Abstract
Introduction
Sub-Saharan Africa has the highest global incidence of cervical cancer. Cervical cancer is the most common cause of cancer morbidity and mortality among women in Zambia. HIV increases the risk for cervical cancer and with a national Zambian adult HIV prevalence of 16%, it is important to investigate the impact of HIV on the progression of cervical cancer. We measured differences in cervical cancer progression between HIV-positive and HIV-negative patients in Zambia.
Methods
This study included 577 stage I and II cervical cancer patients seen between January 2008 and December 2012 at the Cancer Diseases Hospital in Lusaka, Zambia. The inclusion criteria for records during the study period included known HIV status and FIGO stage I and II cervical cancer at initial date of registration in the Cancer Diseases Hospital. Medical records were abstracted for clinical and epidemiological data. Cancer databases were linked to the national HIV database to assess HIV status among cervical cancer patients. Logistic regression examined the association between HIV and progression, which was defined as metastatic or residual tumor after 3 months of initial treatment.
Results
A total of 2451 cervical cancer cases were identified, and after exclusion criteria were performed the final analysis population totaled 537 patients with stage I and II cervical cancer with known HIV status (224 HIV-positive and 313 HIV-negative). HIV-positive women were, on average, 10 years younger than HIV-negative women who had a median age of 42, ranging between 25 and 72. A total of 416 (77.5%) patients received external beam radiation, and only 249 (46.4%) patients received the recommended treatment of chemotherapy, external beam radiation, and brachytherapy. Most patients were stage II (85.7%) and had squamous cell carcinoma (74.7%). HIV-positive patients were more likely to receive lower doses of external beam radiation than HIV-negative patients (47% vs 37%; P<0.05, respectively). The median total dose of external beam radiation for HIV-positive and HIV-negative patients was 46 Gy and 50 Gy, respectively. HIV positivity did not lead to tumor progression (25.4% in HIV-positive vs 23.9% in HIV-negative, OR 1.04, 95% CI [0.57, 1.92]). However, among a subset of HIV-positive patients, longer duration of infection was associated with lower odds of progression.
Conclusion
There was no significant impact on non-metastatic cervical cancer progression by HIV status among patients in Lusaka, Zambia. The high prevalence of HIV among cervical cancer patients suggest that HIV-positive patients should be a primary target group for HPV vaccinations, screening, and early detection.
BACKGROUND
Cervical cancer is the fourth most common malignancy among women worldwide, with the highest incidence and mortality occurring in Sub-Saharan Africa.1 This is a major public health challenge in Zambia, where the cervical cancer annual incidence rate is estimated to be between 33.7 and 58.4 per 100 000 with a mortality rate between 20.7 and 36.2 per 100,000.2–4 While the primary cause for cervical cancer is human papillomavirus (HPV), co-infection with HIV appears to increase the likelihood of progression to cancer.5 Women with HIV who are exposed to HPV are less likely to clear the virus and more likely to develop cervical cancer.6 Zambia has a national adult HIV prevalence of 16%, with an estimated 20.8% prevalence for women 15–49.2 Furthermore, the prevalence of high- risk HPV oncogenic types 16 and 18 each is 21.6% in Zambia.7
The Cancer Diseases Hospital is a specialized hospital for cancer treatment that opened in Lusaka, Zambia in late 2006 and now provides radiotherapy and chemotherapy to cancer patients.2 Due to the national lack of screening and early detection services they report a large number of advanced stage III and IV cervical cancer.8 Also, in 2006 the Center for Infectious Disease Research in Zambia began the first cervical cancer program that has now expanded throughout the country with 49 screening and treatment sites.4 The Center for Infectious Disease Research in Zambia screening sites have since screened more than 275 000 women and have expanded their services to include HIV testing as well.4 In Zambia, HIV-positive patients can receive antiretroviral therapy at the University Teaching Hospital.
Antiretroviral therapy has increased life expectancy among HIV-positive patients.6 This may increase opportunities for HIV-positive women to become coinfected with HPV or develop cervical cancer. The use of antiretroviral therapy among HIV-positive patients is of importance because chemotherapy and antiretroviral therapy drugs are metabolized by a similar cytochrome p450 enzyme pathway.9 This may affect the clearance of chemotherapy drugs, leading to increased toxicity or ineffectiveness.9 Although research has been conducted on acute toxicity of treatment by HIV status, there is little research regarding cervical cancer progression by HIV status, because HIV patients are typically excluded from large randomized studies.6,10 Although it has been suggested that lower CD4 counts may be related to the progression of cervical cancer, the effect of antiretroviral therapy on cervical neoplasia remains unclear.11 The aim of this study was to investigate the association between HIV status on cervical cancer progression among FIGO stage I and II patients. We examine differences in treatment received between HIV-positive and HIV-negative patients, and the effect of duration of infection among a subset of HIV-positive patients.
METHODS
Study design
This retrospective study was conducted at the Cancer Diseases Hospital. Potential subjects were identified using an electronic database created in our previous study to estimate the incidence of cervical cancer in the southern and western provinces of Zambia.2 Paper medical records were abstracted for eligible patients and entered into a newly- created electronic Microsoft ACCESS 2016 database. Information regarding patient HIV treatment was obtained from the national electronic database called the BroadReach SmartCare HIV database which was linked with the newly created Cancer Diseases Hospital cervical cancer database.
Inclusion criteria
All cervical cancer patients seeking diagnosis and/or treatment at the Cancer Diseases Hospital between October 2007 and December 2012 were obtained from the existing electronic database. The inclusion criteria for records included in this analysis were for the periods between January 2008 and December2012 who had known HIV status and FIGO stage I or II cervical cancer at the initial date of registration in the Cancer Diseases Hospital. These dates were chosen as 2007 data did not include variables which were necessary for our inclusion criteria, and 2012 was the last year of complete data available. Patients with unknown HIV status in the Cancer Diseases Hospital records were checked with the BroadReach SmartCare HIV database at the University Teaching Hospital to ascertain the HIV status.
Data collection
Variables abstracted from the Cancer Diseases Hospital paper records included: white blood cell count, platelet count, hemoglobin count, creatinine clearance, HIV status, evaluation of tumor by oncologists at each Cancer Diseases Hospital visit, chemotherapy doses received, radiotherapy doses received, age at diagnosis, cervical cancer FIGO stage, and duration of follow-up. HIV status (where not available, for our primary analysis, in the Cancer Diseases Hospital paper record) and first HIV diagnosis date were obtained from the BroadReach SmartCare HIV national database after linkage between the Cancer Diseases Hospital and HIV database was completed. Data of our secondary analysis were matched by patient first name, last name, residence, date of birth, or age at diagnosis (allowing for an age range that is 5 years over or under the age at diagnosis). All possible matches were reviewed to find true matches based on common factors in both databases, such as diagnoses' dates.
Method of participant identification
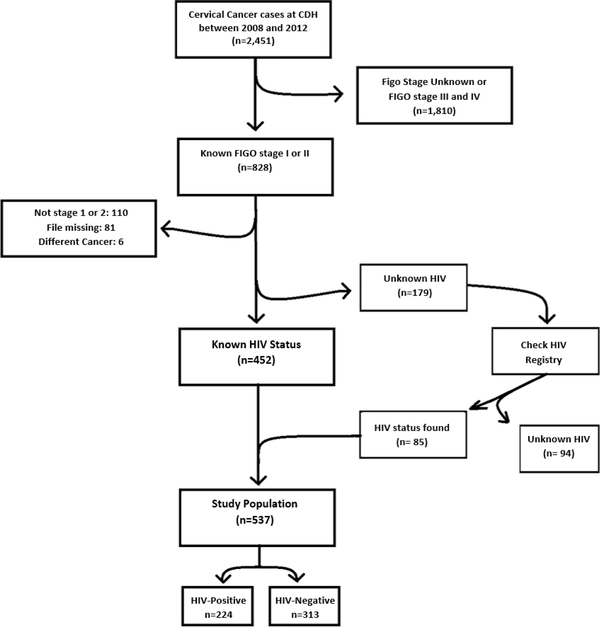
A total of 2451 cervical cancer cases were identified at the Cancer Diseases Hospital between 2008 and 2012. Following the exclusions shown in Figure 1, 828 cases were identified as FIGO stage I or II and were individually reviewed and data abstracted. A total of 179 cases had stage I or II cervical cancer, but unknown HIV status, and were then checked in the BroadReach SmartCare HIV database to ascertain HIV status. Figure 1 shows the case flow which resulted in a final analysis population of 537 stage I and II cervical cancer cases with known HIV status (224 HIV-positive and 313 HIV-negative). A total of 291 cases were excluded for various reasons including not being stage I or II (n=110), missing paper record (n=81), cervical cancer not the primary site (n=6), or unknown HIV status (n=94).
Figure 1.
Case flow diagram for cervical cancer patients seen at the Cancer Diseases Hospital, Lusaka, Zambia.
Outcomes
The outcome of interest was cervical cancer disease progression measured after at least 3 months of follow-up. Disease progression was defined as an oncologist’s clinical evaluation of a patient’s tumor as metastatic or residual tumor that required additional treatment at least 3 months after initial treatment. Participants were classified as having no disease progression if they had a record of medical evaluations that included complete response, no evidence of disease, or any other description that implied complete resolution of tumor from the clinical description. The primary predictor of interest was HIV status (positive vs negative) prior to initiating treatment. A subset of patients had additional HIV diagnosis variables (date of HIV diagnosis and antiretroviral therapy use) available in the BroadReach SmartCare HIV database. Using this subset, we examined the effect of duration of infection (years between HIV and cervical cancer diagnoses) on cervical cancer progression.
Standard treatment at the Cancer Diseases Hospital
The treatment protocols at the Cancer Diseases Hospital for cervical cancer management during the study period were as follows:
46 Gy/23 fractions of external beam radiation therapy plus 6.5 Gy X 4 fractions of high-dose rate brachytherapy OR
50 Gy/25 fractions of external beam radiation therapy plus 8 Gy X 3 fractions of high-dose rate brachytherapy
Concurrent chemotherapy:
80 mg/m2 of cisplatin every 3 weeks if creatinine clearance was ≥60 mL/min and, for HIV- positive patients, if the CD4 count was ≥200 cells/mL
Treatment protocol decisions were made based on clinical evaluations of tumors, where bulky tumors received higher dose of external beam radiation therapy with three fractions of brachytherapy.
Covariates and potential confounders
Covariates and potential confounders included: age (years), marital status (married, widowed, or other), province of residence (Lusaka or other), cervical cancer stage (I or II), baseline height (meters) and weight (kilograms), baseline laboratory measures (baseline creatinine clearance (ml/min), platelet (count ×109/L), hemoglobin (g/dl), and white blood cell counts (count ×109/L)), baseline histology of the tumor (adenocarcinoma, squamous cell carcinoma, or other) and therapy received including brachytherapy doses received (four fractions, three fractions, or other), external beam radiation therapy doses received (25 fractions, 23 fractions, or other), and chemotherapy cycles received (no chemo received, 1–3 cycles, or other amount). Length of follow-up time (days between first tumor evaluation and last visit to the Cancer Diseases Hospital) was calculated.
Statistical analysis
Software used for this study included: SAS 9.4 [Cary, NC] (analysis), ACCESS 2016 [Redmond, WA] (data management), Microsoft SQL Server Management Studio 17 [Redmond, WA] (database linkage), and BroadReach SmartCare [Cape Town, SA] (database linkage). Logistic regression models were used to estimate the effect of HIV status on cervical cancer progression with odds ratios (OR) and 95% confidence intervals (95% CI) as the measure of effect. Linearity in the log odds of continuous predictors was examined graphically using restricted cubic splines (RCS SAS Macro).12 If no statistically significant nonlinearity was found in predictors, they were used linearly. To determine if the effect of HIV (positive vs negative) varied by cervical cancer stage (stage I vs stage II), interaction terms were used and assessed for significance using the likelihood ratio test. The overall fit of the final models was assessed through the Hosmer–Lemeshow test. Discriminative performance was assessed by the c-statistic. Chi-square tests were used to compare treatments received between the two groups.
Logistic regression models were used to estimate the effect of the duration of HIV infection on cervical cancer progression on those patients with duration data. Covariates for this model included age (years) and antiretroviral therapy use (yes or no). Sensitivity analyses were conducted to evaluate the impact of missing data (>18% for missing disease progression). HIV-positive patients were more likely to have missing data. We used multiple imputation by fully conditional specification with 20 iterations: variables considered were those which were related to either the outcome or the missing data. Relatedness was assessed through χ2 tests, t-tests, and Kendall’s Tau correlation coefficient.
Simple imputation sensitivity analysis
We fitted another logistic regression model where we imputed all missing outcome values as progressive disease for HIV-positive patients (50 missing outcome) and as no progression for HIV-negative patients (49 missing outcome). The model adjusted for all of the same variables as our primary adjusted model (age, stage, brachytherapy, external beam radiation, chemotherapy, creatinine clearance, platelet, hemoglobin and white blood cell count, and follow-up time).
Simple imputation combined with multiple imputation
Our next logistic regression model performed multiple imputation by fully conditional specification for all the covariates in the adjusted model and simple imputation for the outcome.
Change in outcome definition
Our final logistic regression model changed the definition of our outcome (progression), to only include those who progressed to metastatic cervical cancer.
Human subjects
This study was reviewed and approved by the University of Arizona Institutional Review Board, the Cancer Diseases Hospital Ethics Committee, ERES Converge IRB in Zambia, and the National Health Research Authority in Lusaka, Zambia.
RESULTS
Baseline descriptive characteristics of this population stratified by HIV status are reported in Table 1. A total of 224 (41.7%) patients were HIV-positive, and HIV-positive women were, on average, 10 years younger than HIV-negative women. Table 1 also shows that 132 (24.6%) women had evidence of cervical cancer progression. However, Table 2 showed no statistically significant effect on cervical cancer progression in either unadjusted or adjusted logistic regression models (unadjusted OR 1.23, 95% CI [ 0.81, 1.86] and adjusted OR 1.04, 95% CI [ 0.57, 1.92]).
Table 1.
Patient demographics and clinical variables by HIV status for cervical cancer patients seen at the Cancer Diseases Hospital, Lusaka, Zambia from 2008 to 2012
| N (%) | HIV-positive n=224 (41.7%) | HIV- negative n=313 (58.3%) | Total n=537 | P-value* |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 42.4 (8.4) | 52.4 (11.8) | 48.3 (11.6) | <0.0001 |
| Baseline FIGO stage | ||||
| I (%) | 35 (15.6) | 42 (13.4) | 77 (14.3) | |
| II (%) | 189 (84.4) | 271 (86.6) | 460 (85.7) | 0.47 |
| Treatment completion | ||||
| 1–3 cycles chemotherapy†(%) | 109 (48.7) | 167 (53.4) | 276 (51.4) | 0.08 |
| EBRT(%) | 177 (79) | 239 (76.4) | 416 (77.5) | 0.02 |
| Brachytherapy (%) | 185 (82.6) | 264 (84.3) | 449 (83.6) | 0.05 |
| BRT + EBRT (%) | 176 (78.6) | 230 (73.4) | 406 (75.6) | 0.70 |
| 1–3 chemo +EBRT (%) | 106 (47.3) | 147 (47) | 253 (47.1) | 0.41 |
| 1–3 chemo+BRT (%) | 108 (48.2) | 159 (50.8) | 267 (49.7) | 0.14 |
| 1–3 chemo+EBRT+ BRT (%) | 106 (47.3) | 143 (45.7) | 249 (46.4) | 0.58 |
| Progressive disease‡ (%) | 57 (25.4) | 75 (23.9) | 132 (24.6) | 0.33 |
All percentages in table 1 are column percentages and patients may be included in multiple groups.
There were 52 patients (28 HIV-positive and 24 HIV-negative) who had all missing treatment information.
P-value for t-test for continuous variables and Chi-square test for categorical variables.
22.5% missing (32% HIV-positive and 16% HIV-negative).
8.4% missing in outcome variable (22.3% HIV-positive and 15.6% HIV-negative).
BRT, brachytherapy; chemo, chemotherapy; EBRT, external beam radiation; FIGO, International Federation of Gynecology and Obstetrics.
Table 2.
Unadjusted and adjusted odds ratios for cervical cancer progression by HIV status among patients seen at the Cancer Diseases Hospital in Lusaka, Zambia (HIV- negative as the reference).
| Model | N | OR (HIV +vs HIV -) | 95% CI | C-statistic |
|---|---|---|---|---|
| Model 1* | 438 | 1.23 | 0.81,1.86 | 0.525 |
| Model 2** | 332 | 1.04 | 0.57,1.92 | 0.786 |
Model 1: Unadjusted with only HIV status included in the model.
Model 2: Adjusted model with HIV status, age, stage, brachytherapy doses received, EBRT doses received, chemotherapy cycles received, baseline creatinine, platelet, hemoglobin and white blood cell count, and follow-up time.
While most patients (460 [85.7%]) were FIGO stage II (Table 1), at least 416 (77.5%) patients received external beam radiation, and only 249 (46.4%) patients received the recommended treatment of chemotherapy, external beam radiation, and brachytherapy. When comparing treatment received by HIV status, the only statistically significant difference was for external beam radiation, with HIV-positive patients more likely to receive a lower dose of radiation over 23 fractions (47% vs 37%; P<0.05). The median total dose of external beam radiation for HIV-positive and HIV-negative patients was 46 Gy, and 50 Gy, respectively. Brachytherapy and chemotherapy had no statistically significant differences in completion, but there was a suggestion that HIV-positive patients were more likely to receive 6.5 Gy X 4 fractions of brachytherapy (48% vs 38%; P=0.05). The median total dose of brachytherapy for HIV-positive and HIV-negative patients was 26 Gy, and 24 Gy, respectively. There was a total of 52 (28 HIV-positive and 24 HIV-negative) patients with all treatment information missing.
Of 224 HIV-positive patients, 32 patients had sufficient data to analyze the effect of duration of HIV infection on cervical cancer progression. The mean duration of HIV infection was 5.3 years (online supplementary table 3). After adjustment for age and antiretroviral therapy use, the odds of cervical cancer progression were statistically significantly reduced per year increase in duration of HIV infection(OR0.66, 95% CI[0.47, 0.92]). Sensitivity analyses evaluated the potential impact of missing data: online supplementary table 4 shows missing data among HIV-positive patients when compared with HIV-negative patients. However, there was no statistically significant effect of HIV status on cervical cancer progression after multiple imputation by fully conditional specification (online supplementary table 5). When performing simple imputation on our outcome our OR of 1.21 (95% CI 0.68, 2.2) remained not significant. This also held true when combining simple imputation of our outcome and multiple imputation by fully conditional specification for our covariates (OR 1.29 [95% CI 0.81, 2.1]). Finally, in our model which changed the outcome definition to include only those who progressed to metastatic cervical cancer our results remained not statistically significant (OR 1.09 [95% CI 0.55,2.17]).
DISCUSSION
The prevalence of HIV in our study was nearly three times higher than the prevalence of HIV in the general population in Zambia.2 This prevalence among cervical cancer patients is higher than the HIV prevalence reported from South Africa.13 It has been shown that HIV-positive patients have a 5–10 times higher risk of developing cervical cancer than HIV-negative patients.14 The higher risk for cervical cancer among HIV-positive patients may be partially explained by the higher prevalence of high-risk oncogenic HPV types among HIV-positive patients in Zambia and the increased likelihood of persistent HPV infection.6,15,16 This finding suggests that HIV-positive patients should be a primary target group for HPV vaccinations, screening, and early detection of cervical cancer because of the significant higher risk induced by HIV.
We observed differences in treatment received by patients based on their HIV status, specifically HIV-positive patients being more likely to receive a lower dose of external beam radiation than HIV-negative patients. HIV-positive patients were more likely to receive chemotherapy than HIV-negative patients. There was a difference in brachytherapy, with HIV-positive patients being more likely to receive 6.5 Gy X four fractions. In countries where HIV prevalence is lower, the treatment of cervical cancer is designed based on tumor stage, however, treatment varies in Sub-Saharan Africa because of the high prevalence of HIV. Although concurrent chemoradiation is recommended, many patients are not able to tolerate treatment due to lower CD4 counts, leukopenia, or other factors. Reasons for treatment difference may require further investigation. It is possible that the treatment differences were due to sub-FIGO staging, where HIV negative patients were more likely to present as FIGO stage IIB. These data, however, were not available in the medical records, and further research is warranted
Of note, there was no evidence of difference in cervical cancer progression by HIV status in this population. Although progression data appeared to be missing (more missing information among the HIV-positive group), there was no difference in disease progression after performing simple imputation where all missing HIV-positive outcomes were imputed as progression and HIV-negative as no progression (online supplemental material). This finding was consistent with a previous study at the Cancer Diseases Hospital that reported no difference in treatment toxicity by HIV status.6,10 However, other studies from South Africa found treatment outcome differences by HIV status.17–19 It is possible that these different findings are due to measurements which were not available in our study, such as viral load and CD4 cell count.
Finally, among a subset of HIV-positive individuals, we found that longer duration of HIV infection reduced the odds of disease progression. This unexpected finding could be due to earlier diagnosis and longer management of HIV infection. A previous study at the Cancer Diseases Hospital showed that patients who started antiretroviral therapy earlier were more likely to complete treatment which could also contribute to the lowered odds of disease progression among HIV-positive patients.10 Those with shorter duration of HIV infection may have been diagnosed later than when they became infected with HIV, thereby leading to a longer period of unmanaged HIV infection. Further research into this topic may be necessary to determine the relationship between HIV infection and tumor progression.
This study contributes to the small body of literature regarding HIV and cervical cancer outcomes.6,10,20 The large number of patients who were lost to follow-up in this country is consistent with findings from previous studies, particularly in Sub-Saharan Africa.6,20–22 The need for increased patient retention methods, particularly among HIV-positive patients, at the Cancer Diseases Hospital are needed. This is evident in the loss to follow-up rate of 20% within the first 3 months (14% among HIV-negative). The strengths of this study include a relatively large sample size in a country with limited prior research but with the availability for data linkage between the cancer and HIV databases which has not been done before. This study is conducted at the only cancer center in Zambia. The high incidence rates of cervical cancer and the high national prevalence of HIV provided an opportunity to investigate the role of HIV cervical cancer progression. Finally, the current screening program in Zambia allowed for a reasonably large sample of non-metastatic cervical cancer. Limitations to this study included the retrospective nature of the study, missing viral loads and CD4 counts, and missing data in regard to cervical cancer progression. While the limitation of missing data was addressed using multiple imputation and the use of the database linkage with the BroadReach SmartCare database, the impact of the missing data may be underestimated. Finally, since the Cancer Diseases Hospital is the only cancer center in the country it is possible that the study population is not representative of the country, but rather of Lusaka.
HIV was not found to have a significant impact on disease progression among non-metastatic cervical cancer patients in Lusaka, Zambia. The difference of treatment based on HIV status and the lack of effect of HIV on progression may relate to differences in response to treatment among HIV-positive and HIV-negative patients, and specific immunological response of HIV-positive patients under treatment. Future studies should investigate the relationship of immunological response to treatment and the relationship between duration of HIV infection and markers of the severity of infection (including viral load and CD4 count) in relation to the progression of cervical cancer.
Supplementary Material
HIGHLIGHTS.
HIV prevalence among cervical cancer patients was nearly three times that of the general population in Zambia.
There was no difference in cervical cancer progression by HIV status in our population.
HIV-positive patients should be a primary target group for HPV vaccinations, screening, and early detection.
Acknowledgements
We thank all of the staff at the Cancer Diseases Hospital who made this work possible. We also thank Juanita Trejo for all of her help with editing the manuscript.
Funding The project described was supported by Grant Number R25CA112383 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement
The deidentified participant data are available upon reasonable request from the corresponding author.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Kalima M, Lishimpi K, Meza JL, et al. Observed and expected incidence of cervical cancer in Lusaka and the southern and western provinces of Zambia, 2007 to 2012. Int J Gynecol Cancer 2015;25:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 4.Parham GP, Mwanahamuntu MH, Kapambwe S, et al. Population-level scale-up of cervical cancer prevention services in a low-resource setting: development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PLoS One 2015;10:e0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lovgren K, Soliman AS, Ngoma T, et al. Characteristics and geographic distribution of HIV-positive women diagnosed with cervical cancer in Dar ES Salaam, Tanzania. Int J STD AIDS 2016;27:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghebre RG, Grover S, Xu MJ, et al. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep 2017;21:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenzie ND, Kobetz EN, Hnatyszyn J, et al. Women with HIV are more commonly infected with non-16 and -18 high-risk HPV types. Gynecol Oncol 2010;116:572–7. [DOI] [PubMed] [Google Scholar]
- 8.Chirwa S, Mwanahamuntu M, Kapambwe S, et al. Myths and misconceptions about cervical cancer among Zambian women: rapid assessment by peer educators. Glob Health Promot 2010;17:47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013;138:103–41. [DOI] [PubMed] [Google Scholar]
- 10.Mdletshe S, Munkupa H, Lishimpi K. Acute toxicity in cervical cancer HIV-positive vs. HIV-negative patients treated by radical chemo-radiation in Zambia. Southern African J Gynaecol Oncol 2016;8:37–41. [Google Scholar]
- 11.Denslow SA, Rositch AF, Firnhaber C, et al. Incidence and progression of cervical lesions in women with HIV: a systematic global review. Int J STD AIDS 2014;25:163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;15:n/a–57. [DOI] [PubMed] [Google Scholar]
- 13.Gichangi PB, Bwayo J, Estambale B, et al. Impact of HIV infection on invasive cervical cancer in Kenyan women. AIDS 2003;17:1963–8. [DOI] [PubMed] [Google Scholar]
- 14.Brickman C, Palefsky JM. Human papillomavirus in the HIV-infected host: epidemiology and pathogenesis in the antiretroviral era. Curr HIV/AIDS Rep 2015;12:6–15. [DOI] [PubMed] [Google Scholar]
- 15.Ng'andwe C, Lowe JJ, Richards PJ, et al. The distribution of sexually-transmitted human papillomaviruses in HIV positive and negative patients in Zambia, Africa. BMC Infect Dis 2007;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham AG, D'Souza G, Jing Y, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr 2013;62:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mangena M, Snyman L, Dreyer G, et al. The impact of HIV infection on women receiving radiation for cervical cancer. South Afr J Gynaecol Oncol 2015;7:44–51. [Google Scholar]
- 18.Simonds HM, Wright JD, du Toit N, et al. Completion of and early response to chemoradiation among human immunodeficiency virus (HIV)-positive and HIV-negative patients with locally advanced cervical carcinoma in South Africa. Cancer 2012;118:2971–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonds HM, Neugut AI, Jacobson JS. Hiv status and acute hematologic toxicity among patients with cervix cancer undergoing radical chemoradiation. Int J Gynecol Cancer 2015;25:884–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira MP, Coghill AE, Chaves CB, et al. Outcomes of cervical cancer among HIV-infected and HIV-uninfected women treated at the Brazilian National Institute of Cancer. AIDS 2017;31:523–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldhaber-Fiebert JD, Denny LE, De Souza M, et al. The costs of reducing loss to follow- up in South African cervical cancer screening. Cost Eff Resour Alloc 2005;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abercrombie PD. Improving adherence to abnormal pap smear follow-up. J Obstet Gynecol Neonatal Nurs 2001;30:80–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The deidentified participant data are available upon reasonable request from the corresponding author.