Abstract
Pyroptosis is a recently discovered inflammatory form of programmed cell death which is mostly triggered by infection with intracellular pathogens and critically contributes to inflammation. Mitigating pyroptosis may be a potential therapeutic target in inflammatory diseases. However, small chemicals to reduce pyroptosis is still elusive. In the present study, we screened 155 chemicals from a microbial natural product library and found Geldanamycin, an HSP90 inhibitor, profoundly rescued THP-1 cells from pyroptosis induced by LPS plus Nigericin treatment. Consistently, other HSP90 inhibitors, including Radicicol, 17-DMAG and 17-AAG, all ameliorated pyroptosis in THP-1 cells by suppressing the inflammasome/Caspase-1/GSDMD signal pathway in pyroptosis. HSP90 inhibition compromised the protein stability of NLRP3, a critical component of the inflammasome. Moreover, up-regulated HSP70 may also contribute to this effect. HSP90 inhibition may thus be a potential therapeutic strategy in the treatment of inflammatory diseases in which pyroptosis plays a role.
Introduction
Inflammation is a crucial pathologic process which participates in a vast variety of critical diseases, including atherosclerosis [1], diabetes [2], asthma [3] and inflammatory bower disease [4]. In inflammation, activated immune cells cause tissue damage in multiple mechanisms and the mitigation of inflammation holds the key to the treatment of these diseases [5].
Pyroptosis is a recently discovered inflammatory form of programmed cell death which is triggered by inflammatory stimuli and eventually exacerbates inflammation [6,7]. When an inflammatory signal is received by the cells, it may activateNACHT-, LRR- and pyrin domain-containing protein 3 (NLRP3), which initiates the assembly of the inflammasome [8]. The inflammasome is a potent pro-inflammatory protease complex. Once it is fully assembled, the outermost component, pro-caspase-1 will be cleaved to its active form caspase-1 [8]. As an inflammatory protease, caspase-1 has two important substrates in inflammation and pyroptosis, pro-interleukin-1β (pro-IL-1β)[8] and gasdermin D (GSDMD) [7].
IL-1β is a potent pro-inflammatory cytokine. It is involved in multiple severe diseases mediated with inflammation [9]. Once synthesized, the full-length protein is stored in the cytoplasm as its latent form, pro-IL-1β [9]. The outmost component of the inflammasome, caspase-1, provides a final cut to pro-IL-1β and converts it into its active form IL-1β, which is ready to be released out of the cells to enhance inflammation [8,9]. The other crucial substrate of caspase-1, GSDMD, is also kept in its latent form in the cytoplasm. When inflammasome is assembled, caspase-1 cleaves it into its active N-terminal. The N-terminal GSDMD then forms pores in the plasma membrane to facilitate the release of IL-1β. Because the pores formed by N-terminal GSDMD connects the material inside and outside of cells, the cells will eventually swell and break, ending up in pyroptosis [6,7]. Thus, in addition to the release of pro-inflammatory cytokine IL-1β, pyroptosis also exacerbates inflammation by releasing damage-associated molecular pattern (DAMP) after cell death [7].
Pyroptosis was first discovered and well-studied in macrophage/monocytes [10]. Many previous studies analyzed pyroptosis pathways in human spontaneously immortalized monocytic cell line THP-1. THP-1 cell line is derived from the peripheral blood of a child with M5 acute monocytic leukemia. It is widely used as a proxy to human monocytes because it mimics many of their crucial functions, including secretion of cytokines upon inflammatory stimulation, assembly of inflammasome, differentiation into macrophages, polarization to M1 and M2 macrophage subtypes, differentiation into mature and immature dendritic cells [11–13]. In the present study, we also use THP-1 cells to gain some clues about pyroptosis in monocytes.
Given the strong pro-inflammatory effect of pyroptosis, reducing the NLRP3/Caspase-1/GSDMD pyroptotic pathway has become a promising therapeutic strategy. Heat shock protein 90 (HSP90) is a well-known chaperon protein in the heat shock protein family. A previous study suggests that NLRP3 may be a client protein of HSP90 [14]. Without HSP90, NLRP3 may misfold before being degraded by the proteasome [15,16]. Therefore, it is possible to attenuate pyroptosis by inhibiting HSP90. Indeed, in the present screen for pyroptosis inhibitors, we found multiple HSP90 inhibitors significantly reduced pyroptosis, probably through the misfolding and degradation of NLRP3.
As an important member of HSP chaperons, loss of HSP90 is frequently reported to increase the expression of HSP70, another member of the same chaperon family as compensation [17]. Paradoxically, HSP70 inhibits inflammasome, the key signal complex of pyroptosis [18]. In our current research, we also found the increase of HSP70 expression in the presence of HSP90 inhibitors. Moreover, inhibiting HSP70 would partially compromise the rescuing effect of some HSP90 inhibitors on pyroptosis. The up-regulation of HSP70 may be another mechanism that HSP90 inhibitors improve THP-1 cells in pyroptosis.
Materials and methods
Cell culture and induction of pyroptosis
THP-1 cells were obtained from ATCC (Manassas, VA) and cultured in RPMI 1640 medium supplemented with 10% FBS, 1 mM Sodium Pyruvate, 25 mM HEPES (all from Gibco, Grand Island, NY) and 1% penicillin/ streptomycin (Corning, Manassas, VA). Pyroptosis was induced according to the previously published procedure [19]. Briefly, 1 × 106/ml cells were cultured with RPMI 1640 medium containing 2% FBS overnight and then treated with 1 μg/ml LPS (Sigma, St. Louis, MO) for 3 h, followed by 10 μg/ml Nigericin (Alfa Aestar, Ward Hill, MA) treatment. Supernatant LDH activity, cell viability and cell protein content were analyzed 1 h following the addition of Nigericin to the culture system unless otherwise indicated.
LDH activity assay
Culture supernatant LDH activity following pyroptosis induction was analyzed with a CytoTox96 Cytotoxicity Assay kit (Promega, Madison, WI) according to the instructions from the manufacturer. Light absorbance was read with Cytation 3 imager (Bio Tek, Winooski, VT).
MTT assay
Cell viability MTT assay was performed after pyroptosis induction by adding 10 μl, 5 mg/ml MTT (Sigma) solution into each well of 96-well-plates. After 3 h of further culture 200 μl DMSO (Fisher, Fair Lawn, NJ) was added to each well and incubated for 15 min in room temperature protected from light. The absorbance of 570 nm wavelength light was then measured with Cytation 3 imager (Bio Tek, Winooski, VT).
Chemical screening and treatment
To screen for inhibitors of pyroptosis, 155 chemicals from microbial natural product library (Target Molecule, Wellesley Hills, MA) were added into individual wells of 96-well-plates at 50 μM 1 h before the induction of pyroptosis. The effect of chemicals on pyroptosis was measured with supernatant LDH activity and cell viability MTT assays.
To test the effect of HSP90 inhibitors on pyroptosis, Geldanamycin, 17-DMAG, 17-AAG and Radicicol (all from Sigma) were added to the culture system 1 h before the induction of pyroptosis with LPS and Nigericin. MG132 (Sigma) was added to the culture 1 h before HSP90 inhibitors if indicated. Ver-155008 (Selleckchem, Huston, TX) was added to the cell culture system simultaneously with HSP90 inhibitors. To test protein expression after HSP90 inhibition, cells were collected and lysed without the induction of pyroptosis at the indicated time after HSP90 inhibitors were added.
Western Blot
Cell lysis was obtained with Ripa buffer and then mixed with 2×Laemmili loading buffer (65.8 mM Tris—HCI, pH 6.8, 2.1% SDS, 26.3% (w/v) glycerol, 0.01% bromophenol blue, 5% 2-mercaptoethanol) before boiled for 10 min. Protein samples and pre-stained protein ruler (Thermo Fisher Scientific, Rockford, IL) were separated by SDS–PAGE in 8%, 10% or 12% resolving gels and then transferred to PVDF membranes (GE, Chicago, IL). After transfer, membranes were blocked with 5% nonfat milk (Millipore, Burlington, MA) in PBS supplemented with 0.1% Tween-20 (Fisher) for 1 h in room temperature. The membranes were then incubated with the following primary antibodies at 4°C overnight, according to the manufacturers’ instruction: Caspase-1, GSDMD, NLRP3, HSP70 (all from Cell Signal Technology, Beverly, MA) HSP90 (Proteintech, Rosemont, IL) and ß-actin (Santa Cruz, Dallas, TX). After three times of wash in PBST, HRP labeled Goat anti-Mouse IgG, (H + L) and Goat anti-Rabbit IgG,(H + L) secondary antibodies (Thermo Fisher Scientific) were used to incubate the membranes. Luminescence was generated after the membranes were exposed to Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) and detected with X-ray film.
Statistics
Statistical analysis was performed with GraphPad Prism software (GraphPad, San Diego, CA). Data were expressed as mean ± SEM. For comparison between two groups, the unpaired Student’s t-test was used. For multiple comparison, one way ANOVA followed by Turkey’s post hoc analysis was used. A value of p < 0.05 was considered significant.
Result
Geldanamycin inhibits pyroptosis
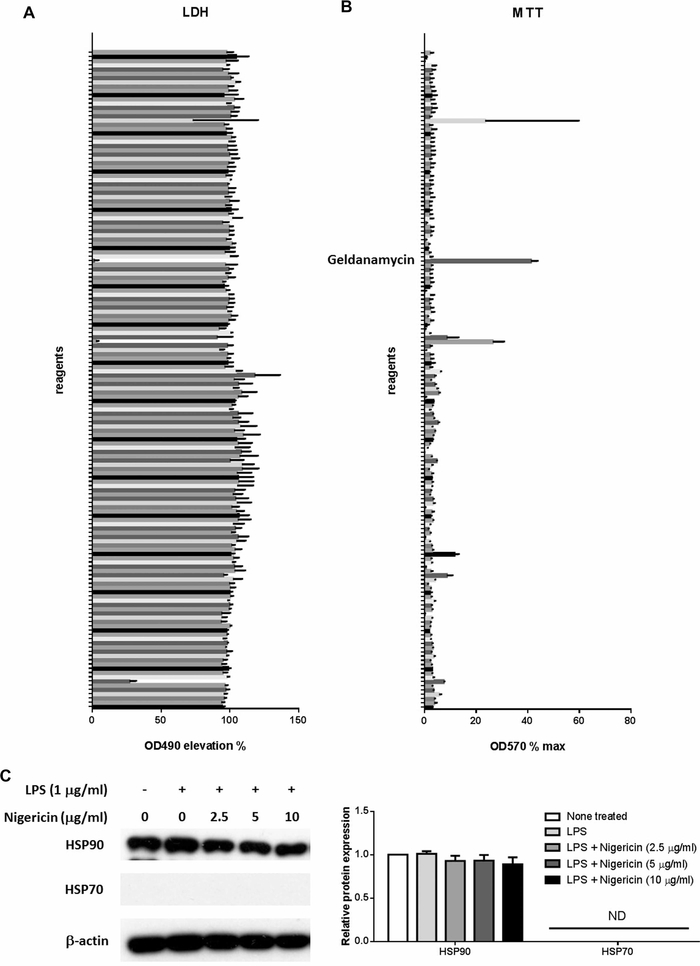
To search for a pyroptosis inhibitor, we screened of 155 chemicals in the microorganism component library according to the effect on LPS plus Nigericin induced pyroptosis in THP-1 cells. Among them, Geldanamycin was the most remarkable to reduce the supernatant LDH activity (Figure 1A) and increase cell viability in MTT assay (Figure 1B). Because Geldanamycin is an inhibitor of HSP90, we further analyzed the expression of HSP90 in THP-1 cells after the stimulation of LPS and Nigericin. We found HSP90 is abundantly expressed in THP-1 cells and is not influenced by LPS or Nigericin. In contrast, HSP70, another important heat shock protein, was not detectible and not induced by LPS or Nigericin (Figure 1C).
Figure 1. Effect of bacterial components on pyroptosis in THP-1 cells.
THP-1 cells were treated with 50 μM of each chemical in the bacterial chemical library for 1 h. After that, pyroptosis was induced with LPS stimulation at 1 μg/ml for 3 h followed by 10 μg/ml treatment of Nigericin for 1 h. (A) LDH activity in the cell culture supernatant was examined. (B) Cell viability was analyzed with MTT conversion. (C) THP-1 cells were stimulated with or without 1 μg/ml LPS for 3 h and Nigericin at the concentration indicated for an additional hour before harvest. The protein expression of HSP90 and HSP70 was analyzed with Western Blot. In (A) and (B), N = 2–3. In (C), data represent one of four independent experiments, N = 4 (ND, not detectible).
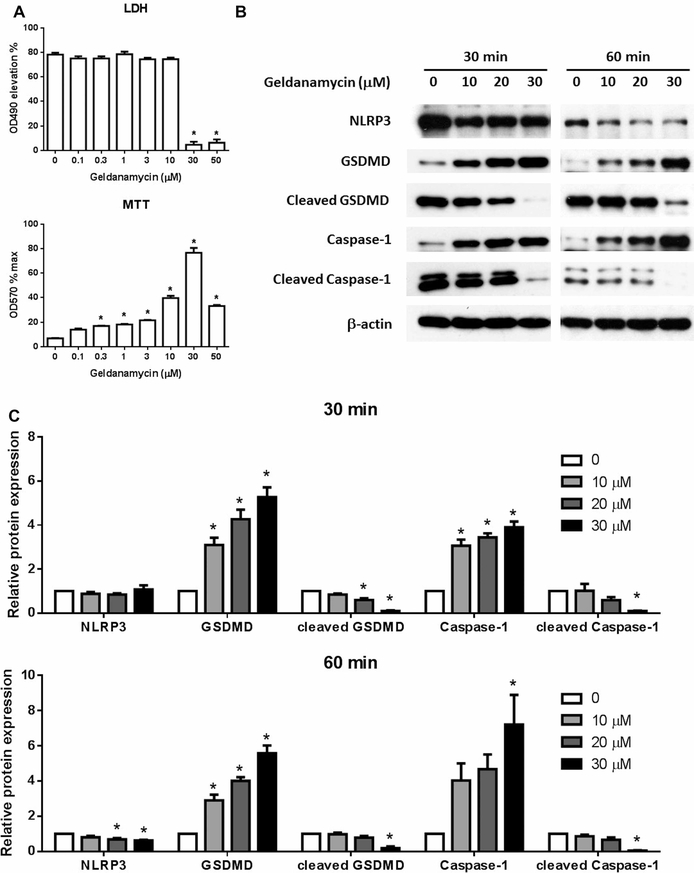
We further found that Geldanamycin dose dependently ameliorates pyroptosis in THP-1 cells with the maximum activity at 30 μM as shown in supernatant LDH activity and cell viability (Figure 2A). Moreover, we examined the classical NLRP3/caspase-1/GSDMD pyroptosis signal pathway and found the protein expression of NLRP3, as well as the activation of caspase-1 and GSDMD through cleavage, were reduced by Geldanamycin in a dose-dependent manner both 30 and 60 min after the treatment of Nigericin (Figure 2B,C). We thus confirm that Geldanamycin ameliorates the pyroptosis induced by LPS plus Nigericin in THP-1 cells.
Figure 2. Geldanamycin inhibits pyroptosis in THP-1 cells.
THP-1 cells were treated with Geldanamycin at different concentration as indicated for 1 h before the induction of pyroptosis with LPS stimulation at 1 μg/ml for 3 h plus 10 μg/ml treatment of Nigericin for 1 h. The cell culture supernatant LDH activity and cell viability were measured (A). 30 and 60 min after Nigericin treatment, cell lysis was obtained and the protein expression of NLRP3, caspase-1, cleaved caspase-1, GSDMD, cleaved GSDMD was examined with Western Blot (B) and statistically analyzed (C). Images represent one of three independent experiments. N = 3. *p < 0.05 versus 0.
HSP90 inhibitors reduce pyroptosis
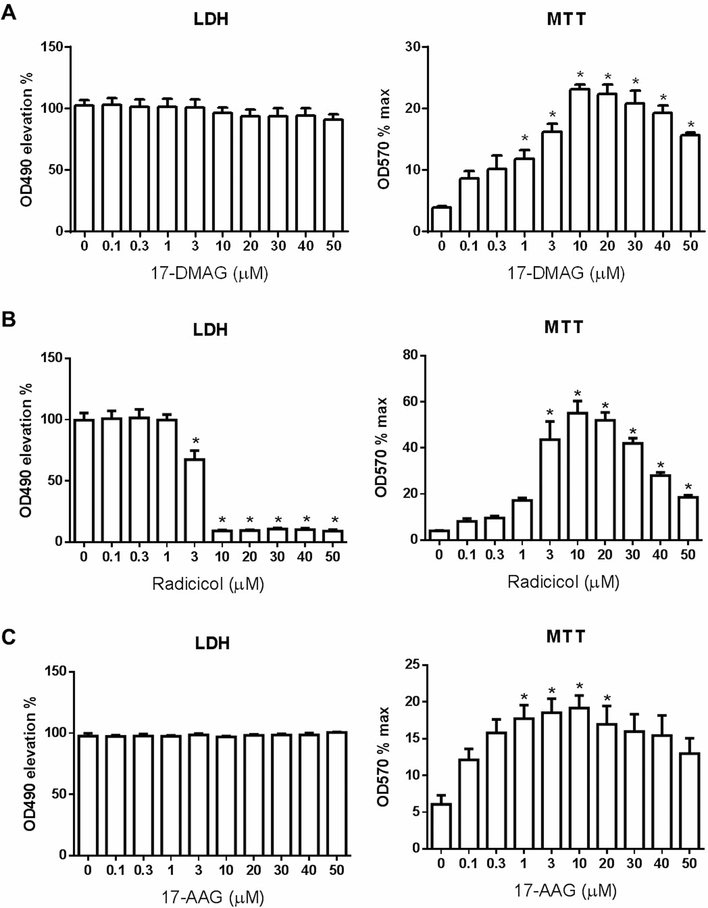
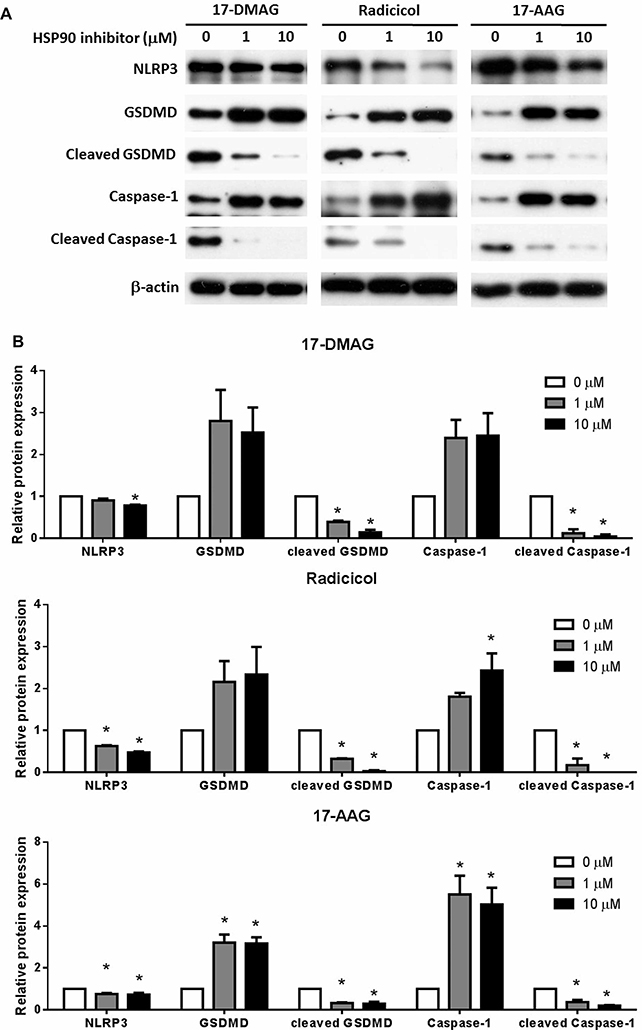
Given Geldanamycin is an HSP90 inhibitor and its potency in reducing pyroptosis, we wonder whether other HSP90 inhibitors would have similar function. Indeed, Radicicol, another HSP90 inhibitor also dose dependently reduced LDH release and increased cell viability. On the other hand, 17-DMAG and 17-AAG significantly improved cell viability but did not ameliorate LDH release (Figure 3). All these three HSP90 inhibitors dose dependently blocked the classical NLRP3/caspase-1/GSDMD pyroptosis signal pathway (Figure 4). These results all indicate that HSP90 inhibition suppress pyroptosis.
Figure 3. HSP90 inhibitors ameliorate pyroptosis in THP-1 cells.
17-DMAG (A), Radicicol (B) and 17-AAG (C) were added to THP-1 cells at indicated concentration 1 h before pyroptosis induction with LPS and Nigericin as in Figure 2. The cell culture supernatant LDH and cell viability were analyzed 60 min after Nigericin treatment. N = 3–4. *p < 0.05 versus 0.
Figure 4. HSP90 inhibitors hinder the pyroptosis signal pathway.
THP-1 cells were treated as in Figure 3 and the cell protein content of NLRP3, caspase-1, cleaved caspase-1, GSDMD, cleaved GSDMD was examined with Western Blot (A) and further statistically analyzed (B). Images represent one of three or four independent experiments. N = 3–4. *p < 0.05 versus 0.
HSP90 inhibitors induce NLRP3 degradation through proteasome
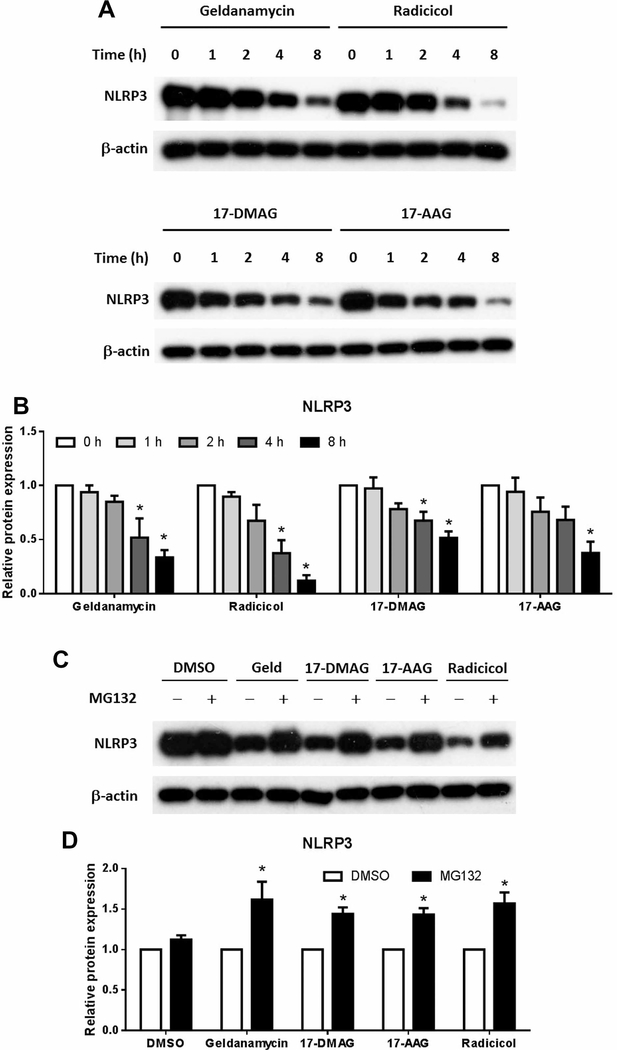
NLRP3 is the central molecule in inflammasome which initiates pyroptosis. In addition, HSP90 has been found to possibly assist the folding of HSP90 protein into the correct structure and prevent its degradation [14]. Since we have already found that HSP90 inhibitors reduce the protein expression of NLRP3, we wonder if its degradation in the absence of HSP90 is the reason. We thus tested the protein expression of NLRP3 in the presence of HSP90 inhibitors without pyroptosis induction and found that all four HSP90 inhibitors time dependently reduced the protein level of NLRP3 (Figure 5A,B). Moreover, this effect was hindered by MG132, a proteasome inhibitor (Figure 5C,D). HSP90 may thus be critical for the protein structure and stability of NLRP3 while its inhibitors may reduce pyroptosis by increasing its misfolding and subsequent degradation through proteasome.
Figure 5. NLRP3 is degraded by the proteasome after HSP90 inhibition.
THP-1 cells were treated with Geldanamycin (30 μM), Radicicol (10 μM), 17-DMAG (10 μM) and 17-AAG (10 μM) for 0, 1, 2, 4 and 8 h. Cell lysis were collected and the protein content of NLRP3 was determined with Western Blot (A) and statistically analyzed (B). MG132 (5 μM) or DMSO was added to THP-1 cells for 1 h before the treatment of HSP90 inhibitors as in (A) for 4 h. The cell protein content of NLRP3 was analyzed with Western Blot (C,D). Images represent one of three or four independent experiments. N = 3–4. *p < 0.05 versus 0 in (B) and versus DMSO in (D).
HSP70 up-regulation may contribute to the rescuing effect of HSP90 inhibitors
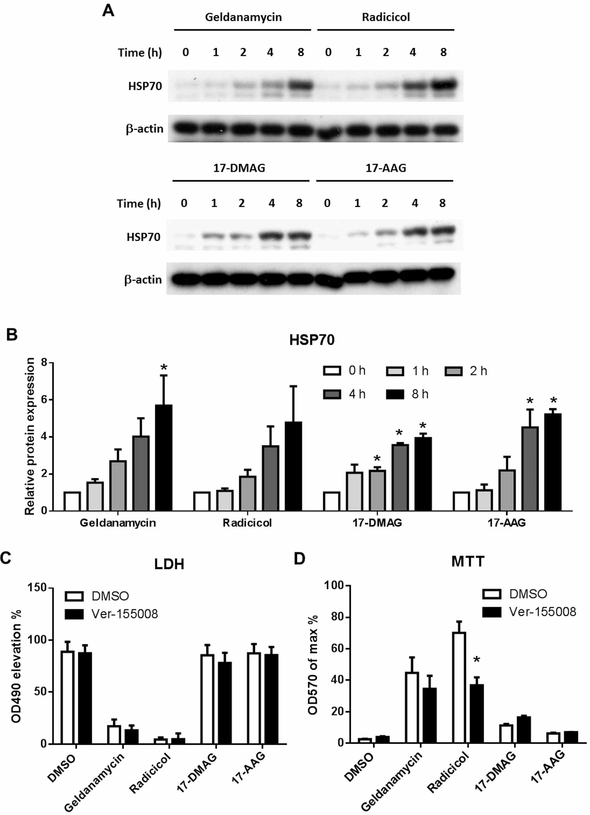
HSP70 is another important chaperon in the family of HSPs. Previous reports suggest that the expression of HSP70 may be up-regulated when HSP90 is inhibited [17]. Because HSP70 is also known to interfere with the signal of the inflammasome [18], we further examined whether HSP70 is involved in the effects of HSP90 inhibitors on pyroptosis. Consistent with the previous reports, in THP-1 cells the expression of HSP70 is time dependently up-regulated by the inhibitors of HSP90 (Figure 6A,B). We thus further tested whether Ver-155008, an HSP70 inhibitor, would interfere with the function of HSP90 inhibitors. In LPS plus Nigericin induced pyroptosis model of THP-1 cells, we found Ver-155008 did not change the LDH reduction of Geldanamycin or Radicicol. However, the cell viability improvement of Radicicol was partially reduced by Ver-155008, while the Geldanamycin effect was also reduced but not reaching a statistical significance (Figure 6C,D). The HSP70 up-regulation is thus possibly involved in the HSP90 inhibiting effects on pyroptosis.
Figure 6. HSP70 up-regulation is involved in the protective effect of HSP90 inhibitors.
THP-1 cells were treated as in Figure 5A and the cell protein content of HSP70 was examined with Western Blot (A) and statistically analyzed (B). HSP70 inhibitor VER-155008 (30 μM) was added together with HSP90 inhibitors 1 h before the induction of pyroptosis with LPS plus Nigericin. Cell culture supernatant LDH (C) and cell viability (D) was analyzed 1 h after Nigericin treatment. Images represent one of three independent experiments. N = 3, *p < 0.05 versus 0 in (B) and versus DMSO in (D).
Discussion
Pyroptosis is a recently discovered type of programmed cell death. It is triggered by inflammation and further enhance inflammation. Given its potent pro-inflammatory function, hampering pyroptosis may be a potential therapeutic strategy for inflammatory diseases. However, the current search for pyroptosis inhibitors is still elusive. In the present study, we screened 155 natural chemicals from microorganisms and found Geldanamycin inhibits pyroptosis in THP-1 cells induced by LPS and Nigericin. Moreover, other HSP90 inhibitors, Radicicol, 17-DMAG and 17-AAG all exhibited similar function as Geldanamycin. Our results indicate that HSP90 is a potential therapeutic target and HSP90 inhibitors may provide therapeutic options to inflammatory diseases where pyroptosis is involved.
Pyroptosis is a type of necrosis like programmed cell death which is triggered by inflammatory stimuli and results in amplifying inflammation [7,20]. First of all, the assembled and activated inflammasome processes nonfunctional pro-IL-1β into bioactive IL-1β, which is a potent pro-inflammatory cytokine [7,21]. Meanwhile, the pores on the plasma membrane formed with activated GSDMD, another substrate of the inflammasome, help to release IL-β out of the cells and stimulate inflammatory signal of other cells. This process simultaneously would release other content of cells, many of which are categorized as DAMPs because of their capacity to stimulate inflammation [7,21]. As pyroptosis is abundantly found in monocytes and macrophages, it may be considered a self-expanding cellular mechanism to exacerbate inflammation [22]. Blocking the signal cascade of pyroptosis is thus probably the key to alleviate inflammatory diseases.
The NLRP3/Caspase-1/GSDMD pathway lies in the center of pyroptosis, and the assembly of NLRP3 inflammasome is an early critical step [23,24]. Therefore, modulating the expression or function of NLRP3 is a potential method to stop pyroptosis. Previous reports show that NLRP3 is a client of chaperon protein HSP90, which helps to form the correct three-dimensional structure [25]. Without HSP90, NLRP3 is probably misfolded and then degraded [15,16]. Consistently, our current study found that HSP90 inhibitors time and dose dependently reduce the protein expression of HSP90, and it is reversed by MG132, a proteasome inhibitor. More importantly, HSP90 inhibitors significantly ameliorate cell death and improve cell viability in LPS plus Nigericin induced pyroptosis, suggesting a promising therapeutic target of HSP90 in pyroptosis and inflammation.
In the present study, we found that MG132 rescued the protein degradation of NLRP3 after HSP90 inhibition. However, because HSP90 is essential for the correct NLRP3 protein folding, it is very likely that NLRP3 goes through misfolding before degradation by the proteasome in the presence of HSP90 inhibitors [15,16]. In such situation, even though MG132 rescues the protein amount of NLRP3, the misfolded protein may not be efficient to assemble inflammasome and mediate pyroptosis. Moreover, MG132 is a well-known inhibitor of NF-κB, which has a complicated regulatory mechanism on inflammasome by both increasing and decreasing its activation [26,27]. The interplay between MG132 and HSP90 inhibitors in pyroptosis warrants a more detailed separate study.
Another intriguing discovery is the involvement of HSP70 in the effect of HSP90 inhibitors on pyroptosis. As another important member of HSP chaperon family, HSP70 is frequently found to be up-regulated when HSP90 function is compromised via inhibitors or genetic modulation, probably as a compensatory response [17]. However, HSP70 was found to regulate NLRP3 in a sharp different way compared with HSP90. Instead of helping folding and stabilizing NLRP3, HSP70 directly binds to NLRP3 and renders it incapable of forming inflammasome in response to inflammatory stimuli [18]. The up-regulation of HSP70 thus makes it more difficult to initiate the NLRP3/Caspase-1/GSDMD pyroptotic pathway. In the present study, we found extremely low expression of HSP70 in THP-1 cells with or without the pyroptotic stimulation. However, all HSP90 inhibitors remarkably increased its expression, providing a possibility to further block pyroptosis via HSP70. Indeed, HSP70 inhibitor Ver-155008 partially reduced the function of Radicicol, suggesting an involvement of HSP70 up-regulation in its effect.
Four HSP90 inhibitors from two distinct structural classes were analyzed in the present study. Among them Geldanamycin, 17-DMAG and 17-AAG belong to class benzoquinone ansamycin polyketide. 17-DMAG and 17-AAG are derived from Geldanamycin by replacing chemical groups on the 17 position. Radicicol, on the other hand, belongs to the class resorcylic acid lactone. The two classes bind to HSP90 ATP/ADP pocket in different ways because of their distinct chemical structure [28]. Albeit their high specificity to HSP90, they also have some off-target effects. For example, 17-DMAG and 17-AAG are found to generate reactive oxygen species (ROS) [29]. In the present study 17-DMAG and 17-AAG drastically reduced the cleavage of Caspase-1 and GSDMD, while the upstream signal NLRP3 was mildly inhibited. This may be a result of some unknown off-target effects. More specific HSP90 inhibitors are to be developed to address the exact mechanisms. HSP90 inhibitors have been reported to alleviate inflammation in vivo in other studies. 17-AAG was found to attenuate LPS induced sepsis and lung injury by inhibiting systemic inflammation [30]. Ganetespib, another HSP90 inhibitor, also improves LPS induced lung injury by suppressing myeloid cell response [31]. In an alcoholic liver disease model, 17-DMAG ameliorates the liver injury by reducing monocyte infiltration and desensitizing liver macrophage to LPS [32]. 17-DMAG also protects streptozotocin-induced diabetic apolipoprotein E-deficient mice from nephropathy and atherosclerosis via suppressing inflammation [33]. Moreover, HSP90 inhibitors also show a protective role in several autoimmunity animal models [34]. Our present study made a connection between HSP90 inhibitors and pyroptosis, an important and powerful contributor of inflammation. Pyroptosis suppression by HSP90 inhibitors may be a mechanism in these in vivo inflammatory studies.
In recent years multiple novel pathways of programmed cell death were discovered. In contrast with apoptosis, some of these pathways manifest necrotic features and enhance inflammation via releasing cell content through the permeabilized cell membrane. Necroptosis, for example, is a type of programmed cell death triggered by death receptors, including Fas, tumor necrotic factor receptors, interferon receptors or toll-like receptors. These receptors go through the caspase-8 signal pathway and activate the RIPK1/3 to eventually phosphorylate and activate the plasma membrane pore-forming molecule MLKL [20,35]. Upon the pore formation in necroptosis, inflammation will be further propagated by the released DAMPs, including HMGB1, ATP and mitochondrial DNA [20,35]. Interestingly, HSP90 is also found indispensable in the signal cascade of necroptosis. Inhibiting HSP90 skews the cell fate from pro-inflammatory necroptosis to anti-inflammatory apoptosis [36]. Added with the current discovery about the role of HSP90 in pyroptosis, HSP90 inhibition may be a potent therapeutic strategy in protecting from the tissue damage raised from overwhelmingly pro-inflammatory cell death.
Conclusion
In the current study, we found HSP90 inhibitors rescue pyroptosis in THP-1 cells induced by LPS plus Nigericin stimulation. HSP90 inhibition compromises the protein stability of NLRP3, a critical component in inflammasome. Up-regulated HSP70 may also be involved in the suppression of pyroptosis.
Acknowledgments
Funding
This work was supported by the National Institute of Health Grant (1R15AI138116 to M.F).
Abbreviation
- HSP90
heat shock protein 90
- HSP70
heat shock protein 70
- LPS
lipopolysaccharides
- GSDMD
gasdermin D
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
- IL-1
interleukin-1
- NLRP3, NACHT
LRR and Pyrin domains-containing protein 3
- DAMP
damage-associated molecular pattern
- 17-DMAG
17-dimethylaminoethylamino-17-demethoxygeldanamycin
- 17-AAG
17-N-allylamino-17-demethoxygeldanamycin
- LDH
lactate dehydrogenase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
Footnotes
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Galkina E and Ley K (2009) Immune and inflammatory mechanisms of atherosclerosis (*). Ann. Rev. Immunol. 27, 165–197 10.1146/annurev.immunol.021908.132620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregor MF and Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Ann. Rev. Immunol. 29, 415–445 10.1146/annurev-immunol-031210-101322 [DOI] [PubMed] [Google Scholar]
- 3.Lambrecht BN and Hammad H (2015) The immunology of asthma. Nat. Immunol. 16, 45–56 10.1038/ni.3049 [DOI] [PubMed] [Google Scholar]
- 4.Silva FA, Rodrigues BL, Ayrizono ML and Leal RF (2016) The immunological basis of inflammatory bowel disease. Gastroenterol. Res. React. 2016, 2097274 10.1155/2016/2097274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock KL, Latz E, Ontiveros F and Kono H (2010) The sterile inflammatory response. Ann. Rev. Immunol. 28, 321–342 10.1146/annurev-immunol-030409-101311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi J, Gao W and Shao F (2017) Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254 10.1016/j.tibs.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 7.Man SM, Karki R and Kanneganti TD (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 277, 61–75 10.1111/imr.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis BK, Wen H and Ting JP (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Ann. Rev. Immunol. 29, 707–735 10.1146/annurev-immunol-031210-101405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Ann. Rev. Immunol. 27, 519–550 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- 10.Miao EA, Rajan JV and Aderem A (2011) Caspase-1-induced pyroptotic cell death. Immunol. Rev. 243, 206–214 10.1111/j.1600-065X.2011.01044.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berges C, Naujokat C, Tinapp S, Wieczorek H, Hoh A, Sadeghi M et al. (2005) A cell line model for the differentiation of human dendritic cells. Biochem. Biophys. Res. Commun. 333, 896–907 10.1016/j.bbrc.2005.05.171 [DOI] [PubMed] [Google Scholar]
- 12.Chanput W, Mes JJ and Wichers HJ (2014) THP-1 cell line: an in vitro cell model for immune modulation approach. Int. Immunopharmacol. 23, 37–45 https://doi.org/10.10167j.intimp.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 13.Bosshart H and Heinzelmann M (2016) THP-1 cells as a model for human monocytes. Ann. Transl. Med. 4, 438 10.21037/atm.2016.08.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayor A, Martinon F, De Smedt T, Petrilli V and Tschopp J (2007) A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat. Immunol. 8, 497–503 10.1038/ni1459 [DOI] [PubMed] [Google Scholar]
- 15.Wu X and Rapoport TA (2018) Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol. 53, 22–28 10.1016/j.ceb.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruggiano A, Foresti O and Carvalho P (2014) Quality control: ER-associated degradation: protein quality control and beyond. J. Cell Biol. 204, 869–879 10.1083/jcb.201312042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudryavtsev VA, Khokhlova AV, Mosina VA, Selivanova EI and Kabakov AE (2017) Induction of Hsp70 in tumor cells treated with inhibitors of the Hsp90 activity: a predictive marker and promising target for radiosensitization. PLoS One 12, e0173640 10.1371/journal.pone.0173640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marline P, Chevriaux A, Derangere V, Apetoh L, Garrido C, Ghiringhelli F et al. (2019) HSP70 is a negative regulator of NLRP3 inflammasome activation. Cell Death Dis. 10, 256 10.1038/s41419-019-1491-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzova JA, Primiano MJ, Jiao A, Stock J, Lee C, Winkler AR et al. (2019) Optimized protocols for studying the NLRP3 inflammasome and assessment of potential targets of CP-453,773 in undifferentiated THP1 cells. J. Immunol. Methods 467, 19–28 10.1016/jjim.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 20.Frank D and Vince JE (2019) Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 26, 99–114 10.1038/S41418-018-0212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergsbaken T, Fink SL and Cookson BT (2009) Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 10.1038/nrmicro2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson N, Ganesan R, Hegedus C, Kovacs K, Kufer TA and Virag L (2019) Programmed necrotic cell death of macrophages: focus on pyroptosis, necroptosis, and parthanatos. Redox Biol. 26, 101239 10.1016/j.redox.2019.101239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H et al. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 24.Swanson KV, Deng M and Ting JP (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 19, 477–489 10.1038/S41577-019-0165-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martine P and Rebe C (2019) Heat shock proteins and inflammasomes. Int. J. Mol. Sci. 20, 4508 10.3390/ijms20184508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J et al. (2016) NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164, 896–910 10.1016/j.cell.2015.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D et al. (2009) Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 10.4049/jimmunol.0901363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitson RR and Moody CJ (2013) Learning from nature: advances in geldanamycin- and radicicol-based inhibitors of Hsp90. J. Org. Chem. 78, 5117–5141 10.1021/jo4002849 [DOI] [PubMed] [Google Scholar]
- 29.Samuni Y, Ishii H, Hyodo F, Samuni U, Krishna MC, Goldstein S et al. (2010) Reactive oxygen species mediate hepatotoxicity induced by the Hsp90 inhibitor geldanamycin and its analogs. Free Radio. Biol. Med. 48, 1559–1563 10.1016/j.freeradbiomed.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee A, Dimitropoulou C, Drakopanayiotakis F, Antonova G, Snead C, Cannon J et al. (2007) Heat shock protein 90 inhibitors prolong survival, attenuate inflammation, and reduce lung injury in murine sepsis. Am. J. Respir. Cit Care Med. 176, 667–675 10.1164/rccm.200702-291OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lilja A, Weeden CE, McArthur K, Nguyen T, Donald A, Wong ZX et al. (2015) HSP90 inhibition suppresses lipopolysaccharide-induced lung inflammation in vivo. PLoS One 10, e0114975 10.1371/journal.pone.0114975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambade A, Catalano D, Lim A, Kopoyan A, Shaffer SA and Mandrekar P (2014) Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury. J. Hepatol. 61, 903–911 10.1016/jjhep.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazaro I, Oguiza A, Redo C, Mallavia B, Madrigal-Matute J, Blanco J et al. (2015) Targeting HSP90 ameliorates nephropathy and atherosclerosis through suppression of NF-κB and STAT signaling pathways in diabetic mice. Diabetes 64, 3600–3613 10.2337/db14-1926 [DOI] [PubMed] [Google Scholar]
- 34.Tukaj S and Wegrzyn G (2016) Anti-Hsp90 therapy in autoimmune and inflammatory diseases: a review of preclinical studies. Cell Stress Chaperones 21, 213–218 10.1007/s12192-016-0670-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi ME, Price DR, Ryter SW and Choi AMK (2019) Necroptosis: a crucial pathogenic mediator of human disease. JCI Insight 4, e128834 10.1172/jci.insight.128834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li D, Li C, Li L, Chen S, Wang L, Li Q et al. (2016) Natural product kongensin A is a non-canonical HSP90 inhibitor that blocks RIP3-dependent necroptosis. Cell Chem. Biol. 23, 257–266 10.1016/j.chembiol.2015.08.018 [DOI] [PubMed] [Google Scholar]