Abstract
Ongoing observational clinical research has prioritized understanding the human immune response to SARS-CoV-2 during the coronavirus disease 2019 (COVID-19) pandemic. Several recent studies suggest that immune dysregulation with early and prolonged adaptive immune system activation can result in cellular exhaustion. In this issue of the JCI, Files et al. compared cellular immune phenotypes during the first two months of COVID-19 in hospitalized and less severe, non-hospitalized patients. The authors utilized flow cytometry to analyze circulating peripheral blood mononuclear cells. Both patient cohorts maintained B and T cell phenotypes consistent with activation and cellular exhaustion throughout the first two months of infection. Additionally, follow-up samples from the non-hospitalized patient cohort showed that activation markers and cellular exhaustion increased over time. These findings illustrate the persistent nature of the adaptive immune system changes that have been noted in COVID-19 and suggest longer term effects that may shape the maintenance of immunity to SARS-CoV-2.
Introduction
The world has been inundated by the effects of a beta coronavirus that emerged in late 2019 and subsequently spread worldwide to cause one of the deadliest viral pandemics of the past century. The number of worldwide deaths attributable to COVID-19, the disease caused by the SARS-CoV-2 virus, currently matches some estimates for the number of deaths attributable to the 1956 to 1958 influenza pandemic and trails only the 1918 to 1920 influenza pandemic and the HIV/AIDS pandemic in the number of estimated worldwide deaths. Although much has been learned over the past 10 months regarding the human immune response to SARS-CoV-2 infection, it is still not clear how these responses evolve over time. Immune response information has become paramount to the pandemic’s management because deaths attributable to COVID-19 will likely continue to increase until widespread immunity is achieved via continued worldwide spread or until an effective vaccine is broadly distributed.
Understanding the human immune response to other viral infections, including influenza infection (1), respiratory syncytial virus infection (2), and Ebola infection (3), has helped inform vaccine design. Presently, ongoing clinical research remains descriptive, providing only details about the natural immune response to SARS-CoV-2 infection, as we eagerly await the evaluation of currently ongoing large-scale COVID-19 vaccine trials. The importance of these studies to provide context for the interpretation of immune responses generated by participants in COVID-19 vaccine trials, including how those responses change over time, cannot be overemphasized. This information will be key in potential modifications to existing COVID-19 vaccines and treatments. Further understanding of the natural immune response to SARS-CoV-2 will help discover correlates of protection that vaccines can target and establish dosing intervals if vaccination redosing is ultimately required.
Prolonged immune activation
Early reports suggested that the immune response to the virus may be impaired to some degree through diminished lymphocyte counts, increased neutrophils, and proinflammatory cytokines in individuals admitted to the intensive care unit (ICU) (4). However, clarity in the exact changes that occur during the first days and weeks following acute infection has come to light more recently (5–7). In this issue of the JCI, Files et al. (5) advance the field’s knowledge by comparing the cellular immune phenotypes of peripheral blood mononuclear cells during the first two months of infection in a cohort of COVID-19 patients requiring hospitalization and a less severe non-hospitalized cohort. The authors further refine the findings of these other recently published studies by exploring the temporal evolution of the immune response in follow-up samples obtained from their non-hospitalized patients (5).
The authors’ principal findings include a notable increase in CD4+ and CD8+ T cell activation in hospitalized versus non-hospitalized patients, including upregulation of CD69, Ox40, HLA-DR, CD154, and CD38. Increases in COVID-19 patients’ activated CD4+ and CD8+ T cells in the context of greater severity of illness has previously been reported (6, 8). However, Files et al. (5) demonstrate that many of these markers of activation were noted to increase in non-hospitalized patients 30 to 45 days after symptom onset. Additionally, HLA-DR, Ox40, and Tim3 were increased over time on CD4+ T cells from non-hospitalized patients, and HLA-DR, TIGIT, and PD-L1 were increased while enduring significant decreases in CD27 and CD28 on the surface of CD8+ T cells on repeat sampling. The expression level of several of the measured surface markers, including PD1, HLA-DR, and TIGIT, had significant positive correlations with age in the cohort on both CD4+ and CD8+ T cells, and the surface expression of CD28 exhibited a significant negative correlation with age on both CD4+ and CD8+ T cells (5). These findings comport with the well-established correlate of increasing T cell exhaustion, defined as depletion in T cell quantitatively or suppression of function following chronic viral infection that occurs with aging, and may guide our understanding of immune responses to a vaccine. The authors also evaluated B cell surface markers and found increased CD69, CD27, and PD1 in hospitalized and non-hospitalized COVID-19 subjects, suggesting increased B cell activation. They further separated out those hospitalized patients admitted to the ICU from those who did not require ICU level of care, demonstrating that many components of increased CD4+ T cell, CD8+ T cell, and B cell activation were more pronounced in the ICU group, with the notable exception of HLA-DR, which showed a lower surface expression on B cells in patients admitted to the ICU.
One limitation in this work as pointed out by the authors is the inability to accurately measure the degree or duration of viremia in individual subjects. Lucas et al. have recently shown that patients with severe illness exhibit prolonged elevated nasopharyngeal viral loads when compared with patients who have moderate illness (7). Furthermore, others have reported prolonged viral shedding in elderly COVID-19 subjects (9), with some reporting nasopharyngeal viral RNA shedding for 30 days or more in small subgroups of patients (10). However, the infectivity of virus associated with these positive viral RNA tests more than 15 days after the onset of symptoms has been questioned (11). Nevertheless, it is certainly apparent that antigens from the SARS-CoV-2 virus may remain longer compared with other common human viruses that do not enter latency within the human host, especially in individuals who are older and those with more severe COVID-19.
The findings by Files et al. (5) are not necessarily unexpected in the setting of what is likely prolonged exposure to antigen in patients with COVID-19. Furthermore, the changes associated with increasing activation in patients who had more severe illness also correlated well with those that would be expected in an individual with a higher viral load and, therefore, more profound exposure to antigen. Nevertheless, the implications for long-term impacts upon the immune system are certainly important. Increasing exposure to antigen leads to increased cellular exhaustion.
Implications for future treatment and vaccines
Understanding that early induction of cellular immune exhaustion may limit T cell and B cell responses in COVID-19 patients has very real impacts in considering the immune response of previously infected patients to subsequent rechallenge with a new infectious dose of virus. While antibody responses in infected human patients appear to be robustly engaged, with more than 90% of infected individuals testing seropositive (12), antigen-specific T cell responses may be limited in quantity or quality upon reexposure to the virus in a subsequent infection. Indeed, some groups have already reported limited induction of antigen-specific CD8+ T cells in some individuals infected with SARS-CoV-2, despite robust antigen-specific CD4+ T cell responses (13).
Many of the current COVID-19 vaccines in the testing pipeline utilize vectors that have been developed to induce both T cell and B cell responses. The successful induction of T cell responses by any of these vaccines may ultimately improve upon protection from severe disease in individuals who receive the vaccine yet become infected. In addition, if natural infection fails to successfully induce large numbers of antiviral CD8+ T cells, then receipt of a vaccine candidate that induces such responses may provide critical protection from severe disease, even for individuals who have already been infected and have substantial neutralizing antibody titers. Increased antiviral T cell quantities may prove more important in elderly individuals, those on immunosuppressive drugs, and other groups with impaired ability to generate T cell responses who may be at higher risk of poor outcome from future infections with SARS-CoV-2.
Unfortunately, early focus in the pandemic has remained on an elusive cytokine storm rather than consideration of an appropriate proinflammatory response (normal HLA-DR expression, elevated IL-6) (14) and subsequent immune suppression. Ongoing data have reiterated the importance of understanding the clear biologic evidence of immunosuppression (low HLA-DR, diminished T cell response with increased CD279 [PD1] expression), smoldering low-level inflammation, and development of secondary infections in patients (14–16). This immune suppression with long-lasting effects up to 45 days as recapitulated by Files et al. (5) (Figure 1) makes a strong argument to support immune-restorative therapies, such as administration of IL-7 or Ox40, while an efficacious vaccine can be developed for large-scale use (16, 17). IL-7 is well established to improve lymphopenia and increase TNF-α and IFN-γ production in patients with COVID-19 (16, 17). Therapies that modify pluripotency (such as IL-7) could be paired with an appropriate vaccine or neutralizing polyclonal antibody combination to reduce illness severity and minimize the temporal immune activation and suppression that render hospitalized and non-hospitalized patients susceptible to secondary infections (18). Approaches that target immune suppression will be vital as relevant vaccines are developed.
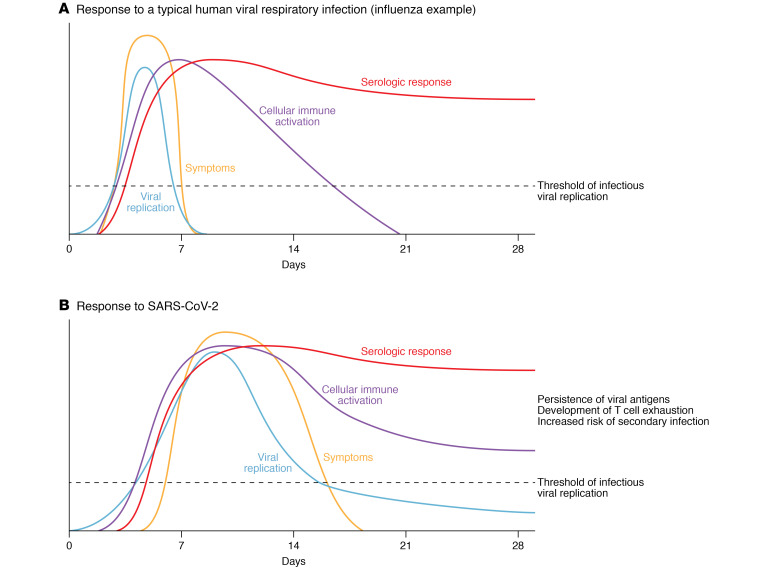
Figure 1. Response to human viral respiratory infections.
(A) In a typical influenza infection, symptoms and viral replication peak within a few days and resolve after the first week; serologic response peaks within the first two weeks and remains high. Notably, with influenza, the cellular immune activation peaks at week one and resolves by week three. (B) In contrast, with SARS-CoV-2 infection, the cellular immune system remains activated despite viral clearance. The persistence of viral antigens, development of T cell exhaustion, and increased risk of secondary infections set SARS-CoV-2 infection apart from other viral respiratory infections.
Conclusion
COVID-19 will undoubtedly persist as a circulating human pathogen during the near term and perhaps into the future if the success of future vaccine campaigns is limited in any way. The insights provided by researchers who have taken on the early burden of rapidly implementing observational studies to describe the natural human immune response to SARS-CoV-2 infection will prove beneficial to the ongoing development of improved diagnostic tests and potential therapies. These findings are needed to further refine relevant immune-modifying therapies that not only neutralize viral entry and/or replication but also reverse persistent adaptive immune suppression that would otherwise predispose individuals to acquiring deleterious secondary infections.
Acknowledgments
PAM is supported by NIH contract HHSN272201400008C. KER is supported by Washington University in St. Louis Institute of Translational Science grant NIH UL1TR000345 and NIH National Institute of General Medical Sciences K08 GM129763.
Version 1. 10/26/2020
In-Press Preview
Version 2. 01/04/2021
Print issue publication
Footnotes
Conflict of interest: PAM is listed as inventor or coinventor on the following applied for, provisional, or full patents: 019387, “Kits and methods for selecting treatments for respiratory infections”; 019488, “Targeted modulation of the inflammatory cascade in patients with severe viral respiratory illness”; and 019454, “Human COVID mAbs for diagnostics.” PAM’s spouse is employed by AbbVie and receives equity in the company through stock options associated with that employment. KER has provisional patents pending: WUSTL 019522/US, 019522/US2, and 019522/US3 titled “ELISpot Assay to Immune Phenotype Patients.”
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(1):e143928. https://doi.org/10.1172/JCI143928.
See the related article at Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection.
Contributor Information
Philip A. Mudd, Email: pmudd@wustl.edu.
Kenneth E. Remy, Email: kremy@wustl.edu.
References
- 1.Ng S, et al. Novel correlates of protection against pandemic H1N1 influenza A virus infection. Nat Med. 2019;25(6):962–967. doi: 10.1038/s41591-019-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Openshaw PJM, Chiu C, Culley FJ, Johansson C. Protective and harmful immunity to RSV infection. Annu Rev Immunol. 2017;35:501–532. doi: 10.1146/annurev-immunol-051116-052206. [DOI] [PubMed] [Google Scholar]
- 3.McElroy AK, et al. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci U S A. 2015;112(15):4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Files JK, et al. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J Clin Invest. 2021;131(1):e140491. doi: 10.1172/JCI140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathew D, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508):eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas C, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sekine T, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158–168. doi: 10.1016/j.cell.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang K, et al. Differences of SARS-CoV-2 shedding duration in sputum and nasopharyngeal swab specimens among adult inpatients with COVID-19. Chest. doi: 10.1016/j.chest.2020.06.015. doi: 10.1016/j.chest.2020.06.015. [published online June 19, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi D, et al. Clinical characteristics and factors associated with long-term viral excretion in patients with SARS-CoV-2 infection: a single center 28-day study. J Infect Dis. 2020;222(6):910–918. doi: 10.1093/infdis/jiaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. doi: 10.1101/2020.06.08.20125310. van Kampen JJ, et al. Shedding of infectious virus in hospitalized patients with coronavirus disease-2019 (COVID-19): duration and key determinants [preprint]. Posted on medRxiv June 9, 2020. [DOI] [PMC free article] [PubMed]
- 12.Gudbjartsson DF, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. doi: 10.1056/NEJMoa2026116. [published online September 1, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grifoni A, et al. Targets of T cell responses to SARS-CoV-2 Coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remy KE, et al. Immunotherapies for COVID-19: lessons learned from sepsis. Lancet Respir Med. 2020;8(10):946–949. doi: 10.1016/S2213-2600(20)30217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mudd PA, et al. Targeted immunosuppression distinguishes COVID-19 from influenza in moderate and severe disease [preprint]. https://www.medrxiv.org/content/10.1101/2020.05.28.20115667v1 Posted on medRxiv May 30, 2020.
- 16.Remy KE, et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. 2020;5(17):140329. doi: 10.1172/jci.insight.140329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laterre PF, et al. Association of interleukin 7 immunotherapy with lymphocyte counts among patients with severe Coronavirus disease 2019 (COVID-19) JAMA Netw Open. 2020;3(7):e2016485. doi: 10.1001/jamanetworkopen.2020.16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horiguchi H, et al. Innate immunity in the persistent inflammation, immunosuppression, and catabolism syndrome and its implications for therapy. Front Immunol. 2018;9:595. doi: 10.3389/fimmu.2018.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]