Abstract
Our continued synthetic interest in this class of retinoids, CD437 and its analogs, against methicillin-resistant Staphylococcus aureus (MRSA) has brought us to explore further isosteric substitutions within the scaffold. Although our previous findings have shown promising activity against gram-positive pathogens, their therapeutic viability remained an issue. Specifically, through preliminary analysis, our best performing compound, analog 2, displayed low solubility within serum as well as high affinity for retinoid binding proteins with a concentration dependent relationship. To circumvent this issue, we proposed a class of analogs containing an azaborine substitution in place of the naphthalene moiety. Azaborines have a nitrogen-boron bond substituting a carbon-carbon double bond that alters the electronics of the parent scaffold. This motif has been explored successfully in cancer research but to the best of our knowledge has yet to be applied to antibiotics. Herein, we describe the synthesis of the desired analogs, antimicrobial activity, and surprising physiochemical properties.
Keywords: CD437, Retinoids, Azaborine, Methicillin-resistant Staphylococcus aureus
Graphical Abstract

Introduction
According to the Centers for Disease Control and Prevention, more than 2.8 million antibiotic resistant infections occur annually in the United States alone.1 One of the most prevalent antibiotic resistant bacteria is methicillin-resistant Staphylococcus aureus (MRSA), which is also known to develop persistent populations. Persister cells are dormant bacterial cells that are genetically identical, but phenotypically different than the wild type.2 These cells have slowed growth rates, diminished metabolism, and are found in many S. aureus populations.3 As most antibiotics target metabolic and growth processes, persister cells are often difficult to eradicate.4
Previously, our laboratory, in collaboration with the Mylonakis laboratory at Brown University, disclosed the potent activity of the synthetic retinoid CD437 (1) at eradicating both wild type and persistent MRSA infections via membrane perturbation with a minimum inhibitory concentration (MIC) of 1 μg/mL (Fig. 1).5 Since this finding in 2018, we have disclosed three generations of analogs to further explore the structure activity relationship of this class of retinoids.6–7 In our investigations, our lead compound was found to be the primary alcohol derivative we deemed analog 2 (2). This compound exhibited an MIC of 2 μg/mL while also reducing the toxicity of the parent compound.
Fig. 1.
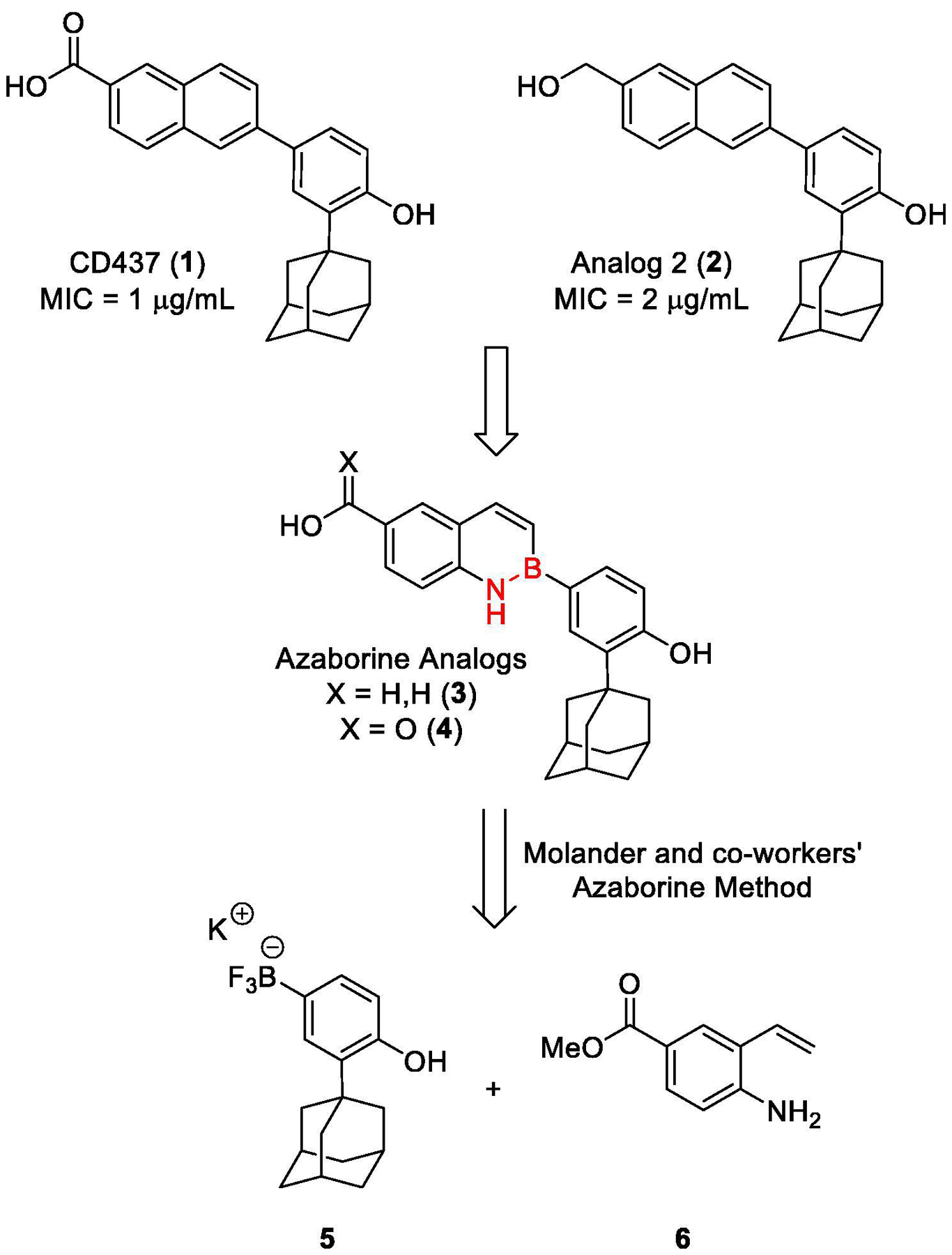
The structure and antimicrobial activity of CD437 (1) and Analog 2 (2) arc shown at the top followed by the proposed azaborine analogs (3. 4) and a retrosynthetic plan invoking the method developed by Molander and co-workers.
Though this compound showed promise, it displayed high levels of binding to plasma binding proteins, namely retinoid binding proteins (unpublished data), thereby reducing its effectiveness in a therapeutic setting. To combat this issue, our laboratory proposed to investigate an isosteric substitution of the naphthalene scaffold known as an azaborine (3, 4). It is well-known that N-B bonds are isosteric to carbon-carbon double bonds. The N-B bond, although isoelectronic, introduces a local dipole moment with the lone pair of the nitrogen donating into the empty p-orbital of boron.8–10 This polarity sharply alters the molecular and solid states of the compound, namely widening the HOMO-LUMO gap due to the lowered energy of the HOMO in comparison to parent C=C bonds.
With physical and molecular changes, N-B isosteres have garnered interest within the medicinal community to expand the structural diversity of therapeutic agents. Beginning in the early 1960s, this isostere was mainly explored in diazaborines (B-N-N), which exhibit antibacterial properties against Gram-negative strains.11–13 Their specific mode of action has been extensively researched and hypothesized to target enoyl reductase; an enzyme involved in fatty acid synthesis.12 Although diazaborines have been studied in bacterial systems, their sister structure, azaborine, to the best of our knowledge has not. To date, azaborines have been applied in various anti-cancer studies with the focus on T-cell lysozyme targets.14 Based on this background we proposed to explore this isosteric model within the antibacterial space.
To achieve this target, we employed a process developed by Molander and co-workers at the University of Pennsylvania.15–16 This method proved to be amenable to our previously disclosed syntheses of the parent compound and allowed us to prepare the azaborine derivative quickly. Herein, we report on the synthesis of the azaborine analog as well as its biological and physiochemical properties in comparison to the parent compounds.
We began by synthesizing the trifluoroborate salt fragment (5) utilizing a previously described route for the synthesis of CD437 (Scheme 1).5–7 Upon achieving the borylated compound, 10 was then subjected to aqueous potassium bifluoride in methanol to afford 5 in 62% yield.17–18 With the trifluoroborate fragment in hand, we then began synthesis of the aminostyrene fragment (6, Scheme 1). Beginning with commercially available 4-amino-3-bromobenzoic acid (11), a methyl protection in the presence of thionyl chloride and methyl iodide afforded 12. This was then subjected to a tin-mediated vinylation to afford 6 in 80% yield.19
Scheme 1.

Synthesis of the trifluoroborate fragment (5), aminostyrene fragment (6), and primary alcohol azaborine analog (3). Attempted conditions to achieve the carboxylic acid analog (4) are described in the text.
With both fragments in hand, we then employed the method developed by Molander and co-workers utilizing silicon tetrachloride and triethylamine (Scheme 1).15–16 This method proved difficult with our scaffold as the MEM-protecting group was subsequently removed during the reaction progress due to the production of hydrochloric acid in situ. We hypothesized that the deprotected free phenol subsequently formed a stable bond with a neighboring boron, eliminating a fluoride anion and thereby limiting the availability of the trifluoroborate in solution. Although triethylamine was added to the reaction to neutralize the hydrochloric acid production, the removal of MEM still occurred regardless of the concentration of triethylamine used. In future use, the addition of a proton sponge would be a potential route to avoid these issues. However, the desired protected azaborine scaffold (13) could still be obtained in 25% yield. Although a change in protecting group strategy could potentially circumvent these issues, we sought to pursue a direct path to the desired analog for biological studies.
In achieving the formation of the desired scaffold, we proceeded with reduction and deprotection pathways to afford our planned primary alcohol and carboxylic acid analogs, respectively. Firstly, protected 13 was subjected to lithium aluminum hydride to afford the desired primary alcohol azaborine (3) in 97% yield. Upon obtaining 3, we then turned to deprotection of the methyl ester 13. Several hydrolysis methods were attempted to achieve the desired carboxylic acid analog (4) including base mediated hydrolysis (NaOH, KOH), acid-mediated hydrolysis (HCl, H2SO4), as well as milder approaches with pig liver esterase (PLE). However, each method proved unsuccessful. The base mediated hydrolysis showed degradation in which the proposed degradative mechanism involved the hydroxide anion attacking the empty p-orbital of the boron, forming a stable O-B bond and eliminating the nitrogen, thereby breaking apart the scaffold. This degraded product was confirmed by NMR spectroscopy (data not shown). Additionally, the acid mediated hydrolysis and the PLE-mediated hydrolysis reactions yielded only recovered starting material.
To avoid degradation of the scaffold, we attempted utilizing oxidation pathways from the primary alcohol 3. First, we implemented a Swern-Pinnick oxidation sequence that yielded starting material as well as minor degradative products. Additionally, we attempted a TEMPO oxidation that yielded only recovered starting material. After these failed attempts to access 4, we decided to focus on the primary alcohol analog (3) as it did mimic our best in class analog, analog 2 (2).
The antimicrobial activity of 3 was analyzed along with 2 against a panel of bacterial strains including three different strains of Staphylococcus aureus (SH1000, ATCC 33591, USA 300–0114), Pseudomonas aeruginosa (PAO1), Enterococcus faecalis (OG1RF), and Escherichia coli (MC4100).20 We chose to use benzalkonium chloride (BAC) as a positive control since it is a common, commercially available quaternary ammonium compound that also induces bacterial killing via membrane perturbation. In addition to antimicrobial activity, the toxicity was analyzed using a red blood cell (RBC) lysis assay utilizing mechanically defibrillated sheep’s blood. These values are indicated as Lysis20 and are measured as the concentration that lyse 20% or less of RBC. Surprisingly, the azaborine analog (3) possessed a higher MIC than its isosteric partner (2, Table 1) across all strains. However, the toxicity was comparable to the parent compound (Table 1).
Table 1.
Antimicrobial activity and toxicity data for analog 2 (2) and azaborine analog (3).
| Compound | Minimum Inhibitory Concentration (μM) | Lysis20 (μM) | |||||
|---|---|---|---|---|---|---|---|
| MSSA | HA-MRSA | CA-MRSA | E. faecalis | P. aeruginosa | E. coli | ||
| BAC | 2 | 4 | 4 | 125 | 125 | 32 | 32 |
| 2 | 2 | 2 | 2 | >250 | >250 | >250 | 125 |
| 3 | 125 | 64 | 250 | >250 | >250 | >250 | 250 |
All MIC and Lysis20 data were acquired through an average of the highest value of three independent trials; all trials were within one dilution.
To further understand this decrease in biological activity, partition coefficients (P) for both the analog 2 (2) and azaborine analog (3) were theoretically calculated via ChemDraw-3D and experimentally determined through extraction protocols with noctanol and deionized water.21–22 This experiment was performed in triplicate, and the average P was determined. From this data, log(base 10) of P was calculated. Somewhat surprisingly, we found that 2 had a logP of 0.93 and 3 had a logP of 0.12 (Table 2). These results indicate that the isosteric substitution in 3 switches the compound to become more hydrophilic in comparison to 2.
Table 2.
Experimentally determined P and logP for analog 2 (2) and azarborine analog (3) as well as quantum mechanical calculated dipole expressed as debye (D).
| 2 | 3 | |
|---|---|---|
| P | 9.16 | 1.39 |
| logP | 0.93 ± 0.20 | 0.12 ± 0.19 |
| cPa | 7.687 | 6.792 |
| clogPa | 0.89 | 0.83 |
| QM Dipole (D) | 2.42 | 1.34 |
Partition coefficients shown as an average over 3 independent trials. logP data shown with + / − standard deviation.
“c” indicates calculated data for the partition coefficient utilizing the ChemDraw3D program.
To further explore this surprising finding, we performed a quantum mechanical analysis with Schrodinger 2020–3 suite of modelling software to analyze the dipole moment of both compounds. Through these calculations, we found that analog 2 had a higher dipole moment of 2.42 D vs 1.34 D for azaborine (Table 2). As was previously disclosed by our group, the proposed mechanism of action of this class of molecules is membrane perturbation via amphiphilic attachment to the phospholipids. We propose that the presence of the N-B bond greatly reduces the molecular dipole moment of the primary alcohol, thereby limiting its amphiphilicity. Additionally, within the dipole analysis it was shown that the phenol dipole moment was greatly reduced in comparison to the parent molecule. This is most likely due to the resonance induction into the boron atom (not shown). Also, as highlighted in Figure 2, the azaborine analog has more hydrophilic regions, explaining the observed differences between clogP and logP (Fig. 2). Therefore, with a more hydrophilic structure and additional points for hydrogen bonding with water, 3 is likely unable to associate strongly with the lipid bilayer thereby reducing its biological activity.
Figure 2.

The structures of Analog 2 and the azaborine analog, displaying contributing resonance structures. Highlighted in red are hydrogen-bond donors and acceptors.
To confirm that the dipole moment, and not chemical stability, was the reason for differential activity we characterized the stability of 3 in water. Toward this end, we suspended the compound in deionized water and heated it to 37 °C for 23 h to mimic the in vitro conditions employed in our biological assays. The resulting mixture was then evacuated and analyzed by NMR spectroscopy, which proved the compound to be stable in neutral water. We recognize that there are limitations in this evaluation as we chose deionized water over the growth media used. However, we wanted a simple system to quantify degradation with minimal background noise in the NMR spectroscopy analysis and did not anticipate the additional nutrients found in growth media to cause further degradation. Overall, we found that the azaborine isosteric substitution is not a viable option for improving the biological activity of our retinoid class of antibacterial agents.
In summary, we successfully completed the synthesis of the first azaborine isostere of a retinoid derivative. Although, the biological activity was inferior to our lead compound we did uncover some surprising physiochemical properties. Namely that the naphthalene to azaborine isosteric substitution increased water solubility, which could find promising applications to other drug candidates. This isosteric substitution warrants further analysis in other antimicrobial natural products to fully understand its availability for drug discovery.
Supplementary Material
Highlights: Synthesis and biological evaluation of an antibacterial azaborine retinoid isostere.
First disclosure of an azaborine isosteric substitution for a retinoid antibiotic.
Discovery of differences in the chemical properties of an azaborine.
The surprising difference in antibacterial activity between these two scaffolds.
Acknowledgments
This work was funded by the National Institute of General Medical Sciences (GM119426) and the Georgia Research Alliance based in Atlanta, Georgia to W.M.W. C.L.S. was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number T32AI106699. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The NMR instruments used in this work were supported by the National Science Foundation (CHE1531620).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.“Biggest Threats and Data” Centers for Disease Control and Prevention, Centers for Disease Control and Prevention, 18 September 2020, www.cdc.gov/drugresistance/biggest-threats.html. [Google Scholar]
- 2.Lewis K Persister cells, dormancy and infectious disease. Nat Rev Microbiol, 2007, 5, 48–56. [DOI] [PubMed] [Google Scholar]
- 3.Prax M and Bertram R Metabolic aspects of bacterial persisters. Front. Cell. Infect. Microbiol, 2014, 4, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fauvart M; De Groote VN; and Michiels J Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. Journal of Medical Microbiology, 2011, 60, 699–709. [DOI] [PubMed] [Google Scholar]
- 5.Kim W; Zhu W; Hendricks GL; Tyne D; et al. , A new class of synthetic retinoid antibiotics effective against bacterial persisters. Nature, 2018, 556, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng AV; Kim W; Escobar IE; Mylonakis E; and Wuest WM Structure-activity relationship and anticancer profile of second-generation anti-MRSA synthetic retinoids. ACS Med. Chem. Lett 2020, 11, 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng AV; Schrank CL; Escobar IE; Mylonakis E; and Wuest WM Addition of ethylamines to the phenols of bithionol and synthetic retinoids does not elicit activity in gram-negative bacteria. Bioorg. Med. Chem. Lett 2020. 30, 127099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Z and Marder TB B-N versus C-C: how similar are they? Angew. Chem. Int. Ed 2008, 47, 242–244. [DOI] [PubMed] [Google Scholar]
- 9.Bonifazi D; Fasano F; Lorenzo-Garcia MM; Marinelli D; Oubaha H; and Tasseroul J Boron–nitrogen doped carbon scaffolding: organic chemistry, self-assembly and materials applications of borazine and its derivatives. Chem. Comm, 2015, 51(83), 15222–15236. [DOI] [PubMed] [Google Scholar]
- 10.Knack DH; Marshall JL; Harlow GP; Dudzik A; et al. BN/CC isosteric compounds as enzyme inhibitors: N- and B-ethyl-1,2-azaborine inhibit ethylbenzene hydroxylation as nonconvertible substrate analogues. Angew Chem Int Ed Engl. 2013, 52(9), 2599–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Högenauer G and Woisetschläger M A diazaborine derivative inhibits lipopolysaccharide biosynthesis. Nature, 1981, 293(5834), 662–664. [DOI] [PubMed] [Google Scholar]
- 12.Baldock C; Rafferty JB; Sedelnikova SE; Baker PJ; Stuitje AR; Slabas AR; Hawkes TR; and Rice DW A Mechanism of Drug Action Revealed by Structural Studies of Enoyl Reductase. Science, 1996, 274(5295), 2107–2110. [DOI] [PubMed] [Google Scholar]
- 13.Baldock C; De Boer GJ; Rafferty JB; Stuitje AR; and Rice DW Mechanism of action of diazaborines. Biochem. Pharma, 1998, 55(10), 1541–1549. [DOI] [PubMed] [Google Scholar]
- 14.Liu L; Marwitz AJ V; Matthews, B. W.; and Liu, S. Boron Mimetics: 1,2-Dihydro-1,2-azaborines Bind inside a Nonpolar Cavity of T4 Lysozyme. Angew Chem Int Ed Engl. 2009, 48(37), 6817–6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisniewski SR; Guenther C L; Argintaru, O.A.; and Molander, G.A. A Convergent, Modular Approach to Functionalized 2,1-Borazaronaphthalenes from 2-Aminostyrenes and Potassium Organotrifluoroborates. J. Org. Chem 2014, 79, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies GHM; Zhou Z; Jouffroy M; and Molander GA Method for Accessing Nitrogen-Containing, B-Heteroaryl-Substituted 2,1-Borazaronaphthalenes. J. Org. Chem, 2017, 82, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos-Filho EF; Sousa JC; Bezerra NMM; Menezes PH; Oliveira RA Environmentally friendly homocoupling reaction of functionalized potassium aryl trifluoroborates salts in aqueous media. Tet Lett, 2011, 52, 5288–5291. [Google Scholar]
- 18.Vedejs E; Fields SC; Hayashi R; Hitchcock SR; Powell DR; and Schrimpf MR Asymmetric Memory at Labile, Stereogenic Boron: Enolate Alkylation of Oxazaborolidinones. J. Am. Chem. Soc 1999, 121, 2460. [Google Scholar]
- 19.Aoyama A; Endo-Umeda K; Kishida K; Ohgane K; et al. Design, Synthesis, and Biological Evaluation of Novel Transrepression-Selective Liver X Receptor (LXR) Ligands with 5,11- Dihydro-5-methyl-11-methylene-6H-dibenz[b,e]azepin-6-one Skeleton. J. Med. Chem 2012, 55, 7360–7377. [DOI] [PubMed] [Google Scholar]
- 20.CLSI. M07-A9: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. 1–88 (2012). [Google Scholar]
- 21.OECD (2004), Test No. 117: Partition Coefficient (noctanol/water), HPLC Method, OECD Guidelines for the Testing of Chemicals, Section 1, OECD Publishing, Paris [Google Scholar]
- 22.Harris MF and Logan JL Determination of log Kow Values for Four Drugs. J. Chem. Educ 2014, 91, 915–918. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


