Abstract
Severe acute respiratory syndrome coronavirus (SARS-CoV-2), a novel corona virus, causing COVID-19 with Flu-like symptoms is the first alarming pandemic of the third millennium. SARS-CoV-2 belongs to beta coronavirus as Middle East respiratory syndrome coronavirus (MERS-CoV). Pandemic COVID-19 owes devastating mortality and destructively exceptional consequences on Socio-Economics life around the world. Therefore, the current review is redirected to the scientific community to owe comprehensive visualization about SARS-CoV-2 to tackle the current pandemic. As systematically shown through the current review, it indexes unmet medical problem of COVID-19 in view of public health and vaccination discovery for the infectious SARS-CoV-2; it is currently under-investigational therapeutic protocols, and next possible vaccines. Furthermore, the review extensively reports the precautionary measures to achieve" COVID-19/Flatten the curve". It is concluded that vaccines formulation within exceptional no time in this pandemic is highly recommended, via following the same protocols of previous pandemics; MERS-CoV and SARS-CoV, and excluding some initial steps of vaccination development process.
Keywords: COVID-19, SARS-CoV-2, Flatten the curve, Vaccination, Viral pandemic, Infectious diseases
Introduction
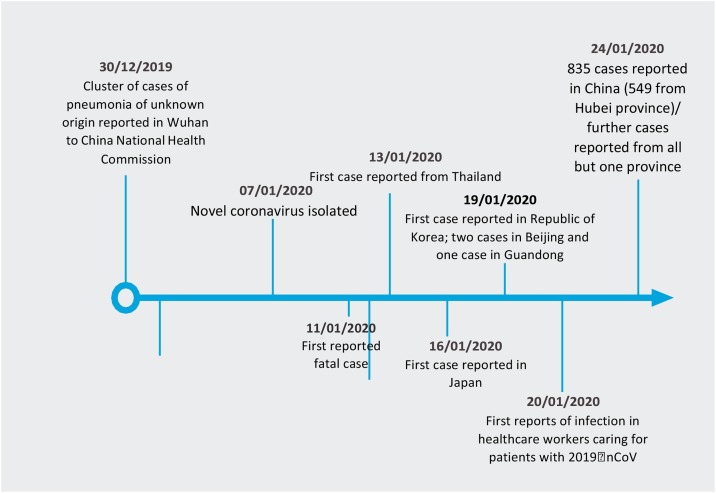
On 31 December 2019, World Health Organization (WHO) was alerted to a cluster of pneumonia patients in Wuhan City, Hubei Province of China. One week later, on 7 January 2020, Chinese authorities confirmed that they had identified a novel (new) coronavirus as the cause of the pneumonia (Chart 1 ). The proposed interim name of the virus is 2019 nCoV [1].
Chart 1.
Timeline stages of COVID-19 outbreak.
Since the first cases were reported, WHO and its partners have been working with Chinese authorities and global experts to learn more about the virus, including how it is transmitted, the populations most at risk, the spectrum of clinical disease, and the most effective ways to detect, interrupt, and contain ”human to human” transmission [2].
This strategic preparedness and response plan outline the public health measures that the international community stands ready to provide to support all countries to prepare for and respond to 2019‑nCoV. The document takes what was learned so far about the virus and translates that knowledge into strategic action that can guide the efforts of all national and international partners when developing context-specific nationally and regionally operational plans [3].
The world is grappling with an unprecedented pandemic. People across the globe fear the impact on the health of individuals and communities, and the global economy is taking a significant hit [4]. As COVID-19 continues to spread, healthcare organizations (HCOs) and Healthcare professionals (HCPs) are doing their parts, which includes not only developing vaccines and treatments, but also keeping the public informed about the work underway [5]. All HCPs and HCOs are committed to supporting patients, healthcare workers, and healthcare authorities as They face this unprecedented health crisis [6]. The top priority is to continue making and delivering medicines that patients rely on while protecting employees, their families, and communities [7]. Equally important, we must ensure that the lessons, we are learning during this pandemic, help spark positive, lasting changes, and stronger health systems [8].
Viruses mutate and can sometimes be harmless or sometimes be dangerous, like COVID-19. A vaccine needs to be tailored made to that strain. Developing a vaccine from scratch takes years, but we can leverage what we have learned from previous efforts and potentially shorten the development timeline [9].
Systematically, the current review discusses COVID-19; in view of viral background, diagnostic symptoms, proposed therapeutic protocols, socio-economic risks of COVID-19, public health manners, COVID-19 progression, the potential role of nanotechnology in COVID-19 therapy, and vaccination promises (Fig. 1 ).
Fig. 1.
Flowchart of the review index.
Background on viruses
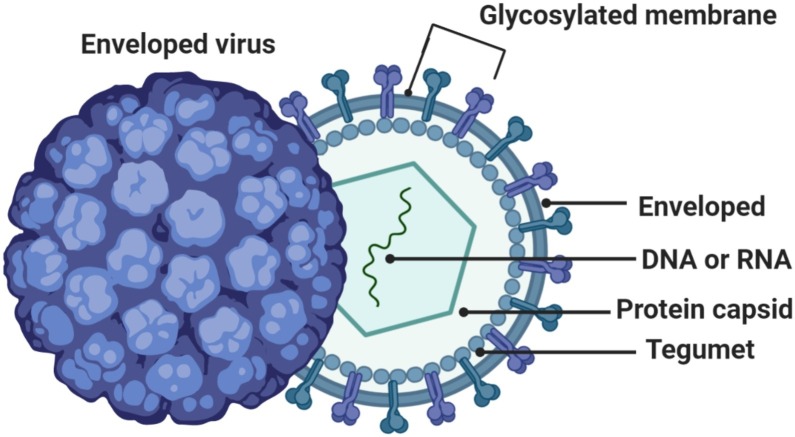
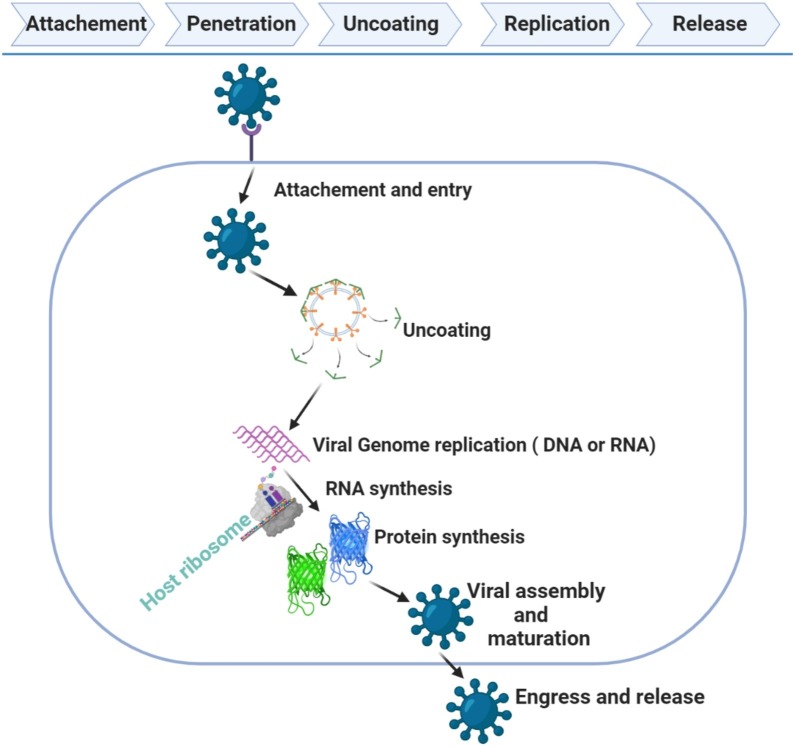
A virus is a microorganism that functions and replicates only inside living cells of other organisms (host cells). Viruses replicate by utilizing part of the host cell’s cellular mechanisms. The specific mechanism depends on multiple factors, such as the individual virus and cellular tropism; ”How viruses have evolved to target specific hosts, tissues, or cell types?” (Fig. 2, Fig. 3 ) [10,11].
Fig. 2.
Structure of a Virus.
Fig. 3.
Lifecycle of a Virus.
Background on coronavirus
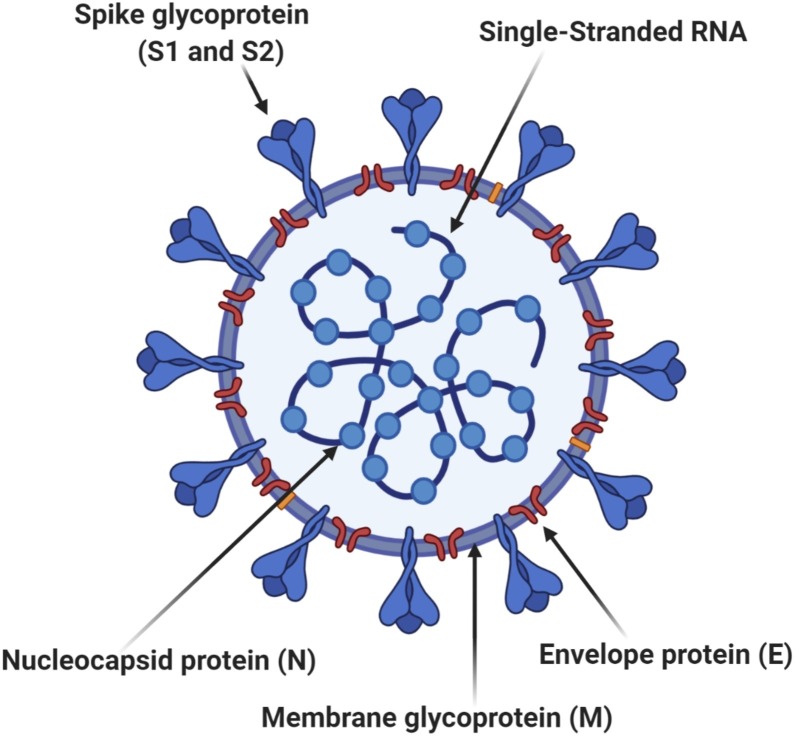
Coronavirus is derived from the Latin corona meaning crown or halo, which refers to the characteristic appearance of virions (the infective form of the virus) by electron microscopy. The morphology is created by the viral spike that determines host tropism. Coronaviruses are enveloped viruses with a positive-sense single- stranded RNA genome (Fig. 4 ). In humans, coronaviruses cause respiratory tract infections that are typically mild, such as some cases of the common cold. Rarer forms such as SARS, MERS, and SARS-CoV-2 can be lethal, typically due to respiratory failure, sepsis, and renal failure [12,13].
Fig. 4.
Cross-sectional Model of Coronavirus.
Background on COVID-19
COVID-19 is a respiratory illness caused by a newly identified coronavirus, SARS-CoV-2 [14,15]. The current COVID-19 outbreak originated in Wuhan, China, in late 2019 [16]. World Health Organization (WHO) has been to characterized the outbreak as a pandemic on 11 March 2020 [17]. The worldwide COVID-19 caseload is rising rapidly on a daily basis, particularly outside of mainland China, where the epicenter of the virus is shifting from China to other regions of the world; United states of America, Brazil, Iran, Europe, South Korea, etc. [18].
Symptoms of COVID-19 vary by case but typically include fever, dry cough, and fatigue as flu-like symptoms (Table 1 ) and some significant symptomatic difference (Table 2 ). Some patients may experience myalgia, nasal congestion, sore throat and/or diarrhea.9 No neurologic symptoms specific to COVID- 19 infection have been reported to date. An onset of symptoms is typically gradual and worsens over time; however, not all infected patients will feel ill or become symptomatic [9].
Table 1.
Similarities Between COVID-19 and the Flu [15].
| Symptoms | Can cause fever, cough, body aches, fatigue, and sometimes GI symptoms. Can be mild or severe, rare fatal cases. Can result in pneumonia. |
|---|---|
| Transmission | Can be spread through droplets in the air (coughing, sneezing, and talking). |
| Treatment | Not treatable with antibiotics. May be treated by addressing symptoms, such as reducing fever. Severe cases may require hospitalization and support such as mechanical ventilation. |
| Prevention | Preventable by frequent, thorough hand washing, coughing into the crook of your elbow, staying home when sick and limiting contact with people who are infected. |
Table 2.
Differences Between COVID-19 and Flu [15].
| Cause | COVID-19: Caused by the novel 2019 coronavirus, now called, SARS-CoV-2. Flu: Caused by any of several different types and strains of influenza. |
|---|---|
| Transmission | Possible difference: COVID-19 might be spread through the airborne route. |
| Antiviral Medications | COVID-19: None currently. Flu: some available; can help manage symptoms/duration. |
| Vaccines | COVID-19: None currently. Flu: vaccine available. |
| Incubation Period | COVID-19 incubation period seems to be ∼14 days. Flu incubation period is 2 ∼ 3 days. |
Research to date suggests that COVID-19 is transmitted primarily through contact with respiratory droplets in the air and on surfaces [18]. There appears to be increased burden of disease with COVID-19 compared to the flu, with potentially higher morbidity based on current numbers. The WHO reports a global mortality rate of 3.4% as of 12 March 2020 [19] making COVID-19 more lethal than most strains of seasonal influenza, which are associated with mortality rates of 0.1−0.2% [20]. However, it is important to note that this is a novel virus and the N of total infected worldwide is not currently known; therefore, additional data are needed to quantify the mortality rate relative to risk following infection. Additionally, some epidemiologists hypothesize that the true CFR of COVID-19 is lower than that reported by the WHO due to overall undercounting/underreporting of COVID-19 cases (because of either mild or unrecognized disease or a lack of available testing [21].
Current data also suggest that risk of complications and death from COVID-19 increases with age and in the presence of comorbidities such as cardiovascular disease, chronic respiratory disease, diabetes and cancer [21,22].
Risk communication and management
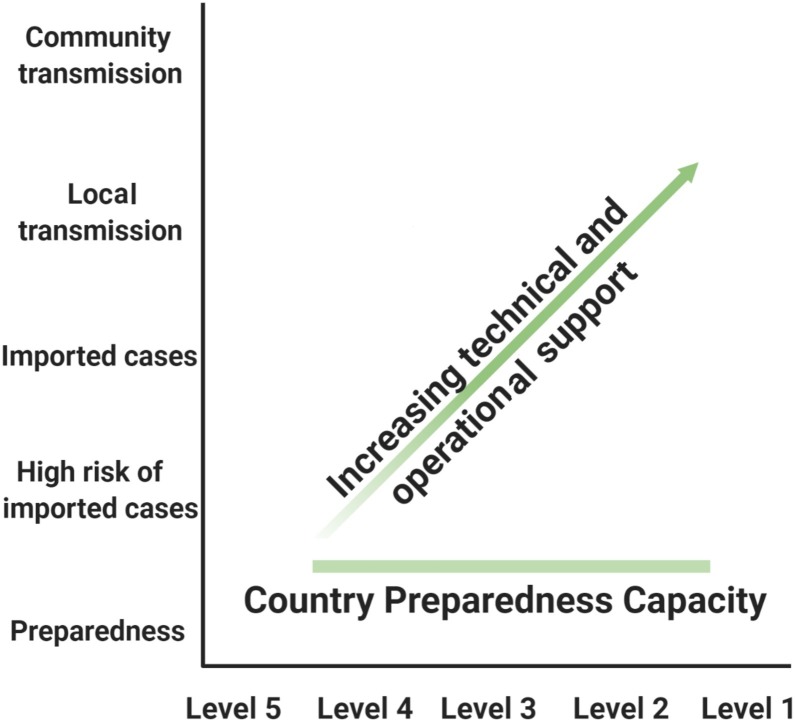
Due to the high demand for timely and trustworthy information about 2019-nCoV, WHO technical risk communication and social media teams have been working closely to track and respond to myths and rumors (Fig. 5 ) [16].
Fig. 6.
Development process of the nanobody vaccination [110].
Fig. 7.
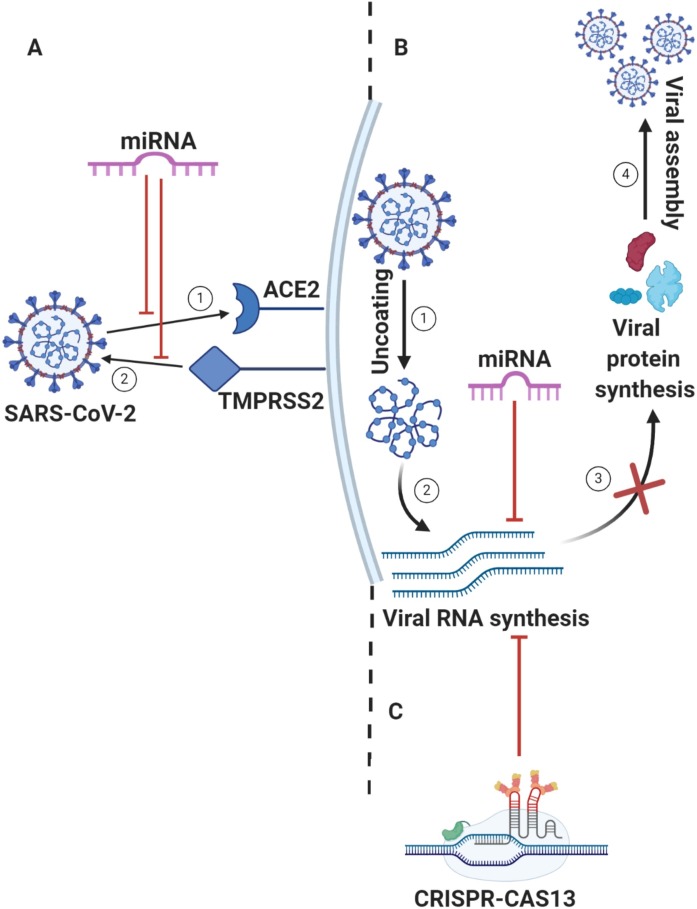
miRNA-based therapy as anti SARS-CoV-2 via: A) Viral proteins crucial in survival and replication of SARS-CoV-2; miRNA targets 1) ACE2 or/and 2) TMPRSS2 which consequently suppress the viral entry to the host cells, B) Host factors of cellular entry and trafficking of SARS-CoV-2; after the virus enters the host cells, it starts 1) Uncoating stage for its RNA release, for 2) Viral RNA synthesis by replication machinery system. Subsequently, 3) Viral protein synthesis of structural and non-structural proteins for 4) Viral assembly and maturation; to stop these stages, miRNA suppress viral protein synthesis by targeting viral RNA synthesis. Eventually, it results in suppression of viral assembly and maturation. C) CRISPR-based therapy; it acts via degradation or mutation of viral RNA.
Fig. 5.
Country risk and vulnerability mapping.
WHO and partners are working 24 h a day to identify the most prevalent rumors that can potentially harm the public’s health, such as false prevention measures or cures. These myths are then refuted with evidence based information. WHO and partners are making public health information and advice on the 2019‑nCoV, including MythBusters, available on social media channels including; Weibo, Twitter, Facebook, Instagram, LinkedIn, Pinterest, and organizational websites [16].
In addition, an expanding group of global response organizations such as the United Nations Children’s Fund (UNICEF) and the International Federation of Red Cross and Red Crescent Societies (IFRC) are coordinating efforts with WHO to ensure that biomedical recommendations can be applied at the community level. These organizations are active at the global, regional, and country level to ensure that affected populations have a voice and are part of the response. Ensuring that global recommendations and communication are tested, adapted, and localized will help countries better control the 2019-nCoV outbreak [23].
Socio-economics consequences of COVID-19
Dramatically, Pandemics such as COVID-19 display catastrophic consequences to the potentiality of the global economies; insurance industry, healthcare systems; to uncover the dually risky crosstalk of COVID-19/Socio-economics consequences [24], as follows; currently, World is facing extraordinary challenges; community quarantines, lockdowns and restrictions of travel. These precautionary measurements have wide economic and social effects. It is reported that strong policies and active actions of some countries demonstrated COVID-19 efficiently and effectively, while huge challenges have been faced by the poor countries that have not good healthcare system. Diseases that are infectious like tuberculosis, malaria and non-communicable like cancer and cardiovascular are high in countries that have low to medium income. Therefore, these countries are more prone to manmade and natural disasters. Lack of coronavirus spread control becomes risk of millions lives [25]. COVID-19 leaves a great impact on the industries. Currently, graph of economic decrease in term of profit, market share, and stable assets while increase for claims for both life and non-life insurance companies which have increased during this pandemic [26].
COVID-19 impact on immigrants
Poverty, lack of affordable, readily available healthcare that is especially relevant during COVID-19 pandemic. Early diagnosis and surveillance of people with COVID19 are important both for maximizing the recovery of the individual patient and for preventing further transmission to the population. There are also disadvantaged immigrants who are under- or uninsured; mostly, immigrants live in a same home or many people lives in a same room. So, there are many chances of spread of COVID-19. Thence, they have a limited proficiency of English language. Therefore, they cannot read the public services massages [27]. Additionally, immigrants play a significant role in the economy by paying taxes; Individual Taxpayer Identification Numbers (ITINs) is assigned to individual. Due to pandemic, individuals did not get benefit any COVID-19 related relief. There is much concern that the COVID-19 pandemic will result in particularly high rates of unemployment and financial strain within immigrant communities. It also affects the people who have jobs of low wage. Workers that were connected with the service of immigrant have badly affected, for the stop of negative impact of COVID-19 on tourism and travel sector. Due to the fears that COVID-19 is transmitted to the workers of food service and immigrants, hotels apply a significant effect on the physical and mental health [28].
COVID-19 impact on employment
Labors have global concerns which are affected by COVID-19. People, having low income jobs, absence of insurance, a limited access of healthcare, and lack of backup savings, are at risk. This implies that already disadvantaged groups will suffer disproportionately from the adverse effects. Policy makers in the Global South have responded to the adverse employment and income effects of the pandemic with a range of measures; cash transfers to the poor and loans to small enterprises to keep them financially afloat [29,30].
COVID-19 with household and poverty
Interruptions in business and shutdowns for the measures of social distance due to COVID-19 pandemic lead to severe economic shock throughout the World. For the estimation of COVD-19 impact on saving, poverty, consumption and the income of household a model is developed known as “A microeconomic model”. It assumes two periods; 1 st is a crisis period, 2nd is a recovery period. This is a first step for the quantification of household scale impacts of COVID-19 to a regional level. This will help to discover the effects that are created by indirect macroeconomic, uncertainty that is in households and exogenous shocks which effects simultaneously [31,32].
Infection prevention and control
Infection prevention and control (IPC) measures are absolutely essential to ensure healthcare workers are protected from infection with 2019‑nCoV and amplification events in healthcare facilities. An Infection prevention and control (IPC) programme at national and facility level with a dedicated and trained team, or at least an IPC focal point, should be in place and supported by the national authorities and facility senior management [33].
In countries where IPC is sub‑optimal or limited, partners will need to support national authorities to ensure that at least minimum requirements for IPC are in place as soon as possible, both at the national and facility level, and to gradually progress to achievement of all requirements of the IPC core components according to local priority plans [34].
Partners should support national authorities to undertake a risk assessment of IPC capacity at all levels of the healthcare system (includes availability of triage and appropriately ventilated isolation rooms) and, on the basis of this assessment, define a referral pathway in collaboration with case management capacity. Particular attention should be given to ensuring IPC compliance with basic IPC principles at the first point of care (usually primary care) [3].
Capacity for triage, early recognition, standard precautions, isolation capacity, and referral procedures should align with WHO IPC guidance in 2019‑nCoV. If supplies are needed to implement recommended protocols (e.g., hand hygiene resources, personal protective equipment, environmental cleaning, and waste management), partners should assist national authorities in procurement and supply where appropriate [35].
Patterns and disruption in drugs Purchase
The risk of shortages of several essential medicines has been increased; it raised the alarm due to COVID-19 pandemic. Contextually, China manufactures raw materials “active pharmaceutical ingredients”, the basic molecules used to make drugs for many key drugs such as antibiotics and statins, and then exported to high populous countries such as India for being formulated into tablets or injections, for sale worldwide. However, these manufacturing capacities have been disrupted by efforts to contain the virus [36,37].
As the manufacture process in China is privileged by being cheap, 70% of the raw materials, procured by the Indian companies, are sourced from China [38]. Notably to be said that during the Chinese COVID-19 epidemic, a significant disruption has been confronted by the Chinese pharmaceutical factories, as a consequence of the Chinese precautionary measures; workers were quarantined and transport between factories was disrupted. It resulted in drastic decline in the Chinese capacities to produce the pharmaceutical raw materials. Hence, the Indian companies have lost its productivity power to formulate and export the same volume of medicines [36]. As a consequent response to shortages, India has taken a decision to prioritize its people with essential medicines first. Recently, India forbidden the exports of the key medicines; antibiotics, statins, and paracetamol. This could wreak havoc, in the long-term, on the National Health Service (NHS) supply line. Despite some Chinese factories have reactivated their productivity capacities, it is not clear when production will be fully restored, or when India will lift its restrictions on drug exports.
In the short-term result of no-deal Brexit planning, the NHS has reserved a supply of drugs to stop shortages from occurring. However, these contingency plans will not persist forever [36]. Past drug shortages were investigated, albeit on a smaller scale; in 2008, there were shortages of raw materials in China, after Chinese factories were closed near Beijing for three weeks during the Olympic Games. In 2019 an epidemic of swine flu in China depleted the global supply of heparin, an essential medicine to prevent heart attacks. In Puerto Rico, Hurricane Maria damaged another factory making heparin, which disrupted supplies of the drug in 2017 [36].
The impacts of COVID-19 pandemic will persist for months or for years. This will expose the vulnerabilities of our drug supply chain. We need to get a new application of new stress tests to the drug supply chain; ”How will the health authorities cope if Chinese supplies are continually disrupted?”, and ”How will it be affected by disruptions to supplies of drugs from other countries, such as Italy?”. For each essential medicine, ”are there alternative suppliers in other countries?”, and ”Are there alternative drugs that patients could take if one drug is no longer available?”. As a previous experience, stress tests were applied to the banking system after the financial crisis of 2008. Our supplies of medicines are equally pivotal and should be considered in similar ways [36].
Instead of relying on imports, countries should re-evaluate the case for manufacturing more medicines Locally, specially countries which have long history of pharmaceutical development [36]. During the Second World War, Glaxo Laboratories in the UK manufactured 7.5 billion tablets of penicillin a year. Now, however, both the UK and US depend on supplies of penicillin from China [36]. The COVID-19 crisis is a chance to do capitalism differently [36].
Human trials for a coronavirus vaccine have been launched in April 2020. However, before a vaccine is ready, we will need to ensure that key drugs to treat the symptoms of coronavirus are available for everyone who needs them. Pilot studies from France and the US suggest that hydroxychloroquine can lessen the severity of coronavirus infection. A clinical trial in Shanghai is currently evaluating whether pirfenidone could improve lung function for people with severe disease. More definitive clinical trial results of these and other drugs are expected in May and June [36].
These drugs are already being manufactured in other countries. But the acute worldwide demand for drugs to treat coronavirus could easily outstrip supply. In times of shortage, other countries could stockpile their supplies to treat their own people. Like India, they may be unwilling to export drugs to the UK [36].
Supply chains are only as strong as their weakest links. If new treatments for coronavirus emerge in the next few months, the UK cannot afford to be the last link in a vulnerable chain. The government has already requested that UK companies manufacture more ventilators and protective masks. It should do the same with the UK pharmaceutical industry, repurposing factories to produce drugs that help treat coronavirus and any other essential medicines at risk of shortage [36].
Treatment options of COVID-19
While a vaccine could be more than a year away to be available, investigating repurposing products “New use of old drugs” for the management of COVID-19, with the appropriate regulatory authorities (Table 3 ) [[39], [40], [41], [42]], as follows:
Table 3.
A list of repurposing drugs (FDA approved, under-clinical trials and new hypothetical approaches) of the treatment options as anti-COVID-19; in view of the drugs main indication/the hypothetical repurposing indication as anti- COVID-19.
| Repurposing products | Main indication/Expected mechanism of action as anti SARS-CoV-2 (Repurposing indication) | Status in COVID-19 management | Refs |
|---|---|---|---|
| VEKLURY® (Remdesivir) |
An adenosine analog with antiviral activity. A hypothetical mechanism of antiviral action of remdesivir is its incorporation into the nascent RNA chain of the virus causing a prematurity for viral cycle termination; it is a post virus entry stage. | FDA approved | [43,44] |
| Aviptadil | A vasoactive intestinal polypeptide (VIP) administered to treat erectile dysfunction/ Block inflammatory cytokines. | FDA approved | [45,46] |
| Dexamethasone | A synthetic potent long-acting broad-spectrum corticosteroid for immune system disorders. Repurposing as anti-COVID-19 (Hypothesis): Potential anti-inflammatory containing the cytokine storm related to COVID-19. |
FDA approved | [[47], [48], [49]] |
| ACTEMRA® (Tocilizumab) KEVZARA® (Sarilumab) SYLVANT® (Siltuximab) |
-Interleukin-6 (IL-6) antagonists blocking the binding of IL-6 (a pro-inflammatory cytokine) to IL-6 receptors resulting in the suppressing of T cell activation, promoting of immunoglobulin secretion, initiation of hepatic acute-phase protein synthesis, and proliferation, differentiation, and stimulation of hematopoietic precursor cell. -Indicated for adults suffering from moderately to severely active rheumatoid arthritis (RA) with an inadequate response or intolerance to ≥1 disease-modifying antirheumatic drugs (DMARDs). Repurposing as anti-COVID-19 (Hypothesis):IL-6- receptor inhibitors; a key cytokine resulting to an inflammatory storm increasing alveolar-capillary blood-gas exchange dysfunction, especially impaired oxygen diffusion, and eventually cause pulmonary fibrosis and organ failure. It has a potential effect of an improvement of immune damaging, lung functional injuries and arterial oxygen saturation. |
Under clinical trials | [[50], [51], [52]] |
| Chloroquine Plaquenil® (Hydroxychloroquine) |
Strong Immunomodulant antimalarial agents with broad-spectrum antiviral activity. Hydroxychloroquine is a common drug in many autoimmune disorders; lupus and rheumatoid arthritis. Repurposing as anti COVID-19 (Hypothesis): Expected to act as anti-COVID-19 by: - Inhibiting pH-dependent viral fusion and replication. - Inhibiting viral assembly in endoplasmic reticulum-Golgi intermediate like structures. - Its immunomodulatory effect through cell signaling pathways and regulating the action of pro-inflammatory cytokines that can enhance its antiviral effect synergistically |
Under clinical trials | [[53], [54], [55]] |
| Corticosteroids and NSAIDs (Ibuprofen) |
Non-selective, reversible inhibition of the cyclooxygenase enzymes COX-1 and COX-2, for the treatment of rheumatoid arthritis (RA) and juvenile rheumatoid arthritis (JRA), juvenile idiopathic arthritis (JIA), pain and fever. Repurposing as anti-COVID-19 (Hypothesis): Used in the management of SARS/MERS-CoV outbreaks via Inhibiting the immune response and so the clearance of COVID-19 could be delayed. Corticosteroids have the disadvantage: - Increasing incidence of secondary bacterial and fungal infections. - Suppressing inflammatory response, highly responsive to the lung damage and acute respiratory distress syndrome (ARDS) during viral infection. |
Under clinical trials | [56,57] |
| DUPIXENT® (Dupilumab) | Fully human monoclonal antibody of an interleukin-4 receptor alpha-antagonist inhibiting IL-4 signaling via the Type I receptor; IL-4 and IL-13 signaling via the Type II receptor resulting in declined IL-4 and IL-13 cytokine-induced responses of the release of pro-inflammatory cytokines, chemokines, and IgE, indicated for the treatment of adult patients suffering from moderate-to-severe atopic dermatitis. Repurposing as anti-COVID-19 (Hypothesis): Via inhibiting IL-4 and IL-13, It is expected to reverse the following immunological reactions which result in lung impairment due to COVID-19: -CD4 + T and CD8 + T cells display a potential antiviral activity via balancing the reaction against SARS-CoV-2. CD4 + T cells induce the production of virus specific antibodies by promoting T-dependent B cells, while CD8 + T cells are cytotoxic killing cells infected by the virus. -Infection promotes T cells to differentiate in various subsets; T-helper1 (Th1), Th17, and subsequent massive release of pro-inflammatory cytokines; interleukin (IL)-1, IL-6, IL-8, IL‐21, TNFβ, and MCP1. The high production of these mediators due to viral persistence (cytokine storm) results inhibiting CD8 + T cells activation. |
Under clinical trials | [[58], [59], [60]] |
| Nafamostat-Mesilate and Gabexate-Mesilate |
A synthetic serine protease, used in trials studying the prevention of Liver Transplantation and Post-reperfusion Syndrome, and as an anticoagulant therapy for patients of continuous renal replacement therapy due to acute kidney injury Repurposing as anti-COVID-19 (Hypothesis): Blocking TMPRSS2-dependent host cell entry of SARS-CoV-2, where the SARS-CoV-2 spike protein (S) undergoes endocytosis into the viral envelope and mediates viral entry into cells; it depends on the cellular enzyme transmembrane protease serine 2 (TMPRSS2) cleaving and promote the S protein. SARS-CoV. Hence, TMPRSS2 is crucial for spread of SARS-CoV. |
Under clinical trials | [[61], [62], [63]] |
| KALETRA® (Lopinavir/Ritonavir) |
Protease Inhibitor; inhibitors of Gag-Pol polyprotein precursor's cleavage causing the formation of immature, noninfectious viral particles where ritonavir in combination with lopinavir act as a pharmacokinetic enhancer by inhibition of lopinavir inactivation metabolism, indicated as anti-HIV. Repurposing as anti-COVID-19: Expected to be anti-protease of SARS-CoV-2, via: Inhibition of the viral activity of 3CLpro, thereby suppressing the process of viral replication and release from host cells |
Under clinical trials | [[64], [65], [66]] |
| Ribavirin | - A nucleoside antihepaciviral agent (anti- hepatitis C virus); Antiviral action against RNA and DNA viruses; inhibition of inosine monophosphate dehydrogenase (IMPDH) cellular protein diminishing intracellular GTP which inhibits RNA replication of viral genomes, so viral growth might be disrupted. - Alternatively, its immunomodulatory effects are by suppression of IL-10. Inhibition of RNA polymerase activity and therefore inhibition of initiation and elongation RNA fragments are inhibited, so viral protein synthesis could be inhibited. |
Under clinical trials | [67,68] |
| Azithromycin | A macrolide antibiotic, indicated for community-acquired pneumonia, pharyngitis/tonsillitis/Expected to decrease the virus entry into cells. | Under clinical trials | [69] |
| Interferon alpha (IFN-α) |
Inhibitor of various stages of viral replication; viral entry, uncoating, mRNA synthesis and protein synthesis, used for hepatitis management. | Under clinical trials | [[70], [71], [72], [73]] |
| Teicoplanin | A glycopeptide antibiotic/Booster for the endosomal pH inhibiting low-pH viral cleavage of spike protein in endosome by Cathepsin L; it results that genomic RNA release and replication of viral life cycle are avoided. | Under clinical trials | [[74], [75], [76]] |
| Favipiravir | RNA-dependent RNA polymerase (RdRp) inhibitor. It displays antiviral effects against variant RNA viruses; Coronaviruses and Influenza viruses via being metabolized to an active form, favipiravir-RTP, as a substrate for viral RNA polymerases. Via binding of favipiravir to viral RNA polymerase, RNA polymerase activity would be inhibited. | Under clinical trials | [77,78] |
| Arbidol® (Umifenovir) |
A broad-spectrum antiviral effective agent against enveloped and non-enveloped RNA or DNA viruses; influenza virus type A and B, respiratory syncytial virus, SARS-CoV, adenovirus, HCV. Repurposing as anti-COVID-19 (Hypothesis): Expected to inhibit viral fusion with targeted membrane preventing the viral entrance to targeted cells. |
Under clinical trials | [[79], [80], [81]] |
| Vitamin C Ascorbic acid |
An antioxidant agent acting as a co-factor of many physiological reactions for immune augmentation; high dose intravenous (IV) vitamin C would be A potential agent in sepsis and septic shock management. Repurposing as anti-COVID-19 (Hypothesis): High dose vitamin C has dual effects; - A pro-oxidant agent for immune cells and as an antioxidant agent for lung epithelial cells. - Inhibition of lactate secretion from immune cells preserving the innate immunity of alveolar epithelial cells type II. |
Under clinical trials | [[82], [83], [84], [85]] |
| Ivermectin (Stromectol) |
A macrocyclic lactones (semi-synthetic derivative of avermectins) causing an influx of Cl- ions via the cell membrane of invertebrates by induction of specific ivermectin-sensitive ion channels; it results in hyperpolarization leads to muscle paralysis treating; nematode parasites, arthropod ectoparasite infestations such as scabies. Repurposing as anti-COVID-19 (Hypothesis): Suppressing the importin (IMP) α/β receptor; this is crucial for transmitting viral proteins into the host cell nucleus. |
Under clinical trials | [[86], [87], [88], [89]] |
| Kineret (Anakinra) |
Blockade of IL-1α and IL-1β act via IL-1 receptor 1 to induce the inflammatory cytokines and TNFα production; it results in the inflammatory cascade. The inflammasome is a complex of distinct proteins converting inactive prointerleukin-1β to active IL-1β, indicated for declining symptoms and delaying the progression of structural damage active rheumatoid arthritis (RA) Repurposing as anti-COVID-19 (Hypothesis): The SARS-CoV-2 causes epithelial damage resulting in the release of IL-1α; it recruits neutrophils and monocytes to the infectious site activating IL-1β in monocyte/macrophages. Additionally, the SARS-CoV-2 induces pro-IL-1β in monocyte/macrophages which induce consequently more IL-1 which recruits and induces further innate immune cells; This autoinflammatory loop, where IL-1α and IL-1β, can promote production and release of further IL-1 has to be regulated as an ongoing loop will induce and recruit furthr innate immune cells independent of the initially primary trigger. Anakinra blocks the IL-1 receptor (IL-1R). Hence, it prevents autoinflammation via blockade activity of IL-1α released from dead epithelial cells, and IL-1β produced by immune cells. IL-1-induced IL-6 will also be suppressed. The autoinflammatory loop can exacerbate from increase innate immune response into uncontrolled macrophage activation syndrome (MAS) a spectrum that associates with increasing ferritin levels. |
Under clinical trials | [[90], [91], [92], [93], [94]] |
| Jakafi® (Ruxolitinib) |
A selective monoclonal antibodies targeting IL-6 and suppressor of JAK1 and JAK2, for the treatment of myelofibrosis (MF), polycythemia vera, and steroid-refractory acute graft-versus-host disease (SR-aGVHD). Repurposing as anti-COVID-19 (Hypothesis): Via inhibitory activity on the cytokine storm associated with COVID-19 given that IL-6. |
Under clinical trials | [[95], [96], [97]] |
| Colchicine | Tubulin-Colchicine Complex; an inhibitor of mitosis and microtubule assembly bound to soluble, non-polymerized tubulin heterodimers to formulate a tubulin-colchicine complex. It interferes with microtubule formation and elongation, and colchicine induces microtubule de-polymerization, indicate for gouty arthritis. Repurposing as anti-COVID-19 (Hypothesis): Coronaviruses, members of the Nidovirales order, are enveloped; positive-sense, single-stranded RNA viruses, redirecting and readjusting host cell membranes for custom as part of the replication of viral genome and transcription; it moves in the cell in a pattern of correspondence to microtubule-associated transport, activating the formulation of double-membrane vesicles in infected cells. The infected cells by coronaviruses include the interaction of the cytoplasmic tail of the spike protein with cytoskeletal proteins; It results in viral entry. Additionally, microtubules are involved in the transport and assembly of spike proteins into virions during the replication cycle. The colchicine-tubulin complex may block viral entry and replication. |
Under clinical trials | [[98], [99], [100], [101], [102]] |
| Convalescent Plasma (FDA approved) | |||
| -Suppressing viremia by antibodies. -Antibody-dependent cellular cytotoxicity, complement activation and phagocytosis (ADCP). -Modification of inflammatory response, accomplished during the early response. -Suggestion: apart from the direct anti-viral properties, plasma components can provide other beneficial actions; restoring coagulation factors. |
[[103], [104], [105], [106], [107]] | ||
| Nano-bodies Therapy (Under clinical trials) Naturally arising single-domain antibody fragments isolated from camelid/ alpaca / llamas variable heavy-chain (VHH) antibodies; they display a uniqueness of biophysical properties of small size and thermo-stability, which permit nebulized administration (Fig. 6). | |||
| An alpaca nano-body | Isolated from an alpaca-derived single domain antibody fragment, Ty1; with specificities to receptor binding domain (RBD) of the SARS-CoV-2 spike, directly suppressing ACE2 engagement, bound to the RBD, obstructing ACE2; it binds to an epitope on the RBD accessible in both the ‘up’ and ‘down’ conformations, sterically lagging RBD-ACE2 binding. Ty1 neutralizes SARS-CoV-2 spike pseudo-virus as a 12.8 kDa nano-body. | [[108], [109], [110], [111]] | |
| MicroRNAs/ siRNA CRISPR/Cas13 |
Nucleic acid-based therapy MicroRNAs/CRISPR -Class of non-coding RNAs of RNAs 18 ∼ 25 nucleotides (nt) in length, with potential roles in post-transcriptional regulation of gene expression via complementary binding to the 3′-untranslated regions (3′-UTRs) of target gene mRNA to promote mRNA degradation and translational repression resulting in protein biosynthesis inhibition. -Clustered Regularly-Interspaced Short Palindromic Repeats (CRISPR) has adapted from the prokaryotic adaptive immune system CRISPR-associated (Cas) system, used as a novel and specific genome editing tool for other organisms. |
[[112], [113], [114], [115], [116]] | |
| Mechanism of action as anti-COVID-19 (Hypothesis), via: Host factors of cellular entry and trafficking of SARS-CoV-2 (Fig. 7A). Viral proteins crucial in survival and replication of SARS-CoV-2 (Fig. 7B). -ACE2 and TMPRSS2 are crucial receptors identified to facilitate the activation and binding of SARS-CoV-2, and its entry to the host cell; host miRNA 27b regulates ACE2 and viral miRNA 147–3p targets TMPRSS2, in case that therapeutic miRNAs can be delivered to the cells, binding of SARS-CoV-2 spike protein to these receptors can be suppressed. -Designing a synthetic miRNA complemented to 3′ translated region (3′ UTR) of Open Reading Frame (ORF-9) of SARS-CoV-2 at encoding a nucleocapsid phosphoprotein (N); it acts a key functional role both structural and non-structural roles in infection via forming the viral capsid and interacts with the viral membrane protein during assembly. Hence, it is hypothetical that targeting this gene may suppress the process of viral particle assembly. -Predictively, Six host miRNAs; let-7a, miR-101, miR-126, miR-23b, miR-378, and miR-98 act as anti-SARS-CoV-2 target genes of nonstructural protein (nsp), nucleocapsid and spike glycoprotein. -Via computational design of specific guide-RNA complementary to a sequence site of the viral RNA, CRISPR displays potentiality of Inhibition of viral function and replication via directly targeting and cleaving all viral positive-sense RNA of non-structural proteins (NSP1-NSP16), accessory proteins, and ORF1−10, and ORF1a/b (Fig. 7C). |
|||
Chloroquine and Hydroxychloroquine: promises and doubts
There has been increased interest by local governments to utilize Plaquenil® (Hydroxychloroquine) as a potential treatment for COVID-19, based on preliminary results from independent studies from countries such as China and France [117]. Plaquenil® has current indications in more than 60 countries [118]. For the Preliminary Study Results; to date, there is insufficient clinical data to draw any final conclusions over the clinical efficacy or safety of hydroxychloroquine (or Chloroquine) in the management of COVID-19. The preliminary results from different independent studies require further analysis and more robust and larger clinical studies to confirm the patient benefit/risk profile of Plaquenil® in COVID-19 [119].
Controversy about Chloroquine in the treatment of Covid-19
Antimalarial drug, chloroquine (CQ) has been shown to have potential anti-SARS-CoV-2 activity and has achieved promising results in clinical treatments [120,121]. Due to its anti-viral properties, CQ has been suggested for the treatment of COVID-19 in many regions and countries [122]. Medical and research field cannot formulate a final decision about Chloroquine in the treatment and prevention of COVID-19 due to lack of knowledge about its safety, efficacy, side effects, dose and combination [123]. These drugs have certain limitations and toxicity, especially on the heart and eyes [124].
CQ, as sulfates and phosphates, is known as antimalarial drug. In recent times, it is widely used for the treatment of auto-immune diseases and has been observed that it shows immunomodulatory, anti-infective and metabolic effects [125]. Despite of its anti-viral effects, the use of chloroquine as a treatment of COVID-19 is still at an issue. The European Medical Agency had allowed the use of CQ only for trials and urgent purposes [126]. This is because; despite of having anti-viral effects chloroquine has many other side effects especially if it is used in higher doses [126]. The patients, who have used CQ for a longer period, were suffered from many irreversible side effects such as retinal diameter defects and retinopathy circular defects. This is because this drug accumulates in the eyes if used for longer period. This can cause irreversible damage to eyesight and even it can cause blindness [127].
Various irreversible and fatal side effects of CQ on heart such as, conduction disorders and cardiomyopathy are also observed. Therefore, the use of CQ in combination with other drugs should be stopped to prevent heart diseases [128]. Further when chloroquine is used, it causes an increase in pH of the human body which affects several organelles. When it enters the cell it causes an acidic pH in organelles such as endosomes, Golgi vesicles and lysosomes, therefore causes destruction of organelles [129,130]. It has been also observed that CQ negatively influences immune system. After its administration during COVID infection the immune effect produce by CQ is still uncertain. Therefore it may be harmful to use it before proper trials [131].
Furthermore, the self-administration of CQ during COVID infection is fatal due to toxicity caused by overdose [132]. The clinical pharmacology and mode of action is unpredictable due to its complex properties [54]. Therefore clinical trials are required before recommending or using CQ for the treatment of COVID infection [133].
As the matter, CQ affects the proper function of cell. Therefore the major medical institutions have recommended its use cautiously by mentioning the dangerous side effects of CQ [134]. Hence, at this point there are no clinical signs that chloroquine has advantageous effects to use it for the treatment of COVID-19. The harmful effects of chloroquine are known and they can be more severe from psychiatric effect to arrhythmia, and may cause sudden death. Therefore until its approval it cannot be recommended [132].
Also the World Health Organization (WHO) still has not recommended any antiviral medicines for the treatment of COVID 19. This provides insufficient evidence regarding effectiveness of chloroquine [135]. The clinicians and health professionals are asking for the evidences regarding the use of chloroquine for COVID-19 treatment. There are very less and weak evidences regarding the use of CQ due to the lack of clinical trials and misinterpretations [131].
Finally, at present there are inadequate evidences to determine that chloroquine is advantageous and not harmful for the treatment of Covid-19. Therefore high quality and deeply investigational clinical trials are required regarding the use of chloroquine [136,137].
The risk of shortage in the outbreak
The situation is evolving very quickly, as many local governments and authorities are currently taking critical decisions to fight against the Covid-19 outbreak [138].
Global community
Legacy of scientific discovery and experience with vaccine development, licensure, and manufacturing that makes some companies more-positioned to respond to emerging public health threats such as COVID-19 [5].
Collaborations with medical institutions like the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services, to leverage previous work on a SARS vaccine and its recombinant vaccine platform to unlock a fast path forward for developing a COVID-19 vaccine [139].
The Coalition for Epidemic Preparedness Innovations (CEPI) which launched in early 2017 in Davos, coordinates the development of future vaccines against targeted epidemic pathogens identified by WHO. Companies will continue to share their vaccine, R&D experience and expertise to CEPI through its Scientific Advisory Committee [140].
Clinical program evaluating KEVZARA® (Sarilumab) in patients hospitalized with severe COVID-19 to evaluate how KEVZARA® may be a potential treatment option for patients [141]. Following preliminary results of studies conducted in China and more recently in France, showing that anti-malaria drug, chloroquine and hydroxychloroquine, can have antiviral activity in COVID-19 infection, companies work with local Health Authorities and scientific experts in countries heavily impacted by the outbreak to investigate the benefit/risk profile of Plaquenil® (Hydroxychloroquine) in the treatment of COVID-19 and if requested by the local governments, to donate the product free of charge [119].
To date, there is insufficient clinical data to draw any conclusions over the clinical efficacy or safety of hydroxychloroquine or chloroquine in the management of COVID-19 [142], therapeutically combined with paracetamol and ibuprofen which are currently used as the main treatments to relieve patients from fever and pain [143].
Transmission
Transmission of COVID-19 occurs primarily through direct contact with an infected individual (e.g. handshake, hug, cohabitating) or through respiratory droplets [144]. Respiratory droplets are defined as particles with a diameter between 5–10 micrometers that originate from the respiratory tract and are produced when a person coughs or sneezes [144]. Transmission via respiratory droplets occurs when a person is within 1 m of an infected individual who does something to create respiratory droplets, and the droplets travel to the uninfected person’s eyes or mouth [144]. Droplets can also land on surfaces and be transmitted to an individual who touches them and then their eyes or mouth before washing their hands [144]. Under ideal conditions the virus can remain viable on a surface for several days, though it is killed almost immediately by household disinfectants or hand washing [145]. There is currently no data to suggest that the virus can become airborne under normal conditions [144]. Airborne transmission occurs when the virus is found in droplet nuclei, which are smaller than respiratory droplets with a diameter of less than 5 micrometers [70]. These smaller droplet nuclei can travel much farther than respiratory droplets and can hang in the air for longer, which makes transmission far more likely [144]. COVID-19 can be aerosolized under specific circumstances, such as during endotracheal intubation, bronchoscopy, when turning a patient prone, performing CPR, etc. [144]. While there is no strong evidence that under normal conditions the virus can aerosolize, research into how long viable viruses can be found in the air is still needed at this time [144]. Both asymptomatic and symptomatic individuals are infections, and there doesn’t appear to be a difference in viral load between them [146].
There has been some evidence that the virus can spread to the intestines and therefore be transmitted via fecal matter [144]. Though there are no cases to date of viral transmission through the fecal-oral route [144].
Patient recovery and disease progression
The most common symptoms of COVID-19 are fever, dry cough, and tiredness [147]. Patients can also experience shortness of breath, aches, and a sore throat [147]. Very rarely will diarrhea, nausea, or a runny nose develop [15,147]. We currently understand that there is a wide range of symptom presentations, ranging from asymptomatic carriers, to Acute Respiratory Distress Syndrome (ARDS) and major organ failure [148].
With around 25-30% of patients requiring intensive care due to complications like ARDS, acute lung injury, shock, and kidney failure [148]. Though this percentage may be higher, as it only includes those who went to the hospital and were tested, and it is possible that there are infected individuals who develop moderate illness and do not seek treatment. In children the disease appears to be mostly mild or asymptomatic [7]. Though, children, younger than a year, have an increased risk of developing a critical condition [149].
There have been cases of pre-symptomatic transmission of COVID19, where a not yet symptomatic patient spread the virus to others. SARS-CoV-2 has an incubation time (time before symptoms present but the virus is in the body), of approximately 3–5 days, and some reports have put the range up to as high as 14 days [[150], [151], [152]]. The incubation time of SARS-CoV-2 appears to be similar to that of SARS-CoV-1 and MERS [152].
The average time for recovery has been estimated to be around 24.7 days, with the average time from onset to death being around 18.8 days [152]. A study of 17 COVID-19 positive patients followed their progression to try and understand how the disease develops. The patients presented with an elevated temperature, heart rate, and low oxygen saturation [153].
Lab tests identified leukopenia in a little over half of their patients (52.9%) and lymphocytopenia in a little less than half (47.1%) [153]. Also, they had elevated C-reactive protein (CRP) levels, erythrocyte sedimentation rate (ESR), alanine aminotransferase, and aspartate aminotransferase levels [153]. High CRP and ESR results are representative of inflammation in the body, probably as a result of the immune response to the virus, while high alanine aminotransferase and aspartate transaminase levels indicate liver damage.
Within the first week of hospital admission the patient’s fever, CRP, ESR, and lymphocyte count improved, and within two weeks as did the heart rate and oxygen saturation [11]. In week two aspartate aminotransferase levels increased and in week three as did levels of alanine transaminase [153].
This study reported that the lesions found on chest CT began as small ground glass opacities (unilaterally, bilaterally, or in the pleural space) in the lower portion of the lungs before developing into a crazy paving pattern [153]. Interestingly, the severity and involvement of the lesions observed on the chest CT increased in weeks one and two, peaked in week two, and began to resolve in weeks three and four [153]. This progression of CT results does not align with the resolution of the fever, laboratory tests, heart rate, and oxygen saturation. This would indicate that the morphological changes in the lungs occur at a different rate than the inflammatory reaction to the virus that has been associated with symptom presentation. This is consistent with another study of 21 patients [154] (who didn’t develop ARDS) that found the highest amount of lung abnormalities on chest CT approx. 10 days after symptoms onset. The lesions began to improve at approx. 14 days [154]. Further research should focus on identifying if there is a correlation between the increase in lesions around week 2, and what this means for patient health.
A very small preliminary study [154], done on 4 healthcare workers who tested positive for COVID-19, found that even after the patients met the criteria for hospital discharge (no clinical symptoms or radiological abnormalities, and two negative Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) tests) they still tested positively 5–13 days later. This indicates that it may be possible for patients to be asymptomatic viral carriers even after their symptoms resolve [154]. It is possible that both the negative tests, needed to allow the patient to return home, were false and that the patients contracted the virus again after they were discharged [154]. This is very early data and more research will be needed to understand how viral load decreases with symptom resolution.
Liver damage has been identified in many COVID-19 patients. During their disease progression, 53% of patients had high levels of alanine aminotransferase and aspartate aminotransferase, which indicates liver damage [155]. It appears that those who have more severe cases of COVID-19 also have higher rates of liver complications [155]. It was investigated that an elevation of AST levels in 8/13 COVID patients, admitted to the Intensive Care Unit (ICU) by 62% and in 7/23 non-ICU patients by 30% [156]. Contextually, a larger investigational study of more than 1000 patients also identified a correlation between severe disease and elevated alanine aminotransferase levels [157]. This damage may be caused by the virus itself directly infecting liver cells. 2-10% of patients present with diarrhea, which may indicate that at some point the virus infects liver cells [157]. The virus has also been found in blood and stool samples in some cases, which may also point to viral infection of the liver [157]. This damage may also be caused by a medication used to treat the patients or inflammatory damage from the body’s immune response to the virus [157].
Secondary bacterial infection (bacterial co‐infections with SARS‐CoV‐2)
Secondary bacterial infections are taken as an important risk factor for higher mortality and severity of the infection in COVID-19 [158]. It is confirmed by epidemiological, laboratory and clinical studies that mortality rate due to any viral disease is increased if bacterial co-infection accompanies [159,160]. Influence of secondary bacterial infections on mortality rate due to viral infection is well previously documented [[161], [162], [163], [164]]. The effects of bacterial co-infections during any epidemic or pandemic mainly in immunosuppressive diseases are irreversible [164] (Table 4 ). It is fact that COVID-19 patients also got bacterial co-infections during hospitalization [[165], [166], [167]].
Table 4.
A Summary of Secondary Bacterial Infection mechanisms, responsible for respiratory viral infections and bacterial confections.
| Mechanism | Description | Refs |
|---|---|---|
| -Virus causing increase in Bacterial attachment | -Receptors are modulated by viruses, hence increasing attachment of bacteria. | [158,168] |
| -Cells are destroyed by enzymes from Viruses. | -Glycoproteins are destroyed by enzymes, especially those that are involved in inhibition of bacterial adhesion. | [158] |
| -Muco-ciliary clearance is reduced. | -Decreased muco-ciliary clearance due to virus causes reduced bacterial material production. | [158] |
| -Reduced Chemotaxis | -Chemotactic factor is reduced by virus hence and decreasing response to invading organisms. | [158] |
| -Straight consequence on phagocytic, initiation of functions of alveolar macrophages after phagocytic | -Many functions like fusion of phagosome and lysosome is stopped by virus. | [158] |
| -Undeveloped phagocytes are induced. | -Macrophages are disrupted by viruses and are replaced with undeveloped phagocytes | [158,169,170] |
| -Decrease in surfactant amount | -Alveolar type 2 pneumocytes’ function is impaired by viruses. | [158,171,172] |
| -Dysbiosis is introduced in microbiome of lower respiratory-tract. | -Immune-response towards respiratory viral-infections is affected by dysbiosis in microbiome. | [173] |
| -Innate and adaptive immunity is dysregulated. | -During the development of apoptosis alverolar macrophages are decreased in number by virus. | [[174], [175], [176], [177]] |
| -Inflammation and apoptosis are modulated. | -Post viral infection secondary-bacterial pneumonia is facilitated by apoptosis and autophagy. | [178] |
| -Immune function of antibacterial is reduced at respiratory-epithelium. | -Viral infections of respiratory-tract causes inclination to secondary bacterial-infection by deviating immune status of respiratory tract. | [158,179,180] |
| -Nutritional immunity is disturbed. | -Some viral infections destabilize nutritional shield and enhance bacterial invasion. | [[181], [182], [183]] |
| -Suppression of immune system | -Some viruses like HIV induce immunosuppression. | [[184], [185], [186]] |
| -Synergistic viral and bacterial co infections | -Co infection’s immuno-pathogenicity is regulated by bacteria as well as viruses. | [159,171,187] |
| -Planktonic bacteria released from biofilms | -Biofilm structure is disrupted by manipulation of many factors like H2O2 and chemokines due to viruses. | [177,[188], [189], [190]] |
Maximum shift time for physicians
At this time, great care is being taken to “flatten the curve” of viral infections by slowing the spread of COVID-19. This is being done to ensure that the number of COVID cases (on top of usual hospital visits that regularly occur outside of a pandemic) do not exceed a level that the healthcare system cannot handle. Even with efforts being taken to slow the spread of the virus, healthcare systems around the world are still overloaded and face shortages of personal protective equipment (PPE), workers, ventilators, and more. There are several things that care centers can do to spread their resources farther, and thus care for more patients.
Limitations on the healthcare system can be broken down into different categories. One factor in a healthcare system’s capacity is patient beds, as some hospitals simply do not have enough room for all of the patients coming in. There are several strategies that hospitals may introduce to conserve such resources. It may be necessary for hospitals to convert single rooms into double rooms, speed up discharges, and slow the rate of patient admission18. Converting catheterization laboratories, hospital lobbies, postoperative care spaces, and waiting rooms into patient care spaces may also be necessary and would increase the physical capacity of the hospital (i.e. number of beds) [191].
Another limiting factor is the amount of medical resources a hospital has access to, including PPE, ventilators, blood, etc. At this time, several methods of increasing supply of ventilators and PPE are underway including retooling factories to produce ventilators and 3D printing PPE (which is discussed further below). There have also been pushes from the public health center to encourage people to donate blood.
What may prove to be the most integral factor in a hospital’s capacity is having enough skilled medical staff to manage these resources. Efforts have been made to rehire retired healthcare workers and some have been rushed through the end of their training to help with the crisis. Beyond these measures, there is no way to produce more healthcare workers, which makes using them effectively vital to patient and hospital survival.
Prior to the current outbreak, there had been many recommendations made regarding the best way to schedule healthcare workers who work on a shift schedule. Such recommendations may need to be modified in order to meet the current demands while also keeping in balance that the healthy and well-rested workers will be more productive and better able to treat patients.
The American College of Emergency Medicine strongly suggests that emergency physicians (and others engage in shiftwork) should be regularly given 24 h off work [192]. This recommendation should not be possible for hospitals at this time, though it should be followed when possible. To preserve sleep quality, it is best to keep workers on long periods of night shifts, or to have them work isolated nights infrequently instead of putting them on and off night shifts [192]. This applies during this outbreak, though the number of times workers are scheduled for night shifts may become more frequent. When scheduling shift time changes (such as what may occur in hospitals during the current crisis), it is preferred to have shifts rotated clockwise (day to evening to night) even when there are days off in between [192]. This will help employees who are overloaded during the COVID crisis as it best preserves circadian rhythms while also allowing them to work extra shifts. If hospitals are able to, offering somewhere for workers to sleep before driving home after a night shift (or after an especially long shift) would be encouraged [192].
It is recommended that those scheduled to work a single night shift should go to sleep as soon as possible after their shift and sleep for four hours [193]. This will allow them to still be tired for their normal bedtime and sleep better at night [193]. Sleeping for longer than four hours after their shift will significantly impact the next night’s sleep and cut down on their total amount of Rapid eye movement sleep (REM) sleep which would be detrimental to the next shift they work, especially if that shift is soon [193]. For those who are on long periods of night shifts (especially those who work infrequent night shifts) it is best to sleep in two, four-hour sections as close to their normal sleep pattern as possible [193]. For example, if you sleep from 10:00 p.m. to 6:00 a.m. but a shift is scheduled for those times, it is best to sleep from 5:30 p.m-9:30 p.m. and then from 6:30 a.m. to 10:30 a.m. to best prevent the disruption of the circadian rhythm [188]. Following such sleep practices will help workers feel rested and more equipped to deal with the challenges that they, and our healthcare system, are currently facing.
In a time when emergency physicians are working more, irregular shifts are ordered to cover the huge influx of patients, it can be useful to maintain “anchor” periods of sleep (when you sleep for four hours at a time, at the same time every day) [193]. In general, persons over the age of 45 don’t adapt as well tonight shifts [193]. Cognitive tests on older shift workers show decreasing functioning the older the subject is [194].
D printing PPE and novel forms of non-official PPE acquisition
Due to the highly infectious nature of COVID-19 having appropriate PPE for frontline workers is essential to their health and the health of their community. The sharp increase in demand for PPE all around the world has created a shortage of such equipment. Hospitals are facing many challenges with PPE acquisition and many have been forced to re-use PPE designed for single use or rely on homemade cloth masks for some of their employees. Due to the lack of widespread testing capabilities in many countries, it is impossible for healthcare centers to know which patients have COVID and thus require full-scale PPE, and which have much-lower risk community-acquired pneumonia or the flu and don’t require such security measures. This not only costs the time required to put on and take off the full equipment, it also puts further strain on dwindling supplies.
In order to fill this shortage, it may be necessary to acquire PPE from outside (i.e. nonmedical) sources such as construction, schools, veterinarians, salons, manufacturing, and dental centers [195]. Obtaining PPE from public sources such as hobbyists, who own masks and respirators, may also be possible [195]. There have also been discussions around reusing PPE and disinfecting the items with heat, UV light, bleach, copper sulfate, ethylene oxide, alcohol, and more. Some have suggested cycling through a 72 -h rotation of equipment (based off of our understanding of viral survival) [195]. Impregnating masks with copper or sodium chloride has been tested in prior epidemics and is a suggested method of disinfecting and extending the life of PPE. Though there are concerns that these methods may breakdown the mask or respirator and thus reduce their ability to filter particles [195].
Possible solutions to the shortage also include using cloth to make re-useable gowns and masks, these items would be cleaned regularly and could replace single-use PPE. Little data is available on how well these homemade items would filter particles, transfer harmful microbes, and stand up to constant reuse. Studies examining the efficacy of cloth PPE are needed at this time [195]. Using filter material intended for non-medical uses, such as Minimum Efficiency Reporting Value (MERV) filters (found in vacuum bags and air conditioning units) has been discussed [190]. Filters found in things like vacuums have a minimum efficiency reporting value of 13 or 14 which can reduce the flow of particles bigger than 0.3 nanometers in diameter by 50-75%, respectively [195]. In comparison, N95 respirators are equivalent to a MERV 16 filter [195]. SARS-CoV-2 is smaller than 0.2 nanometers and so could pass through a 13 or 14 filter, but the respiratory droplets carrying the virus are much larger and may be blocked [195]. Using several layers of MERV 11–16 filter material may be able to block the virus if enough material is used [196].
Currently, there are efforts from both institutions and individuals to make face shields and other forms of PPE using 3D printers. 3D printing PPE such as face masks will provide a physical barrier to the virus, but will not likely provide the same fluid and air barrier as a licensed mask or N95 respirator [197]. The government of Canada has issued guidelines for the production of face shields [197], which seem to be the easiest to create on a 3D printer especially for individuals. Prior to the COVID outbreak, 3D printers had been used to make face seals for Filtering Face piece Respirators which did provide a better fit for the wearer [198].
A recent study from George Washington University [196] designed and tested a re-useable, 3D printed respirator. The design that involved a piece of Minimum Efficiency Reporting Value 16 (MERV16) filter sandwiched between two pieces of MERV13 filter [198]. MERV16 filters often contain fiberglass which would be harmful if inhaled, so the MERV13 filters (which don’t typically contain fiberglass) protect the wearer from inhaling fiberglass filaments [198]. The rest of the mask is made with a 3D printer and the masks were designed to be reused and had refillable filter cartridges [198].
The respirators underwent a negative pressure test where the user put on the mask and inhaled to check for leaks [198]. Successful respirators went to the hospital employee health center for Bittrex testing which including things like moving the head, running in place, deep breathing, etc. to test for leaks [198].
Given that the masks were designed for reuse, the researchers then tested how they held up to sanitization [193]. The masks were cleaned with several rounds of rubbing alcohol followed by soap and water before being sent back out to the hospital, this cleaning process did not appear to negatively affect the performance of the mask [198].
The benefits of this mask design that they are easy to make and clean, can be fit to each individual, and can be sterilized with alcohol, Ultraviolet (UV) electromagnetic radiation, low heat, or soap and water [198].The filtration packs can also be sterilized and reused [198]. Given that MERV filters are also used in furnaces, sterilization with light and heat should not (theoretically) degrade them [198].
One drawback is that these masks need to be individual fit (like N95 respirators) and study participants who didn’t achieve a good suction reported having a hard time fitting the mask around their nose and cheeks [198]. More work on the fit and ease of fitting for the user will be needed before these masks are made on a large scale [198]. Extra testing of MERV filters to ensure that they live up to the manufacturer’s claims will also be needed [198]. Further testing should also ensure that the MERV13 filters catch any fiberglass filaments that the 16 filters may shed [198].
The masks designed by this study can be made at a rate of about 1 per hour per machine (with large 3D printers) and the George Washington University Hospital could produce 70–100/day [198]. Over a thousand filter thousand filter cartridges can be made out of one MERV16 filter and the cost for making each mask is between $2 and $4 depending on type of materials used [198].
Next possible vaccines as landscape for SARS-CoV-2
The scientific/clinical experience is to help develop the COVID-19 vaccine manufacturing process. The animal data gives confidence that the vaccine will generate an immune response. Research and clinical material could be produced relatively quickly leveraging the learning from SARs. Overall, the platform could accelerate availability of large quantities of vaccine [199].
Expectance to have available vaccine
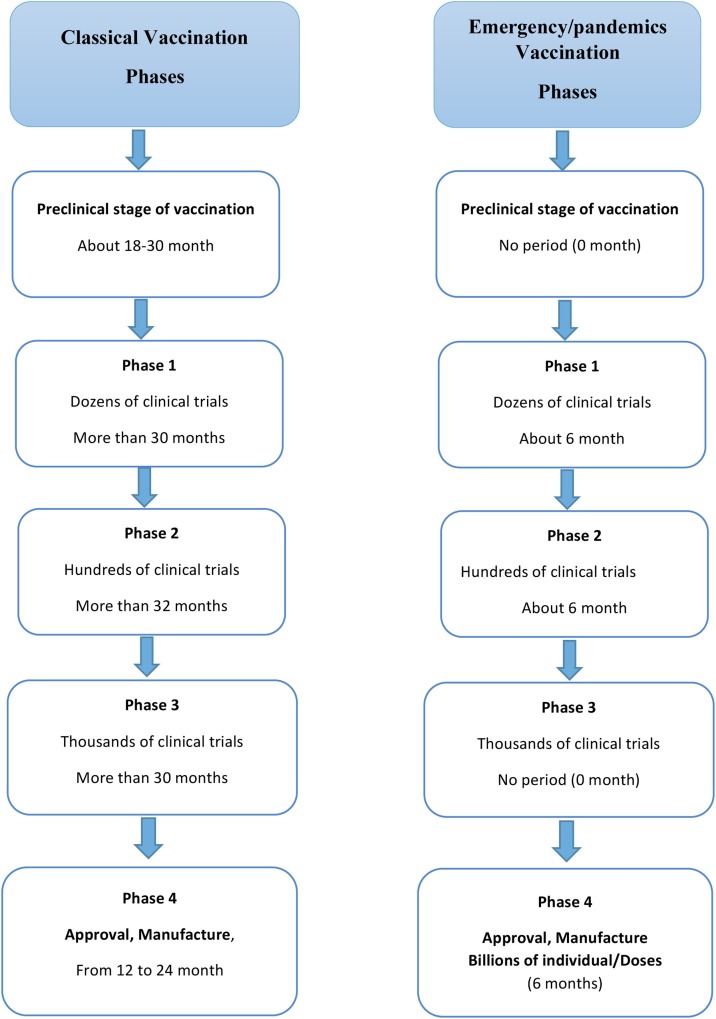
It is estimated that vaccine candidates will be available for in vitro testing within less than six months and potentially enter clinical trials within a year and a half. It is too soon to get an answer for "when a vaccine will be available for wide public use?", because we have not yet entered the clinical trial phase and there are many factors involved including formulation, yields, dosage requirements and other variables [200].
The global availability of COVID-19 vaccine
If a vaccine is emerged with properly activity against COVID-19, then it is thought that 60–70 % of people are in a need to be immunized to the virus in order to stop its spreading (known as herd immunity).
Pharmaceutical companies are committed to supporting the response to this global public health emergency and will work with stakeholders to make the product available where it is needed [201].
Five concepts about COVID-19 vaccine
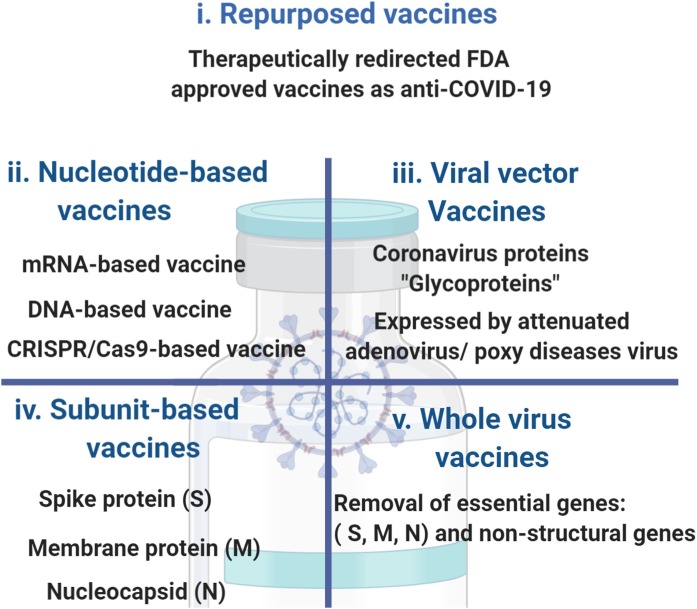
A safe and effective SARS-CoV-2 vaccine is in need to end the global COVID-19 pandemic. An initiation of vaccination candidates is under clinical testing, and many others are in preclinical development. About 1–1.5 year is required to find optimal vaccine for COVID-19. Currently, there are about 140 vaccines for COVID-19, under experimental and clinical investigation. The concepts are built on along human experience in the field of innovative vaccines (Table 5 ) and vaccines already under investigational clinical trials for COVID-19 (Table 6 ). The proposed vaccines [202] are classified into (Fig. 8 ):
-
i
Repurposed vaccines
-
ii
Nucleotide-based vaccines
-
iii
Viral vector vaccines
-
iv
Subunit-based vaccines
-
v
Whole-virus vaccines
Table 5.
Types of Vaccines.
| Vaccine Type | Definition | Examples | Refs | |
|---|---|---|---|---|
| Inactivated vaccines | Produced by killing the micro-organisms | Anthrax, Cholera, typhoid, hepatitis A, hepatitis E, influenza virus A and B vaccine, Rabies. | [203] | |
| Toxoid vaccines | Vaccines contain toxins and chemicals | Whooping cough vaccine (pertussis), Diphtheria, tetanus | [204] | |
| Subunit vaccines | Fragment of the pathogen introduced rather than the whole pathogen into an immune system | meningococcal, and pneumococcal vaccines | ||
| DNA vaccines | Produced by introducing DNA of organism into host body. | [203] | ||
| Recombinant vector vaccines | A weakened bacterium or virus (vector) is used to inject microbial DNA in the body. | Adenovirus and vaccine | [203] | |
| Conjugated vaccines | Vaccine in which a weak antigen and a strong antigen combine as a carrier and the immune system has a strong response towards weak antigen. | Hib-conjugate vaccines | ||
| Live attenuated vaccines |
Produced by weaken or attenuating the micro-organism. | Bacterial vaccines (Bacillus Calmette Guerin Vaccine BCG) Viral vaccines Measles, mumps, polio vaccines) | [203] | |
Table 6.
Vaccines under development using various components of SARS-CoV-2 [205].
| Viral Product used in pre-clinical vaccine trials |
Developer | |
|---|---|---|
| DNA | Inovio pharmaceuticals takis/ Applied DNA sciences Evvivax Zydus Cadila | |
| Inactivated viral products | Sinovac Codagenix / Serum Institute of India | |
| Non replicating viral vector | GeoVax/BravoVax Greffex University of Oxford Altimmune Vaxact Janssen Pharmaceutical companies | |
| Viral protein subunit | ExpreS2ion WRAIR/USAMRIID Clover Biopharmaceuticals Inc/GSK Vaxil Bio AJ Vaccines / EpiVax EpiVax/ Uni of Georgia Sanof Pasteur Novavax Heat Biologics / University of Miami University of Queensland / GSK Baylor college of Medicine Bio/CC-Pharming VIDO-InterVac University of Saskatchewan |
|
| RNA | Fudan University/ Shanghai JiaoTong University RNACure Biopharma China CDC/Tongji University/ Stermina Moderna/ NIAD Arcturus/ Duke-NUS Imperial College London CureVac BioNTech/Fosun Pharma/ pfizerBIOCAD Sanofi Pasteur / Translate Bio | |
Fig. 8.
The vaccination therapeutic approaches of COVID-19.
Repurposed vaccines are FDA-approved vaccines for the infectious diseases and these vaccines are therapeutically redirected to tackle other diseases [206]. For SARS-CoV-2, Bacillus Calmette–Guérin (BCG) vaccination is under clinical investigation [207,208]. BCG as a live attenuate vaccine has been developed against tuberculosis, dates back to the beginning of 20th century. For its mechanism of action; it acts via reprogramming innate immunity via acting as booster for producing pro-inflammatory cytokines of IL-1β, tumor necrosis factor (TNF) and IL-682. It is well hypothesized that BCG could be vital tool against SARS-CoV-281. Because, BCG shows significant reduction in infant mortality, not only as being BCG acts as anti- tuberculosis. However, it is highly expected that BCG acts against viral/bacterial pathogens which are key factors in causing severe acute respiratory syndrome. Ecological observation supports the hypothesis of "BCG as preventive therapy for SARS-CoV-2"; it is that the countries that consider BCG on the followed medical vaccination measures, show low capacity of SARS-CoV-2 from being spread [209].
-
i
Nucleotide-Based vaccine
Biogenetically, Types of Vaccines which are based on expressed chimeric mRNAs containing curated open reading frame (ORF) viral sequences in the cellular cytoplasm, possessing high potentiality to translate directly in the cytoplasm and suppress chromosomal integration [[210], [211], [212]].
Similar to RNA-based vaccines, this promising technique uses genetic material of virus to create a vaccine. “More vaccine doses with DNA can be made than with RNA. But, it is unclear weather the development could be rapid scaled to fullfil the high international demand,” said Dowling. And according to our knowledge, these DNA contained vaccines have not been tested in elderly patients yet.
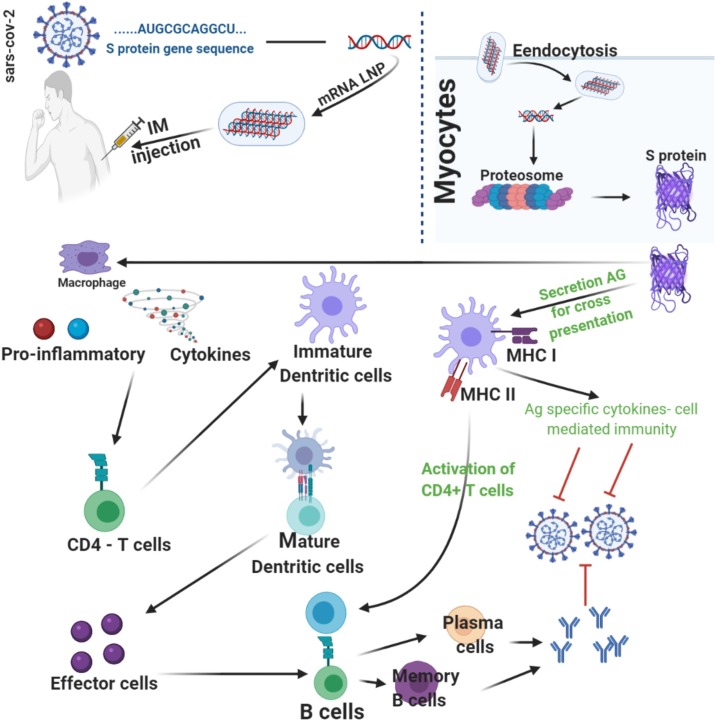
As for COVID-19, mRNA-127387 and BNT162b [213] are promisingly novel lipid nanoparticle (LNP)-encapsulated mRNA-based vaccine [214,215], encoding the spike (S) protein of SARS-CoV-2, acting via the mechanistic action as shown in Fig. 9 .
Fig. 9.
The flowchart of DNA vaccination; mechanism of action [215].
DNA vaccines is to stimulate the immunity system with an infectious agent, genetically modified DNA as Spike protein (S) or Membrane glycoprotein (M), in case of SARS-CoV-2, not to harm or disease is caused, but ensuring that when the host is confronted with that infectious agent, the immune system has an adequate potentiality to neutralize it before it causes disease [216].
This DNA vaccination approach owes potential advantages over conventional approaches, including the stimulation the immune response of such as both B and T-cell, enhancing vaccine stability, the absence of any infectious agent and the relative ease of large-scale manufacture. As an evidence of the principal core of DNA vaccination, immune responses in animals have been obtained using genes from a variety of infectious agents, including influenza virus, hepatitis B virus, human immunodeficiency virus, rabies virus, lymphocytic chorio-meningitis virus, malarial parasites and mycoplasmas. In some cases, protection from disease in animals has also been acquired [216].
RNA-based vaccines; as preliminary concept for conceptual SARS-CoV-2 vaccination, it is RNA power utility. This approach is attractive and innovative. But if it shows potential activity as vaccination, it may be difficult to produce hundreds of millions of doses, and each dose relatively expensive as it may demand a significant quntity of RNA. While experience with human testing is low for this technique, it is possible that adjuvants may enhance the immune response when these vaccines are given to older populations. Consequently, it may decrease the qunatity of RNA required for each vaccine [217].
CRISPR based vaccine; researchers are evaluating previous treatment and try to discover the novel drugs for the COVID-19, which is a global issue, causing high morbidity and mortality. By seeing the huge loses, it is dire need of time to develop a vaccine for SARS-CoV-2 disease (Covid-19) as it is efficient way to control it from the spread and prevent for the future onset. Many attempts have been done and carry on for the design of SARS-CoV-2 vaccine [[218], [219], [220], [221]]. Currently, CRISPR- mediated genome editing approach which does modification in cell by editing or correcting defective. CRISPR, new technique of genome engineering formed of 2 components, having a guide RNA (gRNA) that particular to target RNA or DNA sequence. CRISPR that is nonspecifically associated to endonuclease protein or CRISPR protein (Cas).
Mechanism of action; production of antibodies (Abs) by the activation of B-cells is a fundamental mechanism of many vaccines. These techniques show extraordinary outcomes at the outset. But, they have some impaired impacts pertinent to RNA virus; this issue can be solved by doing modification of B-cells via genome editing technology, CRISPR/Cas9 mediated gene editing. B-cells should be aimed to attain specific properties:
-
•
Specifically expressed antibodies against SARS-CoV-2 epitopes
-
•
No chance of accidental (Ab)
-
•
Developed temporal viability inside the body of the clones of engineered B-cells.
-
•
Salience of being non-oncogenic and benign relatively. The range of such cellular clones can solve the problems of all viral pathogens like SARS-CoV-2.
Basically, vaccines potentiate B-cells to produce Abs against the specific antigens which are known as epitopes of the disease causing pathogens, it is S-spike protein in SARS-CoV-2. This is achieved by B-cells by rearrangement of 3 imperative parts of Ab in their genomes. There are some reasons due to which vaccines fail in the arranging of gene that it does not take proper place effectively; it may be delayed, long lasting and cannot become able to stand a sufficient space. The other critical issue in antibody based vaccine is that level of antibody become low after the short time of injection of vaccine in this case it is repeated.so, it is an additional reason to engineer B-cells in away so that as and when antibody require they going to produce it [[222], [223], [224], [225]].
CRISPR/Cas9 driven genome editing approaches is highly potent for editing the mammalian cells genome sets with utmost and high accuracy and precision. Accordingly, it can be hypothesize that a similar approach would be tried for the alteration and engineering of human B-cells, consequently, specific antibodies with well-arranged expression can be attained under the control of endogenous regulatory elements which is responsible for the production of antibody in these cells. It edits the genomes with the thought to improve and refine the bodily functions. Coinciding with this fact, it can be hypothesized that an analogous approach would be credible to alter and engineer human B-cells. Therefore, well-arranged expression of specific antibodies can be attained under the control of endogenous regulatory elements that are responsible for antibody production (i.e. expression and secretion of normal antibodies) in these cells [226].
Ideas/ways for implementation of hypothesis; we can test the hypothesis in both the human and mice B-cells in a two-facetted outlook. B-cells from mice can be collected and genetically engineered. The successfully engineered B-cells can then be injected into mice which were not previously exposed to the antigen. After exposing the particular mice to the antigen, the antibody titer can be assessed as against the control group (which did not receive the B-cell therapy). The success of the development of B-cells can be confirmed by assessing the quantity of these antibodies against the antigen. Otherwise, B-cells can be harvested from humans and engineered against the viral antigen. Then the successfully engineered cells can be injected into exposed mice. The engineering of the B-cells can be made evident by detecting the antibodies in those mice after antigen exposure. One of the main limitations to consider the genetically engineered B-Cells vaccination specified to SARS-CoV-2 is that it may be rejected by the host immune system. Thence, the allogeneic B-Cells should be dependent on the basis of precision medicine to evoke the immune response [227].
-
iii
Recombinant viral vector vaccines
Recombinant viral vectors acts similarly like an endogenous pathogen, by expressing axenic target protein into the cellular cytoplasm of the host cell. Subsequently, processing of such endogenous antigen, MHC class 1 molecules present them to CD8 + T-lymphocytes causing production of T-cytotoxic cells. This biological pathway, results in establishment of cell-mediated immunity, which is main corner in distracting of virus infected cells [228].
-
iv
Subunit-Based vaccines
As for consideration of the safety efficacy, Subunit-Based Vaccines containing antigens in a pure for are claimed as being advantageous, as the constituent only includes synthetic peptides or recombinant proteins expressing only particularly specific fragments of immunogen, which exclude the participation of contagious viruses. In addition to this, subunit vaccines owe less prone to generate the side effects at the site of inoculation. Due to conditional stability and absolute fragments of pathogen, subunit vaccines accomplish dependable production. AS for all these advantages of subunit vaccines, they are crucial in making it a promising candidate for vaccine. A number of structural proteins of; spike (S), envelope (E), membrane (M), and nucleocapsid (N) are expressed by SARS-CoV that may be subunit vaccination as antigens to activate neutralizing antibodies and generate defensive response [[229], [230], [231], [232], [233]].
-
v
Whole-Virus Vaccines
Whole-Virus vaccines are the vaccination approach where attenuated/killed virus is delivered into the patients for developing the specific immune response for long-lasting immunity. One of under investigational studies, inactivated coronavirus PiCoVacc immunization is a promising candidate as next COVID-19 vaccine [234,235].
Vaccination target group
Age, immunity level, genetic variability, and clinical background are the main factors which are considered for the vaccine injection. There is lot of variations of common infections, high in the infant and low in the adult groups. As from human experience with vaccination, measles, mumps, polio and smallpox are the diseases that are age related [206]. Moreover, each vaccine has different target; in the United States, vaccine against influenza is recommended to individual whose age is more than 6 months. Due to high risk of severe disease in the elderly, children, pregnant women, immune-compromised individual and health care works, vaccination should be prioritized in these groups [236]. In general, vaccine responsiveness and non-responsiveness is evaluated by measuring antibody titer. Timely vaccination of middle-aged adults might progress the immune responses to vaccines, although facts on pathogen-specific immune responses and factors affecting these responses, in middle-aged adults is now limited [237,238]. Therefore, the studies show that Children were the most targeted group, and pregnant women were the least targeted. 90% of vaccine coverage is done on newborns of age 12–24 months. But this coverage is falling in children of 4 years to adolescent. 75% Vaccine coverage is done in adults [239]. Vaccine coverage level decline in adults [240].
Patron of Vaccine in the pandemic condition like COVID-19
Due to the limited supply of the vaccine when it is developed in pandemic condition, it is prefer to inject it to the healthcare workers, as they are in direct contact with the COVID-19 patients, and may be exposed to higher concentrations of the virus. Apart from this check, the patron of pandemic in case of COVID-19 as it mostly targets the older patient. So, they should be prioritized if vaccine is effective in this group [241,242].
Vaccination in view of gender difference
The in vitro study of human and animals reveal that numerous genetic, immunological, hormonal, and environmental factors differ between males and females that contribute to sex- and gender-specific vaccine outcomes and responses (Table 7 ) [243]. Females respond more actively to infections and make more antibodies in the result of infection and vaccination 28. In females, more powerful response of immune system may develop greater risk of autoimmune disease in females and a better ability to fight against infection. Initial studies suggest that there is a high production of certain sub-types of antibodies (IgGs) in females than in males after SARS-CoV-2 infection [244].
Table 7.
Sex and gender as possible modulators of COVID-19.
| Sex Related Factors | |
|---|---|
| Viral receptor | Exposure to virus |
| Distribution of receptor | Symptom reporting |
| Virus reproduction | Access to testing |
| Antibody production | Access to protective equipment |
| Hormonal effects | Compliance with preventive measure |
| Efficacy and side effects of therapy | Recruitment for clinical trails |
As for the design of vaccine issues, specific to the sex like pregnancy, is in need for a consideration in those countries that has limited resources of healthcare and higher fertility rate. Therefore, number of potential pregnant patients of COVID -19 is increasing [245].
Gender differences can influence vaccine design, uptake, responses, and outcome in individuals [[246], [247], [248]]. The high efficacy of vaccines is due to immune response. Females and males are different biologically and contribute to sex-specific vaccine outcomes [247]. It has been studied that females show more vigorous humoral and cell-mediated immune responses to infections and vaccination as compared to males (Table 8 ) [249]. Similarly, many studies provide evidence that females show higher antibody response against vaccine than males. So, the efficacy of vaccines recommended for adults is consistently greater for females than males [249,250]. but in some cases adult women experience more adverse reaction then men due to increased inflammatory cytokine and chemokine and cytokine secretion, including interleukin (IL)-1b, IL-6, and C-X-C motif chemokine 10 (CXCL10) from macrophages, tumor necrosis factor (TNF-a) and dendritic cells in women [250]. The application of yellow fever virus (YFV) vaccine via sub cut route often leads to local inflammation, pain, fever, fatigue and headache [235]. Major factors responsible for sex-based variations in an immune response to vaccination are steroid sex hormones (Estradiol, progesterone and testosterone), genetic and epigenetic regulation, and microbiome [250]. The sequence of cell surface molecules responsible for virus entry can be increased or decreased by Estrogen. For example, the sequence of the CC chemokine receptors C-C motif chemokine receptor (CCR1 and 5) in HIV infection or the integrin αVβ3 that is exploited by adenoviruses for entry changed by Estrogen [251]. Moreover, in men increased levels of testosterone are associated to low levels of antibody titers against influenza [250,251]. Another factor is microbiome that is related to high vaccine response in females. Microbes present in the human skin, oral cavity, gut, genital area and well tolerated by the immune system [252]. Precisely, microbiome composition effected by sex hormones as bacteria can metabolize these hormones [253]. These microbial communities can change the levels of sex steroid hormones which regulate the fate of autoimmune disease and immunological responses against vaccination [254]. So from above discussion, it is concluded that gender in vaccine response and outcome play very crucial role and during vaccine designing, gender differences should be considered for the better functioning of vaccine in corresponding gender [[255], [256], [257]].
Table 8.
Gender response to vaccines.
Vaccination in view of Race related difference
Science has a vital role from development to the phase of vaccination via manufacturing and distribution for effective and safe vaccine of COVID-19, immune response and efficacy of vaccine is directly influenced by all the steps including vaccine type, vector or carrier, excipients, adjuvant, route and dosage, logistics, distribution and mass vaccination [258]. Vaccination trail is done by seeing many factors like existing the condition of disease, Native people, group of people of majority and minority, White people are prefer over black [259]. many studies have reported that clinical cases and greater risk of COVID-19 are more in the people of old age, sex wise to the male [260]. It was reported that out of 1063 patients only 20% Black Americans are included in trail of placebo-controlled Adaptive COVID-19 that was funded by National Institute of Allergy and Infectious Diseases (NIAID). COVID-19 Epidemiological data reveals that this disease is more susceptible in minority groups while there is distribution of racial only to study hospitalized patients [[261], [262], [263], [264], [265], [266]].
Data related to ethnicity and race is incomplete and race and dependent on the information collected at local level [263]. Minority communities are overspread in low wage. Therefore, they are more exposed to the virus, front lines people including essential workforce, health care workers whose income is low whose has to move hospitals, clinics and nursing home homes to complete a living, poor communities cannot keep social distance due to overcrowding and housing density [267].
At a national level, provisional analysis from the UK Office of National Statistics has shown that the risk of death involving COVID-19 has marked ethnic/racial disparities; when considering age into account, Black males were 4.2 times more likely to die from COVID-19 than White males and Black women were 4.3 times more likely to die than White women. These increased risks does not apply to Black ethnicity alone in the UK: Ethnicities of the people of Pakistani, Bangladeshi, Indian, and Mixed had statistically significant raised risk of death during COVID-19 as compared to White ethnicity [265].
It was found that the patients of Black, Asian and Hispanic ethnicity are infected with SARS-CoV-2 in a higher proportional as in comparison to those of White ethnicity, with a possible association of higher risk of ITU admission and death from COVID-19 in Asians, even when most confounders are adjusted for. Interestingly, it is a considerable evidence for citing public health protocols to decline the exposure risk of SARS-CoV-2 in ethnic minority groups, by accessing healthcare resources, and targeting the social determinants, structural racism, and occupational risk underlying inequities [266].
There is need of time to learn lessons from the previous epidemics and their uneven effects on the communities mainly the minorities. We have to need an actual data to overcome this effect and susceptibility, less exposure of people, limitation of health care and guide these efforts, for effective and urgent action as better information. Public and clinical sectors of health should be focused on the underlying conditions like metabolic and cardiovascular that is contributing majorly in the mortality and morbidity in acute cases of COVID-19 [264].
COVID-19 in view of Precision Medicine (PM)
Due to the continuously growing evidences that thepatients of COVID-19 owe heterogeneous disease; differential symptoms in lung compliance and ventilation-to-perfusion mismatch, it can be a key to individualize therapy ”No the same treatment for all”. Clinically a major problem, a large number of thepatients of COVID-19 have shown critically negative trials of care medicine. This results from the heterogeneous patient population; different biological mechanisms and different biological responses to a disease in individual patients. Hence, the conventional trial designs and poor patient selection are not refereed. However, precision medicine has a greater potential for positive results [267].
Better selection of more homogeneous groups of patients can be feasible in COVID-19 compared to other critical illnesses because of the large number of patients needing hospitalization. It is better feasible for the COVID-19 clinical trials to select precisely homogeneous groups of patients according to the specific needs of individual patients. It has benefits of reducing study noise, sample size, and study-associated harm. The precise selection criteria are directed by molecular testing of biomarkers, clinical features of patients; clinical stage of disease, vital signs measurement, laboratory data, and ventilator settings [267,268]. To uncover the power of PM in COVID-19 clinical trial, some examples are under- investigation:
-
•
Hydroxychloroquine in patients of risk of acquiring infection, but not yet infected, those without cardiovascular disease, with normal QTc, avoiding concurrent medications causing a prolong of QT interval, cautiously selected dosing, dose adjusted according to renal function, avoiding and rapidly correcting dyslectrolytemia [269].
-
•
Anti-inflammatory agents (corticosteroids) or anti-cytokine agents (tocilizumab) in patients of risk of endothelial dysfunction, endotheliitis, or in patients of an elevated level of interleukin-6 [270].
-
•
Anticoagulation in patients of sepsis-induced coagulopathy or marked elevation of d-dimer and without contraindication for anticoagulation [271].
Finally, COVID-19 raises a critical question about the role of Precision medicine; ”How the immune system acts differently in view of difference in sex, age and genetic variations?”. Hence, it is recommended that the clinical trials of COVID-19 are investigated in view of Precision medicine and vaccinomics of particular patients.
Previous health condition and other variables vaccine
Mostly COVID-19 hospitalized patients had some preexisting conditions like 50% of hypertension, 48 % of obesity, 35 % of chronic diseases of lung, 28 % of diabetes mellitus, and 28% of cardiovascular conditions are testified in COVID-19 hospitalized patients reported by network of COVID-19 and Centers for Disease Control and Prevention (CDC) associated hospitalization Surveillance [262]. Vaccine should be targeted to priority groups, frontline health workers are at high risk, older adults, age over 60 and people who have diabetes, hypertension, respiratory disease, and heart disease which are underlying condition for COVID-19 [272,273].
Communities that are in minority have underlying conditions like kidney disease, cardiovascular, diabetes and hypertension which are very common in younger age; due to lack of medical management should be focused [264].
Adults having diseases like chronic disease of kidney, cancer, chronic obstructive pulmonary disease (COPD), diseases of heart like state of Immunocompromised and cardiomyopathies, coronary artery disease and heart failure, pregnancy, obesity, smoking and diabetes mellitus type 2 are at high risks, COVID-19 is a new disease therefore, currently there is limited information and data about influence of underlying medical conditions related to COVID-19 [274].
There are challenges and issues from resources to protectionism via manufacturing and distribution. Therefore, application of vaccination programs will be uneven and variable due to numerous platforms of vaccines and strategies. For this, some prosperous countries have procured stocks of vaccines which are still under trails. This vaccine scarcity is key factor to determine a leading nation around the globe [275]. To ensure quick vaccine access to under developed countries, a global collaboration platform, COVID-19 Vaccines Global Access (COVAX), has been devised by the WHO, the Coalition for Epidemic Preparedness Innovations (CEPI) and Gavi.
A working principle has been framed for COVAX under the supervision of WHO by medical experts, scientists and ethicists in order to distribute vaccines to all nations. Vaccines will be introduced in two phases. To immunize most susceptible people in member countries vaccine will be provided to all depending upon size of population. The order of priority for populations is recommended by Strategic Advisory Group of Experts (SAGE) by WHO, suggesting older people, infection dealing health workers and immunocompromised people to be entertained first. The vaccine can be inducted to rest of people in second phase [276].
The time needed to approve the vaccines in normal cases and in emergency cases
Center for Biologics Evaluation and Research (CBER) of FDA is a vaccine regulatory body in United States [277]. Vaccine development follows same principle trials before launching in market as for drugs. In normal circumstances, presenters have to submit an Investigational New Drug application (IND) which describes manufacturing technique, quality control test to release it in the market and also contains information about the safety of vaccine to FDA. In normal circumstances, before releasing a vaccine in the market, it passes through three phases (Table 9 ).
Table 9.
Different phases of vaccination.
| Phases evaluation | Actions | Reference |
|---|---|---|
| Phase 1 | In a small number of closely monitored subjects, immunogenicity and safety studies performed. | [287] |
| Phase 2 | Enroll hundreds of subjects and do dose-ranging studies. | |
| Phase 3 | Provide critical documentation about important and effectiveness additional safety data required for licensing by enrolling thousands of people |
Numerous lives have been saved by vaccination. In this procedure, immune system is recruited and boosted to identify and to cope with targets of choice i.e. bacteria and viruses. Immediate destruction of these germs occurs in encounter if the body is re-infected. This program currently protects 2.3 million people from dying due to measles, influenza, pertussis, tetanus and diphtheria including more than 20 deadly diseases. To get a safer vaccine against COVID-19 a remarkable work is ongoing. Safety index of vaccine is improved by using advance type of vaccines which comprises of a specific antigenic protein or a vector containing disease causing parts of pathogen instead of using them as whole. This type of advancement is achieved after studying modes of the disease production and structural orientations of causative agents. If pathogen is new then this process takes a long time to develop its vaccine. It was our luck that COVID-19 virus’ structure is quite similar to already map the Middle East Respiratory Syndrome Coronavirus (MERSCoV) and SARS-CoV [[278], [279], [280]]. It is really a challenge to develop COVID-19 vaccine in short time as normally it is a long duration of 10−15 years [281], as follows:
-
•
Exploratory stage
Selection of candidate that is known as exploratory stage is the first step of development of vaccine. It helps in the identification of foreign matter like viral or bacterial and their toxin which are causing disease; it may take long time [282]. There are 321 candidate vaccine of COVID-19 up till 3rd September 2020, this number is increasing day by day; some of them are under-investigation in pre-clinical and clinical phase [283].
-
•
Pre-clinical stage
This phase is known is decision making phase either to carry on the vaccine development the process or to stop it. In this stage, synthetic or natural antigen is designed which induce immune reaction in body, basically to determine the boosting effect of immunity on the safety of people. Animals mostly like Monkeys and mice, as they have biological response alike to human, are used during this phase. The progress of pre-clinical phase is clinical Phases [284,285].
-
•
Clinical development
It consists of three phases 1, 2 & 3 (Table 9 and Chart 2 ). In these phases, safety trails are done in small group then on large population. Clinical response is evaluated among 10–100, 100–3000 and at large scale in human subjects in phase 1, 2 & 3, respectively. Phase 1 is known as Safety phase in which appropriate dose, safety and immune response of vaccine is checked by injecting vaccine in immunocompetent and healthy individuals. Phase 2 is known as expanded safety in which same parameters are checked against different groups (age and gender wise). Phase 3 is known as Efficacy in which vaccine is injected to thousands people for the evaluation of efficacy of vaccine; percentage with which reduce incidence of disease rate as compared to placebo. In pandemic diseases like COVID-19 Phase 1 & 2 are combined for the development of vaccine [273,[285], [286], [287]].
-
•
Regulatory review and approval
Chart 2.
Difference between the classical and emergency vaccine phases [278,294].
If a vaccine displays success in these trials, it fills a Biologics License Application (BLA). After that, a team of reviewer of FDA including medical officers, chemist's microbiologists, and biostatisticians check and assess the safety and efficacy information and decide either vaccine is recommended or rejected. Now sponsor and FAD present their review in font of Vaccines and Related Biological Products Advisory Committee (VRBPAC) of FDA. After acceptance from VRBPAC, vaccine is being labeled for getting to understand about proper use of vaccine for consumer [287,288].
-
•
Manufacturing and Quality control
A vaccine’s manufacturing is lengthy, time-consuming and complex process, which is different from the making of other medicines. Generally, the developmental period for a vaccine is about 12−15 years. In traditional vaccines, antigens are injected into the body, obtained from half-active or inactive bacteria or attenuated viruses with various clinical methods. But during emergency situations like COVID-19, the development of vaccine will not trailed repeatedly like in normal vaccine because of the high demand of spontaneous action by the disease and also compatible with the specification of improved strategy WHO for targeted patients (chart 2) [289]. For the infections that show repeated epidemic behavior, the inter-epidemic period can relate to parameters that characterizing the infection like infectious and latent periods, the average age of first infection, this relation are agreed with the data for various childhood diseases. For the eradication of a disease, the criterion is given in terms of the age-specific vaccination schedule and the proportion of the population to be vaccinated [[290], [291], [292], [293]].
A general mechanism of action of vaccine
Basically, a vaccine activates the body immunity, identifies and responds against the antigens. For the activation of immune response, certain substances, which are called as antigen that exists on that specific pathogens are, introduced either in live attenuated or dead form. After this, the immune system easily learns to identify them as foreign invaders and make certain molecules that are known as antibodies and recall them for the future. If that specific pathogen enter again into the body, the immune system identifies this antigens sharply and attack aggressively well, before the infection can spread and cause sickness [295].
Mechanism of action of corona vaccine
For vaccine manufacturing, the current antigen is the coronavirus spike protein, named as because there sits, atop of the spike of a coronavirus particle. This part is the immune system “remembers” of the virus. The immune system made vaccine-induced antibodies against these spike protein and can prevent against infection. In the Precision Vaccines Program (PVP) strategy, we combine these spike protein of coronavirus with adjuvants i.e. little molecules introduced in a vaccine to enhance the immune response of the recipient. This study is also under process to the live animal, mice [296].
COVID-19 vaccine mechanism of action referred with pasteurization
Pasteur Institute is doing this procedure of RNA/DNA based COVID-19 vaccine formation. In this procedure take a virus like measles and change it with the protein of coronavirus [297].
Nanomaterial based composites as antimicrobial and nano vaccine
As nano materials are an ideal for the delivery of antigen as adjuvants, they help in the designing of modern vaccine [298,299]. For the prevention of adhesion of microbes or killing the microorganisms, antimicrobial; Nano compounds are incorporated within, on the surface of materials or coating to the biofilms which represents the best strategy in the challenging field which is increasing [300]. In 2003, it is considered that the updated class of antibiotic was introduced and those diseases that are infectious have become challenged due to the resistance of multidrug [301]. It is highly need of modern strategies against the microbial infection during the era of post antibiotic, when there is resistance of two or more classes of most timely antibiotics [301]. Biomedical devices that have antimicrobial metal nanostructures took the valuable attention not only from pharmaceutical industry but also from academia by providing microbicidal strategy [302]. Antimicrobial nanomaterials are classified into two categories; one is intrinsically antimicrobial second that have both functional properties [300].
Viruses can be considered as nano microorganisms, as they are nanoscale objects. The same scale of length is used in the operate of viruses and nanoparticles; it helps in the development of vaccine and immunoengineering. So, it is a potential characteristic for the use of nanotechnology approaches. The synthetically natural nanoparticles mimic structural feature of viruses; biotechnology, nanochemistry, and chemical biology help in the technologies of the vaccine design, which used in the next generation. From a vaccine technology development point of view, this is an exciting time of nanotechnologically novel approaches poised to make a clinical impact for the first time [298].
Nano- nucleic acid-based vaccines
mRNA vaccines has more stability than DNA as it has no integrating, risk of mutagenesis insertion, and its stability, immunogenicity and half-life can be changed via established modifications [303]. Researchers at the Imperial College of London and Arcturus Therapeutics are trying to enhance the short half-life of the RNA and boosting S protein expression levels via incorporation of self-amplifying RNA technology [304]. Nanotechnology-based methodology solves delivery challenge problems by channelizing the vaccine to targeted sub-cellular and cellular domains. The delivery of DNA vaccines across cell membranes and targeted sites can be done using cationic liposomes and polymeric nanoparticles [305]. Nanotechnology is a platform including liposomes, polysaccharide particles, and cationic nanoemusions; it has been used for the delivery and stability of mRNA based vaccines [306,307].
Nano-Subunit vaccines
Subunit vaccines can also serve as a source of protein nanoparticles or virus-like particles (VLPs). Using recombinant expression, VLP vaccines can be synthesized allowing genetic engineering to incorporate ligands, immunomodulators. Self-assembled nanoparticles and VLPs provide highly organized, stable and monodisperse vaccine formulations in addition to scalable production via fermentation or molecular farming. Contextually, Nicotiana benthamiana is being utilized by Medicago and iBio in order to produce VLPs using the S protein. AdaptVac/ExpreS2ion is manufacturing VLPs from the S2 protein utilizing expression system of insect cell. Their expression display on proteinaceous biomaterial scaffolds such as ferritin, encapsulin, and bacteriophage. VLPs are also being processed to achieve multivalent antigen for the purpose of enhanced immunogenicity [298].
Subunit vaccines can constitute viral proteins incorporated in synthetic nanomaterials, protein cages and VLPs, which serve as adjuvants and delivery vehicles; Influenza virus vaccine Crucell (Janssen, Johnson & Johnson), liposome-base formulation, incorporating influenza protein haemagglutinin is one of its application [308].
Nano-Peptide-based vaccines
The simplest of vaccines, relatively easy to design, readily manufactured and validated are peptide-based vaccine [309]. Their efficacy hinges mainly on availability of adjuvants and delivery system. Nanoparticle vaccine efficacy can be enhanced using various strategies targeting lymph nodes (LNs) or cellular subsets and their immune profiles can be tailored to address specific diseases, like ‘albumin hitchhiking’ strategy utilizes the natural trafficking tendency of albumin to LNs [310].
Peptide-based vaccines can be utilized in formulations of peptides plus adjuvant mixtures, in nucleic-acid vaccines, and for peptide delivery using an appropriate nanocarrier. Several clinical trials regarding peptide-based vaccines as well as peptide–nanoparticle conjugates targeting chronic diseases and cancer are in development [311].
Industries and academic institutes such as OncoGen and University of Cambridge/DIOSynVax are using immunoinformatics-derived peptide sequences of S protein in their vaccine formulations in addition to developing peptide-based COVID-19 vaccine. Pragmatically, use of B-cells epitopes works due to its universality in relation to the HLA-restricted T-cell specific vaccines [312,313].
Update towards COVID-19 vaccination
It is an urgent need for a new method to be emerged, for the assessment of vaccine that is growing with the parameters like immunity. For the better understanding of the response of vaccine heterogenecity with immunity, it is mandatory highlight the molecular signature of vaccine [314].
Critical thinking about vaccine trail
Multiple research groups have designed potential vaccines; however, there is a need of much more work to do. Like during trail, it should be safe, it boost up the immune status of the body, for the use of large scale it have to be developed billions potential doses so overcome the challenges of logistics, it must be approved by the regulators of medicines [315].
Latest update by WHO
The COVAX Facility, a mechanism designed to guarantee rapid, fair and equitable access to COVID-19 vaccines worldwide, secured engagement from more than 150 countries, representing over 60% of the world’s population.
Seventy-five countries have expressed interest in financing the vaccines from their own public finance budgets and partnering with up to 90 lower-income countries that could be supported through the COVAX Advance Market Commitment. The COVAX Facility forms a key part of the Access to COVID-19 Tools (ACT) Accelerator’s vaccines pillar, which is co-led by WHO [316].
Conclusion
Here in this review, it is for a systematic index of the overviewing background of COVID-19 vaccination/public health; I) Discussing of viral structure and life cycle of SARS-CoV-2 as owing a comprehensive visualization about the key factor of the pandemic, II) COVID-19 as in view of clinical symptoms and the diagnostics tools to detect the infection showing the secondary bacterial infection of COVID-19, III) the currently therapeutic protocols for COVID-19 uncovering the potential role of nanotechnology as COVID-19 fighter, as well as, COVID-19 therapy in view of precision medicine, and IV) COVID-19 Communication risks; socio-economic risks of the pandemic, and the socio-medical precautionary measures as means of achieving "Flattening of the pandemic curve", alarming the point of preserving the drugs supplies/purchase. As well as we have integrated the next landscape of vaccination therapeutic approaches as ultimately global seeks to tackle COVID-19 pandemic.
There is a prime requirement to respond for vaccines formulation within no time in this pandemic via following the same protocols for MERS-CoV and SARS-CoV, as an emergent vaccination discovery approach to skip some initial steps of vaccine development. Adoption of pre-established protocols, toxicological and preclinical interpretations of closely related vaccines is possible for developing a new one.
Conflict of interest
Marwan ElBagoury and Yahia Aktham are employees of Sanofi Genzyme.
Acknowledgement
We would like to express our full acknowledgement to BioRender [https://biorender.com/] as online tool for creating the figures in this review.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jiph.2020.12.025.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Organization WH . 2020. Pneumonia of unknown cause – china. Disease Outbreak News. [Google Scholar]
- 2.WHO . WHO Bulletin; 2020. Novel coronavirus (2019-nCoV) pp. 1–7. [Google Scholar]
- 3.Health Organization . Who; 2020. 2019 novel coronavirus (2019-nCoV): strategic preparedness and response plan; p. 28. [Google Scholar]
- 4.Scott J. The economic, geopolitical and health impacts of COVID-19. World Economic Forum. 2020;565:1–5. [Google Scholar]
- 5.World Health Organization . World Health Organisation; 2020. Public statement for collaboration on COVID-19 vaccine development. [Google Scholar]
- 6.OMS . World Health Organization; 2020. WHO coronavirus disease (COVID-19) dashboard. [Google Scholar]
- 7.Wang X.F., Yuan J., Zheng Y.J., Chen J., Bao Y.M., Wang Y.R. Clinical and epidemiological characteristics of 34 children with 2019 novel coronavirus infection in Shenzhen. Zhonghua er ke za zhi = Chinese J. Pediatr. 2020;58:E008. doi: 10.3760/cma.j.issn.0578-1310.2020.0008. [DOI] [PubMed] [Google Scholar]
- 8.Forman R., Atun R., McKee M., Mossialos E. 12 Lessons learned from the management of the coronavirus pandemic. Health Policy (New York) 2020;124:577–580. doi: 10.1016/j.healthpol.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKeever A. National Geographic; 2020. Dozens of COVID-19 vaccines are in development. Here are the ones to follow. Jul 31. [Google Scholar]
- 10.Virus Replication . 2011. Fenner’s veterinary virology; pp. 21–42. [DOI] [Google Scholar]
- 11.Magden J., Kääriäinen L., Ahola T. Inhibitors of virus replication: recent developments and prospects. Appl Microbiol Biotechnol. 2005;66:612–621. doi: 10.1007/s00253-004-1783-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Groot R.J., Baker S.C., Baric R.S., Brown C.S., Drosten C., Enjuanes L. Middle east respiratory syndrome coronavirus (MERS-CoV): announcement of the coronavirus study group. J Virol. 2013;87:7790–7792. doi: 10.1128/jvi.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehr A.R., Perlman S. Vol. 1282. Springer; New York: 2015. Coronaviruses: an overview of their replication and pathogenesis; pp. 1–23. (In coronaviruses: methods and protocols). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Version D. Cross-sectional seroepidemiologic study of MERS-CoV infection in high risk populations in contact with dromedary camels. J Clin Diagn Res. 2013;7:473–479. [Google Scholar]
- 15.Lisa Lockerd Maragakis. Coronavirus Disease 2019 vs. the Flu. Johns Hopkins Medicine.
- 16.WHO . WHO Bulletin; 2020. Novel coronavirus (2019-nCoV) pp. 1–7. [Google Scholar]
- 17.OMS . 2020. Coronavirus disease 2019 (COVID-2019)- Situation report- 51. Situation Report; pp. 1–9. [Google Scholar]
- 18.Organization WH . WHO; 2020. Q&A on coronaviruses (COVID-19) [Google Scholar]
- 19.Practice B.B. World Health Organization 2020; 2019. Coronavirus disease 2019 (COVID-19) Situation Report –23.03.2020; p. 2633. [DOI] [Google Scholar]
- 20.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riou J., Hauser A., Counotte M.J., Althaus C.L. Adjusted age-specific case fatality ratio during the COVID-19 epidemic in Hubei, China. MedRxiv n.d. 10.1101/2020.03.04.20031104. [DOI]
- 22.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P. Comorbidity and its impact on patients with COVID-19. SN Comprehensive Clinical Medicine. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organisation . 2020. Strategic preparedness and response plan; p. 18. [Google Scholar]
- 24.Manjula Bai H. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. ComFin Research. 2020;8:8–17. doi: 10.34293/commerce.v8i4.3293. [DOI] [Google Scholar]
- 25.De Guzman R., Malik M. Dual challenge of Cancer and COVID-19: impact on health care and socioeconomic systems in Asia Pacific. JCO Global Oncology. 2020:906–912. doi: 10.1200/go.20.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babuna P., Yang X., Gyilbag A., Awudi D.A., Ngmenbelle D., Bian D. The impact of covid-19 on the insurance industry. Int J Environ Res Public Health. 2020;17:1–14. doi: 10.3390/ijerph17165766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark E., Fredricksid K., Woc-Colburn L., Bottazzi M.E., Weatherheadid J. Disproportionate impact of the COVID-19 pandemic on immigrant communities in the United States. PLoS Negl Trop Dis. 2020;14:1–9. doi: 10.1371/journal.pntd.0008484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sönmez S., Apostolopoulos Y., Lemke M.K., (Jerrie) Hsieh Y.C. Understanding the effects of COVID-19 on the health and safety of immigrant hospitality workers in the United States. Tourism Manage Perspect. 2020;35 doi: 10.1016/j.tmp.2020.100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danquah M., Schotte S., Sen K. COVID-19 and Employment: Insights from the Sub-Saharan African Experience. Indian J Labour Econ. 2020;63:23–30. doi: 10.1007/s41027-020-00251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youth Team in the Employment, Labour Markets and Youth Branch ILO (ILO) 2020. Youth & Covid-19: impacts on jobs, education, rights and mental well-being; pp. 1–55. [Google Scholar]
- 31.Center on Budget and Policy Priorities . 2020. Tracking the COVID-19 recession’s effects on food, housing, and employment hardships. [Google Scholar]
- 32.Martin A., Markhvida M., Hallegatte S., Walsh B. Socio-economic impacts of COVID-19 on household consumption and poverty. Econ Disasters Clim Chang. 2020;4:453–479. doi: 10.1007/s41885-020-00070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.2020. European centre for disease prevention and control. Infection prevention and control for COVID-19 in healthcare settings. elsevier’s novel coronavirus information center; pp. 3–6. [Google Scholar]
- 34.D.S. H Epidemic and emerging coronaviruses (Severe acute respiratory syndrome and middle east respiratory syndrome) Clin Chest Med. 2017;38:71–86. doi: 10.1016/j.ccm.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ECDC . Elsevier’s Novel Coronavirus Information Center; 2020. Infection prevention and control for COVID-19 in healthcare settings; pp. 3–6. [Google Scholar]
- 36.Badreldin H.A., Atallah B. Research in Social and Administrative Pharmacy; 2020. Global drug shortages due to COVID-19: impact on patient care and mitigation strategies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodcock J. US Food and Drug Administration; 2019. Safeguarding pharmaceutical supply chains in a global economy; pp. 1–13. [Google Scholar]
- 38.Srivastava B.S. . The Economic Times; 2020. India to boost drug ingredient output to pare China reliance. Apr 14. [Google Scholar]
- 39.Singh T.U., Parida S., Lingaraju M.C., Kesavan M., Kumar D., Singh R.K. Drug repurposing approach to fight COVID-19. Pharmacol Rep. 2020 doi: 10.1007/s43440-020-00155-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senger M.R., Evangelista T.C.S., Dantas R.F., Santana MV da S., Gonçalves L.C.S., Neto LR de S COVID-19: molecular targets, drug repurposing and new avenues for drug discovery. Mem Inst Oswaldo Cruz. 2020;115:1–32. doi: 10.1590/0074-02760200254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pawar A.Y. Combating devastating COVID-19 by drug repurposing. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan S.A., Al-Balushi K. Combating COVID-19: The role of drug repurposing and medicinal plants. J Infect Public Health. 2020 doi: 10.1016/j.jiph.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Manli, Cao Ruiyuan, Zhang Leike, Yang Xinglou, Liu Jia, Xu Mingyue. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.FDA . Press Announcements; 2020. Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment; pp. 3–5. [Google Scholar]
- 45.NeuroRx I., SA RTH Intravenous aviptadil for COVID-19 associated acute respiratory distress. ClinicalTrials. 2020:1–8. [Google Scholar]
- 46.Nct. Inhaled Aviptadil for the Prevention of COVID-19 Related ARDS. Https://ClinicaltrialsGov/Show/NCT04536350 2020.
- 47.Ghasemiyeh P., Mohammadi-Samani S. COVID-19 outbreak: challenges in pharmacotherapy based on pharmacokinetic and pharmacodynamic aspects of drug therapy in patients with moderate to severe infection. Heart and Lung. 2020;49:763–773. doi: 10.1016/j.hrtlng.2020.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahase E. Covid-19: Low dose steroid cuts death in ventilated patients by one third, trial finds. BMJ. 2020;369:m2422. doi: 10.1136/bmj.m2422. [DOI] [PubMed] [Google Scholar]
- 49.Hassan M.E., Hasan H.M., Sridharan K., Elkady A., ElSeirafi M.M. Dexamethasone in severe COVID-19 infection: a case series. Respir Med Case Rep. 2020;31 doi: 10.1016/j.rmcr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atal S., Fatima Z. IL-6 Inhibitors in the Treatment of Serious COVID-19: A Promising Therapy? Pharmaceut Med. 2020;34:223–231. doi: 10.1007/s40290-020-00342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samaee H., Mohsenzadegan M., Ala S., Maroufi S.S., Moradimajd P. Tocilizumab for treatment patients with COVID-19: recommended medication for novel disease. Int Immunopharmacol. 2020;89 doi: 10.1016/j.intimp.2020.107018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. The Lancet Rheumatology. 2020;2:e474–84. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou D., Dai S.M., Tong Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667–1670. doi: 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baghaki S., Yalcin C.E., Baghaki H.S., Aydin S.Y., Daghan B., Yavuz E. COX2 inhibition in the treatment of COVID-19: review of literature to propose repositioning of celecoxib for randomized controlled studies. Int J Infect Dis. 2020;101:29–32. doi: 10.1016/j.ijid.2020.09.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Favalli E.G., Ingegnoli F., De Lucia O., Cincinelli G., Cimaz R., Caporali R. COVID-19 infection and rheumatoid arthritis: Faraway, so close! Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Ippolito D., Pisano M. Dupilumab (Dupixent): an Interleukin-4 receptor antagonist for atopic dermatitis. P T. 2018;43:532–535. [PMC free article] [PubMed] [Google Scholar]
- 59.Eshtiaghi P., Gooderham M.J. Dupilumab: an evidence-based review of its potential in the treatment of atopic dermatitis. Core Evid. 2018;13:13–20. doi: 10.2147/ce.s133661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thangaraju P., Venkatesan N., Sudha T.Y.S., Venkatesan S., Thangaraju E. Role of Dupilumab in approved indications of COVID-19 patient: an efficacy-based nonsystematic critical analysis. SN Comprehensive Clinical Medicine. 2020 doi: 10.1007/s42399-020-00510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen C.L., Der Wang S., Zeng Z.Y., Lin K.J., Te Kao S., Tani T. Serine protease inhibitors nafamostat mesilate and gabexate mesilate attenuate allergen-induced airway inflammation and eosinophilia in a murine model of asthma. J Allergy Clin Immunol. 2006;118:105–112. doi: 10.1016/j.jaci.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 62.Hoffmann M., Schroeder S., Kleine-Weber H., Müller M.A., Drosten C., Pöhlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiso M., Sakai-Tagawa Y., Iwatsuki-Horimoto K., Imai M., Takeda M. The anticoagulant nafamostat potently inhibits SARS-CoV-2 S protein-mediated fusion in a cell fusion assay system and viral infection in vitro in a cell-type-dependent manner. Viruses. 2020;12 doi: 10.3390/v12060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.A. C, J. S Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4:1023–1033. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 66.Meini S., Pagotto A., Longo B., Vendramin I., Pecori D., Tascini C. Role of Lopinavir/Ritonavir in the treatment of Covid-19: a review of current evidence, guideline recommendations, and perspectives. J Clin Med. 2020;9:2050. doi: 10.3390/jcm9072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khalili J.S., Zhu H., Mak N.S.A., Yan Y., Zhu Y. Novel coronavirus treatment with ribavirin: groundwork for an evaluation concerning COVID-19. J Med Virol. 2020;92:740–746. doi: 10.1002/jmv.25798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tong S., Su Y., Yu Y., Wu C., Chen J., Wang S. Ribavirin therapy for severe COVID-19: a retrospective cohort study. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sultana J., Cutroneo P.M., Crisafulli S., Puglisi G., Caramori G., Trifirò G. Azithromycin in COVID-19 patients: pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Saf. 2020;43:691–698. doi: 10.1007/s40264-020-00976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gibbert K., Schlaak J.F., Yang D., Dittmer U. IFN-α subtypes: distinct biological activities in anti-viral therapy. Br J Pharmacol. 2013;168:1048–1058. doi: 10.1111/bph.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang B., Li D., Liu T., Wang H., Luo F., Liu Y. Subcutaneous injection of IFN alpha-2b for COVID-19: an observational study. BMC Infect Dis. 2020;20 doi: 10.1186/s12879-020-05425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou Q., Chen V., Shannon C.P., Wei X.S., Xiang X., Wang X. Interferon-α2b treatment for COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pereda R., González D., Rivero H.B., Rivero J.C., Pérez A., López L.D.R. Therapeutic effectiveness of interferon-α2b against COVID-19: the cuban experience. J Interferon Cytokine Res. 2020;40:438–442. doi: 10.1089/jir.2020.0124. [DOI] [PubMed] [Google Scholar]
- 74.Baron S.A., Devaux C., Colson P., Raoult D., Rolain J.M. Teicoplanin: an alternative drug for the treatment of COVID-19? Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C. Glycopeptide antibiotics potently inhibit cathepsin l in the late Endosome/Lysosome and block the entry of ebola, MERS-CoV, and SARS-CoV viruses. J Biol Chem. 2016 doi: 10.1074/jbc.M116.716100. jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang J., Ma X., Yu F., Liu J., Zou F., Pan T. 2020. Teicoplanin potently blocks the cell entry of 2019-nCoV. [DOI] [Google Scholar]
- 77.Agrawal U., Raju R., Udwadia Z.F. Favipiravir: a new and emerging antiviral option in COVID-19. Med J Armed Forces India. 2020;76:370–376. doi: 10.1016/j.mjafi.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shiraki K., Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209 doi: 10.1016/j.pharmthera.2020.107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kiselev O.I., Maleev V.V., Deeva E.G., Leneva I.A., Selkova E.P., Osipova E.A. Clinical efficacy of Arbidol (umifenovir) in the therapy of influenza in adults: preliminary results of the multicenter double-blind randomized placebo-controlled study ARBITR. Ter Arkh. 2015;87:88–96. doi: 10.17116/terarkh201587188-96. [DOI] [PubMed] [Google Scholar]
- 80.Huang D., Yu H., Wang T., Yang H., Yao R., Liang Z. Efficacy and safety of umifenovir for coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoang X., Shaw G., Fang W., Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng R.Z., Kogan M., Davis D. Ascorbate as prophylaxis and therapy for COVID-19—update from Shanghai and U.S. Medical institutions. Glob Adv Health Med. 2020;9 doi: 10.1177/2164956120934768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hemilä H. Vitamin C and SARS coronavirus [6] J Antimicrob Chemother. 2003;52:1049–1050. doi: 10.1093/jac/dkh002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jovic T.H., Ali S.R., Ibrahim N., Jessop Z.M., Tarassoli S.P., Dobbs T.D. Could vitamins help in the fight against covid-19? Nutrients. 2020;12:1–30. doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Canga A.G., Prieto A.M.S., Diez Liébana M.J., Martínez N.F., Sierra Vega M., García Vieitez J.J. The pharmacokinetics and interactions of ivermectin in humans - A mini-review. AAPS J. 2008;10:42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heidary F., Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot. 2020;73:593–602. doi: 10.1038/s41429-020-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C. Glycopeptide antibiotics potently inhibit cathepsin l in the late Endosome/Lysosome and block the entry of ebola, MERS-CoV, and SARS-CoV viruses. J Biol Chem. 2016 doi: 10.1074/jbc.M116.716100. jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rubbert-Roth A., Perniok A. [Interleukin-1 receptor antagonist anakinra (Kineret) for treatment of rheumatic arthritis] Z Rheumatol. 2003;62:367–377. doi: 10.1007/s00393-003-0545-4. [DOI] [PubMed] [Google Scholar]
- 91.Martin-Silva N., de Boysson H., Aouba A. Anakinra for patients with COVID-19. The Lancet Rheumatology. 2020;2:e382–3. doi: 10.1016/S2665-9913(20)30178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Filocamo G., Mangioni D., Tagliabue P., Aliberti S., Costantino G., Minoia F. Use of anakinra in severe COVID-19: a case report. Int J Infect Dis. 2020;96:607–609. doi: 10.1016/j.ijid.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Langer-Gould A., Smith J.B., Gonzales E.G., Castillo R.D., Figueroa J.G., Ramanathan A. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infect Dis. 2020;99:291–297. doi: 10.1016/j.ijid.2020.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020;146:213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raedler L.A. Jakafi (Ruxolitinib): first FDA-Approved medication for the treatment of patients with polycythemia Vera. Am Health Drug Benefits. 2015;8:75–79. [PMC free article] [PubMed] [Google Scholar]
- 96.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146 doi: 10.1016/j.jaci.2020.05.019. 137-146.e3. https://doi.org/10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Booth M. 2020. Incyte announces plans to initiate a phase 3 clinical trial of ruxolitinib (Jakafi®) as a treatment for patients with COVID-19 associated cytokine storm incyte corporation. [Google Scholar]
- 98.Papadopoulos C., Patoulias D., Teperikidis E., Mouselimis D., Tsarouchas A., Toumpourleka M. Colchicine as a potential therapeutic agent against cardiovascular complications of COVID-19: an exploratory review. SN Comprehensive Clinical Medicine. 2020;2:1419–1429. doi: 10.1007/s42399-020-00421-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schlesinger N., Firestein B.L., Brunetti L. Colchicine in COVID-19: an old drug, new use. Curr Pharmacol Rep. 2020;6:137–145. doi: 10.1007/s40495-020-00225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Batya Swift Yasgur . 2020. Colchicine promising in COVID-19 treatment? Medscape pediatrics. [Google Scholar]
- 101.Della-Torre E., Ramirez G.A., Dagna L., Tresoldi M. Colchicine treatment in community healthcare setting to prevent severe COVID-19. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218759. annrheumdis-2020-218759. [DOI] [PubMed] [Google Scholar]
- 102.Deftereos S., Giannopoulos G., Vrachatis D.A., Siasos G., Giotaki S.G., Cleman M. Colchicine as a potent anti-inflammatory treatment in COVID-19: can we teach an old dog new tricks? Eur Heart J Cardiovasc Pharmacother. 2020;6:255. doi: 10.1093/ehjcvp/pvaa033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu S.T.H., Lin H.M., Baine I., Wajnberg A., Gumprecht J.P., Rahman F. Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study. Nat Med. 2020 doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 104.U.S. Food and Drug Administration (FDA) 2020. Recommendations for investigational COVID-19 convalescent plasma. Food and drug administration. [Google Scholar]
- 105.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M. Convalescent plasma: New evidence for an old therapeutic tool? Blood Transfusion. 2016;14:152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pourahmad R., Moazzami B., Rezaei N. Efficacy of plasmapheresis and immunoglobulin replacement therapy (IVIG) on patients with COVID-19. SN Comprehensive Clinical Medicine. 2020;2:1407–1411. doi: 10.1007/s42399-020-00438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Muyldermans S. Nanobodies: Natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–797. doi: 10.1146/annurev-biochem-063011-092449. [DOI] [PubMed] [Google Scholar]
- 109.Konwarh R. Nanobodies: Prospects of Expanding the Gamut of Neutralizing Antibodies Against the Novel Coronavirus, SARS-CoV-2. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martinez-Delgado G. Inhaled nanobodies against COVID-19. Nat Rev Immunol. 2020;20:593. doi: 10.1038/s41577-020-00443-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hanke L., Perez L.V., Sheward D., Das H., Schulte T., Moliner-Morro A. 2020. An alpaca nanobody neutralizes SARS-CoV-2 by blocking receptor interaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hammond S.M. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.El-Nabi S.H., Elhiti M., El-Sheekh M. A new approach for COVID-19 treatment by micro-RNA. Med Hypotheses. 2020;143 doi: 10.1016/j.mehy.2020.110203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Widiasta A., Sribudiani Y., Nugrahapraja H., Hilmanto D., Sekarwana N., Rachmadi D. Potential role of ACE2-related microRNAs in COVID-19-associated nephropathy. Noncoding RNA Res. 2020;5:153–166. doi: 10.1016/j.ncrna.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nalawansha D.A., Samarasinghe K.T.G. Double-barreled CRISPR technology as a novel treatment strategy for COVID-19. Acs Pharmacol Transl Sci. 2020;3:790–800. doi: 10.1021/acsptsci.0c00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lotfi M., Rezaei N. CRISPR/Cas13: A potential therapeutic option of COVID-19. Biomed Pharmacother. 2020;131 doi: 10.1016/j.biopha.2020.110738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Balakrishnan V.S. Increasing accessibility in COVID-19 clinical trials. The Lancet Microbe. 2020;1:e13. doi: 10.1016/s2666-5247(20)30015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sanofi . 2020. COVID-19: sanofi to donate 100 million doses of hydroxychloroquine across 50 countries. Sanofi Press Release. [Google Scholar]
- 119.Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D. 2020. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. [DOI] [Google Scholar]
- 120.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mitjà O., Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health. 2020;8:e639–40. doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saqrane S., El Mhammedi M.A. Review on the global epidemiological situation and the efficacy of chloroquine and hydroxychloroquine for the treatment of COVID-19. New Microbes New Infect. 2020;35 doi: 10.1016/j.nmni.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zou L., Dai L., Zhang X., Zhang Z., Zhang Z. Hydroxychloroquine and chloroquine: a potential and controversial treatment for COVID-19. Arch Pharm Res. 2020 doi: 10.1007/s12272-020-01258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41:919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 125.Plantone D., Koudriavtseva T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin Drug Investig. 2018;38:653–671. doi: 10.1007/s40261-018-0656-y. [DOI] [PubMed] [Google Scholar]
- 126.Agency EM. COVID-19 : chloroquine and hydroxychloroquine only to be used in clinical trials or emergency use programmes 2020;31:1–2.
- 127.Dörner E., Thomas S. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 128.Frisk‐Holmberg M., Bergqvist Y., Englund U. Chloroquine intoxication [letter] Br J Clin Pharmacol. 1983;15:502–503. doi: 10.1111/j.1365-2125.1983.tb01540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Legssyer R., Josse C., Piette J., Ward R.J., Crichton R.R. Changes in function of iron-loaded alveolar macrophages after in vivo administration of desferrioxamine and/or chloroquine. J Inorg Biochem. 2003;94:36–42. doi: 10.1016/S0162-0134(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 130.Gbinigie K., Frie K. Should chloroquine and hydroxychloroquine be used to treat COVID-19? A rapid review. Bjgp Open. 2020 doi: 10.3399/bjgpopen20X101069. bjgpopen20X101069. https://doi.org/10.3399/bjgpopen20x101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guastalegname M., Vallone A. Could Chloroquine /Hydroxychloroquine Be Harmful in Coronavirus Disease 2019 (COVID-19) Treatment? Clin Infect Dis. 2020;71:888–889. doi: 10.1093/cid/ciaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kow C.S., Merchant H.A., Hasan S.S. Is it worth the wait? Should Chloroquine or Hydroxychloroquine be allowed for immediate use in CoViD-19? Br J Pharm. 2020;5 doi: 10.5920/bjpharm.745. [DOI] [Google Scholar]
- 133.Moore N. Chloroquine for COVID-19 infection. Drug Saf. 2020;43:393–394. doi: 10.1007/s40264-020-00933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma A. Chloroquine paradox may cause more damage than help fight COVID-19. Microbes Infect. 2020;22:154–156. doi: 10.1016/j.micinf.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sharma A., Kumar Sharma S., Shi Y., Bucci E., Carafoli E., Melino G. BCG vaccination policy and preventive chloroquine usage: do they have an impact on COVID-19 pandemic? Cell Death Dis. 2020;11 doi: 10.1038/s41419-020-2720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sturrock B.R.H., Chevassut T.J.T. Chloroquine and COVID-19 – a potential game changer? Clinical Medicine, Journal of the Royal College of Physicians of London. 2020;20 doi: 10.7861/clinmed.2020-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.White N.J., Watson J.A., Hoglund R.M., Chan X.H.S., Cheah P.Y., Tarning J. COVID-19 prevention and treatment : a critical analysis of chloroquine and hydroxychloroquine clinical pharmacology Summary points:1–34. [DOI] [PMC free article] [PubMed]
- 138.World OH . 2020. CoVID-19 strategy up date; p. 18. [Google Scholar]
- 139.Sanofi . 2020. Sanofi joins forces with U.S. Department of Health and Human Services to advance a novel coronavirus vaccine. [Google Scholar]
- 140.Bernasconi V., Kristiansen P.A., Whelan M., Román R.G., Bettis A., Yimer S.A. Developing vaccines against epidemic-prone emerging infectious diseases. Bundesgesundheitsblatt Gesundh Gesundh. 2020;63:65–73. doi: 10.1007/s00103-019-03061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Clinical programme to evaluate Kevzara® as COVID-19 treatment begins https://www.europeanpharmaceuticalreview.com/news/115360/clinical-programme-to-evaluate-kevzara-as-covid-19-treatment-begins/ (accessed May 6, 2020).
- 142.Sanofi: Important Information on Plaquenil® and COVID-19 - Sanofi Lebanon https://lb.sanofi.com/en/media/information-on-plaquenil-and-covid-19 (accessed May 6, 2020).
- 143.Hay A.D., Redmond N.M., Costelloe C., Montgomery A.A., Fletcher M., Hollinghurst S. Paracetamol and ibuprofen for the treatment of fever in children: the PITCH randomised controlled trial. Health Technol Assess (Rockv) 2009;13:1–186. doi: 10.3310/hta13270. [DOI] [PubMed] [Google Scholar]
- 144.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cascella M., Rajnik M., Cuomo A., et al. Features, Evaluation, and Treatment of Coronavirus (COVID-19) [Updated 2020 Aug 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK554776/. [PubMed]
- 148.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dong Y., Dong Y., Mo X., Hu Y., Qi X., Jiang F. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 150.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Han X., Cao Y., Jiang N., Chen Y., Alwalid O., Zhang X. Novel coronavirus disease 2019 (COVID-19) pneumonia progression course in 17 discharged patients: comparison of clinical and thin-section computed tomography features during recovery. Clin Infect Dis. 2020;71:723–731. doi: 10.1093/cid/ciaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pan F., Ye T., Sun P., Gui S., Liang B., Li L. Time course of lung changes at chest CT during recovery from Coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR test results in patients recovered from COVID-19. JAMA - Journal of the American Medical Association. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020 doi: 10.1101/2020.02.06.20020974. [DOI] [Google Scholar]
- 158.Mirzaei R., Goodarzi P., Asadi M., Soltani A., Aljanabi H.A., Jeda A.S. Bacterial co‐infections with SARS‐CoV‐2. IUBMB Life. 2020;72(10):2097–2111. doi: 10.1002/iub.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Beadling C., Slifka M.K. How do viral infections predispose patients to bacterial infections? Curr Opin Infect Dis. 2004;17:185–191. doi: 10.1097/00001432-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 160.Metzger D.W., Sun K. Immune dysfunction and bacterial coinfections following influenza. J Immunol. 2013;191(5):2047–2052. doi: 10.4049/jimmunol.1301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jia L., Xie J., Zhao J., Cao D., Liang Y., Hou X. Mechanisms of severe mortality-associated bacterial co-infections following influenza virus infection. Front Cell Infect Microbiol. 2017;(7):338. doi: 10.3389/fcimb.2017.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Katsurada N., Suzuki M., Aoshima M., Yaegashi M., Ishifuji T., Asoh N. Adult Pneumonia Study Group-Japan. The impact of virus infections on pneumonia mortality is complex in adults: a prospective multicentre observational study. BMC Infect Dis. 2017;17(1):755. doi: 10.1186/s12879-017-2858-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Quah J., Jiang B., Tan P.C., Siau C., Tan T.Y. Impact of microbial Aetiology on mortality in severe community-acquired pneumonia. BMC Infect Dis. 2018;18(1):451. doi: 10.1186/s12879-018-3366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.MacIntyre C.R., Bui C.M. Pandemics, public health emergencies and antimicrobial resistance-putting the threat in an epidemiologic and risk analysis context. Arch Public Health. 2017;75(1):1–6. doi: 10.1186/s13690-017-0223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rasmussen S.A., Smulian J.C., Lednicky J.A., Wen T.S., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) and Pregnancy: What obstetricians need to know. Am J Obstet Gynecol. 2020;(222):415–426. doi: 10.1016/j.ajog.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ritchie A.I., Singanayagam A. Immunosuppression for hyperinflammation in COVID-19: a double-edged sword? Lancet. 2020;395:1111. doi: 10.1016/S0140-6736(20)30691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tetro J.A. Is COVID-19 receiving ADE from other coronaviruses? Microbes Infect. 2020;22(2):72–73. doi: 10.1016/j.micinf.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rowe H.M., Meliopoulos V.A., Iverson A., Bomme P., Schultz-Cherry S., Rosch J.W. Direct interactions with influenza promote bacterial adherence during respiratory infections. Nat Microbiol. 2019;4(8):1328–1336. doi: 10.1038/s41564-019-0447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Taneja R., Sharma A.P., Hallett M.B., Findlay G.P., Morris M.R. Immature circulating neutrophils in sepsis have impaired phagocytosis and calcium signaling. Shock. 2008;30(6):618–622. doi: 10.1097/SHK.0b013e318173ef9c. [DOI] [PubMed] [Google Scholar]
- 170.Grudzinska F.S., Brodlie M., Scholefield B.R., Jackson T., Scott A., Thickett D.R. Neutrophils in community-acquired pneumonia: parallels in dysfunction at the extremes of age. Thorax. 2020;75(2):164–171. doi: 10.1136/thoraxjnl-2018-212826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ciencewicki J., Gowdy K., Krantz Q.T., Linak W.P., Brighton L., Gilmour M.I. Diesel exhaust enhanced susceptibility to influenza infection is associated with decreased surfactant protein expression. Inhal Toxicol. 2007;19(14):1121–1133. doi: 10.1080/08958370701665426. [DOI] [PubMed] [Google Scholar]
- 172.Kongchanagul A., Suptawiwat O., Boonarkart C., Kitphati R., Puthavathana P., Uiprasertkul M. Decreased expression of surfactant protein D mRNA in human lungs in fatal cases of H5N1 avian influenza. J Med Virol. 2011;83(8):1410–1417. doi: 10.1002/jmv.22105. [DOI] [PubMed] [Google Scholar]
- 173.Hanada S., Pirzadeh M., Carver K.Y., Deng J.C. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front Immunol. 2018;9:2640. doi: 10.3389/fimmu.2018.02640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Braciale T.J., Sun J., Kim T.S. Regulating the adaptive immune response to respiratory virus infection. Nat Rev Immunol. 2012;12:295–305. doi: 10.1038/nri3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ghoneim H.E., Thomas P.G., McCullers J.A. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol. 2013;191(3):1250–1259. doi: 10.4049/jimmunol.1300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sun K., Metzger D.W. Influenza infection suppresses NADPH oxidase–dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J Immunol. 2014;192(7):3301–3307. doi: 10.4049/jimmunol.1303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bakaletz L.O. Viral–bacterial co-infections in the respiratory tract. Curr Opin Microbiol. 2017;35:30–35. doi: 10.1016/j.mib.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Qin Z., Yang Y., Wang H., Luo J., Huang X., You J. Role of autophagy and apoptosis in the postinfluenza bacterial pneumonia. Biomed Res Int. 2016:2016. doi: 10.1155/2016/3801026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sadler A.J., Williams B.R. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8(7):559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shahangian A., Chow E.K., Tian X., Kang J.R., Ghaffari A., Liu S.Y. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119(7) doi: 10.1172/JCI35412. 1910-20. https://doi.org/10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Melvin J.A., Bomberger J.M. Compromised defenses: exploitation of epithelial responses during viral-bacterial Co-infection of the respiratory tract. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hendricks M.R., Lashua L.P., Fischer D.K., Flitter B.A., Eichinger K.M., Durbin J.E. Respiratory syncytial virus infection enhances pseudomonas aeruginosa biofilm growth through dysregulation of nutritional immunity. Proc Natl Acad Sci USA. 2016;113:1642–1647. doi: 10.1073/pnas.1516979113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hennigar S.R., McClung J.P. Nutritional immunity: starving pathogens of trace minerals. Am J Lifestyle Med. 2016;10(3):170–173. doi: 10.1177/1559827616629117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Siegel S.J., Roche A.M., Weiser J.N. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe. 2014;16(1):55–67. doi: 10.1016/j.chom.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pawlowski A., Jansson M., Sköld M., Rottenberg M.E., Källenius G. Tuberculosis and HIV co-infection. PLoS Pathog. 2012;8(2) doi: 10.1371/journal.ppat.1002464. e1002464. https://doi.org/10.1371/journal.ppat.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vittor A.Y., Garland J.M., Gilman R.H. Molecular diagnosis of TB in the HIV positive population. Ann Glob Health. 2014;80(6):476–485. doi: 10.1016/j.aogh.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 187.Conenello G.M., Zamarin D., Perrone L.A., Tumpey T., Palese P. A single mutation in the PB1-F2 of H5N1 (HK/97) and 1918 influenza A viruses contributes to increased virulence. PLoS Pathog. 2007;3(10):e141. doi: 10.1371/journal.ppat.0030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chattoraj S.S., Ganesan S., Jones A.M., Helm J.M., Comstock A.T., Bright-Thomas R. Rhinovirus infection liberates planktonic bacteria from biofilm and increases chemokine responses in cystic fibrosis airway epithelial cells. Thorax. 2011;66(4):333–339. doi: 10.1136/thx.2010.151431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pettigrew M.M., Marks L.R., Kong Y., Gent J.F., Roche-Hakansson H., Hakansson A.P. Dynamic changes in the Streptococcus pneumoniae transcriptome during transition from biofilm formation to invasive disease upon influenza A virus infection. Infect Immun. 2014;82(11):4607–4619. doi: 10.1128/IAI.02225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chao Y., Marks L.R., Pettigrew M.M., Hakansson A.P. Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front Cell Infect Microbiol. 2015;13(4):194. doi: 10.3389/fcimb.2014.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chopra V., Toner E., Waldhorn R., Washer L. How should U.S. Hospitals prepare for coronavirus disease 2019 (COVID-19)? Ann Intern Med. 2020;172:621–623. doi: 10.7326/M20-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.ACEP news. Journal of the American College of Emergency Physicians 1975;4:75–80. 10.1016/s0361-1124(75)80023-2. [DOI]
- 193.Night S., Rotations S. 2019. How to design the optimal schedule for working shifts; pp. 1–2. [Google Scholar]
- 194.Rouch I., Wild P., Ansiau D., Marquie J.C. Shiftwork experience, age and cognitive performance. Ergonomics. 2005;48:1282–1293. doi: 10.1080/00140130500241670. [DOI] [PubMed] [Google Scholar]
- 195.Livingston E., Desai A., Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic. JAMA - Journal of the American Medical Association. 2020;323:1912–1914. doi: 10.1001/jama.2020.5317. [DOI] [PubMed] [Google Scholar]
- 196.Provenzano D., Rao Y.J., Mitic K., Obaid S.N., Pierce D., Huckenpahler J. Preprints; 2020. Rapid prototyping of reusable 3D-Printed N95 equivalent respirators at the george washington university; pp. 1–9. [DOI] [Google Scholar]
- 197.Government of Canada . 2020. 3D printing and other manufacturing of personal protective equipment in response to COVID-19. [Google Scholar]
- 198.Cai M., Li H., Shen S., Wang Y., Yang Q. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;15:226–234. doi: 10.1080/15459624.2017.1411598. [DOI] [PubMed] [Google Scholar]
- 199.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu R., Wang L., Kuo H.C.D., Shannar A., Peter R., Chou P.J. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Anderson R.M., May R.M. Vaccination and herd immunity to infectious diseases. Nature. 1985;318:323–329. doi: 10.1038/318323a0. [DOI] [PubMed] [Google Scholar]
- 202.Kaur S.P., Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tong C.Y.W., Rosmarin C., Sefton A. 2019. Tutorial topics in infection for the combined infection training programme; p. 482. [Google Scholar]
- 204.Del Giudice G., Rappuoli R. Genetically derived toxoids for use as vaccines and adjuvants. Vaccine. 1999;17 doi: 10.1016/S0264-410X(99)00234-0. [DOI] [PubMed] [Google Scholar]
- 205.World Health Organization . WHO; 2020. Draft landscape of COVID-19 candidate vaccines - 15 May 2020; p. 3. [Google Scholar]
- 206.Cheong K.H. Repurposing vaccines in the fight against COVID-19. BioEssays. 2020;42 doi: 10.1002/bies.202000234. [DOI] [PubMed] [Google Scholar]
- 207.Sharma A.R., Batra G., Kumar M., Mishra A., Singla R., Singh A. BCG as a game-changer to prevent the infection and severity of COVID-19 pandemic? Allergol Immunopathol (Madr) 2020;48:507–517. doi: 10.1016/j.aller.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kamat S., Kumari M. BCG against SARS-CoV-2: second youth of an old age vaccine? Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Melero I., Gato M., Shekarian T., Azna A., Valsesia-Wittmann S., Caux C. Repurposing infectious disease vaccines for intratumoral immunotherapy. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2019-000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Callendret B., Lorin V., Charneau P., Marianneau P., Contamin H., Betton J.M. Heterologous viral RNA export elements improve expression of severe acute respiratory syndrome (SARS) coronavirus spike protein and protective efficacy of DNA vaccines against SARS. Virology. 2007;363:288–302. doi: 10.1016/j.virol.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li L., Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccines. 2016;15:313–329. doi: 10.1586/14760584.2016.1124762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gurunathan S., Klinman D.M., Seder R.A. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000;18:927–974. doi: 10.1146/annurev.immunol.18.1.927. [DOI] [PubMed] [Google Scholar]
- 213.Wang F., Kream R.M., Stefano G.B. An evidence based perspective on mRNA-SARScov-2 vaccine development. Med Sci Monit. 2020;26 doi: 10.12659/MSM.924700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lässer C., O’Neil S.E., Shelke G.V., Sihlbom C., Hansson S.F., Gho Y.S. Exosomes in the nose induce immune cell trafficking and harbour an altered protein cargo in chronic airway inflammation. J Transl Med. 2016;14 doi: 10.1186/s12967-016-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines-a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Calina D., Docea A.O., Petrakis D., Egorov A.M., Ishmukhametov A.A., Gabibov A.G. Towards effective COVID19 vaccines: updates, perspectives and challenges (Review) Int J Mol Med. 2020;46:3–16. doi: 10.3892/ijmm.2020.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lundstrom K. Latest development on RNA-based drugs and vaccines. Future Sci OA. 2018;4 doi: 10.4155/fsoa-2017-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Naresh B.V. A review of the 2019 novel coronavirus (covid-19) pandemic. Asian J Pharm Res. 2020;10:233. doi: 10.5958/2231-5691.2020.00040.4. [DOI] [Google Scholar]
- 219.Kolifarhood G., Aghaali M., Mozafar Saadati H., Taherpour N., Rahimi S., Izadi N. Epidemiological and clinical aspects of COVID-19; a narrative review. Archives of Academic Emergency Medicine. 2020;8:e41. doi: 10.22037/aaem.v8i1.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Faiq M.A., Kumar A., Singh H.N., Pareek V., Qadri R., Raza K. 2020. COVID-19 : a review on molecular basis, pathogenic mechanisms therapeutic aspects and future projections; pp. 1–28. [Google Scholar]
- 221.Hodgson J. The pandemic pipeline. Nat Biotechnol. 2020;38:523–532. doi: 10.1038/d41587-020-00005-z. [DOI] [PubMed] [Google Scholar]
- 222.Shmakov S., Smargon A., Scott D., Cox D., Pyzocha N., Yan W. Diversity and evolution of class 2 CRISPR-Cas systems. Nat Rev Microbiol. 2017;15:169–182. doi: 10.1038/nrmicro.2016.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Smargon A., Cox D., Pyzocha N., Zheng K., Slaymaker I., Gootenberg J. Cas13b is a Type VI-B CRISPR-associated RNA-Guided RNase differentially regulated by accessory proteins Csx27 and Csx28. BioRxiv. 2016 doi: 10.1101/092577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Johnson M.J., Laoharawee K., Lahr W.S., Webber B.R., Moriarity B.S. Engineering of primary human B cells with CRISPR/Cas9 targeted nuclease. BioRxiv. 2018 doi: 10.1101/290973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Chekani-Azar S., Gharib Mombeni E. CRISPR/Cas9 gene editing technology and its application to the coronavirus disease (COVID-19), a review. Journal of Life Science and Biomedicine. 2020;10:1–9. doi: 10.29252/scil.2020.jlsb1. [DOI] [Google Scholar]
- 226.Nalawansha D.A., Samarasinghe K.T.G. Double-barreled CRISPR technology as a novel treatment strategy for COVID-19. Acs Pharmacol Transl Sci. 2020;3:790–800. doi: 10.1021/acsptsci.0c00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Faiq M.A. B-cell engineering: a promising approach towards vaccine development for COVID-19. Med Hypotheses. 2020;144 doi: 10.1016/j.mehy.2020.109948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nascimento I.P., Leite L.C.C. Recombinant vaccines and the development of new vaccine strategies. Braz J Med Biol Res. 2012;45:1102–1111. doi: 10.1590/S0100-879X2012007500142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Bisht H., Roberts A., Vogel L., Bukreyev A., Collins P.L., Murphy B.R. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci USA. 2004;101:6641–6646. doi: 10.1073/pnas.0401939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.He Y., Zhou Y., Liu S., Kou Z., Li W., Farzan M. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: implication for developing subunit vaccine. Biochem Biophys Res Commun. 2004;324:773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Abdelmageed M.I., Abdelmoneim A.H., Mustafa M.I., Elfadol N.M., Murshed N.S., Shantier S.W. Design of a multiepitope-based peptide vaccine against the e protein of human COVID-19: an immunoinformatics approach. Biomed Res Int. 2020;2020 doi: 10.1155/2020/2683286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kalita P., Padhi A.K., Zhang K.Y.J., Tripathi T. Design of a peptide-based subunit vaccine against novel coronavirus SARS-CoV-2. Microb Pathog. 2020;145 doi: 10.1016/j.micpath.2020.104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang N., Shang J., Jiang S., Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11 doi: 10.3389/fmicb.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stefanelli P., Faggioni G., Lo Presti A., Fiore S., Marchi A., Benedetti E. Whole genome and phylogenetic analysis of two SARSCoV-2 strains isolated in Italy in January and February 2020: additional clues on multiple introductions and further circulation in Europe. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.13.2000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dhakal S., Klein S.L. Host factors impact vaccine efficacy: implications for seasonal and universal influenza vaccine programs. J Virol. 2019;93 doi: 10.1128/jvi.00797-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Davies N.G., Klepac P., Liu Y., Prem K., Jit M. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020 doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 238.Ward K., Chow M.Y.K., King C., Leask J. Strategies to improve vaccination uptake in Australia, a systematic review of types and effectiveness. Aust N Z J Public Health. 2012;36:369–377. doi: 10.1111/j.1753-6405.2012.00897.x. [DOI] [Google Scholar]
- 239.Humiston S.G., Rosenthal S.L. Challenges to vaccinating adolescents: vaccine implementation issues. Pediatr Infect Dis J. 2005;24 doi: 10.1097/01.inf.0000166161.12087.94. [DOI] [PubMed] [Google Scholar]
- 240.Liu Y., Yan L.M., Wan L., Xiang T.X., Le A., Liu J.M. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect Dis. 2020;20:656–657. doi: 10.1016/S1473-3099(20)30232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gallagher J. BBC; 2020. Coronavirus vaccine: when will we have one? [Google Scholar]
- 242.Flanagan K.L., Fink A.L., Plebanski M., Klein S.L. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599. doi: 10.1146/annurev-cellbio-100616-060718. [DOI] [PubMed] [Google Scholar]
- 243.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 244.Zeng F., Dai C., Cai P., Wang J., Xu L., Li J. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020 doi: 10.1002/jmv.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Gausman J., Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health. 2020;29:465–466. doi: 10.1089/jwh.2020.8472. [DOI] [PubMed] [Google Scholar]
- 246.Roizen M.F. The Xs and Y of immune responses to viral vaccines. Yearb Anesthesiol Pain Manag. 2011;2011:399–400. doi: 10.1016/j.yane.2011.01.016. [DOI] [Google Scholar]
- 247.Klein S.L., Jedlicka A., Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kleina S.L., Marriott I., Fish E.N. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2014;109:9–15. doi: 10.1093/trstmh/tru167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Fink A.L., Klein S.L. Sex and gender impact immune responses to vaccines among the elderly. Physiology. 2015;30:408–416. doi: 10.1152/physiol.00035.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Fish E.N. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Klein S.L., Pekosz A. Sex-based biology and the rational design of influenza vaccination strategies. J Infect Dis. 2014;209 doi: 10.1093/infdis/jiu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mo R., Chen J., Grolleau-Julius A., Murphy H.S., Richardson B.C., Yung R.L. Estrogen regulates CCR gene expression and function in t lymphocytes. J Immunol. 2005;174:6023–6029. doi: 10.4049/jimmunol.174.10.6023. [DOI] [PubMed] [Google Scholar]
- 254.Ruggierii A., Anticoli S., D’ambrosio A., Giordani L., Mora M. The influence of sex and gender on immunity, infection and vaccination. Ann Ist Super Sanita. 2016;52:198–204. doi: 10.4415/ANN_16_02_11. [DOI] [PubMed] [Google Scholar]
- 255.Thomas S., Izard J., Walsh E., Batich K., Chongsathidkiet P., Clarke G. The host microbiome regulates and maintains human health: a primer and perspective for non-microbiologists. Cancer Res. 2017;77:1783–1812. doi: 10.1158/0008-5472.CAN-16-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.García-Gómez E., González-Pedrajo B., Camacho-Arroyo I. Role of sex steroid hormones in bacterial-host interactions. Biomed Res Int. 2013;2013 doi: 10.1155/2013/928290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Markle J.G.M., Frank D.N., Mortin-Toth S., Robertson C.E., Feazel L.M., Rolle-Kampczyk U. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 258.Wang J., Peng Y., Xu H., Cui Z., Williams R.O. The COVID-19 vaccine race: challenges and opportunities in vaccine formulation. AAPS PharmSciTech. 2020;21 doi: 10.1208/s12249-020-01744-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Chastain D.B., Osae S.P., Henao-Martínez A.F., Franco-Paredes C., Chastain J.S., Young H.N. Racial disproportionality in covid clinical trials. N Engl J Med. 2020;383:e59. doi: 10.1056/nejmp2021971. [DOI] [PubMed] [Google Scholar]
- 260.Webb Hooper M., Nápoles A.M., Pérez-Stable E.J. COVID-19 and Racial/Ethnic disparities. JAMA - Journal of the American Medical Association. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020 doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R. Hospitalization rates and characteristics of patients hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 — COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Quinn S.C., Kumar S., Freimuth V.S., Musa D., Casteneda-Angarita N., Kidwell K. Racial disparities in exposure, susceptibility, and access to health care in the US H1N1 influenza pandemic. Am J Public Health. 2011;101:285–293. doi: 10.2105/AJPH.2009.188029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bibbins-Domingo K. This time must Be different: disparities during the COVID-19 pandemic. Ann Intern Med. 2020;173:233–234. doi: 10.7326/M20-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA - Journal of the American Medical Association. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 266.Sze S., Pan D., Gray L.J., Nevill C.R., Martin C.A., Nazareth J. Ethnicity and clinical outcomes in COVID-19 a systematic review and meta-analysis. MedRxiv. 2020 doi: 10.1016/j.eclinm.2020.100630. 2020.09.05.20188821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Shrestha G.S., Paneru H.R., Vincent J.L. Precision medicine for COVID-19: a call for better clinical trials. Crit Care. 2020;24 doi: 10.1186/s13054-020-03002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Crisci C.D., Ardusso L.R.F., Mossuz A., Müller L. A precision medicine approach to SARS-CoV-2 pandemic management. Curr Treat Options Allergy. 2020;7:422–440. doi: 10.1007/s40521-020-00258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369 doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- 270.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kollias A., Kyriakoulis K.G., Dimakakos E., Poulakou G., Stergiou G.S., Syrigos K. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br J Haematol. 2020;189:846–847. doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gollust S.E., Saloner B., Hest R., Blewett L.A. US Adults’ Preferences for Public Allocation of a Vaccine for Coronavirus Disease 2019. JAMA Network Open. 2020;3:e2023020. doi: 10.1001/jamanetworkopen.2020.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Rod J.E., Oviedo-Trespalacios O., Cortes-Ramirez J. A brief-review of the risk factors for covid-19 severity. Rev Saude Publica. 2020;54 doi: 10.11606/S1518-8787.2020054002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Bollyky T.J., Kickbusch I. Preparing democracies for pandemics. BMJ. 2020;371:m4088. doi: 10.1136/bmj.m4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rab S., Afjal, Javaid M., Haleem A., Vaishya R. An update on the global vaccine development for coronavirus. Diabetes Metab Syndr Clin Res Rev. 2020;14:2053–2055. doi: 10.1016/j.dsx.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.P. L, F D The complementary roles of Phase 3 trials and post-licensure surveillance in the evaluation of new vaccines. Vaccine. 2014 doi: 10.1016/j.vaccine.2014.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.WHO At least 80 million children under one at risk of diseases such as diphtheria, measles and polio as COVID-19 disrupts routine vaccination efforts, warn Gavi, WHO and UNICEF. World Health Organization. 2020:22–25. [Google Scholar]
- 279.Su S., Du L., Jiang S. Learning from the past: development of safe and effective COVID-19 vaccines. Nat Rev Microbiol. 2020 doi: 10.1038/s41579-020-00462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- 281.Han S. Clinical vaccine development. Clin Exp Vaccine Res. 2015;4:46. doi: 10.7774/cevr.2015.4.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Iserson K.V. 2020. Sars-cov-2 (covid-19) vaccine development and production: an ethical way forward. Cambridge Quarterly of Healthcare Ethics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Le T.T., Cramer J.P., Chen R., Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19:667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 284.Rupp R.E., Hawkins B.E. 2014. Clinical evaluation of vaccines. vaccinology: an essential guide; pp. 260–272. https://doi.org/10.1002/9781118638033.ch15. [Google Scholar]
- 285.Sharma O., Sultan A.A., Ding H., Triggle C.R. A review of the progress and challenges of developing a vaccine for COVID-19. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.585354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Singh K., Mehta S. The clinical development process for a novel preventive vaccine: an overview. J Postgrad Med. 2016;62:4–11. doi: 10.4103/0022-3859.173187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Vaccine Product Approval Process. Fda 2020:1–2.
- 288.Kesselheim A.S., Darrow J.J., Kulldorff M., Brown B.L., Mitra-Majumdar M., Lee C.C. An overview of vaccine development, approval, and regulation, with implications for COVID-19. Health Aff (Millwood) 2020 doi: 10.1377/hlthaff.2020.01620. 101377hlthaff202001620. https://doi.org/10.1377/hlthaff.2020.01620. [DOI] [PubMed] [Google Scholar]
- 289.WHO . 2020. Accelerating a safe and effective COVID-19 vaccine. Global Research on Coronavirus Disease (COVID-19) [Google Scholar]
- 290.Shepard C.W., Simard E.P., Finelli L., Fiore A.E., Bell B.P. Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev. 2006;28:112–125. doi: 10.1093/epirev/mxj009. [DOI] [PubMed] [Google Scholar]
- 291.WHO | Regulation and quality control of vaccines. Who 2017.
- 292.Khuroo M.S., Khuroo M.S., Khuroo M.S., Sofi A.A., Khuroo N.S. COVID-19 vaccines: a race against time in the middle of death and devastation! J Clin Exp Hepatol. 2020 doi: 10.1016/j.jceh.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Petersen E., Wejse C., Zumla A. Advancing COVID-19 vaccines – avoiding different regulatory standards for different vaccines and need for open and transparent data sharing. Int J Infect Dis. 2020;98:501–502. doi: 10.1016/j.ijid.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Centers of Disease Control and Prevention. Vaccine Testing and the Approval Process. Https://WwwCdcGov/Vaccines/Basics/Test-ApproveHtml 2020.
- 295.Siegrist C.A., Lambert P.H. second edition. 2016. How vaccines work. The vaccine book; pp. 33–42. [DOI] [Google Scholar]
- 296.Alice McCarthy . 2020. Designing a coronavirus vaccine. The Harvard Gazette Health & Medicine. [Google Scholar]
- 297.Lockey E. COVID-19: the race for a vaccine. JRAAS - Journal of the Renin-Angiotensin-Aldosterone System. 2020;21 doi: 10.1177/1470320320926902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Shin M.D., Shukla S., Chung Y.H., Beiss V., Chan S.K., Ortega-Rivera O.A. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 299.Abd Elkodous M., El-Sayyad G.S., Abdel-Daim M.M. Engineered nanomaterials as fighters against SARS-CoV-2: the way to control and treat pandemics. Environ Sci Pollut Res - Int. 2020 doi: 10.1007/s11356-020-11032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Makvandi P., yu Wang C., Zare E.N., Borzacchiello A., na Niu L., Tay F.R. Metal-based nanomaterials in biomedical applications: antimicrobial activity and cytotoxicity aspects. Adv Funct Mater. 2020;30 doi: 10.1002/adfm.201910021. [DOI] [Google Scholar]
- 301.Makvandi P., Ghaemy M., Mohseni M. Synthesis and characterization of photo-curable bis-quaternary ammonium dimethacrylate with antimicrobial activity for dental restoration materials. Eur Polym J. 2016;1(74):81–90. doi: 10.1016/j.eurpolymj.2015.11.011. [DOI] [Google Scholar]
- 302.Geisinger E., Isberg R.R. Interplay between antibiotic resistance and virulence during disease promoted by multidrug-resistant bacteria. J Infect Dis. 2017;215(1) doi: 10.1093/infdis/jiw402. S9-17. https://doi.org/10.1093/infdis/jiw402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Jiang L., Lin J., Taggart C.C., Bengoechea J.A., Scott C.J. Nanodelivery strategies for the treatment of multidrug-resistant bacterial infections. J Interdiscip Nanomed. 2018;3:111–121. doi: 10.1002/jin2.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zeng C., Hou X., Yan J., Zhang C., Li W., Zhao W. Leveraging mRNAs sequences to express SARS-CoV-2 antigens in vivo. BioRxiv : The Preprint Server for Biology. 2020 doi: 10.1101/2020.04.01.019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lim M., Badruddoza A.Z., Firdous J., Azad M., Mannan A., Al-Hilal T.A. Engineered nanodelivery systems to improve DNA vaccine technologies. Pharmaceutics. 2020;12(1):30. doi: 10.3390/pharmaceutics12010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhang N.N., Li X.F., Deng Y.Q., Zhao H., Huang Y.J., Yang G. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182 doi: 10.1016/j.cell.2020.07.024. 1271-1283.e16. https://doi.org/10.1016/j.cell.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Herzog C., Hartmann K., Künzi V., Kürsteiner O., Mischler R., Lazar H. Eleven years of Inflexal® V-a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27:4381–4387. doi: 10.1016/j.vaccine.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 309.Li W., Joshi M.D., Singhania S., Ramsey K.H., Murthy A.K. Peptide vaccine: progress and challenges. Vaccines. 2014;2:515–536. doi: 10.3390/vaccines2030515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Liu H., Moynihan K.D., Zheng Y., Szeto G.L., Li A.V., Huang B. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Mohsen M.O., Zha L., Cabral-Miranda G., Bachmann M.F. Major findings and recent advances in virus–like particle (VLP)-based vaccines. Semin Immunol. 2017;34:123–132. doi: 10.1016/j.smim.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 312.Grifoni A., Sidney J., Zhang Y., Scheuermann R.H., Peters B., Sette A. A sequence homology and bioinformatic approach can predict candidate targets for immune responses to SARS-CoV-2. Cell Host Microbe. 2020;27:671–680. doi: 10.1016/j.chom.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12(3):254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Gustafson C.E., Kim C., Weyand C.M., Goronzy J.J. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145:1309–1321. doi: 10.1016/j.jaci.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Mukherjee S. 2020. Can a vaccine for Covid-19 Be developed in record time? New york times magazine; pp. 2–4. [Google Scholar]
- 316.CEPI, WHO, Gavi . 2020. More than 150 countries engaged in COVID-19 vaccine global access facility. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.