Abstract
Background
Plasmodium vivax has been recently discovered as a significant cause of malaria in Mauritania, although very rare elsewhere in West Africa. It has not been known if this is a recently introduced or locally remnant parasite population, nor whether the genetic structure reflects epidemic or endemic transmission.
Methodology/Principal findings
To investigate the P. vivax population genetic structure in Mauritania and compare with populations previously analysed elsewhere, multi-locus genotyping was undertaken on 100 clinical isolates, using a genome-wide panel of 38 single nucleotide polymorphisms (SNPs), plus seven SNPs in drug resistance genes. The Mauritanian P. vivax population is shown to be genetically diverse and divergent from populations elsewhere, indicated consistently by genetic distance matrix analysis, principal components analyses, and fixation indices. Only one isolate had a genotype clearly indicating recent importation, from a southeast Asian source. There was no linkage disequilibrium in the local parasite population, and only a small number of infections appeared to be closely genetically related, indicating that there is ongoing genetic recombination consistent with endemic transmission. The P. vivax diversity in a remote mining town was similar to that in the capital Nouakchott, with no indication of local substructure or of epidemic population structure. Drug resistance alleles were virtually absent in Mauritania, in contrast with P. vivax in other areas of the world.
Conclusions/Significance
The molecular epidemiology indicates that there is long-standing endemic transmission that will be very challenging to eliminate. The virtual absence of drug resistance alleles suggests that most infections have been untreated, and that this endemic infection has been more neglected in comparison to P. vivax elsewhere.
Author summary
Plasmodium vivax is a widespread cause of malaria in Mauritania, in contrast to its rarity elsewhere throughout West Africa. To investigate whether the parasite may be recently introduced or epidemic, multi-locus genotyping was performed on 100 Mauritanian P. vivax malaria cases. Analysis of a genome-wide panel of single nucleotide polymorphisms showed the P. vivax population to be genetically diverse and divergent from populations elsewhere, indicating that there has been long-standing endemic transmission. Almost all infections appear to be locally acquired, with the exception of one that was presumably imported with a genotype similar to infections seen in Southeast Asia. The Mauritanian P. vivax population shows no linkage disequilibrium, and very few infections have closely related genotypes, indicating ongoing recombination. The parasite showed no indication of local substructure or epidemic population structure. Drug resistance alleles were virtually absent, suggesting that most infections have been untreated historically. The molecular epidemiology indicates that there has been long-standing endemic transmission of this neglected parasite that requires special attention for control.
Introduction
On the edge of the Sahara in northwest Africa, Mauritania is endemic for malaria, with the majority of cases normally attributed to Plasmodium falciparum [1]. Analysis of parasite DNA from clinical samples indicates that most malaria in the Sahel zone in the south of the country is caused by P. falciparum [2], whereas P. vivax predominates in the central and northern regions of the country within the arid Saharan zone [2–4]. Routine laboratory-based diagnostic testing is conducted in health centres where facilities allow, using either slide microscopy or rapid diagnostic tests, although presumptive clinical diagnosis has also been commonly applied. Chloroquine was first-line treatment for uncomplicated clinical malaria in Mauritania until 2006, with sulphadoxine-pyrimethamine being only occasionally used, and since then artemisinin combination therapy with artesunate-amodiaquine has been officially first-line treatment for all uncomplicated malaria cases, while sulphadoxine-pyrimethamine is reserved for intermittent preventive treatment during pregnancy [3].
There is evidence suggesting that P. vivax in humans emerged in Africa as a spill-over zoonosis from a reservoir in great apes [5,6], but this parasite species is now rare in most of the continent apart from the Horn of East Africa [7,8] and some analyses suggest historical emergence from southeast Asia [9,10], so the origin and epidemiology in northwest Africa remains obscure. It is vital to know whether P. vivax has emerged in Mauritania in the recent past, as was assumed in the initial report of its presence in the country [11], or whether this is a long-established endemic parasite whose presence has only been recognised recently.
Genotypic characterization can provide important information on the origins of parasite populations and their relationships to those elsewhere, and can also illuminate local population structure and variation in transmission [12], as well as the prevalence and spread of drug resistance [13]. Here, we employed multi-locus single nucleotide polymorphism (SNP) analysis of a genome-wide set of loci [14] to analyse P. vivax population genetic structure in patient samples from three areas in Mauritania where this species was noted to be the most common cause of malaria [2], and also analysed candidate drug resistance markers in the same samples. In comparison with genotypic data on P. vivax populations in other countries, the P. vivax population in Mauritania is distinct, and almost all of the infections appeared to have been acquired within the country. The P. vivax population in Mauritania showed no evidence of genetic sub-structure, and is apparently highly recombining, consistent with long-established and ongoing endemicity. Controlling this infection requires characterization of the epidemiology in areas that have had minimal attention previously, and a commitment to radical cure will be important for regional elimination.
Methods
Ethics Statement
All samples were obtained with written informed consent from patients with malaria presenting for treatment, as well as guardians of any patients who were under 18 years of age. The study was approved by ethics committees of the Ministry of Health of Mauritania and the London School of Hygiene and Tropical Medicine (Ethics approval number 6043).
Plasmodium vivax clinical infection samples
P. vivax infections from patients presenting to local health centres and hospitals in different parts of Mauritania in 2012 and 2013 were previously identified by slide microscopy [15] and confirmed by species-specific PCR of genomic DNA from samples collected on filter paper [2]. All cases were local residents who did not report having travelled within the previous two weeks, and who were invited to provide finger-prick blood samples which were collected on filter paper and air-dried prior to storage with desiccant in sealed polythene bags. All samples were obtained with informed consent from patients, and guardians of patients who were under 18 years of age. After molecular confirmation of species, DNA from 100 isolates was analysed successfully by multi-locus SNP genotyping. Most of these (N = 91) were from the capital city Nouakchott, located on the Atlantic coast in the southern edge of the Sahara zone with low annual rainfall (100–200 mm), and dependent on a supply of piped water from the Senegal River basin in the south of the country. A smaller number of genotyped samples (N = 8) were from Zouérat, an iron ore mining town in the extremely arid northern Sahara zone of the country (receiving less than 50 mm annual rainfall), all cases being local residents as the mining operation is long-established and supports a developed urban area with a settled economy and extended families. A single genotyped sample was from N’beika, a small oasis town in the Sahara zone in the central part of the country (receiving 50–200 mm annual rainfall) (Fig 1). All of the P. vivax cases in this study were from individuals with Duffy positive genotypes as determined by PCR analysis previously [2], reflecting that this parasite species is mainly seen in areas of the country where most of the population are of the majority Maure ethnicity.
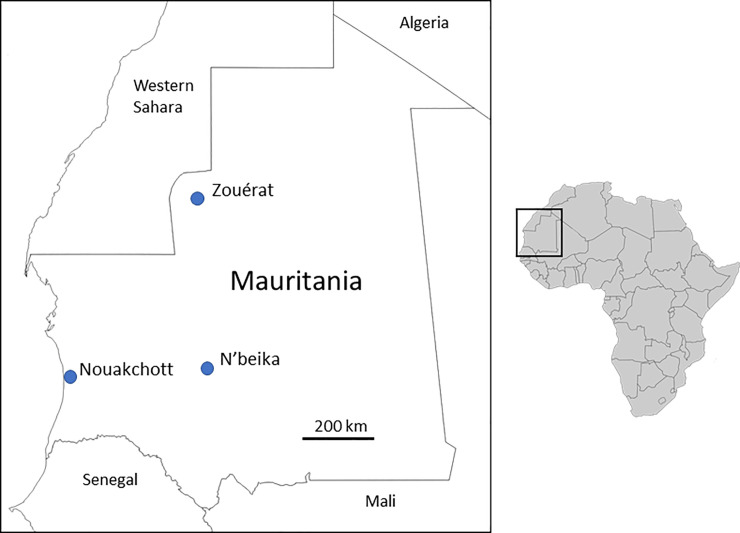
Fig 1. Map showing the location of the sampling sites of 100 P. vivax malaria cases in Mauritania analysed by multi-locus genotyping in this study.
Most cases analysed were from the capital city (Nouakchott, N = 91), while eight were from a northern mining town (Zouérat), and one was from a central oasis town (N’beika). The map of Africa to the right shows the location of Mauritania and surrounding countries in northwest Africa illustrated in the main map.
Multi-locus SNP genotyping
Genotyping was undertaken for a panel of 38 SNP loci from across the P. vivax genome (including markers on all of the 14 chromosomes), representing 90% of the SNPs on a previously described array of putatively neutral markers [14] for which amplicon-based sequencing assays were used in genotyping here (S1 Table). In addition, seven SNPs that have been associated with drug resistance elsewhere were genotyped, in the genes encoding dihydropteroate synthase (dhps, codon polymorphisms A553G and A383G), dihydrofolate reductase (dhfr, codon polymorphisms F57L/I, S58R, T61M and S117N/T), and the multidrug resistance 1 locus (mdr1, codon Y976F) [16]. Genotyping was performed using amplicon-based sequencing on the Illumina MiSeq platform at the Wellcome Sanger Institute, with reference alleles based on the PvP01 P. vivax reference genome sequence [17]. Individual infection samples were considered valid for analysis if clear genotyping scores could be obtained for more than half of the SNP loci, yielding a total of 100 infections for analysis (in most of these samples complete data were obtained for all SNP loci as noted in the Results).
To compare P. vivax from Mauritania with parasite populations in other countries, the SNP genotypes for these same loci were derived using VCFtools from previously published genome sequence data from P. vivax clinical isolates collected in Ethiopia (2013, N = 24), Thailand (2006–2013, N = 104), Papua Province in Indonesia (2011–2014, n = 111) [18–20], Mexico (2000–2007, n = 20) [21], and Colombia (2012–2013, n = 31) [21].
Population genetic and statistical analysis
The population genetic structure analyses focused on the 38 putatively neutral SNPs from throughout the P. vivax genome, while the seven drug resistance SNPs were analysed separately. If mixed alleles at a particular SNP were detected in an infection sample, the major one (with the highest read depth) was scored to contribute to the multi-locus genotype profile, and if this was not clear the SNP call was recorded as missing. A pairwise measure of genetic distance between infections (1-ps) considered the proportion of alleles shared (ps), and a Neighbor-Joining tree of the distance matrix was generated using the ape package in R and iTOL software [22,23]. Similarity of individual samples was also assessed using Principal Component Analysis (PCA) using the ClustVis software (htps://biit.cs.ut.ee/clustvis). The degree of divergence between Mauritania and other geographical populations was assessed using the fixation index (FST), employing the Scikitallel package (https://github.com/cggh/scikit-allel) to determine Hudson’s FST estimator, and using an in-house R script to calculate Weir and Cockerham’s FST as these different estimators focus on slightly different components of the allele frequency spectra [24]. Within-population SNP diversity was assessed using the virtual heterozygosity index (He), the mean of the pairwise differences at each marker between isolates within a given population. A within-population multi-locus measure of statistical linkage disequilibrium (LD) among the SNP loci was provided by the Index of Association (ISA), calculated for samples with complete multi-locus genotype data using LIAN version 3.7 software [25]. This measure of LD was assessed by analysing all samples that included mixed infections, as well as only in samples that had single genotype infections, and with unique multi-locus genotypes. The single genotype infections were defined as those that yielded a Complexity of Infection of 1 using the COIL algorithm [26]. R software was used for statistical tests, including Pearson’s Chi-squared test with Yates’ continuity correction for analysis of categorical variables.
Results
High-quality multi-locus SNP genotyping of Mauritanian P. vivax samples
Of the 100 Mauritanian P. vivax infection samples that had sufficient DNA for genotyping in this study, 84 yielded complete SNP data for all loci (38 loci genome-wide plus seven SNPs in drug resistance genes), and only six samples had >30% SNPs drop out. Twelve of the 100 infection samples contained multiple genotypes at any of the SNP loci, hence the haploid multi-locus genotype could be resolved unequivocally for 88 infections, of which 80 had complete data for all SNPs (all multi-locus genotypes for all individual samples are shown in S1 Datasheet).
Population genetic diversity of P. vivax in Mauritania and divergence from other populations
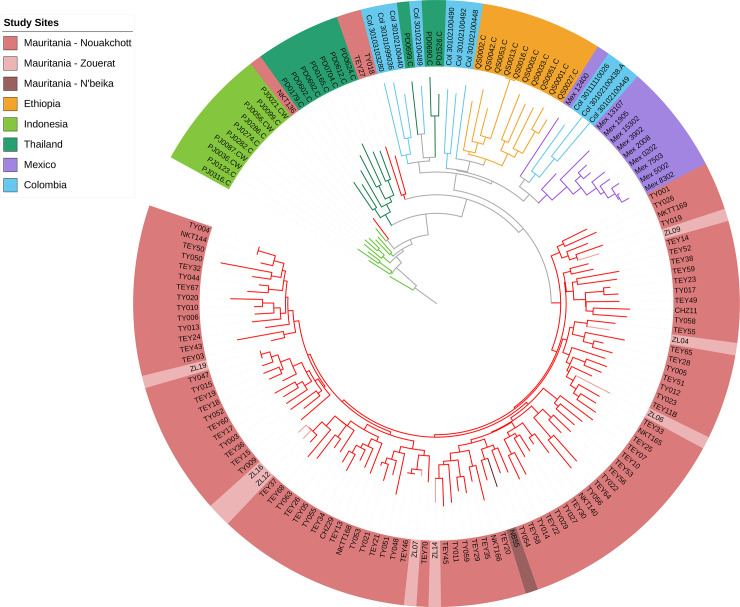
Based on the array of 38 putatively neutral SNP markers from throughout the genome, there was a high level of diversity in Mauritania, assessed by the virtual heterozygosity index of pairwise differences among all infections averaged across all SNPs (He = 0.31). Analysis of the pairwise genetic differences revealed that almost all infections were different from each other. The samples from Zouérat and N’beika were distributed in the same overall range of diversity amongst those from Nouakchott, indicating no genetically separated parasite subpopulations within the country. With exception of three infections from Nouakchott, the Mauritanian samples formed a distinct geographical group, with slightly closer relatedness to previous samples from Ethiopia as well as Central and South America than to most samples from Southeast Asia (Fig 2 and S1 Fig). Of the three outlier infections, two were only slightly outside the grouping of most Mauritanian samples, but one (isolate NKT136 from Nouakchott) was closely related to parasites previously sampled from Southeast Asia.
Fig 2. Neighbor-Joining tree illustrating the genetic relatedness among P. vivax infections in Mauritania in comparison with previous samples from other countries, based on multi-locus genotypes based on a distance matrix using the genome-wide panel of 38 SNPs genotyped in this study.
Data are presented on all samples from Mauritania (n = 100) and a random selection of 10 samples from each of Ethiopia, Thailand, Indonesia, Mexico and Colombia. For visual clarity, a rooted tree is shown using an arbitrarily selected isolate from Indonesia (PJ0316-C) as the ‘root’ (note that this is not a phylogenetic tree but a genetic distance dendrogram of a recombining species, and an unrooted tree has similar topology shown in S1 Fig). Within Mauritania, isolates from the mining town Zouérat and the oasis town N’beika were not genetically separated from samples from the capital city Nouakchott. Mauritanian isolates are diverse but comprise a population distinct from those in the other countries, except for two isolates that are similarly divergent as those from Ethiopia and one isolate (NKT136), showing closer genetic relatedness to the Asian parasites.
Among all of the Mauritanian samples, only three pairs of infections had identical multi-locus genotypes (Fig 2). These were not epidemiologically linked, NKT144 and TY004 having been sampled 123 days apart in Nouakchott, TEY46 and TY048 sampled 312 days apart in Nouakchott, and TY047 and ZL019 respectively sampled 210 days apart in Nouakchott and Zouérat. There was no significant multi-locus linkage disequilibrium in the total population sample from Mauritania (IAS = 0.002, P > 0.05), or separately within Nouakchott (IAS = 0.002, P > 0.05) or Zouérat (IAS = -0.012, P > 0.05). Multi-locus LD remained low in Mauritania when analysis was restricted to single genotype infections (IAS = 0.0004, P > 0.05) and infections with unique multi-locus genotypes (IAS = 0.002, P > 0.05). This is consistent with established endemicity and ongoing genetic recombination in the local P. vivax population, without any evidence of clonal or epidemic population structure which is seen elsewhere when local transmission is unstable (S2 Table).
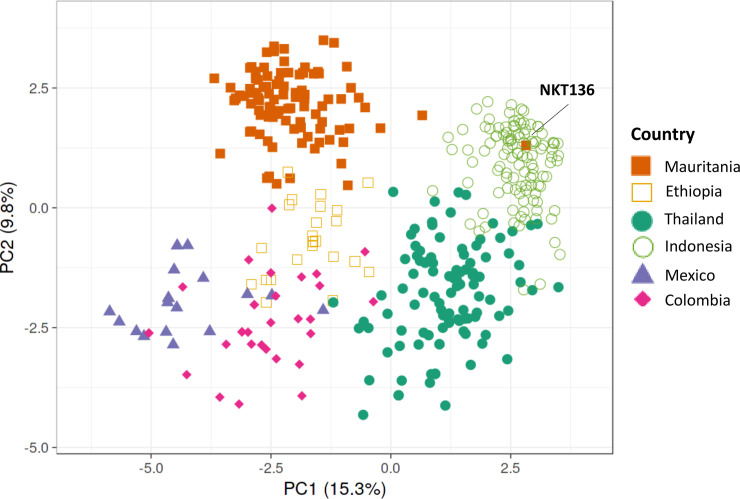
Principal Component Analysis confirmed that almost all of the Mauritanian P. vivax infection samples clustered separately from those previously sampled in other countries, although they were more closely related to those from Ethiopia than from other continents (Fig 3). Consistent with the Neighbor-Joining analysis, it showed that one infection sample (NKT136 from Nouakchott) was genetically similar to parasites from Asia. Further Principal Component Analyses comparing Mauritania with each of the other countries separately, confirmed that all P. vivax samples from Mauritania clustered separately from all others (except isolate NKT136 which consistently clustered with Asian P. vivax samples) (S2 Fig).
Fig 3. Principal Component Analysis (PCA) plot illustrating genetic relatedness among P. vivax infections in Mauritania compared with previous data from other endemic countries [18–20].
The first two principal components are shown, illustrating that the parasite population in Mauritania is distinct from the Southeast Asian and Central and South American populations, and has only a minor degree of overlap with samples from Ethiopia. In agreement with Neighbour-Joining analysis of genetic distance (shown in Fig 2), the single isolate NKT136 from Nouakchott clustered with parasites from Southeast Asia. Further Principal Component Analysis of the Mauritanian data with each of the other populations separately confirm the population distinctness (with the exception of isolate NKT136), and also show that isolates from different sampled areas of Mauritania are not genetically separated (S2 Fig).
The genetic differentiation between the Mauritanian P. vivax population and those from other countries was also estimated using the inter-population fixation index FST based on allele frequencies, revealing a high level of divergence from Ethiopia (FST = 0.22 using Hudson’s estimator) and even higher divergence from populations previously sampled in other continents (S3 Table, also shows FST values using Weir and Cockerham’s estimator with slightly lower values but the same trend).
Extremely low frequency of drug resistance alleles in P. vivax in Mauritania
The proportion of infections containing drug resistance-associated SNP alleles was investigated, and shown to be exceptionally low compared with data from other geographical populations of P. vivax (Table 1). Notably, there were no antifolate resistance-associated alleles at codons 57, 58 and 61 of the dihydrofolate reductase (dhfr) gene in Mauritania (Table 1). Alleles at codon 58 were common in most other P. vivax populations including Ethiopia (P < 1 x 10−15 for each comparison with Mauritania), and at codons 57 and 61 were very common in the Asian populations (P < 1 x 10−15 for each comparison with Mauritania). One infection in Mauritania had a resistance-associated allele at codon 117 of dhfr (S117N), but this was infection sample NKT136 that was shown by the multi-locus analysis of neutral SNPs to be genetically similar to Asian parasites, so the occurrence of this allele is likely to reflect an exceptional case of imported malaria (individuals who had travelled within the two weeks prior to diagnosis were not included in the study but earlier travel was not recorded).
Table 1. Low frequency of SNP variants in P. vivax drug resistance genes in Mauritania compared with other endemic populations.
| Percentage of isolates containing resistance-associated variants (with numbers in brackets) in: | |||||||
|---|---|---|---|---|---|---|---|
| Gene | Codon Allele | Mauritania | Ethiopia | Thailand | Indonesia | Mexico | Colombia |
| dhfr | F57L/I | 0 (0/89) | 0 (0/24) | 91 (88/97) | 82 (77/94) | 0 (0/20) | 0 (0/31) |
| S58R | 0 (0/87) | 94 (17/18) | 100 (104/104) | 99 (104/105) | 0 (0/20) | 97 (30/31) | |
| T61M | 0 (0/90) | 0 (0/24) | 91 (90/99) | 82 (77/94) | 0 (0/20) | 0 (0/31) | |
| S117N/T | 1 (1/98) | 100 (24/24) | 100 (102/102) | 99 (93/94) | 0 (0/20) | 97 (30/31) | |
| dhps | A553G | 0 (0/99) | 0 (0/24) | 98 (98/100) | 16 (16/97) | 100 (20/20) | 100 (31/31) |
| A383G | 2 (2/100) | 17 (4/24) | 100 (104/104) | 97 (106/109) | 0 (0/20) | 84 (26/31) | |
| mdr1 | Y976F | 14 (14/100) | 32 (6/19) | 13 (14/104) | 100 (111/111) | 0 (0/19) | 3 (1/29) |
At the dihydropteroate synthase (dhps) gene, there were no resistance-associated alleles in Mauritania at codon 553 (Table 1), and a resistance-associated allele at codon 383 was only detected in two infections, one of which was NKT136 that has a genotypic profile of Asian P. vivax as noted above. The virtual absence of resistance-associated dhps alleles in Mauritania contrasts with other populations, including Ethiopia where such alleles are of moderately low frequency (P = 0.021), and populations in other continents where they are very common (P < 1 x 10−5 for each comparison, except for codon 383 in Mexico) (Table 1). In the multidrug resistance gene (mdr1), the Y976F variant which has been loosely associated with chloroquine resistance elsewhere [27] was detected in 14% of infections in Mauritania (Table 1).
Discussion
Although P. vivax has only been recognised as a significant cause of malaria in Mauritania recently, this study indicates that the parasite has been long-established in northwest Africa, and that it is a genetically distinctive sub-population compared with parasites from other areas of the world. A genome-wide panel of 38 SNP loci revealed a high level of P. vivax genetic diversity in Mauritania, and no evidence of linkage disequilibrium or population genetic sub-structure within the country. The P. vivax population in Mauritania is clearly genetically divergent from other geographical populations of the parasite, including Ethiopia which is the source of most samples from the African continent that have been analysed by sequencing [19,28,29]. A recent survey of 14 microsatellite loci has also indicated the distinctiveness of P. vivax samples from Mauritania compared to most other sampled populations in Africa, Asia or the Americas [10], further supporting the evidence for an established focus of endemicity.
It is notable that there is no evidence for a clonal or epidemic population genetic structure of P. vivax in Mauritania, whereas such structure has been seen in populations close to elimination, such as described in Malaysia [30] and parts of Central and South America [21]. The areas where P. vivax infections were sampled in Mauritania experience extremely low annual rainfall, within only a few months of the year at most and sometimes none, and this would clearly limit opportunities for transmission by mosquitoes. The other endemic species P. falciparum has been shown to occasionally have an epidemic population structure at a minority of sites sampled further south in the country where rainfall is not quite so low [31]. As P. vivax showed no evidence of a clonal or epidemic structure in extremely dry areas studied here, it is likely that many infections persist for multiple years due to latency of liver-stage parasites, occasional relapses allowing ongoing transmission to occur when conditions allow. For future control and to aim towards eventual elimination, radical cure of P. vivax infections using either primaquine or tafenoquine will be needed, which would require screening for glucose 6-phosphate dehydrogenase deficiency of patients to reduce the risk of haemolytic anaemia as a side effect [32].
This northwest African P. vivax population has probably been present since pre-history, and may have previously been part of a wider geographical population. To the north, P. vivax was eliminated from Morocco in 2010, and used to be present in neighbouring Algeria where it is now almost eliminated [1,33]. This may even be part of the same parasite population that was endemic in Europe until malaria was eliminated in the mid-twentieth century. Sequencing of archival P. vivax from Europe could be performed, as illustrated by analysis of a single specimen so far [34], and once there are data from a range of other samples their relatedness to sequences from parasites in Mauritania should be determined. It is an ongoing priority to increase the amount of P. vivax whole-genome sequence data from infections in Mauritania and other countries where the epidemiology and population history is unclear [35].
To the east and south of Mauritania, a small number of P. vivax infections have been documented in Mali and Senegal respectively [36–38], and it is now considered that this species may occur at low densities more widely in Africa than previously assumed [39,40]. It will be important to genotype P. vivax from other African countries to ascertain if there are any other endemic populations, and any connectivity among them, or if infections elsewhere reflect sporadic introductions. The substantial genetic divergence we show between P. vivax in Mauritania and Ethiopia indicates that there is little gene flow between these two main endemic foci in Africa, consistent with the parasite having only a very sparse and discontinuous endemic distribution elsewhere on the continent.
Analysis of dhfr and dhps genotypes revealed a virtual absence of antifolate resistance-associated variants in P. vivax in Mauritania. Consistent with this, drug resistance genotyping of P. vivax samples previously taken from Nouakchott in 2007–9 and 2013–16 also showed no resistance-associated alleles in dhps [41,42]. However, the same studies reported between 10 and 20% of infections to have double mutant alleles of dhfr (combinations of variants at codons 58, 61 and 117) [41,42], in contrast to results here that showed an absence of resistance-associated alleles at these codons (except for a single variant at codon 117 in infection sample NKT136 that had an overall genotype similar to Asian parasites). Antimalarial antifolate use in Mauritania is now limited to intermittent preventative treatment of pregnant women with sulphadoxine-pyrimethamine [41], although antifolates were previously used for therapy along with chloroquine. Comparing this and previous studies, the frequency of the mdr1 976F allele in Mauritania varied from 28% in 2007–9 to 14% in 2012–13, and 4% in 2013–16 [41,42], which may reflect a decline due to fitness cost after chloroquine use officially stopped following introduction of Artemisinin Combination Therapy in 2006, although the allele is not a marker of chloroquine resistance and is at most a minor modulator [16]. Clinical evidence indicates that most local P. vivax infections are sensitive to chloroquine treatment [43].
In line with political and public health aspirations, Mauritania is aiming to eliminate malaria by 2030, but for this to be attainable a much deeper understanding of P. vivax in the region will be essential, as this species is particularly difficult to eliminate [1,44,45]. The distinct genetic profile of Mauritanian P. vivax compared with parasites elsewhere has important implications. It is highly likely that this population has locally adapted, to maintain parasites in the extreme conditions of the Sahara where opportunities for mosquito vector transmission must be exceedingly rare. Further epidemiological monitoring, clinical studies and genomic analyses of parasites from this unusual population will be necessary to understand this highly important parasite species that has been so long overlooked in Africa.
Supporting information
Note that the topology is similar to the rooted tree in Fig 2, and a single isolate from Mauritania (NKT136) has a genotype similar to Southeast Asian parasites. Note that this is not a phylogenetic tree but a genetic distance dendrogram of a recombining species.
(TIFF)
PCA of P. vivax genotypes (38-SNP array) of individual clinical isolates from different pairs of sites, comparing data from Mauritania in this study with data previously published from elsewhere: (a) Within Mauritania (Zouerat, N’beika and Nouakchott), (b) Mauritania and Ethiopia, (c) Mauritania and Thailand, (d) Mauritania and Indonesia, e) Mauritania and Mexico, f) Mauritania and Colombia. This shows no genetic separation of parasites from different sites within Mauritania, but separation of the Mauritanian population from parasites in each of the other countries (previous data from Ethiopia, Thailand, Indonesia, Mexico and Colombia as cited in the Methods and Results). The outlier isolate NKT136 which shows genetic similarity to those from Southeast Asia is circled.
(TIFF)
(DOCX)
(DOCX)
(DOCX)
(XLS)
Acknowledgments
We are grateful to all patients, and staff of the health facilities for willing participation and support throughout the surveys. We acknowledge the support and encouragement of the Director and other colleagues at the Institut National de Recherche en Santé Publique in Mauritania, and we also thank colleagues at the London School of Hygiene and Tropical Medicine and the Wellcome Sanger Institute for support. We also thank Hidayat Trimarsanto for assistance in compiling published genomic datasets for comparisons.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This study was primarily funded by the UK Medical Research Council (MRC) Project Grant G1100123 to DJC and HB. Parasite genotyping was enabled by the SPOT-Malaria project through the MalariaGEN consortium led by DPK with funding from the Wellcome Trust. RNP is supported by the Wellcome Trust, SA is supported by the Bill and Melinda Gates Foundation, and a Georgina Sweet Award for Women in Quantitative Biomedical Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO. World Malaria Report 2019. World Health Organization; Geneva: 2019. https://www.who.int/publications/i/item/9789241565721 [Google Scholar]
- 2.Ba H, Duffy CW, Ahouidi AD, Deh YB, Diallo MY, Tandia A, et al. Widespread distribution of Plasmodium vivax malaria in Mauritania on the interface of the Maghreb and West Africa. Malar J. 2016;15:80 10.1186/s12936-016-1118-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lekweiry KM, Salem MS, Basco LK, Briolant S, Hafid J, Boukhary AO. Malaria in Mauritania: retrospective and prospective overview. Malar J. 2015;14:100 10.1186/s12936-015-0607-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deida J, Tahar R, Khalef YO, Lekweiry KM, Hmeyade A, Khairy MLO, et al. Oasis Malaria, Northern Mauritania. Emerg Infect Dis. 2019;25:273–80. Epub 2019/01/23. 10.3201/eid2502.180732 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W, Li Y, Shaw KS, Learn GH, Plenderleith LJ, Malenke JA, et al. African origin of the malaria parasite Plasmodium vivax. Nat Commun. 2014;5:3346 Epub 2014/02/22. 10.1038/ncomms4346 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loy DE, Plenderleith LJ, Sundararaman SA, Liu W, Gruszczyk J, Chen YJ, et al. Evolutionary history of human Plasmodium vivax revealed by genome-wide analyses of related ape parasites. Proc Natl Acad Sci U S A. 2018;115:E8450–E9. Epub 2018/08/22. 10.1073/pnas.1810053115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battle KE, Lucas TCD, Nguyen M, Howes RE, Nandi AK, Twohig KA, et al. Mapping the global endemicity and clinical burden of Plasmodium vivax, 2000–17: a spatial and temporal modelling study. Lancet. 2019;394:332–43. Epub 2019/06/24. 10.1016/S0140-6736(19)31096-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twohig KA, Pfeffer DA, Baird JK, Price RN, Zimmerman PA, Hay SI, et al. Growing evidence of Plasmodium vivax across malaria-endemic Africa. PLoS Negl Trop Dis. 2019;13:e0007140 Epub 2019/02/01. 10.1371/journal.pntd.0007140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlton JM, Das A, Escalante AA. Genomics, population genetics and evolutionary history of Plasmodium vivax. Adv Parasitol. 2013;81:203–22. Epub 2013/02/07. 10.1016/B978-0-12-407826-0.00005-9 . [DOI] [PubMed] [Google Scholar]
- 10.Rougeron V, Elguero E, Arnathau C, Acuna Hidalgo B, Durand P, Houze S, et al. Human Plasmodium vivax diversity, population structure and evolutionary origin. PLoS Negl Trop Dis. 2020;14:e0008072 Epub 2020/03/10. 10.1371/journal.pntd.0008072 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes H, Morillas-Marquez F, Valero A. Malaria in Mauritania: the first cases of malaria endemic to Nouakchott. Trop Med Int Health. 2003;8:297–300. 10.1046/j.1365-3156.2003.01029.x . [DOI] [PubMed] [Google Scholar]
- 12.Auburn S, Barry AE. Dissecting malaria biology and epidemiology using population genetics and genomics. Int J Parasitol. 2017;47:77–85. 10.1016/j.ijpara.2016.08.006 . [DOI] [PubMed] [Google Scholar]
- 13.Dalmat R, Naughton B, Kwan-Gett TS, Slyker J, Stuckey EM. Use cases for genetic epidemiology in malaria elimination. Malar J. 2019;18:163 10.1186/s12936-019-2784-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baniecki ML, Faust AL, Schaffner SF, Park DJ, Galinsky K, Daniels RF, et al. Development of a single nucleotide polymorphism barcode to genotype Plasmodium vivax infections. PLoS Negl Trop Dis. 2015;9:e0003539 Epub 2015/03/18. 10.1371/journal.pntd.0003539 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ba H, Ahouidi AD, Duffy CW, Deh YB, Diedhiou C, Tandia A, et al. Evaluation of malaria rapid diagnostic test Optimal-IT(R) pLDH along the Plasmodium falciparum distribution limit in Mauritania [in French]. Bull Soc Pathol Exot. 2017;110:31–7. Epub 2016/12/31. 10.1007/s13149-017-0541-y . [DOI] [PubMed] [Google Scholar]
- 16.Price RN, Auburn S, Marfurt J, Cheng Q. Phenotypic and genotypic characterisation of drug-resistant Plasmodium vivax. Trends Parasitol. 2012;28:522–9. 10.1016/j.pt.2012.08.005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auburn S, Bohme U, Steinbiss S, Trimarsanto H, Hostetler J, Sanders M, et al. A new Plasmodium vivax reference sequence with improved assembly of the subtelomeres reveals an abundance of pir genes. Wellcome Open Res. 2016;1:4 10.12688/wellcomeopenres.9876.1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson RD, Amato R, Auburn S, Miotto O, Almagro-Garcia J, Amaratunga C, et al. Genomic analysis of local variation and recent evolution in Plasmodium vivax. Nat Genet. 2016;48:959–64. 10.1038/ng.3599 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auburn S, Getachew S, Pearson RD, Amato R, Miotto O, Trimarsanto H, et al. Genomic analysis of Plasmodium vivax in southern Ethiopia reveals selective pressures in multiple parasite mechanisms. J Infect Dis. 2019;220:1738–49. 10.1093/infdis/jiz016 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auburn S, Serre D, Pearson R, Amato R, Sriprawat K, To S, et al. Genomic analysis reveals a common breakpoint in amplifications of the Plasmodium vivax multidrug resistance 1 locus in Thailand. J Infect Dis. 2016;214:1235–42. 10.1093/infdis/jiw323 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hupalo DN, Luo Z, Melnikov A, Sutton PL, Rogov P, Escalante A, et al. Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat Genet. 2016;48:953–8. Epub 2016/06/28. 10.1038/ng.3588 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paradis E, Claude J, Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics. 2004;20:289–90. 10.1093/bioinformatics/btg412 . [DOI] [PubMed] [Google Scholar]
- 23.Letunic I, Bork P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–5. 10.1093/nar/gkw290 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhatia G, Patterson N, Sankararaman S, Price AL. Estimating and interpreting FST: the impact of rare variants. Genome Res. 2013;23:1514–21. 10.1101/gr.154831.113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haubold B, Hudson RR. LIAN 3.0: detecting linkage disequilibrium in multilocus data. Linkage Analysis. Bioinformatics. 2000;16:847–8. 10.1093/bioinformatics/16.9.847 . [DOI] [PubMed] [Google Scholar]
- 26.Galinsky K, Valim C, Salmier A, de Thoisy B, Musset L, Legrand E, et al. COIL: a methodology for evaluating malarial complexity of infection using likelihood from single nucleotide polymorphism data. Malar J. 2015;14:4 10.1186/1475-2875-14-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suwanarusk R, Russell B, Chavchich M, Chalfein F, Kenangalem E, Kosaisavee V, et al. Chloroquine resistant Plasmodium vivax: in vitro characterisation and association with molecular polymorphisms. PLoS One. 2007;2:e1089 Epub 2007/11/01. 10.1371/journal.pone.0001089 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford A, Kepple D, Abagero BR, Connors J, Pearson R, Auburn S, et al. Whole genome sequencing of Plasmodium vivax isolates reveals frequent sequence and structural polymorphisms in erythrocyte binding genes. PLoS Negl Trop Dis. 2020;14:e0008234 Epub 2020/10/13. 10.1371/journal.pntd.0008234 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor JE, Pacheco MA, Bacon DJ, Beg MA, Machado RL, Fairhurst RM, et al. The evolutionary history of Plasmodium vivax as inferred from mitochondrial genomes: parasite genetic diversity in the Americas. Mol Biol Evol. 2013;30:2050–64. Epub 2013/06/05. 10.1093/molbev/mst104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auburn S, Benavente ED, Miotto O, Pearson RD, Amato R, Grigg MJ, et al. Genomic analysis of a pre-elimination Malaysian Plasmodium vivax population reveals selective pressures and changing transmission dynamics. Nat Commun. 2018;9:2585 10.1038/s41467-018-04965-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy CW, Ba H, Assefa S, Ahouidi AD, Deh YB, Tandia A, et al. Population genetic structure and adaptation of malaria parasites on the edge of endemic distribution. Mol Ecol. 2017;26:2880–94. 10.1111/mec.14066 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Djigo OKM, Bollahi MA, Hasni Ebou M, Ould Ahmedou Salem MS, Tahar R, Bogreau H, et al. Assessment of glucose-6-phosphate dehydrogenase activity using CareStart G6PD rapid diagnostic test and associated genetic variants in Plasmodium vivax malaria endemic setting in Mauritania. PLoS One. 2019;14:e0220977 Epub 2019/09/17. 10.1371/journal.pone.0220977 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammadi D, Boubidi SC, Chaib SE, Saber A, Khechache Y, Gasmi M, et al. Le paludisme au Sahara Algérien. Bull Soc Pathol Exot. 2009;102:185–92. . [PubMed] [Google Scholar]
- 34.van Dorp L, Gelabert P, Rieux A, de Manuel M, de-Dios T, Gopalakrishnan S, et al. Plasmodium vivax malaria viewed through the lens of an eradicated European strain. Mol Biol Evol. 2019. Epub 2019/11/08. 10.1093/molbev/msz264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daron J, Boissiere A, Boundenga L, Ngoubangoye B, Houze S, Arnathau C, et al. Population genomic evidence of a Southeast Asian origin of Plasmodium vivax. BioRxiv. 2020:doi: 10.1101/2020.04.29.067439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niangaly A, Karthigayan G, Amed O, Coulibaly D, Sa JM, Adams M, et al. Plasmodium vivax Infections over 3 Years in Duffy Blood Group Negative Malians in Bandiagara, Mali. Am J Trop Med Hyg. 2017;97:744–52. 10.4269/ajtmh.17-0254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niang M, Thiam LG, Sow A, Loucoubar C, Bob NS, Diop F, et al. A molecular survey of acute febrile illnesses reveals Plasmodium vivax infections in Kedougou, southeastern Senegal. Malar J. 2015;14:281 10.1186/s12936-015-0808-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niang M, Diop F, Niang O, Sadio BD, Sow A, Faye O, et al. Unexpected high circulation of Plasmodium vivax in asymptomatic children from Kedougou, southeastern Senegal. Malar J. 2017;16:497 10.1186/s12936-017-2146-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howes RE, Battle KE, Mendis KN, Smith DL, Cibulskis RE, Baird JK, et al. Global Epidemiology of Plasmodium vivax. Am J Trop Med Hyg. 2016;95:15–34. 10.4269/ajtmh.16-0141 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmerman PA. Plasmodium vivax infection in Duffy-negative people in Africa. Am J Trop Med Hyg. 2017;97:636–8. 10.4269/ajtmh.17-0461 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mint Deida J, Ould Khalef Y, Mint Semane E, Ould Ahmedou Salem MS, Bogreau H, Basco L, et al. Assessment of drug resistance associated genetic diversity in Mauritanian isolates of Plasmodium vivax reveals limited polymorphism. Malar J. 2018;17:416 10.1186/s12936-018-2548-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mint Lekweiry K, Ould Mohamed Salem Boukhary A, Gaillard T, Wurtz N, Bogreau H, Hafid JE, et al. Molecular surveillance of drug-resistant Plasmodium vivax using pvdhfr, pvdhps and pvmdr1 markers in Nouakchott, Mauritania. J Antimicrob Chemother. 2012;67:367–74. 10.1093/jac/dkr464 . [DOI] [PubMed] [Google Scholar]
- 43.Ould Ahmedou Salem MS, Mohamed Lemine YO, Deida JM, Lemrabott MA, Ouldabdallahi M, Ba MD, et al. Efficacy of chloroquine for the treatment of Plasmodium vivax in the Saharan zone in Mauritania. Malar J. 2015;14:39 10.1186/s12936-015-0563-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–11. 10.1016/S0140-6736(13)60310-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Price RN, Commons RJ, Battle KE, Thriemer K, Mendis K. Plasmodium vivax in the era of the shrinking P. falciparum map. Trends Parasitol. 2020;36:560–70. Epub 2020/05/15. 10.1016/j.pt.2020.03.009 . [DOI] [PMC free article] [PubMed] [Google Scholar]