4):363–9. doi: 10.3109/13697137.2014.899347. [DOI] [PubMed] [Google Scholar]
- 20.Pitsouni E, Grigoriadis T, Tsiveleka A, Zacharakis D, Salvatore S, Athanasiou S. Microablative fractional CO2-laser therapy and the genitourinary syndrome of menopause: An observational study. Maturitas. 2016;94:131–6. doi: 10.1016/j.maturitas.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Behnia-Willison F, Sarraf S, Miller J, Mohamadi B, Care AS, Lam A et al. Safety and long-term efficacy of fractional CO2 laser treatment in women suffering from genitourinary syndrome of menopause. European journal of obstetrics, gynecology, and reproductive biology. 2017;213:39–44. doi: 10.1016/j.ejogrb.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 22.Filippini M, Luvero D, Salvatore S, Pieralli A, Montera R, Plotti F et al. Efficacy of fractional CO2 laser treatment in postmenopausal women with genitourinary syndrome: a multicenter study. Menopause (New York, NY). 2019. doi: 10.1097/gme.0000000000001428. [DOI] [PubMed] [Google Scholar]
- 23.Pagano T, De Rosa P, Vallone R, Schettini F, Arpino G, Giuliano M et al. Fractional microablative CO2 laser in breast cancer survivors affected by iatrogenic vulvovaginal atrophy after failure of nonestrogenic local treatments: a retrospective study. Menopause (New York, NY). 2017. doi: 10.1097/gme.0000000000001053. [DOI] [PubMed] [Google Scholar]
- 24.Pieralli A, Fallani MG, Becorpi A, Bianchi C, Corioni S, Longinotti M et al. Fractional CO2 laser for vulvovaginal atrophy (VVA) dyspareunia relief in breast cancer survivors. Archives of gynecology and obstetrics. 2016;294(4):841–6. doi: 10.1007/s00404-016-4118-6. [DOI] [PubMed] [Google Scholar]
- 25.Quick AM, Zvinovski F, Hudson C, Hundley A, Evans C, Suresh A et al. Fractional CO2 laser therapy for genitourinary syndrome of menopause for breast cancer survivors. 2019. doi: 10.1007/s00520-019-05211-3. [DOI] [PubMed]
- 26.Paraiso MFR, Ferrando CA, Sokol ER, Rardin CR, Matthews CA, Karram MM et al. A randomized clinical trial comparing vaginal laser therapy to vaginal estrogen therapy in women with genitourinary syndrome of menopause: The VeLVET Trial. Menopause (New York, NY). 2019. doi: 10.1097/gme.0000000000001416. [DOI] [PubMed] [Google Scholar]
- 27.Carter J, Stabile C, Seidel B, Baser RE, Goldfarb S, Goldfrank DJ. Vaginal and sexual health treatment strategies within a female sexual medicine program for cancer patients and survivors. Journal of cancer survivorship : research and practice. 2017;11(2):274–83. doi: 10.1007/s11764-016-0585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eaton AA, Baser RE, Seidel B, Stabile C, Canty JP, Goldfrank DJ et al. Validation of Clinical Tools for Vaginal and Vulvar Symptom Assessment in Cancer Patients and Survivors. The journal of sexual medicine. 2017;14(1):144–51. doi: 10.1016/j.jsxm.2016.11.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. Journal of sex & marital therapy. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 30.Baser RE, Li Y, Carter J. Psychometric validation of the female sexual function index (FSFI) in cancer survivors. Cancer. 2012;118(18):4606–18. doi: 10.1002/cncr.26739. [DOI] [PubMed] [Google Scholar]
- 31.Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA. Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourology and urodynamics. 1995;14(2):131–9. [DOI] [PubMed] [Google Scholar]
- 32.https://www.fda.gov/medical-devices/safety-communications/fda-warns-against-use-energy-based-devices-perform-vaginal-rejuvenation-or-vaginal-cosmetic.
- 33.Barber MD, Spino C, Janz NK, Brubaker L, Nygaard I, Nager CW et al. The minimum important differences for the urinary scales of the Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire. American journal of obstetrics and gynecology. 2009;200(5):580.e1–.e5807. doi: 10.1016/j.ajog.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvatore S, Nappi RE, Parma M, Chionna R, Lagona F, Zerbinati N et al. Sexual function after fractional microablative CO(2) laser in women with vulvovaginal atrophy. Climacteric : the journal of the International Menopause Society. 2015;18(2):219–25. doi: 10.3109/13697137.2014.975197. [DOI] [PubMed] [Google Scholar]
- 35.Athanasiou S, Pitsouni E, Grigoriadis T, Zacharakis D, Falagas ME, Salvatore S et al. Microablative fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause (New York, NY). 2018. doi: 10.1097/gme.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 36.Gambacciani M, Levancini M. Vaginal erbium laser as second-generation thermotherapy for the genitourinary syndrome of menopause: a pilot study in breast cancer survivors. Menopause (New York, NY). 2017;24(3):316–9. doi: 10.1097/gme.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 37.Mothes AR, Runnebaum M, Runnebaum IB. Ablative dual-phase Erbium:YAG laser treatment of atrophy-related vaginal symptoms in post-menopausal breast cancer survivors omitting hormonal treatment. Journal of cancer research and clinical oncology. 2018;144(5):955–60. doi: 10.1007/s00432-018-2614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vicariotto F, Raichi M. Technological evolution in the radiofrequency treatment of vaginal laxity and menopausal vulvo-vaginal atrophy and other genitourinary symptoms: first experiences with a novel dynamic quadripolar device. Minerva ginecologica. 2016;68(3):225–36. [PubMed] [Google Scholar]
- 39.Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV et al. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2015;100(11):3975–4011. doi: 10.1210/jc.2015-2236. [DOI] [PubMed] [Google Scholar]
- 40.Minkin MJ, Maamari R, Reiter S. Improved compliance and patient satisfaction with estradiol vaginal tablets in postmenopausal women previously treated with another local estrogen therapy. International journal of women's health. 2013;5:133–9. doi: 10.2147/ijwh.S41897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz VL, Steiner ML, Pompei LM, Strufaldi R, Fonseca FLA, Santiago LHS et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause (New York, NY). 2018;25(1):21–8. doi: 10.1097/gme.0000000000000955. [DOI] [PubMed] [Google Scholar]
- 42.Politano CA, Costa-Paiva L, Aguiar LB, Machado HC, Baccaro LF. Fractional CO2 laser versus promestriene and lubricant in genitourinary syndrome of menopause: a randomized clinical trial. Menopause (New York, NY). 2019;26(8):833–40. doi: 10.1097/gme.0000000000001333. [DOI] [PubMed] [Google Scholar]
- 43.Gaspar A, Brandi H, Gomez V, Luque D. Efficacy of Erbium:YAG laser treatment compared to topical estriol treatment for symptoms of genitourinary syndrome of menopause. Lasers in surgery and medicine. 2017;49(2):160–8. doi: 10.1002/lsm.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon C, Gonzales S, Krychman ML. Rethinking the techno vagina: a case series of patient complications following vaginal laser treatment for atrophy. Menopause (New York, NY). 2019;26(4):423–7. doi: 10.1097/gme.0000000000001293. [DOI] [PubMed] [Google Scholar]
- 45.Kirchheiner K, Fidarova E, Nout RA, Schmid MP, Sturdza A, Wiebe E et al. Radiation-induced morphological changes in the vagina. Strahlentherapie und Onkologie : Organ der Deutschen Rontgengesellschaft [et al]. 2012;188(11):1010–7. doi: 10.1007/s00066-012-0222-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.