Abstract
The microcirculation comprising of arterioles, capillaries and post-capillary venules is the terminal vascular network of the systemic circulation. Microvascular homeostasis, comprising of a balance between vasoconstriction, vasodilation and endothelial permeability in healthy states, regulates tissue perfusion. In severe infections, systemic inflammation occurs irrespective of the infecting microorganism(s), resulting in microcirculatory dysregulation and dysfunction, which impairs tissue perfusion and often precedes end-organ failure. The common hallmarks of microvascular dysfunction in both septic shock and dengue shock, are endothelial cell activation, glycocalyx degradation and plasma leak through a disrupted endothelial barrier. Microvascular tone is also impaired by a reduced bioavailability of nitric oxide. In vitro and in vivo studies have however demonstrated that the nature and extent of microvascular dysfunction as well as responses to volume expansion resuscitation differ in these two clinical syndromes. This review compares and contrasts the pathophysiology of microcirculatory dysfunction in septic versus dengue shock and the attendant effects of fluid administration during resuscitation.
Introduction
The microcirculation is the terminal vascular network of the systemic circulation, whose primary function is to distribute oxygen to, and remove metabolic by-products from living cells. In health, tissue perfusion is regulated by control of microvascular tone and endothelial permeability. In states of shock, however, there is a mismatch between demand for, and delivery or utilisation of oxygen in the tissues1.
The microcirculation comprises arterioles, capillaries and post-capillary venules. The primary component of micro-vessels are endothelial cells; arterioles are also surrounded by vascular smooth muscle cells. Endothelial cells maintain the selective vascular barrier by means of dynamic regulation of inter-endothelial adherens and tight junction integrity, as well as control of cytoskeletal contraction by reorganisation of filamentous actin2. The luminal surface of vascular endothelium is lined with a fine hair-like structure, the glycocalyx, which forms a physical and electrostatic barrier that is important for vascular homeostasis. The glycocalyx is composed of membrane-bound proteoglycans, sulphated-glycosaminoglycans, as well as glycoproteins bearing acidic oligosaccharides and terminal sialic acids3. This elaborate network contains a high density of negatively-charged glycosaminoglycan side-chains that ensure laminar flow is maintained through electrostatic repulsion of intravascular proteins (albumin, globulin, and cellular components) away from the vessel wall towards the centre of the lumen3. Other important functions of the glycocalyx include regulating microvascular tone, inhibiting microvascular thrombosis, regulating leucocyte adhesion on the endothelium, and acting as a bio-sensor for as well as mediating mechanotransduction of shear forces exerted by circulating volume4,5.
In a normal healthy state, the glycocalyx shields the endothelial cells from oxidative stress, and transmits excess luminal shear forces to endothelial cells thus initiating nitric-oxide mediated vasorelaxation4. Vascular homeostasis is also under the control of endothelial-derived prostacyclin, a lipid and endothelin-1, a peptide. Metabolism of arachidonic acid by cyclooxygenase (COX) yields prostaglandin H2, which undergoes further terminal metabolism to produce either prostacyclin (prostaglandin I2) or thromboxane A26.
Prostacyclin is a potent vasodilator of both systemic and pulmonary vasculature as well as an inhibitor of platelet activation7. It acts via G-protein-coupled receptors to stimulate adenylate cyclase and increase the levels of cyclic AMP which in turn activates protein kinase A (PKA) leading to phosphorylation of myosin light chain kinase and platelet inositol 1,4,5-triphosphate8. Thromboxane A2 causes platelet aggregation and vasoconstriction acting synergistically with endothelin-1, a potent endogenous vasoconstrictor secreted by endothelial cells. Endothelin-1 also acts via G-protein-coupled receptors. Endothelin-1 type A receptors are located mostly in vascular smooth muscle cells and are responsible for the vascular contraction, cellular proliferation and proinflammatory effects9. Endothelin-1 type B receptors are located on both endothelial and vascular smooth muscle cells and are thought to play a role in clearance of endothelin-1 as well as release of an endothelium-derived hyperpolarizing factor10,11. The balance of endothelial vasoconstrictors and vasodilators is important in microvascular homeostasis.
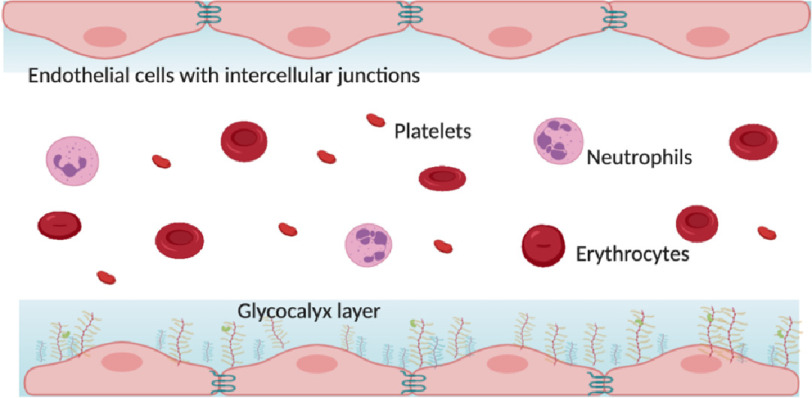
Figure 1 shows the appearance of the microcirculation under normal conditions
Figure 1. Appearance of the microcirculation under normal conditions with intact endothelial cell junctions and glycocalyx lining.
In severe infections, systemic inflammation and direct pathogen-mediated damage can lead to impairment of the microcirculation, which precedes end-organ dysfunction. Progression from severe infection to shock with vasoplegia and multi-organ failure carries a high risk of potentially worse outcomes irrespective of the infecting pathogen12,13. The clinical phenotype of patients with shock caused by bacterial sepsis and viral infections like dengue carry some similarities but also are distinct in their presentation and management. This review therefore compares and contrasts the pathophysiology of microcirculatory dysfunction in septic versus dengue shock and the attendant effects of fluid administration during resuscitation.
Sepsis and Dengue
According to the latest iteration of the Global Burden of Disease study, sepsis affects 50 million people worldwide leading to 11 million deaths14. Despite advances in the understanding of the host-immune responses to sepsis, there has been no translation to new therapies over the last two decades12.
Dengue is an arboviral infectious disease caused by a single-stranded Flavivirus, the dengue virus (DENV) transmitted by Aedes mosquito vectors15. Dengue incidence continues to increase globally, driven in part by globalization, climate change and a lack of effective vaccines or prevention strategies16,17. There is a high disease burden in Southeast Asia, Asia, South America and emerging outbreaks throughout Africa18.
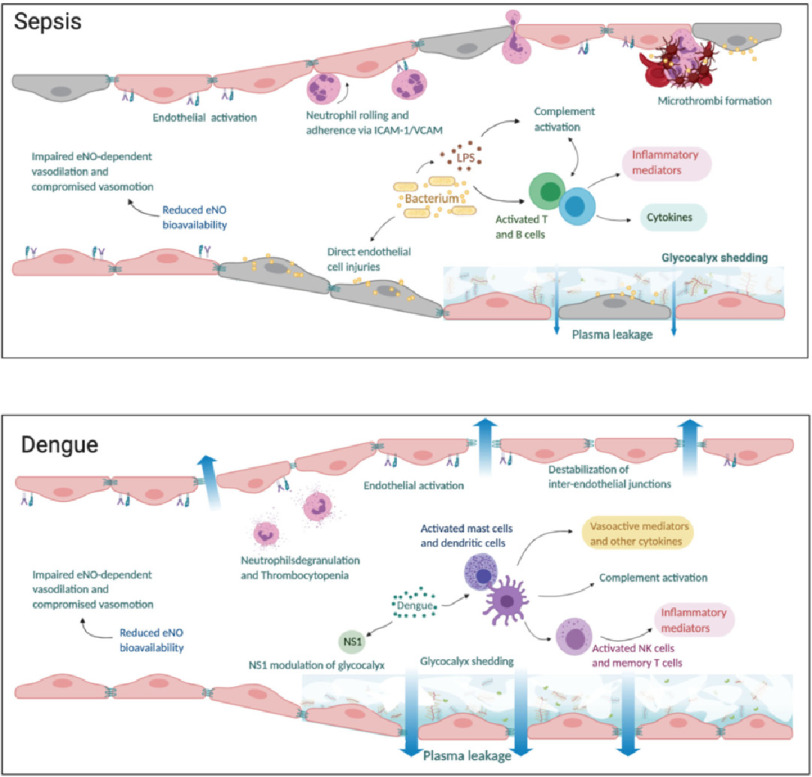
In both septic shock and dengue shock, the common hallmarks of microvascular dysfunction are endothelial cell activation and glycocalyx degradation leading to plasma leak through a disrupted endothelial barrier, together with reduced nitric oxide bioavailability leading to impaired microvascular tone19–22. Figure 2 shows the microcirculation in sepsis and in dengue. Despite similarity of endothelial dysfunction, the responses to fluid therapy in septic and dengue shock are very different. Fluid boluses have been shown to lead to clinical improvement in dengue shock23 but worsening outcomes in septic shock24–27, albeit fluid boluses in dengue shock tend to be of smaller volume.
Figure 2. The microcirculation appearance in (a) sepsis and (b) dengue.
In sepsis, there is inflammation and direct injury of endothelial cells by bacterial proteins and toxins. Endothelial activation leads to neutrophil chemotaxis and adherence to intercellular adhesion molecule- 1 (ICAM-1) and vascular adhesion molecule (VCAM) expressed on endothelial cells. Concurrently, there is activation of complement, T and B immune cells leading to release of inflammatory mediators and cytokines which cause further inflammation and glycocalyx damage. Concurrently, the coagulation cascade is activated, and anticoagulant/fibrinolysis pathways are impaired leading to formation of microthrombi and compromised microvascular flow. In dengue, viral non-structural 1 (NS1) protein triggers hyperpermeability and directly alters the endothelial layer function through the activation of several enzymes responsible for glycocalyx degradation. Activation of immune cells leads to complement activation and release of inflammatory mediators. There is impaired endothelial nitric oxide (eNO) dependent vasodilation due to reduced eNO bioavailability leading to compromised microvascular flow. Plasma leakage in both sepsis and dengue occur as a result of glycocalyx shedding and disruption of tight and adherens junctions between endothelial cells.
While the final common pathway of glycocalyx shedding and microvascular dysfunction is similar in septic and dengue shock, the magnitude of the plasma leak appears to be greater in dengue infections28.
There are several similarities in microvascular dysfunction in septic and dengue shock, yet certain differences are also emerging, including the degree and specific components of glycocalyx disruption. Understanding these pathophysiological mechanisms may lead to improved management in the form of tailored fluid resuscitation and targeted therapeutics.
Measuring microcirculatory dysfunction
No single measurement offers comprehensive evaluation of microvascular function, but combinations of the following techniques can provide insight into the nature of microcirculatory disturbance in septic shock and dengue shock.
Previous methods to quantify perfusion of the microcirculation in vivo used invasive techniques such as intravital microscopy trans-illumination in experimental animals29 or human nail-fold beds30,31 and non-invasive laser speckle and laser Doppler tracking whereby a monochromatic laser light is scattered by red blood cells moving through the microcirculation32,33. However, heterogeneity of microvascular beds in different organs, cost, complexity and invasiveness of assessment precluded routine assessment of the microcirculation in clinical settings using these techniques.
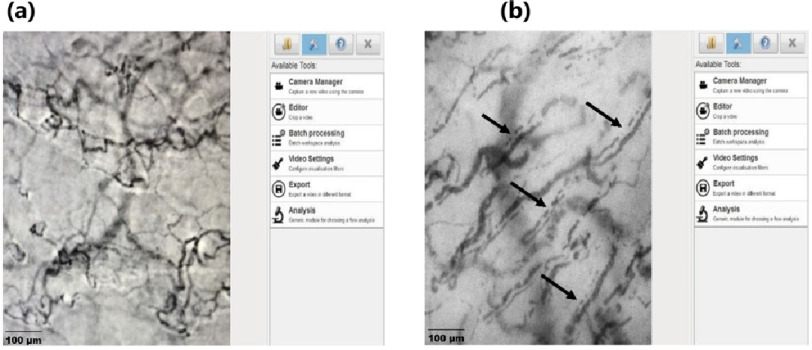
In light of these shortcomings, videomicroscopic techniques based on orthogonal polarisation spectroscopy are increasingly used for direct, non-invasive imaging of the microcirculation. Real-time visualisation of the microcirculation is now possible with dark-field imaging technology. Using side-stream dark-field (SDF) or incident dark-field (IDF) videomicroscopy, it is possible to visualize red blood cells flowing in the microcirculation through mucus membranes34. Hand-held videomicroscopes have made it possible to image the sublingual microcirculation in real-time during critical illness, to measure the proportion of perfused micro-vessels, assess heterogeneity in flow within the microvascular bed, and estimate the depth of the endothelial glycocalyx as a proxy for degradation. Although SDF/IDF videomicroscopy is predominantly a research tool at present, recent advances to automate analysis may make it feasible to evaluate microcirculatory dysfunction and measure response to therapies in the clinical setting35. Figure 3 shows a comparison normal and septic sublingual microcirculation.
Figure 3. Sublingual microcirculation in (a) normal (b) in septic shock.
The micro-vessels in septic shock have a comparatively larger diameter and do not run the entire course resulting in a dotted-line appearance characteristic of impaired microvascular flow in sepsis shown by the arrows in (b). Figures courtesy Critical Care Research Group (CCRG) laboratory, QLD, Australia.
Non-invasive assessment of endothelial nitric oxide dependent vasodilation can be performed using real-time techniques such as brachial artery flow mediated dilatation, and EndoPAT - an automated, user-independent technique22,36.
Biomarkers of glycocalyx degradation and endothelial activation can be measured in circulating plasma; such markers provide an indirect measurement of the mechanism, timing, and extent of glycocalyx and endothelial barrier disruption. In addition, measuring urinary clearance of heparan sulphate can be a useful, non-invasive, indicator of glycocalyx turnover28,37.
Microvascular dysfunction in sepsis
Sepsis is fundamentally an inflammatory disease initiated by host recognition of infection-derived antigen proteins38; however, this host-response to the infection is dysregulated leading, in some cases, to life-threatening organ dysfunction1. Septic shock results from untreated or partially treated sepsis progressing to cause circulatory, cellular and metabolic abnormalities associated with a higher risk of mortality1.
Direct injury of the microvascular endothelium by release of endotoxins, myocardial depressant factors as well as a physiologic cascade of pro-inflammatory cytokines cause dysfunction of the circulatory system in septic shock; these activate a cascade leading to formation of micro-thrombi and impairment of micro-vessel perfusion, endothelial cell activation, disruption of the endothelial-glycocalyx layer and leakage of plasma into the extravascular space. This leads to a drop in the circulating intravascular volume, manifesting clinically as low blood pressure.
Compounding this is a state of reduced nitric oxide bioavailability, resulting in impaired endothelial nitric oxide dependent vasodilation and compromised vasomotion. Nitric oxide is produced by catalytic oxidation of L-arginine by nitric oxide synthase (NOS) enzyme39. Increased arginase activity40 leading to reduced L-arginine is also likely contributory to this hypotensive state in sepsis41.
The cornerstone of resuscitation in sepsis has therefore been fluid administration to restore the circulating volume, with the addition of vasopressors to restore microvascular tone and improve myocardial contractility, if necessary. The use of fluid boluses for resuscitation in septic shock has, however, been challenged by clinical and pre-clinical trials. In 2011, the Fluid Expansion As Supportive Therapy (FEAST) trial, demonstrated a 45% increase in the relative risk of mortality with fluid bolus treatment compared to non-bolus controls (95% CI [1.13–1.86]; p = 0.003)24 with excess mortality following fluid bolus therapy being attributed to fatal cardiovascular collapse42. In 2017, Andrews et al reported a 46% higher in-hospital mortality among predominantly HIV-positive adults with sepsis and hypotension receiving protocolised early intravenous fluids and vasopressors compared to usual care (95% CI [1.04–2.05]; P = 0.03)43. A recent pre-clinical trial revealed a paradoxical increase in vasopressor requirement, a rise in cardiac troponin I and atrial natriuretic peptide as well as increased shedding of hyaluronan, a component of the endothelial-glycocalyx, following fluid bolus resuscitation of endotoxaemic shock compared to non-bolus control44.
Improvements in systemic macro-haemodynamic parameters after resuscitation do not necessarily lead to an improvement in microcirculatory parameters; this ‘loss of haemodynamic coherence’ is an independent predictor of adverse patient outcome45. Indeed, after macrocirculatory optimisation, attempts to directly target the microcirculation with inhaled nitric oxide did not augment microcirculatory perfusion, enhance lactate clearance or reduce organ dysfunction in patients with septic shock46,47. Given these findings, further work is needed to find the best strategies to optimise microcirculatory function in septic shock.
Microvascular dysfunction in severe dengue
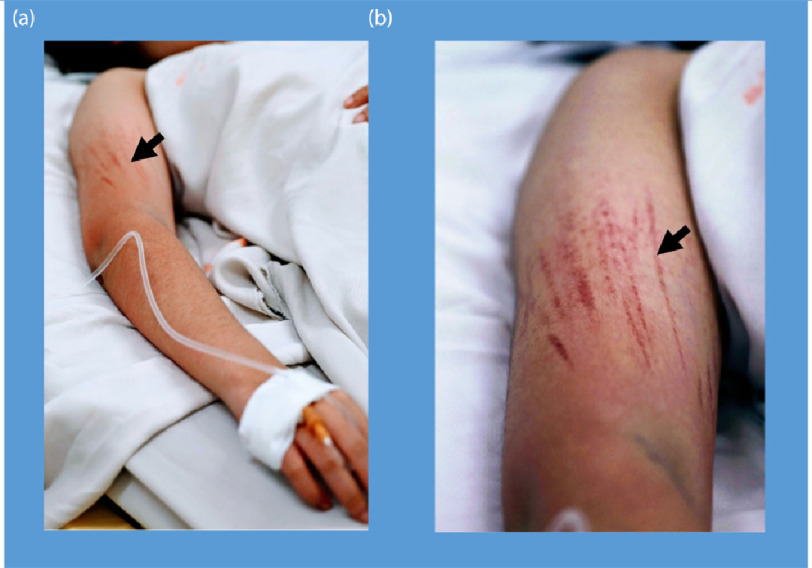
Dengue is a viral illness with a broad spectrum of clinical phenotypes. While the majority of infections are mild and self-limiting, a small proportion of patients develop dengue shock syndrome due to an increase in vascular permeability, profound plasma leakage and subsequent hypovolaemia48,49. Figure 4 shows bleeding manifestations seen clinically in dengue patients.
Figure 4. Bleeding manifestations in dengue shock syndrome showing the appearance of linear petechiae after blood pressure cuff inflation.
Understanding of the mechanisms underlying microcirculatory injury in dengue is still incomplete. The dengue non-structural 1 (NS1) protein, a viral glycoprotein secreted by infected cells, has been shown to cause an increase in endothelial permeability leading to vascular leakage50. Of note, recent findings from Glasner et al have shown that NS1-mediated vascular leak depends on the integrity of endothelial glycocalyx components, both in vitro and in vivo, rather than inflammatory cytokines51. The Dengue-NS1 protein induces expression of several enzymes which degrade glycocalyx components, including heparanases and sialidases52.
In parallel, the host immune response to dengue infection is widely recognised to play a key role in microcirculatory injury53,54,55; excessive pro-inflammatory cytokines, particularly tumour necrosis factor-alpha and other vasoactive mediators, also cause tissue injury resulting in an increase in capillary permeability56.
SDF videomicroscopy has shown reduced perfusion of the sublingual microcirculation in patients with plasma leakage during the critical phase of dengue infection, together with a reduction in glycocalyx depth and elevated plasma levels of glycocalyx components, both most marked in patients with the most severe plasma leak57. Indeed, plasma syndecan-1 levels are several-fold higher in patients with dengue shock versus septic shock, which suggests a key difference in the magnitude or mechanisms underlying microcirculatory damage between the syndromes4,58,59. In vitro studies have also demonstrated rearrangement of intra-cellular actin filaments, enhanced endothelial cytoskeleton contractility, destabilisation of inter-endothelial adherens junctions and decreased expression of tight junction protein ZO-160,61; all of these changes likely contribute to plasma leakage. EndoPAT studies have shown impaired endothelial NO-dependent vasodilation, which might be partly explained by both glycocalyx breakdown and reduced NO bioavailability due to a decrease in L-arginine and increase in arginase during dengue infection22. However, the small proportion of patients who require vasopressors to treat dengue shock compared to septic shock suggests that impairment in microvascular tone is more marked in the latter syndrome62.
At present, the cornerstone of dengue shock management is judicious resuscitation with intravenous fluid, with the occasional need for vasopressors, until the critical phase of plasma leakage ends. Notably, only a minority of children tend to require vasopressor support in dengue shock syndrome, with more adults needing haemodynamic support63. Based on evidence from trials of resuscitation with different types of intravenous fluid, the WHO guidelines recommend the initial use of crystalloid solutions, followed by colloid solutions for patients with unresponsive shock18,23. There is a paucity of evidence from randomised controlled trials on how rate and volume of fluid resuscitation affects outcomes in dengue shock. Learning from research on septic shock, studies are needed to explore whether optimisation of macrocirculatory parameters improves microcirculatory function, or whether current fluid management strategies may be detrimental to an already injured glycocalyx layer in dengue shock.
Summary and future directions
Impairment in perfusion, tone and barrier-function of the microcirculation is central to the pathogenesis of both septic shock and dengue shock; but it is clear from in vitro and clinical studies that the nature and extent of dysfunction differs between the syndromes. These differences may impact the likelihood of responding to treatment; volume expansion, vasopressors to enhance microvascular tone or therapeutics aimed at restoring the glycocalyx layer and endothelial barrier may be differentially effective based on the underlying pathology.
Studies in other conditions investigating different resuscitation fluids on the integrity of the glycocalyx and endothelial function have shown a beneficial effect of fresh frozen plasma (FFP) on glycocalyx restoration and improved vascular permeability64,65. The exact components of FFP and the potential mechanisms responsible for this glycocalyx repair remain to be defined, but preformed SDC1 in the FFP is thought to restore the layer resulting in improved barrier and endothelial cell functions66. In addition, synthetic colloids are thought to transiently restore the capillary permeability barrier properties by the incorporation of small dextran molecules into the glycocalyx layer. In an animal model of ischemic-reperfusion injury, infusion with hydroxyethyl starch (HES) reduced the net coronary fluid filtration67.
Findings from this study suggested that it may be beneficial to give low dose continuous colloid infusion rather than repeated larger colloid boluses in infection related shock syndrome, which could not only reduce the plasma leakage but also allow for lower total intravenous fluid volumes to be infused, potentially avoiding complications associated with fluid overload.
Similarly, Muller et al reported decreased endothelial damage following resuscitation with HES compared to Ringer’s lactate in a clinical sub-study of the Scandinavian Starch for Severe Sepsis/Septic shock (6S) randomized clinical trial (RCT)68. However, overall mortality was higher and there was an increased requirement for dialysis in septic patients who received starch-based resuscitation in the 6S RCT69. This has brought to question the relevance of biomarkers of endothelial damage as surrogates of organ damage and clinical outcomes. The Australian and New Zealand Intensive Care Society Clinical Trial Group (ANZICS-CTG) did not show a difference in the 90-day mortality between HES- and saline-resuscitated critically ill patients, however there was an increased need for dialysis with HES administration70. Findings from these RCTs have resulted in limited use of starch-based solutions for resuscitation in critical illness.
Intravenous fluid resuscitation is likely to remain a cornerstone of management for both syndromes; however, more work is needed to evaluate the impact of liberal versus restrictive fluid resuscitation, optimal choice and rate of fluid administration to prevent further damage to the ailing glycocalyx, and non-invasive methods to assess changes in microvascular function in response to treatment. Aside from using glycocalyx protective or restorative intravenous fluids, interest is emerging in the glycocalyx as a target for novel host-directed therapeutic agents in infectious shock. Progress in this field has thus far been largely confined to pre-clinical studies in animals with induced sepsis. Some strategies which hold potential for translation to humans include; reducing glycocalyx breakdown by downregulating the matrix metalloproteinases responsible for SDC1 shedding (sphingosine-1-phosphate analogues71,72), inhibiting heparanase activity (modified heparins, Sulodexide73, Tie-2 agonists74,75), or accelerating glycocalyx restitution by activating enzymes such as exostosin-1, involved in the heparan sulphate synthesis pathway (fibroblast growth factor76). Progress in screening such glycocalyx-directed therapeutics for dengue has been impeded thus far due to a lack of adequate pre-clinical model for dengue-induced plasma leakage.
Although ultimately the best treatment strategies might be different, sharing lessons learned from studies on the microcirculation in septic shock and dengue shock may help to advance management and improve patient outcomes in a bi-directional manner.
Funding
No funding was received for preparation of this manuscript.
AMB is funded through a Wellcome Trust PhD Fellowship Award [203905/Z/16/Z].
NGO is funded through the DELTAS Africa Initiative [DEL-15-003]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [107769/Z/10/Z] and the UK government.
The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government.
Declarations
SY receives consultancy fees from Janssen pharmaceuticals for dengue antiviral development and as a member of the ROCHE Advisory Board on Severe Dengue.
All other authors declare no conflicts.
Authors contributions
AMB, HQC and NGO wrote the original draft of the manuscript. All authors reviewed, edited and approved submission of the manuscript.
References
- Singer et al. (2016).Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallez & Huber (2008).Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778(3):794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Yuan & Rigor (2010).Yuan SY, Rigor RR. Regulation of endothelial barrier function. edn. San Rafael (CA); 2010. [PubMed] [Google Scholar]
- Uchimido et al. (2019).Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Critical care. 2019;23(1):16. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbell & Cancel (2016).Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med. 2016;280(1):97–113. doi: 10.1111/joim.12465. [DOI] [PubMed] [Google Scholar]
- Caughey et al. (2001).Caughey GE, Cleland LG, Penglis PS, Gamble JR, James MJ. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: selective up-regulation of prostacyclin synthesis by COX-2. J Immunol. 2001;167(5):2831–2838. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- Legr et al. (2019).Legr M, De Backer D, Depret F, Ait-Oufella H. Recruiting the microcirculation in septic shock. Ann Intensive Care. 2019;9(1):102. doi: 10.1186/s13613-019-0577-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebach et al. (2005).Seebach J, Madler HJ, Wojciak-Stothard B, Schnittler HJ. Tyrosine phosphorylation and the small GTPase rac cross-talk in regulation of endothelial barrier function. Thromb Haemost. 2005;94(3):620–629. doi: 10.1160/TH05-01-0015. [DOI] [PubMed] [Google Scholar]
- Kowalczyk et al. (2015).Kowalczyk A, Kleniewska P, Kolodziejczyk M, Skibska B, Goraca A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp (Warsz) 2015;63(1):41–52. doi: 10.1007/s00005-014-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkita et al. (2012).Ohkita M, Tawa M, Kitada K, Matsumura Y. Pathophysiological roles of endothelin receptors in cardiovascular diseases. J Pharmacol Sci. 2012;119(4):302–313. doi: 10.1254/jphs.12r01cr. [DOI] [PubMed] [Google Scholar]
- Kawanabe & Nauli (2011).Kawanabe Y, Nauli SM. Endothelin. Cell Mol Life Sci. 2011;68(2):195–203. doi: 10.1007/s00018-010-0518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (1999).Wang P, Yoo P, Zhou M, Cioffi WG, Ba ZF, Chaudry IH. Reduction in vascular responsiveness to adrenomedullin during sepsis. J Surg Res. 1999;85(1):59–65. doi: 10.1006/jsre.1999.5634. [DOI] [PubMed] [Google Scholar]
- Yacoub et al. (2017).Yacoub S, Trung TH, Lam PK, Thien VHN, Hai DHT, Phan TQ, Nguyet OPK, Quyen NTH, Simmons CP, Broyd C, et al. Cardio-haemodynamic assessment and venous lactate in severe dengue. Relationship with recurrent shock and respiratory distress. PLoS neglected tropical diseases. 2017;11(7):e0005740. doi: 10.1371/journal.pntd.0005740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd et al. (2020).Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn et al. (2002).Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108(5):717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacoub et al. (2011).Yacoub S, Kotit S, Yacoub MH. Disease appearance and evolution against a background of climate change and reduced resources. Philos Trans A Math Phys Eng Sci. 2011;369(1942):1719–1729. doi: 10.1098/rsta.2011.0013. [DOI] [PubMed] [Google Scholar]
- Whitehorn & Yacoub (2019).Whitehorn J, Yacoub S. Global warming and arboviral infections. Clin Med (Lond) 2019;19(2):149–152. doi: 10.7861/clinmedicine.19-2-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2009).Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. edn. Geneva; 2009. [PubMed] [Google Scholar]
- Remick (2007).Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170(5):1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss et al. (2016).Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nature reviews Disease primers. 2016;2:16045. doi: 10.1038/nrdp.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore et al. (2015).Moore JP, Dyson A, Singer M, Fraser J. Microcirculatory dysfunction and resuscitation: why, when, and how. British journal of anaesthesia. 2015;115(3):366–375. doi: 10.1093/bja/aev163. [DOI] [PubMed] [Google Scholar]
- Yacoub et al. (2017).Yacoub S, Lam PK, Huynh TT, Nguyen Ho HH, Dong Thi HT, Van NT, Lien LT, Ha QNT, Le DHT, Mongkolspaya J, et al. Endothelial nitric oxide pathways in the pathophysiology of dengue: a prospective observational study. Clin Infect Dis. 2017;65(9):1453–1461. doi: 10.1093/cid/cix567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills et al. (2005).Wills BA, Nguyen MD, Ha TL, Dong TH, Tran TN, Le TT, Tran VD, Nguyen TH, Nguyen VC, Stepniewska K, et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. The New England journal of medicine. 2005;353(9):877–889. doi: 10.1056/NEJMoa044057. [DOI] [PubMed] [Google Scholar]
- Maitl et al. (2011).Maitl K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, et al. Mortality after fluid bolus in African children with severe infection. The New England journal of medicine. 2011;364(26):2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- Investigators et al. (2014).Investigators A, Group ACT, Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, et al. Goal-directed resuscitation for patients with early septic shock. The New England journal of medicine. 2014;371(16):1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- Pro et al. (2014).Pro CI, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, et al. A randomized trial of protocol-based care for early septic shock. The New England journal of medicine. 2014;370(18):1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouncey et al. (2015).Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, et al. Trial of early, goal-directed resuscitation for septic shock. The New England journal of medicine. 2015;372(14):1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- Wills et al. (2004).Wills BA, Oragui EE, Dung NM, Loan HT, Chau NV, Farrar JJ, Levin M. Size and charge characteristics of the protein leak in dengue shock syndrome. The Journal of infectious diseases. 2004;190(4):810–818. doi: 10.1086/422754. [DOI] [PubMed] [Google Scholar]
- Menger & Lehr (1993).Menger MD, Lehr HA. Scope and perspectives of intravital microscopy–bridge over from in vitro to in vivo. Immunol Today. 1993;14(11):519–522. doi: 10.1016/0167-5699(93)90179-O. [DOI] [PubMed] [Google Scholar]
- Maricq & LeRoy (1973).Maricq HR, LeRoy EC. Patterns of finger capillary abnormalities in connective tissue disease by “wide-field” microscopy. Arthritis Rheum. 1973;16(5):619–628. doi: 10.1002/art.1780160506. [DOI] [PubMed] [Google Scholar]
- Bollinger et al. (1974).Bollinger A, Butti P, Barras JP, Trachsler H, Siegenthaler W. Red blood cell velocity in nailfold capillaries of man measured by a television microscopy technique. Microvasc Res. 1974;7(1):61–72. doi: 10.1016/0026-2862(74)90037-5. [DOI] [PubMed] [Google Scholar]
- Jr & Watkins (1977).Holloway Jr. GA, Watkins DW. Laser Doppler measurement of cutaneous blood flow. J Invest Dermatol. 1977;69(3):306–309. doi: 10.1111/1523-1747.ep12507665. [DOI] [PubMed] [Google Scholar]
- Briers (2001).Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001;22(4):R35–66. doi: 10.1088/0967-3334/22/4/201. [DOI] [PubMed] [Google Scholar]
- van Elteren et al. (2015).van Elteren HA, Ince C, Tibboel D, Reiss IK, de Jonge RC. Cutaneous microcirculation in preterm neonates: comparison between sidestream dark field (SDF) and incident dark field (IDF) imaging. J Clin Monit Comput. 2015;29(5):543–548. doi: 10.1007/s10877-015-9708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovas et al. (2018).Rovas A, Lukasz AH, Vink H, Urban M, Sackarnd J, Pavenstadt H, Kumpers P. Bedside analysis of the sublingual microvascular glycocalyx in the emergency room and intensive care unit - the GlycoNurse study. Scandinavian journal of trauma, resuscitation and emergency medicine. 2018;26(1):16. doi: 10.1186/s13049-018-0483-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis et al. (2009).Davis JS, Yeo TW, Thomas JH, McMillan M, Darcy CJ, McNeil YR, Cheng AC, Celermajer DS, Stephens DP, Anstey NM. Sepsis-associated microvascular dysfunction measured by peripheral arterial tonometry: an observational study. Crit Care. 2009;13(5):R155. doi: 10.1186/cc8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt et al. (2016).Schmidt EP, Overdier KH, Sun X, Lin L, Liu X, Yang Y, Ammons LA, Hiller TD, Suflita MA, Yu Y, et al. Urinary glycosaminoglycans predict outcomes in septic shock and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2016;194(4):439–449. doi: 10.1164/rccm.201511-2281OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi & Akira (2010).Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Knowles & Moncada (1994).Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994;298(Pt 2):249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durante et al. (2007).Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34(9):906–911. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis & Anstey (2011).Davis JS, Anstey NM. Is plasma arginine concentration decreased in patients with sepsis? A systematic review and meta-analysis. Critical care medicine. 2011;39(2):380–385. doi: 10.1097/CCM.0b013e3181ffd9f7. [DOI] [PubMed] [Google Scholar]
- Maitland et al. (2013).Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, Akech SO, Opoka RO, Engoru C, Nyeko R, Mtove G, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC medicine. 2013;11:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews et al. (2017).Andrews B, Semler MW, Muchemwa L, Kelly P, Lakhi S, Heimburger DC, Mabula C, Bwalya M, Bernard GR. Effect of an early resuscitation protocol on in-hospital mortality among adults with sepsis and hypotension: a randomized clinical trial. JAMA. 2017;318(13):1233–1240. doi: 10.1001/jama.2017.10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne et al. (2018).Byrne L, Obonyo NG, Diab SD, Dunster KR, Passmore MR, Boon AC, Hoe LS, Pedersen S, Fauzi MH, Pimenta LP, et al. Unintended consequences: fluid resuscitation worsens shock in an ovine model of endotoxemia. Am J Respir Crit Care Med. 2018;198(8):1043–1054. doi: 10.1164/rccm.201801-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven et al. (2020).Guven G, Hilty MP, Ince C. Microcirculation: physiology, pathophysiology, and clinical application. Blood Purif. 2020;49(1–2):143–150. doi: 10.1159/000503775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerma et al. (2010).Boerma EC, Koopmans M, Konijn A, Kaiferova K, Bakker AJ, van Roon EN, Buter H, Bruins N, Egbers PH, Gerritsen RT, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Critical care medicine. 2010;38(1):93–100. doi: 10.1097/CCM.0b013e3181b02fc1. [DOI] [PubMed] [Google Scholar]
- Trzeciak et al. (2014).Trzeciak S, Glaspey LJ, Dellinger RP, Durflinger P, Anderson K, Dezfulian C, Roberts BW, Chansky ME, Parrillo JE, Hollenberg SM. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis*. Critical care medicine. 2014;42(12):2482–2492. doi: 10.1097/CCM.0000000000000549. [DOI] [PubMed] [Google Scholar]
- Simmons et al. (2012).Simmons CP, Farrar JJ, Nguyen vV, Wills B. Dengue. The New England journal of medicine. 2012;366(15):1423–1432. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- Morra et al. (2018).Morra ME, Altibi AMA, Iqtadar S, Minh LHN, Elawady SS, Hallab A, Elshafay A, Omer OA, Iraqi A, Adhikari P, et al. Definitions for warning signs and signs of severe dengue according to the WHO 2009 classification: Systematic review of literature. Rev Med Virol. 2018;28(4):e1979. doi: 10.1002/rmv.1979. [DOI] [PubMed] [Google Scholar]
- Beatty et al. (2015).Beatty PR, Puerta-Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med. 2015;7(304):304ra141. doi: 10.1126/scitranslmed.aaa3787. [DOI] [PubMed] [Google Scholar]
- Glasner et al. (2017).Glasner DR, Ratnasiri K, Puerta-Guardo H, Espinosa DA, Beatty PR, Harris E. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 2017;13(11):e1006673. doi: 10.1371/journal.ppat.1006673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puerta-Guardo et al. (2016).Puerta-Guardo H, Glasner DR, Harris E. Dengue Virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 2016;12(7):e1005738. doi: 10.1371/journal.ppat.1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson et al. (1997).Anderson R, Wang S, Osiowy C, Issekutz AC. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J Virol. 1997;71(6):4226–4232. doi: 10.1128/jvi.71.6.4226-4232.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolsapaya et al. (2003).Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nature medicine. 2003;9(7):921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- Duangchinda et al. (2010).Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, Mongkolsapaya J, Screaton G. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(39):16922–16927. doi: 10.1073/pnas.1010867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butthep et al. (2012).Butthep P, Chunhakan S, Yoksan S, Tangnararatchakit K, Chuansumrit A. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic Fever. The Pediatric infectious disease journal. 2012;31(12):e232–238. doi: 10.1097/INF.0b013e31826fd456. [DOI] [PubMed] [Google Scholar]
- Yacoub et al. (2016).Yacoub S, Lam PK, Vu le HM, Le TL, Ha NT, Toan TT, Van NT, Quyen NT, Le Duyen HT, Van Kinh N.et al.2016Association of microvascular function and endothelial biomarkers with clinical outcome in dengue: an observational study The Journal of infectious diseases 2145697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwarto et al. (2017).Suwarto S, Sasmono RT, Sinto R, Ibrahim E, Suryamin M. Association of endothelial glycocalyx and tight and adherens junctions with severity of plasma leakage in dengue infection. J Infect Dis. 2017;215(6):992–999. doi: 10.1093/infdis/jix041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride et al. (2020).McBride A, Khanh Lam P, Huynh Thi Le D, Trung TH, Vink H, Wills B, Yacoub S. Visual and biochemical evidence of glycocalyx disruption in human dengue infection, and association with plasma leakage severity. Frontiers in Medicine. 2020;7:642. doi: 10.3389/fmed.2020.545813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewi et al. (2008).Dewi BE, Takasaki T, Kurane I. Peripheral blood mononuclear cells increase the permeability of dengue virus-infected endothelial cells in association with downregulation of vascular endothelial cadherin. J Gen Virol. 2008;89(Pt 3):642–652. doi: 10.1099/vir.0.83356-0. [DOI] [PubMed] [Google Scholar]
- Kanlaya et al. (2009).Kanlaya R, Pattanakitsakul SN, Sinchaikul S, Chen ST, Thongboonkerd V. Alterations in actin cytoskeletal assembly and junctional protein complexes in human endothelial cells induced by dengue virus infection and mimicry of leukocyte transendothelial migration. J Proteome Res. 2009;8(5):2551–2562. doi: 10.1021/pr900060g. [DOI] [PubMed] [Google Scholar]
- McBride et al. (2019).McBride A, Thuy Duong B, Chau Nguyen VV, Thwaites CL, Turner HC, Hao Nguyen V. Catastrophic health care expenditure due to septic shock and dengue shock in Vietnam. Trans R Soc Trop Med Hyg. 2019;113(10):649–651. doi: 10.1093/trstmh/trz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam et al. (2013).Lam PK, Tam DT, Diet TV, Tam CT, Tien NT, Kieu NT, Simmons C, Farrar J, Nga NT, Qui PT, et al. Clinical characteristics of Dengue shock syndrome in Vietnamese children: a 10-year prospective study in a single hospital. Clin Infect Dis. 2013;57(11):1577–1586. doi: 10.1093/cid/cit594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar et al. (2011).Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood-Watson et al. (2011).Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang W, Zaske AM, Menge T, Kozar RA. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar & Pati (2015).Kozar RA, Pati S. Syndecan-1 restitution by plasma after hemorrhagic shock. The journal of trauma and acute care surgery. 2015;78(6 Suppl 1):S83–86. doi: 10.1097/TA.0000000000000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm et al. (2004).Rehm M, Zahler S, Lotsch M, Welsch U, Conzen P, Jacob M, Becker BF. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100(5):1211–1223. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- Muller et al. (2016).Muller RB, Ostrowski SR, Haase N, Wetterslev J, Perner A, Johansson PI. Markers of endothelial damage and coagulation impairment in patients with severe sepsis resuscitated with hydroxyethyl starch 130/0.42 vs Ringer acetate. Journal of critical care. 2016;32:16–20. doi: 10.1016/j.jcrc.2015.11.025. [DOI] [PubMed] [Google Scholar]
- Perner et al. (2012).Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, et al. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. The New England journal of medicine. 2012;367(2):124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- Myburgh et al. (2012).Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass P, Lipman J, Liu B, McArthur C, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. The New England journal of medicine. 2012;367(20):1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- Jiang et al. (2017).Jiang H, Shen SM, Yin J, Zhang PP, Shi Y. Sphingosine 1-phosphate receptor 1 (S1PR1) agonist CYM5442 inhibits expression of intracellular adhesion molecule 1 (ICAM1) in endothelial cells infected with influenza A viruses. PLoS One. 2017;12(4):e0175188. doi: 10.1371/journal.pone.0175188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna et al. (2006).Sanna MG, Wang SK, Gonzalez-Cabrera PJ, Don A, Marsolais D, Matheu MP, Wei SH, Parker I, Jo E, Cheng WC, et al. Enhancement of capillary leakage and restoration of lymphocyte egress by a chiral S1P1 antagonist in vivo. Nat Chem Biol. 2006;2(8):434–441. doi: 10.1038/nchembio804. [DOI] [PubMed] [Google Scholar]
- Song et al. (2017).Song JW, Zullo JA, Liveris D, Dragovich M, Zhang XF, Goligorsky MS. Therapeutic restoration of endothelial glycocalyx in sepsis. J Pharmacol Exp Ther. 2017;361(1):115–121. doi: 10.1124/jpet.116.239509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpers et al. (2011).Kumpers P, Gueler F, David S, Slyke PV, Dumont DJ, Park JK, Bockmeyer CL, Parikh SM, Pavenstadt H, Haller H, et al. The synthetic tie2 agonist peptide vasculotide protects against vascular leakage and reduces mortality in murine abdominal sepsis. Crit Care. 2011;15(5):R261. doi: 10.1186/cc10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han et al. (2016).Han S, Lee SJ, Kim KE, Lee HS, Oh N, Park I, Ko E, Oh SJ, Lee YS, Kim D, et al. Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci Transl Med. 2016;8(335):335ra355. doi: 10.1126/scitranslmed.aad9260. [DOI] [PubMed] [Google Scholar]
- Rizzo & Dudek (2017).Rizzo AN, Dudek SM. Endothelial glycocalyx repair: building a wall to protect the lung during sepsis. Am J Respir Cell Mol Biol. 2017;56(6):687–688. doi: 10.1165/rcmb.2017-0065ED. [DOI] [PMC free article] [PubMed] [Google Scholar]