Abstract
Autism spectrum disorder (ASD) has emerged as one of the most prevalent and poorly understood disorders of our time. The etiology of autism currently remains poorly understood; however, emerging clinical and experimental evidence suggests central roles for maternal immune activation (MIA) during pregnancy in ASD. In particular, children whose mothers suffered from an infectious disease or other inflammatory conditions during pregnancy are at a substantially higher risk of developing ASD. It has been shown that MIA-induced ASD can be modeled by treating pregnant mice with the viral mimetic polyinosinic-polycytidylic acid (PolyI:C) during key neurodevelopmental time points. In this paradigm, PolyI:C treatment induces systemic inflammatory responses that model MIA during viral infections. Offspring from PolyI:C-treated mothers develop many of the defining features of ASD including defects in social interactions, communicative impairments, and repetitive/stereotyped behaviors, as well as neuropathologies that are commonly observed in human ASD. While the early use of this emerging ASD model system has provided important initial insights into the involvement of gestational immune dysfunction in neurodevelopmental disorder pathogenesis, we have only just begun to scratch the surface in our understanding of how MIA affects brain maturation and contributes to neurodevelopmental disease. Here we describe best practices for how the PolyI:C model of MIA can be used to study autism-related disorders in mice.
Keywords: Autism spectrum disorder, maternal immune activation, neuroimmunology, neurodevelopment, behavioral abnormalities, microbiome, communicative deficits, repetitive/stereotyped behaviors, social preference
Introduction
Maternal immune activation (MIA) resulting from infection, autoinflammatory conditions, and autoimmune disease has been increasingly linked to neurodevelopmental disorders such as autism spectrum disorder (ASD) and schizophrenia in humans [1–13]. Some of the first evidence linking gestational immune dysregulation to ASD came from seminal studies conducted by Chess et al. following the 1964 rubella epidemic. In the aftermath of the outbreak, Chess et al. examined children with congenital rubella syndrome (CRS) and found a marked increase in children displaying some degree of autism, identifying 18 cases of 243 examined and suggesting an incidence of 741 autism diagnoses per 10,000 children [6]. Since this original observation, other studies have also linked autism to maternal infections with influenza, measles and mumps, and various bacterial pathogens [6–8]. Of note, one of the largest studies to date, which included over one million children, demonstrated that maternal infection resulting in hospitalization increased the incidence of their children developing ASD by threefold [2].
The animal model of maternal immune activation (MIA) has provided some of the strongest support for maternal immune dysregulation as a contributor to neurodevelopmental abnormalities including schizophrenia and autism. Originally developed as a model for schizophrenia, Zuckerman et al. were interested in investigating if prenatal immune activation with the vial mimetic polyinosinic-polycytidylic acid (PolyI:C) at embryonic day 15 (E15) would lead to schizophrenic behaviors. They found that immune activation during pregnancy can lead to the development of schizophrenia-associated behaviors in the offspring including disrupted latent inhibition (LI) and increased anxiety [14,15]. More recently MIA has been adopted as an experimental model to study the relationship between hyperactive maternal immune responses and autism-related phenotypes in MIA offspring. With minor modifications in the timing of PolyI:C administration, as well as the tests used to assess behavioral abnormalities, it was shown that MIA can be adapted for the study of ASD [16–19]. Indeed, autism-related phenotypes can be induced by treating pregnant mice with PolyI:C at or around E12.5 [16–19]. Importantly, mice that mature in this inflammatory maternal environment develop many of the core symptoms used to diagnose ASD. Namely, PolyI:C treatment during pregnancy causes communicative deficits, abnormalities in social behaviors, and repetitive/stereotyped behaviors in MIA offspring [16–19].
Studies are just now beginning to reveal the specific immunological pathways underlying the abnormal neurodevelopment and behavioral irregularities generated in the MIA model. Early studies identified IL-6 as a critical mediator of MIA-induced neurodevelopmental disorders [17]. In these studies, it was shown that injection of IL-6 into pregnant mothers at E12.5 was sufficient to promote the development of autism-like behaviors in the offspring. Moreover, they demonstrated that ablation of IL-6 signaling with neutralizing antibody treatment provides protection in the PolyI:C-based MIA model of ASD. In more recent years, studies have uncovered additional roles for IL-17a in MIA-induced neurodevelopmental disorders [20–22]. Landmark work by Choi et al discovered that IL-17a production by maternal Th17 cells is a key contributor to the development of behavioral abnormalities and impaired cortical maturation in MIA offspring [20]. Following up on these findings, studies from the Choi and Huh labs, in addition to our group, have shown that maternal microbiota landscape significantly impacts the incidence and severity of MIA-induced neurodevelopmental disorders [22]. In particular, we show that microbiota-mediated calibration of IL-17a responses can dictate neurodevelopmental disorder susceptibility in the MIA model [22]. Besides IL-6 and IL-17a, little is currently known about how other inflammatory mediators or immune-based effector functions affect behavior and neurodevelopment in response to MIA. Therefore, future studies that interrogate the cellular and molecular mechanisms underlying MIA-induced neurodevelopmental disorders are needed to better characterize ASD etiology and to also aid in the identification of potential therapeutic targets for neurodevelopmental disorders.
Materials
Maternal immune activation (MIA) induction
Prepare 10 mg/ml stock of Polyinosinic-polycytidylic acid potassium salt (PolyI:C) (Millipore Sigma P9582) using salt weight in sterile PBS. For one vial of PolyI:C dissolve powder (50 mg) in 5 mL sterile PBS (See Note 1).
Ultrasonic vocalization recording
Microphone capable of recording ultrasonic vocalizations (USVs) between 2 kHz-200 kHz needed (See Note 2).
Software that can detect and analyze USVs (See Note 3).
Glass or plastic container that is capable of fitting microphone and mouse pup (approximately the size of a 500 mL beaker).
Social preference test
Arena for three chamber social preference testing (See Note 4).
Containment cage (See Note 5).
Object that the mouse has not previously encountered (plastic toy).
Software and cameras capable of tracking and analyzing movement of experimental mice (See Note 6).
Marble burying test
Teklad Laboratory Grade Sani-chip bedding.
20 marbles (1.5 cm diameter).
Fecal transplantation
QIAamp DNA Stool Mini Kit for extraction of genomic DNA from fecal samples (Catalog number 51504).
- Segmented Filamentous Bacteria (SFB) forward and reverse primers for confirmation of Taconic microbiome transfer.
- Forward: 5’ GAC GCT GAG GCA TGA GAG CAT 3’
- Reverse: 5’ GAC GGC ACG GAT TGT TAT TCA 3’
- Universal (UNI) forward and reverse primers for total bacteria.
- Forward: 5’ ACT CCT ACG GGA GGC AGC AGT 3’
- Reverse: 5’ ATT ACC GCG GCT GCT GCG 3’
Quantitative real time PCR thermocycler.
Methods
Maternal immune activation
Studies from our lab and others have identified that appropriate gestational inflammatory conditions, such as induction of IL-17a-mediated inflammation, need to be present during pregnancy to consistently promote the development of autism-related phenotypes in MIA offspring [20–22]. Mice from Taconic Biosciences, due to their propensity to generate robust levels of IL-17a in response to immune triggers, produce the most consistent results in the MIA model of neurodevelopmental disease. It should be noted that mice from other vendors can also be used to study MIA-induced neurodevelopmental disorders; however, fecal transplantation with Taconic microbiota or troubleshooting with different dosages of PolyI:C may be required to generate consistent results (See Note 7).
In order to ensure that maternal immune activation occurs at the correct embryonic time points, mice are mated overnight and females are checked twice daily, once in the morning and once in the evening, for the presence of vaginal plugs (Fig. 1). The presence of a plug denotes embryonic day 0.5 (E0.5).
Mice are then left undisturbed until E11.5 when pregnant female mice are weighed and treated with 20 mg/kg PolyI:C or PBS by intraperitoneal (ip) injection (See Note 8).
Females are then subjected to a second dose of PolyI:C (20mg/kg; i.p.) or PBS on E12.5 (See Note 9).
Each dam is returned to its cage and left undisturbed until the birth of its litter. All pups remain with the mother until weaning (typically between postnatal day 21 (P21) and P24), at which time mice can be group housed at 2-5 mice per cage with same-sex littermates (See Note 10).
Figure 1. Calculating date of conception with vaginal plug identification.
(A) White arrow indicates no plug. (B) White arrow indicates plug. Presence of a vaginal plug can be used to mark embryonic day 0.5 (E0.5).
Behavior
In order to ensure reproducible results in behavioral testing, it is important to conduct all behavioral tests under controlled and consistent conditions. The same animal handler should conduct all behavioral tests at the same time of day. It is important to eliminate extraneous noises and to habituate the mice to the room for a least one hour before behavioral tests are conducted. Behavioral equipment should be thoroughly cleaned with 70% ethanol between each experimental mouse to eliminate any urine, feces, or odors. Multiple litters should also be tested to confirm that the behaviors are robust and reproducible. Behavioral abnormalities in the PolyI:C MIA model are typically only observed in male offspring and are absent in their female littermates [23,24]. This sex bias is also observed in human populations where there is a strong bias towards males in both autism and schizophrenia [25,26].
Ultrasonic vocalization recording
On postnatal days 9-11, bring cages containing both mothers and litters to the testing room and allow mice to habituate for 1 hour (See Note 11).
After mice are habituated, remove all male pups from the nest (away from the mother) and put them into a cup with bedding from their cage for 10 minutes without recording.
After the habituation period, individually place mice in a clean holding dish and record mouse pup USVs for 3 min using a microphone capable of recording USVs (e.g., UltraSoundGate GM16/CMPA microphone; AviSoft Bioacoustics) and recording software that can analyze USV data (e.g., SAS Prolab software; AviSoft Bioacoustics) (Fig. 2) (See Note 12). Record USVs that are measured between 25-125 kHz, and exclude background recordings that are shorter than 0.02 milliseconds.
Figure 2. Communication deficits observed in MIA offspring.
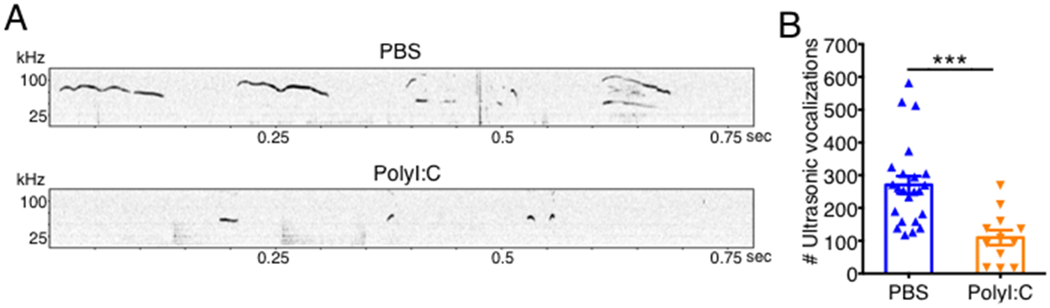
Pregnant C57BL/6 mice were treated with either PBS or 20 mg/kg PolyI:C at embryonic days 11.5 (E11.5) and 12.5 (E12.5). (A-B) Communicative deficits in 10-day-old pups were evaluated through recording of ultrasonic vocalizations (USVs). (A) Representative USV detection plots. (B) Total number of USVs emitted during 3 minutes of recording. Error bars depict mean ± s.e.m. ***P < 0.001 calculated by Student’s t-test. sec, seconds; kHz, kilohertz.
Social Preference Test
Mice can be assessed in the social preference test once they are 8 weeks old. One day prior to testing, experimental mice need to be individually habituated to the three-chamber arena containing empty object containment cages, for two 5-minute sessions in a 3-4 h period (See Note 13).
On the following day, assess social behavior by first placing each mouse in the center chamber without access to either the top or bottom chambers, which contain an unfamiliar C57BL/6 age-matched male mouse in one chamber and an inanimate object (plastic toy) in the other chamber under object containment cages (See Note 14).
After the 5-minute exploration period in the center chamber, remove the barriers to the adjacent chambers, enabling the mouse to explore the top and bottom arenas freely.
Allow the mouse to explore all chambers for an additional 10 min while tracking interaction time as time spent sniffing or approaching ~ 2 cm from the containment cage.
After 10 minutes, experimental and novel mice should be gently removed and returned to home cages, and then all surfaces of the arena, containment cages, and plastic toy should be thoroughly cleaned with 70% ethanol.
Social preference index data can be calculated and plotted as the percentage of time spent investigating the social target (novel mouse) out of the total exploration time of both objects (novel mouse + novel object) (See Note 15) (Fig. 3).
Figure 3. MIA offspring exhibit abnormalities in social behavior.
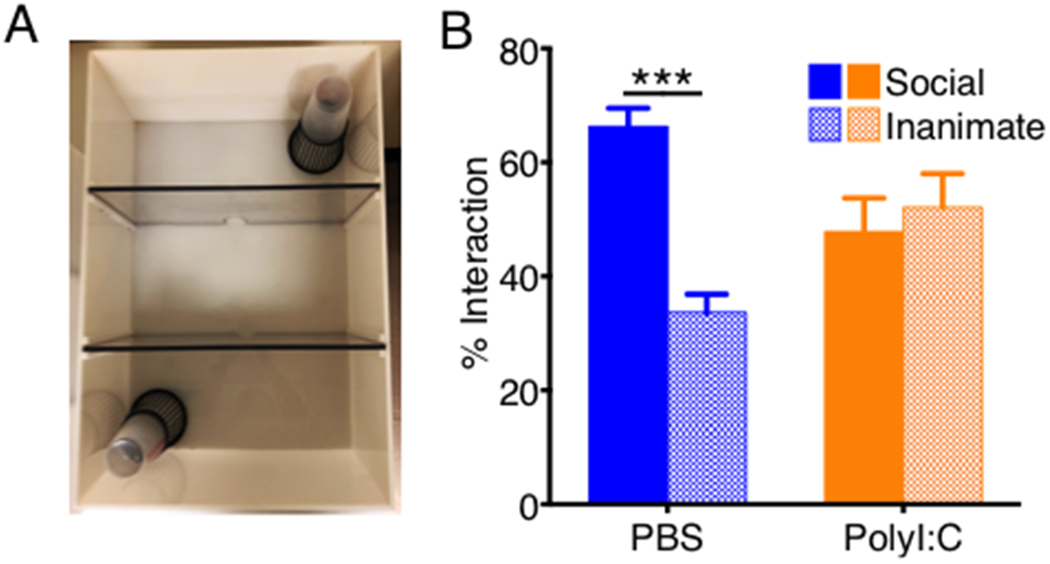
Pregnant C57BL/6 mice were treated with either PBS (n = 15) or 20 mg/kg PolyI:C (n = 14) at E11.5 and E12.5. (A) Representative image depicting setup of the social preference three-chamber arena. (B) Percent interaction with the novel mouse (social) and object (inanimate). Error bars depict mean ± s.e.m. ***P < 0.001 calculated by two-way ANOVA with Tukey post-hoc tests.
Marble burying test
One week following the social preference test, acclimate male mice overnight to autoclaved woodchip bedding (See Note 16).
The following morning, place mice in a clean cage filled with ~2 inches of fresh, autoclaved woodchip bedding containing 20 glass marbles laid out in four rows of five marbles equidistant from one another (Fig. 4).
After a 15-min exploration period, gently remove mice from the testing cages and record the number of marbles buried using the marble burying index: marbles covered >50% by bedding are given a score of 1, ~50% covered are given a score of 0.5, and marbles covered <50% are given a score of 0 [20] (Fig. 4).
Figure 4. MIA offspring display repetitive behaviors in the marble-burying test.
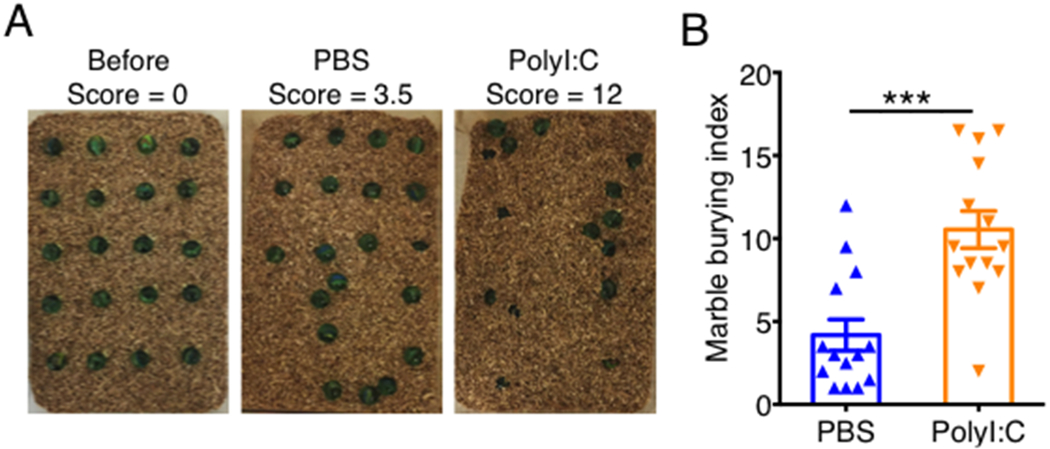
Pregnant C57BL/6 mice were treated with either PBS or 20 mg/kg PolyI:C at E11.5 and E12.5. (A) Representative pictures depicting pretest marble layout and marbles buried by control and MIA mice after 15 minutes. (B) Marble burying index score was calculated based on the following scale: 1 for marbles covered >50% by bedding, 0.5 for ~50% covered, or 0 for anything less. Error bars depict mean ± s.e.m. ***P < 0.001 calculated by Student’s t-test.
Acknowledgement
We thank Tony Filiano and members of the Lukens lab and the Center for Brain Immunology and Glia (BIG) for valuable discussions. This work was supported by The Hartwell Foundation (Individual Biomedical Research Award to J.R.L.), the Owens Family Foundation (J.R.L.), and The Simons Foundation Autism Research Initiative (Pilot Award 515305 to J.R.L.). C.R.L was supported by a NIH National Institute of General Medical Sciences predoctoral training grant (3T32GM008328).
Footnotes
Store PolyI:C stock in small (~200 μL) aliquots at -20°C and avoid freeze-thaw cycles.
USVs displayed in Fig. 2 were recorded using an UltraSoundGateGM16/CMPA microphone (AviSoft Bioacoustics, Glienicke, Germany).
USVs were measured and analyzed with SAS Prolab software (AviSoft Bioacoustics, Glienicke, Germany). When troubleshooting USVs, it is important to confirm counts by hand.
Total arena area 24 x 16 x 9 inches. Dimensions of chambers 8 x 16 x 9 inches (Fig. 3).
Containment cages for the sociability test should enable objects to be clearly seen and smelled, while limiting fighting and mounting.
Social preference data presented in Fig. 3 was tracked and analyzed using TopScan version 3.00.
Fecal microbiota from Taconic mice can be transferred into mice from The Jackson Laboratory or other vendors by exposing mice to feces from Taconic mice for at least two weeks. A small scoop (enough to fill a 50 mL beaker) of Taconic bedding containing feces from sex-matched mice is mixed into cages housing Jackson mice for two weeks, with fresh fecal samples being added every 3 days. SFB transfer can be confirmed by collecting fecal pellets before and after cohousing and assaying for the presence of total bacteria (total 16S rRNA) and SFB by qPCR. Mice can then be mated and subjected to MIA using the same protocol as described in the Methods section. We have confirmed that this experimental strategy is effective in inducing MIA-associated ASD-like phenotypes in Jackson C57BL/6 mice that previously underwent fecal transplantation with microbiota from Taconic C57BL/6 mice. We have not, however, attempted these fecal transfer experiments in mice from other vendors and thus further troubleshooting may be required when using mice from vendors other than Jackson and Taconic.
Pregnant dams injected with PolyI:C should not show overt signs of sickness behavior such as dramatic weight loss or immobility, although they will exhibit reduced weight gain and sometimes minor weight loss for the first few days following PolyI:C injection.
This two-injection MIA protocol (PolyI:C on days E11.5 and E12.5) differs slightly from others in the field, that only rely on a single PolyI:C injection on E12.5. However, through exhaustive troubleshooting that involved various injection regimens, we have found that an injection on both E11.5 and E12.5 produces the most consistent results.
Mice should not be singly housed because this can affect behavior. Studies show that singly housed mice often develop depressive-like behaviors, which can ultimately affect performance in behavioral testing.
Due to differences in USV trends observed between labs, where some labs observe an increase in vocalizations from MIA offspring [20–22] while others detect less [18,19,24], it is helpful to first measure USVs over multiple days (postnatal days 7-14) to detect where the most robust differences are observed.
The microphone should hang ~ 2 inches above the bottom of the container.
Since experimental mice can climb up the containment cages during the test, it helps to have an object on top to prevent the mouse from climbing on top of the containment cage (as seen in Figure 3A).
Before performing social preference testing and while the experimental mice are acclimating to the room, habituate novel mice 2 times (5 minutes each) under the containment cages.
It is important to also track distance traveled during both the habituation and social preference tests to ensure that any observed differences in social preference are not due to overall lack of activity or alertness.
If mice are already housed in woodchip bedding, this step is not necessary. The same cages can be used for mice from the same litter; however, it is important to change the woodchip bedding between each mouse regardless of if they are littermates.
Competing Interests
The authors declare no competing financial interests.
References
- 1.Atladottir HO, Pedersen MG, Thorsen P, Mortensen PB, Deleuran B, Eaton WW, Parner ET (2009) Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics 124 (2):687–694. doi: 10.1542/peds.2008-2445 [DOI] [PubMed] [Google Scholar]
- 2.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET (2010) Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord 40 (12):1423–1430. doi: 10.1007/s10803-010-1006-y [DOI] [PubMed] [Google Scholar]
- 3.Patterson PH (2011) Maternal infection and immune involvement in autism. Trends Mol Med 17 (7):389–394. doi: 10.1016/j.molmed.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mednick SA, Machon RA, Huttunen MO, Bonett D (1988) Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry 45 (2):189–192 [DOI] [PubMed] [Google Scholar]
- 5.Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, Susser ES (2000) Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull 26 (2):287–295 [DOI] [PubMed] [Google Scholar]
- 6.Chess S (1971) Autism in children with congenital rubella. J Autism Child Schizophr 1 (1):33–47 [DOI] [PubMed] [Google Scholar]
- 7.Chess S (1977) Follow-up report on autism in congenital rubella. J Autism Child Schizophr 7 (1):69–81 [DOI] [PubMed] [Google Scholar]
- 8.Chess S, Fernandez P, Korn S (1978) Behavioral consequences of congenital rubella. J Pediatr 93 (4):699–703 [DOI] [PubMed] [Google Scholar]
- 9.Brimberg L, Sadiq A, Gregersen PK, Diamond B (2013) Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Mol Psychiatry 18 (11):1171–1177. doi: 10.1038/mp.2013.101 [DOI] [PubMed] [Google Scholar]
- 10.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP (2014) Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol 10 (11):643–660. doi: 10.1038/nrneurol.2014.187 [DOI] [PubMed] [Google Scholar]
- 11.Estes ML, McAllister AK (2015) Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci 16 (8):469–486. doi: 10.1038/nrn3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braunschweig D, Van de Water J (2012) Maternal autoantibodies in autism. Arch Neurol 69 (6):693–699. doi: 10.1001/archneurol.2011.2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deykin EY, MacMahon B (1979) Viral exposure and autism. Am J Epidemiol 109 (6):628–638 [DOI] [PubMed] [Google Scholar]
- 14.Zuckerman L, Rehavi M, Nachman R, Weiner I (2003) Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology 28 (10):1778–1789. doi: 10.1038/sj.npp.1300248 [DOI] [PubMed] [Google Scholar]
- 15.Zuckerman L, Weiner I (2005) Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res 39 (3):311–323. doi: 10.1016/j.jpsychires.2004.08.008 [DOI] [PubMed] [Google Scholar]
- 16.Shi L, Fatemi SH, Sidwell RW, Patterson PH (2003) Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci 23 (1):297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH (2007) Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 27 (40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH (2012) Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun 26 (4):607–616. doi: 10.1016/j.bbi.2012.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155 (7):1451–1463. doi: 10.1016/j.cell.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR (2016) The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351 (6276):933–939. doi: 10.1126/science.aad0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin Yim Y, Park A, Berrios J, Lafourcade M, Pascual LM, Soares N, Yeon Kim J, Kim S, Kim H, Waisman A, Littman DR, Wickersham IR, Harnett MT, Huh JR, Choi GB (2017) Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549 (7673):482–487. doi: 10.1038/nature23909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR (2017) Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature 549 (7673):528–532. doi: 10.1038/nature23910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Cazakoff BN, Thai CA, Howland JG (2012) Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology 62 (3):1299–1307. doi: 10.1016/j.neuropharm.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaafsma SM, Gagnidze K, Reyes A, Norstedt N, Mansson K, Francis K, Pfaff DW (2017) Sex-specific gene-environment interactions underlying ASD-like behaviors. Proc Natl Acad Sci U S A 114 (6):1383–1388. doi: 10.1073/pnas.1619312114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Werling DM, Geschwind DH (2013) Sex differences in autism spectrum disorders. Curr Opin Neurol 26 (2):146–153. doi: 10.1097/WCO.0b013e32835ee548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abel KM, Drake R, Goldstein JM (2010) Sex differences in schizophrenia. Int Rev Psychiatry 22 (5):417–428. doi: 10.3109/09540261.2010.515205 [DOI] [PubMed] [Google Scholar]


