Abstract
Background
Cigarette smoking is one of the most critical risk factors for peripheral arterial disease (PAD) and inversely correlated Vitamin C. Here we determine whether serum vitamin C correlates with the risk of PAD, especially among current smokers.
Methods
A cross-sectional analysis of 2383 individuals ≥40 y was performed from the U.S. National Health and Nutrition Examination Survey (NHANES 2003–2004), including measurement of ankle-brachial index (ABI), smoking status and serum vitamin C. We examined the interactions between plasma vitamin C and exposure to smoking on the risk of PAD.
Results
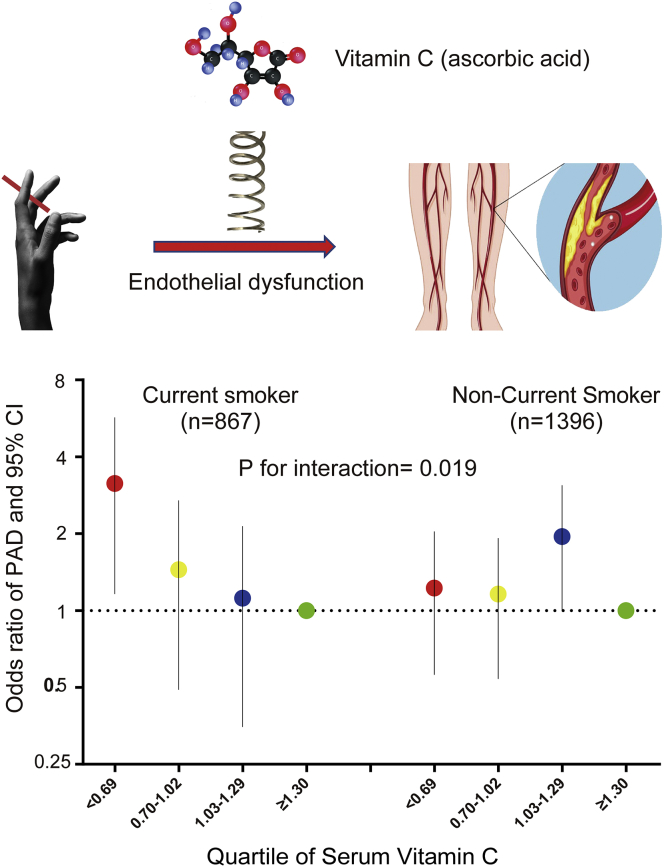
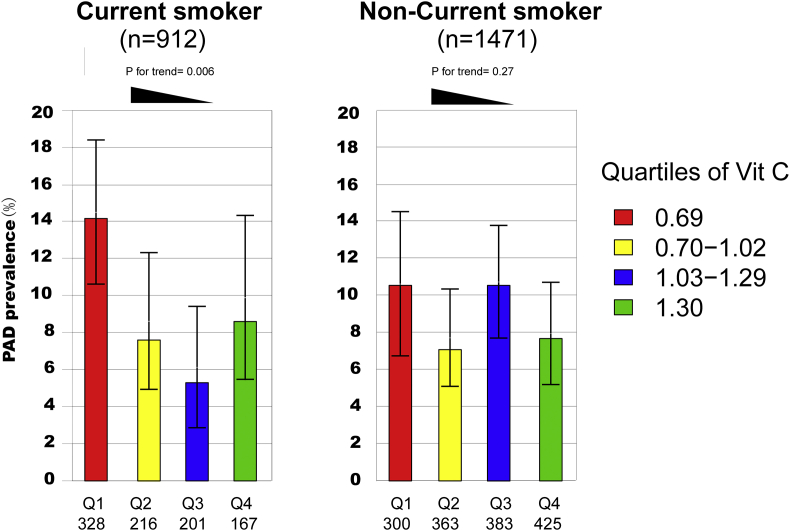
912 (38.2%) were current smokers while 207 participants were diagnosed with PAD based on ABI(ABI≤0.9). Current smokers in the lowest vitamin C quartile had the highest prevalence of PAD (14.1%) compared to other quartiles. However, this trend was not significant in nonsmokers. Current smokers in the lowest quartile had a 2.32-fold risk (95% CI, 1.03–5.32; P = 0.04) for PAD after weighted adjustment for potential confounders, including vitamin D and C-reactive protein. In contrast, non-smokers did not have a differing risk of PAD as a function of vitamin C (P for interaction = 0.019).
Conclusions
As an anti-oxidant and anti-inflammatory, low serum vitamin C appears to associates with the risk of PAD in smokers. A relationship between PAD and vitamin C in non-current smokers is not apparent. Modulating vitamin C in current smokers may help mitigate the risk of PAD and should be a target of mechanistic study.
Keywords: Serum vitamin C, Smoking, Peripheral arterial disease, Interaction
1. Introduction
Peripheral artery disease (PAD) is responsible for significant morbidity and mortality from cardiovascular disease (CVD) [1,2]. Globally, over 200 million people were diagnosed with PAD in 2015 [3]. Current smoking is the most influential risk factor for PAD as well as former smoking, diabetes, hypertension, total cholesterol, and history of cardiovascular disease [1]. Furthermore, smoking in PAD results in substantial increases in PAD-related hospitalizations, coronary heart disease, and PAD procedures, consequently a higher economic burden. However, the global effect of smoking is unlikely to abate substantially. Recent predictions suggest that the smoking population will grow from 794 million in 2010 to 872 million in 2030 [4]. Attenuating smoking-associated PAD risk is an important public health issue.
Observational studies demonstrate an anti-inflammatory role for serum Vitamin C. Vitamin C concentration is inversely associated with markers of inflammation and endothelial dysfunction, and plays a protective role in chronic cardiovascular conditions [5], and mortality [6]. Patients with PAD have a lower concentration of serum vitamin C, which is associated with inflammation and severity of atherosclerosis [7]. Systemic oxidative stress and inflammation are confirmed in smokers by numerous population-based studies [8]. However, it is not known whether vitamin C modulates the risk of PAD, especially in smokers. Smoking has been strongly associated with lower concentrations [9] and bioavailability [10] of vitamin C. Since serum vitamin C modulates oxidative stress and inflammation, both of which are high in smokers, we hypothesized that serum vitamin C might limit the risk of PAD in smokers especially. To test our hypothesis, we analyzed data from a nationally representative sample of the United States adult population aged ≥40 years in NHANES (National Health and Nutrition Examination Survey) 2003–2004.
2. Methods
2.1. Study population
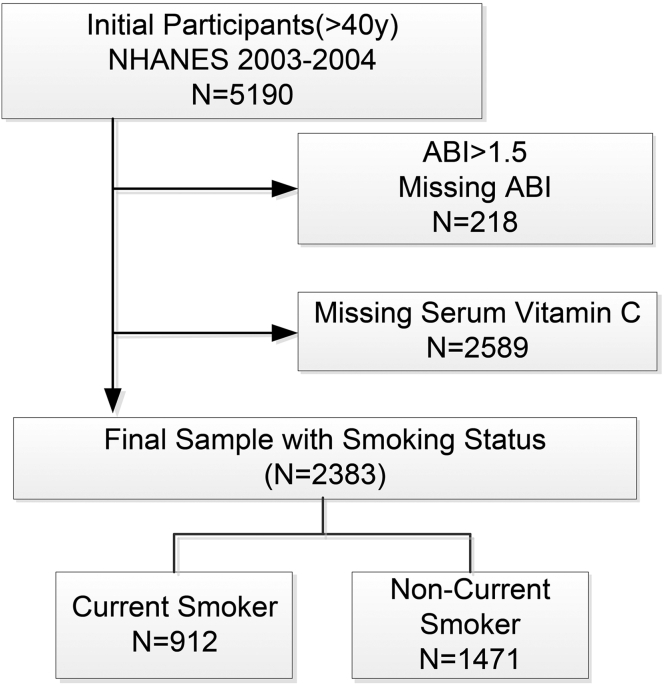
Data were retrieved from the NHANES 2003–2004, a multistage, stratified probability sample of non-institutionalized US adults conducted by the National Center for Health Statistics. In total, 5190 participants conducted standardized interviews, physical examinations, and laboratory testing. Participants who completed a medical evaluation at the NHANES mobile examination center had complete information on ankle-brachial index (ABI) measurements and serum vitamin C (n = 2283) were included. All participants provided written informed consent, and the National Center for Health Statistics institutional review board approved each NHANES cycle [11](Supplemental Fig. 1). The manuscript followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline for cross-sectional studies [12].
2.2. Diagnosing for PAD
The ankle-brachial index (ABI) exam was performed by trained technicians using an equipped room within the mobile examination center (MEC) as described. In brief, systolic pressure is assessed on three sites (the right arm for brachial artery and both ankles for posterior tibial arteries). Systolic blood pressure is measured twice at each site for participants aged 40–59 years and once at each site for participants aged 60 years and older [13]. ABI was calculated according to the ratio of the average ankle systolic blood pressure to arm systolic blood pressure. The larger of the two measurements was considered the ABI for this study. Participants with ABI over 1.5 may have severe arterial rigidity and were therefore excluded from all analyses. PAD was defined as ABI <0.9 [14].
2.3. Serum vitamin C evaluation
In NHANES 2003–2004, 4438 adults aged ≥40 y had total serum concentrations of ascorbic acid (oxidized and reduced) measured. Within 30 min of separation from the clot, one part serum was treated with four parts of 6% metaphosphoric acid in the MEC. The stabilized serum samples were stored frozen, then shipped to the Centers for Disease Control and Prevention in Atlanta, where they were stored at −20 °C until tested. An isocratic reverse-phase HPLC method with electrochemical detection of ascorbic acid was used for NHANES 2003–2004 [15].
2.4. Ascertainment of smoking status
NHANES obtained smoking history with the following question [16]: ‘‘Have you smoked at least 100 cigarettes in your entire life?’’ [16]. Participants who responded affirmatively were regarded as ever smokers and were queried on current smoking status with the following question ‘‘Do you now smoke cigarettes?’‘. A response of ‘‘Every day’’ or ‘‘Some days’’ was coded as a current smoker, while the rest of the participants were coded as non-current smokers. The non-smoker and past smoker were categorized into non-current smokers.
Cotinine concentration was another marker used to assess current smoking status. Cotinine is a major metabolite of nicotine that may be used as a marker for active smoking. We therefore also coded participants with a serum cotinine over 10 ng/mL as a current smoker [17]. Serum cotinine was detected by isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry; cotinine concentrations were derived from the ratio of native to labeled cotinine in the sample by comparisons to a standard curve. The laboratory limit for the detection of cotinine was 0.05 ng/mL [18].
2.5. Covariates
We collected covariates that are linked to PAD or CVD risk according to the previous studies. In the NHANES study, data were collected through a participant questionnaire and medical evaluation. The participant questionnaire collected self-reported data on age, race/ethnicity, sex, education, physical activity, history of a diagnosis of diabetes mellitus, hypertension, myocardial infarction, angina, and coronary heart disease. In our study, cardiovascular disease included self-reported diagnoses of coronary heart disease, stroke, or congestive heart failure. The NHANES medical evaluation included measurements of height and weight that were used to calculate body mass index. A blood sample was collected for measurement of serum creatinine, glycosylated hemoglobin (hemoglobinA1c), C-reactive protein, vitamin D, and lipid profile [19].
2.6. Statistical analysis
Statistical analysis was performed according to the guidelines of Centers for Disease Control and Prevention (CDC) (https://wwwn.cdc.gov/nchs/nhanes/tutorials/default.aspx). All analyses accounted for the complex survey design and NHANES probabilistic sampling weights using the Survey design commands in statistical software package R-3.4.3 (http://www.R-project.org, The R Foundation) and Empower-Stats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). Continuous variables were represented as mean ± standard deviation, while categorical variables were represented as percentages. Weighted linear regression models were used to calculate the differences in continuous variables among different groups of independent variables (age, gender, race/ethnicity, and the ratio of family income to poverty), while weighted chi-square tests were used to calculate the differences in categorical variables among different groups of those dependent variables. A P value less than 0.05 (two-sided) was considered statistically significant.
Weighted generalized additive models were used to describe the dose-response relationship between serum vitamin C and risk of PAD both in current smokers and non-current smokers defined by self-report and serum cotinine. All models were adjusted for sex, age, race/ethnicity.
Three sequential multivariable logistic regression models were employed to calculate odds ratios (ORs) for the relation of vitamin C on the risk of PAD both in current smokers and non-current smokers. The second model adjusted for age, sex, race/ethnicity, hypertension, BMI, physical activity, education, diabetes, CAD; hypertension, total cholesterol and triglycerides (mg/dL) following the unadjusted model. The final model with adjusted covariates in model 2 plus CRP and serum vitamin D. Potential effect modification of smoking was evaluated by multiplicative interaction terms, with statistical significance determined by Wald's test. A p interaction value of less than 0.10 (two-sided) was considered statistically significant.
3. Results
3.1. Baseline participant characteristics defined by smoking status in NHANES2003-2004
After excluding participants without ABI or serum vitamin C data, a total of 2383 participants were included in the final analysis (Supplemental Fig. 1); 912 (38.2%) were current smokers. 1228 (51.28%) were male, and the mean ± SD age was 61.05 ± 13.2 years. Mean serum vitamin C level was 0.99 ± 0.55 mg/dL overall, and was lower in current smoking (0.86 ± 0.54) than non-current smoking (1.07 ± 0.55) participants. The prevalence of PAD was 9.69% (84/912)in current smokers and 8.81% (123/1228) in non-smokers (p = 0.48). Baseline characteristics by smoking status are summarized in Table 1. Briefly, current smokers tended to be younger, male, African-American, have a lower level of formal education, and were less physically active. Additionally, smokers had less prevalence of CHD history, a higher CRP, and lower vitamin D concentration. Total cholesterol, glycohemoglobin, and creatinine level were comparable between current smokers and non-current smokers (all p<0.05; Table 1).
Table 1.
Participants characteristics by smoking status; NHANES 2003–2004.
| Characteristics | Overall |
Non-current smoker |
Current smokers |
P-value |
|---|---|---|---|---|
| 2383 | 1471 | 912 | ||
| Age (years) | 61.05 ± 13.22 | 63.13 ± 13.19 | 57.70 ± 12.56 | <0.001 |
| Male sex, % | 1222 (51.28%) | 725 (49.29%) | 497 (54.50%) | 0.013 |
| Race/ethnicity, % | ||||
| Non-Hispanic White | 1376 (57.74%) | 867 (58.94%) | 509 (55.81%) | 0.133 |
| Black | 405 (17.00%) | 215 (14.62%) | 190 (20.83%) | <0.001 |
| Mexican American | 453 (19.01%) | 299 (20.33%) | 154 (16.89%) | 0.037 |
| Other Hispanic | 62 (2.60%) | 33 (2.24%) | 29 (3.18%) | 0.163 |
| Other Race/Ethnicity | 87 (3.65%) | 57 (3.87%) | 30 (3.29%) | 0.459 |
| Body mass index (kg/m2) | 28.50 ± 5.53 | 28.80 ± 5.48 | 28.01 ± 5.57 | <0.001 |
| History of CHD, % | 170 (7.18%) | 117 (8.00%) | 53 (5.85%) | 0.049 |
| Any Diabetes (FBG≥126 mg/dL or self-report) | 393 (16.65%) | 253 (17.40%) | 140 (15.45%) | 0.217 |
| Any Hypertension? (BP≥139/90 or self-report) | 1144 (48.11%) | 727 (49.52%) | 417 (45.82%) | 0.079 |
| Education, % | 0.003 | |||
| Less than high school | 716 (30.11%) | 437 (29.79%) | 279 (30.63%) | |
| High school only | 607 (25.53%) | 344 (23.45%) | 263 (28.87%) | |
| More than high school | 1055 (44.37%) | 686 (46.76%) | 369 (40.50%) | |
| Physical Activity (MET-based rank) | 0.047 | |||
| 0 | 527 (23.41%) | 303 (21.70%) | 224 (26.20%) | |
| 1 | 632 (28.08%) | 387 (27.72%) | 245 (28.65%) | |
| 2 | 418 (18.57%) | 271 (19.41%) | 147 (17.19%) | |
| 3 | 674 (29.94%) | 435 (31.16%) | 239 (27.95%) | |
| Total cholesterol (mg/dL) | 207.14 ± 41.96 | 207.07 ± 42.10 | 207.25 ± 41.75 | 0.920 |
| Systolic blood pressure (mmHg) | 123.01 ± 15.46 | 123.29 ± 15.11 | 122.60 ± 15.95 | 0.373 |
| Glycohemoglobin (%) | 5.78 ± 1.02 | 5.79 ± 0.97 | 5.77 ± 1.11 | 0.661 |
| C-reactive protein (mg/dL) | 0.46 ± 0.87 | 0.42 ± 0.65 | 0.53 ± 1.13 | 0.002 |
| Creatinine (mg/dL) | 0.95 ± 0.38 | 0.95 ± 0.36 | 0.94 ± 0.40 | 0.399 |
| Ankle brachial index (Left) | 1.10 ± 0.14 | 1.11 ± 0.14 | 1.10 ± 0.15 | 0.210 |
| Ankle brachial index (Right) | 1.09 ± 0.14 | 1.10 ± 0.14 | 1.09 ± 0.15 | 0.017 |
| Vitamin D (ng/mL) | 23.37 ± 9.28 | 23.84 ± 8.92 | 22.60 ± 9.80 | 0.001 |
| LDL-cholesterol (mg/dL) | 120.66 ± 35.55 | 121.13 ± 35.54 | 119.86 ± 35.59 | 0.564 |
| Triglycerides (mg/dL) | 144.30 ± 116.23 | 138.45 ± 86.91 | 153.69 ± 151.51 | 0.002 |
| Vitamin C (mg/dL) | 0.99 ± 0.55 | 1.07 ± 0.55 | 0.86 ± 0.54 | <0.001 |
| Vitamin C quartiles | <0.001 | |||
| ≤0.69 | 300 (20.39%) | 328 (35.96%) | ||
| 0.70–1.02 | 363 (24.67%) | 216 (23.68%) | ||
| 1.03–1.29 | 383 (26.03%) | 201 (22.03%) | ||
| ≥1.30 | 425 (28.89%) | 167 (18.31%) | ||
| PAD prevalence (%) | 207 (9.15%) | 123 (8.81%) | 84 (9.69%) | 0.481 |
Abbreviations: NHANES: National Health and Nutrition Examination Survey; PAD: peripheral artery disease; CHD:coronary heart diseases.
3.2. ABI positively associated with vitamin C in current smokers
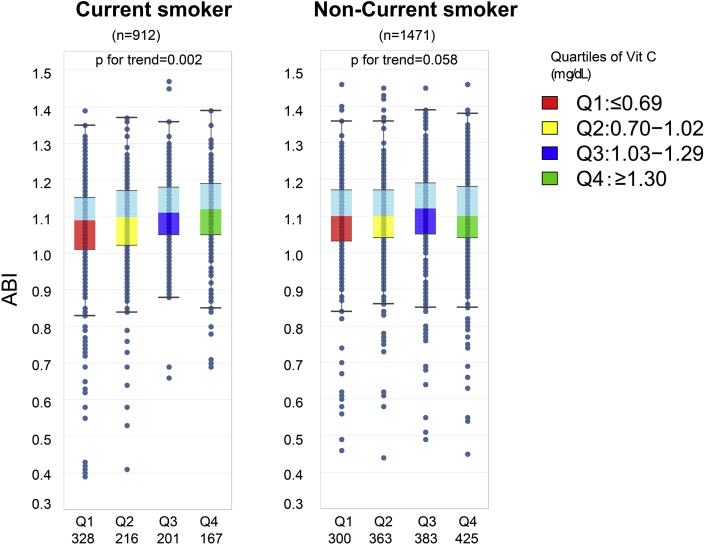
Participants were divided into four groups according to vitamin C quartiles (Q1:≤0.69, Q2: 0.70–1.02; Q3:1.03–1.30; Q4≥1.30 mg/dL). Compared to non-current smokers, participants with the lowest quartile of vitamin C were more commonly defined as current smokers (Supplemental Fig. 2). As shown in Fig. 2, both the right and left ABI increased with an increase in serum vitamin C concentration. Smokers demonstrated a consistent trend linking serum vitamin C and ABI (ptrend = 0.002) among four vitamin C quartiles. However, there was not a consistent increase between vitamin C and ABI in non-current smokers (ptrend = 0.058).
Fig. 2.
Graphic abstract: Vitamin C modulates the risk of PAD in Current Smokers. Up: Vitamin C modulates the risk of PAD in Current Smokers by endothelial dysfunction. Bottom: Graph comparing the risk of PAD across vitamin C quartiles both in current smokers and non-smokers. The participants in the lowest vitamin C quartile have the greatest risk of PAD in current smokers. However, this is not the case for the nonsmokers. (P for interaction = 0.019).
3.3. Vitamin C associated with PAD risk in current smokers
To further evaluate the interaction between current smoking and vitamin C on the risk of PAD, we observed the prevalence of PAD among smoking and nonsmoking participants within varying vitamin C quartiles. In total, 207 participants were diagnosed with PAD. As shown in Fig. 1, current smokers with the lowest vitamin C level had the highest prevalence of PAD (14.1%) when compared with participants in other quartiles. However, for non-smokers, this trend was not significant.
Fig. 1.
PAD prevalence among vitamin C quartiles by smoking status. The percentages are shown (mean and 95% confidence intervals) for PAD prevalence among vitamin C quartiles by smoking status in NHANES 2003 to 2004. Current smokers in the lowest vitamin C quartiles had the highest prevalence of PAD (14.1%) when compared with participants in other quartiles (p for trend = 0.006). However, for non-smokers, the prevalence of PAD is comparable among vitamin C quartiles. (p for trend = 0.27).
3.4. Dose-response relationship between serum vitamin C and risk of PAD
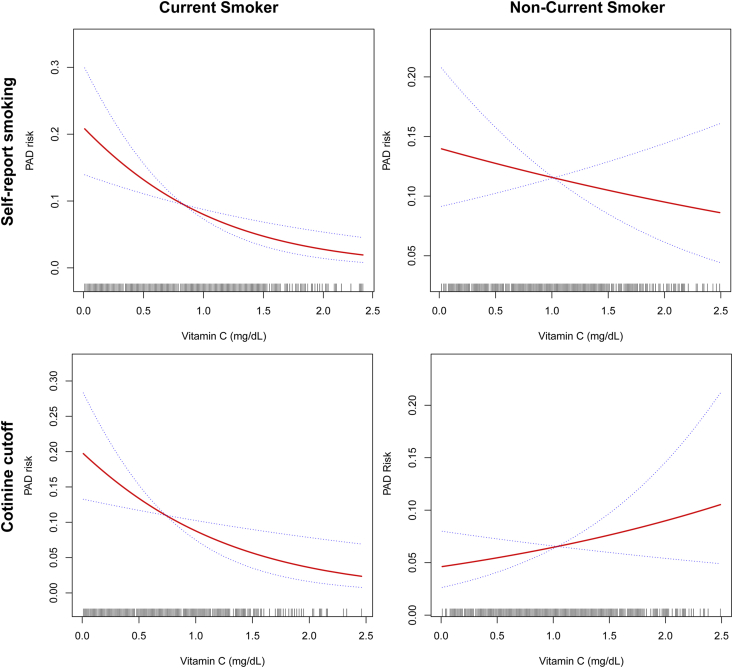
There was an “L”- shaped association between serum vitamin C and PAD risk in current smokers. For the questionnaire identified current smokers, the prevalence of PAD was consistently decreased with the increase of serum vitamin C after adjusting age, sex, and race. However, this trend was not significant for non-smokers. After adjusting for age, sex, and race, the risk of PAD in self-reported smokers was lower as vitamin C concentration was increased. Similarly, in current smokers defined by serum cotinine levels, an increase in vitamin C concentration was associated with a lower risk of PAD. This association between PAD and vitamin C concentration was not seen in patients with normal cotinine levels (Supplemental Fig. 3).
3.5. Serum vitamin C may limit the risk of PAD among current smokers
After adjusting for traditional risk factor in model 1, smokers in the lowest quartile of Vitamin C had a 2.55-fold risk for PAD. Because inflammation [7] and serum vitamin D [20,21] are two important confounders for the association among serum vitamin C, we included C-reactive protein as an inflammation biomarker and serum vitamin D in the final multiple logistic models. There was a 2.32-fold risk for PAD in the lowest quartile of Vitamin C for current smokers. In contrast, non-smokers did not have a differing risk of PAD as a function of vitamin C (Pinteraction = 0.019; Table 2).
Table 2.
Vitamin C and risk of PAD by smoking status.
| PAD | Overall |
Non-current smoker |
Current smokers |
|---|---|---|---|
| OR (95% CI) P value | OR (95% CI) P value | OR (95% CI) P value | |
| Non-adjusted | |||
| Serum vitamin C quartiles | |||
| ≥1.30 | 1.0 | 1.0 | 1.0 |
| 1.03–1.29 | 1.08 (0.70, 1.65) 0.7373 | 1.39 (0.85, 2.29) 0.1939 | 0.56 (0.24, 1.30) 0.1790 |
| 0.70–1.02 | 0.92 (0.59, 1.42) 0.6979 | 0.93 (0.54, 1.60) 0.7829 | 0.88 (0.41, 1.85) 0.7278 |
| ≤0.69 | 1.61 (1.08, 2.40) 0.0190 | 1.40 (0.82, 2.38) 0.2168 | 1.69 (0.90, 3.19) 0.1056 |
| Adjust I | |||
| Serum vitamin C quartiles | |||
| ≥1.30 | 1.0 | 1.0 | 1.0 |
| 1.03–1.29 | 1.43 (0.89, 2.29) 0.1420 | 1.76 (1.00, 3.11) 0.0512 | 0.87 (0.35, 2.15) 0.7555 |
| 0.70–1.02 | 1.10 (0.67, 1.81) 0.7108 | 0.98 (0.52, 1.85) 0.9473 | 1.16 (0.50, 2.72) 0.7311 |
| ≤0.69 | 1.65 (1.02, 2.68) 0.0420 | 1.04 (0.54, 2.00) 0.9083 | 2.55 (1.15, 5.66) 0.0212 |
| Adjust II | |||
| Serum vitamin C quartiles | |||
| ≥1.30 | 1.0 | 1.0 | 1.0 |
| 1.03–1.29 | 1.35 (0.84, 2.18) 0.2212 | 1.70 (0.96, 3.02) 0.0692 | 0.75 (0.29, 1.91) 0.5440 |
| 0.70–1.02 | 1.03 (0.62, 1.72) 0.8940 | 0.93 (0.49, 1.78) 0.8290 | 1.05 (0.45, 2.49) 0.9055 |
| ≤0.69 | 1.53 (0.94, 2.51) 0.0902 | 1.00 (0.51, 1.96) 0.9990 | 2.32 (1.03, 5.23) 0.0431 |
Model Ⅰ: age (years); gender; Race/Ethnicity; Body Mass Index (kg/m∗∗2); Physical Activity; Education; Diabetes; CAD; hypertension, total cholesterol, and triglycerides (mg/dL).
ModelⅡ: age (years); gender; Race/Ethnicity; Body Mass Index (kg/m∗∗2); Physical Activity; Education; Diabetes; CAD; hypertension, total cholesterol, triglycerides (mg/dL); Vitamin D; C-reactive protein(mg/dL).
4. Discussion
In this analysis of 5190 participants from the NHANES2003-2004, we observed remarkable differences in the risk of PAD among subgroups by smoking status and vitamin C. In both self-reported and biochemically confirmed current smokers, PAD was observed with a prevalence of 14.1% in those with the lowest serum vitamin C concentrations. Compared to participants whose vitamin C levels were in the upper quartiles, this prevalence was significantly higher. However, for non-current smokers, the prevalence of PAD was comparable across all concentrations of vitamin C. The most considerable risk of PAD for current smokers was observed in the participants within the lowest serum vitamin C quartiles. A nearly 3-fold risk of PAD was revealed in the lowest vitamin C quartiles when compared with the highest serum vitamin C quartiles for the current smokers. In contrast, serum vitamin C had no protective role on PAD in non-current smokers. In summary, our data indicate that among American adults, current smokers with low serum vitamin C had the highest risk of PAD. As an antioxidant and anti-inflammatory, serum vitamin C may attenuate the risk of PAD in smokers, but not in nonsmokers. These findings suggest that smokers, who are already at high risk of PAD, may benefit from strategies to increase serum vitamin C concentrations. Modulating serum vitamin C by consuming more vegetables and fruits may help mitigate the risk of PAD in smokers, and should be a target of mechanistic study.
The protective role of serum vitamin C in cardiovascular disease and PAD [7] has been reported in previous observational studies. A meta-analysis of randomized controlled trials showed that the benefit of vitamin C is dependent on health status, with stronger effects in those with a higher risk for cardiovascular disease. It was also reported that diet vitamin C estimated by questionnaire was positively associated with ABI in the Edinburgh Artery study [22]. Furthermore, Heffron and his colleagues reported that a higher frequency and amount of intake of fruit and vegetables was associated with a lower risk of PAD in a large community sample of nearly 4,000,000 individuals. The association was only present in current and former smokers in the subgroup analysis [23]. However, that study did not evaluate the serum vitamin C concentrations and their effects on PAD risk. Serum vitamin C is a well-established surrogate biomarker of fruit and vegetable consumption [24] and can be altered by smoking status [9,25]. Our results provide direct evidence that serum vitamin C is protective against PAD in current smokers.
Our study differed from previous studies in several important aspects. In regards to study design, our study utilized data collected from NHANES, a program of assessments specifically designed to assess the health and nutritional status of adults and children in the United States. Using a new statistical analysis of independent data within an established database, we were able to assess the association between serum vitamin C and PAD risk and determine that this relationship is most salient in current smokers. Second, we were able to obtain detailed information on potentially confounding covariates and systematically utilize them within the analysis. To address the independent role of serum vitamin C on PAD, we adjusted for variables recognized widely as potential confounders for PAD risk, including serum vitamin D and CRP. Both inflammation [26] and serum vitamin D [20] contributes to the underlying association between vitamin C and PAD. Therefore, in our final model, CRP and serum vitamin D were added as covariates. Even after controlling for both CRP and serum vitamin D, the association between vitamin C and PAD was still significant in current smokers. Our data indicated that the protective role of serum vitamin C on the prevalence of PAD was independent of serum vitamin D and CRP as a marker of inflammation. Third, in our study, we confirmed the diagnosis of PAD using ABI measurements rather than anecdotal information. ABI is a Class I recommendation for diagnosing PAD in the current management guidelines [14].
It has been well established that smoking modulates biomarkers of oxidative stress, inflammation, and endothelial dysfunction, thereby increasing the risk of cardiovascular disease [27]. In vivo and in vitro studies show that the inflammatory response and oxidative damage induced by smoking can be inhibited by vitamin C intervention [28,29]. For example, Heit et al. reported that vitamin C improves endothelial dysfunction by endothelium-dependent and independent vasodilation in chronic smokers [30]. Furthermore, meta-analyses from 44 RCT clinical trials suggested vitamin C supplementation improved endothelial function detected by flow-mediated dilation [31]. Vitamin C supplementation exerted stronger effects in those at higher cardiovascular disease risk such as atherosclerotic, diabetes and heart failure patients [31]. However, this study showed no effect of vitamin C supplementation on endothelial dysfunction in smokers [31].
5. Limitations
Limitations of our study include the cross-sectional design, which permits the detection of associations, but neither a temporal nor causal relationship. We cannot establish a cause-effect association between vitamin C and PAD risk. Furthermore, information regarding the nutrient intake of vitamin C was not analyzed. We cannot tell whether low serum vitamin C is a result from decreased fruit and vegetable intake. However, the dietary intake of vitamin C supplements has a only a moderate relationship with its serum level [32] and did not associate with the risk of PAD in other studies [33]. According to our data, serum vitamin C is a biomarker of PAD in current smokers. However, we cannot determine whether supplementing vitamin C will reduce the incidence of PAD, and if so, what form it should be provided in. Further studies are warranted to assess whether nutritional supplementation of vitamin C will be beneficial in modulating the risk of PAD in smokers.
6. Conclusion
Among adult participants from NHANES2003-2004, we found that current smokers with the lowest level of serum vitamin C had the highest prevalence of PAD, which represents nearly three folds extra risk of PAD for current smokers (Fig. 2). Our results have important clinical and public health significance because the prevalence of PAD still is increasing globally. Moreover, smoking is still the most critical risk factor for the development of PAD and at least doubles the risk compared with that of a nonsmokers [2]. According to our data, identification of the individuals who have vitamin C deficiency may help reduce the risk of PAD smokers.
CRediT authorship contribution statement
Guangzhi Cong: Conceptualization, Methodology, Data curation, Writing - original draft. Ru Yan: Methodology, Software, Data curation. Ulka Sachdev: Conceptualization, Methodology, Writing - review & editing, Supervision.
Declaration of Competing Interest
None declared.
Acknowledgments
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R01HL136556 to US. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijchy.2020.100037.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
Study sample selection flowchart. NHANES: National Health and Nutrition Examination Survey. Participants' data from NHANES2003-2004 were retrieved. Participants were included for this study when data on ABI measurement, serum vitamin C, and smoking status according to the questionnaire and serum cotinine level were available. We also excluded the participants’ data when ABI is more than 1.5. In total, there are 2383 participants in our final analysis. Among them, 912 participants were coded a current smoker, and 1471 participants were coded a non-current smoker 1
figs2.
ABI distribution among vitamin C quartiles by smoking status. Box-and-whisker plot diagram showing ABI distribution by median, first and third quartile, upper/lower whisker. Dots indicate individual values. In current smokers, there is a concentration-dependent of linear ABI increases across vitamin C quartiles(p for trend=0.002). However, this is not t the case for nonsmokers(p for trend=0.058) 2
figs3.
Adjusted dose-response between vitamin C and PAD risk by smoking status. Evaluation of the dose-response relationship between serum vitamin C and risk of PAD by generalized additive models adjusted by age, sex, and race. The x-axes represent serum vitamin C levels in mg/dL. The vertical scale can be interpreted as the relative prevalence of PAD as a function of serum vitamin C. Both for questionnaire and serum cotinine determined current smokers, there is an L shaped association between vitamin C and prevalence of PAD. Nevertheless, this is not the case for the nonsmokers 3
References
- 1.Fowkes F.G., Aboyans V., Fowkes F.J., McDermott M.M., Sampson U.K., Criqui M.H. Peripheral artery disease: epidemiology and global perspectives. Nat. Rev. Cardiol. 2017;14:156–170. doi: 10.1038/nrcardio.2016.179. [DOI] [PubMed] [Google Scholar]
- 2.Criqui M.H., Aboyans V. Epidemiology of peripheral artery disease. Circ. Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 3.Song P., Rudan D., Zhu Y., Fowkes F.J.I., Rahimi K., Fowkes F.G.R. Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis. The Lancet Global Health. 2019;7:e1020–e1030. doi: 10.1016/S2214-109X(19)30255-4. [DOI] [PubMed] [Google Scholar]
- 4.Mendez D., Alshanqeety O., Warner K.E. The potential impact of smoking control policies on future global smoking trends. Tobac. Contr. 2013;22:46–51. doi: 10.1136/tobaccocontrol-2011-050147. [DOI] [PubMed] [Google Scholar]
- 5.Simon J.A., Hudes E.S., Browner W.S. Serum ascorbic acid and cardiovascular disease prevalence in U.S. adults. Epidemiology. 1998;9:316–321. [PubMed] [Google Scholar]
- 6.Khaw K.T., Bingham S., Welch A., Luben R., Wareham N., Oakes S. Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study. European Prospective Investigation into Cancer and Nutrition. Lancet. 2001;357:657–663. doi: 10.1016/s0140-6736(00)04128-3. [DOI] [PubMed] [Google Scholar]
- 7.Langlois M., Duprez D., Delanghe J., De Buyzere M., Clement D.L. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001;103:1863–1868. doi: 10.1161/01.cir.103.14.1863. [DOI] [PubMed] [Google Scholar]
- 8.Yanbaeva D.G., Dentener M.A., Creutzberg E.C., Wesseling G., Wouters E.F.M. Systemic effects of smoking. Chest. 2007;131:1557–1566. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 9.McCall S.J., Clark A.B., Luben R.N., Wareham N.J., Khaw K.T., Myint P.K. Plasma vitamin C levels: risk factors for deficiency and association with self-reported functional health in the European prospective investigation into cancer-norfolk. Nutrients. 2019;11 doi: 10.3390/nu11071552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conney A.H., Reidenberg M.M. Cigarette smoking, coffee drinking, and ingestion of charcoal-broiled beef as potential modifiers of drug therapy and confounders of clinical trials. J. Pharmacol. Exp. Therapeut. 2012;342:9–14. doi: 10.1124/jpet.112.193193. [DOI] [PubMed] [Google Scholar]
- 11.Johnson C.L., Paulose-Ram R., Ogden C.L., Carroll M.D., Kruszon-Moran D., Dohrmann S.M. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. 2013;2:1–24. [PubMed] [Google Scholar]
- 12.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 13.The National Health and Nutrition Examination Survey (NHANES) 2005 Dec. Analytic and Reporting Guidelines.http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf [Google Scholar]
- 14.Gerhard-Herman M.D., Gornik H.L., Barrett C., Barshes N.R., Corriere M.A., Drachman D.E. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2017;135:e686–e725. doi: 10.1161/CIR.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCoy L.F., Bowen M.B., Xu M., Chen H.P., Schleicher R.L. Improved HPLC assay for measuring serum vitamin C with 1-methyluric acid used as an electrochemically active internal standard. Clin. Chem. 2005;51:1062–1064. doi: 10.1373/clinchem.2004.046904. [DOI] [PubMed] [Google Scholar]
- 16.National Center for Health Statistics . 2004. NHANES 2003–2004: Smoking - Cigarette/Tobacco Use - Adult (SMQ_C)https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/SMQ_C.htm Available from: [Google Scholar]
- 17.Kim S. Overview of cotinine cutoff values for smoking status classification. Int. J. Environ. Res. Publ. Health. 2016;13 doi: 10.3390/ijerph13121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benowitz N.L., Bernert J.T., Caraballo R.S., Holiday D.B., Wang J.T. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am. J. Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics . 2004. NHANES 2003–2004: Laboratory Data.https://wwwn.cdc.gov/nchs/nhanes/Search/DataPage.aspx?Component=Laboratory&CycleBeginYear=2003 Available from: [Google Scholar]
- 20.Melamed M.L., Muntner P., Michos E.D., Uribarri J., Weber C., Sharma J. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from NHANES 2001 to 2004. Arterioscler. Thromb. Vasc. Biol. 2008;28:1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapson I.R., Michos E.D., Alonso A., Hirsch A.T., Matsushita K., Reis J.P. Serum 25-hydroxyvitamin D is associated with incident peripheral artery disease among white and black adults in the ARIC study cohort. Atherosclerosis. 2017;257:123–129. doi: 10.1016/j.atherosclerosis.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donnan P.T., Thomson M., Fowkes F.G.R., Prescott R.J., Housley E. Diet as a risk factor for peripheral arterial-disease IN the general-population - the Edinburgh-artery-study. Am. J. Clin. Nutr. 1993;57:917–921. doi: 10.1093/ajcn/57.6.917. [DOI] [PubMed] [Google Scholar]
- 23.Heffron S.P., Rockman C.B., Adelman M.A., Gianos E., Guo Y., Xu J.F. Greater frequency of fruit and vegetable consumption is associated with lower prevalence of peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2017;37:1234–1240. doi: 10.1161/ATVBAHA.116.308474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mielgo-Ayuso J., Valtuena J., Huybrechts I., Breidenassel C., Cuenca-Garcia M., De Henauw S. Fruit and vegetables consumption is associated with higher vitamin intake and blood vitamin status among European adolescents. Eur. J. Clin. Nutr. 2017;71:458–467. doi: 10.1038/ejcn.2016.232. [DOI] [PubMed] [Google Scholar]
- 25.Schectman G., Byrd J.C., Gruchow H.W. The influence of smoking on vitamin C status in adults. Am. J. Publ. Health. 1989;79:158–162. doi: 10.2105/ajph.79.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauley J.A., Kassem A.M., Lane N.E., Thorson S. Prevalent peripheral arterial disease and inflammatory burden. BMC Geriatr. 2016;16:213. doi: 10.1186/s12877-016-0389-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messner B., Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014;34:509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 28.Ray T., Maity P.C., Banerjee S., Deb S., Dasgupta A.K., Sarkar S. Vitamin C prevents cigarette smoke induced atherosclerosis in Guinea pig model. J. Atherosclerosis Thromb. 2010;17:817–827. doi: 10.5551/jat.2881. [DOI] [PubMed] [Google Scholar]
- 29.Panda K., Chattopadhyay R., Chattopadhyay D.J., Chatterjee I.B. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic. Biol. Med. 2000;29:115–124. doi: 10.1016/s0891-5849(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 30.Heitzer T., Just H., Munzel T. Antioxidant vitamin C improves endothelial dysfunction in chronic smokers. Circulation. 1996;94:6–9. doi: 10.1161/01.cir.94.1.6. [DOI] [PubMed] [Google Scholar]
- 31.Ashor A.W., Lara J., Mathers J.C., Siervo M. Effect of vitamin C on endothelial function in health and disease: a systematic review and meta-analysis of randomised controlled trials. Atherosclerosis. 2014;235:9–20. doi: 10.1016/j.atherosclerosis.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Dehghan M., Akhtar-Danesh N., McMillan C.R., Thabane L. Is plasma vitamin C an appropriate biomarker of vitamin C intake? A systematic review and meta-analysis. Nutr. J. 2007;6:41. doi: 10.1186/1475-2891-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naqvi A.Z., Davis R.B., Mukamal K.J. Nutrient intake and peripheral artery disease in adults: key considerations in cross-sectional studies. Clin. Nutr. 2014;33:443–447. doi: 10.1016/j.clnu.2013.06.011. [DOI] [PubMed] [Google Scholar]