Abstract
Diabetes mellitus (DM) is a risk factor for cancer. The role of DM-induced hyperglycemic (HG) stress in blood cancer is poorly understood. Epidemiologic studies show that individuals with DM are more likely to have a higher rate of mutations in genes found in pre-leukemic hematopoietic stem and progenitor cells (pre-LHSPCs) including TET2. TET2-mutant pre-LHSPCs require additional hits to evolve into full-blown leukemia and/or an aggressive myeloproliferative neoplasm (MPN). Intrinsic mutations have been shown to cooperate with Tet2 to promote leukemic transformation. However, the extrinsic factors are poorly understood. Using a mouse model carrying Tet2 haploinsufficiency to mimic the human pre-LHSPC condition and HG stress, in the form of an Ins2Akita/+ mutation, which induces hyperglycemia and type 1 DM, we show that the compound mutant mice developed a lethal form of MPN and/or acute myeloid leukemia (AML). RNA-Seq revealed that this was due in part to upregulation of proinflammatory pathways, thereby generating a feed-forward loop, including expression of the antiapoptotic, long noncoding RNA (lncRNA) Morrbid. Loss of Morrbid in the compound mutants rescued the lethality and mitigated MPN/AML. We describe a mouse model for age-dependent MPN/AML and suggest that hyperglycemia acts as an environmental driver for myeloid neoplasms, which could be prevented by reducing expression levels of the inflammation-related lncRNA Morrbid.
Keywords: Hematology, Inflammation
Keywords: Diabetes, Leukemias
Introduction
Cancer and diabetes are prevalent diseases that share multiple common risk factors such as metabolic dysfunction, aging, and chronic inflammation. It has been hypothesized that diabetes is a risk factor for some types of solid cancers. More important, and relevant to this study, are strong epidemiological findings showing a link between diabetes and leukemia. Studies have shown an increased risk of developing leukemia for patients with type 1 or type 2 diabetes (1, 2). Given the large number of individuals with diabetes worldwide, this disease has now become a tremendous public health concern. The unaddressed questions in the field have to do with whether hyperglycemia contributes to leukemia and/or myeloproliferative neoplasm (MPN) initiation and development and the what the possible mechanisms are by which this occurs. Although epidemiologic findings clearly suggest a correlation between diabetes mellitus (DM) and blood cancers, no animal models have been developed to interrogate their causative relationship, which will be critical to understanding the mechanisms of hyperglycemia-induced leukemogenesis and, consequently, for developing appropriate therapies.
In an effort to directly examine the correlation between DM and blood cancers, a recent meta-analysis using exome-sequencing on peripheral blood (PB) cells from individuals with DM and healthy individuals revealed that people at an older age or with DM have a higher rate of gene mutations in blood cancer genes compared with controls (3). The meta-study also revealed that genes encoding 2 epigenetic regulators, TET2 and DNMT3A, are among the top genes mutated in elderly and DM individuals, consistent with the association of TET2 mutations in age-related clonal hematopoiesis (ARCH) in healthy elderly individuals (3, 4). Not only are TET2 mutations prevalent in healthy elderly individuals manifesting ARCH, but TET2 is also one of the most highly mutated genes in MPNs, chronic myelomonocytic leukemia (CMML), myelodysplasia syndrome (MDS), secondary acute myeloid leukemia (AML), and de novo AML (3, 4).
Murine models for studying the biological function of Tet2 have been established. These mice show (a) increased hematopoietic stem cell (HSC) self-renewal, (b) myeloid lineage skewing, and (c) age-dependent leukocytosis but not AML development (5). This manifestation is mostly seen with close to full penetrance, when both alleles of Tet2 are deleted (Tet2–/–), but not when a single allele of Tet2 is deleted (Tet2+/–). In the majority of patients with AML, CMML, or MPN, with TET2 mutations, only 1 allele of TET2 is mutated. These findings suggest that secondary hits in Tet2+/– HSCs/progenitor cells (HSPCs) are necessary for the development of AML and/or full-blown MPN. To this end, little is known about the impact of extrinsic factors on the modulation of Tet2-deficient cells and on overall disease pathogenesis. We and others have recently shown that LPS or bacterial challenge of Tet2-deficient mice induces a strong inflammatory response (6–8). On the basis of these findings, we hypothesized that chronic inflammation due to HG stress (high glucose exposure) may potentiate and exacerbate the Tet2+/– pre-leukemic hematopoietic stem and progenitor cells (pre-LHSPCs) phenotype, resulting in the development of AML and/or severe MPN. Using a mouse model bearing haploinsufficiency of Tet2 (Tet2+/–) to mimic the human pre-LHSPC condition and hyperglycemic (HG) stress, in the form of an Ins2Akita/+ mutation, which induces hyperglycemia and type 1 DM (T1DM) in mice, we show that the compound mutant mice developed a lethal form of MPN/AML driven in part by upregulation of proinflammatory pathways, including expression of the antiapoptotic long noncoding RNA (lncRNA) Morrbid.
Results and Discussion
Ins2Akita/+-induced HG stress and Tet2 heterozygosity cooperate to drive aggressive MPN/AML in a mouse model.
Ins2Akita/+ mice carry a point mutation in the insulin-encoding gene insulin 2 (Ins2), which leads to insufficient insulin production and apoptosis in pancreatic cells. This strain is widely used as a genetic murine model for T1DM research and is pathologically comparable but more stable than the streptozotocin-induced (STZ-induced) chemical diabetes model (9). Ins2Akita/+ mice (both males and females) were bred with Tet2+/– (male and female) mice to obtain Ins2Akita/+ Tet2+/– compound mutant mice and controls (Figure 1A). Like the Ins2Akita/+ mutants, the compound mutant mice showed increased blood glucose levels and reduced body weight — 2 typical physiological characteristics of T1DM (Figure 1E). We found no hematological abnormalities in any of the experimental groups until 6 months of age, when mice from the Tet2+/– Ins2Akita/+ group started to succumb to the disease. All of the Tet2+/– Ins2Akita/+ mice died between 6 and 9 months of age, with a median survival of 221 days, whereas all mice in the other cohorts survived beyond 300 days (Figure 1B).
Figure 1. An age-dependent MPN/AML model driven by HG stress and preleukemic mutation.
(A) Schematic for the generation of compound mutant Tet2+/– Ins2Akita/+ mice along with WT, Tet2+/–, and Ins2Akita/+ controls. (B) Kaplan-Meier survival curve for the compound mutant Tet2+/– Ins2Akita/+ mice and controls. Only compound mutant Tet2+/– Ins2Akita/+ mice showed early mortality, between 6 and 9 months of age, with 100% penetration. The median survival for the compound mutant mice was approximately 7 months (221 days), whereas all control mice survived beyond 300 days, after which the experiment was discontinued. (C) At the end stage of life (6–9 months of age), all the compound mutant Tet2+/– Ins2Akita/+ mice displayed a visible myeloid sarcoma under the skin around the ocular area on 1 or both sides (red arrow), as well as pale bones (blue arrow) and splenomegaly. (D) Spleen weights at 6–9 months of age, when the compound mutant mice became moribund. (E) Mutant Ins2Akita/+ and Tet2+/– Ins2Akita/+ mice manifested an increase in blood glucose levels and a decrease in body weight compared with WT and Tet2+/– mice throughout all stages. The plots show data for the mice at 5 months of age. (F) Hematologic analysis of PB using the automatic blood cell counter Element HT5. NE, neutrophil. (G) Analysis of myeloid cell compartments by flow cytometry using staining with Ly6G and Mac1 antibodies. (H) Analysis of BM mononuclear cells by flow cytometry using antibodies that recognize cKit and Mac1. (I) Quantification of the frequency of HSPCs and mature cells in the BM. n = 4–10 mice. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, by 1-way ANOVA (D–I). Data in B were analyzed with GraphPad Prism 6, using the Kaplan-Meier survival package. Freq., frequency.
In addition to the typical features of myeloid neoplasia including splenomegaly and pale bones, we observed myeloid sarcoma–like aberrant tissue growth in all of the Tet2+/– Ins2Akita/+ moribund mice (Figure 1, C and D). Hematologic analysis of PB samples demonstrated that the moribund Tet2+/– Ins2Akita/+ mice had an emergency granulopoiesis or severe MPN-like phenotype, including an increase in the absolute counts and percentages of neutrophils associated with a significant reduction in RBCs (Figure 1F). Flow cytometric analysis of PB and bone marrow (BM) from these mice revealed a significant increase in the frequency of Mac-1+ mature myeloid cells and a profound reduction of CD19+ B cells, as well as a significant increase in the frequency of AML blasts, characterized by the expression of cKit+Mac-1+ cells and Ly6G+Mac1+ cells (Figure 1, G–I, and Supplemental Figure 1, A and B; supplemental material available online with this article; https://doi.org/10.1172/JCI140707DS1). Furthermore, in the BM, we found that the frequencies of lineage-negative, Sca-1+, and cKit+ (LSK) cells, HSCs (LSK CD48–CD150+), granulocyte-macrophage progenitors (GMPs) (LSK CD16+CD34+), as well as more mature myeloid cells (Mac-1+) were significantly increased in the compound mutants compared with frequencies in the other genotypes, whereas B cell development was repressed (Figure 1I and Supplemental Figure 1A). The cells in the myeloid sarcoma were dominated by Mac1+Ly6G+ (data not shown). Taken together, these results demonstrate that Ins2Akita/+-induced HG stress cooperated with Tet2 heterozygosity in mice to induce severe MPN/AML-like disease between 6 and 9 months of age.
The presence of a severe MPN/AML-like phenotype in compound mutant mice is largely driven by progressive inflammation and the inflammatory cytokine–induced lncRNA Morrbid.
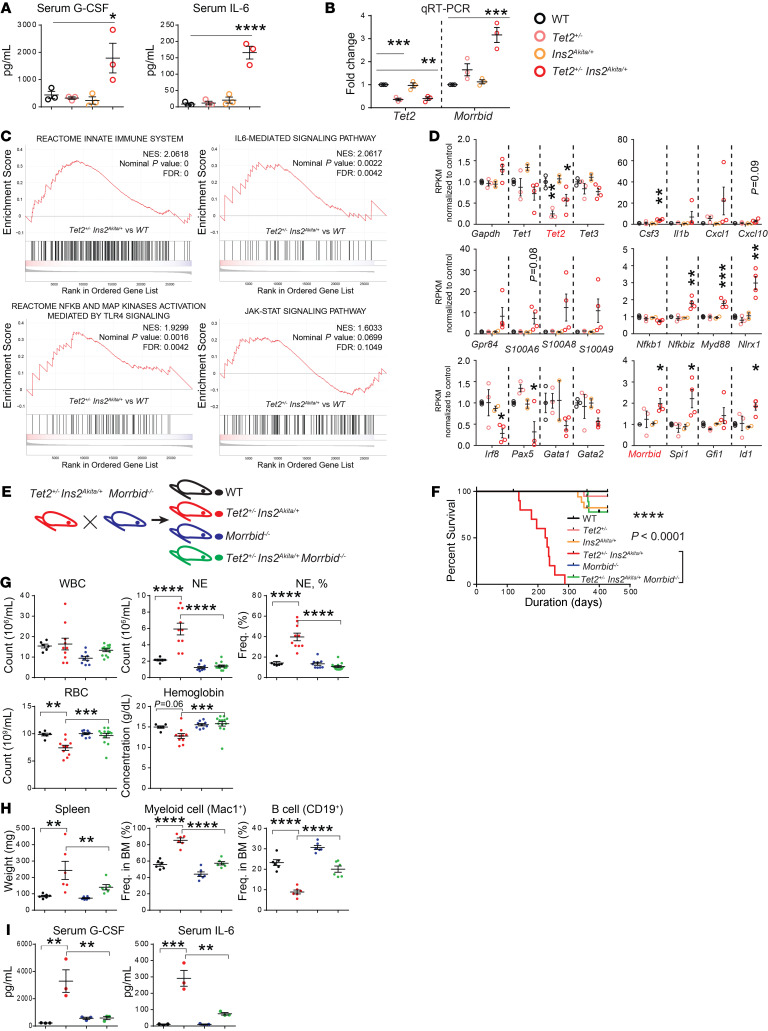
To characterize the pathology and potential driver or drivers of mortality in Tet2+/– Ins2Akita/+ compound mutant mice, we performed histological analysis of various tissues, serological analysis of cytokines, and RNA-Seq analysis of Lin– hematopoietic progenitors. Except for more blast-like cells in PB of the compound mutants, we found no obvious alterations in the histology of other tissues (Supplemental Figure 1D and data not shown). Serum cytokine analysis revealed a significant increase in the presence of proinflammatory cytokines such as IL-6 as well as in the levels of cytokines involved in driving emergency granulopoiesis such as granulocyte CSF (G-CSF) (Figure 2A). Furthermore, bioinformatics analysis of the RNA-Seq data revealed important new information with regard to global unbiased changes in gene expression, which were quite dramatic in the Lin– cells derived from Ins2Akita/+ Tet2+/– mice relative to the controls. Gene set enrichment analysis (GSEA) revealed that pathways involved in the innate immune reactome — IL-6–mediated signaling and TLR4-NF-κB signaling — were significantly altered in Ins2Akita/+ Tet2+/– mice compared with controls (P < 0.0025, Figure 2C). Such significant changes were not observed between Tet2+/– and WT mice or between Ins2Akita/+ and WT mice at a similar time point (data not shown). Although, not statistically significant, the JAK/STAT pathway also appeared to be upregulated in Ins2Akita/+ Tet2+/– mice relative to controls (P = 0.0699, Figure 2C).
Figure 2. The MPN/AML-like phenotype in compound mutants is largely driven by progressive inflammation and inflammatory cytokine–induced lncRNA Morrbid.
(A) Serological analysis of PB samples from various genotypes showed an increase in G-CSF and IL-6 in compound mutant mice. (B) qRT-PCR analysis was performed to detect the expression of Tet2 and Morrbid in lineage-negative cells from mice of various genotypes. (C) GSEA derived from RNA-Seq data set on lineage-negative BM cells from 4 groups of mice (6–8 month of age). Comparisons between Tet2+/– Ins2Akita/+ and WT revealed significant changes in the innate immune pathway, the IL-6–mediated signaling pathway, and the TLR4/NF-κB signaling pathway. In addition, the JAK/STAT pathway demonstrated a trend toward upregulation in Tet2+/– Ins2Akita/+ mice compared with WT. NES, normalized enrichment score. (D) Representative quantitative graphs of various innate immune response–related genes derived from the RNA-Seq data set, including the Bim-targeting lncRNA Morrbid (shown in the bottom right panel). (E) Schematic for the generation of triple-mutant Tet2+/– Ins2Akita/+ Morrbid–/– mice along with WT, Tet2+/– Ins2Akita/+, and Morrbid–/– controls. (F) Loss of Morrbid in compound mutants rescued the lethality associated with them compared with controls. A total of 9 mice were analyzed in the Tet2+/– Ins2Akita/+ Morrbid–/– cohort, and 10 mice in each of the other cohorts. (G) Comparison of hematological values between Tet2+/– Ins2Akita/+ Morrbid–/– and Tet2+/– Ins2Akita/+ mice. (H) Splenomegaly and an aberrant myeloid cell/B cell imbalance in compound heterozygous mutants was reversed in Tet2+/– Ins2Akita/+ Morrbid–/– mice. (I) Serum levels of G-CSF and IL-6. Mice in G–I were approximately 6–8 months old. n = 2–13 mice. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, by 1-way ANOVA (A, B, D, and G–I). Data in F were analyzed with GraphPad Prism 6, using the Kaplan-Meier survival package.
We also examined the expression of individual genes by plotting their RPKM values (reads per kilobase of transcript, per million mapped reads), normalized to the RPKM for WT controls. We confirmed that Tet2 expression was reduced to approximately 50% of WT levels in Tet2+/– and Ins2Akita/+ Tet2+/– mice, which was verified by quantitative reverse transcription PCR (qRT-PCR) analysis (Figure 2, B and D). Consistent with the overall evaluation by the GSEA shown in Figure 2C, a profound upregulation in the production of proinflammatory cytokines, including Csf3, Il1b, Cxcl1, and Cxcl10, and the components associated with the innate immune response, including the expression of ligands for the TLR S100A family of ligands, was detected in Ins2Akita/+ Tet2+/– mice relative to controls (Figure 2D). An increase in the expression of downstream innate immune signaling molecules from TLRs such as Nfkbiz and Myd88 in these mutants was also observed and verified by qRT-PCR (Figure 2D and Supplemental Figure 1E). Furthermore, consistent with the observed reduction in the frequency of CD19+ B cells in the BM, we found that expression of the B cell–specific transcription factor Pax5 (encoded by Pax5) was also significantly reduced in the compound mutants (Figure 2D). The increase in myeloid cells correlated with an increase in the myeloid-specific transcription factor PU.1 (encoded by Spi1). Importantly, the Gata-2 locus has recently been shown to be affected in Tet2–/– Flt3ITD mice, which develop AML associated with an increased presence of cKit+Mac-1+ blasts (10). We, too, observed a reduction in the expression of Gata-2 in Tet2+/– Ins2Akita/+ compound mutant mice (Figure 2D). Interestingly, RNA-Seq analysis revealed a significant upregulation in expression of the antiapoptotic lncRNA Morrbid in Tet2+/– Ins2Akita/+ compound mutant mice (Figure 2D), which we verified by qRT-PCR (Figure 2B). We recently demonstrated an increase in the expression of MORRBID in human AML cells, which is associated with poor patient survival (11).
Morrbid is specifically expressed in myeloid cells and uniquely represses expression of the proapoptotic gene Bim via a DNA loop in cis to regulate the lifespan of myeloid cells (12). Morrbid–/– mice manifest an inefficient production of innate immune cells (~50% of WT levels) but have normal activity and lifespan (12). Since Morrbid is specifically expressed in myeloid cells (i.e., neutrophils, monocytes, and eosinophils), but not in other cell types, under physiologic conditions, the uniqueness of its expression pattern and function offer a great advantage and opportunity to test whether it is required for the onset and progression of the aggressive MPN/AML-like phenotype in Tet2+/– Ins2Akita/+ compound mutant mice.
Given the presence of elevated IL-6 and increased Morrbid expression in Tet2+/– Ins2Akita/+ compound mutants, combined with the manifestation of a hyperinflammatory condition, we hypothesized that inflammation-induced Morrbid expression significantly contributes to mortality and manifestation of the severe MPN/AML phenotype driven via the cooperative interaction between Tet2+/– and Ins2Akita/+ in Tet2+/– Ins2Akita/+ mice. To test this, we generated Tet2+/– Ins2Akita/+ Morrbid–/– triple-mutant mice along with controls (Figure 2E). As expected, we observed that Morrbid loss completely reversed the early lethality in Tet2+/– Ins2Akita/+ mice (Figure 2F). Although no significant difference in the content of BM progenitors was observed between Tet2+/– Ins2Akita/+ and Tet2+/– Ins2Akita/+ Morrbid–/– mice at 1 time point, we observed a significant correction in splenomegaly and anemia as well as in the content of mature myeloid cells in the PB and BM of Tet2+/– Ins2Akita/+ Morrbid–/– mice relative Tet2+/– Ins2Akita/+ mice (6–8 months of age) (Figure 2, G and H). Furthermore, although slightly higher than the levels seen in WT mice, serum levels of G-CSF and IL-6 were dramatically decreased in Tet2+/– Ins2Akita/+ Morrbid–/– mice compared with Tet2+/– Ins2Akita/+ mice (6–8 months of age) (Figure 2I). Taken together, a “rescue experiment” via Morrbid loss strongly suggested that aberrant inflammation contributed to the early mortality and severe MPN/AML-like phenotype observed in the aged Tet2+/– Ins2Akita/+ mice.
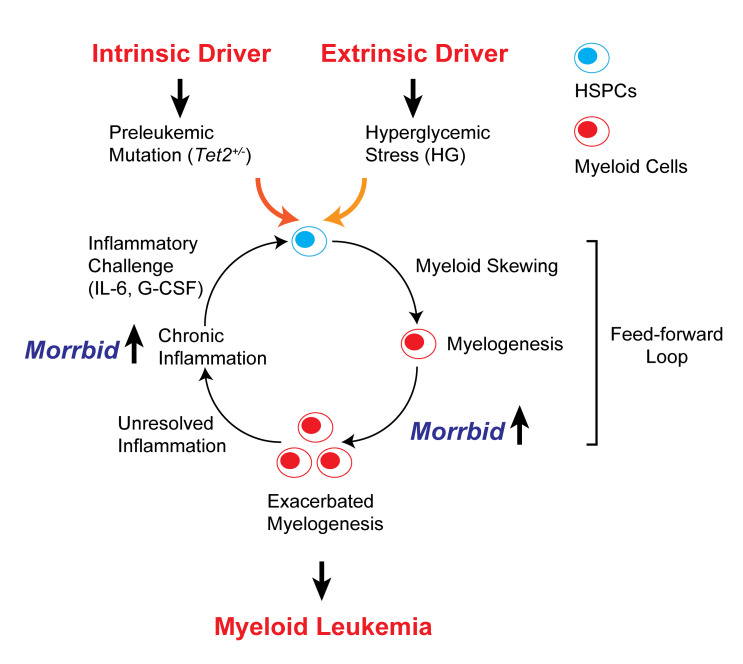
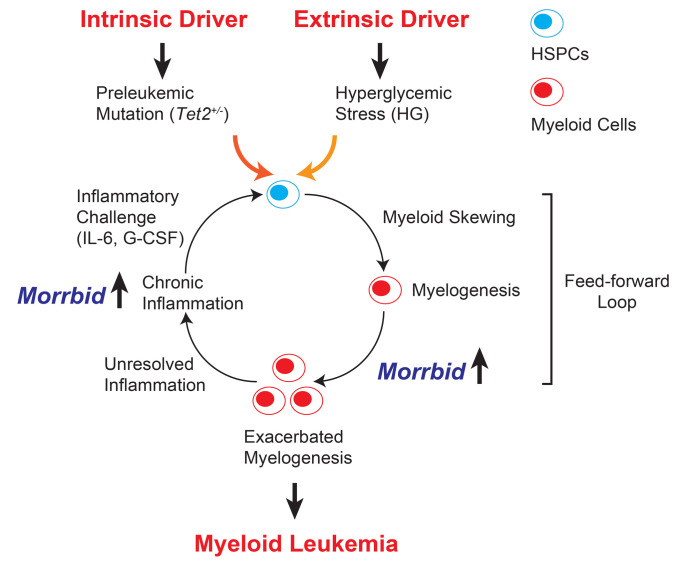
A model is depicted in Figure 3 to summarize our findings. We suggest that Tet2 mutation (an intrinsic factor) cooperates with HG stress (an extrinsic factor) to induce a higher grade of inflammation, perpetuating a feed-forward loop and resulting in leukemogenesis over time. The myeloid-specific survival regulator lncRNA Morrbid contributes to 2 critical steps induced by the cooperation of Tet2 mutation and hyperglycemia: (a) exacerbation of myelogenesis and (b) chronic inflammation. Loss of Morrbid effectively breaks the feed-forward loop. In line with this model, we have recently demonstrated that high expression levels of MORRBID are associated with TET2 mutations in human AML and are associated with a poor prognosis (11). It will be intriguing to determine whether such a correlation also exists in patients with AML who have diabetes. Additionally, although the present study suggests that inflammation plays an important role in the development of hyperglycemia-induced leukemia, it does not rule out the possibility that hyperglycemia can directly induce epigenetic changes and promote leukemogenesis, as suggested by a recent study demonstrating that hyperglycemia can alter the stability of the TET2 protein via the AMPK signaling pathway (13). Future studies are required to test whether hyperglycemia changes TET protein levels and/or activity in the hematopoietic system. Of note, although survival was greatly improved in Tet2+/– Ins2Akita/+ Morrbid–/– mice, 2 of the 9 mice that died had splenomegaly, indicating the development of leukemia in these 2 mice. In our recent study, a similar scenario was observed in a model of juvenile myelomonocytic leukemia (JMML) driven by Shp2E76K, suggesting that loss of Morrbid only mitigates or delays leukemia development rather than completely preventing it (14).
Figure 3. A “feed-forward loop” model for cooperation between Tet2+/– preleukemic mutation and HG stress.
Tet2 heterozygosity (Tet2+/–) defines a preleukemic condition in HSCs and intrinsically promotes HSC self-renewal and myeloid skewing under normal physiological conditions. Consequently, Tet2+/– mice develop a low-grade MPN. Extrinsic stress induced by HG exacerbates systematic inflammation and cooperates with Tet2 heterozygosity to perpetuate myeloid skewing/myelogenesis, chronic inflammation, and production of proinflammatory cytokines such as IL-6 and G-CSF. The myeloid-specific survival regulator lncRNA Morrbid mediates myelogenesis and chronic inflammation and also functions as a downstream effector of IL-6 and G-CSF. Consequently, loss of Morrbid mitigates the feed-forward loop observed in compound mutant mice.
In conclusion, the present study not only describes an important animal model in which to study the relationship between hematologic malignancies and diabetes, but also identifies a strong role for inflammation in driving early mortality in this model. Thus, we believe that Tet2+/– Ins2Akita/+ and Tet2+/– Ins2Akita/+ Morrbid–/– mice can serve as a tremendous resource for studying the relationship between diabetes, inflammation, and myeloid neoplasia.
Methods
Mice.
C57/B6 mice (CD45.2+) were procured from the Indiana University School of Medicine core facility and used as WT controls. Tet2+/– and Morrbid–/– mice have been previously described (5, 12), and Ins2Akita/+ mice were obtained from The Jackson Laboratory (catalog 003548). Flow cytometric analysis in mouse experiments was described in detail in our recent study (6). Measurement of blood glucose was performed at Indiana University Diabetes Research Center.
RNA-Seq and GSEA.
Total RNA from Lin– cells was purified using the RNeasy Mini Kit (catalog 74104, QIAGEN) and used for RNA-Seq analysis at the Indiana University Center for Medical Genomics. The sequencing data were deposited in the NCBI’s Gene Expression Omnibus (GEO) public database (GEO GSE158351). For GSEA, differential gene expression was computed using DESeq2. Pathway enrichment analysis was computed using the Msigdb v6 canonical pathway, with P < 0.005 (or any other cutoff) as the significant cutoff. The log fold change of the expression level of each gene was used for GSEA. For RPKM plotting, the individual gene’s RPKM for the study groups was normalized to that of the WT group.
Statistics.
All experimental procedures using mice were run in parallel with proper controls (sex- and age-matched littermate controls when possible) to detect experimental variabilities. Analysis of grouped data was not blinded, and no samples were excluded. Error bars indicate the mean ± SEM. With the exception of the GSEA, statistical analysis was performed using GraphPad Prism 6 (GraphPad Software), with ordinary 1-way ANOVA for multiple comparisons and Kaplan-Meier survival analysis for survival comparisons.
Study approval.
The animal experiments were approved by the IACUC of the Indiana University School of Medicine (protocol 11424), and the animals were maintained in the Laboratory Animal Resource Center at the Indiana University School of Medicine.
Author contributions
ZC, LH, and RK conceived and designed the experiments, analyzed data, and wrote the manuscript. ZC performed most of the experiments and acquired the data. XL, CZ, FF, and KN collected the RNA-Seq raw data and performed pathway analysis. SN, FA, BR, and AH assisted with certain experiments and acquired a portion of the data. JJK, AW, and JHM contributed reagents.
Supplementary Material
Acknowledgments
We thank our colleagues for their technical support, for critically reading our manuscript, and for their suggestions to improve it. This work was supported in part by grants from the NIH (R01-CA134777, R01-HL140961, R01-HL146137, and R01-CA173852, to RK) and by funding from the Riley Children’s Foundation (to RK). ZC is supported by funding from the NIH (T32HL007910). We would also like to thank Tracy Winkle for her administrative support.
Version 1. 10/22/2020
In-Press Preview
Version 2. 01/04/2021
Print issue publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(1):e140707.https://doi.org/10.1172/JCI140707.
Contributor Information
Zhigang Cai, Email: zcai@iupui.edu.
Xiaoyu Lu, Email: Lu14@iu.edu.
Chi Zhang, Email: czhang87@iu.edu.
Sai Nelanuthala, Email: snelanut@iu.edu.
Fabiola Aguilera, Email: faguiler@indiana.edu.
Abigail Hadley, Email: abihadle@iu.edu.
Baskar Ramdas, Email: ramdasb@iupui.edu.
Fang Fang, Email: ffang@indiana.edu.
Adam Williams, Email: adam.williams@jax.org.
Jorge Henao-Mejia, Email: jhena@mail.med.upenn.edu.
Reuben Kapur, Email: rkapur@iupui.edu.
References
- 1.Shu X, Ji J, Li X, Sundquist J, Sundquist K, Hemminki K. Cancer risk among patients hospitalized for Type 1 diabetes mellitus: a population-based cohort study in Sweden. Diabet Med. 2010;27(7):791–797. doi: 10.1111/j.1464-5491.2010.03011.x. [DOI] [PubMed] [Google Scholar]
- 2.Castillo JJ, Mull N, Reagan JL, Nemr S, Mitri J. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: a meta-analysis of observational studies. Blood. 2012;119(21):4845–4850. doi: 10.1182/blood-2011-06-362830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaiswal S, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genovese G, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood. 2011;118(17):4509–4518. doi: 10.1182/blood-2010-12-325241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Z, et al. Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal hematopoiesis. Cell Stem Cell. 2018;23(6):833–849.e5. doi: 10.1016/j.stem.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meisel M, et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature. 2018;557(7706):580–584. doi: 10.1038/s41586-018-0125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Q, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525(7569):389–393. doi: 10.1038/nature15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagareddy PR, et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013;17(5):695–708. doi: 10.1016/j.cmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shih AH, et al. Mutational cooperativity linked to combinatorial epigenetic gain of function in acute myeloid leukemia. Cancer Cell. 2015;27(4):502–515. doi: 10.1016/j.ccell.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai Z, et al. Targeting Bim via a lncRNA morrbid regulates the survival of preleukemic and leukemic cells. Cell Rep. 2020;31(12):107816. doi: 10.1016/j.celrep.2020.107816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotzin JJ, et al. The long non-coding RNA Morrbid regulates Bim and short-lived myeloid cell lifespan. Nature. 2016;537(7619):239–243. doi: 10.1038/nature19346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu D, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559(7715):637–641. doi: 10.1038/s41586-018-0350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai Z, Zhang C, Kotzin JJ, Williams A, Henao-Mejia J, Kapur R. Role of lncRNA Morrbid in PTPN11(Shp2)E76K-driven juvenile myelomonocytic leukemia. Blood Adv. 2020;4(14):3246–3251. doi: 10.1182/bloodadvances.2020002123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.