Abstract
The genetic factors that determine a patient’s risk for developing the acute respiratory distress syndrome (ARDS) remain understudied. In this issue of the JCI, Reilly and colleagues analyzed data from three cohorts of critically ill patients and observed an association between the ABO allele A1 and the onset of moderate-severe ARDS. This association was most notable in patients with non-pulmonary sepsis (an indirect, vasculature-targeted mechanism of lung injury) and persisted in patients who lacked epithelial expression of the A antigen, suggesting an endothelial mechanism of A1-associated ARDS susceptibility. Critically ill patients with blood type A had increased circulating concentrations of endothelium-derived glycoproteins such as von Willebrand factor and soluble thrombomodulin, and marginal lungs from blood type A donors were less likely to recover function during ex vivo perfusion. These findings implicate A antigen glycosylation of endothelial cells as a critical, genetically determined risk factor for indirect lung injury that may contribute to the mechanistic heterogeneity of ARDS.
Diverse roles for ABH glycosylation
The COVID-19 pandemic has prompted intense investigation of both genetic and environmental risk factors for severe SARS-CoV-2 infection and its consequences, such as the acute respiratory distress syndrome (ARDS). Multiple cohort studies have demonstrated that patients with blood type A experience increased severity of illness with viral infections, such as the severe acute respiratory syndrome virus (SARS-CoV-1) and SARS-CoV-2 (1, 2). Indeed, blood type is a known risk factor for multiple infectious diseases. Compared with patients with blood type O, patients with type A blood are at increased risk for malaria infection due to Plasmodium falciparum but decreased risk for both Helicobacter pylori gastritis and Vibrio cholerae (3, 4). Intriguingly, many of these ABO associations with disease are not directly mediated by erythrocytes. Rather, an individual’s ABO blood type is a reflection of the highly complex processes of cell-surface glycosylation employed by that individual across numerous cell types, in the never-ending attempt to evade pathogen recognition (5).
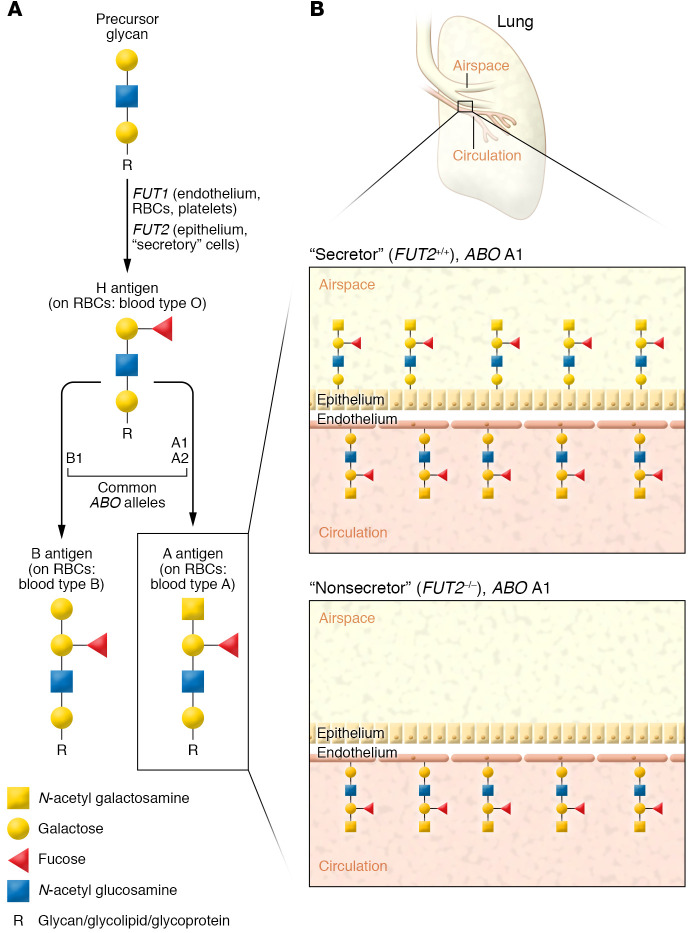
The histo-blood groups ABO (9q34.1), H (now known as FUT1, 19q13.3), and Se (now known as FUT2, 19q13.3) are a set of genes that encode enzymes that catalyze the addition of monosaccharide units to precursor oligosaccharide chains. Sequential action of these glycosyltransferases creates the ABH glycan antigens displayed on erythrocytes (responsible for ABO blood types) as well as numerous other cell types (Figure 1A). An individual’s allelic variation at these genes dictates the repertoire of glycan structures that their body can produce (6). ABH antigen synthesis begins with the production of the H antigen by the action of a fucosyltransferase (such as FUT1), which adds a fucose monosaccharide (α1-2 fucose) to the nonreducing end of a glycan precursor anchored to glycoprotein or glycolipid (6). This H antigen is subsequently modified by glycosyltransferases encoded by the ABO gene. ABO A101 (A1) or A102 (A2) alleles encode a glycosyltransferase (α1-3 N-acetyl galactosamine transferase) that creates the A glycan epitope, with A1 associated with greater glycosyltransferase activity and thus increased A antigen density. The ABO B101 (B1) allele encodes an alternative glycosyltransferase (α1-3 galactosyltransferase) responsible for B glycan formation (6). The ABO O101 (O) allele has a frameshift mutation that leads to the production of a functionally inactive glycosyltransferase, which results in persistence of the H antigen without additional glycan modifications; on red blood cells, this imparts blood type O (7). Notably, the mechanisms responsible for ABH antigen production are cell type specific. Epithelial cells do not express FUT1 and instead rely upon a glycosyltransferase encoded by the FUT2 gene to catalyze H antigen production. However, in 20% of European populations, FUT2 is nonfunctional, and these individuals do not display ABH antigens on their epithelium or within mucosal secretions (ref. 8 and Figure 1B).
Figure 1. Cell-specific expression of ABH antigens.
(A) Cell type–specific fucosyltransferases (FUT1 in multiple cell types; FUT2 in epithelial and secretory cells) create H antigens by adding a fucose monosaccharide to the nonreducing (free) ends of glycans anchored to glycolipids or glycoproteins. H antigens are then modified by glycosyltransferases encoded by the ABO genes. ABO allele A1 produces a highly active glycosyltransferase that imparts high A antigen density on cell surfaces. ABO allele O produces a nonfunctional glycosyltransferase, leaving the H antigen unmodified. A, B, or H antigens on red blood cells (RBCs) impart blood types A, B, or O, respectively. Simplified schema does not show nature of glycan linkages (i.e., α2 linkage of fucose). (B) Approximately 20% of European populations have loss-of-function mutations in FUT2, leading to an inability to form H antigens (and consequently, A or B antigens) on epithelial surfaces. As such, FUT2–/– individuals with ABO A1 alleles will express a high density of A antigens on erythrocytes and endothelial cells but have no expression of A antigens on epithelial surfaces or mucosal secretions. Simplified schema excludes underlying glycan core structures. A antigens can be further modified into hyperglycosylated A1 and A2 antigen subtypes; only A antigens are shown for simplicity.
In addition to their role in host-pathogen interactions, which are largely mediated by ABH glycan expression on epithelial cells, ABH antigens are also expressed on endothelial cells and platelets, where they influence endothelial activation and coagulation (9). The importance of the ABH antigens in these processes is highlighted by the well-established increase in risk of both cardiovascular and thromboembolic disease in patients with non-O blood types (10, 11). The underlying mechanisms are likely multifactorial, as ABH antigens have been shown to mediate von Willebrand factor (vWF) expression, density of glycan antigen loading, and metabolism, as well as the expression of multiple genes involved in microvascular permeability and inflammation, including P selectin, E selectin, and soluble intercellular adhesion molecule (12–14). The established role of many of these proteins in inflammatory vascular leak led to the hypothesis that ABO genotype may alter ARDS risk and contribute to heterogeneity of pulmonary responses to inflammatory stimuli.
Endothelial ABH glycosylation status mediates ARDS risk
Reilly and colleagues first reported an association between blood type and ARDS risk in a cohort of 732 critically ill trauma and sepsis patients. In that cohort, patients of European descent with blood type A — representative of a propensity for glycosylation of cell surfaces with the A antigen — showed an association with increased risk of ARDS (15). This association was not seen with patients of African descent. Blood type A was similarly associated with the development of acute kidney injury in sepsis and trauma patients of European descent but not those of African descent (16). The rationale for the ethnicity-based disparity in ABO blood type risk is uncertain. Interestingly, a polymorphism of the Duffy antigen/chemokine receptor gene, which encodes another blood type glycosyltransferase, is associated with ARDS risk in patients who are African American (17).
In this issue of the JCI, Reilly and colleagues add mechanistic insights to their previously reported association between blood type A and ARDS. In this study the authors leverage the statistical power of three large independent cohorts of critically ill sepsis (from the Molecular Epidemiology of SepsiS in the ICU [MESSI] and Identification of SNPs Predisposing to Altered ALI Risk [iSPAAR] studies) and trauma (Penn Trauma Organ dysfunction cohort study) patients to demonstrate an impressive dose-dependent relationship between the density of A antigen expression and risk of ARDS (18). In all three cohorts, the possession of any copy of the A1 allele, which encodes a version of the A glycosyltransferase with increased catalytic activity, was associated with increased moderate or severe ARDS risk (18). Interestingly, in both cohorts of sepsis patients (MESSI and iSPAAR), the association between blood type A and ARDS was present in patients with non-pulmonary sepsis but not pulmonary sepsis, suggesting that ABH antigens may specifically shape the pulmonary response to a vasculature-targeted injury (18). Furthermore, analysis of “nonsecretor” (FUT2–/–) patients, who do not express ABH antigens on epithelial cells or their secretions, maintained this association with ARDS, suggesting that the propensity for ARDS is not mediated by epithelial glycosylation status. The authors also demonstrated that blood type A patients had increased levels of the endothelium-derived glycoproteins vWF and thrombomodulin, suggesting a potential vasculature-mediated mechanism underlying these effects. Shedding of these endothelial glycoproteins may contribute to dysregulated coagulation, as the presence of an A1 allele was associated with increased risk of disseminated intravascular coagulation in patients with sepsis. Finally, the importance of A antigen density was demonstrated in donated human lungs undergoing ex vivo perfusion with an erythrocyte-free buffer in the attempt to improve their transplantation candidacy. The authors observed that lungs from donors with blood type A (indicating A antigen glycosylation of pulmonary cells) were less likely to recover lung function with ex vivo perfusion than lungs from type O donors. These findings, derived from erythrocyte-free lungs, provide further evidence that the effects of blood type on inflammatory lung injury are mediated by parenchymal glycosylation of endothelial cells, rather than the glycosylation of erythrocytes or other blood cells.
Future directions
Reilly et al. (18) add key mechanistic insights into the pathobiology of ARDS, demonstrating the complex contributions of both genetics and glycobiology. Additionally, the association of blood type A (indicative of A antigen expression) and ARDS in patients with non-pulmonary sepsis, but not pulmonary sepsis, highlights the heterogeneity of ARDS and suggests that there are key mechanistic differences between systemic insults, often termed “indirect lung injury,” and pulmonary insults, termed “direct lung injury.” Prior work has demonstrated that patients with ARDS due to indirect lung injury have elevated markers of endothelial injury (angiopoietin-2, vWF, IL-6, and IL-8) whereas patients with ARDS due to direct lung injury have elevated markers of epithelial injury (surfactant protein D) (19). This study adds additional layers of complexity by illuminating the interactions between genetic and injury-specific sources of ARDS heterogeneity. Given these clear mechanistic differences, it is likely that subgroups of patients may require different treatment approaches (20, 21). For example, ARDS endotypes that incorporate the patient’s blood type may inform clinical trial enrollment with tailored therapeutics aimed at glycoproteins processing the ABO antigens. Additionally, although this study indicated that endothelial ABH glycosylation status mediates ARDS risk, the role of epithelial glycosylation has not yet been fully characterized. Epithelial cell glycosylation may be more important in direct lung injury, and the study of this process may require sampling airspace fluid directly (22). Future research is necessary to fully elucidate the complex interplay between genetic and injury-specific risk factors with the goal of identifying subgroups of patients that may benefit from mechanism-targeted therapies.
Acknowledgments
ANR was supported by NIH T32 HL007085. EPS was supported by NIH R01 GM125095, R01 HL149422, and R01 HL125371.
Version 1. 11/03/2020
In-Press Preview
Version 2. 01/04/2021
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(1):e144075. https://doi.org/10.1172/JCI144075.
Contributor Information
Alicia N. Rizzo, Email: Alicia.Rizzo@cuanschutz.edu.
Eric P. Schmidt, Email: eric.schmidt@ucdenver.edu.
References
- 1. Zhao J, et al. Relationship between the ABO blood group and the COVID-19 susceptibility [published online August 4, 2020]. Clin Infect Dis. https://doi: 10.1093/cid/ciaa1150.
- 2.Cheng Y, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293(12):1450–1451. doi: 10.1001/jama.293.12.1450-c. [DOI] [PubMed] [Google Scholar]
- 3.Stowell CP, Stowell SR. Biologic roles of the ABH and Lewis histo-blood group antigens. Part I: infection and immunity. Vox Sang. 2019;114(5):426–442. doi: 10.1111/vox.12787. [DOI] [PubMed] [Google Scholar]
- 4.Cserti CM, Dzik WH. The ABO blood group system and Plasmodium falciparum malaria. Blood. 2007;110(7):2250–2258. doi: 10.1182/blood-2007-03-077602. [DOI] [PubMed] [Google Scholar]
- 5.Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126(5):841–845. doi: 10.1016/j.cell.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 6. Stanley P, Cummings RD. Structures common to different glycans. In: Varki A, et al., eds. Essentials of Glycobiology. Cold Spring Harbor Laboratory Press; 2015:161–178. [PubMed] [Google Scholar]
- 7.Hosoi E. Biological and clinical aspects of ABO blood group system. J Med Invest. 2008;55(3–4):174–182. doi: 10.2152/jmi.55.174. [DOI] [PubMed] [Google Scholar]
- 8.Kelly RJ, Rouquier S, Giorgi D, Lennon GG, Lowe JB. Sequence and expression of a candidate for the human Secretor blood group α(1,2)fucosyltransferase gene (FUT2). Homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J Biol Chem. 1995;270(9):4640–4649. doi: 10.1074/jbc.270.9.4640. [DOI] [PubMed] [Google Scholar]
- 9.Stowell SR, Stowell CP. Biologic roles of the ABH and Lewis histo-blood group antigens part II: thrombosis, cardiovascular disease and metabolism. Vox Sang. 2019;114(6):535–552. doi: 10.1111/vox.12786. [DOI] [PubMed] [Google Scholar]
- 10.Vasan SK, et al. ABO blood group and risk of thromboembolic and arterial disease: a study of 1.5 million blood donors. Circulation. 2016;133(15):1449–1457. doi: 10.1161/CIRCULATIONAHA.115.017563. [DOI] [PubMed] [Google Scholar]
- 11.Zhong M, et al. ABO blood group as a model for platelet glycan modification in arterial thrombosis. Arterioscler Thromb Vasc Biol. 2015;35(7):1570–1578. doi: 10.1161/ATVBAHA.115.305337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morelli VM, et al. ABO blood group genotypes, plasma von Willebrand factor levels and loading of von Willebrand factor with A and B antigens. Thromb Haemost. 2007;97(4):534–541. doi: 10.1160/TH06-09-0549. [DOI] [PubMed] [Google Scholar]
- 13.Barbalic M, et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum Mol Genet. 2010;19(9):1863–1872. doi: 10.1093/hmg/ddq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paterson AD, et al. Genome-wide association identifies the ABO blood group as a major locus associated with serum levels of soluble E-selectin. Arterioscler Thromb Vasc Biol. 2009;29(11):1958–1967. doi: 10.1161/ATVBAHA.109.192971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reilly JP, et al. ABO blood type A is associated with increased risk of ARDS in whites following both major trauma and severe sepsis. Chest. 2014;145(4):753–761. doi: 10.1378/chest.13-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilly JP, et al. The ABO Histo-Blood Group and AKI in critically ill patients with trauma or sepsis. Clin J Am Soc Nephrol. 2015;10(11):1911–1920. doi: 10.2215/CJN.12201214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kangelaris KN, et al. The association between a Darc gene polymorphism and clinical outcomes in African American patients with acute lung injury. Chest. 2012;141(5):1160–1169. doi: 10.1378/chest.11-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reilly JP, et al. The ABO Histo-Blood Group, endothelial activation, acute respiratory distress syndrome risk in critical illness. J Clin Invest. 2021;131(1):e139700. doi: 10.1172/JCI139700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calfee CS, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147(6):1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthay MA, McAuley DF, Ware LB. Clinical trials in acute respiratory distress syndrome: challenges and opportunities. Lancet Respir Med. 2017;5(6):524–534. doi: 10.1016/S2213-2600(17)30188-1. [DOI] [PubMed] [Google Scholar]
- 21.Reilly JP, Calfee CS, Christie JD. Acute respiratory distress syndrome phenotypes. Semin Respir Crit Care Med. 2019;40(1):19–30. doi: 10.1055/s-0039-1684049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haeger SM, et al. Epithelial heparan sulfate contributes to alveolar barrier function and is shed during lung injury. Am J Respir Cell Mol Biol. 2018;59(3):363–374. doi: 10.1165/rcmb.2017-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]