Summary
Background:
Early rapid weight gain (RWG) increases, whereas longer durations of breastfeeding decreases, odds for later obesity.
Objectives:
To determine the independent and interactive effects of early weight gain and diet on infant weight status trajectories and odds for overweight at 1 year.
Methods:
We conducted secondary analysis on data from two longitudinal trials with repeated anthropometric measures. One trial consisted of predominantly or exclusively breastfed (BF, n = 97) infants, whereas the other consisted of exclusively formula-fed (FF, n = 113) infants. Weight-for-length z-score (WLZ) change from 0.5 to 4.5 months was used to categorize early weight gain as slow (<−0.67; SWG), normal (−0.67 to 0.67; NWG) or rapid (>0.67; RWG). Linear-mixed effects models were fit to examine the independent effects and interaction of early diet (BF, FF) and weight gain (SWG, NWG, RWG) groups on WLZ trajectories; logistic regression was used to assess odds for overweight at 1 year.
Results:
While similar percentages (41%) of BF and FF infants experienced RWG, we found a significant diet × early weight gain group interaction (P < .001) on weight status. At 1 year, the WLZ of FF infants with RWG (1.57 ± 0.99) was twice that of BF infants with RWG (0.83 ± 0.92). Using BF infants with NWG as the reference group, FF infants with RWG had increased odds [OR: 25.3 (95% CI: 3.21, 199.7)] for overweight at 1 year, whereas BF infants with RWG did not.
Conclusions:
Early diet interacts with early weight gain and influences weight status trajectories and overweight risk at 1 year.
Keywords: breastfeeding, early rapid weight gain, feeding styles, infant formula
1 ∣. INTRODUCTION
Infancy is regarded as a sensitive period when nutrition and accelerated weight gain program risks for later diseases.1 A convergence of evidence from an international body of randomized control trials, prospective and retrospective studies, systematic reviews and meta-analyses, indicates that the patterning of early weight gain affects later weight status. More specifically, early rapid weight gain (RWG) associates with greater weight gain trajectories (ie, weight for length Z-scores [WLZ] in infancy and childhood, and greater odds for later overweight, obesity and adverse metabolic and cardiovascular profiles).2-10
Early RWG has been defined a variety of ways, including relatively higher gains in weight during the first week, 2 months or 4 months; increases in weight-for-age (WAZ) or WLZ scores from birth to 4 or 6 months; or increases in WAZ or WLZ greater than 0.67 standard deviations (SD) from birth to varying ages during childhood.4,6,8,9 Driven in part by overnutrition,11 RWG is influenced by both maternal (eg, pre-pregnancy body mass index [BMI],12 income13) and infant (eg, birth weight,14 formula feeding on a schedule,15 postnatal diet14-17) factors.
During early life, the postnatal diet is unique in that it is typically delivered as a liquid consisting of breast milk, breast milk substitutes (ie, infant formula) or a combination of both (mixed). In general, infant formula feeding is a risk factor for RWG in infancy.14-17 Longer duration of breastfeeding, which is a corollary to shorter durations of formula feeding, has been associated with lower odds of early RWG.14,15 Particularly when exclusive (ie, no other food or drink, not even water, except breast milk with vitamins, minerals and medicines18), or predominant (ie, breastmilk predominant with water, nutritive liquids, vitamins, minerals and medicines18), longer duration of breastfeeding is also associated with lower weight status during childhood14,19 and lower odds for obesity across the lifespan.14,17,19-21
The present article focuses on the interaction between these two known risk factors: early weight gain patterns and the postnatal diet. To this end, we analyzed a unique data set from two longitudinal trials that lasted from 0.5 months to 1 year. One trial was comprised of exclusively formula-fed infants (FF),22 whereas the other consisted of infants who were either exclusively or predominantly breastfed (BF) for at least the first 4.5 months, with breastfeeding lasting for more than 10.5 months with no formula supplementation for the majority.23 We aimed to determine whether the weight status (WLZ) trajectories during the first year and the risk for overweight at 1 year, differed based on the interaction between early weight gain and the type of diet. Based on the evidence of protective effects of breastfeeding,14,17,19-21 we hypothesized that increases in WLZ (0.5 months to 1 year) would be lower in BF infants with early RWG, when compared to FF infants with RWG. We also hypothesized that FF infants with RWG would have greater odds for overweight at 1 year, whereas BF infants with RWG would not, when compared to the reference group that consisted of BF infants with NWG.
2 ∣. METHODS
2.1 ∣. Participants and overview of trials
Data from two longitudinal trials were combined for secondary data analyses. While both trials focused on the growth of healthy term infants living in the Philadelphia area, the type of diet experienced differed substantially and resulted in two distinct groups.
One trial was designed to determine the effects of infant formula composition on energy balance and growth among exclusively FF infants during the first year22 (NCT01700205; years of study: 2012-2015). Mothers were recruited after parturition and enrolled only if their decision not to breastfeed was established at 2 weeks postpartum. In this trial, 113 FF infants were randomized to be exclusively fed either cow milk formula (CMF; Enfamil, Mead Johnson Nutrition; N = 59) or an isocaloric, extensive protein hydrolysate formula (EHF; Nutramigen, Mead Johnson Nutrition; N = 54).
The other trial was designed to determine the effects of maternal diet on the BF infants' (N = 97) acceptance of vegetables at weaning23 (NCT01667549; years of study: 2012-2015; Figure S1). Pregnant or recently parturient women who intended to breastfeed their infants exclusively (with no formula supplementation) were recruited. This inclusion criteria resulted in a group of infants for which breast milk was the exclusive or predominant source of nourishment for the first 4.5 months. After 4.5 months, the majority of BF infants continued to be breastfed such that 81% were still breastfeeding and 62% had never been fed infant formula at 10.5 months. In sum, the grouping based on early diet was dichotomous: the FF infants were never breastfed and BF infants never or rarely fed infant formula during the time period in which they were classified into early weight gain groups (0.5-4.5 months) or months thereafter.
Inclusion criteria for both trials included that infants were born healthy and at term (37-42 weeks), with body weights that were considered typical (2500-4500 g).24 Exclusion criteria included maternal gestational diabetes or infant congenital malformations, systemic or congenital infections, or family history of atopy. Anthropometric data were obtained from FF infants on 13 separate occasions, at monthly intervals from 0.5 to 12.5 months and from BF infants on eight occasions; monthly from 0.5 to 4.5 months, at the 3-days vegetable acceptance test at ~7.5 months (6.5-9.5 months) and then again at two follow-up visits: 10.5 months and ~1 year (11.5-15.5 months; see Figure S1). There was variability in the timing of the latter two visits. The evaluation of infants' acceptance of pureed vegetables (ie, ~7.5 months) and vegetable juices (~1 year) was dependent upon when the mothers decided to complement breastfeeding with solid foods and when they gave their child a sippy cup, respectively. Mothers were not instructed by study personnel on how or how much infant formula to feed their infants (FF group), when or how long to breastfeed (BF group), or when or how to complement their infants' diets with solid foods (FF and BF groups). Both trials were reviewed and approved by the Institutional Review Board of the University of Pennsylvania and were conducted in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration. Written informed consent was obtained from each mother prior to study entry.
2.2 ∣. Anthropometry, weight gain and overweight status
Infant birth weight was obtained by maternal report. Beginning when the infant was 0.5 month, weight was measured to the nearest 0.001 kg and length was measured to the nearest 0.1 cm in triplicate at each study visit, by research personnel trained and certified in standard anthropometric techniques, using calibrated equipment. The digital pediatric scale (Scale-Tronix, White Plains, NY) and infantometer (Harpenden 702; Crymych, Dyfed, UK), accurate to 0.001 kg and 0.1 cm, respectively. From these data, anthropometric measures were converted to Z-scores using World Health Organization (WHO) growth standards25: Z-scores for weight for age (WAZ), length for age (LAZ), and weight for length (WLZ).
Because changes in WLZ (or in older children weight-for-height Z) have been shown to have a strong effect on prevalence ratios of later obesity and overweight26 and because of the precedence in the literature (eg, Taveras et al,27; Hawkins et al,28; Wills et al29), we used WLZ changes to define early weight gain groups and overweight status at 1 year, and report on WLZ changes from 0.5 months to 1 year. Early weight gain groups were determined based on changes in WLZ from baseline (0.5 month) to 4.5 months: WLZ changes <−0.67 SD were categorized as slow weight gain (SWG); between −0.67 and 0.67 SD, as normal weight gain (NWG); and >0.67 SD, as RWG.30,31 Overweight status at 1 year was defined as WLZ > 85th percentile (WLZ > 1.0364).
2.3 ∣. Secondary outcomes
In addition to early diet and early weight gain, a number of potential confounders,10,12,13,32,33 determined a priori, that could impact overweight status at 1 year were assessed: infant birth weight, maternal age, pre-pregnancy weight and height, gestational weight gain, race, infant sex, parity, household income and maternal education. While pre- and peri-pregnancy anthropometric measures were obtained from maternal reports, each woman was weighed (kg) and height (m) was measured at enrolment in duplicate by trained research personnel, while wearing light clothing and no shoes. To gain insight into the influence of the complementary diet and maternal feeding beliefs on infant weight status, we analyzed the caloric content of the complementary diet (ie, solid foods and beverages excluding formula or breastmilk) fed to infants and the feeding beliefs of the mothers, as measured by the Infant Feeding Style Questionnaire (IFSQ).34 We chose the age of 10.5 months because complementary foods had been introduced to the diet of all infants and both studies had a visit that occurred at this time.
With respect to diet, mothers recorded all foods and beverages ingested by their infants for a 24-hour period. These data were analyzed using Nutrient Data System for Research (NDS-R; University of Minnesota, Minneapolis, Minnesota) to determine available energy intake (EI) from the complementary diet for the infants. Data were not available on the amount of, or energy content of breast milk for the BF group. All diet records were reviewed by registered dietitians, and diet records with physiologically implausible energy intakes, either based on estimated energy needs or because incomplete, were eliminated.
With respect to feeding beliefs, mothers completed the IFSQ,34 a validated questionnaire. From these data, we report on five constructs that have been associated with infant feeding and growth,35,36 laissez-faire (ie, mother has little interaction during feeding and does not believe child should have limits on food), pressuring (ie, mother believes child should finish feed, should be fed to soothe or help fall asleep), restrictive (ie, mother believes she should limit quantity and control quality of food given to child), responsive (ie, mother believes she should encourage exploration and attend to child cues) and indulgent (ie, mother does not believe in setting limits on quality or quantity of food given to child). For each question, if the mother deemed it applicable, values ranged from 1 to 5; higher scores reflect more of that belief. Cronbach α coefficients for each construct were calculated to determine internal consistency, which was acceptable for the restrictive, pressuring and indulgent scales (α ≥ .70) but questionable for laissez-faire and responsive (α ≥ .42).
2.4 ∣. Statistical analyses
Infant and maternal characteristics at baseline were evaluated two ways. First, we focused on the FF infants only and compared characteristics between the type of infant formula group: (CMF vs EHF). Because we found no significant differences in baseline characteristics between formula groups (CMF, EHF), we combined them into a single formula fed (FF) diet group. Second, we compared characteristics by diet group: FF vs BF. Two-sample t-tests were used for continuous variables and Chi-squared or Fisher Exact probability (if one cell <5) tests were used for categorical variables.
Consistent with prior research, we used a class-based approach3,4,14,17,26,31,37 and grouped infants based on early diet, early weight gain, and their combinations. We then fit a linear-mixed effects model with a random intercept for infant and a random slope for time to examine predictors of WLZ score over the first year. Time was measured in monthly intervals from 0.5 to 12.5 months for FF infants and 0.5 to 4.5, 7.5 (±1), 10.5 and 12.5 (±1) months (hereafter referred to as 1 y) for BF infants. Fixed effects in the model included time, diet group (BF, FF), early weight gain group (SWG, NWG, RWG), and a diet group × early weight gain group interaction that compared infants WLZ over time across diet and early weight gain groups, using BF infants with NWG as the reference group. An unstructured correlation matrix was chosen for the correlation among repeated WLZ measurements per infant after comparison of models with other correlation matrices. Marginal means and standard error of the means (SEM) were generated from the linear-mixed effect model and utilized to demonstrate differences in WLZ growth over time by diet group × early weight gain group combination.
Next we examined odds for overweight at 1 year. We identified potential confounders by determining whether baseline infant and maternal characteristics differed between overweight and non-overweight infants. Logistic regression models were fit to estimate the odds ratio for overweight at 1 year associated with diet group (BF, FF) and early weight gain group (SWG, NWG, RWG). To examine the combined effects of diet and early weight gain groups, an interaction term was created that resulted in six dummy variables (BF infants with SWG, BF infants with NWG, BF infants with RWG, FF infants with SWG, FF infants with NWG, FF infants with RWG). For each model, the group of BF infants with NWG was the reference group against which the other five groups were compared. Two overall models were then fit. Model 1 examined the crude association of the diet (BF, FF) and early weight gain group (NWG, SWG, RWG). Model 2 adjusted for infant and maternal characteristics that differed between overweight and non-overweight status at 1 year. For each model, the overall effect of the interaction between diet group and early weight gain group was evaluated using a Wald chi-squared test statistic.
Finally, as an exploratory aim, separate general linear models were used to examine differences in the caloric content of the complementary diet (kcal/d) or maternal feeding beliefs (ie, laissez-faire, restrictive, pressuring, responsive, indulgent) at 10.5 months by diet group (BF, FF), early weight gain group (NWG, SWG, RWG), and their interaction. All statistical tests and corresponding P-values less than .05 were considered statistically significant.
3 ∣. RESULTS
3.1 ∣. Study populations and characteristics
The study populations consisted of 113 FF (n = 59 CMF, n = 54 EHF) infants and 97 BF infants at enrolment (0.5 month). As shown in Figure S1, 81% of FF infants and 84% of BF infants completed the 4.5 month visit, and thus were categorized into early weight gain (NWG, SWG, RWG) groups. The completion rates were 73% (n = 83) at 12.5 months for the FF trial and 79% (n = 77) for the ~1 year follow-up visit for the BF trial (n = 58 infants tested at 11.5-13.5 months; n = 19 infants tested at 14.5-15.5 months).
As shown in Figure 1A, the percentage of infants in each of the early weight gain groups (ie, SWG, NWG, RWG) differed by infant formula type (CMF, EHF) and BF groups (Chi-squared test, P = .005). Overall, EHF fed infants were over-represented in the SWG category and under-represented in RWG category. Comparing the BF group with the FF group (Figure 1B), the percentage of infants in each of the early weight gain groups did not differ between BF infants and FF infants as a group (Chi-squared test, P = .298).
FIGURE 1.
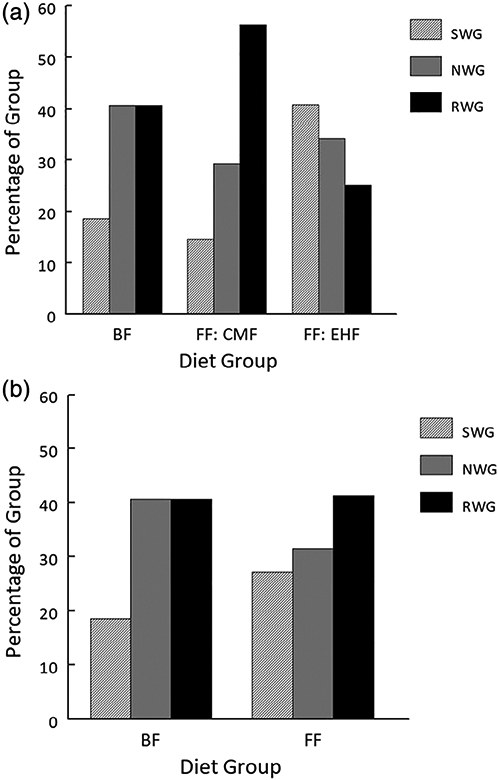
A, Percentage (%) of infants breastfed (BF), fed cow milk formula (CMF), or extensive protein hydrolysate formula (EHF) in each early weight gain category [slow (SWG), normal (NWG) and rapid (RWG)] differed by infant formula type (CMF, EHF) and BF groups (Chi-squared test, P = .005); based on contributions to the overall Chi-squared, EHF fed infants were over-represented in SWG and under-represented in RWG categories. B, Percentage (%) of breastfed (BF) and formula-fed (FF) infants as a group, in each early weight gain category (SWG, NWG, RWG) did not differ (Chi-squared test, P = .298)
3.2 ∣. Infant and maternal characteristics by diet group
Baseline infant and maternal characteristics by infant formula type (CMF, EHF) group and by diet (BF, FF) group are shown in Table 1. The two formula type groups were combined into a single FF diet group because there were no significant differences in these characteristics. When compared to the BF group, the FF group had a lower percentage of white infants and higher percentage of black infants. FF infants had lower WAZ and LAZ at birth and lower WAZ and LAZ at 0.5 month compared to BF infants. While FF infants had a higher WLZ at birth than BF infants, there was no significant group difference at baseline (0.5 months). Mothers of FF infants were significantly younger and had higher BMI pre-pregnancy and at baseline, lower household incomes and lower education levels, and a greater percentage were multiparous compared to mothers of BF infants. Although smoking rates were low (<15%) in both groups, a greater percentage mothers of FF infants smoked during pregnancy.
TABLE 1.
Dyad baseline characteristics by formula type groups and by diet groups
| Formula type groups |
Diet groups |
|||||
|---|---|---|---|---|---|---|
| Characteristics | CMF infants (n = 59) |
EHF infants (n = 54) |
CMF vs EHF, P-valuea |
Combined FF group (n = 113) |
BF group (n = 97) |
FF vs BF, P-valuesa |
| Infants | ||||||
| Female | 28 (47%) | 29 (54%) | .51 | 57 (50%) | 50 (52%) | .873 |
| Race | .14 | <.001 | ||||
| Black | 35 (59%) | 35 (65%) | 70 (62%) | 31 (32%) | ||
| White | 17 (29%) | 8 (15%) | 25 (22%) | 49 (51%) | ||
| Other/more than one race | 7 (12%) | 11 (20%) | 18 (165) | 17 (18%) | ||
| Anthropometry, Z-scores | ||||||
| WAZ, birth | −0.03 ± 0.88 | 0.01 ± 0.91 | .81 | −0.01 ± 0.89 | 0.46 ± 0.91 | <.001 |
| LAZ, birth | 0.45 ± 1.08 | 0.39 ± 1.15 | .79 | 0.42 ± 1.11 | 1.33 ± 1.18 | <.001 |
| WLZ, birth | −0.54 ± 1.07 | −0.40 ± 1.21 | .52 | −0.47 ± 1.13 | −0.91 ± 1.29 | .010 |
| WAZ, 0.5 month | −0.36 ± 0.87 | −0.25 ± 0.81 | .49 | −0.30 ± 0.84 | 0.11 ± 0.87 | <.001 |
| LAZ, 0.5 months | −0.49 ± 1.01 | −0.46 ± 1.08 | .91 | −0.48 ± 1.04 | 0.11 ± 1.00 | <.001 |
| WLZ, 0.5 months | −0.26 ± 0.84 | −0.12 ± 1.00 | .43 | −0.19 ± 0.92 | −0.26 ± 1.05 | .619 |
| Mothers | ||||||
| Age, years | 27.1 ± 6.0 | 27.0 ± 5.4 | .88 | 27.1 ± 5.7 | 30.8 ± 5.4 | <.001 |
| Gestational weight gainb, kg | 13.0 ± 9.4 | 13.8 ± 8.8 | .65 | 13.4 ± 9.1 | 14.4 ± 5.7 | .342 |
| Body mass index, pre-pregnancyb, kg/m2 | 30.1 ± 8.6 | 28.2 ± 8.2 | .25 | 29.2 ± 8.4 | 25.8 ± 6.3 | .001 |
| Body mass index at 0.5 monthsb, kg/m2 | 30.7 ± 7.4 | 31.2 ± 8.4 | .75 | 30.9 ± 7.9 | 28.3 ± 6.2 | .008 |
| Primiparous | 12 (20%) | 11 (20%) | 1.00 | 23 (20%) | 33 (34%) | .026 |
| Household incomeb | .70 | <.001 | ||||
| <$35 000 | 44 (76%) | 37 (70%) | 81 (73%) | 40 (41%) | ||
| $35 000 to 75 000 | 5 (9%) | 7 (13%) | 12 (11%) | 21 (22%) | ||
| >$75 000 | 9 (15%) | 9 (17%) | 18 (16%) | 36 (37%) | ||
| Education level | .21 | <.001 | ||||
| Primary school | 12 (20%) | 5 (9%) | 17 (155) | 5 (5%) | ||
| High school/technical school | 32 (54%) | 36 (67%) | 68 (60%) | 32 (33%) | ||
| College degree or higher | 15 (26%) | 13 (24%) | 28 (25%) | 60 (62%) | ||
| Smoked during pregnancy | 8 (14%) | 8 (15%) | .85 | 16 (14%) | 2 (2%) | .001 |
Note: Values are mean ± SD or n (%).
Abbreviations: BF, breastfed; CMF, cow milk formula; EHF, extensively protein hydrolyzed formula; FF, formula fed; LAZ, length for age Z-score; WAZ, weight for age Z-score; WLZ, weight for length Z-score.
P-values for main effect of infant formula (CMF vs EHF) or diet (FF vs BF) groups obtained from Pearson Chi-squared or Fisher exact probability (if one cell <5) tests or general linear models with group as the between-subject factor.
Values do not sum to total because of missing data: gestational weight gain (FF: n = 111; BF: n = 96); body mass index, pre-pregnancy (FF: n = 112; BF: n = 95); body mass index, 0.5 months (BF: n = 96); household income (FF: n = 111).
3.3 ∣. Infant and maternal characteristics by weight status at 1 year
All analyses on weight status at 1 year included the 83 FF infants who completed the 12.5 month visit (Figure S1). However, because of the variability in the BF infants' ages at testing, we included only those BF infants who completed the follow-up visit at 12.5 ± 1 months. Table 2 summarizes the baseline characteristics of the dyads by infant weight status at 1 year. Infants who were overweight at 1 year had higher WLZ at birth and at 0.5 month when compared to non-overweight infants. There were no between-group differences in other infant or in maternal characteristics by weight status group (overweight, non-overweight). We repeated the analysis and included the BF infants who were not tested until 14.5 to 15.5 months and the results were unchanged.
TABLE 2.
Dyad baseline characteristics by infant weight status at 1 year
| Infant weight statusa at 1 year |
|||
|---|---|---|---|
| Characteristics | Non-overweight (n = 99) | Overweight (n = 41) | P-valuesb |
| Infants | |||
| Female | 48 (48%) | 23 (55%) | .495 |
| Race | |||
| Black | 49 (49%) | 17 (40.5%) | 538 |
| White | 31 (31%) | 17 (40.5%) | |
| Other/more than one race | 19 (19%) | 8 (19%) | |
| Anthropometry Z-scores | |||
| WAZ, birth | 0.10 ± 0.92 | 0.31 ± 0.88 | .207 |
| LAZ, birth | 0.82 ± 1.17 | 0.66 ± 1.12 | .432 |
| WLZ, birth | −0.82 ± 1.20 | −0.24 ± 0.98 | .007 |
| WAZ, 0.5 months | −0.20 ± 0.85 | 0.00 ± 0.77 | .186 |
| LAZ, 0.5 months | −0.21 ± 1.05 | −0.28 ± 0.96 | .745 |
| WLZ, 0.5 months | −0.35 ± 0.93 | 0.04 ± 0.89 | .022 |
| Mothers | |||
| Age, years | 28.9 ± 5.6 | 28.8 ± 6.2 | .948 |
| Gestational weight gain, kg | 13.9 ± 8.6 | 12.9 ± 7.4 | .496 |
| Body mass index, pre-pregnancy, kg/m2 | 26.6 ± 6.2 | 28.3 ± 8.5 | .198 |
| Body mass index, 0.5 months, kg/m2 | 29.8 ± 7.3 | 30.3 ± 7.9 | .719 |
| Primiparous | 26 (26%) | 12 (29%) | .778 |
| Household income | |||
| <$35 000 | 58 (59%) | 25 (60%) | .689 |
| $35 000 to 75 000 | 17 (17%) | 5 (12%) | |
| >$75 000 | 24 (24%) | 12 (29%) | |
| Education level | |||
| Primary school | 9 (9%) | 1 (2%) | .097 |
| High school/technical school | 48 (48%) | 28 (67%) | |
| College degree or higher | 42 (43%) | 13 (31%) | |
| Smoked during pregnancy | 9 (9%) | 4 (10%) | .935 |
Note: Values are mean ± SD or n (%).
Abbreviations: LAZ, length for age Z-score; WAZ, weight for age Z-score; WLZ, weight for length Z-score.
Overweight at 1 y defined as WLZ > 85th percentile.
P-Values for main effect of infant weight status grouping obtained from Pearson chi-squared or Fisher exact probability (if one cell <5) tests, or general linear models, with group as the between-subject factor.
3.4 ∣. Changes in WLZ over time
The linear-mixed effects model revealed a statistically significant diet group × early weight gain group interaction on longitudinal changes in WLZ during the first year (P-for-interaction <.001; Figure 2). Compared to BF infants with NWG, BF and FF infants with RWG had a greater increase in WLZ over the study period (BF infants with RWG: β = 0.36; 95% CI: 0.00, 0.72; FF infants with RWG: β = 0.65; 95% CI: 0.30, 0.99). However at 1 year, the WLZ of FF infants with RWG (1.57 ± 0.99, mean ± SD) was twice that of BF infants with RWG (0.83 ± 0.92). As a reference, the WLZ of BF infants with NWG at 1 year was 0.50 ± 0.79. We repeated WLZ trajectory modeling and included the BF infants who were not tested until 14.5 to 15.5 months and the results were unchanged. Compared to BF-NWG infants, BF-RWG infants and FF-RWG infants, had increased WLZ over the study period (BF-RWG: β = 0.34; 95% CI: −0.02, 0.70; FF-RWG: β = 0.67; 95% CI: 0.33, 1.02, respectively).
FIGURE 2.
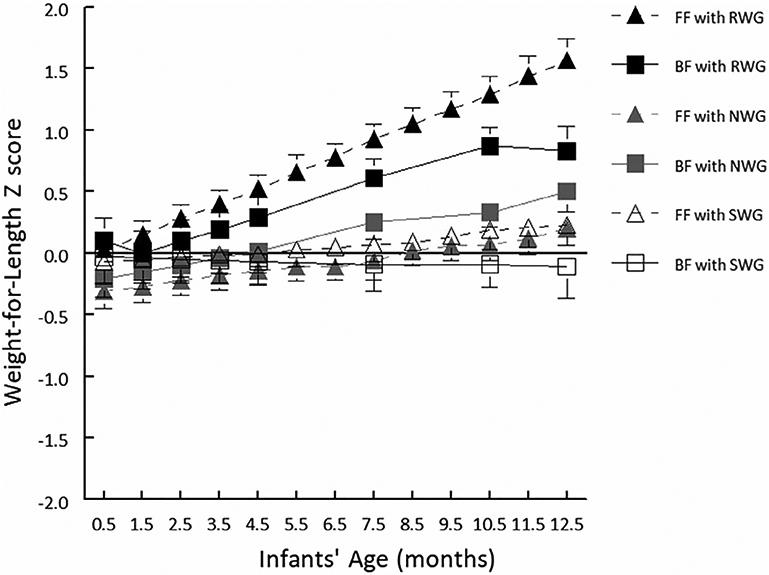
Weight-for-length Z-scores (WLZ; based on World Health Organization growth standards) by diet group (breastfed [BF]: n = 59, formula fed [FF]: n = 83) and early weight gain category (rapid [RWG], normal [NWG], slow [SWG]). Linear-mixed effects model revealed a statistically significant interaction between diet group and early weight gain category on longitudinal changes in WLZ during the first year (P < .001). Data are least-square means ± SE of the means. At 1 year, FF infants with RWG had an average WLZ score (1.57 ± 0.99) twice that of the BF infants with RWG (0.83 ± 0.92); as a reference, BF infants with NWG had an average WLZ of 0.50 ± 0.79
3.5 ∣. Odds for overweight at 1 year
In model 1, a statistically significant interaction between diet group (BF, FF) and early weight gain group (SWG, NWG, RWG) was found for odds for overweight at 1 year (Chi-squared = 26.7; P < .001). Compared to the reference group (BF infants with NWG), FF infants with RWG had 25 times greater odds for overweight at 1 year, whereas BF infants with RWG did not have increased odds (Table 3). This association remained after adjustment for characteristics (ie, WLZ at 0.5 month) that differed between overweight and non-overweight status at 1 year (model 2). Although the mean WLZ at birth also differed, it was highly correlated with WLZ at 0.5 month and thus was not included in model 2 due to multicollinearity. A statistically significant diet group × early weight gain group interaction was found (Chi-squared = 30.2, P < .001). When compared to the reference group (BF infants with NWG), FF infants with RWG had 31 times greater odds for overweight at 1 year (OR: 30.9 [95% CI: 3.1, 305.5]).
TABLE 3.
Odds for overweight at 1 year
| Odds ratioa | 95% Confidence interval | |
|---|---|---|
| Breastfed infants | ||
| NWG | 1.00 | - |
| SWG | 1.11 | (0.23, 5.47) |
| RWG | 0.56 | (0.12, 2.55) |
| Formula-fed Infants | ||
| NWG | 0.43 | (0.19, 1.97) |
| SWG | 1.92 | (0.21, 17.81) |
| RWG | 25.3 | (3.21, 199.70) |
Abbreviations: NWG, normal weight gain; SWG, slow weight gain; RWG, rapid weight gain.
Crude model coefficients from logistic regression using breastfed infants with NWG as reference group.
3.6 ∣. Maternal feeding style beliefs and the complementary diet of infants
There was a main effect of diet group (BF, FF) on maternal feeding style beliefs, but no main effect of early weight gain group or diet group × early weight gain group interaction. The laissez-faire and restrictive feeding belief scores did not differ between mothers in each diet group; however, FF mothers' belief scores regarding pressuring (P < .001) and indulgence (P < .001) were higher and belief scores regarding responsiveness (P < .001) was lower than those among BF mothers. Similarly, there was a main effect of diet group (BF, FF) on the energy intake from complementary foods. FF infants had a significantly higher energy intake from complementary foods (537 ± 421 kcal/d; P < 0.001) compared to BF infants (322 ± 194 kcal/d). There was no main effect of early weight gain groups or diet group × early weight gain group interaction on energy intake from the complementary diet.
4 ∣. DISCUSSION
Whether the source of early nutrition during the sensitive period of the first 4.5 months consisted solely of breast milk or infant formula, just under half of the BF (41%) and FF (41%) infants experienced early RWG. However, the consequence of the early RWG on weight trajectories and odds for overweight differed based on the type of early diet (BF, FF). While both BF and FF infants with RWG had greater increases in WLZ over time compared to the reference group of BF infants with NWG, the mean WLZ of BF infants with RWG was 0.74 SD lower than FF with RWG infants. Additionally, FF infants with RWG had significantly higher odds of overweight at 1 year, whereas BF infants with RWG did not. While there were no interactions between diet and early weight gain groups in the mothers' feeding belief scores over time or in the infants' energy intake from solid foods, mothers of BF infants were less indulgent or pressuring and more responsive and fed their infants a complementary diet that was lower in energy than mothers who formula fed their infants.
These findings, which come from two dichotomous early diet groups consisting of exclusively FF and predominantly BF infants, build upon prior research that shows that early RWG increases,2-7,10 whereas longer durations of breastfeeding decreases,14,20,38 risks for later obesity. In examining the interaction of these two known risk factors, we found that FF infants with RWG were at the highest risk for being overweight at 1 year and thus may be particularly vulnerable to longer term consequences of RWG. Several explanations, not mutually exclusive, may contribute to the protective effects of breastfeeding among infants with RWG.
First, there are marked differences in the composition of the breast milk and infant formula. The metabolizable energy content of mature human milk39,40 is lower than that of most infant formulas for term infants, which may lead to lower energy intake in the breastfed infant.40 Moreover, the total protein concentration of mature human milk decreases to from ~13 g/L at 1 to 2 months to ~11 g/L at 5 to 6 months postpartum.41 In contrast, FF infants are consistently exposed to a protein concentration of 13 to 14 g/L and hence higher protein intake.42 Protein intake in excess of requirements may drive higher weight gain in the FF infant, as higher concentrations of fasting43 and postprandial44-46 branched chain amino acids may stimulate increased insulin concentrations and, in turn, increase cellular glucose uptake.45,47
Second, the higher weight status of FF infants with RWG may be due to the differences in the complementary diet fed to them. Although we focused on energy intake from complementary foods at 10.5 months only, energy intake from the complementary was higher in FF compared to BF infants. Research has shown that FF infants are introduced to complementary foods at an earlier age,48 and their total food49 and energy intake from formula and complementary foods42 is greater in the first year when compared to BF infants.
Third, the differential weight gain may be due to the differences in the feeding beliefs between lactating and formula feeding mothers. We found that lactating mothers were less indulgent or pressuring and more responsive in their feeding beliefs than mothers of FF infants, a finding consistent with prior research.50-52 A recent intervention trial by Savage, Paul, Birch and colleagues10 that investigated whether a multi-component, responsive parenting practices intervention differed between FF and BF infants, found that responsive parenting was equally effective in lowering the incidence of overweight status at 1 y among BF and FF infants. These findings suggest that mothers of FF infants with RWG during early infancy may be important targets for such preventative interventions.
Strengths of this study include that all infants were born term and were unique in that their early diet consisted of the sole source of nutrition. That is, FF infants were exclusively formula fed and all BF infants were exclusively or predominantly fed breastmilk for the first 4.5 months, with the majority of BF infants continuing to be breastfed and not formula fed during the second half of the first year. Given that 47% of infants in the United States are exclusively breastfed through 3 months,53 the incidence of breastfeeding in our cohort is above that reported nationally. Additionally, anthropometric measures were by trained research personnel, and BF and FF infants were recruited from the same geographic area and during the same time period.
The study had limitations. First, the small sample size limits the precision of our estimated effects and the models presented may be overfit. However, we underscore that the present discovery of an interaction between early diet and early weight gain on odds for overweight at 1 year, should be considered in the context of the large body of research54 demonstrating the relationship between early RWG and later life weight status.3,4,14,17,26,31,55-59 Second, inherent to research that compares outcomes of BF and FF infants is the heterogeneity of the study populations due in part to the sociocultural influences on infant feeding.60,61 While we adjusted for maternal and infant characteristics that differed by infant overweight status at 1 year, we recognize that there is the potential for unmeasured or residual confounding that could influence the relationship among early RWG, diet and odds for overweight. Third, we acknowledge that there are other parental (eg, food insecurity,62 self-efficacy63) and infant (eg, appetite,64 temperament63) factors that may be on the causal pathway to overweight status at 1 year and beyond.64,65 Understanding how such factors protect or exacerbate weight status as the child grows, especially among those who experience early RWG, is an important area for further research.
5 ∣. CONCLUSIONS
What infants are fed interacts with how rapidly they gain weight during the sensitive period of the first 4.5 months and influences weight status trajectories and risk for overweight at 1 year. While a similar proportion of BF and FF infants demonstrated early RWG, the WLZ of FF infants with RWG was twice that of BF infants with RWG and they had greater odds for overweight at 1 year. While no similar interactions were found between early diet and weight gain for the energy intake from complementary foods or the feeding belief scores of the mothers, formula feeding mothers, as a group, fed their infants a complementary diet higher in energy and were more indulgent and less responsive in feeding beliefs. These data highlight the importance of this early sensitive period and the need for a better understanding of the influences and interactions of the composition of the diet (infant formula, breast milk, complementary foods), timing and duration of exposure to the diet, and caregiver feeding beliefs interact on later obesity risks which will, in turn, inform healthy weight gain initiatives.
Supplementary Material
ACKNOWLEDGEMENTS
J.C.T., J.A.M. and V.A.S. conceptualized and designed the study, supervised data collection and analyses, critically reviewed the manuscript drafted by J.C.T. M.A.P., J.C.T. and A.D.S. conducted the statistical analyses. J.R.E. entered dietary intake data into a nutrient data composition database and conducted initial analyses. All authors approved the final manuscript as submitted. We acknowledge the expert technical assistance of Loma Inamdar, Naomi Pressman, Ashley Reiter and Loran Pryor. We dedicate this manuscript in memory of our dear colleague, Leann L. Birch. This research was supported by NIH grants R01HD072307 and R03HD094908 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) awarded to J.C.T. and J.A.M., and NIH grant R01HD037119 from NICHD awarded to J.A.M. A.D.S. was supported by T32DC000014-38 and F32DC018710-01 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding information
National Institute of Child Health and Human Development, Grant/Award Numbers: R01HD037119, R01HD072307, R03HD094908; National Institute on Deafness and Other Communication Disorders, Grant/Award Numbers: F32DC018710-01, T32DC000014-38
Abbreviations:
- BF
breastfed
- BMI
body mass index
- CI
confidence interval
- CMF
cow milk formula
- EHF
extensive protein hydrolysate formula
- FF
formula fed
- LAZ
length-for age z-score
- NWG
normal weight gain
- OR
odds ratio
- RWG
rapid weight gain
- SD
standard deviation
- SWG
slow weight gain
- WAZ
weight-for-age z-score
- WHO
World Health Organization
- WLZ
weight-for-length z-score
Footnotes
CONFLICT OF INTEREST
The authors have no financial relationships to disclose. J.C.T. consults on nutrition clinical trial design for Paidion Research, Inc. and ByHeart, Inc. and V.A.S. consults for AbbVie, Nestlé Medical Nutrition, Danone and Regeneron. J.A.M., M.A.P., A.D.S. and J.R.E. have no conflicts of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Barker DJ, Osmond C, Law CM. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health. 1989;43(3):237–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch dis Child. 2012;97(12):1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karaolis-Danckert N, Buyken AE, Bolzenius K, Perim de Faria C, Lentze MJ, Kroke A. Rapid growth among term children whose birth weight was appropriate for gestational age has a longer lasting effect on body fat percentage than on body mass index. Am J Clin Nutr. 2006;84(6):1449–1455. [DOI] [PubMed] [Google Scholar]
- 4.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fabricius-Bjerre S, Jensen RB, Faerch K, et al. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adolescence. PLoS One. 2011;6(6):e20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life--a systematic review. Obes Rev. 2005;6(2):143–154. [DOI] [PubMed] [Google Scholar]
- 7.Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity–a systematic review. Int J Obes Relat Metab Disord. 2004;28(10):1247–1256. [DOI] [PubMed] [Google Scholar]
- 8.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95(8):904–908. [DOI] [PubMed] [Google Scholar]
- 9.Rzehak P, Oddy WH, Mearin ML, et al. Infant feeding and growth trajectory patterns in childhood and body composition in young adulthood. Am J Clin Nutr. 2017;106(2):568–580. [DOI] [PubMed] [Google Scholar]
- 10.Savage JS, Birch LL, Marini M, Anzman-Frasca S, Paul IM. Effect of the INSIGHT responsive parenting intervention on rapid infant weight gain and overweight status at age 1 year: a randomized clinical trial. JAMA Pediatr. 2016;170(8):742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stunkard AJ, Berkowitz RI, Stallings VA, Schoeller DA. Energy intake, not energy output, is a determinant of body size in infants. Am J Clin Nutr. 1999;69(3):524–530. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90(5):1303–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois L, Girard M. Early determinants of overweight at 4.5 years in a population-based longitudinal study. Int J Obes (Lond). 2006;30(4):610–617. [DOI] [PubMed] [Google Scholar]
- 14.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihrshahi S, Battistutta D, Magarey A, Daniels LA. Determinants of rapid weight gain during infancy: baseline results from the NOURISH randomised controlled trial. BMC Pediatr. 2011;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rzehak P, Sausenthaler S, Koletzko S, et al. Short- and long-term effects of feeding hydrolyzed protein infant formulas on growth at < or = 6 y of age: results from the German Infant Nutritional Intervention Study. Am J Clin Nutr. 2009;89(6):1846–1856. [DOI] [PubMed] [Google Scholar]
- 17.Bell KA, Wagner CL, Feldman HA, Shypailo RJ, Belfort MB. Associations of infant feeding with trajectories of body composition and growth. Am J Clin Nutr. 2017;106(2):491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. The World Health Organization's Infant Feeding Recommendation. Geneva, Switzerland: WHO; 2002. [Google Scholar]
- 19.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162(5):397–403. [DOI] [PubMed] [Google Scholar]
- 20.Kramer MS, Guo T, Platt RW, et al. Feeding effects on growth during infancy. J Pediatr. 2004;145(5):600–605. [DOI] [PubMed] [Google Scholar]
- 21.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–1377. [DOI] [PubMed] [Google Scholar]
- 22.Mennella JA, Inamdar L, Pressman N, et al. Type of infant formula increases early weight gain and impacts energy balance: a randomized controlled trial. Am J Clin Nutr. 2018;108(5):1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mennella JA, Daniels LM, Reiter AR. Learning to like vegetables during breastfeeding: a randomized clinical trial of lactating mothers and infants. Am J Clin Nutr. 2017;106(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin JA, Hamilton BE, Osterman MJK. Births in the United States. NCHS Data Brief. 2018;2019(346):1–8. [PubMed] [Google Scholar]
- 25.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 26.Monteiro PO, Victora CG, Barros FC, Monteiro LM. Birth size, early childhood growth, and adolescent obesity in a Brazilian birth cohort. Int J Obes Relat Metab Disord. 2003;27(10):1274–1282. [DOI] [PubMed] [Google Scholar]
- 27.Taveras EM, Rifas-Shiman SL, Sherry B, et al. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–998. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins SS, Rifas-Shiman SL, Gillman MW, Taveras EM. Racial differences in crossing major growth percentiles in infancy. Arch dis Child. 2018;103(8):795–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wills AK, Strand BH, Glavin K, Silverwood RJ, Hovengen R. Regression models for linking patterns of growth to a later outcome: infant growth and childhood overweight. BMC Med Res Methodol. 2016;16:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang G, Johnson S, Gong Y, et al. Weight gain in infancy and overweight or obesity in childhood across the gestational spectrum: a prospective birth cohort study. Sci Rep. 2016;6:29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salgin B, Norris SA, Prentice P, et al. Even transient rapid infancy weight gain is associated with higher BMI in young adults and earlier menarche. Int J Obes (Lond). 2015;39(6):939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen SS, Alexander DD, Krebs NF, et al. Factors associated with breastfeeding initiation and continuation: a meta-analysis. J Pediatr. 2018;203:190–196.e121. [DOI] [PubMed] [Google Scholar]
- 33.Regnault N, Gillman MW. Importance of characterizing growth trajectories. Ann Nutr Metab. 2014;65(2–3):110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson AL, Mendez MA, Borja JB, Adair LS, Zimmer CR, Bentley ME. Development and validation of the infant feeding style questionnaire. Appetite. 2009;53(2):210–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hittner JB, Johnson C, Tripicchio G, Faith MS. Infant emotional distress, maternal restriction at a home meal, and child BMI gain through age 6years in the Colorado Adoption Project. Eat Behav. 2016;21:135–141. [DOI] [PubMed] [Google Scholar]
- 36.Thompson AL, Adair LS, Bentley ME. Pressuring and restrictive feeding styles influence infant feeding and size among a low-income African-American sample. Obesity (Silver Spring). 2013;21(3):562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multitrajectory modeling. Stat Methods Med Res. 2018;27(7):2015–2023. [DOI] [PubMed] [Google Scholar]
- 38.Dewey KG, Peerson JM, Brown KH, et al. Growth of breast-fed infants deviates from current reference data: a pooled analysis of US, Canadian, and European data sets. World Health Organization Working Group on Infant Growth. Pediatrics. 1995;96(3) Pt 1:495–503. [PubMed] [Google Scholar]
- 39.Reilly JJ, Ashworth S, Wells JC. Metabolisable energy consumption in the exclusively breast-fed infant aged 3-6 months from the developed world: a systematic review. Br J Nutr. 2005;94(1):56–63. [DOI] [PubMed] [Google Scholar]
- 40.Hester SN, Hustead DS, Mackey AD, Singhal A, Marriage BJ. Is the macronutrient intake of formula-fed infants greater than breast-fed infants in early infancy? J Nutr Metab. 2012;2012:891201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng P, Gao M, Burgher A, Zhou TH, Pramuk K. A nine-country study of the protein content and amino acid composition of mature human milk. Food Nutr Res. 2016;60:31042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heinig MJ, Nommsen LA, Peerson JM, Lonnerdal B, Dewey KG. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: the DARLING Study. Am J Clin Nutr. 1993;58(2):152–161. [DOI] [PubMed] [Google Scholar]
- 43.Picone TA, Benson JD, Moro G, et al. Growth, serum biochemistries, and amino acids of term infants fed formulas with amino acid and protein concentrations similar to human milk. J Pediatr Gastroenterol Nutr. 1989;9(3):351–360. [DOI] [PubMed] [Google Scholar]
- 44.Trabulsi J, Capeding R, Lebumfacil J, et al. Effect of an alpha-lactalbumin-enriched infant formula with lower protein on growth. Eur J Clin Nutr. 2011;65(2):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fleddermann M, Demmelmair H, Grote V, et al. Role of selected amino acids on plasma IGF-I concentration in infants. Eur J Nutr. 2017;56(2):613–620. [DOI] [PubMed] [Google Scholar]
- 46.Tikanoja T, Simell O. Plasma amino acids after a feed of human milk or formula at three months of age. J Pediatr Gastroenterol Nutr. 1983;2(2):252–255. [PubMed] [Google Scholar]
- 47.Ginsburg BE, Lindblad BS, Lundsjo A, Persson B, Zetterstrom R. Plasma valine and urinary C-peptide in breast-fed and artificially fed infants up to 6 months of age. Acta Paediatr Scand. 1984;73(2):213–217. [DOI] [PubMed] [Google Scholar]
- 48.Barrera CM, Hamner HC, Perrine CG, Scanlon KS. Timing of introduction of complementary foods to US infants, National Health and Nutrition Examination Survey 2009-2014. J Acad Nutr Diet. 2018;118(3):464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iguacel I, Monje L, Cabero MJ, et al. Feeding patterns and growth trajectories in breast-fed and formula-fed infants during the introduction of complementary food. Nutr Hosp. 2019;36(4):777–785. [DOI] [PubMed] [Google Scholar]
- 50.Ventura AK, Garcia P, Schaffner AA. Associations between bottle-feeding intensity and maternal encouragement of bottle-emptying. Public Health Nutr. 2017;20(17):3090–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiSantis KI, Hodges EA, Fisher JO. The association of breastfeeding duration with later maternal feeding styles in infancy and toddlerhood: a cross-sectional analysis. Int J Behav Nutr Phys Act. 2013;10:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Black MM, Aboud FE. Responsive feeding is embedded in a theoretical framework of responsive parenting. J Nutr. 2011;141(3):490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention. In: Author, ed. Breastfeeding Report Card: United States, 2018. Atlanta, GA; 2019. https://www.cdc.gov/breastfeeding/data/reportcard.htm. [Google Scholar]
- 54.Oakes LM. Sample size, statistical power, and false conclusions in infant looking-time research. Inf Dent. 2017;22(4):436–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paul IM, Savage JS, Anzman-Frasca S, et al. Effect of a responsive parenting educational intervention on childhood weight outcomes at 3 years of age: the INSIGHT randomized clinical trial. JAMA. 2018;320(5):461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cameron N, Pettifor J, De Wet T, Norris S. The relationship of rapid weight gain in infancy to obesity and skeletal maturity in childhood. Obes Res. 2003;11(3):457–460. [DOI] [PubMed] [Google Scholar]
- 57.Goodell LS, Wakefield DB, Ferris AM. Rapid weight gain during the first year of life predicts obesity in 2-3 year olds from a low-income, minority population. J Community Health. 2009;34(5):370–375. [DOI] [PubMed] [Google Scholar]
- 58.Tang A, Slopen N, Nelson CA, Zeanah CH, Georgieff MK, Fox NA Catch-up growth, metabolic, and cardiovascular risk in post-institutionalized Romanian adolescents. Pediatr Res. 2018;84(6):842–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heck KE, Braveman P, Cubbin C, Chavez GF, Kiely JL. Socioeconomic status and breastfeeding initiation among California mothers. Public Health Rep. 2006;121(1):51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh GK, Kogan MD, Dee DL. Nativity/immigrant status, race/-ethnicity, and socioeconomic determinants of breastfeeding initiation and duration in the United States, 2003. Pediatrics. 2007;119(Suppl 1):S38–S46. [DOI] [PubMed] [Google Scholar]
- 62.Gross RS, Mendelsohn AL, Messito MJ. Additive effects of household food insecurity during pregnancy and infancy on maternal infant feeding styles and practices. Appetite. 2018;130:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anzman-Frasca S, Stifter CA, Paul IM, Birch LL. Infant temperament and maternal parenting self-efficacy predict child weight outcomes. Infant Behav Dev. 2013;36(4):494–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Jaarsveld CH, Boniface D, Llewellyn CH, Wardle J. Appetite and growth: a longitudinal sibling analysis. JAMA Pediatr. 2014;168(4):345–350. [DOI] [PubMed] [Google Scholar]
- 65.Kong KL, Anzman-Frasca S, Feda DM, et al. Infant temperament is associated with relative food reinforcement. Child Obes. 2016;12(6):411–417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


