Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD, dioxin) and structurally related halogenated aromatics modulate gene expression and induce biochemical and toxic responses that are mediated by initial binding to the aryl hydrocarbon receptor (AhR). The AhR also binds structurally diverse compound including pharmaceuticals, endogenous biochemicals, health promoting phytochemicals and microbial metabolites. Many of these AhR ligands do not induce TCDD-like toxic responses and some AhR ligands such as microbial metabolites of tryptophan play a role in maintaining gut health and protecting against intestinal inflammation and cancer. Many AhR ligands exhibit tissue- and response- specific AhR agonist or antagonist activities, and act as selective AhR modulators (SAhRMs) and this SAhRM-like activity has also been observed in AhR-ligand mediated effects in the intestine. This review summarizes studies showing that several AhR ligands including phytochemicals and TCDD protect against dextran sodium sulphate-induced intestinal inflammation. In contrast, AhR ligands such as oxazole compounds enhance intestinal inflammation suggesting that AhR-mediated gut health can be enhanced or decreased by selective AhR modulators and this needs to be considered in development of AhR ligands for therapeutic applications in treating intestinal inflammation.
Keywords: Ah receptor, intestine, AhR ligands, IL-22, inflammation, protective effects
1. Introduction
The aryl hydrocarbon receptor (AhR) was first identified by Poland and coworkers as the intracellular protein that bind 2,3,7,8–tetrachlorodibenzo-p-dioxin (TCDD), a toxic industrial by-product identified as a contaminant in the herbicide 2,4,5–trichlorophenoxyacetic acid ( 2,4,5 – T) and Agent Orange (Poland et al. 1976; Poland and Knutson 1982). TCDD and related halogenated compounds are also formed as products of combustion of organic materials (e.g.: wastes) and this also includes their formation during forest fires (Hites 2011). The AhR binds to TCDD with KD values in the 10−12 M range (Bradfield and Poland 1988) and the AhR also binds structurally related halogenated aromatics including other polychlorinated dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), biphenyls (PCBs) and selected polynuclear aromatic hydrocarbons (PAHs) such as benzo[a]pyrene (Piskorska-Pliszczynska et al. 1986; Safe 1990). Mechanistic studies using induction of CYP1A1 gene expression as a model showed that TCDD bound the cytosolic AhR which subsequently formed a complex with the AhR nuclear translocator (Arnt) protein. Interaction of the ligand-bound nuclear AhR-Arnt heterodimer with cognate cis-promoter elements results in induction of gene expression. It was observed that for a specific toxic or biochemical response within a species there was a correlation between the rank order binding affinities of TCDD and related halogenated aromatics with their relative toxic and biochemical potencies including the induction of CYP1A1 gene expression (Poland et al. 1976; Poland and Knutson 1982; Safe 1990). This observation lead to the development and application of toxic equivalency factors (TEFs) for several “dioxin-like” PCDDs, PCDFs, PCBs and polybrominated aromatics and this value defines the potency of a specific compound relative to TCDD which has been assigned a TEF or 1.0 (Van den Berg et al. 1998; Van den Berg et al. 2006; van den Berg et al. 2013). Thus, TEFs can be used to estimate the toxic or dioxin equivalence of a mixture of these compounds and despite pit falls in the TEF approach (Safe 1998), it had provided useful guidance on determining permissible levels of exposure to dioxin-like compounds and has led to regulations that resulted in decreased emissions and decreased environmental levels of these compounds. Initial studies with TCDD as a model compound showed that there was a wide range of species-specific differences in lethality which range from the sensitive guinea pig (LD50 = 0.6–2.1 µg/kg) to the less sensitive hamster (LD50 = 1157–5000 µg/kg) (Pohjanvirta and Tuomisto 1994). The rank order sensitivity of different species to the lethality of TCDD is not observed for other toxic and biochemical response induced by this compound. Thus, the TEF approach for risk assessment does not predict interspecies sensitivities to dioxin-like compounds.
It was hypothesized and subsequently confirmed in AhR knockout mice (AhR-/-) that the AhR mediated the diverse age/species/sex-specific toxicities induced by TCDD and related compounds thus linking the AhR and its functions with toxic endpoints (Fernandez-Salguero et al. 1996; Schmidt and Bradfield 1996; Mimura et al. 1997; Lund et al. 2003; Lahvis et al. 2005). The linkage of the AhR with the toxicity of dioxin-like compounds was accompanied by observations that the AhR also plays an important role in maintaining homeostasis in mice. Firstly, close examination of AhR knockout mice showed that they exhibit decreased fertility, reproductive tract problems, altered portal duct fibrosis, problems in liver and stem cell development, uric stone formation in the bladder and ocular and immune functional deficits (Fernandez-Salguero et al. 1995; Fernandez-Salguero et al. 1997; Benedict et al. 2000; Benedict et al. 2003; Lund et al. 2003; Baba et al. 2005; Lahvis et al. 2005; Quintana et al. 2008; Veldhoen et al. 2008; Wang et al. 2010; Sauzeau et al. 2011; Singh KP et al. 2011; Butler et al. 2012; Chevallier et al. 2013; Stockinger et al. 2014; Esser and Rannug 2015). It was also reported that the AhR not only interacted with TCDD and related compounds but also bound structurally diverse phytochemicals (polyphenolics and heterocyclic compounds), pharmaceuticals, of tryptophan and other microbial metabolites, and endogenous biochemicals including leukotrienes, bilirubin and 7-ketocholesterol (Rannug et al. 1987; Denison et al. 1998; Denison and Nagy 2003; Denison and Faber 2017). Figure 1 illustrates the diverse structures of some AhR ligands that have been used to investigate their effects in mouse models of intestinal inflammation and these include the phytochemical indole-3-carbinol, a weak AhR ligand that forms more potent AhR-active compounds in the gut, indigo, two microbial metabolites 1,4-dihydroxy-2-naphthoic acid (DHNA) and indole-3-aldehyde, and two endogenous ligands 6-formylindolo [3,2-b] carbazole (FICZ) and 2-(1´-H-indole-3´-carbonyl)thiazole-4-carboxylic acid methyl ester (ITE) and two synthetic compounds, β-naphthoflavone (βNF) and TCDD. With the exception of TCDD these compounds do not induce the pattern of toxicities observed for dioxin-like compounds. There is also evidence that AhR ligands, like ligands for the estrogen receptor (ER) and other nuclear receptors (Katzenellenbogen et al. 1996; Jordan and O’Malley 2007) are selective AhR modulators (SAhRMs) that exhibit tissue –specific AhR agonist or antagonist activities (Lu et al. 1996; McDougal et al. 2001; Flaveny et al. 2009; Murray et al. 2010; Zhao et al. 2010; Smith et al. 2011; Soshilov and Denison 2014; Ehrlich et al. 2018; Jin et al. 2018; Safe 2018) and this will be discussed in more detail in section 3.
Figure 1.
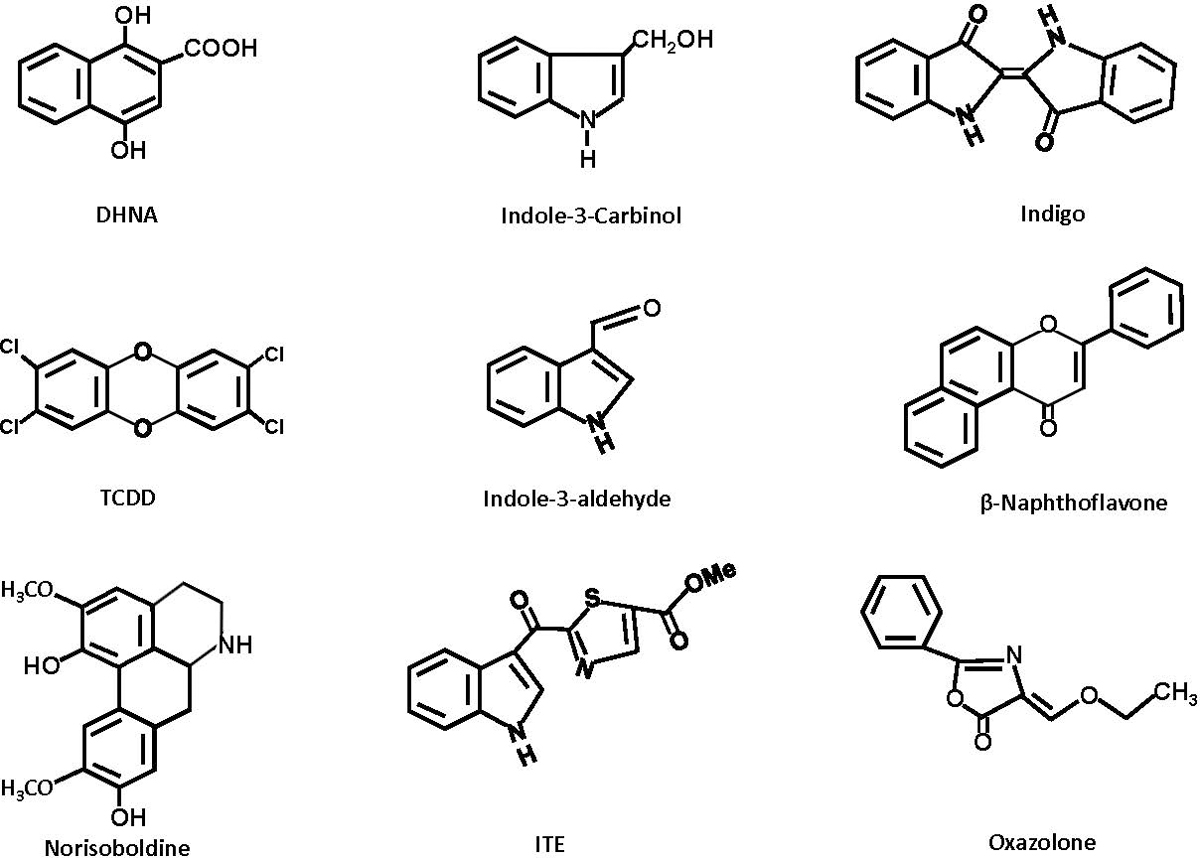
Structurally diverse ligands that inhibit intestinal colitis/inflammation (Table 1) include 1,4-dihydroxy-2-napthoic acid (DHNA), indole-3-carbinol (I3C), indigo, TCDD, indole-3-aldehyde, ?-naphthoflavone, norisoboldine, 2-(1´-H-indole-3´-carbonyl) thiazole-4-carboxylic acid methyl ester (ITE), and oxazalone.
2. Role of the AhR in gut homeostasis and disease
Colitis.
AhR knockout mouse models have been extensively used to identify and characterize the functions of the AhR in maintaining gut homeostasis and the potential for using the AhR as a drug target for treating diseases. Several studies show that the AhR plays an important role in inflammation-induced gastrointestinal (GI) diseases such as colitis, colon cancer and effects of AhR ligands in ameliorating these effects have also been investigated (Arsenescu et al. 2011; Benson and Shepherd 2011; Furumatsu et al. 2011; Li et al. 2011; Singh NP et al. 2011; Zelante et al. 2013; Fukumoto et al. 2014; Lv et al. 2015; Diaz-Diaz et al. 2016; Goettel et al. 2016; Monteleone et al. 2016; Hubbard et al. 2017; Islam et al. 2017; Kawai et al. 2017; Ye et al. 2017; Iyer et al. 2018; Lv et al. 2018). Figure 2 illustrates the diverse roles of the AhR in maintaining gut health and this includes enhancing barrier function, protecting against microbial infection, inhibition of inflammation through induction of multiple genes including interleukin-22 (IL-22), and protecting against development of metabolic syndrome and colon cancer. Structurally diverse AhR ligands enhance AhR-mediated responses and the potential for AhR ligand-dependent enhancement and inhibition of these pathways will be discussed in section 3. In one study it was shown that administration of 1, 2 and 3% dextran sodium sulfate (DSS) over a period of 5 days had minimal effects on body weights in wild-type mice but there was a dose-dependent increase in colon damage in AhR-/- mice based on histological scores (Furumatsu et al. 2011). In AhR-/- mice body weight loss was decreased in the 2 and 3% DSS treated group (not in the 1% DSS group) and histological damage was higher in AhR-/- mice compared to wild-type mice. These results demonstrated that loss of the AhR enhanced DSS-induced damage which increased with increasing levels of DSS. A second study (Arsenescu et al. 2011) used 3.5% DSS (7 days) and compared responses in wild-type, AhR-/+ and AhR-/- mice. All of the AhR-/- mice died within the 7 days during the DSS treatment period but > 80% of wild-type and AhR-/+ mice survived. Surprisingly, most of the measures of inflammation and colonic damage were lower in AhR-/+ compared to wild-type mice (Arsenescu et al. 2011). This observation using AhR-/+ mice was unique compared to studies in AhR-/- mice and needs to be re-examined. The reasons for the latter response are unclear but suggest that an AhR and DSS dosages may also be important in mediating induced inflammatory responses in the gut. There was a decrease in expression of this receptor in intestinal tissues of patients with inflammatory bowel disease (Monteleone et al. 2016) suggesting that the AhR is protective in humans as observed in animal models, however, this needs to be further investigated and verified.
Figure 2.
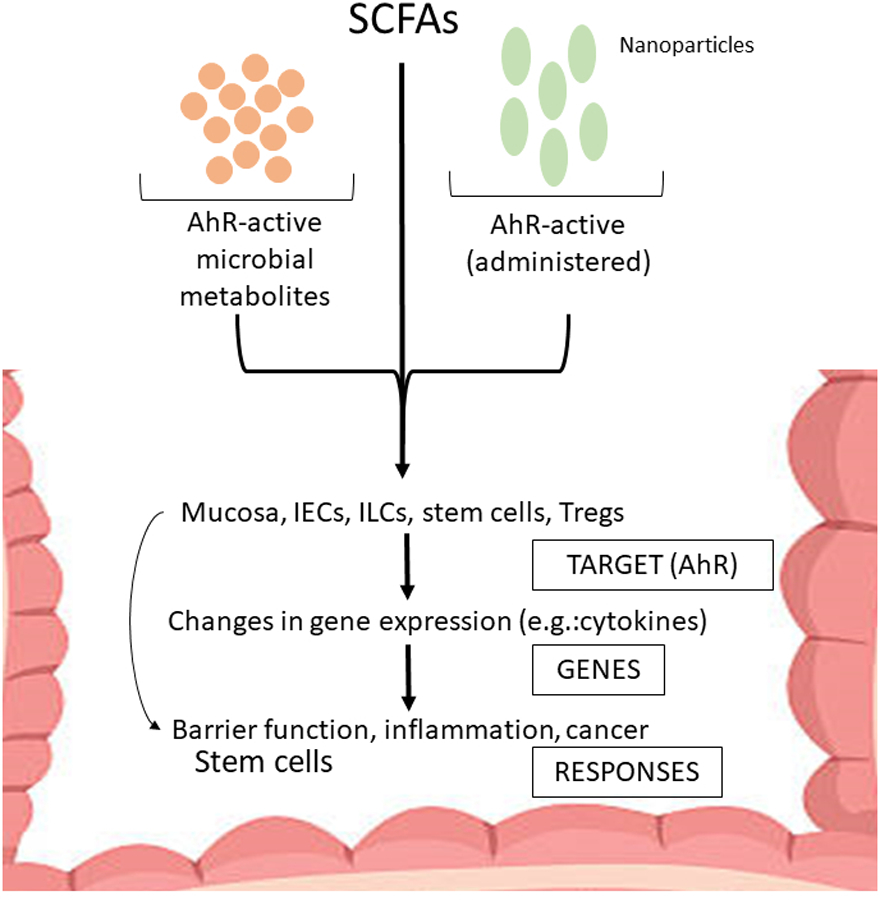
Model for interactions of AhR ligands and SCFAs with AhR-expressing cells in the intestine. Microbiota- and dietary-derived AhR ligand and SCFAs target intestinal AhR-responsive cell types resulting in changes in gene expression which subsequently impacts intestinal barrier function, inflammation, stem cells, and carcinogenesis. The illustrated impacts of the diverse AhR ligands are primarily observed when these compounds are AhR agonists which protect against gut inflammation and cancer.
Cancer.
The AhR is expressed in multiple tumor types and exhibits tumor specific anti-cancer and tumor promoter-like activities (Safe et al. 2013). Expression of the AhR plays an important role in development of colon cancer in various mouse models. Kawajiri and co-workers (2009) initially reported that AhR-/- mice spontaneously developed colon tumors near the cecum whereas these tumors were not observed in heterozygous AhR-/+ or wild-type AhR+/+ mice. A similar pattern of tumor development was observed in mice with APC mutations (APCmin/+) that results in spontaneous development of colon tumors. The loss of the AhR in the compound APCmin/+•AhR mice resulted in earlier tumor development compared to mice with only one genetic defect (Kawajiri et al. 2009). Interestingly, the deletion of the apoptosis-associated speck-like protein containing a caspase recruitment gene (ASC) protected AhR-/- and APCmin/+ mice from cecal tumor development and tumor development in germ free AhR-/- mice was also decreased (Ikuta et al. 2013). It was assumed that germ free conditions would decrease expression of inflammatory microbial metabolites (Ikuta et al. 2013) however, this needs to be further examined since levels of “protective” AhR active microbial metabolites may also be decreased. Loss of the AhR also significantly enhanced tumorigenesis in an inflammation-induced mouse model in which animals are treated with DSS and the carcinogen azoxymethane (Diaz-Diaz et al. 2016). Using a similar approach in which the AhR was deleted only in the intestinal epithelium, there was also an increased tumor burden in these mice compared to wild-type mice demonstrating the critical role of intestinal epithelial AhR as an inhibitor of intestinal tumor formation (Metidji et al. 2018). Thus, the AhR is protective with respect to development of colon cancer and colitis, and this is consistent with the role of colitis as a risk factor for colon cancer.
Inflammation, immune responses and infection.
Loss of the AhR has profound effects on gut inflammation and resistance to infection and this is due, in part, to a compromised immune system (Gutierrez-Vazquez and Quintana 2018) (Fig. 2). The lamina propria is a layer of loose connective tissue that contains multiple cell types including fibroblasts and diverse immune cells. This thin multicomponent layer along with intestinal epithelial and basement membrane cells form the intestinal mucosa and play an important role in modulating inflammation and serving as a barrier to microbial infection. Innate lymphoid cells (ILCs) are also present in the lamina propria and are important for maintaining barrier function and gut health (Kawajiri et al. 2009; Ikuta et al. 2013; Zelante et al. 2013; Diaz-Diaz et al. 2016; Monteleone et al. 2016). ILCs expressing RORγt+ induce formation of intestinal lymphoid follicles postnatally and the AhR is required for this response and for expression of IL-22 and Kit genes which are required for ILC homeostasis (Kiss et al. 2011). Loss of the AhR resulted in aberrant lymphoid follicles and increased susceptibility to infection by C. rodentium (Kiss et al. 2011). ILC3s are primarily produced in the gastrointestinal tract and loss of the AhR results in decreased IL-22 secretion and increased intestinal Th17 cells. There is also evidence that the AhR cooperatively regulates IL-22 gene expression in association with RORγt in ILC3 cells, and this contributes to the IL-22 dependent anti-inflammatory responses in the gut and protection from C. rodentium infection (Qiu et al. 2012; Qiu et al. 2013; Li et al. 2017). In contrast, the AhR suppresses ILC2 function and expression of ILC2-regulated expression of IL-5 and IL-13 genes and this also results in enhanced anti-helminth immunity (Li et al. 2018). Thus, the AhR plays an important role in regulating transcriptional programs in ILC-2 and ILC3 cells and this in turn contributes to resistance from intestinal infections.
Metidji and coworkers (2018) utilized mouse-derived organoid cell culture models with AhR deficiency only in intestinal epithelial cells (IECs) and showed that the AhR played multiple roles in the gut. For example, loss of the AhR decreased resistance to the intestinal pathogen C. rodentium and decreased expression of IL-22. In vivo studies showed that expression of the AhR in IECs is critical for many of the AhR-mediated protective effects in the colon. For example, in mice with AhR-deficient IECs there was impaired barrier function and resistance to C. rodentium, enhanced stem cell proliferation (in vivo and in vitro) and enhanced azoxymethane (± inflammation) -induced colon tumorigenesis compared to wild-type (AhR+/+) mice. This study (Metidji et al. 2018) also reported many other roles for IECs that are AhR-dependent and these include; regulation of Wnt-β-catenin signaling, decreased inflammation-induced colon carcinogenesis and decreased intestinal stem cell proliferation.
One of the early hallmarks of research on the endogenous functions of the AhR was the discovery that this receptor played an important role in the induction of T-regulatory (Treg) cells, particularly FoxP3-expressing Treg cells and this is accompanied by AhR–dependent modulation of TH17 cell differentiation (Quintana et al. 2008; Veldhoen et al. 2008). The AhR and its ligands and their regulation of Tregs in the intestine contribute to the Tregs – mediated anti-inflammatory pathways (Fig. 2) including inhibition of induced colitis. The role of the AhR in regulating Tregs in various tissues was also investigated using a knocked-in constitutively active AhR (CA-AhR) mouse model which expresses FoxP3. Both genes expressed different fluorescent tags and it was shown that AhR in Tregs was detected in multiple tissues but the highest levels of AhR expression was observed in gut peripheral Tregs (Ye et al. 2017). Deletion of the AhR in Tregs significantly affected peripheral Tregs. Results of studies in AhR+/+, AhR-/+ and AhR-/- mice show that the AhR promoted the gut homing of Tregs, suppressed pro-inflammatory cytokine production and protected against gut histopathological changes as well as inflammation. In summary, expression of the AhR in multiple sub-types of colonic immune and epithelial cells are critical for maintaining gut health by inhibiting inflammatory pathways, strengthening barrier function and modulating intestinal stem cell expansion resulting in decreased inflammation and infection and decreased colon tumor development as illustrated in Figure 2.
3. Effects of AhR ligands on gut resilience
AhR ligands as selective receptor modulators (SAhRMs).
Many studies on the role of the AhR in maintaining gut health also report the effects of various AhR ligands (Fig. 1) which protect against intestinal infection, inflammation and tumorigenesis. Figure 2 illustrates the effects of both endogenous (microbial-derived) and dietary-derived AhR ligands, their various cellular targets in the gut and the resulting responses which include their inhibition of gut inflammation and cancer. However, the potential future development of AhR-active drugs for treating intestinal diseases will have to take into account that AhR ligands are selective AhR modulators (SAhRMs) (Flaveny et al. 2009; Boitano et al. 2010; Murray et al. 2010). The term selective receptor modulators (SRMs) has been extensively used for steroid hormone receptor ligands and particularly those that bind estrogen receptor α (ERα) (Jordan et al. 2007; Katzenellenbogen et al. 1996). 17β-Estradiol (E2) and tamoxifen both bind ERα but in humans tamoxifen acts as an antagonist in breast cancer and as an agonist in the uterus. The selectivity of receptor ligands in terms of their agonist or antagonist activities is due to multiple factors including; ligand structure-dependent conformational changes in the bound receptor which affect interactions with nuclear co-activators/co-factors and with cis-elements (Katzenellenbogen et al. 1996). Since chromatin structure (e.g.: promoter DNA) and nuclear co-factor expression are highly variable in different tissues/organs and species, the antagonist or agonist activities of receptor ligands are difficult to predict and need to be determined directly in an appropriate diagnostic assay.
The concept of selective receptor modulators and the differences in their ligand-dependent functional activity is due, in part, to the assumption that ligands induce different conformations of their cognate receptors and this has been verified for ERα binding to selective ER modulators (SERMs). The crystal structure of the AhR ligand binding domain in the absence or presence of ligands has not been determined, however, the crystal structure of the AhR-Arnt heterodimer bound to the cis-dioxin responsive element (DRE) has been reported (Seok et al. 2017). Their results suggest a dynamic structural hierarchy and this could be related to the ability of structurally diverse ligands to bind and activate the receptor. Modeling approaches coupled with site-directed mutagenesis have also been used to investigate ligand structure-dependent binding to the AhR (Flaveny et al. 2009; Xing et al. 2012; Perkins et al. 2014; Shiizaki et al. 2014; Soshilov and Denison 2014; Jin et al. 2018; Giani Tagliabue et al. 2019). One study using mouse AhR showed the importance of Q377 and G298 for the binding and activity of TCDD and benzo[a]pyrene respectively (Xing et al. 2012). Another approach used both modeling and site directed mutagenesis to characterize AhR ligands into three distinct classes, namely, dioxin-like halogenated aromatics, polynuclear aromatic hydrocarbons and benzoflavones/heteroaromatics. Ligands in the three classes bound to distinct regions of the mouse AhR ligand binding pocket (Soshilov and Denison 2014; Giani Tagliabue et al. 2019). For example, FICZ, but not other ligands activated mAhR containing the I319K mutation (Soshilov and Denison 2014) and a recent modeling study showed that even among structurally related flavonoids (quercetin and apigenin) there were differences in their interactions with amino acids in the human AhR binding pocket (Jin et al. 2018).
There is increasing evidence that AhR ligands are selective receptors modulators or SAhRMs (Murray, Krishnegowda, et al. 2010; Murray, Morales, et al. 2010; Zhao et al. 2010; Li et al. 2018; Safe et al. 2018). TCDD and structurally related halogenated aromatic compounds induce comparable toxic responses, however even among dioxin-like compounds genomic studies indicate that these compounds are SAhRMs (Safe et al. 2018). For example, TCDD and 2,3,7,8-tetrachlorodibenzofuran (TCDF) induced dose-response curves for 1027 and 837 genes respectively in mouse liver; however, only 373 of these genes were enhanced by both compounds indicating AhR ligand-dependent selective activation of genes (Burgoon et al. 2009). Species-dependent differences in the effects of TCDD were examined in mouse hepatocytes expressing mouse (mAhR) and human (hAhR) receptor. TCDD induced/repressed 1752/1100 genes in mAhR hepatocytes and 1186/779 genes in hAhR hepatocytes and the number of common genes induced and repressed by TCDD in the two cell types was 265 (18%) and 462 (49%) respectively (Flaveny et al. 2010). In addition, several reports on structurally diverse AhR ligands including 6-methyl-1,3,8-trichlorodibenzofuran, 3’-methoxy-4’-nitro-flavone, α-naphthoflavone and several other flavonoids and tryptophan metabolites and 3,3’-diindolylmethane (DIM) show that these compounds exhibit tissue and responsive-specific AhR agonist or antagonist activities (Santostefano et al. 1993; Chen et al. 1996; Lu et al. 1996; McDougal et al. 2001; Denison and Nagy 2003; Zatloukalova et al. 2007; Burgoon et al. 2009; Flaveny et al. 2009; Boitano et al. 2010; Flaveny et al. 2010; Murray, Krishnegowda, et al. 2010; Murray, Morales, et al. 2010; Zhao et al. 2010; Murray et al. 2011; Smith et al. 2011; Soshilov and Denison 2014; Cheng et al. 2015; Denison and Faber 2017; Jin et al. 2017; Ehrlich et al. 2018; Jin et al. 2018; Muku et al. 2018; Safe et al. 2018; Singh et al. 2019). Recent studies reported that urothilin A, a microbial metabolite of ellagic acid exhibited AhR antagonist activity in human HepG2 but not mouse Hepa1.1 hepatoma cell using a DRE-luc reporter gene assay (Muku et al. 2018). In contrast, urothilin A induced CYP1A1 in HT-29 colon cancer cells (mRNA and protein) and induced CYP1A1 in the colon and liver of C57BL/6 mice and exhibited AhR agonist activity (Muku et al. 2018). Studies with flavonoids show that in Caco2 colon cancer cells that some tetrahydroxy flavones such as luteolin (5,7,3´,4´-tetrahydroxyflavone) are AhR antagonists for induction of CYP1A1 mRNA and AhR agonists for induction of UGT1A1 mRNA (Jin et al. 2018).
The concept of SAhRMs includes all structural classes of AhR ligands, however, development of potentially therapeutic AhR ligands would require compounds that have recently been designated rapidly metabolized AhR ligands (RMAhRLs) (Ehrlich and Kerkvliet 2017; Dolciami et al. 2020). These compounds would include most structural classes of AhR ligands but not dioxin-like compounds which are poorly metabolized and bioaccumlate and this persistence coupled with their high receptor binding affinity contributes to their toxicity. However, although RMAhRLs generally do not induce the toxic responses observed for TCDD and related halogenated aromatics it cannot be assumed that RMAhRLs will not induce some AhR-dependent toxic responses. For example, AhR active oxazoles (RMAhRLs) enhance intestinal inflammation (Iyer et al. 2018) whereas TCDD inhibited induced intestinal inflammation (Benson and Shepherd 2011). Thus, RMAhRLs are also SAhRMs and exhibit tissue- and response- specific effects which in some cases may be an adverse response.
Short chain fatty acids (SCFAs) and their interactions with AhR ligands.
Fibre-derived short chain fatty acids (SCFAs) including acetate, propionate and butyrate also play a role in gut resiliency and there is evidence that SCFAs exhibit anti-inflammatory activity, enhance intestinal barrier function and protect against infection and development of colon cancer (Pool-Zobel et al. 2005; Russo et al. 2012; Singh et al. 2014; Kaiko et al. 2016; van der Beek et al. 2017). Moreover, butyrate suppresses stem cell expansion in the crypt during mucosal injury (van der Beek et al. 2017) and many of the functional effects of butyrate resemble those reported for the AhR and its ligands. There are reports that SCFAs enhance AhR and AhR ligand-induced responses and possible mechanisms include SCFA-induced histone deacetylase (HDAC) activity (Jin et al. 2017) and direct binding (of butyrate) to the AhR (Marinelli et al. 2019). SCFA-AhR ligand interactions are response-specific and their combined effects on various parameters of gut resiliency remains to be determined. Thus, phytochemicals, microbial-derived SCFAs and tryptophan-derived microbial metabolites exhibit gene-specific AhR agonist or antagonist activities and this is consistent with the designation of these compounds as SAhRMs. All of these AhR ligands individually and in combination with SCFAs have the potential to impact AhR-dependent gut resilience. However, it is unlikely that the commonly used assays for induction of drug metabolizing enzymes will predict their agonist/antagonist activities and their enhancement or inhibition of AhR-dependent gut resiliency. The following section examines AhR-ligand-dependent effects on gut inflammation, infection and tumorigenesis with a focus on their possible SAhRM-like effects. Modulation of these responses by SCFAs is a possibility that should be investigated in the future.
AhR ligands and gut resiliency – are there SAhRM-like effects?
Most studies characterizing AhR ligands as SAhRMs have used induction or inhibition of drug-metabolizing enzymes as end points. However, initial studies on AhR ligand-dependent protection from autoimmune disease in mouse models reported that TCDD but not FICZ induced Treg cells which inhibit T cell proliferation and cytokine production and decreased the severity of experimental autoimmune encephalomyelitis (EAE) in mice (Quintana et al. 2008; Veldhoen et al. 2008). Subsequent studies showed that FICZ partially decreased EAE pathology and the effects of TCDD involved AhR-dependent activation of both T cells and dendritic cells (Duarte et al. 2013). Since the AhR is functional in multiple cell types in the intestine, the activity of AhR ligands as SAhRMs may include their AhR agonist or antagonist activities of ligands which may vary in different cell types. It has been reported that a limited number of structurally-diverse AhR ligands inhibit both DSS- and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced inflammation in rodent models (Table 1). AhR ligands that inhibit colitis and associated inflammatory responses (Fig. 2) include synthetic compounds (TCDD and BNF), endogenous AhR – active compounds (2-1H-indol-3-carbonyl-4-thiazolecarboxylic and, ITE) phytochemicals and plant extracts (I3C, norisoboldine, indigo, indigo naturalis, and broccoli extracts) and microbial metabolites such as tryptophan metabolites and 1,4-dihydroxy-2-naphthoic acid (DHNA) (Benson and Shepherd 2011; Furumatsu et al. 2011; Li et al. 2011; Singh NP et al. 2011; Zelante et al. 2013; Fukumoto et al. 2014; Lv et al. 2015; Diaz-Diaz et al. 2016; Goettel et al. 2016; Hubbard et al. 2017; Islam et al. 2017; Kawai et al. 2017) (Fig. 2). In addition, there is also evidence that AhR ligands such as urothilin A and its derivatives (Muku et al. 2018) and FICZ (Liu et al. 2018; Yu et al. 2018) modulate expression of multiple genes that enhance epithelial barrier function by maintaining tight junction integrity and this is observed in both in vivo and cell culture models. All of these compounds have previously been reported as AhR agonists although I3C exhibited some partial AhR antagonist activity in breast cancer cells (Chen et al. 1996).
Table 1.
Summary of AhR ligands that protect against or induce colitis in mouse models
| Colitis Model | Ah Receptor Ligand | Ref AhR deletion/inhibition |
|---|---|---|
| DSS induced | DHNA | Fukumoto et al. 2014; CH223191 |
| DSS induced | TCDD | Singh et al. 2011; AhR-/- |
| DSS induced | Norisoboldine | Lv et al. 2015; CH223191 |
| DSS induced | b-Naphthoflavone | Furumatsu et al. 2011; AhR-/- |
| DSS induced | Indole-3-carbinol | Li et al. 2011; AhR-/- |
| DSS induced | Tryptophan (dietary) | Islam et al. 2017; AhR-/- |
| DSS induced | Broccoli | Hubbard et al. 2017; AhRb/b AhRd/d |
| DSS induced | Indole-3-carbinol | Diaz-Diaz et al. 2016; AhR+/+ AhRΔ2/Δ2** |
| DSS induced | Ginger ELNs | Deng et al. 2017; |
| TNBS induced | Indigo naturalis/indigo | Kawai et al. 2017; AhR-/- |
| TNBS induced | ITE | Goettel et al. 2016; humanized N5GabcDR1* |
| TNBS induced | TCDD | Benson and Shepherd et al. 2011; AhR+/+ |
| TMO and oxazolone | 2,4,5-trimethyl-2,5-dihydro-1,3-oxazole (TMO), oxazolone | Iyer et al. 2018; AhR+/+, AhRΔIEC |
Driven by human CD4+ T cells
AhRΔ2/Δ2; exon2 AhR knockout mice
A recent study showed that oxazalone and structurally related compounds induced AhR-responsive luciferase activity and CYP1A1 gene expression in immortalized mouse intestinal epithelial (MODE-K) cells (Iyer et al. 2018). Previous studies demonstrated that oxazalone induces CD1d-dependent inflammation in mouse models of colitis (Colgan et al. 1999; Heller et al. 2002; Olszak et al. 2014). Iyer and coworkers (2018) demonstrated that oxazolone-induced colitis was dependent on both CD1d and AhR expression in intestinal epithelial cells (IECs). Moreover, the endogenous AhR ligand ITE did not enhance or inhibit the inflammatory effects of oxazoles. One of the hallmarks of oxazolone-induced inflammation is AhR-dependent downregulation of the anti-inflammatory cytokine IL-10. Oxazolone also induced formation of the tryptophan metabolites kynurenic acid and xanthurenic acid, which enhanced oxazolone-mediated downregulation of the anti-inflammatory cytokine IL-10 (Iyer et al. 2018). In contrast, the AhR ligand indole-3-aldehyde inhibited gut inflammation and colitis and induced anti-inflammatory IL-22 expression via AhR-dependent pathways (Zelante et al. 2013). Interestingly, indigo naturalis an AhR-active mixture which inhibited TNBS-induced colitis (Kawai et al. 2017), provided some protection against oxazolone-induced dermatitis but enhanced oxazolone-induced colitis through alteration of gut microflora (Adachi et al. 2017). In contrast, other studies on colitis show that ITE induced IL-10 gene expression in T-cells treated with TGFβ (Goettel et al. 2016) and IL-10 expression in the stomach and esophagus was AhR-dependent (Zelante et al. 2013).
These studies show significant differences in the effects of tissue-specific versus whole body loss of AhR on IECs (in the absence of ligand). In mice with intestinal loss of AhR, there were no effects on colitis scoring, weight loss or IL-10 expression (Iyer et al. 2018). In contrast, the whole body AhR-/- mice exhibited decreased resistance to C. rodentium and enhanced inflammation-induced colon cancer (Zelante et al. 2013)suggesting that loss of the AhR in multiple cell types (other than intestinal epithileum) contribute to the colon phenotype. Moreover, there were differences in the effects of the two AhR ligands indole-3-aldehyde and oxazole compounds to protect against or exacerbate “adverse” intestinal responses in the mouse models. These differences may be due to several factors and need to be further investigated by comparing results of parallel studies in both animal models with whole body and tissue specific AhR knockdown. It was also reported that the yeast malassezia restricta enhances colitis in mice (Limon et al. 2019) and this yeast also produces several AhR ligands (Wille et al. 2001; Mexia et al. 2015) and it is possible that like oxazolone, these compounds may also enhance colon inflammation. Figure 2 summarizes the multiple effects of AhR ligands on gut resiliency and their possible interactions with SCFAs. However, the indicated positive AhR-mediated effects of these compounds is structure-dependent due to their activity as SAhRMs.
Diet-induced modulation of AhR metabolites and their effects.
The studies outlined above focused primarily on direct effects of dietary/supplemented AhR ligands on subcellular targets in the intestine and not on their changes to the gut microbial populations and their metabolites. There is evidence that the environment (primarily diet) is a major factor in influencing gut health and gut distal organ interactions (Ooi et al. 2014; Khalili et al. 2018; Rothschild et al. 2018). Several studies have clearly shown that botanicals or dietary compounds containing different AhR ligands can modulate the microbiota composition and elicit a range of responses due to the administered compound. For example, feeding broccoli extracts (Hubbard et al. 2017) decreases levels of Erysipelotrichaceae species and also increases AhR responsiveness. Members belonging to this family of pathogens have been shown previously to be increased in immune-mediated inflammatory diseases such as multiple sclerosis in Mu2-knock-out mice and in murine colitis models (Osaka et al. 2017). These observations suggest that the AhR active-components in broccoli could promote protection from inflammatory stimuli through modulating levels of Erysipelotrchaceae. In a different study, diets supplemented with broccoli or blueberries decreased Lactobacilius spp and lactic acid levels (Paturi et al. 2012). Since lactic acid is a substrate for sulfate reducing bacteria (SRB), the decrease in lactic acid could result in decreased production of hydrogen sulfide and reduced intestinal epithelial cell toxicity. Mice that were fed the broccoli-supplemented diet also exhibited increased cecal levels of butyrate but reduced abundance of Faecalibacterium prausnitzii, which is a well-established butyrate producer (Zhou et al. 2018).
The AhR active indigo naturalis exhibits opposing effects on colitis in two murine models. While administration of indigo naturalis ameliorated DSS-induced colitis in mice, (Kawai et al. 2017) it also exacerbated oxazolone-induced colitis by increasing members of the Firmicutes phylum and decreasing bacteria belonging to the phylum Bacteriodetes. Thus, the effect of different AhR active foods and compounds on intestinal health also depends on the relative changes in the compositions of intestinal microbial populations. More importantly, the levels of AhR-active metabolites produced by the microbiota are also likely to play a key role in the observed effects. This can also explain the observation that administration of broccoli extracts increased butyrate production while decreasing the abundance of a known butyrate producer, suggesting that the increase in butyrate is likely due to increased abundance of other butyrate producing bacteria. These observations further underscore the importance of considering functional redundancy in the microbiota while analyzing changes in the abundance of specific species and correlating them to intestinal resilience and responses.
Zhang and coworkers have examined specific mechanisms of diet-derived effects on gut health using plant exosome-like nanoparticles (ELNs)-derived from ginger and broccoli extracts which contain AhR-active compounds (Deng et al. 2017; Teng et al. 2018). It was reported that ginger-derived ELNs contain plant-derived exosomal microRNAs. These were taken up by Lactobacillus rhamnosus (LGG) and directly influenced expression of specific mRNAs and the subsequent formation of corresponding gene products. For example, microRNA7267-3P expressed in ginger ELNs targeted the monooxygenase yanE resulting in increased formation of indole-3-aldehyde. Enhanced formation of indole-3-aldehyde induces AhR-dependent expression of IL-22 and inhibition of DSS-induced colitis. In this unique study, mice administered AhR-active ginger-derived ELNs were protected from inflammation and colitis due to factors (e.g.: RNAs) within the ELNs which altered microbial metabolism of tryptophan (Teng et al. 2018). If diet-microbiota interactions involve a genetic component associated with ELN RNAs and their uptake by specific microorganisms then understanding and predicting dietary effects on gut microbial populations and their metabolites will require more detailed insights on diet-derived ELNs and their functions.
4. Summary and Conclusion
The aryl hydrocarbon receptor (AhR) is a ligand activated receptor that was initially identified as the intracellular receptor that mediates the effects of TCDD and structurally-related toxic halogenated aromatics. Subsequent studies with AhR knockout mice confirmed the role of this receptor in mediating the toxicity of TCDD and related compounds, however, it was also observed that AhR-/- mice exhibit multiple tissue and organ –specific deficits. Studies in mouse models for cancer and non-cancer endpoints demonstrate that the AhR is important for maintaining gut resiliency and loss of this receptor results in enhanced intestinal inflammation and colitis and increased carcinogen /genetic-induced intestinal tumor formation. AhR expression in multiple intestinal cell types plays a critical role in influencing microbiota-derived metabolite formation and their AhR-dependent protection from intestinal infection and maintaining barrier function.
Intestinal microbiota and microbial metabolites are influenced by multiple factors including the diet and drugs and these changes can also impact multiple distal organs/tissues (Maruvada et al. 2017). Moreover, very little is known about dose-response and metabolic effects of microbial metabolites and their interactions with more than one signaling pathway. For example, gut microbiota-derived tryptophan metabolites such as indole-3-acetate and tryptamine modulate inflammatory responses in the liver that are AhR dependent (Krishnan et al. 2018). Moreover, AhR-active tryptophan metabolites also attenuated central nervous system inflammation and experimental autoimmune encephalomyelitis in mouse models and demonstrate a powerful AhR-dependent influence on the gut-brain axis (Rothhammer et al. 2016; Rothhammer et al. 2018). Thus, the AhR is targetable not only in the gut for treating gut inflammatory diseases but also in distal organs where microbial formation of AhR active metabolites can be therapeutic. Despite these observations, progress on the development and clinical applications of AhR-active drugs or dietary methods to enhance production of AhR active microbial metabolites has been limited. One notable exception is the AhR-active drug laquinimod which has been in phase III clinical trials for treatment of multiple sclerosis (Comi et al. 2012). One reason for the slow development of AhR-active drugs has been the association of the AhR with toxic outcomes since this receptor was first identified and characterized as the “dioxin (TCDD) receptor”. Another reason for caution in developing drugs that act through the AhR is based on evidence demonstrating that AhR ligands are SAhRMs which exhibit structure-dependent and tissue-specific AhR agonist or antagonist activities. For example, in the DSS/TNBS – induced colitis models in mice both non-toxic dietary/microbial-derived AhR ligands and TCDD exhibit anti-inflammatory activity. Evidence for SAhRM-like activity of AhR ligands in the gut includes studies showing that tryptophan metabolites and indole-3-carbinol act as inhibitors of colitis and gut inflammation in mouse models whereas oxazolone and related AhR active compounds induce gut inflammation. Thus, development of AhR ligands for enhancing gut resilience needs to assess their possible SAhRM-like adverse responses.
5. Acknowledgements
The authors greatly appreciate all of the comments provided by the Reviewers. These have been taken into account in the revision of the manuscript.
Footnotes
Declaration of Interest
The authors of the manuscript declare that they have no conflict of interest and are solely responsible for the contents and opinions that appear in the manuscript. All three authors are employed as Faculty at Texas A&M University, and the views expressed are solely those of the authors and not the University Financial support from the NIH, Cancer Prevention Research Institute of Texas, Texas A&M Agrilife and chair endowment funds have been acknowledged, however these funding sources did not influence the content or opinions expressed in the manuscript. Drs. Safe, Chapkin and Jayaraman have no declarations of interest in any legal, advocacy or regulatory activities linked to the contents and opinions expressed in this manuscript.
7. References
- Adachi S, Hoshi N et al. 2017. Indigo Naturalis Ameliorates Oxazolone-Induced Dermatitis but Aggravates Colitis by Changing the Composition of Gut Microflora. Int Arch Allergy Immunol 173(1):23–33. [DOI] [PubMed] [Google Scholar]
- Arsenescu R, Arsenescu V et al. 2011. Role of the Xenobiotic Receptor in Inflammatory Bowel Disease. Inflammatory Bowel Diseases. 17(5):1149–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Mimura J et al. 2005. Intrinsic Function of the Aryl Hydrocarbon (Dioxin) Receptor as a Key Factor in Female Reproduction. Molecular and Cellular Biology. 25(22):10040–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict JC, Lin TM et al. 2000. Physiological Role of the Aryl Hydrocarbon Receptor in Mouse Ovary Development. Toxicological Sciences. 56(2):382–388. [DOI] [PubMed] [Google Scholar]
- Benedict JC, Miller KP et al. 2003. Aryl Hydrocarbon Receptor Regulates Growth, but Not Atresia, of Mouse Preantral and Antral Follicles. Biology of Reproduction. 68(5):1511–1517. [DOI] [PubMed] [Google Scholar]
- Benson JM, Shepherd DM. 2011. Aryl Hydrocarbon Receptor Activation by Tcdd Reduces Inflammation Associated with Crohn’s Disease. Toxicological Sciences. 120(1):68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitano AE, Wang J et al. 2010. Aryl Hydrocarbon Receptor Antagonists Promote the Expansion of Human Hematopoietic Stem Cells. Science. 329(5997):1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield CA, Poland A. 1988. A Competitive Binding Assay for 2,3,7,8-Tetrachlorodibenzo-P-Dioxin and Related Ligands of the Ah Receptor. Mol Pharmacol 34(5):682–688. [PubMed] [Google Scholar]
- Burgoon LD, Ding Q et al. 2009. Automated Dose-Response Analysis of the Relative Hepatic Gene Expression Potency of Tcdf in C57bl/6 Mice. Toxicological Sciences. 112(1):221–228. [DOI] [PubMed] [Google Scholar]
- Butler R, Inzunza J et al. 2012. Uric Acid Stones in the Urinary Bladder of Aryl Hydrocarbon Receptor (Ahr) Knockout Mice. Proceedings of the National Academy of Sciences USA. 109(4):1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I, Safe S et al. 1996. Indole-3-Carbinol and Diindolylmethane as Aryl Hydrocarbon (Ah) Receptor Agonists and Antagonists in T47d Human Breast Cancer Cells. Biochem Pharmacol 51(8):1069–1076. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Jin UH et al. 2015. Aryl Hydrocarbon Receptor Activity of Tryptophan Metabolites in Young Adult Mouse Colonocytes. Drug Metab Dispos 43(10):1536–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier A, Mialot A et al. 2013. Oculomotor Deficits in Aryl Hydrocarbon Receptor Null Mouse. PloS One. 8(1):e53520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Hershberg RM et al. 1999. Ligation of Intestinal Epithelial Cd1d Induces Bioactive Il-10: Critical Role of the Cytoplasmic Tail in Autocrine Signaling. Proc Natl Acad Sci USA. 96(24):13938–13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comi G, Jeffery D et al. 2012. Placebo-Controlled Trial of Oral Laquinimod for Multiple Sclerosis. N Engl J Med 366(11):1000–1009. [DOI] [PubMed] [Google Scholar]
- Deng Z, Rong Y et al. 2017. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell Amp-Activated Protein Kinase. Mol Ther 25(7):1641–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Faber SC. 2017. And Now for Something Completely Different: Diversity in Ligand-Dependent Activation of Ah Receptor Responses. Curr Opin Toxicol 2:124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. 2003. Activation of the Aryl Hydrocarbon Receptor by Structurally Diverse Exogenous and Endogenous Chemicals. Annu Rev Pharmacol Toxicol 43:309–334. [DOI] [PubMed] [Google Scholar]
- Denison MS, Seidel SD et al. 1998. Natural and Synthetic Ligands for the Ah Receptor In: Puga A, Kendall KB, editors. Molecular Bioloy Approaches to Toxicology. London: Taylor and Francis; p. 3–33. [Google Scholar]
- Diaz-Diaz CJ, Ronnekleiv-Kelly SM et al. 2016. The Aryl Hydrocarbon Receptor Is a Repressor of Inflammation-Associated Colorectal Tumorigenesis in Mouse. Ann Surg 264(3):429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolciami D, Ballarotto M et al. 2020. Targeting Aryl Hydrocarbon Receptor for Next-Generation Immunotherapies: Selective Modulators (Sahrms) Versus Rapidly Metabolized Ligands (Rmahrls). Eur J Med Chem 185:111842. [DOI] [PubMed] [Google Scholar]
- Duarte JH, Di Meglio P et al. 2013. Differential Influences of the Aryl Hydrocarbon Receptor on Th17 Mediated Responses in Vitro and in Vivo. PloS One. 8(11):e79819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich AK, Kerkvliet NI. 2017. Is Chronic Ahr Activation by Rapidly Metabolized Ligands Safe for the Treatment of Immune-Mediated Diseases? Curr Opin Toxicol 2:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich AK, Pennington JM et al. 2018. Tcdd, Ficz, and Other High Affinity Ahr Ligands Dose-Dependently Determine the Fate of Cd4+ T Cell Differentiation. Toxicological sciences. 161(2):310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C, Rannug A. 2015. The Aryl Hydrocarbon Receptor in Barrier Organ Physiology, Immunology, and Toxicology. Pharmacol Rev 67(2):259–279. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Hilbert DM et al. 1996. Aryl Hydrocarbon Receptor-Deficient Mice Are Resistant to 2,3,7,8-Tetrachlorodibenzo-P-Dioxin-Induced Toxicity. Toxicol Appl Pharmacol 140:173–179. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T et al. 1995. Immune System Impairment and Hepatic Fibrosis in Mice Lacking the Dioxin-Binding Ah Receptor. Science. 268(5211):722–726. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Ward JM et al. 1997. Lesions of Aryl-Hydrocarbon Receptor-Deficient Mice. Veterinary Pathology. 34(6):605–614. [DOI] [PubMed] [Google Scholar]
- Flaveny CA, Murray IA et al. 2009. Ligand Selectivity and Gene Regulation by the Human Aryl Hydrocarbon Receptor in Transgenic Mice. Mol Pharmacol 75(6):1412–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaveny CA, Murray IA et al. 2010. Differential Gene Regulation by the Human and Mouse Aryl Hydrocarbon Receptor. Toxicological Sciences. 114(2):217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Toshimitsu T et al. 2014. Identification of a Probiotic Bacteria-Derived Activator of the Aryl Hydrocarbon Receptor That Inhibits Colitis. Immunology and Cell Biology. 92(5):460–465. [DOI] [PubMed] [Google Scholar]
- Furumatsu K, Nishiumi S et al. 2011. A Role of the Aryl Hydrocarbon Receptor in Attenuation of Colitis. Digestive Diseases and Sciences. 56(9):2532–2544. [DOI] [PubMed] [Google Scholar]
- Giani Tagliabue S, Faber SC et al. 2019. Modeling the Binding of Diverse Ligands within the Ah Receptor Ligand Binding Domain. Sci Rep 9(1):10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goettel JA, Gandhi R et al. 2016. Ahr Activation Is Protective against Colitis Driven by T Cells in Humanized Mice. Cell Rep 17(5):1318–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Vazquez C, Quintana FJ. 2018. Regulation of the Immune Response by the Aryl Hydrocarbon Receptor. Immunity. 48(1):19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller F, Fuss IJ et al. 2002. Oxazolone Colitis, a Th2 Colitis Model Resembling Ulcerative Colitis, Is Mediated by Il-13-Producing Nk-T Cells. Immunity. 17(5):629–638. [DOI] [PubMed] [Google Scholar]
- Hites RA. 2011. Dioxins: An Overview and History. Environ Sci Technol 45(1):16–20. [DOI] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA et al. 2017. Dietary Broccoli Impacts Microbial Community Structure and Attenuates Chemically Induced Colitis in Mice in an Ah Receptor Dependent Manner. J Funct Foods. 37:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta T, Kobayashi Y et al. 2013. Asc-Associated Inflammation Promotes Cecal Tumorigenesis in Aryl Hydrocarbon Receptor-Deficient Mice. Carcinogenesis. 34(7):1620–1627. [DOI] [PubMed] [Google Scholar]
- Islam J, Sato S et al. 2017. Dietary Tryptophan Alleviates Dextran Sodium Sulfate-Induced Colitis through Aryl Hydrocarbon Receptor in Mice. J Nutr Biochem 42:43–50. [DOI] [PubMed] [Google Scholar]
- Iyer SS, Gensollen T et al. 2018. Dietary and Microbial Oxazoles Induce Intestinal Inflammation by Modulating Aryl Hydrocarbon Receptor Responses. Cell. 173(5):1123–1134 e1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Cheng Y et al. 2017. Short Chain Fatty Acids Enhance Aryl Hydrocarbon (Ah) Responsiveness in Mouse Colonocytes and Caco-2 Human Colon Cancer Cells. Sci Rep 7(1):10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Park H et al. 2018. Structure-Dependent Modulation of Aryl Hydrocarbon Receptor-Mediated Activities by Flavonoids. Toxicological Sciences. 164(1):205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan VC, O’Malley BW. 2007. Selective Estrogen-Receptor Modulators and Antihormonal Resistance in Breast Cancer. Journal of Clinical Oncology. 25(36):5815–5824. [DOI] [PubMed] [Google Scholar]
- Kaiko GE, Ryu SH et al. 2016. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell. 167(4):1137. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen JA, O’Malley BW et al. 1996. Tripartite Steroid Hormone Receptor Pharmacology - Interaction with Multiple Effector Sites as a Basis for the Cell- and Promoter-Specific Action of These Hormones. Mol Endocrinol 10(2):119–131. [DOI] [PubMed] [Google Scholar]
- Kawai S, Iijima H et al. 2017. Indigo Naturalis Ameliorates Murine Dextran Sodium Sulfate-Induced Colitis Via Aryl Hydrocarbon Receptor Activation. J Gastroenterol 52(8):904–919. [DOI] [PubMed] [Google Scholar]
- Kawajiri K, Kobayashi Y et al. 2009. Aryl Hydrocarbon Receptor Suppresses Intestinal Carcinogenesis in Apcmin/+ Mice with Natural Ligands. Proc Natl Acad Sci USA. 106(32):13481–13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili H, Chan SSM et al. 2018. The Role of Diet in the Aetiopathogenesis of Inflammatory Bowel Disease. Nat Rev Gastroenterol Hepatol 15(9):525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C et al. 2011. Natural Aryl Hydrocarbon Receptor Ligands Control Organogenesis of Intestinal Lymphoid Follicles. Science. 334(6062):1561–1565. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Ding Y et al. 2018. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep 23(4):1099–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis GP, Pyzalski RW et al. 2005. The Aryl Hydrocarbon Receptor Is Required for Developmental Closure of the Ductus Venosus in the Neonatal Mouse. Mol Pharmacol 67(3):714–720. [DOI] [PubMed] [Google Scholar]
- Li S, Bostick JW et al. 2018. Aryl Hydrocarbon Receptor Signaling Cell Intrinsically Inhibits Intestinal Group 2 Innate Lymphoid Cell Function. Immunity. 49(5):915–928 e915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Bostick JW et al. 2017. Regulation of Innate Lymphoid Cells by Aryl Hydrocarbon Receptor. Front Immunol 8:1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Innocentin S et al. 2011. Exogenous Stimuli Maintain Intraepithelial Lymphocytes Via Aryl Hydrocarbon Receptor Activation. Cell. 147(3):629–640. [DOI] [PubMed] [Google Scholar]
- Limon JJ, Tang J et al. 2019. Malassezia Is Associated with Crohn’s Disease and Exacerbates Colitis in Mouse Models. Cell Host Microbe 25(3):377–388 e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li L et al. 2018. Aryl Hydrocarbon Receptor Activation Maintained the Intestinal Epithelial Barrier Function through Notch1 Dependent Signaling Pathway. Int J Mol Med 41(3):1560–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YF, Santostefano M et al. 1996. Substituted Flavones as Aryl Hydrocarbon (Ah) Receptor Agonists and Antagonists. Biochem Pharmacol 51(8):1077–1087. [DOI] [PubMed] [Google Scholar]
- Lund AK, Goens MB et al. 2003. Cardiac Hypertrophy in Aryl Hydrocarbon Receptor Null Mice Is Correlated with Elevated Angiotensin Ii, Endothelin-1, and Mean Arterial Blood Pressure. Toxicology and Applied Pharmacology. 193(2):177–187. [DOI] [PubMed] [Google Scholar]
- Lv Q, Qiao SM et al. 2015. Norisoboldine Ameliorates Dss-Induced Ulcerative Colitis in Mice through Induction of Regulatory T Cells in Colons. Int Immunopharmacol 29(2):787–797. [DOI] [PubMed] [Google Scholar]
- Lv Q, Wang K et al. 2018. Norisoboldine, a Natural Ahr Agonist, Promotes Treg Differentiation and Attenuates Colitis Via Targeting Glycolysis and Subsequent Nad(+)/Sirt1/Suv39h1/H3k9me3 Signaling Pathway. Cell Death Dis 9(3):258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli L, Martin-Gallausiaux C et al. 2019. Identification of the Novel Role of Butyrate as Ahr Ligand in Human Intestinal Epithelial Cells. Sci Rep 9(1):643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruvada P, Leone V et al. 2017. The Human Microbiome and Obesity: Moving Beyond Associations. Cell Host Microbe 22(5):589–599. [DOI] [PubMed] [Google Scholar]
- McDougal A, Wormke M et al. 2001. Tamoxifen-Induced Antitumorigenic/Antiestrogenic Action Synergized by a Selective Ah Receptor Modulator. Cancer Res 61:3901–3907. [PubMed] [Google Scholar]
- Metidji A, Omenetti S et al. 2018. The Environmental Sensor Ahr Protects from Inflammatory Damage by Maintaining Intestinal Stem Cell Homeostasis and Barrier Integrity. Immunity. 49(2):353–362 e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mexia N, Gaitanis G et al. 2015. Pityriazepin and Other Potent Ahr Ligands Isolated from Malassezia Furfur Yeast. Arch Biochem Biophys 571:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura J, Yamashita K et al. 1997. Loss of Teratogenic Response to 2,3,7,8-Tetrachlorodibenzo-P-Dioxin (Tcdd) in Mice Lacking the Ah (Dioxin) Receptor. Genes to Cells. 2(10):645–654. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Zorzi F et al. 2016. Aryl Hydrocarbon Receptor-Driven Signals Inhibit Collagen Synthesis in the Gut. Eur J Immunol 46(4):1047–1057. [DOI] [PubMed] [Google Scholar]
- Muku GE, Murray IA et al. 2018. Urolithin a Is a Dietary Microbiota-Derived Human Aryl Hydrocarbon Receptor Antagonist. Metabolites. 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IA, Flaveny CA et al. 2011. Suppression of Cytokine-Mediated Complement Factor Gene Expression through Selective Activation of the Ah Receptor with 3’,4’-Dimethoxy-Alpha-Naphthoflavone. Mol Pharmacol 79(3):508–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IA, Krishnegowda G et al. 2010. Development of a Selective Modulator of Aryl Hydrocarbon (Ah) Receptor Activity That Exhibits Anti-Inflammatory Properties. Chem Res Toxicol 23(5):955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray IA, Morales JL et al. 2010. Evidence for Ligand-Mediated Selective Modulation of Aryl Hydrocarbon Receptor Activity. Mol Pharmacol 77(2):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszak T, Neves JF et al. 2014. Protective Mucosal Immunity Mediated by Epithelial Cd1d and Il-10. Nature. 509(7501):497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi JH, Waddell A et al. 2014. Dominant Effects of the Diet on the Microbiome and the Local and Systemic Immune Response in Mice. PloS One. 9(1):e86366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka T, Moriyama E et al. 2017. Meta-Analysis of Fecal Microbiota and Metabolites in Experimental Colitic Mice During the Inflammatory and Healing Phases. Nutrients. 9(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paturi G, Mandimika T et al. 2012. Influence of Dietary Blueberry and Broccoli on Cecal Microbiota Activity and Colon Morphology in Mdr1a(-/-) Mice, a Model of Inflammatory Bowel Diseases. Nutrition. 28(3):324–330. [DOI] [PubMed] [Google Scholar]
- Perkins A, Phillips JL et al. 2014. A Structural Switch between Agonist and Antagonist Bound Conformations for a Ligand-Optimized Model of the Human Aryl Hydrocarbon Receptor Ligand Binding Domain. Biology (Basel). 3(4):645–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskorska-Pliszczynska J, Keys B et al. 1986. The Cytosolic Receptor Binding Affinities and Ahh Induction Potencies of 29 Polynuclear Aromatic Hydrocarbons. Toxicol Lett 34(1):67–74. [DOI] [PubMed] [Google Scholar]
- Pohjanvirta R, Tuomisto J. 1994. Short-Term Toxicity of 2,3,7,8-Tetrachlorodibenzo-P-Dioxin in Laboratory Animals: Effects, Mechanisms, and Animal Models. Pharmacol Rev 46(4):483–549. [PubMed] [Google Scholar]
- Poland A, Glover E et al. 1976. Stereospecific, High Affinity Binding of 2,3,7,8-Tetrachlorodibenzo-P-Dioxin by Hepatic Cytosol: Evidence That the Binding Species Is Receptor for Induction of Aryl Hydrocarbon Hydroxylase. J Biol Chem 251:4936–4946. [PubMed] [Google Scholar]
- Poland A, Knutson JC. 1982. 2,3,7,8-Tetrachlorodibenzo-P-Dioxin and Related Halogenated Aromatic Hydrocarbons: Examination of the Mechanism of Toxicity. Annu Rev Pharmacol Toxicol 22:517–554. [DOI] [PubMed] [Google Scholar]
- Pool-Zobel BL, Selvaraju V et al. 2005. Butyrate May Enhance Toxicological Defence in Primary, Adenoma and Tumor Human Colon Cells by Favourably Modulating Expression of Glutathione S-Transferases Genes, an Approach in Nutrigenomics. Carcinogenesis 26(6):1064–1076. [DOI] [PubMed] [Google Scholar]
- Qiu J, Guo X et al. 2013. Group 3 Innate Lymphoid Cells Inhibit T-Cell-Mediated Intestinal Inflammation through Aryl Hydrocarbon Receptor Signaling and Regulation of Microflora. Immunity. 39(2):386–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Heller JJ et al. 2012. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunity. 36(1):92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS et al. 2008. Control of Treg and T(9)H17 Cell Differentiation by the Aryl Hydrocarbon Receptor. Nature. 453(7191):65–71. [DOI] [PubMed] [Google Scholar]
- Rannug A, Rannug U et al. 1987. Certain Photooxidized Derivatives of Tryptophan Bind with Very High Affinity to the Ah Receptor and Are Likely to Be Endogenous Signal Substances. J Biol Chem 262(32):15422–15427. [PubMed] [Google Scholar]
- Rothhammer V, Borucki DM et al. 2018. Microglial Control of Astrocytes in Response to Microbial Metabolites. Nature. 557(7707):724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Mascanfroni ID et al. 2016. Type I Interferons and Microbial Metabolites of Tryptophan Modulate Astrocyte Activity and Central Nervous System Inflammation Via the Aryl Hydrocarbon Receptor. Nat Med 22(6):586–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild D, Weissbrod O et al. 2018. Environment Dominates over Host Genetics in Shaping Human Gut Microbiota. Nature. 555(7695):210–215. [DOI] [PubMed] [Google Scholar]
- Russo I, Luciani A et al. 2012. Butyrate Attenuates Lipopolysaccharide-Induced Inflammation in Intestinal Cells and Crohn’s Mucosa through Modulation of Antioxidant Defense Machinery. PloS One. 7(3):e32841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Safe S 1990. Polychlorinated Biphenyls (Pcbs), Dibenzo-P-Dioxins (Pcdds), Dibenzofurans (Pcdfs), and Related Compounds: Environmental and Mechanistic Considerations Which Support the Development of Toxic Equivalency Factors (Tefs). Critical Reviews in Toxicology. 21(1):51–88. [DOI] [PubMed] [Google Scholar]
- Safe S, Han H, Goldsby J, Mohankumar K, Chapkin RS 2018. Aryl Hydrocarbon Recpetor (Ahr) Ligands as Selective Ahr Modulators: Genomic Studies. Curr Opin Toxicol 10–20. [DOI] [PMC free article] [PubMed]
- Safe S, Lee SO et al. 2013. Role of the Aryl Hydrocarbon Receptor in Carcinogenesis and Potential as a Drug Target. Toxicological Sciences. 135(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe SH. 1998. Development Validation and Problems with the Toxic Equivalency Factor Approach for Risk Assessment of Dioxins and Related Compounds. J Anim Sci 76(1):134–141. [DOI] [PubMed] [Google Scholar]
- Santostefano M, Merchant M et al. 1993. Alpha-Naphthoflavone-Induced Cyp1a1 Gene Expression and Cytosolic Aryl Hydrocarbon Receptor Transformation. Mol Pharmacol 43(2):200–206. [PubMed] [Google Scholar]
- Sauzeau V, Carvajal-Gonzalez JM et al. 2011. Transcriptional Factor Aryl Hydrocarbon Receptor (Ahr) Controls Cardiovascular and Respiratory Functions by Regulating the Expression of the Vav3 Proto-Oncogene. J Biol Chem 286(4):2896–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. 1996. Ah Receptor Signaling Pathways. Annu Rev Cell Dev Biol 12:55–89. [DOI] [PubMed] [Google Scholar]
- Seok SH, Lee W et al. 2017. Structural Hierarchy Controlling Dimerization and Target DNA Recognition in the Ahr Transcriptional Complex. Proc Natl Acad Sci USA. 114(21):5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiizaki K, Ohsako S et al. 2014. Identification of Amino Acid Residues in the Ligand-Binding Domain of the Aryl Hydrocarbon Receptor Causing the Species-Specific Response to Omeprazole: Possible Determinants for Binding Putative Endogenous Ligands. Mol Pharmacol 85(2):279–289. [DOI] [PubMed] [Google Scholar]
- Singh KP, Garrett RW et al. 2011. Aryl Hydrocarbon Receptor-Null Allele Mice Have Hematopoietic Stem/Progenitor Cells with Abnormal Characteristics and Functions. Stem Cells and Development. 20(5):769–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Gurav A et al. 2014. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity. 40(1):128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Singh UP et al. 2011. Activation of Aryl Hydrocarbon Receptor (Ahr) Leads to Reciprocal Epigenetic Regulation of Foxp3 and Il-17 Expression and Amelioration of Experimental Colitis. PloS One. 6(8):e23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Chandrashekharappa S et al. 2019. Enhancement of the Gut Barrier Integrity by a Microbial Metabolite through the Nrf2 Pathway. Nat Commun 10(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Murray IA et al. 2011. Identification of a High-Affinity Ligand That Exhibits Complete Aryl Hydrocarbon Receptor Antagonism. The Journal of Pharmacology and Experimental Therapeutics. 338(1):318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soshilov AA, Denison MS. 2014. Ligand Promiscuity of Aryl Hydrocarbon Receptor Agonists and Antagonists Revealed by Site-Directed Mutagenesis. Molecular and Cellular Biology. 34(9):1707–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Di Meglio P et al. 2014. The Aryl Hydrocarbon Receptor: Multitasking in the Immune System. Annu Rev Immunol 32:403–432. [DOI] [PubMed] [Google Scholar]
- Teng Y, Ren Y et al. 2018. Plant-Derived Exosomal Micrornas Shape the Gut Microbiota. Cell Host Microbe 24(5):637–652 e638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum L et al. 1998. Toxic Equivalency Factors (Tefs) for Pcbs, Pcdds, Pcdfs for Humans and Wildlife. Environmental Health Perspectives. 106(12):775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS et al. 2006. The 2005 World Health Organization Reevaluation of Human and Mammalian Toxic Equivalency Factors for Dioxins and Dioxin-Like Compounds. Toxicological Sciences. 93(2):223–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M, Denison MS et al. 2013. Polybrominated Dibenzo-P-Dioxins, Dibenzofurans, and Biphenyls: Inclusion in the Toxicity Equivalency Factor Concept for Dioxin-Like Compounds. Toxicological Sciences. 133(2):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Beek CM, Dejong CHC et al. 2017. Role of Short-Chain Fatty Acids in Colonic Inflammation, Carcinogenesis, and Mucosal Protection and Healing. Nutr Rev 75(4):286–305. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Hirota K et al. 2008. The Aryl Hydrocarbon Receptor Links Th17-Cell-Mediated Autoimmunity to Environmental Toxins. Nature. 453(7191):106–109. [DOI] [PubMed] [Google Scholar]
- Wang Y, Fan Y et al. 2010. Dioxin Exposure Disrupts the Differentiation of Mouse Embryonic Stem Cells into Cardiomyocytes. Toxicological Sciences. 115(1):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille G, Mayser P et al. 2001. Malassezin--a Novel Agonist of the Arylhydrocarbon Receptor from the Yeast Malassezia Furfur. Bioorg Med Chem 9(4):955–960. [DOI] [PubMed] [Google Scholar]
- Xing Y, Nukaya M et al. 2012. Identification of the Ah-Receptor Structural Determinants for Ligand Preferences. Toxicological Sciences. 129(1):86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Qiu J et al. 2017. The Aryl Hydrocarbon Receptor Preferentially Marks and Promotes Gut Regulatory T Cells. Cell Rep 21(8):2277–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Wang Q et al. 2018. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int J Biol Sci 14(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatloukalova J, Svihalkova-Sindlerova L et al. 2007. Beta-Naphthoflavone and 3’-Methoxy-4’-Nitroflavone Exert Ambiguous Effects on Ah Receptor-Dependent Cell Proliferation and Gene Expression in Rat Liver ‘Stem-Like’ Cells. Biochem Pharmacol 73(10):1622–1634. [DOI] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG et al. 2013. Tryptophan Catabolites from Microbiota Engage Aryl Hydrocarbon Receptor and Balance Mucosal Reactivity Via Interleukin-22. Immunity. 39(2):372–385. [DOI] [PubMed] [Google Scholar]
- Zhao B, Degroot DE et al. 2010. Ch223191 Is a Ligand-Selective Antagonist of the Ah (Dioxin) Receptor. Toxicological Sciences. 117(2):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhang M et al. 2018. Faecalibacterium Prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflammatory Bowel Diseases. [DOI] [PubMed]


