Abstract
Silver nanoparticles (AgNP) exhibit size and concentration dependent toxicity to terrestrial plants, especially crops. AgNP exposure could decrease seed germination, inhibit seedling growth, affect mass and length of roots and shoots. The phytotoxic pathway has been partly understood. Silver (as element, ion or AgNP) accumulates in roots/leaves and triggers the defense mechanism at cellular and tissue levels, which alters metabolism, antioxidant activities and related proteomic expression. Botanical changes (either increase or decrease) in response to AgNP exposure include reactive oxygen species generation, superoxide dismutase activities, H2O2 level, total chlorophyll, proline, carotenoid, ascorbate and glutathione contents, etc. Such processes lead to abnormal morphological changes, suppression of photosynthesis and/or transpiration, and other symptoms. Although neutral or beneficial effects are also reported depending on plant species, adverse effects dominate in majority of the studies. More in depth research is needed to confidently draw any conclusions and to guide legislation and regulations.
Keywords: Silver nanoparticles, seed germination, terrestrial plants, phytotoxic mechanism, nano-bio interaction
1. Introduction
The market size for metal nanoparticles is estimated to be USD 25 billion by 2022 worldwide.1 As a representative, silver nanoparticles (AgNP) will reach a market of USD 3 billion by 2024. Its application covers many industries, including pharmaceutical, healthcare, electrical, chemical, personal care, and cosmetics. Started in 2003, AgNP has been investigated for applications in food and agriculture industry; and since then, hundreds of commercial agricultural product containing or based on AgNP have been marketed.2–4 It is estimated that by 2024, AgNP application in food and beverage industry will surpass USD 300 million.1 Therefore, AgNP will be released into the environment in the near future and the majority will end up in the terrestrial environment. It thus brings concerns on potential adverse impact on the ecosystem and the food supplies. There is an urgent need to understand their impact on terrestrial plants.
In the past decade, many studied the effect of nanoparticles on terrestrial plants including agricultural plants, annual herb, grass and flowering plants. Few studies focus on perennial trees. However, this list is far from complete. For instance, many crucial experimental details or parameters are missing in the reports.
Under this background, the impact of AgNP in literature is comprehensively reviewed from several standpoints, seed germination, seedling growth, and overall botanical health, including morphological change, cytotoxicity and genotoxicity. Possible mechanism is discussed from Ag intake, metabolic and proteomic response upon AgNP exposure. This piece of information provides crucial and fundamental guidance and reference for scientists, product developers, government agencies, and policy makers to design, make, legislate, regulate, market, and dispose metal nanoparticle containing products in an environment friendly manner.
The current report addressed the influence of AgNP on three common beans, Black eyed (Vigna unguiculata L.), Garbanzo (Cicer arietinum L.) and Lentil (Lens culinaris) beans, to observe their seed germination, elongation and biomass of both root and shoot of seedlings, and Ag intake by seeds upon AgNP response. These common beans are commercially grown all over the world and occupy a huge market in agricultural industry.
2. AgNP inhibit seed germination and seedling growth
The majority of literatures report adverse effects of AgNP on seed germination and seedling growth while no effect and some beneficial aspects are mentioned. Such influence mainly depends on the concentration of AgNP and plant species. Higher concentration of AgNP leads to more severe inhibitory influence, but root growth inhibition can be induced at a concentration as low as 0.001 ppm in onion and radish.5 Effect of AgNP varies, as listed in Table 1. The main reason is that AgNP are coated or mixed with many other materials. The observed outcome is not solely owing to core AgNP but also to a comprehensive list of all the co-existing substances, which will be discussed in section 2.3.
Table 1.
Examples of toxic effects of various AgNP on terrestrial plants, including decreased germination, inhibited growth, reduced root and shoot length.
| AgNP (resource, size, coating and [AgNP] in ppma) | Type of seeds | Main effect(s) of AgNP | Ref. |
|---|---|---|---|
| Synthesized using trisodium citrate, 0–50 in deionized water | Black eyed (Vigna unguiculata), Garbanzo (Cicer arietinum), Lentil bean (Lens culinaris) | Decrease in germination rate of Garbanzo, slow seedling growth. | This study |
| Nanosilver adsorbed on fumed silica, 0–10,000 in deionized water | Onion (Allium cepa L), radish (Raphanus sativus L.) | Induce root growth inhibition at a concentration as low as 0.001 ppm. | [5] |
| Purchased from Sigma Aldrich, <100 nm, 0–20 in deionized water | Lycopersicum esculentum, Zea mays | Inhibit root length and germination. | [8] |
| Purchased from NanoComposix®, 5–75 nm, PVP-coated, 0–100 in distilled water | Allium cepa | Reduce germination in a diameter-dependent manner. Smaller size had stronger inhibition. | [9] |
| Chemical synthesized, 50 nm, sodium citrate coated, 0–100 in water | Seven Varieties of tomato (Lycopersicon esculentum Mill) | Shorten sprout time, decrease germination percentage, vigor index, tolerance index, root and shoot length. | [10] |
| Different sizes, 20, 30–60, 70–120 and 150nm, 0–1000 in water | Jasmine rice (Oryza sativa L. cv. KDML 105) | Decrease seed germination and subsequent seedling growth. | [11] |
| Purchased from Natural Path/Silver Wings, 1–10nm, 0–500 in deionized water | Radish (Raphanus sativus) | Reduce the root and shoot lengths and the seedlings had less Ca, Mg, B, Cu, Mn, and Zn elements. | [12] |
| Purchased from Purest Colloids (MesoSilver, 0.6–2 nm) and Quantum Sphere (Ag-QSI, 20 nm); 0–100 in water with 0.1% (v/v) Tween 20 | Flax (Linum usitatissimum L., cv. Electra), ryegrass (Lolium perenne L, cv. Tove), two-rowed barley (Hordeum vulgare L, cv. Annabell) | Inhibit seed germination and never completely impede germination. Reduction in shoot growth. | [13] |
| Purchased from Sigma-Aldrich, PVP coated, 0–540 in distilled water | Wheat (Triticum aestivum) | A significant inhibitory effect on root and shoot length of seedlings. | [14] |
| Purchased from Shanghai Science & Technology, 30 nm and 70 nm, 100 in deionized water | Lettuce (Lactuca sativa L.) | Decrease seed germination and seedling development indices. Larger AgNP exerted stronger toxicity. | [15] |
| Purchased from RAS AG (Regensburg, Germany), 15 nm, contains Tagat Tween 20, 0–2000 in MES buffer (pH 6.0–6.1) | Zea mays L. | Affect seed germination and shoot length negatively in a dose-dependent manner. AgNP retarded root growth. | [16] |
| Microwave assisted reduction using Cassia auriculata leaf extract, 13nm, 0–50 in deionized water | Pearl millet (Pennisetum glaucum) | Higher concentration of AgNP decreased the root, shoot and total seedling length. | [17] |
| Provided by ABC Nanotech (Daejeon, Korea), Citrate-coated, 5–25 nm, 0–40 in deionized water | Phaseolus radiates, Sorghum bicolor | Seedling growth was adversely affected. | [18] |
| Biologically synthesized, 0–500 in sterile deionized water | Chinese cabbage (Brassica rapa ssp. pekinensis) | Reduce root, shoot growth, and fresh biomass. | [19] |
| Purchased from XFNANO Materials Technology (China), 17nm, PVP coated, 0–9.2 in medium with Ca(NO3)2, KNO3, MgSO4, KH2PO4 (pH 6.0) | Wheat (Triticum aestivum L.) | Decrease in relative root elongation and root weight, which could be alleviated by extracellular polymeric substances isolated from Pseudomonasputida. | [20] |
| Synthesized using leaf extract of Aloe vera plants, 22 nm, 0–324 in deionized water | Pea (Pisium sativum) | Decline growth parameters, photosynthetic pigments and chlorophyll fluorescence. | [21] |
| Obtained from Ted Pella Inc. (Reading, US), 20 nm, 0–50 in ½ MS agar medium | Mung bean (Vigna radiata L.) | Significant reduction in shoot length, root elongation and weight. | [22] |
| Synthesis from isolate B. marisflavi., 1–10 in deionized water | turnip (Brassica rapa ssp. rapa L.) | 5 and 10 ppm AgNP decreased the plant growth, biomass, and chlorophyll content. | [23] |
| Obtained from Biopure AG10, Nanocomposix (San Diego, USA), 10nm, PVP coated, 1–10 in deionized water with/without PVP | Wheat (Triticum aestivum) | Adversely affect the seedling growth and induced morphological modifications in root tip cells. | [24] |
| Reduced using sodium borohydride and coated by Tween-20, 1000 | Oryza sativa | Deposit inside the root cells by damaging the cell wall and vacuoles to enter. | [25] |
| Obtained from Attostat Inc. (West Jordan, UT, US), 10 nm, 0 – 5 in sand | Wheat (Triticum aestivum L.) | Reduce the length of shoots and roots in a dose-dependent manner. | [26] |
| Obtained from US Research Nanomaterials Inc (Texas, US), 20 nm diameter, 0–790 in soil | Bishop pine (Pinus muricata D. Don) | Reduce the root length but have a small effect on ground plant biomass. | [27] |
| Chemical synthesized, 5–50 nm sphere, 100–900 in soil | Faba bean (Vicia faba L.) | The germination declined by 40% when exposed to 800 ppm AgNP. | [28] |
| Purchased from U.S. Research Nanomaterials, 20 nm, 0–2500 | Corn (Zea mays L.) | Toxic effect on corn root elongation. | [29] |
Concentration range is converted to ppm for a better comparison. Density of all solutions is considered as 1.0 g/mL.
Abbreviations: DDAB, didecyldimethylammonium bromide; PVP, polyvinylpyrrolidine.
2.1. Effects of AgNP vary but toxicity dominates
There are three main types of AgNP: chemically synthesized, green synthesized using natural product extract, and commercially purchased. Each type of AgNP has unique profile depending on their form, size, shape, coating and additives,6 which makes it complicated to study their interaction with seeds and plants. Considering varieties of terrestrial plants on earth, there are theoretically countless combinations of studies in this field. This topic is still in its infancy and gained attention only ten years ago in 2009 when Stampoulis et al.7 evaluated the effects of five nanomaterials, including AgNP, on seed germination and seedling growth of Cucurbita pepo (zucchini). Ever since, it has grown to a long list of such studies shown in Table 1.
2.2. Crops are the most studied among terrestrial plants
Majority of studies focus on agricultural plants (at least 43 out of 53 publications, some are shown in Table 1). Some focus on flowering/medicinal plants that are commonly available in fields and agricultural lands (like Arabidopsis thaliana,30 Thymus vulgaris L. and Thymus daenensis Celak,31 Thymus kotschyanus,32 Artemisia absinthium33). Few focus on trees including orange trees (Citrus reticulate34), pine (Pinus muricata,27 Pinus sylvestris L.35) and oak (Quercus robur L.,36 Quercus robur L.35).
Among the crops, common wheat (Triticum aestivum) is the most studied with at least 8 publications, followed by maize/corn (Zea mays) with at least five reports. Rice (Oryza sativa) has also been included in at least three reports. According to the Food and Agriculture Organization,37 corn, rice, and wheat are three most produced crops in the world that are directly consumed by humans and they have received the most attention regarding their response to AgNP exposure.
Other studied crops include zucchini (C. pepo),7 mung bean (Phaseolus radiatus),18 great millet (Sorghum bicolor),18 common bean (Phaseolus vulgaris L.),38 castor bean (Ricinus communis),39 pearl millet (Pennisetum glaucum),17 tomato (Solanum lycopersicum,40 Lycopersicon esculentum Mill10), radish sprouts,12 pea (Pisum sativum),41 lettuce,15 pigeon pea (Cajanus cajan L),42 cabbage (Brassica oleracea var. capitata L.)43 and onion (Allium cepa L.).5
2.3. AgNP as potential threat to terrestrial plant community
As shown in Table 1, various ambient conditions are covered. Tested concentration ranges from 0 (control) to 10,000 ppm (10,000 mg/L−1).5 Suspension solvents include different types of water (deionized, distilled, etc.), medium (1/2 MS medium, MS medium and other artificial medium) and buffers. The toxic effect of AgNP is concentration dependent. A high AgNP concentration exerts more adverse influence. In the practical situation, one excuse for the low or non-toxicity of AgNP to the plants is the low concentration available in the soil since the portion of nanoparticle in actual products is low that is further distributed into massive of soil. This assumption does not consider the potential bioaccumulation of Ag element. Besides, many studies in Table 1 covering AgNP concentration of 1.0 ppm and lower observed adverse influence toward seed germination and seedling growth of terrestrial plants.
Overall, adverse effects dominate when compare acute impact of AgNP on terrestrial plants. Particularly, varieties of plants coexist in the same community and ecosystem. When AgNP are harmful to most or even some species of plants, they are threat to the whole community and to the environment and ecosystem. Some studies reported that no obvious adverse effects on germination rate and seedling growth,44,45 and even a stimulatory effect.46–48 A dose-dependent manner is concluded by many reports.17,23,38,49,50 Low concentrations of AgNP exerted no or mild toxic activity while increasing concentration induced a significant inhibitory effect.
2.4. AgNP as a mixture plays the key role
In the case of wheat, tested AgNP are not exactly the same and the resulting effects are also different. Five commercial AgNP from Sigma-Aldrich (St. Louis, Missouri, US),14 RAS AG (Regensburg, Germany),16 XFNANO Materials Technology (Nanjing, China),20 Biopure Nanocomposix (San Diego, CA)24 and Attostat (West Jordan, UT, US)26 all showed adverse effect on germination and shoot and root growth of seedlings, regardless of the size and coating of AgNP. AgNP produced using the high voltage physics arcing method did not affect germination but damaged root cells.51 Green synthesized AgNP using Laminaria japonica algal extract49 had no impact on seed germination, increased root length at low concentration but inhibited both root and shoot growth at high concentrations.
The case of wheat is a good representative that AgNP with different parameters could have distinct impacts on the seed germination and growth of wheat. It is the same for corn and other plants. For instance, inhibitory effect,16 or both positive and negative effects29,38 were reported for corn, depending on the AgNP parameters and its concentrations. Overall, adverse influence dominates in majority of reports.
All parameters of AgNP play some role when they interact with seeds and seedling. Blending chemically synthesized AgNP with nicotinic acid and KNO3 exerted a beneficial effect by increasing length and fresh weight of shoot and root.52 This means introducing new chemicals to the AgNP could potentially alleviate the negative influence of AgNP. It was reported that cytotoxicity and phytotoxicity of the green synthesized AgNP are significantly less than wet-chemistry synthesized AgNP.53 The underlying mechanism is not clarified but new chemical species could replace coating agent, interact with AgNP core, and trigger a series of down-stream reactions, i.e., AgNP transformation. AgNP could be altered before they interact with seeds or plants so that distinct outcome is observed. It is highly possible because of high reactivity of AgNP cores, similar with synergistic antibacterial mechanism of AgNP combine with tetracycline.54
It also provides a potential strategy to reconcile the phytoxicity of AgNP by introducing one or more particular substance(s), which needs to be further studied.
Size is another important factor whereas its impact is found different. Smaller sized AgNP had stronger inhibition on A. cepa.9 No clear size dependent effect was reported on Flax, ryegrass and barley.13 However, AgNP with larger sizes exerted stronger toxicity in both Lettuce (Lactuca sativa L.)15 and Jasmine rice (O. sativa L.).11
Instead of regarding them as “inconsistent” or even “contradictory” findings, the difference among the finds is due to different parameters of AgNP. It was pointed out that AgNP and the majority of other metal (oxide) nanoparticles, are a complicated mixture during toxicity studies.6 Variable intrinsic crystal structure of Ag(0) core, starting materials and coating agents (it is impossible to get rid of all materials, instead “a minimal concentration” is more appropriate), co-existing species and transformation products (i.e., Ag2O and released Ag+ ions) plus the change of ambient conditions (temperature, concentration, etc.), all can contribute to these differences. Every single parameter that is ignored could cause significant differences.
2.5. General method – three common beans as an example
A general routine to study effect of AgNP on seed germination is to soak seeds first in AgNP suspension, followed by germination process in petri dish (in the lab, with or without agar) or in pots (in green houses or field, with or without soil). AgNP treatment can also be achieved by soaking direct contact with AgNP suspension or mixing AgNP with agar or soil. Different methods lead to different observations too, like in agar and soil.13,18,55 A universal direct contact method was employed to evaluate influence of chemically synthesized AgNP on seed germination rate, length and mass of root and shoot of the three common beans. AgNP were chemically synthesized and used as described in our previous reports.54,56,57 Sodium borohydride was used to reduce silver nitrate, followed by citrate mediated reduction and growth.58,59 Such chemically synthesized AgNP have a characteristic extinction peak at 398 nm revealed by UV–vis spectroscopy and a spherical morphology of an average diameter of 20 ± 4 nm as revealed by transmission electron microscopy. To determine the concentration of AgNP, its solution was added with nitric acid to completely oxidize all Ag atoms to Ag+ ion, followed by dilution and analysis with inductively coupled plasma mass spectrometry (ICP-MS). This protocol is applied to all Ag samples that are subject to ICP-MS characterization. All these characterization details are the same as used in our previous reports.54,56,60
AgNP solutions of 0, 2, 5, 10, 20 and 50 ppm were prepared in deionized water and used to soak the three types of bean seeds: Black eyed (V. unguiculata L.) Garbanzo (C. arietinum L.) and Lentil (L. culinaris) beans. Sodium citrate of 1.3 ppm, that is, the minimal sodium citrate concentration in AgNP solution,56 was used as control group to soak bean seeds as well. Each group contained 10–15 randomly chosen seeds. After soaking for 24 h under room temperature (20 °C), all the seeds were rinsed with deionized water and then transferred onto moist filter paper and kept in dark under room temperature for germination purpose. The seeds were checked daily and were taken out for characterization on the 3rd day. Roots and shoots of all groups were collected. The length and mass of the root and shoot were recorded. Each experiment was replicated three times. The results are expressed as mean ± standard deviation (SD). To confirm statistical significance between data obtained from AgNP-treated groups and water control groups, one-way ANOVA in Statistical Package for Social Sciences (SPSS) software was employed and a p < 0.05 is used for significant responses.
2.5.1. AgNP affects seed germination rate
Seed germination rates of the three beans are shown in Table 2. Sodium citrate of 1.3 ppm does not affect germination rate. Treatment of AgNP shows no obvious impact on germination rate of black eye beans, but slightly inhibited garbanzo bean germination by 5.2–9.9% and enhanced lentil bean germination by 16.0–21.0%. These effects are not AgNP concentration dependent in the tested range.
Table 2.
Seed germination rates of the three beans under 20 °C for 3 days.
| Black eye (%) | Garbanzo (%) | Lentil (%) | ||
|---|---|---|---|---|
| Control | Water | 96.7 ± 1.3 | 94.5 ± 1.5 | 65.9 ± 2.0 |
| Citrate | 94.3 ± 1.7 | 95.0 ± 2.4 | 65.1 ± 2.3 | |
| [AgNP], ppm | 2 | 96.8 ± 1.7 | 89.3 ± 2.1* | 84.7 ± 1.6* |
| 5 | 94.2 ± 2.0 | 86.1 ± 1.5* | 86.9 ± 1.4* | |
| 10 | 93.8 ± 2.3 | 87.9 ± 2.2* | 85.6 ± 1.5* | |
| 20 | 93.2 ± 1.3 | 84.6 ± 2.8* | 84.3 ± 2.2* | |
| 50 | 95.3 ± 1.7 | 88.4 ± 1.8* | 81.9 ± 2.0* |
Notes: Sodium citrate (citrate) of 1.3 ppm is used as control group.
Significance is confirmed by one-way ANOVA, p < 0.05.
2.5.2. AgNP influence root length and mass
As listed in Table 3, among the three beans, black eye has the longest root length and heaviest root mass. Both length and mass steadily increase with the increase of AgNP concentration, although the increase is not significant until AgNP concentration reaches 50 ppm. Compared to control, black eye beans treated with AgNP had greater root length and mass. Garbanzo roots were not significantly affected by AgNP treatment but the root mass increased with 20 and 50 ppm AgNP treatment compared to control. Length of lentil roots significantly increased by 3.6–7.2% at AgNP concentrations below 50 ppm. However, when treated with 50 ppm, there was a reduction in root length, which was significantly different from control. The AgNP had no significant effect on the root mass of Garbanzo beans regardless of the concentration. Length and mass of black eye bean are proportional to AgNP concentration while length of lentil bean is inversely proportional to AgNP concentration.
Table 3.
Root length and mass of three beans in presence of AgNP solutions.
| Root length (mm) | Root mass (mg) | ||||||
|---|---|---|---|---|---|---|---|
| Black eye | Garbanzo | Lentil | Black eye | Garbanzo | Lentil | ||
| Control | Water | 28.4 ± 2.2 | 16.6 ± 2.0 | 19.8 ± 1.5 | 91.2 ± 9.9 | 45.8 ± 7.8 | 27.0 ± 4.8 |
| Citrate | 27.6± 1.8 | 16.4 ± 2.0 | 19.1 ± 2.2 | 78.4 ± 7.9 | 44.6 ± 6.7 | 25.7 ± 3.7 | |
| [AgNP], ppm | 2 | 30.5 ± 2.4 | 16.7 ± 2.4 | 27.0 ±2.0* | 89.0 ± 12.2 | 43.2 ± 7.5 | 30.0 ± 5.8 |
| 5 | 30.6± 1.8 | 16.5 ± 2.3 | 26.3 ± 2.0* | 95.3 ± 10.2 | 47.6 ± 5.9 | 27.1 ± 5.1 | |
| 10 | 31.5 ± 2.6 | 16.3 ± 2.2 | 24.8 ± 1.6* | 102.3 ± 11.7 | 49.4 ± 6.7 | 28.3 ± 6.6 | |
| 20 | 32.5 ± 2.5 | 18.6 ± 2.5 | 23.4 ± 1.8* | 104.1 ± 14.1 | 60.5 ± 8.0 | 24.7 ± 7.1 | |
| 50 | 34.1 ± 1.8* | 17.9 ± 2.4 | 22.5 ± 2.3 | 117.6 ± 12.0* | 56.2 ± 8.2 | 24.9 ± 5.4 | |
Note: Same volume of water and sodium citrate (citrate) solution are used as control.
Significance is confirmed by one-way ANOVA, p < 0.05, as compared to control groups.
2.5.3. AgNP influence shoot length and mass
AgNP treatment exhibits no significant impact on length or mass of both black eye and lentil beans shoots, as shown in Table 4. Shoot of garbanzo bean seeds did not fully develop on the third day of germination because of the relative low temperature, 20 °C. Setting temperature to 25 °C using an incubator could greatly improve the level of shoot development (results not shown). In term of shoot rate, black eye beans treated with AgNP were inhibited by 5.1–8.5% while lentil beans were significantly enhanced by10.9–18.4% when compared to control. The shoot rate of lentil beans decreased with the increase of AgNP concentration.
Table 4.
Shoot length, mass and percentage of beans in presence of AgNP solutions.
| Shoot length (mm) | Shoot mass (mg) | Rate (%) | |||||
|---|---|---|---|---|---|---|---|
| Black eye | Lentil | Black eye | Lentil | Black eye | Lentil | ||
| Control | Water | 12.0 ± 2.1 | 18.8 ± 1.9 | 19.0 ± 1.9 | 16.0 ± 1.8 | 96.7 ± 3.3 | 65.9 ± 3.0 |
| Citrate | 11.2 ± 1.4 | 17.7 ± 2.0 | 18.5 ± 1.7 | 14.8 ± 2.0 | 94.0 ± 2.4 | 65.0 ± 1.8 | |
| [AgNP], ppm | 2 | 10.3 ± 1.8 | 19.1 ± 1.5 | 16.6 ± 2.1 | 16.1 ± 1.8 | 91.6 ± 2.9 | 83.0 ± 1.1* |
| 5 | 10.5 ± 1.7 | 19.0 ± 2.1 | 20.0 ± 2.1 | 14.8 ± 1.9 | 89.2 ± 3.1* | 84.3 ± 2.1* | |
| 10 | 11.1 ± 2.5 | 21.0 ± 2.0 | 18.4 ± 2.3 | 16.4 ± 1.2 | 88.2 ± 3.0* | 78.7 ± 1.7* | |
| 20 | 11.5 ± 2.1 | 21.2 ± 2.2 | 17.4 ± 2.3 | 15.3 ± 1.9 | 89.4 ± 2.7* | 77.9 ± 2.3* | |
| 50 | 10.9 ± 1.8 | 18.6 ± 2.4 | 20.0 ± 2.3 | 16.8 ± 1.7 | 90.5 ± 1.2* | 76.8 ± 2.8* | |
Note: Water and sodium citrate (citrate) solution are used as control.
Significance is confirmed by one-way ANOVA, p < 0.05.
In control groups, the germination rate, root development and shoot rate are identical for both citrate and water-treated black eyed and lentil bean seeds. However, in the presence of AgNP, shoot rate of both black eye and lentil beans becomes smaller than root rate, indicating that some seeds germinated but the development of shoot is slowed down. AgNP treatment delays or inhibits shoot development even though seed germination rate is not affected (black eye bean) or even enhanced (lentil bean).
3. AgNP cause morphological, metabolic and proteomic changes – phytotoxicity mechanism
To understand phytotoxicity mechanisms of AgNP, more and more studies have been digging deeper to unveil how AgNP interact with terrestrial plants at tissue and cellular level. The morphological, metabolic and proteomic changes upon exposure to AgNP are summarized in Table 5. Each finding provides a piece of information regarding the mechanism puzzle of AgNP phytotoxicity. Details will be discussed in terms of uptake of Ag, role of AgNP and Ag+ ions, morphological, metabolic and proteomic response.
Table 5.
Phytotoxic pathways of AgNP on plants.
| Plants | Main action of AgNP* | Ref. |
|---|---|---|
| Zucchini | Decrease in plant biomass and transpiration. | [7] |
| Jasmine rice | AgNP trapped in the roots rather than transported to the leaves. Cause leaf cell deformation. | [11] |
| Wheat | Suppress the photosynthetic activity with a destruction of photosystems. Lead an improper regulation of PSI electron transport. | [14] |
| Maize | AgNP reduced transpiration and assimilation rate. | [16] |
| Cabbage | Increase ROS generation, malondialdehyde production, anthocyanin biosynthesis, and decrease chlorophyll content. Induce most of the genes related to secondary metabolism (glucosinolates, anthocyanin) and antioxidant activities. | [19] |
| Pea | Stimulate the activities of SOD and ascorbate peroxidase while inhibited activities of glutathione reductase and dehydroascorbate reductase. Declined the total ascorbate and glutathione contents and severely damage leaf and root anatomical structures. | [21] |
| Mung bean | Reduced Total chlorophyll content but increased proline content. Increased H2O2 level and lipid peroxidation levels in roots. | [22] |
| Turnip | Anthocyanin, malondialdehyde, and hydrogen peroxide levels were increased. ROS production and DNA damage were significantly elevated. | [23] |
| Wheat | Accumulation of Ag in the shoots. AgNP caused oxidative stress in roots, as indicated by accumulation of oxidized glutathione, and induced expression of a gene encoding a metallothionein involved in detoxification by metal ion sequestration. | [26] |
| Thale cress | Accumulate in leaves. Disrupt the thylakoid membrane structure and decrease chlorophyll content. Alter the transcription of antioxidant and aquaporin genes, changing the balance between the oxidant and antioxidant systems. | [30] |
| Wormwood | Enhance secondary metabolites production. Increase total phenolic AND flavonoid contents, antioxidant activity and SOD activity. | [33] |
| Kinnow | Enhance antioxidant activity and SOD activity. Total phenolic and flavonoid contents were significantly high. | [34] |
| Pine, Oak | Chloroplasts shape changed from lenticular to round. AgNP-treated oaks contained large starch granules while treated pines exhibit plastoglobules. | [35] |
| English oak | Disturbances in the shape of plastids, plastoglobules and the starch content of oak leaves. Showed the high degree of mycorrhization. | [36] |
| Castor bean | Cause an enhanced enzymatic activity of ROS enzymes and phenolic content in seedlings. | [39] |
| Maize | A significant change in metaxylem count. | [43] |
| Wheat | Increase in the quantum efficiency of energy trapping in the PSII reaction center. Accumulate in roots of seedlings and translocation to aerial parts. | [51] |
| Wheat | Proteins related to photosynthesis and protein synthesis increased, while glycolysis, signaling, and cell wall related proteins decreased. Proteins related to redox and mitochondrial electron transport chain also decreased. Glycolysis associated proteins (glyceraldehyde-3-phosphate dehydrogenase) increased, while phosphoenol pyruvate carboxylase decreased. | [52] |
| Rice | Increase in chlorophyll a and carotenoid contents. A low level of ROS concomitant with decreased amount of lipid peroxidation and H2O2 content. | [61] |
| Mustard | Improved photosynthetic quantum efficiency and higher chlorophyll contents in leaves. Levels of malondialdehyde and hydrogen peroxide decreased in the seedlings. Reduce ROS levels and decrease proline content. | [62] |
| Wheat | Morphological changes induced oxidative stress in plant cells as a consequence of the osmotic stress action. | [63] |
| Scots pine | High concentration inhibited the formation of mycorrhizae. Low concentration increased mycorrhizal colonization and the dry mass of roots. | [64] |
| Wheat | The wheat variety sensitive to pathogen action, showed a substantial increase in the TBARS contents, while other varieties showed less changes. | [65] |
| Rice | An enhancement in the activities of the antioxidant enzymes catalase, superoxide dismutase, ascorbate peroxidase, glutathione reductase and glutathione peroxidase generally occurred in primed seedlings. | [66] |
| Soybean | The abundances of glyoxalase II 3 and fermentation related proteins were time-dependently decreased. The alcohol dehydrogenase 1 and pyruvate decarboxylase 2 genes were downregulated. | [67] |
| Arabidopsis | AgNP were taken up by the root and primarily localized at the cell wall and intercellular spaces. Auxin accumulation was reduced in the root tips. AgNP inhibited root gravitropism with a reduction in auxin accumulation and expression of auxin receptors. | [68] |
| Common grass | Increased root and shoot Ag content but inhibited seedling growth. Seedlings fail to develop root hairs, with highly vacuolated and collapsed cortical cells and broken epidermis and root cap. | [69] |
Main actions include accumulation in roots and/or leaves, suppression of photosynthesis and/or transpiration, alteration of metabolism and proteomic expression.
Abbreviations: ROS, reactive oxygen species; SOD, superoxide dismutase; TBARS, 2-thiobarbituric acid reactive substances.
3.1. Uptake of Ag
It is a fact that AgNP treatment leads to uptake of Ag element through root system, followed by translocation and accumulation in specific parts of the plant. So that bio–nano interaction3,70 can take place and exhibit phytotoxicity. Detailed pathway of AgNP uptake is not certain but can be one or more of the following:71,72 (1) direct uptake by root hair cells; (2) cross cell wall due to small size (despite the pore size barrier); (3) internalization by endocytosis; (4) endocytosis involving Clathrin dependent and independent pathway; (5) either Symplastic or Appoplastic mechanism; and (6) through Xylem as the conducting vessel.
In the case of terrestrial plants, both Ag+ ions and AgNP can be absorbed through root system as is evident from many studies. For instance, Ag+ ion exposure (AgNO3 treatment) causes thinner and irregular root cells of Zea mays,43 indicating root tips can absorb soluble Ag+ ions. AgNP are also found in Lolium multiflorum root hairs69 and accumulated in root cap of wall cress (A. thaliana).73 These findings demonstrate that uptake of Ag is in the form of both nanoparticle and Ag+ ions, since Ag+ ions always co-exist with AgNP. One evidence is that both AgNP and soluble Ag are recovered from water extracts of the sand after growth of plants.26
Uptake of Ag by the three common beans is also confirmed by ICP-MS. Total amount and percentage of Ag intake by seed(s) is estimated in Table 6. For black eye bean, both intake mass and percent increased steadily with the increase in AgNP concentration. Three types of seeds showed different uptake pattern. Garbanzo seeds absorbed more Ag when AgNP concentration increased but percentage of Ag lies in 33–47%, approximately 2.2–3.2% by every single Garbanzo bean seed. It is proportional to AgNP concentration. Lentil seeds absorbed a relatively constant mass of Ag, 10 μg to 15 μg under AgNP concentration ranged 2–10 ppm, which further increased to 49 μg and 159 μg under AgNP of 20 ppm and 50 ppm respectively. The percentage of Ag remains constant between 12–17%, accounting for 0.8–1.2% for each lentil seed, except for the case of 2 ppm AgNP that has a percent of 52% that is equivalent to 3.5% for a single lentil bean seed.
Table 6.
Mass and percent of Ag intake by three common bean seeds.
| [AgNP], ppm | Black eye | Garbanzo | Lentil | |||
|---|---|---|---|---|---|---|
| Mass (μg) | Percent, | Mass (μg) | Percent | Mass (μg) | Percent | |
| 2 | 4.6 ± 0.7 | 17.9 ± 2.8 | 16.9 ± 1.5 | 47.3 ± 4.1 | 13.3 ± 1.2 | 52.1 ± 4.7 |
| 5 | 19.5 ± 2.4 | 24.8 ± 3.1 | 39.6± 3.3 | 38.1 ± 3.2 | 10.5 ± 1.4 | 16.6 ± 2.2 |
| 10 | 55.4 ± 6.9 | 29.5 ± 3.7 | 70.1 ± 4.5 | 37.8± 2.4 | 14.8 ± 2.4 | 11.8 ± 1.9 |
| 20 | 152.8 ± 16.0 | 44.0 ± 4.6 | 133.1 ± 11.6 | 33.3 ± 2.9 | 48.8 ± 5.9 | 17.4 ± 2.1 |
| 50 | 488.3 ± 48.9 | 43.9 ± 4.4 | 566.3 ± 51.1 | 42.1 ± 3.8 | 158.5 ± 20.2 | 15.7 ± 2.0 |
| 2 | 0.3 | 1.2 | 1.1 | 3.2 | 0.9 | 3.5 |
| 5 | 1.3 | 1.7 | 2.6 | 2.5 | 0.7 | 1.1 |
| 10 | 3.7 | 2.0 | 4.7 | 2.5 | 1.0 | 0.8 |
| 20 | 10.2 | 2.9 | 8.9 | 2.2 | 3.3 | 1.2 |
| 50 | 32.6 | 2.9 | 37.8 | 2.8 | 10.6 | 1.0 |
Top half of the table shows the mass and percentage of Ag intake by a group of seeds (average ± SD, 15 seeds) while bottom part shows the average mass and percent of Ag intake by a single seed. Percent is estimated based on total mass of initial dose.
During the soaking procedure, over 70% of lentil seed shell and around 25% of black eye bean seed shell fell off. Garbanzo bean seeds remained intact. This indicates that shells of the tested bean seeds do not protect seeds from absorbing Ag from solution and whether the shell fell off or not had no impact on Ag intake. Instead, the appearance and smell of the solutions resulted from soaking seeds demonstrate the release of some organic matter from the seeds. It is hard to draw any conclusion on the intake behavior of Ag by these three common beans whereas each type of bean shows a unique pattern of Ag intake when soaked in AgNP solutions.
3.2. Translocation and accumulation
Traces of AgNP has been found in root, leaves and stems, mainly in vascular system,74,75 which could explain how AgNP travel through different parts of the plant. The driving force and kinetics for translocation is not yet very clear but it is species dependent. Only pieces of information can be found in literature. For example, AgNP accumulate in root cap of wall cress (A. thaliana),73 in the shoots of wheat (T. aestivum L.) seedlings26 and in leaves of A. thaliana.30 In the case of Jasmine rice (O. sativa L.),11 AgNP is trapped in the roots rather than transported to the leaves.
Accumulation often triggers robust interactions between AgNP and local tissue, leading to acute toxicity to the plant.3,76 Different consequences can be observed at tissue, cellular and molecular level, i.e., biomass change (weight loss), root elongation, alterations in the shape and size of the cells43 and damage of vascular cylinder, cortex and epidermis of root tips as well as metaxylem vessel.3,75,77–80
3.3. AgNP vs. Ag+ ions
Albeit both AgNP and soluble Ag+ ions show toxicity to living organisms it is difficult to distinguish their toxicity profiles. Hence this has been an enigma for a long time.81–85 Released Ag+ ions from AgNP are generally considered culprits for high toxicity of AgNP to mammal cells and microbe.54,83,85,86 In terrestrial plants, effect of AgNP and AgNO3 has been compared extensively but has ended in disagreements, even in the same plant species.
AgNP is reported to exhibit more toxicity than equivalent Ag+ ion in terms of inhibition to seed germination and seedling growth of L. esculentum, maize (Zea mays)8 and A. thaliana.30 One evidence is provided by adding CaCl2, where the negative effects disappeared in maize. This indicates that acute negative effects can be largely attributed to free Ag+ ions rather than AgNP.16 In contrast, germination and root elongation revealed lower toxicity in AgNP than ions in both maize and cabbage.43 One explanation is that AgNP exposure resulted in lower Ag biouptake. Also, no significant difference between AgNP and Ag+ ions is reported in wheat.63 Both AgNP and AgNO3 cause a significant change in metaxylem count in both maize and cabbage,43 and enhanced enzymatic activity of reactive oxygen species (ROS) enzymes and phenolic content in seedlings.39 Both AgNP and AgNO3 treatments cause changes in proteins involved in the redox regulation and in the sulfur metabolism that are essential to maintain cellular homeostasis.87
Yin et al.69 compared the action of AgNP and Ag+ ions in common grass (Lolium multiflorum). Seedlings exposed to AgNP failed to develop root hairs, accompanied by highly vacuolated and collapsed cortical cells and broken epidermis and root cap. However, identical concentrations of AgNO3 showed no such abnormalities. Adding cysteine (which binds Ag+) mitigated the effects of AgNO3 but did not reduce the toxicity of AgNP treatments. This means effects of AgNP are not simply due to the release of Ag ions, in accordance with findings in Eruca sativa.87
Insight into their action pathways could somehow reconcile the disagreement. Soluble Ag+ ions seems to be much easily absorbed by root systems of plants and traveled throughout the plants. Instead, AgNP normally resulted in lower Ag bio uptake.43 AgNP toxicity is often mainly caused by interaction with adjacent biomolecules and the main factor responsible appears to be total surface area. Smaller AgNP (6 nm) seems to inhibit the growth of grass more strongly as compared to larger AgNP (25 nm)69 due to the larger surface area.
3.4. Morphologic changes
Visible consequences caused by AgNP exposure include inhibited germination and growth, root and shoot elongation, shape and size change of cells (under the microscope) and overall health to name a few. AgNP blended with nicotinic acid and KNO3 exerted stimulatory effect on the number of grains/spike, 100-grains weight and yield of wheat without any negative influence on growth and morphology of next generation wheat plants.52
The growth of black eye bean treated with AgNP was also monitored. Experiment groups had the seeds soaked in 20 ppm AgNP and watered with 50 mL of 20 ppm AgNP solution 3 times a week (4 plants per pot). Several morphological observations included: (1) Seedling growth of beans soaked in AgNP was much slower and were substantially shorter than seeds soaked in water in the same environmental conditions. (2) AgNP irrigated bean seedlings tended to have irregular leaf shapes. (3) AgNP-treated plants were more sensitive and vulnerable to change of environmental parameters. For instance, in a case of temperature drop, AgNP-treated black eye beans exhibited symptoms of freezing and drying of leaves before the control group. (4) AgNP treatment altered the soil conditions in many ways, which could further cause growth differences or health issues of the plant. Another interesting observation was that soil irrigated with AgNP solution dried out much faster as compared to soil irrigated with water.
3.5. Metabolic and proteomic response
Interaction between AgNP and plants could trigger a series of metabolic and proteomic response.88–90 Using general engineered nanomaterials as reference, such consequence could possibly include: (1) altered stomatal conductivity resulting in decreased photosynthesis; (2) chloroplast disorganization reducing photosynthetic activity; (3) altered acquisition of water and nutrients (via the changes of epidermis, cortex, pitch, cambium, xylem and/or phloem); (4) impediment in the generation of secondary metabolites; (5) histological alteration (some are listed above); (6) alteration in the normal electron transfer signal pathways; (7) changes in cellular structure and metabolism caused by ROS; (8) negative influence on plant genome system, either up- or downregulated certain genes; and (9) certain protein get expressed or repressed.
Phytotoxicity study showed that AgNP could induce stress in plants by manipulating the endogenous mechanisms. For instance, oxidative stress,26 osmotic stress action,63 oxygen-deprivation stress,67 as stated in Table 5. In response to these stresses, plants release various defensive compounds, known as antioxidant secondary metabolites,91 accompanied by altered enzyme activity as well as regulated gene expression. Several representatives are shown below.
In E. sativa, AgNP caused changes in proteins involved in the redox regulation and in the sulfur metabolism as well as proteins related to the endoplasmic reticulum and vacuole, indicating that these two organelles are targets of the AgNP action.87
In rice (O. sativa L., cv. Swarna),61 antioxidative enzyme activity and related gene expression level is changed. Elevated levels of catalase, ascorbate peroxidase and glutathione reductase activities were recorded with improved seedling growth. Enzymes of the ascorbate cycle, such as ascorbate peroxidase and glutathione reductase were more active in ensuring protection against oxidative damage since the activity of superoxide dismutase (SOD) was not significantly altered at low concentration of AgNP. There was significant upregulation of catalase and ascorbate peroxidase gene expressions in seedlings exposed to AgNP. Acceleration of seedling growth was due to increased efficiency of redox reactions, revealed by the antioxidant enzyme activities and gene expression patterns coupled with the levels of H2O2 and lipid peroxidation.
Besides proteins related to defense mechanism (like oxidative stress), proteins involved in photosynthesis appears to have interested many investigators. Jhanzab et al.52 carried out proteomic analysis in wheat (T. aestivum L.) where growth was promoted by AgNP blended with nicotinic acid and KNO3. This was achieved by the regulation of energy metabolism due to suppression of glycolysis. Increased proteins included those associated with photosynthesis and glycolysis (such as glyceraldehyde-3-phosphate dehydrogenase). Antioxidant enzyme activities such as SOD, catalase, and peroxidase are promoted. Suppressed proteins were those related to glycolysis, signaling, cell wall, redox and mitochondrial electron transport chain as well as phosphoenol pyruvate carboxylase.
Besides metabolism, modifications in plant growth were also noticed. AgNP increased seedling biomass at low concentrations but reduces seedling size at high concentration in Physalis peruviana L. Interestingly, no change in the seedlings antioxidant metabolism was observed.92 Pyrrolizidine alkaloids, as a common naturally occurring alkaloid, were widely identified as capping agents during green synthesis of AgNP.93,94 Metabolism of pyrrolizidine alkaloids produced a series of DNA reactive pyrrolic metabolites95–99 that could lead to complicated interactions with AgNP as well.
3.6. Role of soil
The role of soil was not our focus but it could not be ignored during the evaluation of the influence of AgNP on terrestrial plants. The soil itself is a complicated system containing mineral, organic matter, air, water and numerous microbial cells. Each component could potentially interact with AgNP and alter their properties before AgNP can actually be absorbed by plant roots. Meanwhile, each component also determines the growth status of the plants. Thus, the essential question is how much AgNP the soil could hold and how these AgNP would impact on soil components (specifically soil microbial).
3.6.1. AgNP holding capacity of normal garden soil – 12.3 μg/g of dry soil
A device was designed to test the amount of AgNP that normal garden soil could retain in the current experimental conditions, as shown in Figure 1. A cylinder with diameter of 6.5 cm and height of 7.0 cm contained approximately 30.0 g of completely dried soil and was slowly irrigated with 50 mL deionized water with or without AgNP. Filtrate was collected for further ICP-MS characterization. To mimic the practical agricultural condition, the soil was neither flooded nor dried out, but saturated with deionized water. The depth was kept at 7.5 cm to mimic the depth of bean roots.
Figure 1.
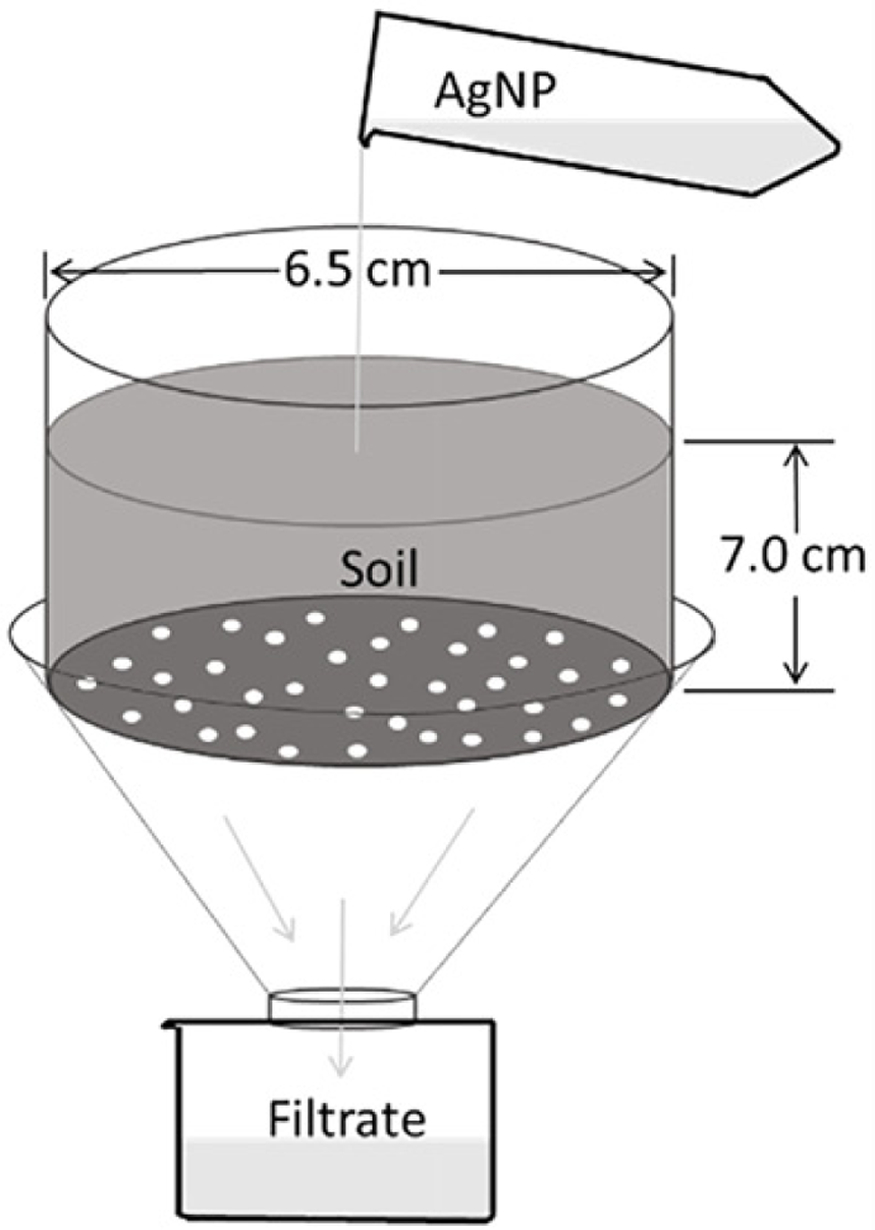
Schematic drawing of device designed to evaluate AgNP retention by soil.
Results showed that 30.0 g of dried garden soil could retain 73.8 ±11.9% of total Ag, when slowly irrigated with 50 mL of 50 ppm AgNP solution. This percent was obtained from the initial two irrigation, which yielded an AgNP holding capacity of at least 12.3 μg Ag per g of dried soil (or 0.16 μg Ag per cm3 of dried soil). The AgNP retention was dependent on soil type and property.100
It has to be mentioned that in the control group where the soil was treated with deionized water (before treatment of AgNP solution), 21.8 μg of Ag was washed out by 50 mL of water. This indicated that original commercial garden soil contains traceable Ag elements although the form is not clear.
The presence of soil has proven to have a modest influence on AgNP toxicity and inhibitory effect of AgNP is less pronounced compared to other media (aqueous solution or agar).13,18,55 One illustration is that soil contains varieties of organic components that have high binding affinity to AgNP. Thus, AgNP is more likely to be “trapped” in the soil and its toxicity is reconciled.
3.6.2. AgNP affect soil microbials activity
AgNP is well known owing for its antimicrobial activity101–104 and could possibly pose a threat to the microorganism community in the soil. Recent studies105–108 provide evidence that AgNP contamination exerted a significant negative effect on soil microbial biomass, relative abundance, growth, diversity and some enzyme activity. Meanwhile, no significant effect was also reported in long time exposure109 or during the field study.110
AgNP could simultaneously impact plant pathogens. Seedling blight of wheat caused by Fusarium culmorum infection has been reduced by AgNP treatment51 while AgNP did not appear to affect the growth and development of P. herpotrichoides,65 a plant pathogen infecting rye and wheat. So far, however, data is not convincing enough to draw any safe conclusions.
4. Perspective
Many reports have focused on the phytotoxicity of AgNP on terrestrial plants but without providing enough information about the mechanisms of action. At least three aspects need to be more comprehensively addressed.
4.1. Provision of more rigorous and comprehensive data
AgNP show phytotoxicity to terrestrial plants, indicating as a potential threat to ecosystem. Similar effects have been found in other engineered nanomaterials, as extensively reviewed.71–73,79,80,111 There is no convincing evidence to draw any clear conclusion on phytotoxic pathways. AgNP exert unique influence on a terrestrial plant of a specific class, family, genus, or even same species but at different stages of growth.7,10,49,112 Each evaluation should be dealt as a case-by-case scenario that is evidenced by the results from three type of beans. This indicates that a database containing such massive information is the need and would benefit a broad range of people from academics and industry, including scientists, product developers, government agencies, and policy makers. With the assistance of big data or other computer based techniques, this goal could be achieved consuming much less effort and cost.
The main challenges are the complexity of both nanoparticle and botanic systems together with the complicated ambient conditions. Too many variables are involved in each study, i.e., nanoparticles (metal core, synthesis method, coating agents, size, shape, etc.), concentration of nanoparticles, ways and time of treatment, exposure time, ambient conditions (temperature, humidity, etc.) and of course species of plants. However, some important details were found to be ambiguous or completely missing in several references, including AgNP size, shape, coating agents, suspension solvent and concentration. Any of these factors could potentially contribute to significant and varied outcomes. Therefore, a concern is also raised that rigorous studies are needed to comprehensively include all these crucial parameters. Besides, a proper control must also be placed in experimental design.
4.2. In-depth study of bio–nano interaction continues to be a frontier topic
Investigations on the impact of AgNP on terrestrial plants began around a decade ago. It has blossomed roughly five years ago, with a shift of research focus from morphological observations to mechanistic studies, i.e., bio–nano interactions. Bio–nano interaction determines the behavior of biomolecules near AgNP surface, transformation and fate of AgNP, as well as impact on plants/environment.3,56,113 In depth study of bio–nano interaction serves as the most promising pathway to understand mechanism of nanomaterials action, which will further guide fabrication and disposal of metal nanoparticle containing products to ensure their safe application.
As summarized above, many aspects regarding the bio–nano interactions at various levels in terrestrial plants have been studied. However, more puzzles remain to be solved and this will continue to be a hot research field in near future.
4.3. Moving policy and regulation forward for nanotechnology applications
The main driving force for research in this field is the huge economic market of metal nanoparticles.1,2 AgNP in particular shows the promising applications including the agriculture and food industry. Industries are proposing more and more AgNP containing products to be marketed that has to be first approved by government agencies. However, current legislation and regulation regarding engineered nanomaterial is very general and limited and many details remain blank since each evaluation could be unique.114–116 Therefore, it is extremely essential to move policy and regulation forward for nanotechnology applications particularly in agriculture.117 On the other hand, experimental findings and phytotoxicity data serve as fundamental guidance and reference to make policy and regulations. Therefore, research on the influence of AgNP on terrestrial plants is of urgent need and it lays corner stones to move legislation and regulations forward.
Acknowledgment
The authors wish to thank the Army Research Office for funding through a cooperative agreement with Johns Hopkins University (W911NF-122-0022) and the National Institute of Minority Health and Health Disparities (NIMHD) for the cooperative agreement 1U54MD013376-01A1, which supported the core research facilities used for this research.
Footnotes
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/lesc.
References
- 1.Market MN. Metal Nanoparticles Market by metal (Platinum, Gold, Silver, Iron, Titanium, Copper, Nickel), End-use industry (Pharmaceutical & healthcare, Electrical & electronics, Catalyst, Personal care & cosmetics), and Region – Global Forecast to 2022, Top Market Reports; 2018. [Google Scholar]
- 2.He X, Hwang H-M. Nanotechnology in food science: functionality, applicability, and safety assessment. J Food Drug Anal. 2016;24(4):671–681. doi: 10.1016/j.jfda.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He X, Fu P, Aker WG, et al. Toxicity of engineered nanomaterials mediated by nano–bio–eco interactions. J Environ Sci Health C. 2018;36(1):21–42. doi: 10.1080/10590501.2017.1418793. [DOI] [PubMed] [Google Scholar]
- 4.He X, Deng H, Hwang H-M. The current application of nanotechnology in food and agriculture. J Food Drug Anal. 2019;27(1):1–21. doi: 10.1016/j.jfda.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittol M, Tomacheski D, Simões DN, et al. Macroscopic effects of silver nanoparticles and titanium dioxide on edible plant growth. Environ Nanotechnol Monit Manage. 2017;8:127–133. doi: 10.1016/j.enmm.2017.07.003. [DOI] [Google Scholar]
- 6.Deng H, Zhang Y, Yu H. Nanoparticles considered as mixtures for toxicological research. J Environ Sci Health C. 2018;36(1):1–20. doi: 10.1080/10590501.2018.1418792. [DOI] [PubMed] [Google Scholar]
- 7.Stampoulis D, Sinha SK, White JC. Assay-dependent phytotoxicity of nanoparticles to plants. Environ Sci Technol. 2009;43(24):9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- 8.Ravindran A, Prathna TC, Verma VK, et al. Bovine serum albumin mediated decrease in silver nanoparticle phytotoxicity: root elongation and seed germination assay. Toxicol Environ Chem. 2012;94(1):91–98. doi: 10.1080/02772248.2011.617034. [DOI] [Google Scholar]
- 9.Scherer MD, Sposito JCV, Falco WF, et al. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: a close analysis of particle size dependence. Sci Total Environ. 2019;660:459–467. doi: 10.1016/j.scitotenv.2018.12.444. [DOI] [PubMed] [Google Scholar]
- 10.Karami Mehrian S, Heidari R, Rahmani F, et al. Effect of chemical synthesis silver nanoparticles on germination indices and seedlings growth in seven varieties of Lycopersicon esculentum Mill (tomato) plants. J Clust Sci. 2016;27(1):327–340. doi: 10.1007/s10876-015-0932-4. [DOI] [Google Scholar]
- 11.Thuesombat P, Hannongbua S, Akasit S, et al. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol Environ Saf. 2014;104(1):302–309. doi: 10.1016/j.ecoenv.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Zuverza-Mena N, Armendariz R, Peralta-Videa JR, et al. Effects of silver nanoparticles on radish sprouts: root growth reduction and modifications in the nutritional value. Front Plant Sci.. 2016;7:1–11. doi: 10.3389/fpls.2016.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Temsah YS, Joner EJ. Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol. 2012;27(1):42–49. doi: 10.1002/tox.20610. [DOI] [PubMed] [Google Scholar]
- 14.Rastogi A, Zivcak M, Tripathi DK, et al. Phytotoxic effect of silver nanoparticles in Triticum aestivum: improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica. 2019;57(1):209–216. doi: 10.32615/ps.2019.019. [DOI] [Google Scholar]
- 15.Wang C, Jiang K, Wu B, et al. Silver nanoparticles with different particle sizes enhance the allelopathic effects of Canada goldenrod on the seed germination and seedling development of lettuce. Ecotoxicology. 2018;27(8):1116–1125. doi: 10.1007/s10646-018-1966-9. [DOI] [PubMed] [Google Scholar]
- 16.Fellmann S, Eichert T. Acute Effects of Engineered Nanoparticles on the Growth and Gas Exchange of Zea mays L.—what are the Underlying Causes? Water Air Soil Pollut. 2017;228(5):176. [Google Scholar]
- 17.Parveen A, Rao S. Effect of nanosilver on seed germination and seedling growth in Pennisetum glaucum. J Clust Sci. 2015;26(3):693–701. doi: 10.1007/s10876-014-0728-y. [DOI] [Google Scholar]
- 18.Lee W-M, Kwak JI, An Y-J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: media effect on phytotoxicity. Chemosphere. 2012; 86(5):491–499. doi: 10.1016/j.chemosphere.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 19.Baskar V, Venkatesh J, Park SW. Impact of biologically synthesized silver nanoparticles on the growth and physiological responses in Brassica rapa ssp. pekinensis. Environ Sci Pollut Res. 2015;22(22):17672–17682. doi: 10.1007/s11356-015-4864-1. [DOI] [PubMed] [Google Scholar]
- 20.Li CC, Wang YJ, Dang F, et al. Mechanistic understanding of reduced AgNP phytotoxicity induced by extracellular polymeric substances. J Hazard Mater. 2016; 308:21–28. doi: 10.1016/j.jhazmat.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 21.Tripathi DK, Singh S, Singh S, et al. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol Biochem. 2017;110:167–177. doi: 10.1016/j.plaphy.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Nair PMG, Chung IM. Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna radiata L.). Acta Physiol Plant. 2015;37(1):1719. [Google Scholar]
- 23.Thiruvengadam M, Gurunathan S, Chung IM. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma. 2015;252(4):1031–1046. doi: 10.1007/s00709-014-0738-5. [DOI] [PubMed] [Google Scholar]
- 24.Vannini C, Domingo G, Onelli E, et al. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J Plant Physiol. 2014; 171(13):1142–1148. doi: 10.1016/j.jplph.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Mazumdar H, Ahmed GU. Phytotoxicity effect of silver nanoparticles on Oryza sativa. Int J ChemTech Res. 2011;3(3):1494–1500. [Google Scholar]
- 26.Dimkpa CO, McLean JE, Martineau N, et al. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol. 2013;47(2): 1082–1090. doi: 10.1021/es302973y. [DOI] [PubMed] [Google Scholar]
- 27.Sweet MJ, Singleton I. Soil contamination with silver nanoparticles reduces Bishop pine growth and ectomycorrhizal diversity on pine roots. J Nanopart Res. 2015; 17(11):1–8. doi: 10.1007/s11051-015-3246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abd-Alla MH, Nafady NA, Khalaf DM. Assessment of silver nanoparticles contamination on faba bean-Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: implications for induction of autophagy process in root nodule. Agric Ecosyst Environ. 2016;218:163–177. doi: 10.1016/j.agee.2015.11.022. [DOI] [Google Scholar]
- 29.Almutairi ZM, Alharbi A. Effect of silver nanoparticles on seed germination of crop plants. J Adv Agric. 2015;4(1):283–288. [Google Scholar]
- 30.Qian H, Peng X, Han X, et al. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J Environ Sci (China). 2013;25(9):1947–1956. doi: 10.1016/S1001-0742(12)60301-5. [DOI] [PubMed] [Google Scholar]
- 31.Ghavam M Effect of silver nanoparticles on seed germination and seedling growth in Thymus vulgaris L. and Thymus daenensis Celak under salinity stress. J Rangeland Sci. 2018;8(1):93–100. [Google Scholar]
- 32.Khalaki MA, Ghorbani A, Moameri M. Effects of silica and silver nanoparticles on seed germination traits of Thymus kotschyanus in laboratory conditions. J Rangeland Sci. 2016;6(3):221–231. [Google Scholar]
- 33.Hussain M, Raja NI, Mashwani ZUR, et al. In vitro seed germination and biochemical profiling of Artemisia absinthium exposed to various metallic nanoparticles. 3 Biotech. 2017;7(2):101. doi: 10.1007/s13205-017-0741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain M, Raja NI, Iqbal M, et al. Seed germination and biochemical profile of Citrus reticulata (Kinnow) exposed to green synthesised silver nanoparticles. IET Nanobiotechnol. 2018;12(5):688–693. doi: 10.1049/iet-nbt.2017.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aleksandrowicz-Trzcińska M, Bederska-Blaszczyk M, Szaniawski A, et al. The effects of copper and silver nanoparticles on container-grown Scots pine (Pinus sylvestris L.) and pedunculate oak (Quercus robur L.) seedlings. Forests. 2019;10(3): 269. doi: 10.3390/f10030269. [DOI] [Google Scholar]
- 36.Olchowik J, Bzdyk RM, Studnicki M, et al. The effect of silver and copper nanoparticles on the condition of English oak (Quercus robur L.) seedlings in a container nursery experiment. Forests. 2017;8(9):310. doi: 10.3390/f8090310. [DOI] [Google Scholar]
- 37.FAO, World Food and Agriculture – Statistical Pocketbook 2018. Rome, Italy: FAO; 2018. [Google Scholar]
- 38.Salama HM. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int Res J Biotechnol. 2012;3(10):190–197. [Google Scholar]
- 39.Yasur J, Rani PU. Environmental effects of nanosilver: impact on castor seed germination, seedling growth, and plant physiology. Environ Sci Pollut Res. 2013; 20(12):8636–8648. doi: 10.1007/s11356-013-1798-3. [DOI] [PubMed] [Google Scholar]
- 40.Almutairi ZM. Influence of silver nano-particles on the salt resistance of tomato (Solanum lycopersicum) during germination. Int J Agric Biol. 2016;18(2):449–457. doi: 10.17957/IJAB/15.0114. [DOI] [Google Scholar]
- 41.Barabanov PV, Gerasimov AV, Blinov AV, et al. Influence of nanosilver on the efficiency of Pisum sativum crops germination. Ecotoxicol Environ Saf. 2018;147: 715–719. doi: 10.1016/j.ecoenv.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 42.Yadu B, Chandrakar V, Korram J, et al. Silver nanoparticle modulates gene expressions, glyoxalase system and oxidative stress markers in fluoride stressed Cajanus cajan L. J Hazard Mater. 2018;353:44–52. doi: 10.1016/j.jhazmat.2018.03.061. [DOI] [PubMed] [Google Scholar]
- 43.Pokhrel LR, Dubey B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci Total Environ. 2013;452–453: 321–332. doi: 10.1016/j.scitotenv.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 44.Jo YK, Cromwell W, Jeong HK, et al. Use of silver nanoparticles for managing Gibberella fujikuroi on rice seedlings. Crop Prot. 2015;74:65–69. doi: 10.1016/j.cropro.2015.04.003. [DOI] [Google Scholar]
- 45.Liu G, Zhang M, Jin Y, et al. The effects of low concentrations of silver nanoparticles on wheat growth. Seed Qual Soil Microb Commun Water Air Soil Pollut. 2017;228(9):348. [Google Scholar]
- 46.Thangavelu RM, Munisamy B, Krishnan K. Effect of deoxycholate capped silver nanoparticles in seed dormancy breaking of Withania somnifera. Curr Sci. 2019; 116(6):952–958. [Google Scholar]
- 47.Aleksandrowicz-Trzcińska M, Olchowik J, Studnicki M, et al. Do silver nanoparticles stimulate the formation of ectomycorrhizae in seedlings of pedunculate oak (Quercus robur L.)? Symbiosis. 2019;79(1):89. doi: 10.1007/s13199-019-00628-0. [DOI] [Google Scholar]
- 48.Iram F, Iqbal MS, Athar MM, et al. Glucoxylan-mediated green synthesis of gold and silver nanoparticles and their phyto-toxicity study. Carbohydr Polym. 2014; 104(1):29–33. doi: 10.1016/j.carbpol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Kim DY, Saratale RG, Shinde S, et al. Green synthesis of silver nanoparticles using Laminaria japonica extract: characterization and seedling growth assessment. J Clean Prod. 2018;172:2910–2918. doi: 10.1016/j.jclepro.2017.11.123. [DOI] [Google Scholar]
- 50.Maity A, Natarajan N, Pastor M, et al. Nanoparticles influence seed germination traits and seed pathogen infection rate in forage sorghum (Sorghum bicolour) and cowpea (Vigna unguiculata). Indian J Exp Biol. 2018;56(6):363–372. [Google Scholar]
- 51.Gorczyca A, Pociecha E, Kasprowicz M, et al. Effect of nanosilver in wheat seedlings and Fusarium culmorum culture systems. Eur J Plant Pathol. 2015;142(2): 251–261. doi: 10.1007/s10658-015-0608-9. [DOI] [Google Scholar]
- 52.Jhanzab HM, Razzaq A, Bibi Y, et al. Proteomic analysis of the effect of inorganic and organic chemicals on silver nanoparticles in wheat. IJMS. 2019;20(4):825. doi: 10.3390/ijms20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amooaghaie R, Saeri MR, Azizi M. Synthesis, characterization and biocompatibility of silver nanoparticles synthesized from Nigella sativa leaf extract in comparison with chemical silver nanoparticles. Ecotoxicol Environ Saf. 2015;120:400–408. doi: 10.1016/j.ecoenv.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 54.Deng H, McShan D, Zhang Y, et al. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ Sci Technol. 2016;50(16):8840–8848. doi: 10.1021/acs.est.6b00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yin L, Colman BP, McGill BM, et al. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE. 2012;7(10):e47674. doi: 10.1371/journal.pone.0047674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deng H, Yu H. Self-assembly of rhodamine 6G on silver nanoparticles. Chem Phys Lett. 2018;692(16):75–80. doi: 10.1016/j.cplett.2017.12.003. [DOI] [Google Scholar]
- 57.Deng H, Gao Y, Dasari TPS, et al. A facile 3D construct of graphene oxide embedded with silver nanoparticles and its potential application as water filter. J Miss Acad Sci. 2016;61(2):190–197. [Google Scholar]
- 58.Tejamaya M, Römer I, Merrifield RC, et al. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol.. 2012; 46(13):7011–7017. doi: 10.1021/es2038596. [DOI] [PubMed] [Google Scholar]
- 59.Sun Y, Xia Y. Shape-controlled synthesis of gold and silver nanoparticles. Science. 2002;298(5601):2176–2179. doi: 10.1126/science.1077229. [DOI] [PubMed] [Google Scholar]
- 60.Deng H, Yu H. Silver nanoparticle surface enabled self-assembly of organic dye molecules. Materials. 2019;12(16):2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta SD, Agarwal A, Pradhan S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: an insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol Environ Saf. 2018;161:624–633. doi: 10.1016/j.ecoenv.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 62.Sharma P, Bhatt D, Zaidi MGH, et al. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl Biochem Biotechnol. 2012;167(8):2225–2233. doi: 10.1007/s12010-012-9759-8. [DOI] [PubMed] [Google Scholar]
- 63.Barbasz A, Kreczmer B, Oćwieja M. Effects of exposure of callus cells of two wheat varieties to silver nanoparticles and silver salt (AgNO 3). Acta Physiol Plant. 2016; 38(3):1–11. [Google Scholar]
- 64.Aleksandrowicz-Trzcińska M, Szaniawski A, Studnicki M, et al. The effect of silver and copper nanoparticles on the growth and mycorrhizal colonisation of scots pine (Pinus sylvestris l.) in a container nursery experiment. IForest. 2018;11(5):690–697. doi: 10.3832/ifor2855-011. [DOI] [Google Scholar]
- 65.Belava VN, Panyuta OO, Yakovleva GM, et al. The effect of silver and copper nanoparticles on the wheat—Pseudocercosporella herpotrichoides Pathosystem. Nanoscale Res Lett. 2017;12(1):250. doi: 10.1186/s11671-017-2028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thuesombat P, Hannongbua S, Ekgasit S, et al. Effects of silver nanoparticles on hydrogen peroxide generation and antioxidant enzyme responses in rice. J Nanosci Nanotechnol.. 2016;16(8):8030–8043. doi: 10.1166/jnn.2016.12754. [DOI] [Google Scholar]
- 67.Mustafa G, Sakata K, Hossain Z, et al. Proteomic study on the effects of silver nanoparticles on soybean under flooding stress. J. Proteom 2015;122:100–118. doi: 10.1016/j.jprot.2015.03.030. [DOI] [PubMed] [Google Scholar]
- 68.Sun J, Wang L, Li S, et al. Toxicity of silver nanoparticles to Arabidopsis: inhibition of root gravitropism by interfering with auxin pathway. Environ Toxicol Chem. 2017;36(10):2773–2780. doi: 10.1002/etc.3833. [DOI] [PubMed] [Google Scholar]
- 69.Yin L, Cheng Y, Espinasse B, et al. More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol. 2011;45(6):2360–2367. doi: 10.1021/es103995x. [DOI] [PubMed] [Google Scholar]
- 70.Nel AE, Mädler L, Velegol D, et al. Understanding biophysicochemical interactions at the nano–bio interface. Nat Mater. 2009;8(7):543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 71.Tripathi DK, Singh S, Singh S, et al. An overview on manufactured nanoparticles in plants: uptake, translocation, accumulation and phytotoxicity. Plant Physiol Biochem. 2017;110:2–12. [DOI] [PubMed] [Google Scholar]
- 72.Rico CM, Majumdar S, Duarte-Gardea M, et al. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem. 2011;59(8):3485–3498. doi: 10.1021/jf104517j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ma X, Geiser-Lee J, Deng Y, et al. Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ. 2010;408(16):3053–3061. doi: 10.1016/j.scitotenv.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 74.Kumari M, Mukherjee A, Chandrasekaran N. Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ. 2009;407(19):5243–5246. doi: 10.1016/j.scitotenv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 75.Nair R, Varghese SH, Nair BG, et al. Nanoparticulate material delivery to plants. Plant Sci. 2010;179(3):154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- 76.Ray PC, Yu H, Fu PP. Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C. 2009;27(1):1–35. doi: 10.1080/10590500802708267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geisler-Lee J, Wang Q, Yao Y, et al. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology. 2012;7(3):323–337. doi: 10.3109/17435390.2012.658094. [DOI] [PubMed] [Google Scholar]
- 78.Lin D, Xing B. Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol. 2008;42(15):5580–5585. doi: 10.1021/es800422x. [DOI] [PubMed] [Google Scholar]
- 79.Miralles P, Church TL, Harris AT. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ Sci Technol. 2012;46(17): 9224–9239. doi: 10.1021/es202995d. [DOI] [PubMed] [Google Scholar]
- 80.Yang J, Cao W, Rui Y. Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J Plant Interact. 2017;12(1):158–169. doi: 10.1080/17429145.2017.1310944. [DOI] [Google Scholar]
- 81.Chernousova S, Epple M. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed.. 2013;52(6):1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 82.Ivask A, ElBadawy A, Kaweeteerawat C, et al. Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver. Acs Nano. 2014;8(1): 374–386. doi: 10.1021/nn4044047. [DOI] [PubMed] [Google Scholar]
- 83.Kittler S, Greulich C, Diendorf J, et al. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater. 2010;22(16):4548–4554. doi: 10.1021/cm100023p. [DOI] [Google Scholar]
- 84.van der Zande M, Vandebriel RJ, Van Doren E, et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano. 2012;6(8):7427–7442. doi: 10.1021/nn302649p. [DOI] [PubMed] [Google Scholar]
- 85.Beer C, Foldbjerg R, Hayashi Y, et al. Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicol Lett. 2012;208(3):286–292. doi: 10.1016/j.toxlet.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 86.Sotiriou GA, Pratsinis SE. Antibacterial activity of nanosilver ions and particles. Environ Sci Technol. 2010;44(14):5649–5654. doi: 10.1021/es101072s. [DOI] [PubMed] [Google Scholar]
- 87.Vannini C, Domingo G, Onelli E, et al. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE. 2013;8(7): e68752. doi: 10.1371/journal.pone.0068752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Farooqui A, Tabassum H, Ahmad A, et al. Role of nanoparticles in growth and development of plants: a review. Int J Pharm Bio Sci. 2016;7(4):P22–P37. doi: 10.22376/ijpbs.2016.7.4.p22-37. [DOI] [Google Scholar]
- 89.Cox A, Venkatachalam P, Sahi S, et al. Reprint of: silver and titanium dioxide nanoparticle toxicity in plants: a review of current research. Plant Physiol Biochem. 2017;110:33–49. doi: 10.1016/j.plaphy.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Rai PK, Kumar V, Lee S, et al. Nanoparticle-plant interaction: implications in energy, environment, and agriculture. Environ Int. 2018;119:1–19. doi: 10.1016/j.envint.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 91.Zaka M, Abbasi BH, Rahman LU, et al. Synthesis and characterisation of metal nanoparticles and their effects on seed germination and seedling growth in commercially important Eruca sativa. IET Nanobiotechnol. 2016;10(3):134–140. doi: 10.1049/iet-nbt.2015.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Timoteo CO, Paiva R, dos Reis MV, et al. In vitro growth of Physalis peruviana L. affected by silver nanoparticles. 3 Biotech. 2019;9(4):145. doi: 10.1007/s13205-019-1674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jacob SJP, Finub J, Narayanan A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf B Biointerf. 2012;91:212–214. doi: 10.1016/j.colsurfb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 94.Borase HP, Salunke BK, Salunkhe RB, et al. Plant extract: a promising biomatrix for ecofriendly, controlled synthesis of silver nanoparticles. Appl Biochem Biotechnol.. 2014;173(1):1–29. doi: 10.1007/s12010-014-0831-4. [DOI] [PubMed] [Google Scholar]
- 95.He X, Xia Q, Gamboa da Costa G, et al. 1-Formyl-7-hydroxy-6, 7-dihydro-5H-pyrrolizine (1-CHO-DHP)—a potential proximate carcinogenic metabolite of pyrrolizidine alkaloids. Chem Res Toxicol.. 2019;32(6):1193. doi: 10.1021/acs.chemrestox.9b00038. [DOI] [PubMed] [Google Scholar]
- 96.He X, Xia Q, Fu PP. 7-Glutathione-pyrrole and 7-cysteine-pyrrole are potential carcinogenic metabolites of pyrrolizidine alkaloids. J Environ Sci Health C. 2017; 35(2):69–83. doi: 10.1080/10590501.2017.1298358. [DOI] [PubMed] [Google Scholar]
- 97.He X, Ma L, Xia Q, et al. 7-N-acetylcysteine-pyrrole conjugate—a potent DNA reactive metabolite of pyrrolizidine alkaloids. J Food Drug Anal. 2016;24(4): 682–694. doi: 10.1016/j.jfda.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He X, Xia Q, Ma L, et al. 7-Cysteine-pyrrole conjugate: a new potential DNA reactive metabolite of pyrrolizidine alkaloids. J Environ Sci Health C. 2016;34(1): 57–76. doi: 10.1080/10590501.2015.1135593. [DOI] [PubMed] [Google Scholar]
- 99.Xia Q, Ma L, He X, et al. 7-Glutathione pyrrole adduct: a potential DNA reactive metabolite of pyrrolizidine alkaloids. Chem Res Toxicol. 2015;28(4):615–620. doi: 10.1021/tx500417q. [DOI] [PubMed] [Google Scholar]
- 100.Rahmatpour S, Shirvani M, Mosaddeghi MR, et al. Retention of silver nano-particles and silver ions in calcareous soils: influence of soil properties. J Environ Manag. 2017;193:136–145. doi: 10.1016/j.jenvman.2017.01.062. [DOI] [PubMed] [Google Scholar]
- 101.Kumar A, Vemula PK, Ajayan PM, et al. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat Mater. 2008;7(3):236–241. doi: 10.1038/nmat2099. [DOI] [PubMed] [Google Scholar]
- 102.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y, Shareena Dasari TP, Deng H, et al. Antimicrobial activity of gold nanoparticles and ionic gold. J Environ Sci Health C. 2015;33(3):286–327. doi: 10.1080/10590501.2015.1055161. [DOI] [PubMed] [Google Scholar]
- 104.Deshmukh S, Patil S, Mullani S, et al. Silver nanoparticles as an effective disinfectant: a review. Mater Sci Eng C. 2018;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Das P, Barua S, Sarkar S, et al. Mechanism of toxicity and transformation of silver nanoparticles: inclusive assessment in earthworm-microbe-soil-plant system. Geoderma. 2018;314:73–84. doi: 10.1016/j.geoderma.2017.11.008. [DOI] [Google Scholar]
- 106.Grün AL, Straskraba S, Schulz S, et al. Long-term effects of environmentally relevant concentrations of silver nanoparticles on microbial biomass, enzyme activity, and functional genes involved in the nitrogen cycle of loamy soil. J Environ Sci (China). 2018;69:12–22. doi: 10.1016/j.jes.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 107.Kim MJ, Ko D, Ko K, et al. Effects of silver-graphene oxide nanocomposites on soil microbial communities. J Hazard Mater. 2018;346:93–102. doi: 10.1016/j.jhazmat.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 108.Samarajeewa AD, Velicogna JR, Schwertfeger DM, et al. Effect of silver nanoparticle contaminated biosolids on the soil microbial community. NanoImpact. 2019; 14:100157. doi: 10.1016/j.impact.2019.100157. [DOI] [Google Scholar]
- 109.Huang J, Xiao J, Chen M, et al. Fate of silver nanoparticles in constructed wetlands and its influence on performance and microbiome in the ecosystems after a 450-day exposure. Bioresour Technol. 2019; 281:107–117. doi: 10.1016/j.biortech.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 110.Maity A, Natarajan N, Vijay D, et al. Influence of metal nanoparticles (NPs) on germination and yield of oat (Avena sativa) and berseem (Trifolium alexandrinum). Proc Natl Acad Sci India, Sect B Biol Sci. 2018;88(2):595–607. doi: 10.1007/s40011-016-0796-x. [DOI] [Google Scholar]
- 111.Navarro E, Baun A, Behra R, et al. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology. 2008;17(5): 372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 112.Rizwan M, Ali S, Qayyum MF, et al. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater. 2017;322:2–16. doi: 10.1016/j.jhazmat.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 113.Pulido-Reyes G, Leganes F, Fernández-Piñas F, et al. Bio-nano interface and environment: a critical review. Environ Toxicol Chem.. 2017;36(12):3181–3193. doi: 10.1002/etc.3924. [DOI] [PubMed] [Google Scholar]
- 114.He X, Deng H, Aker WG, et al. Regulation and safety of nanotechnology in the food and agriculture industry In: Molina I, Pelissari FM, Asiri AM, eds. Food Applications of Nanotechnology. Boca Raton: Taylor & Francis Group; 2019:12. [Google Scholar]
- 115.Cushen M, Kerry J, Morris M, et al. Nanotechnologies in the food industry—recent developments, risks and regulation. Trends Food Sci Technol. 2012;24(1):30–46. doi: 10.1016/j.tifs.2011.10.006. [DOI] [Google Scholar]
- 116.Arnaldi S, Muratorio A. Nanotechnology, uncertainty and regulation. A guest editorial. NanoEthics. 2013;7(3):173–175. doi: 10.1007/s11569-013-0185-3. [DOI] [Google Scholar]
- 117.Mitter N, Hussey K. Moving policy and regulation forward for nanotechnology applications in agriculture. Nat Nanotechnol. 2019;14(6):508. doi: 10.1038/s41565-019-0464-4. [DOI] [PubMed] [Google Scholar]


