Abstract
BACKGROUND
The ABO histo-blood group is defined by carbohydrate modifications and is associated with risk for multiple diseases, including acute respiratory distress syndrome (ARDS). We hypothesized that genetically determined blood subtype A1 is associated with increased risk of ARDS and markers of microvascular dysfunction and coagulation.
METHODS
We conducted analyses in 3 cohorts of critically ill trauma and sepsis patients (n = 3710) genotyped on genome-wide platforms to determine the association of the A1 blood type genotype with ARDS risk. We subsequently determined whether associations were present in FUT2-defined nonsecretors who lack ABO antigens on epithelium, but not endothelium. In a patient subgroup, we determined the associations of blood type with plasma levels of endothelial glycoproteins and disseminated intravascular coagulation (DIC). Lastly, we tested whether blood type A was associated with less donor lung injury recovery during human ex vivo lung perfusion (EVLP).
RESULTS
The A1 genotype was associated with a higher risk of moderate to severe ARDS relative to type O in all 3 populations. In sepsis, this relationship was strongest in nonpulmonary infections. The association persisted in nonsecretors, suggesting a vascular mechanism. The A1 genotype was also associated with higher DIC risk as well as concentrations of thrombomodulin and von Willebrand factor, which in turn were associated with ARDS risk. Blood type A was also associated with less lung injury recovery during EVLP.
CONCLUSION
We identified a replicable association between ABO blood type A1 and risk of ARDS among the critically ill, possibly mediated through microvascular dysfunction and coagulation.
FUNDING
NIH HL122075, HL125723, HL137006, HL137915, DK097307, HL115354, HL101779, and the University of Pennsylvania McCabe Fund Fellowship Award.
Keywords: Pulmonology
Keywords: Coagulation, Epidemiology, endothelial cells
Introduction
Acute respiratory distress syndrome (ARDS) is a common complication of critical illness characterized by lung inflammation, epithelial and endothelial dysfunction, alveolar capillary leak, and microthrombosis (1, 2). Clinically, it is recognized as diffuse noncardiogenic pulmonary edema and severe hypoxemia leading to acute respiratory failure in the setting of a precipitating illness (3). It is estimated that ARDS affects at least 190,000 people in the United States annually, and mortality ranges from 30% to 50% (4). Currently, ARDS therapies are largely supportive, including lung-protective mechanical ventilation, fluid restrictive management, and prone positioning (5–7). Despite many clinical trials, however, no pharmacological therapy aimed at its pathogenesis has been consistently shown to either prevent ARDS or reduce mortality (8–11). One potential explanation for this failure of translation from promising preclinical therapeutics to human disease is pathogenic heterogeneity of ARDS, in which genetic factors may play a role (12–16).
We previously identified an association between ABO histo-blood type A and an increased risk of ARDS in trauma and sepsis populations (17). The ABO histo-blood group is genetically determined by the ABO gene, which encodes a family of glycosyltransferases that catalyze specific carbohydrate modifications on glycans and glycoproteins (18). In addition to RBCs, the ABO glycans are present on the surface of platelets, endothelium, epithelium, circulating solubilized glycoproteins, and epithelial secretions. The genetic diversity seen in the ABO gene has been shaped by evolutionary host-pathogen interactions that resulted in selective advantages over infectious diseases, particularly malaria, but may now contribute to altered risk for inflammatory conditions such as ARDS (19–21). More recently, the ABO locus has been identified to be among the most pleiotropic loci in the genome, influencing circulating blood levels of a host of glycoproteins (22–25). These include endothelium-derived proteins such as von Willebrand factor (vWF), soluble intercellular adhesion molecule-1 (sICAM-1), and soluble thrombomodulin (sTM) (26–31). Plasma vWF, sICAM-1, and sTM are elevated in critical illness and are markers of processes implicated in ARDS pathogenesis (32–35). Convergent with our findings in ARDS, ABO blood type A has also been associated with increased risk of vascular diseases, including myocardial infarction and venous thromboembolism (36, 37).
Although we previously identified an association between ABO blood type A and ARDS, routine antigen testing does not reflect the full antigen complexity of the ABO histo-blood group. Within blood type A, heterozygotes (A/O) express lower A antigen density relative to (A/A) homozygotes (38, 39). Additionally, there are 2 common genetically determined A subtypes, A1 and A2, which are distinguished by a 30–50-fold difference in A transferase activity. We reasoned that identifying a dose-response relationship of A antigen density with ARDS would provide stronger evidence of a causal link. Higher ARDS risk in A/A homozygotes compared with A/O heterozygotes and in patients with the A1 versus A2 genotype would provide evidence of such a relationship. Furthermore, the related FUT2 “secretor” gene encodes a fucosyltransferase that is necessary for the expression of ABO(H) antigens on epithelium and in secretions, but is not required for expression on endothelium, RBCs, or platelets. A common loss-of-function mutation in FUT2 results in the absence of ABO antigens (“nonsecretor” status) in lung epithelium, saliva, and gut and is present in approximately 20% of individuals of European ancestry (40). Determining whether an association between ABO blood type and ARDS risk exists within nonsecretors could localize the underlying association to the vasculature. Lastly, genome-wide association studies of ambulatory patients have found associations between ABO genetic variation and plasma concentrations of multiple endothelium-derived glycoproteins, including vWF, sICAM-1, E-selectin, and sTM (27–29, 41, 42). These and other markers of endothelial activation have been implicated in ARDS pathogenesis and could represent a mechanistic link between ABO blood type and ARDS risk. In critical illness, however, these glycoproteins are secreted or cleaved from endothelial cells and increased dramatically in circulation. It remains unknown whether ABO blood type is associated with the critical illness–evoked phenotype of endothelial activation and glycoprotein release, as well as whether this mediates ARDS risk.
We hypothesized that an individual’s ABO blood type phenotype influences endothelial activation and subsequent microvascular coagulation in critical illness, resulting in altered risk of ARDS. Specifically, we hypothesized that there is a dose-response relationship, whereby genotypes that result in a higher density of A antigen, such as the A1 subtype of blood type A, will have the highest risk of moderate and severe ARDS. We also hypothesized that ABO blood type A1 is associated with elevated plasma biomarkers of endothelial activation and disseminated intravascular coagulation (DIC), resulting in increased ARDS risk. In order to test these hypotheses, we utilized 3 large observational studies of critical illness; 2 prospective cohort studies of trauma and sepsis conducted at the Hospital of the University of Pennsylvania, and a multicenter case-control study of sepsis-associated ARDS. Lastly, in an ex vivo lung perfusion (EVLP) model, we determined whether ABO blood type A was associated with a lower recovery of donor organs for transplantation compared with ABO blood type O.
Results
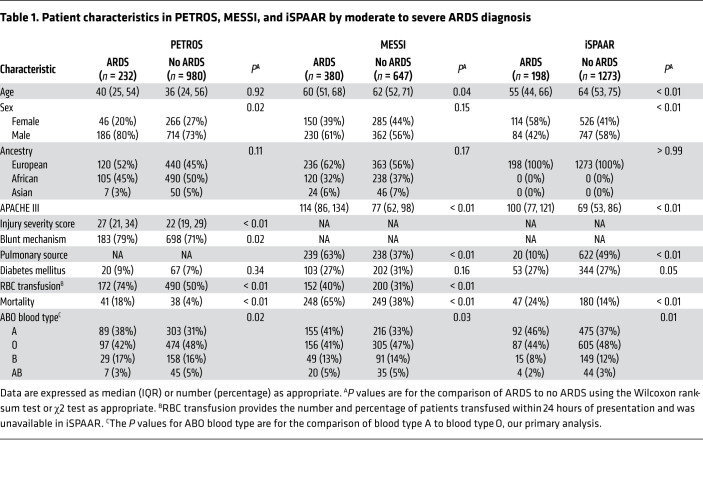
Study populations.
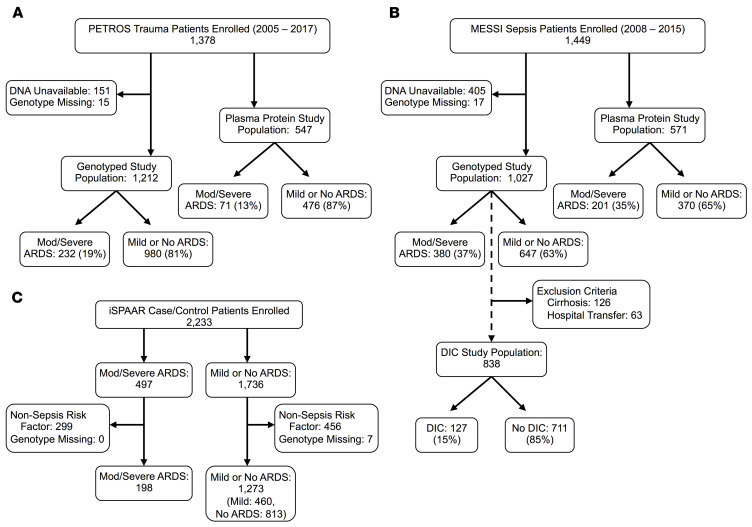
We included patients enrolled in 3 parent studies in the analyses presented in this manuscript (Figure 1 and Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI139700DS1). The first study population included 1212 subjects who were genotyped and 547 subjects with plasma protein testing prospectively enrolled in the Penn Trauma Organ dysfunction cohort study (PETROS) between 2005 and 2017 (13, 43). PETROS is a cohort of critically ill severe trauma patients presenting to the University of Pennsylvania’s level I trauma center within 24 hours of injury. In PETROS, 232 of 1212 (19%) patients developed moderate or severe ARDS within 6 days, and 79 (7%) died while hospitalized. The second study population included 1027 subjects who were genotyped and 571 subjects with plasma protein testing prospectively enrolled in the Molecular Epidemiology of SepsiS in the ICU (MESSI) cohort study between 2008 and 2015 (44, 45). MESSI is a cohort of critically ill sepsis patients admitted to the medical intensive care unit (ICU) of the Hospital of the University of Pennsylvania. In MESSI, 380 of 1027 (37%) patients developed moderate or severe ARDS within 6 days and 497 (48%) died within 30 days. The third study population included 198 sepsis subjects with moderate or severe ARDS, and 1273 septic controls (460 with mild ARDS and 813 with no ARDS) enrolled in the multicenter Identification of SNPs Predisposing to Altered Acute Lung Injury Risk (iSPAAR) case-control study (45, 46). Characteristics of patients included in each population are detailed in Table 1. In all populations, trained investigators individually reviewed chest radiographs and arterial blood gases to accurately phenotype the ARDS outcome (3).
Figure 1. Study populations.
(A) PETROS, (B) MESSI, (C) iSPAAR. DNA was unavailable in some patients because a whole blood sample was missed or minimal DNA was present in the whole blood sample (e.g., patient had leukopenia secondary to chemotherapy administration).
Table 1. Patient characteristics in PETROS, MESSI, and iSPAAR by moderate to severe ARDS diagnosis.
Genetically determined ABO blood type is associated with ARDS risk in critical illness.
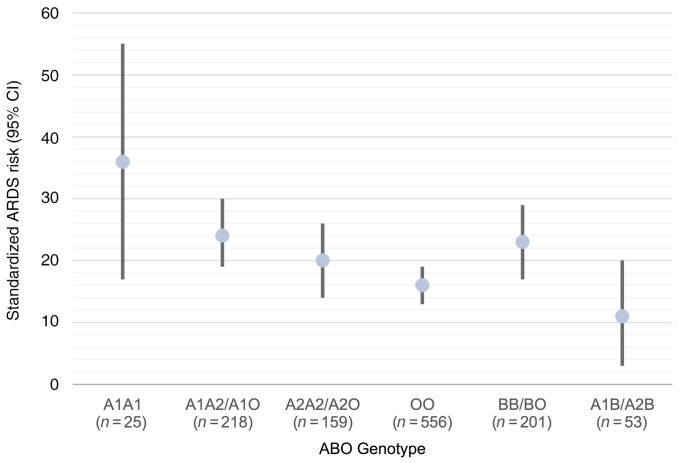
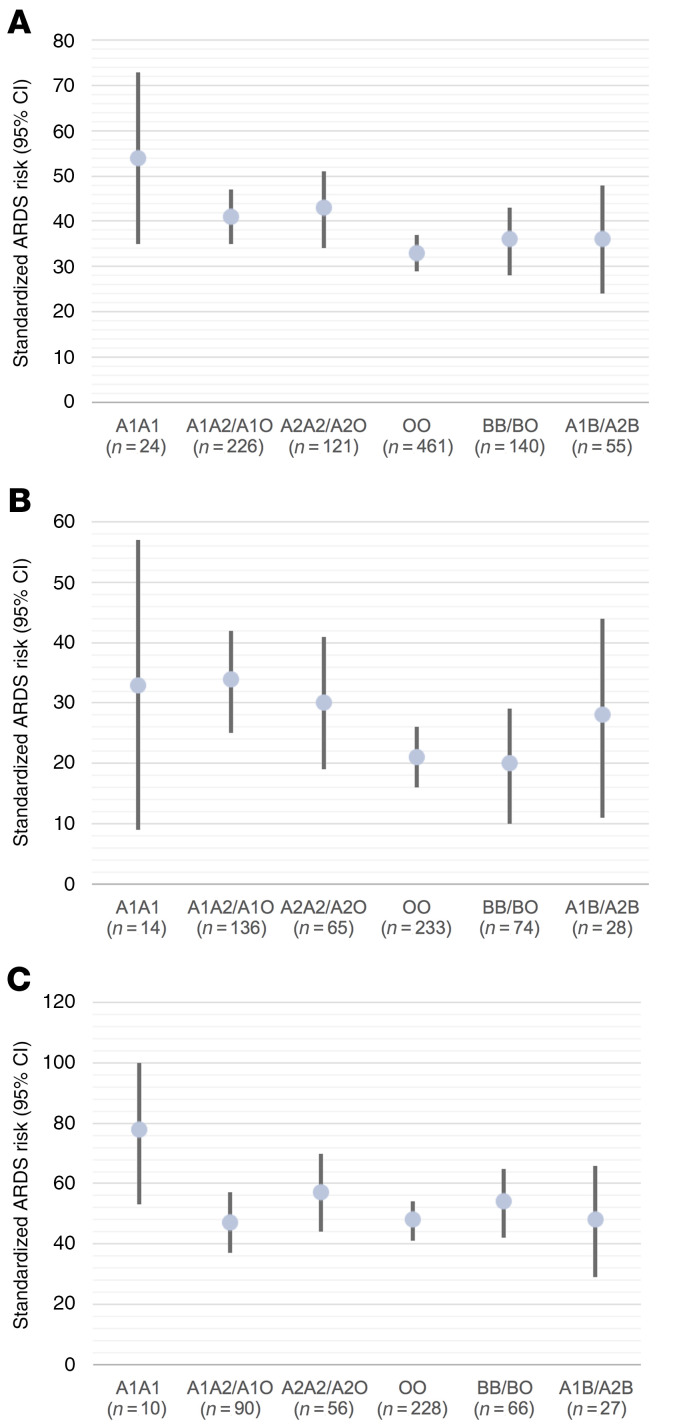
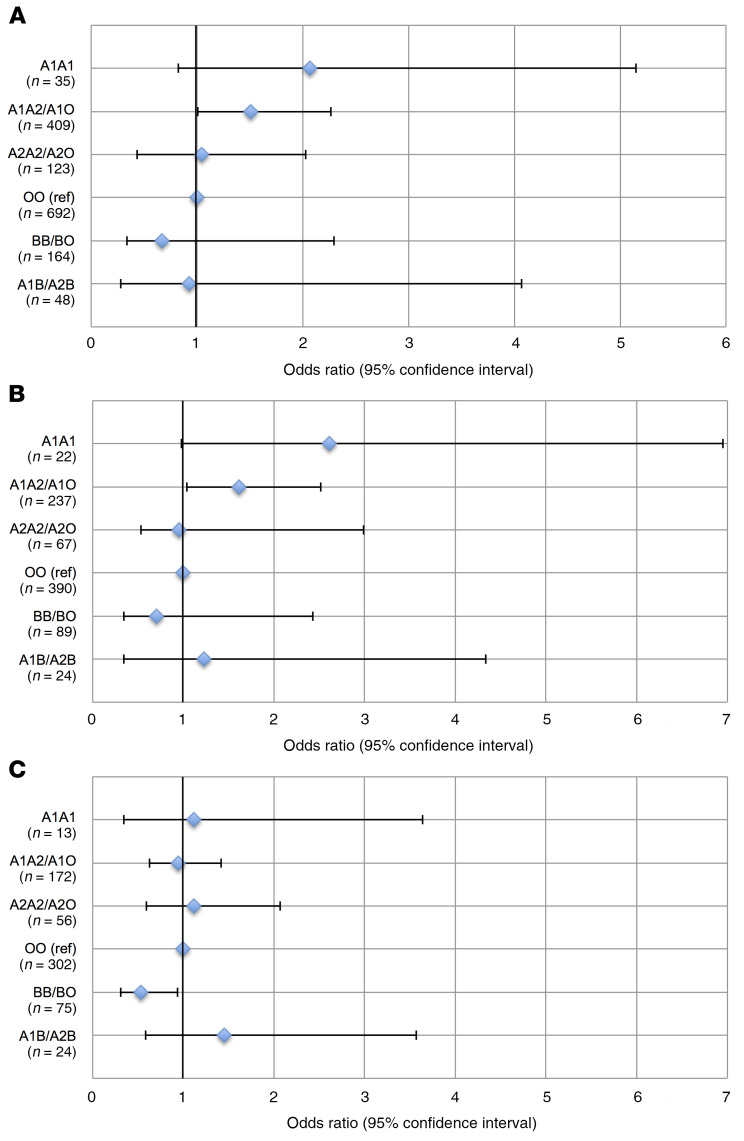
ABO blood type, including the A1 and A2 subtypes, was inferred using a haplotype created by multiple SNPs in the ABO gene (28, 47, 48). In PETROS and MESSI, subjects were genotyped using the Affymetrix Axiom Txv1 array including more than 750,000 SNPs. Three SNPs in the ABO gene were used to infer the 2 ABO blood type alleles (Supplemental Table 2) with more than 91% concordance with blood bank–determined blood type (Supplemental Table 3). Our primary outcome was moderate to severe ARDS in order to avoid including milder cases that do not have the characteristic pathobiology of ARDS (49). Supplemental Table 4 provides the unadjusted distributions of moderate to severe ARDS risk by ABO genotype in PETROS and MESSI. The possession of any copy of the A1 haplotype relative to genetically determined blood type O was associated with increased moderate or severe ARDS risk (PETROS: OR 1.67; 95% CI 1.14–2.46; P = 0.008, MESSI: OR 1.53; 95% CI 1.08–2.17; P = 0.015), adjusting for potential confounders and population stratification. Including mild ARDS patients in the outcome slightly attenuated the findings (PETROS: OR 1.56; 95% CI 1.09–2.25; P = 0.016, MESSI: OR 1.38; 95% CI 0.98–1.93; P = 0.064). Results were similar after excluding patients previously included in our prior nongenetic study (Supplemental Table 5 and ref. 17). Results were also similar comparing patients with an A1 genotype to all patients without an A1 genotype (Supplemental Table 5). The standardized risk of ARDS ranged from 16% and 33% in ABO blood type O to 36% and 54% in the A1 homozygous blood type in PETROS and MESSI, respectively (Figure 2 and Figure 3A). Genotypes that result in a higher A antigen density were consistently associated with a higher ARDS risk.
Figure 2. Standardized risk of moderate or severe ARDS by ABO genotype grouped by expected expression of A and B antigens in the PETROS trauma cohort.
The dot represents the standardized ARDS risk adjusted for injury severity score, age, sex, mechanism of trauma, and population stratification. The bars represent the 95% CI around the standardized risk. ARDS risk decreases when comparing ABO genotypes predicted to confer a higher density of A antigens (i.e., A1A1 has the highest A antigen density and ARDS risk, then A1A2/A1O, followed by A2A2/A2O, and finally OO).
Figure 3. Standardized risk of moderate or severe ARDS in the MESSI sepsis cohort.
Standardized risk of moderate or severe ARDS by ABO genotype grouped by expected expression of A and B antigens in the (A) overall MESSI population, (B) MESSI nonpulmonary sepsis, and (C) MESSI pulmonary sepsis. The dot represents the standardized ARDS risk adjusted for age, sex, RBC transfusion, diabetes, hematologic malignancy, source of sepsis, and population stratification. The bars represent the 95% CI around the standardized risk. ARDS risk generally decreased when comparing ABO genotypes predicted to confer a higher density of A antigens (i.e., A1A1 has the highest A antigen density and ARDS risk, then A1A2/A1O, followed by A2A2/A2O, and finally OO).
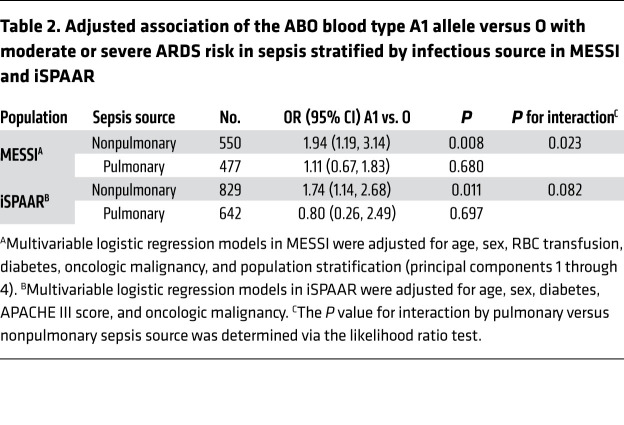
In iSPAAR, subjects were genotyped with the Illumina Human610-Quad Bead array of more than 500,000 SNPs, and 3 SNPs in the ABO gene were used to infer ABO blood type alleles (Supplemental Table 2). Blood bank–determined ABO blood type was unavailable. The distribution of genotypes in cases and controls is provided in Supplemental Table 6. The possession of an A1 allele was again independently associated with an increased risk of moderate or severe ARDS relative to the genetically inferred O blood type (OR 1.51; 95% CI 1.01–2.27; P = 0.044). Similar to MESSI and PETROS, this association was attenuated by the addition of mild ARDS cases (OR 1.28; 95% CI 0.99–1.65; P = 0.056). Figure 4A provides the OR (95% CIs) for the association of ABO genotypes grouped by estimated A1 antigen density and ARDS relative to the genetically inferred O blood type.
Figure 4. Odd ratios for the association of ABO genotypes and moderate or severe ARDS in iSPAAR.
Odds ratios for the association of ABO genotypes grouped by estimated antigen density and moderate or severe ARDS in the (A) overall iSPAAR sepsis case-control study and (B) nonpulmonary source and (C) pulmonary source of sepsis in the iSPAAR study. The point estimate represents the comparison of each ABO genotype grouping to the reference group, genetically inferred O blood type, adjusted for age, sex, acute physiology, and chronic health evaluation (APACHE) III score, history of diabetes, and history of malignancy. The error bars represent the 95% CI around each odds ratio. The iSPAAR study is a case-control study, not a cohort study, so we are unable to calculate standardized risks.
The ABO and ARDS association is driven by nonpulmonary sources of sepsis.
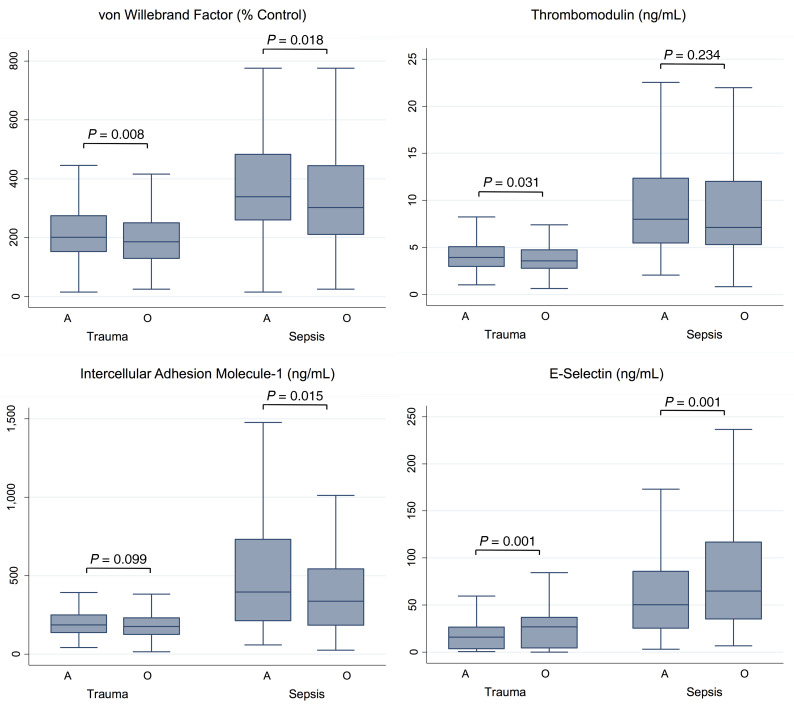
Having validated the genetic association in 3 critically ill populations, we next sought to determine whether this association was present in both pulmonary and nonpulmonary sepsis. There is significant evidence for “ARDS heterogeneity” in sepsis based on the source of infection. Specifically, nonpulmonary (nonpneumonia) sepsis has been associated with higher levels of endothelial biomarkers, suggesting a more significant systemic vascular insult relative to pulmonary sepsis (50, 51). Given this evidence, we a priori planned to test for a statistical interaction in the association between ABO genotype and moderate to severe ARDS by source of sepsis. In MESSI and iSPAAR, the association between the A1 genotype and higher risk of ARDS relative to the O genotype was only present in patients with a nonpulmonary source of sepsis. The P values for statistical interaction were 0.023 and 0.082 for MESSI and iSPAAR, respectively. Given that these P values were less than 0.10, we additionally present the results of the association between the A1 genotype relative to O and ARDS stratified by source of sepsis (Table 2; Figure 3, B and C; Figure 4, B and C).
Table 2. Adjusted association of the ABO blood type A1 allele versus O with moderate or severe ARDS risk in sepsis stratified by infectious source in MESSI and iSPAAR.
FUT2-determined secretor status does not modify the ABO and ARDS association.
Nonsecretors do not have ABO(H) antigens on epithelium or in secretions and can be identified using the rs601338 SNP in the FUT2 gene (40, 52). In order to determine whether epithelial secretor status was relevant to the ABO and ARDS association, we tested the association between ABO genotype and ARDS among genetically determined nonsecretors. Given the autosomal dominant inheritance of secretor status, subjects homozygous for the minor allele were FUT2-null and thus nonsecretors. In order to have sufficient power, we combined the nonsecretors from the PETROS and nonpulmonary sepsis MESSI cohorts who represented 25% and 24% of the overall cohorts, respectively. The A1 allele relative to the O allele was still associated with increased ARDS risk (OR 2.15; 95% CI 1.18–3.92; P = 0.012; n = 432) in nonsecretors adjusting for age, sex, population stratification, and sepsis versus trauma. This finding suggests that the presence of ABO antigens on epithelium is not necessary to influence ARDS risk. Supplemental Table 7 provides the association of the A1 allele versus O allele with moderate or severe ARDS risk, individually in MESSI stratified by sepsis source and PETROS among nonsecretors.
ABO blood type is associated with biomarkers of endothelial activation in sepsis and trauma.
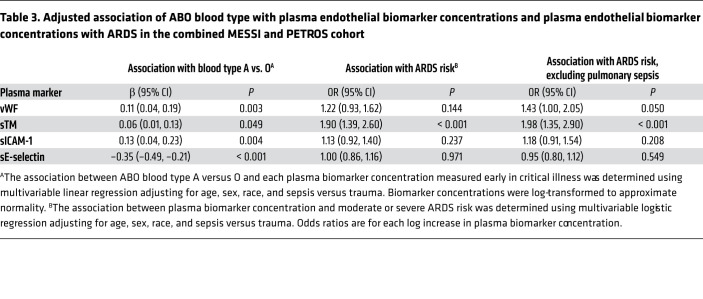
In ambulatory patients, ABO blood type A is associated with higher plasma levels of vWF and lower levels of sTM, sICAM-1, and E-selectin (26–28, 30–35, 41). However, critical illness is an evoked phenotype whereby plasma concentrations of endothelium-derived glycoproteins increase dramatically and are associated with the development of ARDS. We measured plasma concentrations of these 4 endothelium-derived glycoproteins in more than 500 PETROS and MESSI subjects. The 4 endothelium-derived glycoproteins demonstrated mild to moderate correlation with each other (Supplemental Table 8). Given that approximately 32% of patients with biomarker measurements did not have genotypes because of insufficient DNA quantity or quality, our primary analysis compared biomarker levels between blood bank–determined ABO blood types. ABO blood type A relative to O was associated with higher concentrations of vWF, sTM, and sICAM-1 and lower concentrations of E-selectin (Figure 5 and Supplemental Table 9). When MESSI and PETROS patients were combined, these associations were statistically significant and robust to adjustment for age, sex, race, and sepsis versus trauma (Table 3). Additionally, in multivariable logistic regression models, higher plasma sTM concentrations were associated with moderate to severe ARDS risk. Higher plasma vWF concentrations were associated with moderate to severe ARDS when excluding pulmonary sepsis. Plasma E-selectin and sICAM-1 were not associated with ARDS in our cohorts. There was no effect modification by race or mechanism of trauma.
Figure 5. Box-and-whisker plots comparing the median concentrations of vWF, sTM, sICAM-1, and sE-selectin of ABO blood type A to O separately in trauma and sepsis.
The box-and-whisker plots display the median value as a line within the boxes, the bounds of the box representing the IQR, and the whiskers representing the range of the data. P values are for the unadjusted comparison of ABO blood type A to O using the Wilcoxon rank-sum test. Blood type A and O sample sizes for each biomarker are vWF: 450 trauma, 465 sepsis; sTM: 451 trauma, 465 sepsis; sICAM-1: 449 trauma, 454 sepsis; sE-selectin: 451 trauma, 453 sepsis.
Table 3. Adjusted association of ABO blood type with plasma endothelial biomarker concentrations and plasma endothelial biomarker concentrations with ARDS in the combined MESSI and PETROS cohort.
ABO blood type is also associated with risk of sepsis-induced DIC.
DIC is a systemic disruption of coagulation homeostasis and commonly occurs as a consequence of the widespread endothelial activation of sepsis (53). Among the 1027 subjects enrolled in MESSI, we phenotyped DIC according to the International Society on Thrombosis and Haemostasis score within the first 5 days after ICU admission, with a score higher than 4 indicating DIC. Patients were excluded if they had a history of cirrhosis or hepatic failure to avoid misclassification bias given their inability to produce coagulation factors (Figure 1). Subjects who were transferred from another hospital more than 24 hours after admission were also excluded. DIC developed in 127 (15%) of the 838 remaining subjects. The risk of moderate or severe ARDS was 54% in patients who developed DIC and 30% in patients who did not (P < 0.001). Possession of the ABO allele A1 relative to genetically determined ABO blood type O was associated with the development of DIC in sepsis independent of age, sex, history of hematologic malignancy, and genetically determined population stratification (OR 1.66; 95% CI 1.02, 2.69; P = 0.042).
In an EVLP model, ABO blood type A is associated with reduced organ recovery for transplantation.
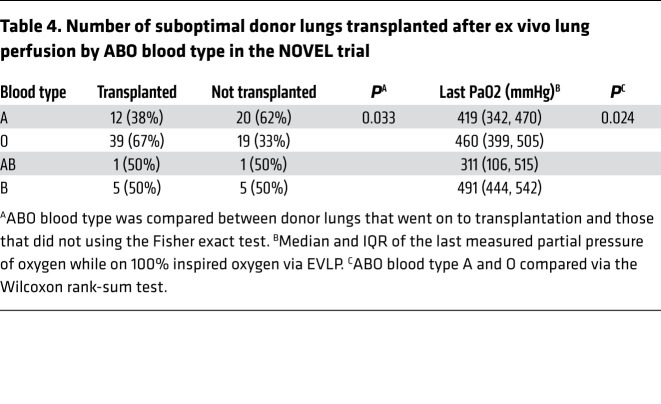
Utilizing data from a multicenter study of EVLP for lung transplantation, we determined whether ABO blood type was associated with likelihood for marginal donor lungs to recover and be transplantable after treatment with EVLP. Marginal donor lungs were defined as lungs with evidence of acute lung injury on radiology, visible inspection, or by a moderately reduced PaO2 to FiO2 ratio, but without chronic lung disease, severe hypoxia, or significant pneumonia. EVLP-treated lungs were considered to have their acute lung injury “recovered” if 2 consecutive partial pressures of arterial oxygen were greater than 350 mmHg on 100% supplemental oxygen with stable or improving pulmonary vascular resistance, lung compliance, and airway pressure. Of the 102 donor lungs treated with EVLP between 2010 and 2014, 57 (56%) were subsequently transplanted into a recipient. Table 4 displays the distribution of ABO blood type among the donor lungs that recovered and were transplanted versus those that did not sufficiently recover their lung injury to qualify for transplant. In a multivariable conditional logistic regression model adjusting for age, sex, race, and transplant center, ABO blood type A was associated with a significantly reduced likelihood of lung injury recovery relative to blood type O (OR 0.20; 95% CI 0.06, 0.64; P = 0.007). Additionally, the last measured partial pressures of arterial oxygen on EVLP were statistically higher in blood type O relative to A (Table 4).
Table 4. Number of suboptimal donor lungs transplanted after ex vivo lung perfusion by ABO blood type in the NOVEL trial.
Discussion
In this study, we identified a consistent association between genetically determined ABO blood type A and higher ARDS risk in 3 critically ill populations, including a total of 3710 closely phenotyped patients. This relationship was driven by the A1 subtype of blood type A, which confers a significantly higher density of A antigens than the A2 subtype. In sepsis, subjects with a nonpulmonary source of infection drove the ABO and ARDS association. We also demonstrated that the association between ABO blood type A and ARDS was present in FUT2-determined nonsecretors, suggesting that the presence of A antigens on epithelium is not required to alter ARDS risk. ABO blood type A was also associated with higher plasma levels of endothelial and coagulation biomarkers, sTM, vWF, and sICAM-1. Plasma levels of sTM and vWF were also associated with ARDS risk, providing evidence that endothelial activation and microvascular coagulation is important in the ABO-ARDS relationship. ABO blood type A was also associated with a higher risk of clinically determined DIC, which in turn conveyed a significantly higher risk of ARDS. Lastly, we demonstrated in an EVLP model that acutely injured lungs suboptimal for transplantation were less likely to recover their lung injury during EVLP if the donor blood type was A. Taken together, our study provides strong evidence that the ABO blood type A glycan confers a higher ARDS risk relative to blood type O mediated through microvascular activation and coagulation, suggesting a population that may particularly benefit from therapies targeting plasma glycoproteins such as sTM and vWF.
Although the ABO histo-blood group was first discovered more than 100 years ago, its role in human biology outside of transfusion medicine is incompletely understood (54). The antigenic diversity of the ABO histo-blood group has been linked to susceptibility and outcomes in a host of inflammatory and vascular diseases ranging from infections (e.g., severe malaria and Vibrio cholerae) to thrombotic disorders (e.g., myocardial infarction, venous thromboembolism, and stroke) (20, 21, 55). Evolutionary host-pathogen interactions are believed to underlie the genetic diversity seen in the human ABO gene, likely resulting in altered risk to historic infections as well as modern inflammatory diseases, such as ARDS.
In our study, sTM and vWF were the proteins most strongly associated with both ABO blood type and ARDS risk in the setting of critical illness. Although our study cannot definitely prove causality, it is possible that these proteins mechanistically link ABO blood type and ARDS. Therefore, biological pathways that include sTM and/or vWF may be therapeutic targets in a critical illness endotype defined by ABO blood type A and high risk for ARDS. VWF is a large multimeric glycoprotein that is produced predominately by endothelial cells and released into circulation alongside other proteins from the Weibel-Palade bodies (30). The protein has a major role in microvascular coagulation by binding factor VIII and localizing it to sites of injury as well as coupling platelets to endothelial surfaces. Although it is not definitively known whether ABO antigens affect vWF secretion, function, or clearance, ABO(H) antigens are known to be present on the vWF molecule and are believed to alter the ability of ADAMTS13 to degrade vWF multimers. It is possible future therapies aimed at vWF, such as recombinant ADAMTS13, will be proven effective in ARDS if aimed at an endotype defined by blood type. Thrombomodulin is a glycoprotein present on the cell surface of endothelial cells and serves as a receptor for thrombin and accelerates thrombin-induced activation of protein C, leading to an anticoagulation effect (56). In the setting of critical illness, thrombomodulin is cleaved from the endothelial cell surface and released into circulation. The mechanisms linking sTM to ABO blood type remain unclear; however, recombinant human thrombomodulin was recently studied in a large randomized clinical trial in sepsis-associated coagulopathy (57). In this study, coagulopathy was defined as an elevated international normalized ratio (INR) or thrombocytopenia believed to be secondary to sepsis. The clinical trial did not identify a mortality benefit to thrombomodulin therapy; however, it is possible that the population studied was not the ideal population to therapeutically benefit. Understanding ABO blood type A and sTM association may allow for future predictive enrichment in the population most likely to benefit from therapies targeting coagulation, such as recombinant sTM (58).
We also identified an association between ABO blood type A and lower concentrations of plasma E-selectin in sepsis and trauma. Plasma E-selectin concentration was not associated with ARDS risk in our cohorts. In ambulatory patients, ABO blood type A is consistently associated with lower concentrations of plasma E-selectin (26, 59, 60). We hypothesize that the associations between ABO blood type and plasma E-selectin level identified in our cohorts represent the baseline relationship between blood type and E-selectin, rather than an evoked phenotype with relevance to ARDS. These findings suggest a specific endothelial injury pattern relevant to certain endothelium-derived glycoproteins, including vWF and sTM, but not all markers of endothelial activation.
In sepsis, the associations between genetically determined ABO blood type A1 and ARDS risk were only present in nonpulmonary sepsis. Additionally, the statistical tests of interaction between pulmonary and nonpulmonary sepsis were significant in both sepsis cohorts, suggesting a true difference in the ABO and ARDS relationship based on infectious source. This finding is consistent with previous studies that have demonstrated higher systemic markers of endothelial activation and microvascular coagulation in nonpulmonary sepsis and our data linking ABO blood type A to higher vWF and sTM (50, 51). The interaction finding is also consistent with our data demonstrating an association between ABO blood type and ARDS even among nonsecretors who lack ABO(H) antigens on epithelium but not in the vasculature. In our previous study, the association between clinically determined ABO blood type and ARDS was only present in individuals of European ancestry and not African ancestry (17). In our current study using genetically determined blood type, individuals of African ancestry also demonstrated an association between ABO blood type A1 and ARDS. Possible reasons for this difference include differences in the distribution of the ABO blood type A subtypes between races, which were determined in this study but not our previous study; increased power in our current study; adjustment for population stratification; or an increased severity of illness observed in our current study populations.
Recently, a large genome-wide association study identified an association between the genetic variation that determines ABO blood type A and increased risk of severe COVID-19, the current global disease pandemic resulting from SARS-CoV-2 infection (61). These findings are consistent with a small study published in 2005 reporting an association between ABO blood type A and increased risk of infection with SARS-CoV (55). ARDS is the primary manifestation of COVID-19 in the critically ill, and a large number of patients who did not survive COVID-19 manifested overt DIC in addition to severe ARDS (62, 63). Although COVID-19 is a pulmonary form of ARDS, autopsy reports demonstrate profound microvascular coagulation, suggesting a vascular mechanism of disease that may overlap with other subtypes of nonpulmonary ARDS (64). Our study was conducted prior to the COVID-19 pandemic and did not include COVID-19 patients. Future research is needed to determine whether our findings are relevant to the association between ABO blood type and COVID-19 disease.
Our study has several strengths. First, we utilized 3 large critically ill populations that were extensively phenotyped for sepsis, severe trauma, and ARDS outcome. Second, we genotyped the ABO gene to determine ABO blood type, including the A1 and A2 subtypes that are predicted to have distinct A antigen expression. Third, our analyses were adjusted for genetic population stratification via principal component analyses rather than patient-reported race. Fourth, we supported our primary genetic findings with data suggesting vWF and sTM may underlie the association between ABO blood type and ARDS, and that ABO blood type may affect lung recovery on EVLP. Finally, we used clinical data to phenotype DIC in the MESSI population and identified a potentially novel association between ABO blood type A and increased risk of sepsis-associated DIC.
Our study also has several limitations. First, despite providing strong evidence to direct future research, we cannot definitively identify the mechanism linking ABO blood type A and increased ARDS risk. The lack of a mouse model of ABO glycobiology, since mice lack ABO expression, significantly limits our ability to closely study the mechanism in animals, leaving our human epidemiological research as an important first step. Future research should focus on in vitro cell models aimed at refining mechanistic associations. Second, despite including 3 cohorts with over 3000 closely phenotyped patients, we are underpowered to compare all individual genotypes, including the rarer blood type B genotypes. Third, the ARDS phenotype is difficult to identify consistently, and it is possible some degree of misclassification of our outcome occurred; however, each individual patient was closely phenotyped by trained clinician investigators with adjudication for disagreements. Additionally, the DIC phenotype relied on labs drawn for clinical purposes with missing lab values assumed to be normal, possibly introducing misclassification and an underestimate of the true incidence of DIC. However, the DIC outcome misclassification is likely nondifferential by ABO blood type and therefore would bias us to the null.
In summary, we have demonstrated a strong reproducible association between genetically determined ABO blood type A1 and increased risk of ARDS mediated through microvascular dysfunction and coagulation. Our findings suggest that ABO blood type A defines a unique endotype of critical illness that may benefit from therapies targeting the microvasculature.
Methods
Study populations.
Three study populations were included in the reported analyses (Supplemental Table 1). PETROS and the MESSI cohort studies are ongoing prospective cohort studies enrolling at the University of Pennsylvania (13, 17, 43–45). iSPAAR is a multicenter case-control study (45, 46). Of the PETROS and MESSI patients included in this study, 39% and 41% were also enrolled in our prior nongenetic study identifying an association of ABO blood type A and ARDS risk (17).
In PETROS, all acutely injured trauma patients presenting to the level I trauma center of the University of Pennsylvania and admitted to the surgical ICU are screened. Subjects are enrolled if they were injured within 24 hours of presentation and have an injury severity score greater than 15 (65). Subjects are excluded if they die or are discharged from the ICU within 24 hours, have isolated severe head trauma, or are incarcerated. Subjects are enrolled with a waiver of informed consent with approval of the IRB of the University of Pennsylvania. Extensive data including mechanism of injury, underlying comorbidities, therapeutic interventions, and outcomes are collected on standardized case report forms by study personnel.
In MESSI, all admissions to the medical ICU are screened for criteria for sepsis. Subjects are enrolled if they meet sepsis-2 criteria for severe sepsis or septic shock and have a primary indication for ICU admission of sepsis (66). Subjects are excluded if they are admitted from long-term acute care facilities or have a do-not-intubate order at the time of enrollment. Subjects are enrolled with a waiver of timely informed consent with approval of the IRB of the University of Pennsylvania. Subjects or their surrogates are approached as soon as possible for consent, and if consent is not obtained, all biospecimens and data are discarded.
In iSPAAR, cases were obtained from subjects enrolled in NIH-sponsored ARDS Network trials and ARDS subjects enrolled in the Molecular Epidemiology of ARDS (MEARDS) cohort at Massachusetts General Hospital (6, 46, 67–69). Controls were obtained from the MEARDS cohort, which included a critically ill at-risk population. For the purposes of this study, only subjects with sepsis as their primary ARDS risk factor were included. Other at-risk populations enrolled in iSPAAR, such as aspiration- and transfusion-related acute lung injury, were excluded.
Genotyping.
DNA was extracted from whole blood collected at the time of enrollment using standard methods in all 3 cohorts. In PETROS and MESSI, subjects were genotyped using the Affymetrix Axiom Txv1 array including 785,194 SNPs. In iSPAAR, subjects were genotyped with the Illumina Human610-Quad Bead array of more than 500,000 SNPs. Principal component analysis was performed using all genotyped SNPs that passed quality control. Quality control included filtering SNPs with a minor allele frequency less than 5%, with missing calls for more than 10% of the populations, and SNPs displaying Hardy Weinberg disequilibrium (P < 10-6). All SNPs used to infer genetically determined ABO blood type and FUT2 secretor status passed quality control.
ARDS outcome ascertainment.
In PETROS and MESSI, ARDS was phenotyped within 6 days of presentation based on the Berlin definition with the added requirement for invasive mechanical ventilation (3, 70). Two physician investigators trained in identifying ARDS reviewed all chest radiographs ordered for clinical purposes, with consensus discussion for any disagreements. Arterial blood gases ordered for clinical purposes were used to determine the ratio of arterial partial pressure of oxygen to fraction of inhaled oxygen, with a ratio less than or equal to 300 constituting ARDS and less than or equal to 200 moderate to severe ARDS. If an arterial blood gas was unavailable within 24 hours of a positive chest radiograph, the ratio of the oxygen saturation to fraction of inhaled oxygen was used based on previously published methods (71).
In iSPAAR, ARDS cases were phenotyped based on the American European Consensus Criteria definition of ARDS given that the cases were drawn from clinical trials that predate the Berlin definition (72).
Plasma biomarker measurements in PETROS and MESSI.
Residual citrated plasma was collected from patients at emergency room/trauma bay presentation in patients admitted through the emergency department and at the point closest to ICU admission in patients transferred from the hospital ward. Plasma was centrifuged within 30 minutes of blood draw, used for clinical testing, and then refrigerated at 4°C. Residual plasma was then collected within 24 hours, placed in aliquots, and frozen at –80°C until biomarker testing was performed. This approach allowed us to obtain plasma from the earliest possible time point in patients’ presentation regardless of availability of research personnel. Plasma concentrations of sICAM-1, vWF, sTM, and E-selectin were then measured using commercially available ELISAs optimized for human plasma (vWF: Diagnostica Stago, 00942; sICAM-1: eBioscience, BMS201INSTCE; sTM, E-selectin: R & D Systems, DTHBD0 and DSLE00, respectively).
DIC phenotype.
In the MESSI cohort, subjects were determined to have DIC based on an International Society of Thrombosis and Haemostasis (ISTH) score greater than or equal to 5 based on labs drawn for clinical purposes within the first 6 days of admission (53). The lowest platelet count and fibrinogen and highest prothrombin time and D-dimer for each ICU day were utilized for ISTH score calculation. Because fibrinogen and D-dimer are often only clinically measured if DIC is suspected, missing values were assumed to be normal. No subjects were missing platelet counts or prothrombin time. We excluded patients with a history of cirrhosis or hepatic failure to avoid misclassifying patients who cannot produce coagulation factors as patients with DIC who are consuming coagulation factors.
EVLP experiments.
We tested the association between ABO blood type and recovery rate of suboptimal donor lungs for transplantation in a prospective cohort of donor lungs treated with EVLP as part of the 6-center NOVEL lung trial (73). In the NOVEL lung trial, EVLP was considered if the donor lungs had evidence of acute lung injury. Specifically, donor lungs demonstrated a PaO2/FiO2 less than or equal to 300 mmHg or a PaO2/FiO2 greater than 300 mmHg, but the donor had any of the following risk factors: multiple blood transfusions, pulmonary edema, donation after cardiac death, or the investigator deemed the donor lung quality as poor. Donor lungs with chronic lung disease, pneumonia, gastric acid aspiration, or significant barotrauma were excluded. After procurement, donor lungs were placed in a cold preservation solution and transported to a study site. Lung grafts were then placed on a mechanical ventilator and EVLP perfusion circuit and rewarmed. Lungs were perfused with an acellular perfusate, including balanced electrolytes and protein. EVLP was maintained for 4 hours and the grafts underwent serial assessments for improvement in physiological parameters. Once placed on EVLP, lungs were considered transplantable if they had 2 consecutive PaO2 greater than 350 on 100% FiO2 and had stable or improving pulmonary vascular resistance, compliance, and airway pressure. EVLP cases were performed on the XVIVO Perfusion System platform. ABO blood type was determined for each lung by blood bank typing of the donor because genetically determined ABO blood type was unavailable.
Statistics.
Study characteristics were compared between patients who developed ARDS and those who did not using the χ2 test for categorical variables and Wilcoxon rank-sum test for continuous variables. In the primary analyses, multivariable logistic regression was used to determine the association of genetically determined blood type A1 versus O with moderate or severe ARDS risk, adjusting for a prespecified list of potential confounders and population stratification. Population stratification was determined via principal component analyses and all multivariable models were adjusted for principal components 1 through 4. Our primary comparison was between ABO blood type A1 and O because it is unclear whether rarer blood types exhibit a phenotype closer to A1 or O, and we are underpowered to evaluate such differences. We used moderate to severe ARDS as the primary outcome because these patients are more likely to have diffuse alveolar damage, the pathological correlate to ARDS, as their underlying pathology (49). We also prespecified secondary analyses stratified by source of sepsis in the 2 sepsis cohorts. We tested for statistical interaction by source of sepsis, race, and mechanism of trauma using the likelihood ratio test. Biomarker concentrations were compared between blood types first in MESSI and PETROS separately using the Wilcoxon rank-sum test. We then combined the cohorts to conduct adjusted analyses using multivariable linear regression. For regression models, biomarker concentrations were first log-transformed to approximate a normal distribution. Biomarker concentrations were then compared between ARDS and no ARDS using the Wilcoxon rank-sum test followed by multivariable logistic regression adjusting for potential confounders. Multivariable logistic regression was also used to test the association of blood type and DIC, as well as blood type and EVLP recovery. A P value less than 0.05 was considered significant.
Study approval.
Participants enrolled in PETROS were enrolled under a waiver of informed consent granted with approval from the IRB of the University of Pennsylvania. Participants enrolled in MESSI were enrolled with a waiver of timely informed consent granted with approval from the IRB of the University of Pennsylvania. All subjects or their surrogates subsequently provided written informed consent.
Author contributions
JPR, NJM, MPR, and JDC designed the studies included in the manuscript. JPR, NJM, MGSS, BJA, CI, CF, PNL, and JDC designed and conducted the prospective cohort studies MESSI and PETROS at the University of Pennsylvania. JPR, NJM, MGSS, BJA, CI, TGD, BL, CF, MPB, EK, PNL, and JDC acquired data for MESSI and PETROS, including clinical data, outcome phenotypes, and plasma biomarker measurements. NJM, CSC, MAM, CM, KW, JR, DCC, MMW, and JDC designed, conducted, and acquired data for the iSPAAR case-control study. EC acquired data from the NOVEL lungs study. JPR, NJM, MPB, RF, EC, NSM, and JDC analyzed data. JPR initially drafted the manuscript with all authors editing and approving the final version.
Supplementary Material
Acknowledgments
We would like to acknowledge our funding sources for this research including NIH grants HL122075 (to JPR), HL125723 (to JPR), HL137915 (to NJM), DK097307 (to MGSS), HL115354 (to JDC), HL101779 (to MMW), and the University of Pennsylvania McCabe Fund Fellowship Award (to JPR). We would also like to acknowledge the patients enrolled in the studies included in this manuscript and their families for their participation.
Version 1. 09/15/2020
In-Press Preview
Version 2. 01/04/2021
Print issue publication
Footnotes
Conflict of interest: NJM reports grants from Athersys Inc., Biomarck Inc., and the Marcus Foundation. CSC reports grants and personal fees from Bayer, Roche/Genentech, Prometic, Quark Pharmaceuticals, GEn1E Life Sciences, and Vasomune. MAM reports grants from Bayer Pharmaceuticals and Roche Genentech and personal fees from GenLife Science and Citius Pharmaceuticals. JDC reports grants from GlaxoSmithKline and Bristol Myers Squibb and personal fees from Onspira.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(1):e139700.https://doi.org/10.1172/JCI139700.
See the related Commentary at ABO blood type: a window into the genetics of acute respiratory distress syndrome susceptibility.
Contributor Information
John P. Reilly, Email: John.Reilly@pennmedicine.upenn.edu.
Nuala J. Meyer, Email: nuala.meyer@uphs.upenn.edu.
Michael G.S. Shashaty, Email: shashatm@mail.med.upenn.edu.
Brian J. Anderson, Email: brian.anderson@uphs.upenn.edu.
Caroline Ittner, Email: Caroline.Ittner@uphs.upenn.edu.
Thomas G. Dunn, Email: tdunn@pennmedicine.upenn.edu.
Brian Lim, Email: blim0121@gmail.com.
Caitlin Forker, Email: cforker@bu.edu.
Michael P. Bonk, Email: bonk-michael@cooperhealth.edu.
Rui Feng, Email: ruifeng@mail.med.upenn.edu.
Edward Cantu, Email: edward.cantu@pennmedicine.upenn.edu.
Nilam S. Mangalmurti, Email: nspatel@pennmedicine.upenn.edu.
Carolyn S. Calfee, Email: Carolyn.Calfee@ucsf.edu.
Michael A. Matthay, Email: michael.matthay@ucsf.edu.
Carmen Mikacenic, Email: cmikacen@uw.edu.
Keith R. Walley, Email: Keith.Walley@hli.ubc.ca.
David C. Christiani, Email: dchris@hsph.harvard.edu.
Mark M. Wurfel, Email: mwurfel@medicine.washington.edu.
Paul N. Lanken, Email: paul.lanken@uphs.upenn.edu.
Muredach P. Reilly, Email: mpr2144@cumc.columbia.edu.
Jason D. Christie, Email: jchristi@upenn.edu.
References
- 1.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellani G, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 3.The ARDS Definition Task Force, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Rubenfeld GD, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.The Acute Respiratory Distress Syndrome Network, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 7.Guérin C, et al. Prone positioning in the acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 8.Rubenfeld GD. Confronting the frustrations of negative clinical trials in acute respiratory distress syndrome. Ann Am Thorac Soc. 2015;12 Suppl 1:S58–S63. doi: 10.1513/AnnalsATS.201409-414MG. [DOI] [PubMed] [Google Scholar]
- 9.Spragg RG, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351(9):884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 10.McAuley DF, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371(18):1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 11.Kor DJ, et al. Effect of aspirin on development of ARDS in at-risk patients presenting to the emergency department: The LIPS-A Randomized Clinical Trial. JAMA. 2016;315(22):2406–2414. doi: 10.1001/jama.2016.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5(6):524–534. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- 13.Reilly JP, et al. Heterogeneous phenotypes of acute respiratory distress syndrome after major trauma. Ann Am Thorac Soc. 2014;11(5):728–736. doi: 10.1513/AnnalsATS.201308-280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reilly JP, Christie JD, Meyer NJ. Fifty years of research in ARDS. Genomic contributions and opportunities. Am J Respir Crit Care Med. 2017;196(9):1113–1121. doi: 10.1164/rccm.201702-0405CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calfee CS, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calfee CS, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reilly JP, et al. ABO blood type A is associated with increased risk of ARDS in whites following both major trauma and severe sepsis. Chest. 2014;145(4):753–761. doi: 10.1378/chest.13-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Storry JR, Olsson ML. The ABO blood group system revisited: a review and update. Immunohematology. 2009;25(2):48–59. [PubMed] [Google Scholar]
- 19.Cserti CM, Dzik WH. The ABO blood group system and Plasmodium falciparum malaria. Blood. 2007;110(7):2250–2258. doi: 10.1182/blood-2007-03-077602. [DOI] [PubMed] [Google Scholar]
- 20.Stowell SR, Stowell CP. Biologic roles of the ABH Lewis histo-blood group antigens part II: thrombosis, cardiovascular disease metabolism. Vox Sang. 2019;114(6):535–552. doi: 10.1111/vox.12786. [DOI] [PubMed] [Google Scholar]
- 21.Stowell CP, Stowell SR. Biologic roles of the ABH and Lewis histo-blood group antigens Part I: infection and immunity. Vox Sang. 2019;114(5):426–442. doi: 10.1111/vox.12787. [DOI] [PubMed] [Google Scholar]
- 22.Emilsson V, et al. Co-regulatory networks of human serum proteins link genetics to disease. Science. 2018;361(6404):769–773. doi: 10.1126/science.aaq1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun W, et al. Common genetic polymorphisms influence blood biomarker measurements in COPD. PLoS Genet. 2016;12(8):e1006011. doi: 10.1371/journal.pgen.1006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao C, et al. Genome-wide mapping of plasma protein QTLs identifies putatively causal genes and pathways for cardiovascular disease. Nat Commun. 2018;9(1):3268. doi: 10.1038/s41467-018-05512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suhre K, et al. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun. 2017;8:14357. doi: 10.1038/ncomms14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiechl S, et al. Association of variation at the ABO locus with circulating levels of soluble intercellular adhesion molecule-1, soluble P-selectin, and soluble E-selectin: a meta-analysis. Circ Cardiovasc Genet. 2011;4(6):681–686. doi: 10.1161/CIRCGENETICS.111.960682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barbalic M, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19(9):1863–1872. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paré G, et al. Novel association of ABO histo-blood group antigen with soluble ICAM-1: results of a genome-wide association study of 6,578 women. PLoS Genet. 2008;4(7):e1000118. doi: 10.1371/journal.pgen.1000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morelli VM, et al. ABO blood group genotypes, plasma von Willebrand factor levels and loading of von Willebrand factor with A and B antigens. Thromb Haemost. 2007;97(4):534–541. [PubMed] [Google Scholar]
- 30.Franchini M, Capra F, Targher G, Montagnana M, Lippi G. Relationship between ABO blood group and von Willebrand factor levels: from biology to clinical implications. Thromb J. 2007;5:14. doi: 10.1186/1477-9560-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blann AD, Daly RJ, Amiral J. The influence of age, gender and ABO blood group on soluble endothelial cell markers and adhesion molecules. Br J Haematol. 1996;92(2):498–500. doi: 10.1046/j.1365-2141.1996.d01-1486.x. [DOI] [PubMed] [Google Scholar]
- 32.Claus RA, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost. 2009;101(2):239–247. doi: 10.1160/TH08-03-0161. [DOI] [PubMed] [Google Scholar]
- 33.Calfee CS, et al. Soluble intercellular adhesion molecule-1 and clinical outcomes in patients with acute lung injury. Intensive Care Med. 2009;35(2):248–257. doi: 10.1007/s00134-008-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fremont RD, et al. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma. 2010;68(5):1121-1127. doi: 10.1097/TA.0b013e3181c40728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ware LB, Eisner MD, Thompson BT, Parsons PE, Matthay MA. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004;170(7):766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 36.Reilly MP, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: two genome-wide association studies. Lancet. 2011;377(9763):383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu O, Bayoumi N, Vickers MA, Clark P. ABO(H) blood groups and vascular disease: a systematic review and meta-analysis. J Thromb Haemost. 2008;6(1):62–69. doi: 10.1111/j.1538-7836.2007.02818.x. [DOI] [PubMed] [Google Scholar]
- 38.O’Donnell J, Boulton FE, Manning RA, Laffan MA. Amount of H antigen expressed on circulating von Willebrand factor is modified by ABO blood group genotype and is a major determinant of plasma von Willebrand factor antigen levels. Arterioscler Thromb Vasc Biol. 2002;22(2):335–341. doi: 10.1161/hq0202.103997. [DOI] [PubMed] [Google Scholar]
- 39.Daniels G. The molecular genetics of blood group polymorphism. Hum Genet. 2009;126(6):729–742. doi: 10.1007/s00439-009-0738-2. [DOI] [PubMed] [Google Scholar]
- 40.Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. Sequence and expression of a candidate for the human Secretor blood group alpha(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270(9):4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 41.Paré G, et al. Genome-wide association analysis of soluble ICAM-1 concentration reveals novel associations at the NFKBIK, PNPLA3, RELA, and SH2B3 loci. PLoS Genet. 2011;7(4):e1001374. doi: 10.1371/journal.pgen.1001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lieb W, et al. Genome-wide association study for endothelial growth factors. Circ Cardiovasc Genet. 2015;8(2):389–397. doi: 10.1161/CIRCGENETICS.114.000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reilly JP, et al. Low to moderate air pollutant exposure and acute respiratory distress syndrome after severe trauma. Am J Respir Crit Care Med. 2019;199(1):62–70. doi: 10.1164/rccm.201803-0435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reilly JP, et al. Neutropenic sepsis is associated with distinct clinical and biological characteristics: a cohort study of severe sepsis. Crit Care. 2016;20(1):222. doi: 10.1186/s13054-016-1398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reilly JP, et al. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: evidence from Mendelian randomization and mediation analysis. Intensive Care Med. 2018;44(11):1849–1858. doi: 10.1007/s00134-018-5328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhatraju PK, et al. Acute kidney injury subphenotypes based on creatinine trajectory identifies patients at increased risk of death. Crit Care. 2016;20(1):372. doi: 10.1186/s13054-016-1546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paterson AD, et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29(11):1958–1967. doi: 10.1161/ATVBAHA.109.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markt SC, et al. ABO blood group alleles and prostate cancer risk: results from the breast and prostate cancer cohort consortium (BPC3) Prostate. 2015;75(15):1677–1681. doi: 10.1002/pros.23035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thille AW, et al. Comparison of the Berlin definition for acute respiratory distress syndrome with autopsy. Am J Respir Crit Care Med. 2013;187(7):761–767. doi: 10.1164/rccm.201211-1981OC. [DOI] [PubMed] [Google Scholar]
- 50.Calfee CS, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147(6):1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tejera P, et al. Distinct and replicable genetic risk factors for acute respiratory distress syndrome of pulmonary or extrapulmonary origin. J Med Genet. 2012;49(11):671–680. doi: 10.1136/jmedgenet-2012-100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Velkova A, et al. The FUT2 secretor variant p.Trp154Ter influences serum vitamin B12 concentration via holo-haptocorrin, but not holo-transcobalamin, and is associated with haptocorrin glycosylation. Hum Mol Genet. 2017;26(24):4975–4988. doi: 10.1093/hmg/ddx369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bakhtiari K, Meijers JC, de Jonge E, Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32(12):2416–2421. doi: 10.1097/01.CCM.0000147769.07699.E3. [DOI] [PubMed] [Google Scholar]
- 54.Landsteiner K. Über Agglutinationserscheinungen normalen menschlichen Blutes. Wien Klin Wochenschr. 1901;14:1132–1134. [PubMed] [Google Scholar]
- 55.Cheng Y, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293(12):1450–1451. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- 56.Loghmani H, Conway EM. Exploring traditional and nontraditional roles for thrombomodulin. Blood. 2018;132(2):148–158. doi: 10.1182/blood-2017-12-768994. [DOI] [PubMed] [Google Scholar]
- 57.Vincent JL, et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. JAMA. 2019;321(20):1993–2002. doi: 10.1001/jama.2019.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reilly JP, Calfee CS, Christie JD. Acute respiratory distress syndrome phenotypes. Semin Respir Crit Care Med. 2019;40(1):19–30. doi: 10.1055/s-0039-1684049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paterson AD, et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29(11):1958–1967. doi: 10.1161/ATVBAHA.109.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi L, et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum Mol Genet. 2010;19(9):1856–1862. doi: 10.1093/hmg/ddq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellinghaus D, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. doi: 10.1056/NEJMoa2020283. [published online ahead of print June 17, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou F, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker SP, O’Neill B, Haddon W, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. doi: 10.1097/00005373-197403000-00001. [DOI] [PubMed] [Google Scholar]
- 66. Levy MM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. [DOI] [PubMed] [Google Scholar]
- 67.Rice TW, et al. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.National Heart, Lung, Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, et al. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei Y, et al. Platelet count mediates the contribution of a genetic variant in LRRC16A to ARDS risk. Chest. 2015;147(3):607–617. doi: 10.1378/chest.14-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah CV, et al. An alternative method of acute lung injury classification for use in observational studies. Chest. 2010;138(5):1054–1061. doi: 10.1378/chest.09-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rice TW, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 72. Bernard GR, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 pt 1):818–824. [DOI] [PubMed] [Google Scholar]
- 73.Leiva-Juárez MM, et al. Extended post-ex vivo lung perfusion cold preservation predicts primary graft dysfunction and mortality: Results from a multicentric study. J Heart Lung Transplant. 2020;39(9):954–961. doi: 10.1016/j.healun.2020.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.