Abstract
Regulatory T cells (Treg cells) are a small subset of immune cells that are dedicated to curbing excessive immune activation and maintaining immune homeostasis. Accordingly, deficiencies in Treg cell development or function result in uncontrolled immune responses and tissue destruction and can lead to inflammatory disorders such as graft-versus-host disease, transplant rejection and autoimmune diseases. As Treg cells deploy more than a dozen molecular mechanisms to suppress immune responses, they have potential as multifaceted adaptable smart therapeutics for treating inflammatory disorders. Indeed, early-phase clinical trials of Treg cell therapy have shown feasibility, tolerability and potential efficacy in these disease settings. In the meantime, progress in the development of chimeric antigen receptors and in genome editing (including the application of CRISPR–Cas9) over the past two decades has facilitated the genetic optimization of primary T cell therapy for cancer. These technologies are now being used to enhance the specificity and functionality of Treg cells. In this Review, we describe the key advances and prospects in designing and implementing Treg cell-based therapy in autoimmunity and transplantation.
The adaptive immune system has evolved to recognize and destroy a virtually infinite variety of pathogens while remaining unresponsive towards self-tissues; this state is known as immune tolerance. Immune tolerance is maintained by a multilayered, interconnected and redundant array of dominant and recessive mechanisms, ensuring that immune responses are regulated in an effective and timely manner1,2. Recessive immune tolerance mechanisms are cell intrinsic and include the deletion of self-reactive immune cells, rendering them non-functional (that is, subject to anergy) and increasing the number of inhibitory receptors on immune cells to increase their activation threshold. By contrast, dominant immune tolerance mechanisms are cell extrinsic and are carried out by subsets of specialized immune cells that actively suppress the activation, expansion and function of other immune cells, thereby regulating the intensity and the duration of immune responses.
Anergy.
Peripheral mechanism for tolerizing T cells in which they are blocked at the G1 phase of the cell cycle and unable to proliferate.
Autoimmune disorders arise from defects in immune tolerance and affect more than 50 million individuals in the United States alone and more than 4% of the world population. Progress in treating individuals with these diseases has been slow owing to the complex mechanisms underlying the balance between immune reactivity and immune tolerance. Although small-molecule and biologic treatments can alleviate symptoms, they are often non-specific, require long-term administration (and thus long-term exposure to the toxic effects associated with them) and do not account for variability in underlying disease pathogenesis and drug responses. For instance, the mainstay treatment for severe systemic lupus erythematosus, an autoimmune disorder caused by autoreactive B cells, is steroids, which non-specifically suppress inflammation. Living drugs, such as regulatory T cells (Treg cells), may have greater specificity and more complex therapeutic benefits than conventional immunosuppressive drugs (such as steroids and ciclosporin), biologics (such as rituximab and belimumab), antimetabolites (such as azathioprine and methotrexate) and alkylating agents (such as cyclophosphamide), amongst others, and could potentially cure disease by restoring immune tolerance.
T cell-based antigen-specific immune tolerance was first postulated in 1970 (REF.1). However, Treg cells were not identified as the main cell type responsible for this phenomenon until the 1990s2. Treg cells constitute 5% of circulating CD4+ T cells and can be identified by the lineage marker forkhead box protein P3 (FOXP3). Mutations in the gene encoding FOXP3 (a transcription factor), as well as in genes encoding other molecules that modulate Treg cell function, such as the surface receptors cytotoxic T lymphocyte protein 4 (CTLA-4) and CD25 (also known as IL-2 receptor subunit-α, part of the trimeric high-affinity IL-2 receptor) and the transcription factor signal transducer and activator of transcription 5 (STAT5), lead to the development of severe autoimmune polyendocrine syndromes; the best known example of these syndromes is immunodysregulation polyendocrinopathy enteropathy X-linked syndrome3. Moreover, Treg cells can become unstable, losing FOXP3 expression and immunosuppressive function, converting into effector T cells (Teff cells) under extreme inflammatory conditions4,5. The inability of Treg cells to produce IL-2 while expressing high levels of CD25 is a cardinal feature of Treg cells. Thus, in the absence of IL-2 produced by other cell subtypes, or signalling by its receptor, there is a decrease in the number and functional activity of the Treg cells, leading to inflammation and autoimmunity6,7. Expression of CD127 (also known as IL-7 receptor subunit-α) is inversely correlated with the expression of FOXP3 and the suppressive function of human Treg cells, and is currently used in conjunction with CD25 as a phenotypic marker in the purification of Treg cells, defined as CD4+CD25+CD127low T cells8,9. However, the best indicator of a stable Treg cell lineage is demethylation of the Treg cell-specific demethylated region (TSDR), an evolutionarily conserved non-coding regulatory sequence in the FOXP3 locus; demethylation of the TSDR ensures high, stable levels of FOXP3 (REF.10).
Systemic lupus erythematosus.
A group of chronic autoimmune disorders defined by inflammation affecting various connective tissues in various organs, including the skin, joints, kidney, lung, nervous system or haematopoietic system.
Autoimmune polyendocrine syndromes.
A group of diseases characterized by loss of tolerance and inflammation in endocrine glands, including the thyroid, parathyroid and adrenal glands or the pancreas. They are frequently associated with alopecia, vitiligo, coeliac disease and autoimmune gastritis.
Immunodysregulation polyendocrinopathy enteropathy X-linked syndrome.
Specific form of an inherited autoimmune polyendocrine syndrome characterized by a mutation or mutations in the master transcription factor forkhead box protein P3 gene (FOXP3), leading to regulatory T cell dysfunction.
Type 1 T helper cell.
(TH1 cell). A type of CD4+ T helper cell expressing TBET as a key transcription factor and defined by its ability to preferentially secrete interferon-γ and induce CD8+T cell and macrophage activation.
Type 2 T helper cell.
(TH2 cell). A type of CD4+ T helper cell expressing GATA3 as a key transcription factor and defined by its ability to preferentially secrete Il-4, IL-5 and IL-13 and promote B cell expansion and antibody class switching.
IL-17-producing T helper cell.
(TH17 cell). A type of CD4+ T helper cell expressing RORγt as a key transcription factor and defined by the production of IL-17, a cytokine important for maintaining mucosal barrier integrity and clearing helminth infections.
The dominance and durability of Treg cell-mediated immune tolerance is underscored by two main features: bystander suppression and infectious tolerance. During bystander suppression, which was first described by Weiner and colleagues11 in 1991, Treg cells activated by one antigen suppress immune responses against other antigens. During infectious tolerance, a term coined by Gershon and Kondo12 in 1971 and expanded by Waldmann and colleagues13 in 1993, suppressive capacity is transferred from one cell population to another. This process is thought to occur mainly via the production of inhibitory cytokines by Treg cells; these cytokines block dendritic cell (DC) maturation and migration, creating a local tolerogenic environment in which Teff cells undergo apoptosis and naive T cells are converted to induced Treg cells. Molecularly, Treg cells act through pleiotropic mechanisms, depending on their target cells and whether they are in lymphoid organs or in non-lymphoid tissues14. Furthermore, studies in mice and humans show that Treg cells become specialized, converting into cells with type 1 T helper cell (TH17 cell)-like, type 2 T helper cell (TH2 cell)-like and IL-17-producing T helper cell (TH17 cell)-like phenotypes, characterized by distinct patterns of chemokine receptor, cytokine and transcription factor expression15–18. Genetic deletion experiments in vivo indicate that, by expressing chemokine receptors similar to those on specific T helper cell subsets, specialized Treg cells can more efficiently suppress their targets19. In addition to suppressing immune responses, murine Treg cells promote tissue repair following viral infection19.
Of note, CD4+FOXP3+ Treg cells are not the only immunosuppressive cells. Other cell types with immunosuppressive functions include CD8+ Treg cells, IL-10-producing type 1 Treg cells (TR1 cells), transforming growth factor-β (TGFβ)-producing CD4+ TH3 cells, regulatory γδ T cells, regulatory B cells (Breg cells), myeloid-derived suppressor cells, immunosuppressive plasmocytes, regulatory invariant natural killer (NK) T cells and even subsets of innate lymphoid cells (BOX 1). Yet, to date, FOXP3+ Treg cells are the only known cell lineage arising in the thymus that is exclusively dedicated to inducing and maintaining immune tolerance. Moreover, almost all ongoing clinical trials using cell therapy to induce immune tolerance use CD4+FOXP3+ Treg cells. Hence, in this Review, we focus on strategies to engineer CD4+FOXP3+ Treg cells as the next generation of living drugs for treatment of autoimmune and inflammatory diseases. First, we critically assess the current status of Treg cell therapy, including challenges in the isolation and manufacture of Treg cells, finding the best disease indication and the potential crosstalk of Treg cell therapy with other immunosuppressive treatments. Then we discuss the prospects of tailoring Treg cell specificity and function using genome editing and synthetic biology.
Box 1 |. The increasing diversity of immunosuppressive cell types.
A successful immune response entails the eradication of the pathogen and the timed contraction of activated immune cell populations to avoid excessive tissue damage. The best understood subsets of immunoregulatory cells that, in addition to CD4+FoXP3+ regulatory T cells (Treg cells), play a role in suppressing immune responses and maintaining immune homeostasis are briefly summarized here.
Type 1 Treg cells
Inducible type 1 Treg cells (TR1 cells) are CD4+ FoXP3− T cells that secrete the immunosuppressive molecule IL-10. Some TR1 cells also express granzyme B and can kill myeloid cells213. TR1 cells are enriched by the simultaneous expression of CD49b and lymphocyte activation gene 3 protein (LAG3)214. In vitro, TR1 cell expansion protocols use vitamin D3, dexamethasone215 or IL-10-producing antigen-presenting cells to generate antigen-specific TR1 cells216. In 2012, in the first open-label uncontrolled, multicentre, single-infusion dose-escalation phase I/IIa clinical trial using TR1 cells, ovalbumin-specific TR1 cells were injected into 20 patients with refractory Crohnʼs disease; the injection was well tolerated and showed dose-related efficacy217. A multicentre phase II trial using TR1 cells was completed in 2016 (NCT02327221; results not yet available). TR1 cell-based cell therapy has also been successful in haematological diseases, preventing graft-versus-host disease and improving immune reconstitution218.
CD8+ Treg cells
In 1970 one group established that T cells could suppress antibody responses1, and hypothesized that suppressor T cells existed219. T cells expressing CD8α (Ly-2 at the time) and CD8β (Ly-3), but not CD4 (Ly-1), were later found to have suppressive functions220. CD8+ Treg cells were thus the first suppressor cells to be identified. CD8+ Treg cells share markers with activated conventional CD8+ T cells, making it difficult to isolate them or dissect their function. Nevertheless, human CD8+FoXP3+CD45RClow Treg cells are potent suppressive cells in graft-versus-host disease and solid organ transplantation221, and CD8+FoXP3+CD25+TNFR2+ Treg cells are suppressive in patients with type 1 diabetes treated with teplizumab, a humanized anti-CD3 antibody222. FoXP3 expression may allow the identification of bona fide human CD8+ Treg cells. In mice, the transcription factor HELIOS is required for the stability of both CD4+ and CD8αα+ Treg cells in a proinflammatory milieu223. CD8αα+ Treg cells reside in the intestine, recognize the nonclassical major histocompatibility complex molecule Qa1 (HLA-E in humans) and protect against CD4+ T cell-mediated colitis224,225. CD8+ Treg cells may primarily suppress activated T cells by killing them directly or by secreting inhibitory cytokines.
Type 3 T helper cells
Transforming growth factor-β (TGFβ)-producing type 3 T helper cells (TH3) were identified when SJL mice, which are susceptible to experimental autoimmune encephalomyelitis (EAE), were fed myelin basic protein (MBP), and MBP-specific CD4+ T cell clones were isolated from mesenteric lymph nodes226. These clones secreted low levels of interferon-γ (a hallmark of TH1 cells), IL-4 (a hallmark of TH2 cells) and IL-10 and high levels of TGFβ. Adoptive transfer of these T cell clones suppressed EAE in mice immunized with MBP in a TGFβ-dependent manner. The study authors named these mucosal regulatory cells TH3 cells226. TH3 cell-derived TGFβ can prevent and reverse autoimmune encephalomyelitis by inducing Treg cell differentiation227. TH3 cells may have an important role in controlling autoimmunity and allergy in humans.
Regulatory B cells
Regulatory B cells (Breg cells) secrete anti-inflammatory cytokines, mainly IL-10, and suppress the proliferation of lymphocytes, including effector T cells228. B cell-mediated tolerance was originally hypothesized in the 1970s229, but it was not until 1996, when the genetic ablation of B cells was shown to decrease the frequency of spontaneous recovery from EAE, that a regulatory role for Breg cells was proposed230. B cells in mice that recovered from EAE were found to secrete IL-10 in response to self-antigen, and the genetic ablation of IL-10 in B cells prevented spontaneous recovery from EAE231. Inflammation seems to trigger the induction of Breg cells through the production of IL-10 by suppressive cells at the inflamed site232. Accordingly, individuals with chronic inflammation and autoimmunity display deficiencies in the number and function of suppressive cells circulating and at the inflamed site233. Importantly, Breg cells skew T cell differentiation towards Treg cells234,235 and promote Treg cell expansion236,237, suggesting that they act by promoting Treg cell activity.
Fundamentals of Treg cells as a therapy
Treg cells are an attractive therapeutic candidate for restoring immune tolerance in autoimmune and autoinflammatory diseases, and thus for reducing or replacing immunosuppressive drugs. Treg cells are also being considered as a therapy for inducing tolerance to allogeneic cells and tissues upon the transplantation of haematopoietic stem cells and solid organs. As of July 2019, 51 clinical trials using Treg cells had been registered in ClinicalTrials.gov, of which six have been completed (NCT01634217, NCT00602693, NCT01210664, NCT02166177, NCT02244801 and NCT02129881), five terminated (NCT02428309, NCT00725062, NCT01050764, NCT00376519 and NCT01818479), four suspended (NCT02494492, NCT02991898, NCT02526329 and NCT03773328) and two withdrawn (NCT02118311 and NCT01163201) (FIG. 1). Overall, these studies demonstrate the feasibility and safety of Treg cell infusion, although their small size, in general, limited the opportunity to assess efficacy. The relatively high rate of prematurely terminated, suspended or withdrawn trials mostly reflects challenges in manufacturing Treg cells and in selecting and recruiting patients, as discussed later in this Review.
Fig. 1 |. Registered clinical trials using regulatory T cells.
The number and status of registered clinical trials based on regulatory T cell (Treg cell) infusion were searched for in ClinicalTrials.gov and are summarized here (the numbers represent the data available in July 2019). The large circle shows the overall number of registered trials, divided into coloured segments that represent the proportion of these clinical trials with their status defined as not yet recruiting (blue), recruiting (green), active not recruiting (yellow), terminated or completed (pink), withdrawn or suspended (light grey) and unknown (dark grey). Unknown status corresponds to studies, the last known status of which was recruiting, not yet recruiting, or active, not recruiting, but that have passed their completion date but have not had their status verified within the past 2 years (http://clinicaltrials.gov). Small circles are categorized by indication: hematopoietic stem cell transplantation or graft-versus-host disease, solid organ transplantation and autoimmune disease.
Treg cells as living drugs
In traditional pharmacology, a drug is characterized according to its pharmacodynamics, that is, its effect in the body (such as its on-target and off-target interactions), and its pharmacokinetics, that is, the effect of the body on its absorption, distribution and metabolism. Several properties are key to successfully using Treg cells as living drugs, and these can be summed up as the four S’s: suppression, survival, stability and specificity (FIG. 2).
Fig. 2 |. Regulatory T cells as living drugs.
Four key properties are needed to successfully use regulatory T cells (Treg cells) as living drugs, summed up as the four S’s: suppression, specificity, stability and survival. In terms of suppression, Treg cells act through multiple suppression mechanisms (including IL-2 deprivation from the milieu, secretion of inhibitory cytokines and interactions with antigen-presenting cells (APCs)), which could be tailored to specific conditions or diseases by, for example, forcing the expression of specific transcription factors. Regarding specificity, it is possible to create Treg cells with a desired specificity using T cell receptor (TCR) gene transfer or artificial immune receptors, such as chimeric antigen receptors (CARs). Specificity can be made conditional by using synthetic Notch (SynNotch) receptors. Overexpressing transcription factors characteristic of T helper cell subsets can also enhance the specificity of Treg cells. With respect to stability, forkhead box protein P3 (FOXP3) expression is central to the lineage of Treg cells. Strategies to increase Treg cell stability include the ectopic expression of the transcription factors FOXP3, HELIOS and BACH2 or of a constitutively active form of signal transducer and activator of transcription 5 (STAT5-CA), as well as the ablation of carboxy terminus of Hsp70-interacting protein (CHIP), deleted in breast cancer gene 1 protein (DBC1) or protein kinase C-θ (PKCθ) to prevent the degradation of FOXP3. Finally, the survival of Treg cells depends on exogenous IL-2, metabolic requirements and tonic signalling mediated by the TCR and costimulatory molecules. Targeting Treg cell metabolic requirements or manipulating the phosphatidylinositol 3-OH kinase (PI3K)–AKT or JUN amino-terminal kinase 1 (JNK1) signalling pathways may increase Treg cell survival after infusion. MHC, major histocompatibility complex; RE, regulatory element.
Treg cell-meditated immunosuppression
The main goal of Treg cell therapy is to induce or re-establish immune tolerance; this goal requires the suppressive function of Treg cells, which is influenced by factors such as their activation status, their cytokine milieu, the availability of antigen and the affinity of the T cell receptors (TCRs) for the recognized antigens. Although continuous TCR signalling is required for the repressive function of Treg cells20, and Treg cells are highly sensitive to activation by the recognized antigen21, the impact of TCR affinity on Treg cell-mediated suppression is unclear. Some studies suggest that Treg cells expressing high-affinity TCRs have a more potent suppressive function than Treg cells expressing low-affinity TCRs22,23, whereas others have found that Treg cells expressing TCRs with affinities that differ by several orders of magnitude have a similar suppressive function24.
Mixed lymphocyte reactions.
In vitro tests consisting of mixing different subsets of T cells together in the presence of antigen-presenting cells.
There are several mechanisms of Treg cell-mediated suppression, including IL-2 deprivation (wherein Treg cells act as an IL-2 sink, reducing the primary growth and survival factor for Teff cells), the secretion of inhibitory cytokines (such as IL-10 and TGFβ) and the acquisition of costimulatory molecules from antigen-presenting cells (APCs) via high-affinity binding to CTLA-4 (REF.25). Currently, the suppressive function of human Treg cells is primarily quantified by measuring the degree to which they inhibit Teff cell proliferation in vitro or prevent graft-versus-host disease (GvHD) in humanized mice. Additional in vitro assays include measuring Treg cell-mediated suppression of mixed lymphocyte reactions, which depends on the interaction of CTLA-4 on Treg cells with DCs26, and the inhibition of inflammatory cytokine production by Teff cells27.
Different subsets of Treg cells specialize in suppressing specific T helper cell subsets: TH1 cell-like Treg cells, which have a similar gene signature to TH1 cells, are most effective in inhibiting TH1 cells, TH2 cell-like Treg cells are most effective in inhibiting TH2 cells, and so on14,16. Furthermore, studies in mice have demonstrated that the expression of chemokine receptors on Treg cell subsets must mimic the expression of chemokine receptors on T helper cell subsets for specialized Treg cells to preferentially migrate and suppress specific T helper cell-driven immune responses14. The approach to maximize the suppressive function of Treg cells will likely need to be tailored for each therapeutic application.
Treg cell survival
Unlike small molecules and biologics, living cells can change their identity or undergo apoptosis in response to the levels of oxygen, nutrients and signalling molecules in their new environment. Indeed, Treg cells infused in humans and in non-human primates rapidly (that is, within 2 weeks) decreased in number28–30. However, using deuterium to label infused Treg cells, a later study showed that a marked percentage of infused Treg cells are detected in some patients up to 1 year following infusion31; these data indicate that better techniques, such as the use of gene-modified cells, are needed to assess, and increase, the survival of human Treg cells in patients. Circulating Treg cells constantly require exogenous IL-2 for their survival, as they do not produce this cytokine themselves6. Nevertheless, tissue-resident Treg cells can become IL-2 independent, relying instead on IL-7 and IL-33 for their survival and stability32,33. New insights into the metabolic requirements and signalling circuitry of Treg cells may yield strategies to enhance Treg cell survival.
Treg cell stability
FOXP3 expression is central to the Treg cell lineage. Loss of FOXP3 expression and/or ablation of the TSDR in the FOXP3 locus results in loss of Treg cell identity and systemic autoimmunity3,34. In response to proinflammatory or otherwise inhospitable conditions, Treg cells transdifferentiate into Teff cells (also known as ‘ex-Treg cells’), including pathogenic TH1 cells, TH2 cells and TH17 cells4,5,35. Thus, once administered, antigen-specific Treg cells targeting a specific tissue could convert into pathogenic cells with the same specificity and further exacerbate tissue damage. Strategies to increase Treg cell stability include the ectopic expression of the transcription factors FOXP3, HELIOS and BACH2 (REFS36–38), which activate or repress gene transcription, or of a constitutively active form of STAT5 (REF.6), as well as the genetic ablation of PRKCQ (encoding protein kinase C-θ (PKCθ); PKCθ is found in the immune synapse and controls early T cell activation events critical for Treg cell stability)39,40, STUB1 (encoding the E3 ubiquitin-protein ligase carboxy terminus of Hsp70-interacting protein (CHIP)) or CCAR2 (encoding deleted in breast cancer gene 1 protein (DBC1)); CHIP and DBC1 promote FOXP3 degradation41,42.
Treg cell specificity
Most clinical trials conducted to date using Treg cell therapy have used ex vivo expanded polyclonal Treg cells. Yet, antigen-specific Treg cells are superior to their polyclonal counterparts in their migration to, and persistence in, the target tissue, and in their execution of a local immunosuppressive response43,44. These properties of antigen-specific Treg cells will allow, compared with polyclonal Treg cells, the use of fewer cells, greatly reducing the risk of inducing unwanted, widespread, non-specific immunosuppression. In addition to isolating and expanding endogenous antigen-specific Treg cells, it is also possible to create Treg cells with a desired specificity by TCR gene transfer23,45–47. The use of artificial immune receptors, such as chimeric antigen receptors (CARs), to redirect Treg cell specificity towards pathogenic T cells or the affected tissue has expanded this approach48,49.
Manufacturing Treg cells for therapy
Manufacturing Treg cells involves choosing the source from which Treg cells should be isolated as well as the methods for purifying and expanding Treg cells, product specification and release criteria. Detailed information on protocols for producing Treg cells is often only partially provided in the literature. A ‘minimum information about T regulatory cells’ document has been generated in a first but critical step towards process reproducibility and standardization50.
Sources of human Treg cells
Several sources of human Treg cells have been explored. Peripheral blood is the most accessible, and often the only, option for autologous applications. Umbilical cord blood (UCB) has been successfully tested in GvHD using partially human leukocyte antigen (HLA)-matched Treg cells from non-autologous UCB donors30,51,52. Treg cells can also be isolated from discarded thymuses removed during paediatric cardiac surgery. Approximately 300 × 106 CD4+CD25+ Treg cells can be isolated from thymuses from one donor53, which is equivalent to the number of Treg cells in the entire blood volume of an adult donor. Thus, paediatric thymuses may be an attractive source of non-autologous Treg cells, even though this source is yet to be tested in clinical trials or multiple preclinical models53.
Methods of purifying Treg cells
If Treg cells are to be expanded in vitro before infusion, the starting Treg cell population must be of high purity. This requirement is because Treg cells proliferate slowly when compared with conventional CD4+ T cells and CD8+ T cells. Minor conventional CD4+ T cell and CD8+ T cell contaminants at the outset of the expansion can allow cells other than Treg cells to overtake the culture, preventing the generation of a pure Treg cell product. The markers initially used to isolate Treg cells were CD4 and CD25. Although expression of these markers is sufficient for isolating Treg cells from UCB, it was not sufficient for isolating Treg cells from adult peripheral blood containing activated antigen-experienced conventional T cells expressing CD25 (REF.54). Selection of the T cells on the basis of low CD127 expression greatly increased Treg cell purity and recovery from peripheral blood and lymphoid tissue8,9. Additional Treg cell markers are likely to result from efforts aimed at pinpointing the molecular signatures of bona fide Treg cells55,56.
Two methods have been used to purify Treg cells: magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS). Although MACS-based approaches are good manufacturing practice (GMP) compliant and can process billions of cells simultaneously, they are imprecise and often result in Treg cells with insufficient purity, a low rate of Treg cell recovery or both. By contrast, FACS allows the precise identification of Treg cells through the use of multiple markers, ensuring high purity and high recovery; however, the process is slow and limits the number of cells that can be processed to ~109 peripheral blood mononuclear cells (PBMCs) per day, consequently limiting the dose that can be manufactured. In addition, FACS instrumentation, for example, FACSAria (Becton Dickinson), is typically not GMP compliant owing to open air processing and unexchangeable parts that are in contact with cells. Exceptions include the new FX500 fluidics cell sorter (Sony) and the three-laser MACSQuant Tyto cell sorter (Miltenyi Biotec), closed on-chip sorting systems with exchangeable parts used to enrich and purify the final population. Nonetheless, both MACS and FACS are currently being used to manufacture Treg cells for use in the clinic; the regulatory environment of the country and financial resources of the investigators often dictate which method is chosen.
Methods of expanding Treg cells
Most Treg cell therapies aim to increase, albeit transiently, the number of Treg cells to reset the inflammation-prone state of the recipient. This inhibitory effect is estimated to require an infusion of millions to billions of Treg cells57 and, thus, requires the expansion of Treg cells before infusion. So far, Treg cells used in clinical trials have been mostly expanded by polyclonal stimulation; that is, anti-CD3 and anti-CD28 beads30,31,58–60. To prevent the outgrowth of conventional T cells and to maintain high FOXP3 expression during Treg cell expansion, many centres culture the cells in the presence of rapamycin, an inhibitor of the mechanistic target of rapamycin (mTOR). Treg cells are less sensitive to phosphatidylinositol 3-OH kinase (PI3K) activity (an upstream kinase in the signalling pathway that activates mTOR) than conventional T cells, making them markedly less sensitive to the anti-proliferative effect of rapamycin than Teff cells61–63. Including rapamycin in cell cultures is especially important when Treg cells are purified by MACS as it helps to compensate for the considerably reduced initial purity of the Treg cells. Importantly, however, rapamycin also suppresses the expansion of Treg cells, leading to longer culture durations that require repeated stimulation with anti-CD3 and anti-CD28 beads, and sometimes with artificial APCs, to produce sufficient doses of Treg cells. Our laboratories have shown that rapamycin is not needed when Treg cells are purified by FACS with selection for CD4+CD25+CD127low Treg cells58. Antigen-specific approaches for expanding Treg cell populations are being developed and used. These approaches include the manufacture of clinical grade human alloantigen-reactive Treg cells following expansion in the presence of donor-derived stimulated B cells, which selectively increases the pool of alloantigen-reactive Treg cells that is naturally present in the blood64,65. Of note, DCs66 and K562 cell-based artificial APCs67 have also been used to expand human Treg cells ex vivo.
K562 cell-based artificial APCs.
K562 cells are an immortalized human leukocyte antigen (HLA)-deficient cell line initially isolated from a chronic myelogenous leukaemia. Artificial antigen-presenting cells (APCs) are K562 cells that have been gene edited to express CD80 and/or CD86 and specific HLA alleles to function as APCs.
As Treg cells proliferate poorly in vitro, most protocols for expanding Treg cells rely on the use of strong stimulants to drive cell proliferation and on high concentrations of IL-2 to sustain Treg cell expansion65. Yet, considerable variability in the proliferative potential of Treg cells has been observed during ex vivo expansion68. This variability may relate to the activation state of the cells, which is reported to be affected by the age of the Treg cell donor, as well as the donor’s immunological experiences, inflammatory conditions and current medications. For example, CD45RA-expressing naive Treg cells are in a resting state when isolated, yet readily expand upon in vitro stimulation and maintain their Treg cell lineage after expansion more efficiently than CD45RA− Treg cells58,69,70. Among CD45RA− Treg cells, recently activated effector Treg cells negative for CC-chemokine receptor type 7 (CCR7) have the least potential for expansion and are the most likely to lose expression of FOXP3 (REF.71). Consistent with these observations, UCB-derived Treg cells are mainly naive, bestowing them with a growth advantage over their peripheral blood counterparts72. Unexpectedly, Treg cells isolated from infant thymuses, although mostly naive, do not proliferate as effectively as Treg cells from adult peripheral blood53.
Product specification and release criteria for Treg cells
Most centres conducting Treg cell therapy trials agree that the expanded Treg cell product should remain CD4+CD25+FOXP3highCD127low, and these markers are widely used to define product identity. Activated conventional human T cells also show a transient increase in FOXP3 expression in response to TCR–CD28 signals, limiting the reliability of this marker alone for the identification of expanded human Treg cells and explaining why lineage-committed Treg cells are identified by demethylation at TSDRs. In activated conventional T cells that transiently upregulate FOXP3, the TSDR remains methylated73,74. The ongoing development of validated and quantitative standardized assays for measuring TSDR demethylation in lymphocytes, such as the one performed by Epiontis75, could soon allow the routine inclusion of this important criterion for Treg cell product release.
Lessons from Treg cell therapy trials
Taming transplant rejection
Thus far, excellent safety profiles have been consistently shown in patients receiving Treg cells14. Treg cell therapy was first applied in GvHD, supported by striking efficacy data from preclinical models in the early years of the first decade of this century76,77. In 2009, a team in Gdansk, Poland, published the first report of Treg cell therapy in humans. In this study, ex vivo expanded CD4+CD25+CD127low Treg cells were infused into one patient with acute (grade IV) GvHD and two patients with chronic GvHD. Treg cells alleviated symptoms, and pharmacological immunosuppression could be reduced in chronic, but not acute, GvHD59. Subsequent studies provided encouraging results regarding the use of Treg cells to prevent and treat acute and chronic GvHD30,51,78–80, although two clinical trials have been withdrawn (NCT01163201, NCT02118311). These studies were halted for reasons other than safety, including logistics, trial design and replacement with a new study.
Alloantigen-reactive Treg cells can attenuate donor-reactive T cells in preclinical models of transplantation, providing robust evidence to justify their evaluation in clinical trials64,81,82. As a result, they are being tested in several phase I studies in kidney and liver transplantation60,83,84 (FIG. 1). Overall, Treg cell infusions have been safe and well tolerated and, although manufacturing the cells in the context of chronic immunosuppressive treatments remains challenging, these studies provide enough safety data to advance Treg cell therapy to phase II trials. Data from these trials, which will include efficacy, will be unveiled in the coming years.
Autoimmune and autoinflammatory disease
There is much interest in using Treg cells to treat autoimmune diseases, particularly patients with type 1 diabetes (T1D). Strong preclinical data were obtained in the non-obese diabetic (NOD) mouse model43. A single infusion of ex vivo expanded islet-specific Treg cells prevented autoimmunity and restored sustained self-tolerance in mice with recent diabetes onset. The natural pool of Treg cells in NOD mice is numerically normal, preferentially expands in the pancreatic lymph nodes of prediabetic mice and migrates to inflamed islets. Yet, Treg cells fail to control islet destruction owing to a survival disadvantage in chronically inflamed islets85. Moreover, NOD Teff cells are more resistant to Treg cell-mediated suppression than their B6 mouse counterparts86. The first clinical trial testing polyclonal Treg cell therapy in T1D showed Treg cell administration to be safe and well tolerated, and the children into whom Treg cells had been infused had markedly higher C-peptide levels and lower insulin requirements than children in the untreated group28. In a second phase I clinical trial conducted in the United States, ex vivo expanded polyclonal Treg cells were infused into 14 patients with T1D in doses ranging from 5 × 106 to 2.6 × 109 cells. Infusions were well tolerated, and C-peptide levels in most patients remained stable for 1 year, although efficacy could not be conclusively shown. Notably, cell pharmacokinetic analysis of Treg cells labelled with deuterium during ex vivo expansion showed that a small but visible percentage of the infused Treg cells persisted in peripheral blood for at least 1 year without evidence of deuterium-positive Teff cells; this finding demonstrates that the Treg cells remained phenotypically stable after infusion31. Nonetheless, a phase II clinical trial (NCT02691247) performed by Caladrius Biosciences, in which 113 newly diagnosed (that is, less than 100 days since T1D diagnosis) adolescents with T1D into whom autologous ex vivo polyclonally expanded Treg cells had been infused failed to show that Treg cell infusion led to preservation of C-peptide production 1 year after the start of treatment (see Related links). The trial is ongoing but these negative results are in agreement with the finding in preclinical models that antigen-specific, and not polyclonal, Treg cells are required to reverse T1D as the frequency of islet-specific Treg cells in blood is extremely low. One strategy is to artificially redirect peripheral blood Treg cells to specific antigens (see later).
C-peptide.
Short polypeptide connecting the A chain of proinsulin to the B chain. After packaging in vesicles in pancreatic beta cells, C-peptide is removed from proinsulin, leaving the A chain and the B chain linked by a disulfide bridge. Blood C-peptide levels are used to monitor endogenous insulin expression in patients with diabetes.
Amyotrophic lateral sclerosis.
Neurodegenerative disorder characterized by the progressive loss of motor neurons, causing muscle weakness, atrophy and eventually death.
Pemphigus vulgaris.
Group of skin disorders characterized by the formation of blisters induced by autoantibodies targeting intercellular adhesion molecules on keratinocytes.
Guillain–Barré syndrome.
Immune-mediated polyneuropathy, usually started after an infection, sharing cross-reactive epitopes with peripheral nerves (myelin or axonal membrane).
Alzheimer’s disease.
Neurogenerative disorder leading to progressive dementia associated with the accumulation of amyloid-β plaques in nervous cells of the central nervous system.
Marginal zone.
High-transit area constituted by B cells, macrophages, dendritic cells and granulocytes, interposed between lymphoid tissues and the circulation, serving as a sentinel for blood-borne antigens.
Two smaller studies assessing the use of Treg cells in the treatment of autoimmune and autoinflammatory diseases have also been reported. The first study focused on one patient with systemic lupus erythematosus and active skin disease who received 1 × 108 autologous Treg cells expanded by polyclonal stimulation87. The Treg cells migrated into the affected areas of the skin and markedly attenuated the activity of the interferon-γ (IFNγ) pathway while enhancing the activity of the IL-17 pathway87. Of note, IL-17 may be involved in barrier homeostasis. Thus, a shift from IFNγ to IL-17 may lead to reduced skin inflammation and tissue repair. In the second study, three patients with amyotrophic lateral sclerosis, who received eight consecutive infusions of Treg cells while undergoing IL-2 therapy, showed reduced disease progression88. In short, the field is steadily moving forward, with several phase I clinical trials aiming to test Treg cell therapy in autoimmune hepatitis (NCT02704338), pemphigus vulgaris (NCT03239470), inflammatory bowel disease (NCT03185000), Guillain–Barré syndrome (NCT03773328) and Alzheimer disease (NCT03865017).
Co-medication and Treg cell therapy
IL-2 therapy and Treg cells
In the 1990s, and counterintuitively at the time, mice deficient in the crucial T cell growth factor IL-2 displayed uncontrolled T cell activation and succumbed to widespread autoimmunity89. Later experiments revealed that IL-2 is required for the development, homeostasis and suppressive function of Treg cells90, explaining this observation and suggesting that IL-2 could be used to expand Treg cells in vivo in patients with autoimmune disease6. Although other immune cells — such as conventional T cells, NK cells and some subsets of innate lymphoid cells — express the IL-2 receptor, as Treg cells constitutively express CD25 (a component of the high-affinity IL-2 receptor), low-dose IL-2 should preferentially affect Treg cells91. Thus far, the best evidence that this phenomenon occurs in humans comes from an uncontrolled phase I/IIa clinical trial in patients with hepatitis C-mediated vasculitis that is resistant to treatment with IFNα and the antiviral ribavirin92. Low-dose IL-2 treatment had efficacy in eight out of ten patients, as judged by an increase in Treg cell frequency and a concomitant decrease in the proportion of marginal zone B cells92. A concurrent study revealed that daily low-dose IL-2 treatment increased Treg cell numbers and alleviated chronic GvHD symptoms in 12 of the 23 patients treated93. Finally, an uncontrolled trial using a low dose of IL-2 to treat patients with systemic lupus erythematosus also showed efficacy, with the expansion of Treg cells, T follicular helper cells and TH17 cells, but not of TH1 cells or TH2 cells, along with decreased disease activity94.
Currently, nine trials are actively testing the efficacy of low-dose IL-2 treatment for several autoimmune diseases. The TRANSREG study goes even further, comparing, in a multicentre, uncontrolled, open-label study, 14 autoimmune diseases (NCT01988506). In T1D, low-dose IL-2 therapy is safe and augments Treg cell numbers; however, the numbers of NK cells and eosinophils were also increased, and efficacy is yet to be demonstrated95–97. Hence, IL-2 therapy could be combined with Treg cell therapy to increase efficacy, and three clinical trials are currently testing this hypothesis in the treatment of T1D (NCT02772679), steroid refractory chronic GvHD (NCT01937468) and amyotrophic lateral sclerosis (NCT03241784). Future approaches may include the use of IL-2–anti-IL-2 complexes that selectively expand Treg cells98. Alternatively, engineering an orthogonal IL-2 molecule specific to an orthogonal IL-2 receptor is feasible and would allow the selective expansion and survival of the infused engineered cells99.
Crosstalk with immunosuppressive regimens
Patients with autoimmune disease are often treated with several immunosuppressive drugs, including corticosteroids (such as prednisone), antimetabolites (such as methotrexate, azathioprine, mycophenolate mofetil and leflunomide), alkylating agents (such as cyclophosphamide), blocking monoclonal antibodies that target cytokines or their receptors (such as anti-tumour necrosis factor (TNF), anti-IL-1β, anti-IL-4 receptor subunit-α (which simultaneously inhibits the IL-4 and IL-13 pathways), anti-IL-5, anti-IL-6 and anti-IL-17), abatacept (which blocks CD80 and CD86 in APCs), and B cell-depleting antibodies (such as rituximab and belimumab). In the setting of transplantation, the interactions of different immunosuppressive drugs (for example, ciclosporin, rapamycin, anti-CD25 and thymoglobulin) with Treg cells need also to be evaluated, as reviewed elsewhere100. Immunosuppressive drugs may impact the number, expansion and function of Treg cells. If fewer Treg cells can be isolated and expanded from patients with autoimmune disease and transplant recipients compared with healthy individuals, the minimum number of Treg cells required for product release, a typical hurdle in trials for Treg cell therapy in transplantation, may not be achieved101. Furthermore, the dosage of these treatments can vary widely during disease treatment, likely with variable effects on the Treg cell compartment. Encouraging preliminary results obtained from patients who underwent allogeneic kidney transplantation suggest that dialysis, immunosuppression and acute rejection episodes affect Treg cell maturation, resulting in a reduced percentage of mature Treg cells in these patients, but do not preclude ex vivo Treg cell expansion and the generation of a final Treg cell product with therapeutic properties102. Future research will shed light on this potential limitation as we gather data on the unwarranted interactions between immunosuppressive drug regimens and infused Treg cells.
Engineering Treg cell specificity
Treg cells could potentially be manipulated to restore immune tolerance in the treatment of autoimmunity. Treg cells must migrate to appropriate sites and respond to their cognate antigen to effectively suppress immune responses. Studies in transgenic NOD mice genetically engineered to express the BDC2.5 diabetogenic TCR revealed that relatively small numbers of antigen-specific Treg cells, but not of polyclonal Treg cells, are sufficient to prevent and even reverse T1D43. Strikingly, in a B6 mouse model where insulitis and autoimmune diabetes were induced by expression of the proinflammatory cytokine TNF in islets, as few as 2,000 Treg cells isolated from the pancreatic draining lymph node could prevent diabetes103. These seminal studies established the importance of using antigen-specific Treg cells for therapy. Yet, only minute numbers of antigen-specific Treg cells relevant for a given condition are found in peripheral blood. Moreover, most antigen-specific Treg cells reside in tissues, making them difficult to isolate and their cognate antigens laborious to identify. These obstacles have fuelled interest in devising strategies to artificially direct Treg cells to a desired target (FIG. 3).
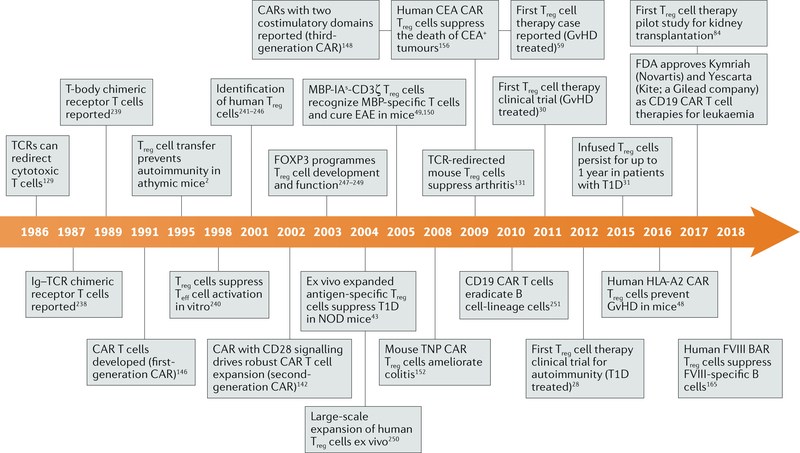
Fig. 3 |. Timeline of events in the development of regulatory T cell therapy.
A timeline of key developments leading to the use of regulatory T cell (Treg cell) therapy in the clinic. BAR, B cell-targeting antibody receptor; CAR, chimeric antigen receptor; CEA, carcinoembryonic antigen; EAE, experimental autoimmune encephalomyelitis; FOXP3, forkhead box protein P3; FVIII, factor VIIII; GvHD, graft-versus-host disease; HLA, human leukocyte antigen; IA, murine major histocompatibility complex class II molecule I-A; Ig, immunoglobulin; MBP, myelin basic protein; NOD, non-obese diabetic; T1D, type 1 diabetes; TCR, T cell receptor; Teff cell, effector T cell; TNP, 2,4,6-trinitrophenyl.
HLA restriction.
The presentation of a peptide by a specific human leukocyte antigen (HLA) to a T cell’s receptor.
Currently, engineered TCRs and CARs are the main receptors employed to impart Treg cell specificity. TCRs have lower affinity than CARs yet can recognize just one molecule per target cell104, whereas cells must have at least 100 target molecules for CARs to recognize them105. However, CAR targets tend to be moderately to highly expressed surface proteins. Thus, the difference in affinity between TCRs and CARs is compensated for by different expression levels of the ligands on target tissues. CARs are not subject to HLA restriction or dependent on a co-receptor, but whether the high affinity and downstream signalling of CARs are ideal for Treg cell function is unclear. The relative advantages and disadvantages of each receptor continue to be explored (TABLE 1).
Table 1 |.
Advantages and disadvantages of TCRs and CARs in engineering regulatory T cell specificity
| Property | TCRs | CARs |
|---|---|---|
| Specificity | Peptide–HLA complex | Any surface antigen or multivalent soluble antigen |
| HLA restriction | Yes | No |
| Co-receptor required | Yes (CD4 or CD8) | No |
| Affinitya | KD = 10−6–10−4 M252 | KD = 10−10–10−6 M253 |
| Sensitivity | <10 molecules per target cell104,254 | 100–10,000 molecules per target cell105,255–257 |
| Signalling | Via endogenous CD3 complex (comprising six chains) | Via synthetic modular signalling domain |
| Expression challenges | Endogenous TCR might pair with exogenous TCR chains | Protein aggregation; aggregation of single-chain variable fragments leading to tonic signalling |
CAR, chimeric antigen receptor; TCR, T cell receptor.
KD, equilibrium dissociation constant; the lower the KD value, the higher the affinity.
Targeting TCRs to engineer Treg cells
The right TCR for a Treg cell
Perhaps the most straight-forward way to redirect Treg cell specificity is to engineer a TCR recognizing a peptide of interest in the target tissue (FIG. 4). The TCR is a heterodimer comprising an α-chain and a β-chain, each of which is composed of a variable region (VDJ) and a constant region. Each T cell expresses a unique TCR, owing to stochastic V(D)J recombination, which can generate up to 1 × 1061 different TCR sequences in humans106; detailed amino acid sequences of each individual region of many TCRs can be found in the IMGT database. Although most T cells with strong reactivity against self-peptide–major histocompatibility complex (MHC) class II complexes are clonally depleted in the thymus (an example of recessive immune tolerance), a subset of these cells acquire FOXP3 expression when the CNS2 enhancer in the first intron of the FOXP3 gene is demethylated107,108. Thus, the TCR repertoire of thymically derived Treg cells is distinct from that of Teff cells, which have been selected for weak peptide-bound MHC–TCR interactions. Autoimmune regulator (AIRE), a transcriptional regulator driving tissue-specific antigen expression in the thymus, promotes Treg cell development by skewing autoreactive T cells towards a Treg cell phenotype109,110. Still, some autoreactive Teff cells escape clonal deletion and they may share a common pool of self-reactive TCRs with Treg cells111. Distinct Treg cell TCRs can be specific to non-overlapping peptides derived from the self-antigen that is targeted by autoantibodies, suggesting that Treg cells recognize only a few proteins deemed most susceptible to autoimmune recognition112.
Fig. 4 |. Redirecting regulatory T cells by engineering T cell receptors.
A tissue biopsy sample is taken from a patient with an autoimmune disease (step 1) and subjected to single-cell paired T cell receptor (TCR) sequencing to characterize the TCR repertoire of the regulatory T cells (Treg cells) or, alternatively, of the autoimmune T cells (step 2). On the basis of algorithms identifying conserved motifs and complementarity-determining regions, pathogenic epitopes are predicted (step 3) and selected TCR ultramer templates are synthesized (step 4). Using a non-viral approach, the ultramer and a Cas9 ribonucleoprotein complex targeting the endogenous TCR via homology repair are electroporated into peripheral blood-derived Treg cells (step 5). Edited Treg cells are further expanded in vitro (step 6) and finally reinfused into the patient (step 7).
As peptides from any protein (including intracellular proteins) can potentially be bound by MHC and recognized by the TCR, TCRs can be isolated or designed to recognize an almost infinite number of targets. Another advantage of the TCR is that the peptides it recognizes can be derived from proteins that are post-translationally modified. Post-translational modification by oxidation113, deamidation114, citrullination115 or phosphorylation116 generates unique epitopes that typically circumvent central T cell deletion117,118. Hybrid peptides (that is, products of the fusion of the amino terminus of one peptide to the carboxy terminus of another peptide by a peptide bond) have also been uncovered as important self epitopes119,120.
Importantly, how TCRs can be engineered to impart Treg cell specificity may be different depending on whether Treg cells function primarily in lymphoid organs to restore immune tolerance or in tissues to suppress autoinflammation or induce tissue repair. In the mouse, continuous, steady-state TCR stimulation in lymphoid organs mediates suppression20,121, whereas in tissues that lack TCR stimulation and have low levels of IL-2, CD44hiCD62LlowCCR7low Treg cells depend mainly on costimulation through inducible T cell costimulator (ICOS) for their maintenance71,122. Additional signals that are important for the maintenance of tissue-resident murine Treg cells have been identified; Treg cells in adipose tissue rely on IL-33 (REF.33), whereas memory Treg cells in the skin require IL-7 for survival32.
In inflammatory conditions, such as in murine autoimmune diabetes, Treg cells specific to islet-derived antigens clonally expand in inflamed islets but not in the spleen or lymph nodes123. Treg cells with high-affinity or low-affinity TCRs can be found in islets, and evidence supports the hypothesis that these Treg cell subsets have complementary roles in immune homeostasis22. High-affinity Treg cells expressed high levels of suppressive molecules (namely IL-10, LAG3 and TIGIT), whereas low-affinity Treg cells expressed amphiregulin, a key factor in tissue repair22. Furthermore, Treg cell subsets characterized by distinct cytokine profiles and transcription factor dependencies have been shown to feature TCR signalling of differing strengths124.
TCR immunosequencing as a route to engineering TCRs
Progress in engineering TCRs will also depend on advances in TCR immunosequencing, at the single-cell level, and thus in predicting which peptides they recognize. High-throughput immunosequencing of the TCRα and TCRβ chains of CD4+ T cells, CD8+ T cells and Treg cells, in pancreatic tissues and blood, have uncovered a population of pancreas antigen-specific T cells selectively in the pancreatic islet tissue125. The local accumulation of these T cells suggests that they are reactive to islet antigens, making their TCRs potential candidates for redirecting Treg cells to peptides selectively expressed in the pancreas. However, knowledge of the sequence of both TCRα and TCRβ and of how they are paired to form heterodimers in a single T cell is needed to reconstitute TCR specificity. The cost of single-cell paired TCR sequencing technology is rapidly decreasing, permitting the routine use of this approach for characterizing TCR repertoires on various T cell subsets, including Teff cells and Treg cells. Additionally, a high-throughput method to pair TCRα and TCRβ genes without the need for single-cell technologies has been reported126. These new technologies will help rigorously define the TCR repertoire of Treg cells and, eventually, of autoimmune T cells, which can be used to engineer T cells for therapeutic purposes (FIG. 4). However, predicting the epitope specificity of TCRs will be key, and several algorithms can now cluster TCR sequences on the basis of conserved motifs and complementarity-determining regions127,128.
As had been previously shown with conventional T cells129, introducing a TCR from another antigen-specific T cell to Treg cells can effectively redirect them towards a known antigen specificity. In one example, Treg cells were transduced with TCRs that conferred them with specificity for a transplant antigen. Specifically, a TCR specific for a peptide derived from an MHC class I molecule (H-2Kd from the BALB/c strain) presented by an MHC class II molecule (H-2Ab from the B6 strain) was transduced into B6 Treg cells, leading to peptide–MHC specific cells that induced the long-term survival of fully MHC-mismatched (MHC class I and MHC class II) BALB/c heart grafts in immunocompetent B6 mice130. In another study, Treg cells from chicken ovalbumin (OVA) antigen-specific, OT-II, transgenic mice, suppressed the in vitro proliferation of Teff cells on stimulation with DCs presenting the relevant OVA peptide. Moreover, in an in vivo model of rheumatoid arthritis, redirected Treg cells from the same OT-II mice homed to the inflamed joint (following an intra-articular injection of OVA in the knee), reduced the number of TH17 cells in the draining lymph node and decreased inflammation and bone damage131.
Complementarity-determining regions.
Parts of the variable chain of the T cell receptor or of an antibody that determine specificity to their cognate antigen.
To date, several TCRs have been successfully used to redirect the specificity of human Treg cells while preserving their in vitro suppressive function. Target antigens include insulin, glutamic acid decarboxylase and factor VIII23,45,46. Treg cells transduced with a high-affinity TCR have superior suppressive function compared with Treg cells transduced with a low-affinity TCR specific for the same peptide23. Strikingly, Treg cells transduced with a high-avidity HLA-A2-restricted tyrosinase-specific TCR from CD8+ T cells recognized an MHC class I-presented antigen while maintaining their capacity to suppress Teff cell responses against that same antigen47. This result suggests that TCRs for translational applications using Treg cells can be obtained from cells other than Treg cells or conventional CD4+ T cells.
Chimeric TCRs
TCRs are complex to engineer, in part owing to their heterodimeric nature; introduced subunits can recombine with the endogenous TCRα or TCRβ chain. This complexity raises the risk of generating TCRs with unknown specificities and off-target effects. Several strategies have been devised to address this problem. One approach is to insert extra cysteine residues in the constant region of engineered TCRα and TCRβ chains to form a disulfide bridge between the two to promote preferential pairing of the transduced TCR132,133. Another strategy is to replace the human constant region in TCR chains with a mouse constant region, as interspecies pairing between a murine TCR chain and a human TCR chain has never been observed. This approach also facilitates signal transduction, as the mouse constant region has higher affinity for the TCR–CD3 complex than its human counterpart. Building on this approach to avoid xenogeneic immunogenicity, two independent groups systematically identified the minimum set of residues in the murine TCR constant chains required to ‘murinize’ the human TCR constant chain. The resulting transgenic TCR had enhanced stability and avidity134,135. A final strategy is to use vectors with strong promoters to express the engineered TCR136.
Currently, the most promising approach to prevent mixed TCR dimer formation is to use genome editing to engineer the endogenous TCR locus. One or both endogenous TCR chains could be knocked out before a new antigen receptor is introduced137. A more elegant method, however, would be to replace the endogenous TCR with the new TCR by knocking it into the TCRα constant region locus (TRAC), ablating expression of the native TCR and endowing the new TCR with the same genomic location and transcriptional regulation as the endogenous gene. Indeed, this was accomplished with reasonable efficiency in primary human T cells by CRISPR–Cas9-mediated editing coupled with a double-stranded DNA template coding for a tumour antigen (NY-ESO)-specific TCR for homology-directed repair138.
An additional challenge inherent in engineering TCRs is that they require costimulation to fully activate downstream signalling. To overcome this issue, two groups have generated chimeric TCRs. One group linked a melanoma antigen-specific TCR to the transmembrane and intracellular portions of CD28 followed by CD3ε, observing enhanced expression of TCR at the cell surface, the formation of immune synapses, IL-2 secretion, survival of the engineered T cell and tumour clearance139. Another group fused a soluble TCR to a CAR transmembrane and signalling domain, demonstrating that this TCR–CAR was functional in primary T cells and in an NK cell line140.
Engineered CAR Treg cells
Overview of early CAR Treg cell milestones
Next-generation Treg cell therapy will undoubtedly benefit from the field of immuno-oncology, in particular CAR T cell therapy. Since 2017, when the FDA approved CD19 CAR T cell therapy for paediatric acute lymphoblastic leukaemia and non-Hodgkin lymphoma, there have been 241 CAR T cell therapy clinical trials worldwide (91 in the United States; 14 in Europe, 128 in China, 3 in Canada, 2 in Japan; 2 in Australia and New Zealand, and 1 in Israel) as listed in ClinicalTrials.gov as of January 2018 (REF.141). The first clinical trial using CAR T cells targeted the GP120 region of the HIV envelope glycoprotein and was completed 2 years before CD19 CAR T cells were reported to eradicate tumours in animal models142. The GP120 CAR consisted of the CD4 co-receptor (which binds HIV GP120) fused to CD3ζ143,144. However, although HIV-specific cytotoxicity was shown in vitro, GP120 CAR T cell therapy failed to reduce viral load in patients with HIV infection145.
As mentioned earlier, robust T cell activation requires the interaction of the TCR with its cognate antigen and the binding of a costimulatory receptor (such as CD28) to its ligand (such as CD80 or CD86) on the surface of an APC. First-generation CARs contained solely a TCR CD3ζ endodomain, which, despite being able to induce T cell activation, could not drive robust T cell expansion146. Including the CD28 signalling domain in second-generation CARs allowed sustained CAR T cell expansion on repeated exposure to antigen, and these CARs displayed superior cytolytic function142. Incorporating an additional costimulatory domain (whether it be 4–1BB (also known as CD137), OX40 or another costimulatory domain) into a CD28–CD3ζ CAR creates a third-generation CAR (containing two costimulatory domains, as opposed to only one in second-generation CARs), further fine-tuning CAR signalling; addition of the intracellular domain of 4–1BB minimizes CAR-induced exhaustion147,148, and including the intracellular domain of OX40 (a costimulatory molecule belonging to the TNF receptor family) decreases CAR-induced IL-10 secretion, limiting the inhibition of antitumour activity149.
Engineering murine CAR Treg cells
The first described efforts to engineer Treg cell specificity involved creating a transgenic mouse line expressing an artificial chimeric receptor comprising an extracellular peptide-bound MHC complex linked to an intracellular TCR ζ-chain signalling domain49. The peptide antigen chosen was MBP89–101, an autoantigen derived from myelin basic protein (MBP) that induces experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis. This receptor design allowed CD4+CD25+ Treg cells to directly recognize and be activated by the TCR of pathogenic self-reactive T cells49. Upon activation by MBP89–101-reactive T cells, these engineered Treg cells secreted high levels of anti-inflammatory IL-10 and TGFβ and low levels of proinflammatory IFNγ, consistent with a regulatory function. Moreover, the level of cytokines produced in response to anti-CD3ε or MBP89–101-reactive T cell-mediated activation were similar, indicating that the chimeric receptor mimics physiological TCR signalling49. Strikingly, adoptive transfer of engineered Treg cells not only prevented MBP89–101-induced EAE but also treated it when performed 11 days or even 31 days (after epitope spreading) following disease induction in an example of bystander suppression49. Subsequent work revealed that these Treg cells expressing chimeric receptors functioned primarily by inducing MBP-specific T cells to produce IL-10. The adoptive transfer of non-transgenic MBP-specific T cells from mice treated with engineered Treg cells could prevent MBP89–101-induced EAE in naive mice, indicating that pathogenic T cells differentiated into antigen-specific Treg cells in an example of infectious tolerance150.
A different chimeric receptor design emerged a few years later in 2009, aimed at engineering Treg cells to prevent colitis151. Intrarectal administration of 2,4,6-trinitrobenzenesulfonic acid (TNBS) is a common strategy to induce colitis in mice. Ethanol disrupts the intestinal barrier, allowing TNBS to interact with proteins and render them immunogenic, generating the hapten 2,4,6-trinitrophenyl (TNP). The study authors created a tripartite chimeric receptor in which a recognition unit (an antibody single-chain variable fragment (scFv) specific for the hapten TNP) was fused to the transmembrane and cytoplasmic domains of CD28 and to the signalling domain of Fc receptor-γ. Unlike the aforementioned work, this approach allowed Treg cells to directly recognize a disease-specific tissue antigen. TNBS-induced colitis resulted in only 10% mortality in transgenic mice expressing the receptor in T cells, compared with 50% mortality in control mice. Moreover, Treg cells expressing the chimeric receptor were activated specifically by TNPylated cells and suppressed Teff cell proliferation in vitro. When adoptively transferred into wild-type mice 16 h after colitis induction, transgenic Treg cells migrated to the site of colonic damage and prevented colitis. Even though transgenic Treg cells could suppress colitis induced only by TNBS, indicative of their antigen specificity, they could also ameliorate colitis induced by other agents when trace amounts of TNBS were present, which is a hallmark of bystander suppression152. Of note, mice treated with transgenic Treg cells also displayed increased survival after a second round of TNBS-induced colitis (25% mortality compared with 66% mortality in controls). Whether this phenomenon was due to persistence of the modified cells or infectious tolerance was not explored151.
These initial chimeric receptor designs gave way to the next generation CARs first used in Teff cells for cancer therapy, with two groups generating CD28–CD3ζ CAR Treg cells specific for carcinoembryonic antigen (CEA)153,154. CEA expression is upregulated in benign colon inflammation (ulcerative colitis), as well as in colon cancer in humans155, and the first report focused on induced colitis153. Similarly to the TNP-specific modified Treg cells developed by the same group151,152, CEA CAR Treg cells localized to the colon, suppressed the production of inflammatory cytokines and prevented T cell-mediated colitis in mice when coadministered with CEA CAR CD4+ Teff cells153. As CEA is expressed in the lungs as well as the intestine, the second group tested the function of CEA CAR Treg cells in asthma154. Using a transgenic mouse model in which the human CEA gene promoter drives CEA expression in the pulmonary and gastrointestinal epithelium, the study authors induced allergic airway inflammation via immunization with OVA. CEA CAR Treg cells potently secreted IL-10 and suppressed Teff cell proliferation in vitro, and localized to the lung on intravenous administration into CEA transgenic mice. Moreover, administration of CEA CAR Treg cells 1 week after initial sensitization, followed by four consecutive days of challenge with OVA, almost completely prevented airway hyper-reactivity and reduced mucus production, eosinophil lung infiltration and the production of TH2 cell-type cytokines154.
Engineering human CAR Treg cells
These encouraging results in mouse disease models moved the field to work in human cells. The first study using a CAR to redirect human Treg cells also involved CEA. However, instead of preventing or alleviating disease in the intestine or the lung, human CEA CAR Treg cell-mediated suppression in vivo was demonstrated via partial protection of a CEA-expressing tumour from CEA CAR T cell-mediated death in immunodeficient mice156. One immediate caveat of this setting was that both CAR Treg cells and CAR T cells, which were administered at a 1:1 ratio, targeted the same antigen156. Thus, the observed partial protection could have been due to competition between these cell types for antigen rather than due to true suppression. Shortly afterwards, a different group generated human CD19 CAR Treg cells that suppressed the proliferation and cytotoxic activity of CD19 CAR Teff cells in vitro and migrated to CD19-expressing B cell-derived tumours in mice, preventing CD19 CAR Teff cell-mediated tumour killing in vivo at a ratio as low as one CAR Treg cell per 16 CAR Teff cells157.
The most immediate application of CAR Treg cells is poised to be in GvHD and organ transplant rejection. Unlike in most autoimmune disorders, there are very clear targets in transplantation in the form of HLA molecules. Moreover, CARs remove the need to activate recipient Treg cells with donor-derived APCs158. In 2016, the first HLA-A2 CAR Treg cells were reported; as HLA-A2 is present in 50% of the population, creating tolerance for this allele is advantageous. The study authors demonstrated that HLA-A2 CAR Treg cells suppress Teff cell proliferation and prevent HLA-A2+ PBMC-mediated GvHD in immunodeficient NSG mice. HLA-A2 CAR Treg cells did not kill HLA-A2+ cells in short-term in vitro assays but completely prevented HLA-A2+ PBMC engraftment, leaving open the possibility that they might kill HLA-A2+ cells in vivo48. Two subsequent studies also generated HLA-A2 CAR Treg cells159,160 and introduced new models to test HLA-A2 CAR Treg cells in vivo. One group demonstrated that HLA-A2 CAR Treg cells suppressed a mixed lymphocyte reaction between HLA-A2+ and HLA-A2− PBMCs in vivo, reducing ear swelling and preserving HLA-A2+ cells in mice. Of note, this result was accomplished at an HLA-A2 CAR Treg cell to HLA-A2+ PBMC ratio of 1:10, indicating that CAR-mediated suppression of GvHD is similar in potency to TCR-mediated suppression of GvHD161. Moreover, HLA-A2 CAR Treg cells prevented the rejection of HLA-A2+ skin grafts by HLA-2– PBMCs159. The other group also used HLA-A2+ skin graft rejection as a model, and found that HLA-A2 CAR Treg cells reduced alloimmune damage, as assessed by a reduction in the number of keratinocytes in the graft and increased blood vessel integrity160. Recently, a panel of fully humanized HLA-A2 CARs was generated and tested in Treg cells. Several humanized HLA-A2 CAR Treg cells rapidly migrated to, and persisted in, HLA-A2+ skin grafts, delaying HLA-A2 skin graft rejection162.
Introducing B cell-targeting antibody receptor Treg cells
New applications for CAR Treg cells are still emerging. B cell-targeting antibody receptor (BAR) Treg cells have been described recently. Human factor VIII (FVIII) injections are used to treat patients with haemophilia A. Over time, anti-FVIII neutralizing antibodies develop, causing morbidity and death163. A BAR containing an immunodominant domain of FVIII, either A2 or C2, as the extracellular domain, was designed to target FVIII-specific B cells; the intracellular signalling domain remained as CD28–CD3ζ as in a previous FVIII CAR design by the same group164. Strikingly, in vitro, human BAR Treg cells displaying either the A2 domain or the C2 domain suppressed antibody production by splenocytes isolated from mice that had been immunized with recombinant FVIII. Furthermore, intravenous administration of human FVIII BAR Treg cells into mice the day before immunization with recombinant FVIII prevented anti-FVIII antibody formation in vivo, whereas infusion of the cells into mice already producing anti-FVIII antibodies decreased antibody titres. BAR Treg cells could thus suppress antibody production even in a xenogeneic setting, both in vitro and in vivo. Whether these cells prevented T follicular helper cells from helping B cells, directly targeted FVIII-specific memory B cells or interacted with APCs to indirectly affect B cells was unclear. To address these questions, T cells and B cells were isolated from mice immunized with FVIII and treated with either BAR Treg cells or control OVA BAR Treg cells. B cells isolated from mice treated with control Treg cells produced anti-FVIII antibodies regardless of the source of the T cells, whereas B cells from mice treated with FVIII BAR Treg cells did not. BAR Treg cells are thus likely to act directly on B cells, either by suppressing them or by killing them165.
Alternative targets for CAR Treg cells
CAR Treg cell targets need not be present on the cell membrane. Our laboratories generated a CAR specific for citrullinated vimentin, a modified protein unique to the inflamed joints of more than 50% of patients with rheumatoid arthritis166. Citrullinated vimentin CAR Treg cells recognized and were activated by citrullinated vimentin present in synovial fluid from patients with rheumatoid arthritis; this approach may provide a strategy to restore homeostasis at the site of inflammation167. Currently, citrullinated autoantigens are thought to be present in the extracellular milieu primarily as a result of neutrophil extracellular trap (NET)-associated cell death (NETosis). A unique form of programmed cell death, NETosis involves intracellular content extrusion, particularly of genomic DNA-rich NETs of neutrophils infiltrated in synovial tissue168.
Adapting CAR signalling for Treg cells
Early experiments in the field found that incorporating CD28 signalling into CD19 CAR T cells, by switching from CD3ζ alone to a bipartite CD28–CD3ζ intracellular domain, made these CAR T cells resistant to Treg cell-mediated suppression, resulting in higher proliferation of CAR T cells and increased CAR T cell-mediated killing of B cell-derived leukaemia cells169. Yet, subsequent experiments reported that CEA CAR T cells containing a CD28–CD3ζ intracellular domain were more susceptible to Treg cells in a solid tumour compared with CAR T cells containing CD3ζ only. Deleting the binding site for the tyrosine-protein kinase LCK on the CD28 moiety of the CEA CAR ablated IL-2 secretion while supporting CAR T cell proliferation and preventing suppression by tumour-infiltrating Treg cells170. The seemingly contradictory results of the two studies might reflect how CAR T cells interact with liquid tumours that express CD80 and CD86 (CD19 CAR for B cell-derived leukaemia) and with solid tumours that do not (CEA CAR for non-blood cancer).
Efforts have been made to find the ideal CAR signalling architecture for CAR T cells by comparing how different costimulatory domains induce CAR T cell proliferation and longevity, as well as their ability to secrete specific cytokines and to kill tumour cells (reviewed elsewhere171). However, this is not the case for CAR Treg cells. After the first chimeric receptor design for Treg cells, in which CD3ζ alone was used as a signalling moiety49, subsequent CAR Treg cell studies in mice and humans used the prototypical CD28–CD3ζ CAR, with the exception of the use of CD3ζ alone in CD19 CAR Treg cells157. Yet, different CAR signalling modalities are now being tested in human Treg cells. One group constructed a CAR recognizing dextran and tested the function of different signalling domains in it using 4–1BB expression as a marker of Treg cell activation172. Curiously, 4–1BB–CD3ζ CARs and OX40–CD3ζ CARs induced CAR Treg cell activation, whereas CD28–CD3ζ CARs and CARs containing other signalling architectures did not172. This observation is at odds with results obtained with a ‘universal’ CAR (in which the scFv binds to a targeting module, which in turn recognizes a target antigen) featuring CD3ζ, CD28–CD3ζ or 4–1BB–CD3ζ as a signalling domain173. All three CAR Treg cell populations suppressed Teff cell proliferation with the same efficiency and were not cytotoxic in vitro. However, CD28–CD3ζ CAR Treg cells uniquely secreted detectable IFNγ and TNF and were activated to a greater extent than 4–1BB–CD3ζ CAR Treg cells, as assessed by expression of the CD69 early T cell activation marker. 4–1BB–CD3ζ CAR Treg cells efficiently protected a tumour from CAR T cell-mediated killing in a humanized mouse model173. In contrast, a study by a different group found that 4–1BB–CD3ζ CAR Treg cells are poor suppressors of Teff cells in vitro and fail to protect skin xenografts in vivo, whereas CD3ζ CAR Treg cells and CD28–CD3ζ CAR Treg cells performed well in both assays. All three CARs activated Treg cells to the same extent, as assessed by their upregulation of CD69 expression174. The discrepancies observed in these preliminary studies may be due to the use of different experimental models and also to the different activation markers used; the levels of 4–1BB, the activation marker used in the first study172, may be more sensitive to activation downstream of TNF receptor family members than the levels of CD69, which was the activation marker used in the second and third studies173,174. Current work in our laboratories focuses on systematically studying the impact of different signalling domains on CAR Treg cell and CAR Teff cell survival, stability and function175. From a clinical standpoint, it would be valuable to develop a CAR that maximizes the properties of Treg cells and is simultaneously a poor inducer of cytotoxic and proinflammatory responses even if it is inserted in a Teff cell; such responses could contaminate Treg cell preparations and thus jeopardize the efficacy and safety of CAR Treg cell therapy.
Finally, the choice of scFv binding domain may also impact CAR signalling as varying the affinity of scFv modulates CAR T cell function176. Work comparing a CD19 CAR with a GD2 CAR (GD2 is a disialoganglioside expressed in neuroblastomas) found that the latter is substantially more prone to aggregation, tonic signalling and subsequent T cell exhaustion than the former147. Thus, tailored signalling architectures may be required to accommodate differences in the affinity of, and the propensity for self-aggregation amongst, scFv chains. Indeed, 4–1BB costimulation was superior to CD28 costimulation in diminishing GD2 CAR tonic signalling-induced exhaustion147. Curiously, in the studies discussed above, CEA CD28–CD3ζ CAR Treg cells were used to suppress CEA CD28–CD3ζ CAR T cells156, whereas CD19 CD3ζ CAR Treg cells were used to suppress CD19 CD28–CD3ζ CAR Teff cells in a different study157. The rationale behind using different CAR signalling domains for the same scFv in Treg cells and Teff cells in the second article was not given. Intriguingly, Treg cells expressing only anti-HLA-A2 scFv migrated to and protected an HLA-A2+ skin graft in NSG mice to the same extent as CD28–CD3ζ HLA-A2 CAR Treg cells, possibly indicating the occurrence of bystander suppression via polyclonal endogenous TCR signalling160. Whether CAR-mediated signalling is always required for Treg cell function, whether there is an ideal signalling architecture for CAR Treg cells and whether such a signalling architecture is dependent on a specific scFv require further investigation.
Tonic signalling.
Low level of signalling independent of activating antigen in resting T cells.
Next-generation Treg cell engineering
Synthetic biology
Developing Treg cells as living drugs for autoimmune diseases need not be limited to use of TCRs and CARs. Synthetic immunology has produced a number of artificial receptors and systems that warrant testing in Treg cells. These systems include a T cell antigen coupler that recruits the endogenous TCR complex to a non-MHC target via a linked scFv177, CARs optimized to bind and be activated by soluble ligands178, and a split, universal and programmable (SUPRA) CAR system that fine-tunes the strength of T cell activation179. CAR variants may be indispensable in some of these systems. For instance, the SUPRA CAR system encompasses a receptor that recognizes multiple targets and is activated only if all of the targets are present. This feature is especially important when one is targeting tissue-specific antigens and solid tumours, as discovering a single antigen that is uniquely expressed in that tissue is highly unlikely. Another hurdle to optimal CAR function is tonic signalling and concomitant exhaustion. Synthetic Notch is a gene circuit in which binding to one target elicits translocation of an engineered Notch transcription factor into the nucleus, which in turn activates the transcription of a CAR receptor that recognizes a different antigen180. This system thus allows for greater specificity, by requiring two distinct antigens to elicit T cell activation, and prevents tonic signalling and undesired binding to CAR by only expressing CAR if the first antigen is recognized.
Rewiring cytokine signalling
Not surprisingly, cytokines play a key part in the outcome of an immune response. Treg cells constitutively express CD25, the high-affinity chain of the IL-2 receptor, effectively depriving Teff cells of IL-2. With this fact in mind, Treg cells have been used to deprive Teff cells of additional cytokines by expressing chimeric high-affinity cytokine receptors in engineered Treg cells. Use of receptors in which the extracellular domain of one cytokine receptor is fused to the intracellular domain of a different receptor prevents the corresponding proinflammatory cytokine from having biological efficacy. This strategy successfully converted IL-4 signalling, which limits T cell persistence and effector function in the tumour microenvironment, into IL-7 signalling, augmenting antitumour activity181. Converting proinflammatory cytokine signalling into IL-2 or IL-10 signalling in engineered Treg cells could increase the suppression of inflammation182. Indeed, designer cells that could sense TNF and IL-22, which in turn elicited secretion of IL-4 and IL-10, resolved local inflammation in a mouse model of psoriasis183. Ultimately, Treg cells could incorporate entire synthetic gene circuits that secrete anti-inflammatory cytokines in response to proinflammatory cytokines, effectively remodelling the cytokine milieu.
Genome editing
Genome editing, which uses engineered DNA endo-nucleases programmed to recognize and bind to a specific site in the genome, allows genomes to be precisely modified and holds promise in next-generation cell therapy. The past two decades have witnessed the development of several generations of engineered nucleases that offer great opportunities for gene therapy, including zinc-finger nucleases and transcription activator-like effector nucleases, although these technologies are laborious to implement. The field changed dramatically with the advent of CRISPR as a tool to edit the genome of human cells184. The CRISPR–Cas9 system includes the Cas9 nuclease, which induces double-stranded DNA breaks at specific locations in the genome; Cas9 is directed to target locations by a single guide RNA, which can be designed to interact with any 20-base-pair (bp) DNA sequence upstream of an NGG (where N is any nucleobase and G is guanine) protospacer-associated motif through Watson–Crick base pairing. These properties make CRISPR–Cas9 the cheapest, most efficient and most scalable genome editing technology to date.
Since the first demonstration that genes in primary human T cells could be edited with use of CRISPR–Cas9 (REF.185), tremendous progress has been made in our capacity to perform CRISPR-mediated gene modifications in human T cells; the achievements include biallelic gene knockout with efficiencies greater than 80%186, for example, of the TRAC locus, and the knock-in of multiple genes at precise genomic locations in primary human T cells138. This progress has been achieved in part by improved delivery methods; electroporation of Cas9 protein complexed with guide RNA (ribonucleoprotein (RNP)) is the delivery method of choice for T cells.
Several preclinical studies using CRISPR–Cas9 to disrupt genes in human T cells have been published. These include knocking out the gene encoding C-C chemokine receptor type 5 (CCR5) in CD4+ T cells, which generated T cells that were resistant to HIV infection185; knocking out the gene encoding CD7 in CD7 CAR T cells, which prevented fratricide, as T cells themselves express CD7 (REF.187); knocking out the gene encoding programmed cell death protein 1 (PD1) in CD19 CAR T cells, which improved tumour clearance in a humanized mouse model188; and a step towards the generation of ‘universal’ exhaustion-resistant CAR T cells by ablation of the genes encoding β2-microglobulin, TCR and PD1 (REF.189). Clinical trials using CRISPR technology in the United States started recently. The first CRISPR clinical trial in the United States, launched by CRISPR Therapeutics and Vertex, is treating patients with the blood disorder β-thalassaemia (NCT03655678) (see Related links). The first T cell cancer immunotherapy trial is being conducted by the University of Pennsylvania in partnership with Tmunity and the Parker Institute for Cancer Immunotherapy (NCT03399448). These studies have proceeded despite recent reports that CRISPR–Cas9 editing can provoke large genomic deletions and rearrangements away from the target site190. Efforts towards engineering high-fidelity versions of Cas9 and alternative CRISPR–Cas systems are under way. Of note, CRISPR-mediated editing is not limited to altering genomic sequence; new CRISPR–Cas systems and its variants have been engineered for targeted DNA methylation, gene activation and direct RNA editing191–193.
Improving delivery
Currently, the manufacture of CAR T cells uses retroviral and lentiviral transduction to deliver and integrate genetic material into T cells (FIG. 5). Lentiviruses, which are members of the retrovirus family, have a diploid RNA genome. Safe use of such viruses is achieved by splitting the three main coding regions of the genome — gag (encoding structural proteins), pol (encoding reverse transcriptases and integrases) and env (encoding virus envelopes) — into separate packaging plasmids that are combined only during the transfection process for viral production. This strategy allows the gene of interest, flanked by long terminal repeat sequences, to be integrated into the genome of T cells while prohibiting viral replication194. Despite progress in the use of lentiviruses to genetically modify T cells, this approach features several drawbacks, including the random integration of the gene of interest, a different number of gene copies per cell and the use of a constitutive and strong promoter to drive gene expression, resulting in non-physiological gene regulation. Indeed, CAR expression levels in cells transduced by lentiviruses differ between patients and are often supraphysiological, prompting CAR T cell exhaustion195. Inserting the CAR into the TRAC locus by means of CRISPR–Cas9 normalized CAR expression between patients, averted tonic CAR signalling and allowed the internalization and re-expression of the CAR on the cell surface upon CD19 recognition, mimicking the surface expression dynamics of physiological TCR195.
Fig. 5 |. Methods for delivering genetic material to engineer regulatory T cells.
a | The first FDA-approved method for delivering genetic material to engineer regulatory T cells uses retroviruses or lentiviruses, which are pseudodiploid single-stranded RNA viruses. Genomic integration of the gene cassette of interest is random, resulting in non-physiological gene regulation with often multiple copies of the gene per cell. The second FDA-approved method uses recombinant adenoassociated viruses (AAVs); these viruses persist inside the cell as a double-stranded DNA episome that is not integrated into the genome and will therefore be diluted over time in replicating cells. Many groups combine use of recombinant AAVs with use of CRISPR-based technologies, as the episome can serve as a homology repair (HR) template, an approach recently approved by the FDA for clinical trials. b | A third method, which is purely non-viral but not FDA approved yet, electroporates the desired cassette as double-stranded DNA or an ultramer (single-stranded DNA) template together with a Cas9–ribonucleoprotein (RNP) complex. Although this method leads to a single copy of the gene being precisely integrated at a desired location in the genome, it also typically results in lower modification efficiencies.
In the study that inserted the CAR into the TRAC locus195, recombinant AAV (rAAV) was used to deliver the CAR DNA homology repair template into the T cells (FIG. 5). AAVs are single-stranded linear DNA viruses that can be safely produced, similarly to lentiviruses, by splitting the main coding regions — rep (encoding replication proteins), cap (encoding capsid proteins) and aap (encoding assembly proteins) — into separate packaging plasmids during transfection for virus production. In contrast to lentiviruses, however, after transduction, the inverted terminal repeat-flanked transgene of the rAAV persists inside the cell as an episome rather than being integrated into the genome196 (FIG. 5). rAAV is therefore lost over time in replicating cells, making it ideal to deliver a homology repair template to introduce specific mutations or knock in a gene cassette of interest.
There are nine serotypes of rAAV (rAAV1–rAAV9), each of which has a different tropism. Currently, rAAV6 is the most efficient serotype for T cell transduction138,197. Several groups are working on bioengineering the rAAV capsid via multiplexed sequential directed evolution screens using capsid libraries with the goal of making rAAVs with novel cell specificities198,199. Thus, in the future, specific rAAVs could be made to selectively target distinct T cell (or even Treg cell) subtypes, vastly improving the precision and safety of gene editing for T cell therapy.
A non-viral approach to knock in DNA sequences more than 1 kilobase (kb) long at a specific genomic site in human T cells has also been reported138 (FIG. 5). Contrary to expectation, double-stranded DNA templates were not toxic when co-electroporated with CRISPR RNPs. This method corrected a pathogenic CD25 mutation in cells from patients with a monogenic autoimmune disease, inserted GFP downstream of several genes and inserted a transgenic TCR into the TRAC locus of Teff cells, demonstrating its potential138. This approach holds great promise, as manufacturing and testing clinical grade lentivirus or rAAV is time-consuming, expensive and plagued by safety issues. However, this approach is limited by the fact that editing efficiency decreases as the size of DNA templates increases, limiting the electroporated construct to an ~1.5-kb insert, which includes two homology arms of 300 bp long. CAR and TCR constructs are ~1.5 kb long and may therefore be too large to be efficiently integrated into DNA by this method; a GFP tag, by contrast, is only 750 bp long. Single-stranded rAAV6 templates have a packaging capacity of 4.7 kb, leaving up to 3.6 kb available for the gene construct after exclusion of the homology arms and inverted terminal repeat regions200. In conclusion, the methods used for gene editing differ depending on cell type, the insert size and the editing efficiency required. Furthermore, viral approaches can be combined with CRISPR RNP complexes to disrupt genes and/or to insert new cassettes, such as CAR or TCR transgenes.
Designer Treg cells
CRISPR–Cas9 genome editing could potentially knock in antigen receptors in precise genomic locations while simultaneously editing multiple genes that regulate Treg cell function (FIG. 2). As discussed earlier, one hurdle facing Treg cell therapy is ensuring the survival of the cells following infusion. Deleting the gene encoding JUN amino-terminal kinase 1 (JNK1) in murine Treg cells was shown to make them resistant to apoptosis, and JNK1-deficient Treg cells secreted higher levels of IL-10 and TGFβ and protected transplanted islets from rejection 100 days longer than their wild type counterparts201. Additionally, Treg cells can become unstable and transdifferentiate into pathogenic TH17 cells in inflammatory milieus4. PKCθ, the most abundant PKC isoform in T cells, is activated downstream of TCR and CD28, resulting in the induction of nuclear factor of activated T cells and nuclear factor-κB. Deletion of PRKCQ, which codes for PKCθ, or pharmacological inhibition of PKCθ reduced the propensity of Treg cells to differentiate to TH17 cells while preserving the suppressive function of Treg cells in mice and humans39,40. A complementary strategy could involve stabilizing high FOXP3 levels. Treg cells can have diminished levels, or even loss, of FOXP3 expression as a result of increased methylation of the TSDR locus over prolonged periods of in vitro culture202, or because of the CHIP-dependent ubiquitylation of FOXP3 in response to proinflammatory cytokines41. Indeed, genetically ablating STUB1 (the gene encoding CHIP) in murine Treg cells prevented FOXP3 degradation, whereas STUB1 overexpression abrogated Treg cell function in vitro and in vivo41. DBC1 also promotes FOXP3 destabilization by associating with FOXP3 to trigger its degradation in response to TNF by activating caspase 8. Accordingly, Treg cells in DBC1-deficient mice were present in larger numbers, and were more potent suppressors of EAE and colitis upon adoptive transfer, than Treg cells from wild-type mice42. Several other proteins interact with FOXP3 (REF.203), and the list continues to grow. Future mechanistic studies are likely to yield additional targets for modulating FOXP3 stability. Finally, an alternative approach would be to enforce the expression of factors shown to preserve Treg cell identity. One example is BACH2, a transcriptional repressor that reduces the differentiation of naive T cells into TH1, TH2 and TH17 cells38. Another example is STAT5-CA, a constitutively active form of signal transducer and activator of transcription 5, a key molecule that is activated downstream of IL-2 signalling and is essential for the suppressive function of Treg cells204.
As well as optimizing the suppressive properties of Treg cells, genome editing could be used to tailor Treg cells to different needs. In particular, there is growing evidence that Treg cells have an important role in tissue repair by producing amphiregulin and keratinocyte growth factor, amongst other molecules205,206. Including these and other factors in engineered Treg cells can further increase their therapeutic value. Finally, the recent generation of hypoimmunogenic human pluripotent stem cells via genome editing207,208, coupled with ongoing efforts to differentiate stem cells into Treg cells209, could revolutionize engineered Treg cell therapy.
Conclusion
Improper immune reactivity and inflammation underlie many currently incurable diseases. Using synthetic bio logy to fine-tune and augment the properties of immune cells will accelerate the implementation of curative cell-based therapies for autoimmunity and, ultimately, help achieve one of the most elusive goals in immunology: immune tolerance.
Currently, Treg cell manufacturing is still not ideal, largely due to the lack of tailored instruments and reagents. A process that combines MACS with FACS would allow the bulk processing and precision isolation of highly pure Treg cells as a starting material for manufacturing. The low proliferation rates of Treg cells in vitro are in sharp contrast to their highly proliferative state in vivo210–212, suggesting that poor proliferation is not an intrinsic characteristic of human Treg cells but likely a result of the suboptimal conditions currently used to expand Treg cells. Culture media, growth factors and stimulants that are suited for Treg cell biology and that can accommodate donor variability are yet to be developed. Moreover, current Treg cell manufacturing processes are costly and labour-intensive. Maximizing automation will not only decrease costs but will also improve reproducibility and lead to standardization.
It is the right time to design therapies using Treg cells. Recent decades have witnessed dramatic progress in our knowledge of basic Treg cell biology and autoimmune diseases, and also in our capacity to build artificial immune receptors and edit the genome of primary immune cells. However, many questions remain in the field of Treg cell biology and Treg cell therapy. For example, it will be important to identify markers that can distinguish lineage-committed thymic Treg cells from peripheral Treg cells and to determine whether certain Treg cell subsets are more suited to engineering for Treg cell therapy than others. Indeed, it is currently unclear whether the plasticity of Treg cells reflects the initial heterogeneity of a Treg cell population and whether only a subset of Treg cells (or all Treg cells) can become Teff cells. The origin of Treg cells that control local inflammation (that is, whether they are tissue resident or circulating) also needs to be determined. Moving into the clinic, a better understanding of how Treg cells maintain tissue integrity during homeostasis and in autoimmunity and organ transplantation, whether (and how) Treg cells change their identity in autoimmunity and whether Treg cells from patients with autoimmune disease are intrinsically defective, and thus unsuitable for therapeutic use, will also be critical.
To optimize Treg cell engineering, we must ascertain whether there is a minimal set of signalling pathways that, when incorporated into an artificial immune receptor such as a CAR, guarantees the survival, stability and suppressive function of engineered antigen-specific Treg cells. Advancing the field of engineered CAR Treg cell therapy also requires a better understanding of whether the inflamed tissue, the pathogenic T cells causing the immune response or the APCs activating the pathogenic T cells are the best target for engineered Treg cells, and whether the target molecule on these cells can be a soluble antigen instead of a surface molecule.
In the next decade, as some of these outstanding questions are addressed, we can expect to see continued improvements in the manufacture of Treg cells and an increased alliance with synthetic biology and genome editing to tailor Treg cell therapies to a growing number of conditions.
Acknowledgements
L.M.R.F. is the Jeffrey G. Klein Family Diabetes Fellow. Y.D.M. was supported by the Swiss National Science Foundation (Advanced Postdoctoral Mobility grant no. P300PB_174500). J.A.B. acknowledges support of the Sean N. Parker Autoimmune Laboratory. The authors apologize to all of the researchers whose work they could not cite owing to reference number limitations.
Competing interests
J.A.B. and Q.T. acknowledge support from the Juvenile Diabetes Research Foundation, Caladrius Biosciences, Becton, Dickinson and Company (BD), Juno Therapeutics and Pfizer. L.M.R.F. has patents and pending patents on genome editing of T cells, haematopoietic stem cells and pluripotent stem cells. J.A.B. and Q.T. have patents and pending patents on polyclonal and antigen-specific regulatory T cells and con- sult for Third Rock Ventures and Juno Therapeutics in this area. J.A.B. serves on the board of Pfizer. Y.D.M. has no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Caladrius Biosciences Treg cell phase ii trial: https://www.evaluate.com/vantage/articles/interviews/caladrius-refuses-give-tregs-diabetes
ClinicalTrials.gov: https://clinicaltrials.gov/
CRisPR Therapeutics press release (25 February 2019): http://ir.crisprtx.com/news-releases/news-release-details/crispr-therapeutics-and-vertex-announce-progress-clinical
IMGT: http://www.imgt.org
References
- 1.Gershon RK. & Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 18, 723–737 (1970). [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Sakaguchi N, Asano M, Itoh M & Toda M Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 (1995). [PubMed] [Google Scholar]
- 3.Husebye ES, Anderson MS & Kampe O Autoimmune polyendocrine syndromes. N. Engl. J. Med. 378, 1132–1141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komatsu N et al. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 20, 62–68 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Zhou X et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007 (2009).This study reveals that Treg cells can become unstable, losing FOXP3 expression and converting into pathogenic T cells (‘ex-Treg cells’) in vivo.
- 6.Abbas AK, Trotta E, D RS, Marson A & Bluestone JA Revisiting IL-2: biology and therapeutic prospects. Sci. Immunol. 3, eaat1482 (2018).A comprehensive review discussing the discovery, biology and therapeutic potential of the cytokine IL-2.
- 7.Fan MY et al. Differential roles of IL-2 signaling in developing versus mature Tregs. Cell Rep. 25, 1204–1213.e1204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 203, 1701–1711 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seddiki N et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203, 1693–1700 (2006).Together with Liu et al. (2006) this article describes the use of CD127 (IL-7 receptor subunit-α) as a surface marker, the exclusion of which dramatically enhances peripheral blood human Treg cell purity after FACS.
- 10.Ohkura N, Kitagawa Y & Sakaguchi S Development and maintenance of regulatory T cells. Immunity 38, 414–423 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Miller A, Lider O & Weiner HL Antigen-driven bystander suppression after oral administration of antigens. J. Exp. Med. 174, 791–798 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gershon RK & Kondo K Infectious immunological tolerance. Immunology 21, 903–914 (1971).Seminal experimental article establishing one of the basic tenets of dominant immune tolerance: infectious tolerance.
- 13.Qin S et al. “Infectious” transplantation tolerance. Science 259, 974–977 (1993). [DOI] [PubMed] [Google Scholar]
- 14.Esensten JH, Muller YD, Bluestone JA & Tang Q Regulatory T cell therapy for autoimmune and autoinflammatory diseases: the next frontier. J. Allergy Clin. Immunol. (2018). [DOI] [PubMed] [Google Scholar]
- 15.Koch MA et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10, 595–602 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duhen T, Duhen R, Lanzavecchia A, Sallusto F & Campbell DJ Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood 119, 4430–4440 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhry A et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326, 986–991 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng Y et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control TH2 responses. Nature 458, 351–356 (2009).Together with Koch et al. (2009) and Chaudhry et al. (2009) this article reveals that Treg cells can adopt the expression of master transcription factors of T helper cell subsets (TH1, TH17 and TH2) and this results in enhanced Treg cell migration and suppression specifically of the mimicked T helper cells.
- 19.Sharma A & Rudra D Emerging functions of regulatory T cells in tissue homeostasis. Front. Immunol. 9, 883 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine AG, Arvey A, Jin W & Rudensky AY Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 15, 1070–1078 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi T et al. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 10, 1969–1980 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Sprouse ML et al. Cutting edge: low-affinity TCRs support regulatory T cell function in autoimmunity. J. Immunol. 200, 909–914 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeh WI et al. Avidity and bystander suppressive capacity of human regulatory T cells expressing de novo autoreactive T-cell receptors in type 1 diabetes. Front. Immunol. 8, 1313 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plesa G et al. TCR affinity and specificity requirements for human regulatory T-cell function. Blood 119, 3420–3430 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Q & Bluestone JA The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat. Immunol. 9, 239–244 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y et al. Acquisition of suppressive function by activated human CD4+ CD25− T cells is associated with the expression of CTLA-4 not FoxP3. J. Immunol. 181, 1683–1691 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberle N, Eberhardt N, Falk CS, Krammer PH & Suri-Payer E Rapid suppression of cytokine transcription in human CD4+CD25 T cells by CD4+Foxp3+ regulatory T cells: independence of IL-2 consumption, TGF-beta, and various inhibitors of TCR signaling. J. Immunol. 179, 3578–3587 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Marek-Trzonkowska N et al. Administration of CD4+CD25highCD127-regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes Care 35, 1817–1820 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh K et al. Superiority of rapamycin over tacrolimus in preserving nonhuman primate Treg half-life and phenotype after adoptive transfer. Am. J. Transpl. 14, 2691–2703 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunstein CG et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 117, 1061–1070 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bluestone JA et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl Med. 7, 315ra189 (2015).First clinical trial demonstrating up to 1 year survival of infused Treg cells in patients with T1D.
- 32.Gratz IK et al. Cutting edge: memory regulatory T cells require IL-7 and not IL-2 for their maintenance in peripheral tissues. J. Immunol. 190, 4483–4487 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasanthakumar A et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 16, 276–285 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Bluestone JA FOXP3, the transcription factor at the heart of the rebirth of immune tolerance. J. Immunol. 198, 979–980 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Van Gool F et al. A mutation in the transcription factor Foxp3 drives T helper 2 effector function in regulatory T cells. Immunity 50, 362–377 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passerini L et al. CD4+ T cells from IPEX patients convert into functional and stable regulatory T cells by FOXP3 gene transfer. Sci. Transl Med. 5, 215ra174 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Takatori H et al. Helios enhances Treg cell function in cooperation with FoxP3. Arthritis Rheumatol. 67, 1491–1502 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Roychoudhuri R et al. BACH2 represses effector programs to stabilize Treg-mediated immune homeostasis. Nature 498, 506–510 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He X et al. Targeting PKC in human T cells using sotrastaurin (AEB071) preserves regulatory T cells and prevents IL-17 production. J. Invest. Dermatol. 134, 975–983 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Kwon MJ, Ma J, Ding Y, Wang R & Sun Z Protein kinase C-theta promotes Th17 differentiation via upregulation of Stat3. J. Immunol. 188, 5887–5897 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Z et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity 39, 272–285 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y et al. Inflammation negatively regulates FOXP3 and regulatory T-cell function via DBC1. Proc. Natl Acad. Sci. USA 112, E3246–E3254 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Q et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 199, 1455–1465 (2004).Seminal study showing that antigen-specific Treg cells can be expanded ex vivo and cure T1D in mice.
- 44.Masteller EL et al. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J. Immunol. 175, 3053–3059 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Hull CM et al. Generation of human islet-specific regulatory T cells by TCR gene transfer. J. Autoimmun. 79, 63–73 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Kim YC et al. Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses. Blood 125, 1107–1115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brusko TM et al. Human antigen-specific regulatory T cells generated by T cell receptor gene transfer. PLOS ONE 5, e11726 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacDonald KG et al. Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J. Clin. Invest. 126, 1413–1424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mekala DJ & Geiger TL Immunotherapy of autoimmune encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Blood 105, 2090–2092 (2005).First study redirecting Treg cells using a chimeric receptor, designed to recognize autoreactive T cells in EAE.
- 50.Fuchs A et al. Minimum information about T regulatory cells: a step toward reproducibility and standardization. Front. Immunol. 8, 1844 (2017). This article provides guidelines on the minimum information required about Treg cells for therapy, in an effort to help standardize Treg cell manufacture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunstein CG et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 127, 1044–1051 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seay HR et al. Expansion of human Tregs from cryopreserved umbilical cord blood for GMP-compliant autologous adoptive cell transfer therapy. Mol. Ther. Methods Clin. Dev. 4, 178–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dijke IE et al. Discarded human thymus is a novel source of stable and long-lived therapeutic regulatory T cells. Am. J. Transpl. 16, 58–71 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Miyara M et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 (2009). [DOI] [PubMed] [Google Scholar]
- 55.Cuadrado E et al. Proteomic analyses of human regulatory T cells reveal adaptations in signaling pathways that protect cellular identity. Immunity 48, 1046–1059 (2018).A large study using transcriptomic and proteomic analyses of human Treg cells and Teff cells to derive a signature that uniquely identifies Treg cells that found that FOXP3 is the only molecule enriched in Treg cells at both the transcript level and the protein level.
- 56.Miragaia RJ et al. Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity 50, 493–504.e497 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang Q & Lee K Regulatory T-cell therapy for transplantation: how many cells do we need? Curr. Opin. Organ. Transpl. 17, 349–354 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Putnam AL et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 58, 652–662 (2009).This article describes the good manufacturing practice manufacture of Treg cells isolated from patients with T1D for Treg cell therapy.
- 59.Trzonkowski P et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127-T regulatory cells. Clin. Immunol. 133, 22–26 (2009). [DOI] [PubMed] [Google Scholar]
- 60.Mathew JM et al. A phase I clinical trial with ex vivo expanded recipient regulatory T cells in living donor kidney transplants. Sci. Rep. 8, 7428 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coenen JJ, Koenen HJ, van Rijssen E, Hilbrands LB & Joosten I Rapamycin, and not cyclosporin a, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells.Blood 107, 1018–1023 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Haxhinasto S, Mathis D & Benoist C The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205, 565–574 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Battaglia M, Stabilini A & Roncarolo MG Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105, 4743–4748 (2005). [DOI] [PubMed] [Google Scholar]
- 64.Lee K, Nguyen V, Lee KM, Kang SM & Tang Q Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am. J. Transpl. 14, 27–38 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Putnam AL et al. Clinical grade manufacturing of human alloantigen-reactive regulatory T cells for use in transplantation. Am. J. Transpl. 13, 3010–3020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM & Dhodapkar KM Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood 108, 2655–2661 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Golovina TN et al. CD28 costimulation is essential for human T regulatory expansion and function. J. Immunol. 181, 2855–2868 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Afzali B et al. Comparison of regulatory T cells in hemodialysis patients and healthy controls: implications for cell therapy in transplantation. Clin. J. Am. Soc. Nephrol. 8, 1396–1405 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Canavan JB et al. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut 65, 584–594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoffmann P et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood 108, 4260–4267 (2006). [DOI] [PubMed] [Google Scholar]
- 71.Smigiel KS et al. CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J. Exp. Med. 211, 121–136 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santner-Nanan B et al. Accelerated age-dependent transition of human regulatory T cells to effector memory phenotype. Int. Immunol. 20, 375–383 (2008). [DOI] [PubMed] [Google Scholar]
- 73.Miyao T et al. Plasticity of Foxp3+ T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 36, 262–275 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Ohkura N et al. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Sehouli J et al. Epigenetic quantification of tumor-infiltrating T-lymphocytes. Epigenetics 6, 236–246 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen JL, Trenado A, Vasey D, Klatzmann D & Salomon BL CD4+CD25+ immunoregulatory T cells: new therapeutics for graft-versus-host disease. J. Exp. Med. 196, 401–406 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Edinger M et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat. Med. 9, 1144–1150 (2003). [DOI] [PubMed] [Google Scholar]
- 78.Martelli MF et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood 124, 638–644 (2014). [DOI] [PubMed] [Google Scholar]
- 79.Theil A et al. Adoptive transfer of allogeneic regulatory T cells into patients with chronic graft-versus-host disease. Cytotherapy 17, 473–486 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Di Ianni M et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 117, 3921–3928 (2011). [DOI] [PubMed] [Google Scholar]
- 81.Joffre O et al. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat. Med. 14, 88–92 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang Q & Bluestone JA Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb. Perspect. Med. 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Safinia N et al. Cell therapy in organ transplantation: our experience on the clinical translation of regulatory T cells. Front. Immunol. 9, 354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chandran S et al. Polyclonal regulatory T cell therapy for control of inflammation in kidney transplants. Am. J. Transpl. 17, 2945–2954 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang Q et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 28, 687–697 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.D’Alise AM et al. The defect in T-cell regulation in NOD mice is an effect on the T-cell effectors. Proc. Natl Acad. Sci. USA 105, 19857–19862 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dall’Era M et al. Adoptive Treg cell therapy in a patient with systemic lupus erythematosus. Arthritis Rheumatol. 71, 431–440 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thonhoff JR et al. Expanded autologous regulatory T-lymphocyte infusions in ALS: A phase I, first-in-human study. Neurol. Neuroimmunol. Neuroinflamm. 5, e465 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sadlack B et al. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur. J. Immunol. 25, 3053–3059 (1995). [DOI] [PubMed] [Google Scholar]
- 90.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N & Lafaille JJ Interleukin 2 signaling is required for CD4+ regulatory T cell function. J. Exp. Med. 196, 851–857 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang Q Therapeutic window of interleukin-2 for autoimmune diseases. Diabetes 64, 1912–1913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saadoun D et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N. Engl. J. Med. 365, 2067–2077 (2011). [DOI] [PubMed] [Google Scholar]
- 93.Koreth J et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N. Engl. J. Med. 365, 2055–2066 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He J et al. Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic lupus erythematosus. Nat. Med. 22, 991–993 (2016). [DOI] [PubMed] [Google Scholar]
- 95.Todd JA et al. Regulatory T cell responses in participants with type 1 diabetes after a single dose of interleukin-2: a non-randomised, open label, adaptive dose-finding trial. PLOS Med. 13, e1002139 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenzwajg M et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J. Autoimmun. 58, 48–58 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hartemann A et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 1, 295–305 (2013). [DOI] [PubMed] [Google Scholar]
- 98.Trotta E et al. A human anti-IL-2 antibody that potentiates regulatory T cells by a structure-based mechanism. Nat. Med. 24, 1005–1014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sockolosk JT. et al. Selective targeting of engineered T cells using orthogonal IL-2 cytokine-receptor complexes. Science 359, 1037–1042 (2018).This study reports the generation of an orthogonal IL-2–IL-2 receptor pair that could be used to trigger IL-2 signalling specifically in infused engineered Treg cells.
- 100.Furukawa A, Wisel SA & Tang Q Impact of immune-modulatory drugs on regulatory T cell. Transplantation 100, 2288–2300 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang Q & Vincenti F Transplant trials with Tregs: perils and promises. J. Clin. Invest. 127, 2505–2512 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Landwehr-Kenzel S et al. Ex vivo expanded natural regulatory T cells from patients with end-stage renal disease or kidney transplantation are useful for autologous cell therapy. Kidney Int. 93, 1452–1464 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Green EA, Choi Y & Flavell RA Pancreatic lymph node-derived CD4+CD25+ Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity 16, 183–191 (2002).This article demonstrates that small numbers (2,000 cells) of pancreatic antigen-specific Treg cells exposed to TNF receptor signalling in vivo can prevent autoimmune diabetes in mice.
- 104.Huang J et al. A single peptide-major histocompatibility complex ligand triggers digital cytokine secretion in CD4+ T cells. Immunity 39, 846–857 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bluhm J et al. CAR T Cells with enhanced sensitivity to B cell maturation antigen for the targeting of B cell non-Hodgkin’s lymphoma and multiple myeloma. Mol. Ther. 26, 1906–1920 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Elhanati Y, Sethna Z, Callan CG Jr., Mora T & Walczak AM Predicting the spectrum of TCR repertoire sharing with a data-driven model of recombination. Immunol. Rev. 284, 167–179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klein L, Kyewski B, Allen PM & Hogquist KA Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat. Rev. Immunol. 14, 377–391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jordan MS et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 301–306 (2001). [DOI] [PubMed] [Google Scholar]
- 109.Malchow S et al. Aire enforces immune tolerance by directing autoreactive T cells into the regulatory T cell lineage. Immunity 44, 1102–1113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Anderson MS et al. Projection of an immunological self shadow within the thymus by the Aire protein. Science 298, 1395–1401 (2002). [DOI] [PubMed] [Google Scholar]
- 111.Hsieh CS, Zheng Y, Liang Y, Fontenot JD & Rudensky AY An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7, 401–410 (2006). [DOI] [PubMed] [Google Scholar]
- 112.Leonard JD et al. Identification of natural regulatory T cell epitopes reveals convergence on a dominant autoantigen. Immunity 47, 107–117 e108 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang M et al. Cutting edge: processing of oxidized peptides in macrophages regulates T cell activation and development of autoimmune arthritis. J. Immunol. 199, 3937–3942 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Moss CX, Matthews SP, Lamont DJ & Watts C Asparagine deamidation perturbs antigen presentation on class II major histocompatibility complex molecules. J. Biol. Chem. 280, 18498–18503 (2005). [DOI] [PubMed] [Google Scholar]
- 115.Valesini G et al. Citrullination and autoimmunity. Autoimmun. Rev. 14, 490–497 (2015). [DOI] [PubMed] [Google Scholar]
- 116.Patterson H, Nibbs R, McInnes I & Siebert S Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin. Exp. Immunol. 176, 1–10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Delong T et al. Diabetogenic T-cell clones recognize an altered peptide of chromogranin A. Diabetes 61, 3239–3246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McGinty JW, Marre ML, Bajzik V, Piganelli JD & James EA T cell epitopes and post-translationally modified epitopes in type 1 diabetes. Curr. Diab. Rep. 15, 90 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jin N et al. N-terminal additions to the WE14 peptide of chromogranin A create strong autoantigen agonists in type 1 diabetes. Proc. Natl Acad. Sci. USA 112, 13318–13323 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Delong T et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351, 711–714 (2016).This article shows that some diabetogenic T cells recognize epitopes formed by fusion of proinsulin peptides to other peptides that are found in the secretory vesicles of beta cells.
- 121.Liu Z et al. Immune homeostasis enforced by co-localized effector and regulatory T cells. Nature 528, 225–230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moran AE et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spence A et al. Revealing the specificity of regulatory T cells in murine autoimmune diabetes. Proc. Natl Acad. Sci. USA 115, 5265–5270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wei X et al. Reciprocal expression of IL-35 and IL-10 defines two distinct effector Treg subsets that are required for maintenance of immune tolerance. Cell Rep. 21, 1853–1869 (2017). [DOI] [PubMed] [Google Scholar]
- 125.Seay HR et al. Tissue distribution and clonal diversity of the T and B cell repertoire in type 1 diabetes. JCI Insight 1, e88242 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Howie B et al. High-throughput pairing of T cell receptor alpha and beta sequences. Sci. Transl Med. 7, 301ra131 (2015). [DOI] [PubMed] [Google Scholar]
- 127.Glanville J et al. Identifying specificity groups in the T cell receptor repertoire. Nature 547, 94–98 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dash P et al. Quantifiable predictive features define epitope-specific T cell receptor repertoires. Nature 547, 89–93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dembic Z et al. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature 320, 232–238 (1986). [DOI] [PubMed] [Google Scholar]
- 130.Tsang JY et al. Conferring indirect allospecificity on CD4+CD25+ Tregs by TCR gene transfer favors transplantation tolerance in mice. J. Clin. Invest. 118, 3619–3628 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wright GP et al. Adoptive therapy with redirected primary regulatory T cells results in antigen-specific suppression of arthritis. Proc. Natl Acad. Sci. USA 106, 19078–19083 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cohen CJ et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–3903 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kuball J et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 109, 2331–2338 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sommermeyer D & Uckert W Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J. Immunol. 184, 6223–6231 (2010). [DOI] [PubMed] [Google Scholar]
- 135.Bialer G, Horovitz-Fried M, Ya’acobi S, Morgan RA & Cohen CJ Selected murine residues endow human TCR with enhanced tumor recognition. J. Immunol. 184, 6232–6241 (2010). [DOI] [PubMed] [Google Scholar]
- 136.Uckert W & Schumacher TN TCR transgenes and transgene cassettes for TCR gene therapy: status in 2008. Cancer Immunol. Immunother. 58, 809–822 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Torikai H et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19- specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119, 5697–5705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roth TL et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Govers C et al. TCRs genetically linked to CD28 and CD3epsilon do not mispair with endogenous TCR chains and mediate enhanced T cell persistence and anti-melanoma activity. J. Immunol. 193, 5315–5326 (2014). [DOI] [PubMed] [Google Scholar]
- 140.Walseng E et al. A TCR-based chimeric antigen receptor. Sci. Rep. 7, 10713 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Elahi R, Khosh E, Tahmasebi S & Esmaeilzadeh A Immune cell hacking: challenges and clinical approaches to create smarter generations of chimeric antigen receptor T cells. Front. Immunol. 9, 1717 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maher J, Brentjens RJ, Gunset G, Riviere I & Sadelain M Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta/ CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002). [DOI] [PubMed] [Google Scholar]
- 143.Deeks SG et al. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol. Ther. 5, 788–797 (2002). [DOI] [PubMed] [Google Scholar]
- 144.Walker RE et al. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood 96, 467–474 (2000). [PubMed] [Google Scholar]
- 145.Leibman RS et al. Supraphysiologic control over HIV-1 replication mediated by CD8 T cells expressing a re-engineered CD4-based chimeric antigen receptor. PLOS Pathog. 13, e1006613 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Irving BA & Weiss A The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64, 891–901 (1991).Seminal study reporting the first CAR.
- 147.Long AH et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Carpenito C et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl Acad. Sci. USA 106, 3360–3365 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hombach AA, Heiders J, Foppe M, Chmielewski M & Abken H OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4+ T cells. Oncoimmunology 1, 458–466 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mekala DJ, Alli RS & Geiger TL IL-10-dependent infectious tolerance after the treatment of experimental allergic encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Proc. Natl Acad. Sci. USA 102, 11817–11822 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elinav E, Adam N, Waks T & Eshhar Z Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology 136, 1721–1731 (2009). [DOI] [PubMed] [Google Scholar]
- 152.Elinav E, Waks T & Eshhar Z Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology 134, 2014–2024 (2008). [DOI] [PubMed] [Google Scholar]
- 153.Blat D, Zigmond E, Alteber Z, Waks T & Eshhar Z Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol. Ther. 22, 1018–1028 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Skuljec J et al. Chimeric antigen receptor-redirected regulatory T cells suppress experimental allergic airway inflammation, a model of asthma. Front. Immunol. 8, 1125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Smithson JE, Warren BF, Young S, Pigott R & Jewell DP Heterogeneous expression of carcinoembryonic antigen in the normal colon and upregulation in active ulcerative colitis. J. Pathol. 180, 146–151 (1996). [DOI] [PubMed] [Google Scholar]
- 156.Hombach AA, Kofler D, Rappl G & Abken H Redirecting human CD4+CD25+ regulatory T cells from the peripheral blood with pre-defined target specificity. Gene Ther. 16, 1088–1096 (2009). [DOI] [PubMed] [Google Scholar]
- 157.Lee JC et al. In vivo inhibition of human CD19- targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 71, 2871–2881 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ferreira LM & Tang Q Generating antigen-specific regulatory T cells in the fast lane. Am. J. Transpl. 17, 851–853 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Noyan F et al. Prevention of allograft rejection by use of regulatory T cells with an MHC-specific chimeric antigen receptor. Am. J. Transpl. 17, 917–930 (2017). [DOI] [PubMed] [Google Scholar]
- 160.Boardman DA et al. Expression of a chimeric antigen receptor specific for donor HLA Class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am. J. Transpl. 17, 931–943 (2017). [DOI] [PubMed] [Google Scholar]
- 161.Semple K, Yu Y, Wang D, Anasetti C & Yu XZ Efficient and selective prevention of GVHD by antigen- specific induced Tregs via linked-suppression in mice. Biol. Blood Marrow Transpl. 17, 309–318 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dawson NA et al. Systematic testing and specificity mapping of alloantigen-specific chimeric antigen receptors in regulatory T cells. JCI Insight 4, 123672 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Peyvandi F, Garagiola I & Young G The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet 388, 187–197 (2016). [DOI] [PubMed] [Google Scholar]
- 164.Yoon J et al. FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood 129, 238–245 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang AH, Yoon J, Kim YC & Scott DW Targeting antigen-specific B cells using antigen- expressing transduced regulatory T cells. J. Immunol. 201, 1434–1441 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mathsson L et al. Antibodies against citrullinated vimentin in rheumatoid arthritis: higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 58, 36–45 (2008). [DOI] [PubMed] [Google Scholar]
- 167.Raffin C et al. Development of citrullinated-vimentin- specific CAR for targeting Tregs to treat autoimmune rheumatoid arthritis. J. Immunol. 196, 210.19 (2016). [Google Scholar]
- 168.Khandpur R et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl Med. 5, 178ra140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Loskog A. et al. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia 20, 1819–1828 (2006). [DOI] [PubMed] [Google Scholar]
- 170.Kofler DM et al. CD28 costimulation impairs the efficacy of a redirected T-cell antitumor attack in the presence of regulatory T cells which can be overcome by preventing Lck activation. Mol. Ther. 19, 760–767 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.van der Stegen SJ, Hamieh M & Sadelain M The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 14, 499–509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nowak A et al. CD137+CD154- expression as a regulatory T cell (Treg)-specific activation signature for identification and sorting of stable human Tregs from in vitro expansion cultures. Front. Immunol. 9, 199 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Koristka S et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J. Autoimmun. 90, 116–131 (2018). [DOI] [PubMed] [Google Scholar]
- 174.Boroughs AC et al. Chimeric antigen receptor costimulation domains modulate human regulatory T cell function. JCI Insight 5, 126194 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ferreira LM et al. Tailoring a new generation of chimeric antigen receptors for regulatory T cells. J. Immunol. 200, 176.115 (2018). [Google Scholar]
- 176.Hudecek M et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 19, 3153–3164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Helsen CW et al. The chimeric TAC receptor co-opts the T cell receptor yielding robust anti-tumor activity without toxicity. Nat. Commun. 9, 3049 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chang ZL et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat. Chem. Biol. 14, 317–324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cho JH, Collins JJ & Wong WW Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173, 1426–1438 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Roybal KT et al. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 167, 419–432.e416, (2016).This article describes the development of synthetic Notch signalling to control gene circuits in human T cells.
- 181.Leen AM et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 22, 1211–1220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chaudhry A et al. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34, 566–578 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schukur L, Geering B, Charpin-El Hamri G & Fussenegger M Implantable synthetic cytokine converter cells with AND-gate logic treat experimental psoriasis. Sci. Transl Med. 7, 318ra201 (2015). [DOI] [PubMed] [Google Scholar]
- 184.Hsu PD, Lander ES & Zhang F Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mandal PK et al. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell 15, 643–652 (2014).First report of CRISPR–Cas9 genome editing in human T cells.
- 186.Schumann K et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl Acad. Sci. USA 112, 10437–10442 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gomes-Silva D et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 130, 285–296 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Rupp LJ et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci. Rep. 7, 737 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu X et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res. 27, 154–157 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kosicki M, Tomberg K & Bradley A Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 36, 765–771 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Konermann S et al. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 173, 665–676 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu XS et al. Editing DNA methylation in the mammalian genome. Cell 167, 233–247.e217, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Matharu N et al. CRISPR-mediated activation of a promoter or enhancer rescues obesity caused by haploinsufficiency. Science 363, eaau0629 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Milone MC & O’Doherty U Clinical use of lentiviral vectors. Leukemia 32, 1529–1541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Eyquem J et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).This study shows that knocking the CAR gene cassette into the endogenous TCR locus in T cells leads to CAR T cells with physiologically regulated CAR expression, less variability in CAR expression levels across patients and reduced exhaustion upon repeated antigen stimulation.
- 196.Naso MF, Tomkowicz B, Perry WL 3rd & Strohl WR Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 31, 317–334 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang J et al. Highly efficient homology-driven genome editing in human T cells by combining zinc-finger nuclease mRNA and AAV6 donor delivery. Nucleic Acids Res. 44, e30 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Paulk NK et al. Bioengineered AAV capsids with combined high human liver transduction in vivo and unique humoral seroreactivity. Mol. Ther. 26, 289–303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Deverman BE, Ravina BM, Bankiewicz KS, Paul SM & Sah DWY Gene therapy for neurological disorders: progress and prospects. Nat. Rev. Drug Discov. 17, 641–659 (2018). [DOI] [PubMed] [Google Scholar]
- 200.Bak RO, Dever DP & Porteus MH CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat. Protoc. 13, 358–376 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tripathi D et al. c-Jun N-terminal kinase 1 defective CD4+CD25+FoxP3+ cells prolong islet allograft survival in diabetic mice. Sci. Rep. 8, 3310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hoffmann P et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur. J. Immunol. 39, 1088–1097 (2009). [DOI] [PubMed] [Google Scholar]
- 203.Rudra D et al. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat. Immunol. 13, 1010–1019 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chinen T et al. An essential role for the IL-2 receptor in Treg cell function. Nat. Immunol. 17, 1322–1333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang C et al. ‘Repair’ Treg cells in tissue injury. Cell Physiol. Biochem. 43, 2155–2169 (2017). [DOI] [PubMed] [Google Scholar]
- 206.Arpaia N et al. A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Deuse T et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Han X et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl Acad. Sci. USA 116, 10441–10446 (2019).This article describes the development of gene-edited hypoimmunogenic human pluripotent stem cells, which could in the future be used as an inexhaustible source for universally compatible Treg cells.
- 209.Haque R et al. Programming of regulatory T cells from pluripotent stem cells and prevention of autoimmunity. J. Immunol. 189, 1228–1236 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Booth NJ et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J. Immunol. 184, 4317–4326 (2010). [DOI] [PubMed] [Google Scholar]
- 211.Gavin MA, Clarke SR, Negrou E, Gallegos A & Rudensky A Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat. Immunol. 3, 33–41 (2002). [DOI] [PubMed] [Google Scholar]
- 212.Walker LS, Chodos A, Eggena M, Dooms H & Abbas AK Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J. Exp. Med. 198, 249–258 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gregori S & Roncarolo MG Engineered t regulatory type 1 cells for clinical application. Front. Immunol. 9, 233 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gagliani N et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19, 739–746 (2013). [DOI] [PubMed] [Google Scholar]
- 215.Barrat FJ et al. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 195, 603–616 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gregori S, Roncarolo MG & Bacchetta R Methods for in vitro generation of human type 1 regulatory T cells. Methods Mol Biol 677, 31–46 (2011). [DOI] [PubMed] [Google Scholar]
- 217.Desreumaux P et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology 143, 1207–1217.e1202 (2012). [DOI] [PubMed] [Google Scholar]
- 218.Bacchetta R et al. Immunological outcome in haploidentical-HSC transplanted patients treated with IL-10-anergized donor T cells. Front. Immunol. 5, 16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gershon RK, Cohen P, Hencin R & Liebhaber SA Suppressor T cells. J. Immunol. 108, 586–590 (1972). [PubMed] [Google Scholar]
- 220.Cantor H, Shen FW & Boyse EA Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J. Exp. Med. 143, 1391–1340 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bezie S et al. Ex vivo expanded human non-cytotoxic CD8+CD45RClow/- Tregs efficiently delay skin graft rejection and GVHD in humanized mice. Front. Immunol. 8, 2014 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ablamunits V, Bisikirska B & Herold KC Acquisition of regulatory function by human CD8+ T cells treated with anti-CD3 antibody requires TNF. Eur J. Immunol. 40, 2891–2901 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim HJ et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 350, 334–339 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Poussier P, Ning T, Banerjee D & Julius M A unique subset of self-specific intraintestinal T cells maintains gut integrity. J. Exp. Med. 195, 1491–1497 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tang X et al. Regulation of immunity by a novel population of Qa-1-restricted CD8alphaalpha+ TCRalphabeta+ T cells. J. Immunol. 177, 7645–7655 (2006). [DOI] [PubMed] [Google Scholar]
- 226.Chen Y, Kuchroo VK, Inobe J, Hafler DA & Weiner HL Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science 265, 1237–1240 (1994). [DOI] [PubMed] [Google Scholar]
- 227.Carrier Y, Yuan J, Kuchroo VK & Weiner HL Th3 cells in peripheral tolerance. I. Induction of Foxp3- positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J. Immunol. 178, 179–185 (2007). [DOI] [PubMed] [Google Scholar]
- 228.Rosser EC & Mauri C Regulatory B cells: origin, phenotype, and function. Immunity 42, 607–612 (2015). [DOI] [PubMed] [Google Scholar]
- 229.Katz SI, Parker D & Turk JL B-cell suppression of delayed hypersensitivity reactions. Nature 251, 550–551 (1974). [DOI] [PubMed] [Google Scholar]
- 230.Wolf SD, Dittel BN, Hardardottir F & Janeway CA Jr. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J. Exp. Med. 184, 2271–2278 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D & Anderton SM B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3, 944–950 (2002). [DOI] [PubMed] [Google Scholar]
- 232.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS & Bhan AK Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16, 219–230 (2002). [DOI] [PubMed] [Google Scholar]
- 233.Nathan C & Ding A Nonresolving inflammation. Cell 140, 871–882 (2010). [DOI] [PubMed] [Google Scholar]
- 234.Carter NA et al. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J. Immunol. 186, 5569–5579 (2011). [DOI] [PubMed] [Google Scholar]
- 235.Flores-Borja F et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci. Transl Med. 5, 173ra123 (2013). [DOI] [PubMed] [Google Scholar]
- 236.Ray A, Basu S, Williams CB, Salzman NH & Dittel BN A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J. Immunol. 188, 3188–3198 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lee KM et al. TGF-beta-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J. Immunol. 44, 1728–1736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kuwana Y et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 149, 960–968 (1987). [DOI] [PubMed] [Google Scholar]
- 239.Gross G, Waks T & Eshhar Z Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10024–10028 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Thornton AM & Shevach EM CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 188, 287–296 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Levings MK, Sangregorio R & Roncarolo MG Human Cd25+Cd4+ T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J. Exp. Med. 193, 1295–1302 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jonuleit H. et al. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J. Exp. Med. 193, 1285–1294 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dieckmann D, Plottner H, Berchtold S, Berger T & Schuler G Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 193, 1303–1310 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ng WF et al. Human CD4+CD25+ cells: a naturally occurring population of regulatory T cells. Blood 98, 2736–2744 (2001). [DOI] [PubMed] [Google Scholar]
- 245.Stephens LA, Mottet C, Mason D & Powrie F Human CD4+CD25+ thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur. J. Immunol. 31, 1247–1254 (2001). [DOI] [PubMed] [Google Scholar]
- 246.Baecher-Allan C, Brown JA, Freeman GJ & Hafler DA CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167, 1245–1253 (2001).Together with Levings et al. (2001), Jonuleit et al. (2001), Dieckmann et al. (2001), Ng et al. (2001) and Stephens et al. (2001), this article is a seminal study, the first to describe Treg cells in humans.
- 247.Khattri R, Cox T, Yasayko SA & Ramsdell F An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 4, 337–342 (2003). [DOI] [PubMed] [Google Scholar]
- 248.Hori S, Nomura T & Sakaguchi S Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003). [DOI] [PubMed] [Google Scholar]
- 249.Fontenot JD, Gavin MA & Rudensky AY Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4, 330–336 (2003). [DOI] [PubMed] [Google Scholar]
- 250.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R & Edinger M Large-scale in vitro expansion of polyclonal human CD4+CD25high regulatory T cells. Blood 104, 895–903 (2004). [DOI] [PubMed] [Google Scholar]
- 251.Kochenderfer JN et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Stone JD, Chervin AS & Kranz DM T-cell receptor binding affinities and kinetics: impact on T-cell activity and specificity. Immunology 126, 165–176 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Liu X et al. Affinity-tuned ErbB2 or EGFR chimeric antigen receptor T cells exhibit an increased therapeutic index against tumors in mice. Cancer Res. 75, 3596–3607 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Irvine DJ, Purbhoo MA, Krogsgaard M & Davis MM Direct observation of ligand recognition by T cells. Nature 419, 845–849 (2002). [DOI] [PubMed] [Google Scholar]
- 255.Watanabe K et al. Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. J. Immunol. 194, 911–920 (2015). [DOI] [PubMed] [Google Scholar]
- 256.Stone JD, Aggen DH, Schietinger A, Schreiber H & Kranz DM A sensitivity scale for targeting T cells with chimeric antigen receptors (CARs) and bispecific T-cell engagers (BiTEs). Oncoimmunology 1, 863–873 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Walker AJ et al. Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Mol. Ther. 25, 2189–2201 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]